Dear Editor,
We read with interest the recent report on the development of antibodies to SARS-CoV-2 [1]. Such studies provide clear evidence of seroconversion in the majority of patients hospitalized with COVID-19, and kinetics of IgM and IgG antibody development similar to that of other respiratory virus infections. While such reports provide specific antibody measurements, they have not assessed possible cross-reactive antibodies that have been recognised for some time [2], [3], [4].
Recent reports raise concerns about the possibility of vaccine-related antibody-dependent enhancement (ADE) between the various coronaviruses, including SARS-CoV-2 [5], [6], [7]. According to these concerns, there is a potential risk of enhanced pathology caused by interactions between non-neutralising antibodies binding to the spike of SARS-CoV-2. Such antibodies could be induced by certain vaccines, or – in theory- from seasonal or ‘common cold’ coronavirus infections [5], [6], [7] – the latter of which are currently mostly relatively benign and self-limiting [8]. Whether these concerns are valid remains to be determined, but it is clear that understanding the potential interactions will be important in the months and years to come.
Here we present some older and more recent seasonal coronavirus positivity data, compared with contemporaneous seasonal influenza A and B, to set the epidemiological context for SARS-CoV-2 immunological responses in new host populations. The data was obtained from a larger study from diagnostic laboratories from various countries (Vancouver, Edmonton, Halifax, Canada; Leicester, UK; Rotterdam, The Netherlands; Ulaanbaatar, Mongolia; Kuala Lumpur, Malaysia; Singapore; Christchurch, New Zealand), where seasonal coronaviruses are tested routinely in their respiratory PCR panels.
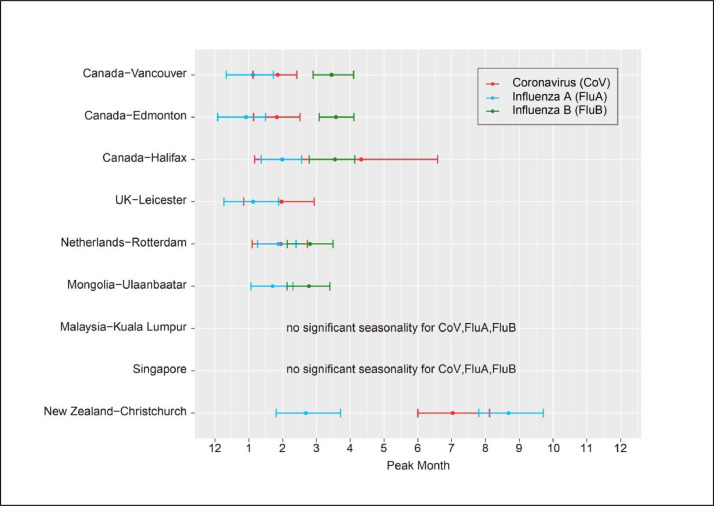
The data has been plotted as monthly %positive samples. Fig. 1 summarises and compares the seasonality of the seasonal coronaviruses across these countries. Fig. 2 gives an example of the individual country wavelet plots, with additional Online Material showing additional wavelet (Figure S1) and linear (Figure S2) plots for individual viruses at each site, as previously described [9].
Fig. 1.
Comparative seasonality of influenza A, B and coronavirus infections across seven countries. The absence of the influenza B coloured bars in the data UK-Leicester and New Zealand-Christchurch is due to the absence of a regular peak for this virus over the samplnig period.
Fig. 2.
Example of wavelet plot for Leicester UK, 2016-2019 (an expandable higher resolution version is available online, Figure S1D). Note that the timing of the major peak for influenza A (Flu A), B (Flu B) and CoV (seasonal coronaviruses: CoV-OC43, CoV-229E, CoV-NL63, CoV-HKU1) are virtually coincident during this period, but that the CoV signal is dominated by the peak positivity of CoV-OC43.
The wavelet plots offer a higher degree of temporal resolution and highlight major and minor periods of enhanced periodicity for each virus. Although there is often a major peak (marked in red in the Figure S1 panels), there are usually multiple minor peaks around this, which show that these viral infections can be ongoing over a longer period though at lower levels. Despite this and the variability in the overall period of data capture for each site (mainly due to the availability of a multiplex respiratory virus PCR containing the coronavirus targets), there are several fairly clear seasonal patterns.
In most temperate sites (Canada, UK, The Netherlands, New Zealand), the coronavirus peak occurs either slightly before or simultaneously with the influenza A and B peaks. In Singapore, there is no consistent relationship between the influenza A, B and coronavirus peaks. In Mongolia and Kuala Lumpur, the coronavirus peak appears to precede the influenza A peaks by about 4 months.
The potential for ADE has been recognised with previous viral vaccines, particularly with RSV (respiratory syncytial virus) and dengue [10]. Further investigations into these issues are needed as the SARS-CoV-2 trial vaccines go through their testing phases. If there is a potential ADE issue with any SARS-CoV-2 vaccine with the common cold coronaviruses, recognising and understanding their local circulation patterns can help us avoid SARS-CoV-2 vaccination at times when such ADE complications may be worst – especially where such complications are correlated to specific levels of vaccine-induced SARS-CoV-2 antibodies that may wane over time.
Declaration of Competing Interest
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2020.04.032.
Appendix. Supplementary materials
References
- 1.Xiao D.A.T., Gao D.C., Zhang D.S. Profile of Specific Antibodies to SARS-CoV-2: The First Report. J Infect. 2020 Mar 21 doi: 10.1016/j.jinf.2020.03.012. pii: S0163-4453(20)30138-9[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan K.H., Cheng V.C., Woo P.C., Lau S.K., Poon L.L., Guan Y., Seto W.H., Yuen K.Y., Peiris J.S. Serological responses in patients with severe acute respiratory syndrome coronavirus infection and cross-reactivity with human coronaviruses 229E, OC43, and NL63. Clin Diagn Lab Immunol. Nov 2005;12(11):1317–1321. doi: 10.1128/CDLI.12.11.1317-1321.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Che X.Y., Qiu L.W., Liao Z.Y., Wang Y.D., Wen K., Pan Y.X., Hao W., Mei Y.B., Cheng V.C., Yuen K.Y. Antigenic cross-reactivity between severe acute respiratory syndrome-associated coronavirus and human coronaviruses 229E and OC43. J Infect Dis. 2005 Jun 15;191(12):2033–2037. doi: 10.1086/430355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer B., Drosten C., Müller M.A. Serological assays for emerging coronaviruses: challenges and pitfalls. Virus Res. 2014 Dec 19;194:175–183. doi: 10.1016/j.virusres.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 Vaccines at Pandemic Speed. N Engl J Med. 2020 Mar 30 doi: 10.1056/NEJMp2005630. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Tetro J.A. Is COVID-19 receiving ADE from other coronaviruses? Microbes Infect. Mar 2020;22(2):72–73. doi: 10.1016/j.micinf.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peeples L.Proceedings of the National Academy of Sciences (USA). News Feature: Avoiding pitfalls in the pursuit of a COVID-19 vaccine. https://www.pnas.org/content/early/2020/03/27/2005456117 [DOI] [PMC free article] [PubMed]
- 8.Lau S.K., Woo P.C., Yip C.C., Tse H., Tsoi H.W., Cheng V.C., Lee P., Tang B.S., Cheung C.H., Lee R.A., So L.Y., Lau Y.L., Chan K.H., Yuen K.Y. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol. Jun 2006;44(6):2063–2071. doi: 10.1128/JCM.02614-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam T.T., Tang J.W., Lai F.Y., Zaraket H., Dbaibo G., Bialasiewicz S., Tozer S., Heraud J.M., Drews S.J., Hachette T., Chan P.K., Koay E.S., Lee H.K., Tee K.K., Liu Y., Fraaij P., Jennings L., Waris M., Krajden M., Corriveau A., Jalal H., Nishimura H., Nymadawa P., Badarch D., Watanabe A., Kabanda A., Sloots T., Kok J., Dwyer D.E., Koopmans M. INSPIRE (International Network for the Sequencing of Respiratory Viruses). Comparative global epidemiology of influenza, respiratory syncytial and parainfluenza viruses, 2010-2015. J Infect. Oct 2019;79(4):373–382. doi: 10.1016/j.jinf.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ubol S., Halstead S.B. How innate immune mechanisms contribute to antibody-enhanced viral infections. Clin Vaccine Immunol. Dec 2010;17(12):1829–1835. doi: 10.1128/CVI.00316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.