The COVID-19 pandemic has claimed over 150,000 lives worldwide. Infection with SARS-CoV-2 results in a broad spectrum of disease, with in excess of 80% of patients having few or no symptoms. What is striking about COVID-19 is the enormous variation in the reported case fatality rate between countries and between regions in the same country. While these differences may in part be related to variations in case definitions, reporting and surveillance, they may also be attributed to underlying physiological reasons. It is likely that a number of factors, including age, co-morbidities, race, access to healthcare and genetic factors (and the complex interactions between these factors), determine the clinical course after exposure to SARS-CoV-2. We postulate that vitamin D status may influence the risk of dying from SARS-CoV-2.
Vitamin D deficiency is a major global public health problem in all age groups [1]. It has been estimated that in excess of one billion people worldwide have vitamin D deficiency [2]. Very few foods naturally contain vitamin D; dermal synthesis is the major source of the vitamin. Vitamin D3 is synthesized non-enzymatically in skin during exposure to ultraviolet B (UVB) radiation in sunlight. Vitamin D3 is inactive and requires enzymatic conversion in the liver and kidney to form the active form, 1,25-dihydroxyvitamin D. Increased skin pigmentation reduces the efficacy of UVB because melanin functions as a natural sunblock. In addition, aging decreases the ability of the skin to produce vitamin D3 [3]. During the winter months at latitudes of >40°, little or no UVB radiation reaches the surface of the earth. Therefore, residence at high latitude increases the risk of vitamin D deficiency during the winter. This is likely compounded by age and skin pigmentation. However, residence at low latitude does not guarantee adequate vitamin D levels. Social and cultural norms may limit sun exposure. Vitamin D deficiency is particularly common in Middle Eastern girls and women [1]. Furthermore, despite abundant sunlight throughout the year in Ecuador, vitamin D deficiency was reported to be common among elderly women [4].
Vitamin D is a pluripotent hormone that modulates the innate and adaptive immune response [5]. Vitamin D influences several immune pathways, with the net effect of boosting mucosal defenses while simultaneously dampening excessive inflammation [6,7]. Vitamin D deficiency is a risk factor for and/or a driver of the exaggerated and persistent inflammation that is a hallmark of acute respiratory distress syndrome (ARDS) [8,9]. Vitamin D deficiency has been associated with an increased risk of respiratory infections such as respiratory syncytial virus infection, tuberculosis and influenza [10,11]. The winter incidence of influenza closely correlates with seasonal serum vitamin D levels [12]. In a meta-analysis of randomized controlled clinical trial, Bergman and colleagues demonstrated that prophylactic vitamin D reduced the risk of developing respiratory tract infections (OR, 0.64; 95%; CI, 0.49 to 0.84) [13]. In this study, the optimal dose was between 1000 IU to 4000 IU/day and the benefit was greatest in those living at latitudes greater than 40o. Vitamin D deficiency likely adversely affects the outcome of viral infections. Grant and Giovannucci reported a strong inverse correlation between UVB dose and the case fatality during the 1918–1919 influenza pandemic [14]. As vitamin D deficiency enhances the cytokine storm [6,7], it may be particularly lethal in patients with SARS-CoV-2 infection.
The United States is a vast country extending from 30o latitude in the South to 50o latitude in the North. Based on publically available data (COVID-19 Dashboard by the Center for Systems Science and Engineering at Johns Hopkins University), we calculated the case fatality rate (CFR, i.e., number of deaths/reported number of confirmed cases) for each of the 50 states in the USA (Fig. 1 ). This figure tends to show an increasing mortality with increasing latitude. Furthermore, the cumulative summary case fatality rate was significantly greater for Northern states (> 40o latitude) as compared to Southern States (6.0% vs. 3.5%, P < .001). However, this association is imperfect, with some Northern Sates (i.e., Wyoming and South Dakota) having low mortality rates while Louisiana has a high rate. Additional factors, such as racial makeup, population density, adherence to social distancing, use of vitamin supplements, and access to quality medical care, etc., likely play an additional role in explaining these geographical variations. In addition, we have assumed that the difference of test methods and statistics of all states are statistically insignificant; this assumption may not be entirely correct.
Fig. 1.
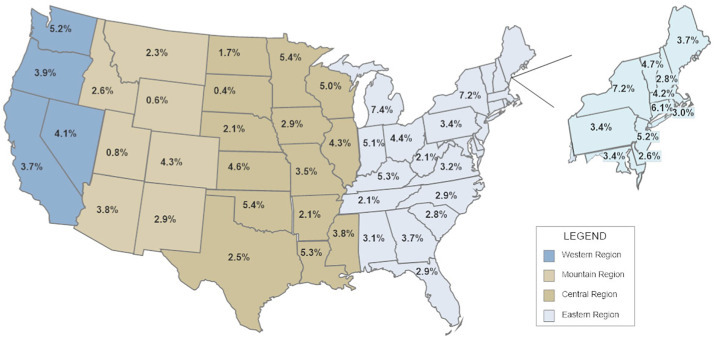
The reported case fatality rate (number of deaths/number of confirmed cases) for each of the 50 states in the USA as reported on 4/19/2020 by the Center for Systems Science and Engineering at Johns Hopkins University.
Our data are supported by the paper by Rhodes et al. They tabulated the mortality for COVID-19 around the world and demonstrated that the mortality was relatively low for countries below 35o latitude [15]. Similarly, Daneshkhah and coworkers demonstrated that the age-specific case fatality rate of COVID-19 was highest in Italy, Spain, and France, European countries with the highest incidence severe vitamin D deficiency [16]. Our findings suggest that vitamin D deficiency may partly explain the geographic variations in the reported case fatality rate of COVID-19, implying that supplementation with vitamin D may reduce the mortality from this pandemic. However, as commonly suggested in the lay press, high-dose vitamin D appears to have a limited role in the treatment of patients with severe COVID-19 disease. The National Heart, Lung, and Blood Institute (NHBLI) performed a randomized controlled trial evaluating the role of high dose vitamin D (single dose of 540,000 IU of vitamin D3) in critically ill patients who were vitamin D deficient (25-hydroxyvitamin D level < 50 nmol/l) [17], the study failed to demonstrate any benefit from high dose vitamin D. This implies that higher doses than common recommendations are not supported by clinical evidence at present; therefore we advise a vitamin D dosage at what would be considered a standard nutritional supplement that may be sufficient in providing clinical benefits. Additional studies are required to further validate our hypothesis and translate this into an effective intervention for COVID-19.
Conflict of Interest
The authors have no conflict of interest to declare.
Footnotes
For the Front Line COVID-19 Critical Care Working Group.
References
- 1.Palacios C., Gonzalez L. Is vitamin D deficiency a major global health problem. J Steroid Biochem Mol Biol. 2014;144:138–145. doi: 10.1016/j.jsbmb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van schoor N.M., Lips P. Worldwide vitamin D status. Best Pract Res Clin Endocrinol Metab. 2011;25:671–680. doi: 10.1016/j.beem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin J., Holick M.F. Aging decreases the capacity of human skin to produce Vitamin D3. J Clin Invest. 1985;76:1536–1538. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orces C.H. Vitamin D status among older adults residing in Littoral and Andes Mountains in Ecuador. Scientific World Journal. 2015;2015:545297. doi: 10.1155/2015/545297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rezaei R. Immunomodulatory effects of Vitamin D in influenza infection. Curr Immunol Rev. 2018;14:40–49. [Google Scholar]
- 6.Khare D., Godbole N.M., Pawar S.D. Calcitriol [1, 25[OH]2 D3] pre- and post-treatment suppresses inflammatory response to influenza A (H1N1) infection in human lung A549 epithelial cells. Eur J Nutr. 2013;52:1405–1415. doi: 10.1007/s00394-012-0449-7. [DOI] [PubMed] [Google Scholar]
- 7.Parlak E., Erturk A., Cag E. The effect of inflammatory cytokines and the level of vitamin D on prognosis in Crimean-Congo hemorrhagic fever. Int J Clin Exp Med. 2015;8:18302–18310. [PMC free article] [PubMed] [Google Scholar]
- 8.Dancer R.C., Parekh D., Lax S. Vitamin D deficiency conributes directly to the acute respiratory distress syndrome (ARDS) Thorax. 2015;70:617–624. doi: 10.1136/thoraxjnl-2014-206680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parekh D., Thickett D.R., Turner A.M. Vitamin D deficiency and acute lung injury. Inflamm Allergy Drug Targets. 2013;12:253–261. doi: 10.2174/18715281113129990049. [DOI] [PubMed] [Google Scholar]
- 10.Berry D.J., Hesketh K., Power C. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br J Nutr. 2011;106:1433–1440. doi: 10.1017/S0007114511001991. [DOI] [PubMed] [Google Scholar]
- 11.Cannell J.J., Vieth R., Umhau J.C. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginde A.A., Mansbach J.M., Camargo C.A. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergman P., Lindh A.U., Bjorkhem-Bergman L. Vitamin D and respiartory tract infections: A systematic review and meta-analysis of randomized controlled trials. PloS ONE. 2013;8 doi: 10.1371/journal.pone.0065835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant W.B., Giovannucci E. The possible roles of solar ulraviolet-B radiation and vitamin D in reducing case fatality rates from the 1918-1919 influenza pandemic in the United States. Dermato-Endocrinology. 2009;1:215–219. doi: 10.4161/derm.1.4.9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhodes J.M., Subramanian S., Laird E. Editorial: low population mortality from COVID-19 in countries south of 35 degrees North - supports vitamin D as a factor determining severity. Aliment Pharmacol Ther. 2020 doi: 10.1111/apt.15777. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daneshkhah A., Eshein A., Subramanian H. The role of vitamin D in suppressing cytokine storm of COVID-19 patients and associated mortality. medRxiv. 2020 [Google Scholar]
- 17.Early high-dose vitamin D3 for critically ill, vitamin D-deficient patientsN Engl J Med. 2019;381:2529–2540. doi: 10.1056/NEJMoa1911124. [DOI] [PMC free article] [PubMed] [Google Scholar]


