Abstract
This study uses data from the publicly available Centers for Medicare & Medicaid Services (CMS) Inventory Tool to determine the proportion of CMS quality measures that are used or finalized for use in a CMS program, under development or consideration for use, or not in use.
In the US, the Centers for Medicare & Medicaid Services (CMS) is increasingly tying reimbursement to the value of care, resulting in the rapid proliferation of quality measures to evaluate clinician and health system performance. Quality measurement can improve patient care, but there is growing concern that many measures may not be meaningful and that the administrative and financial burden placed on clinicians to report quality measures is substantial.1,2,3 Less is known about the current landscape of CMS quality measures and the magnitude of spending on measure development. Understanding this landscape is important for efforts that aim to enhance the value of quality measurement and improvement.
Therefore, this study aimed to answer 3 questions. First, how many quality measures are currently available in the inventory of the CMS? Second, how many of these measures are used in CMS programs, are under development or consideration, or are not in use? Third, how much has the CMS invested in the development of quality measures?
Methods
The publicly available CMS Inventory Tool, which includes a compilation of CMS quality measures, was reviewed as of December 2019 to determine the percentage of measures that (1) had been implemented or finalized for use in a CMS program, (2) were under development, proposal, or consideration for use, or (3) were not in use. We also characterized the domains of quality assessed by measures that have been implemented or finalized in CMS programs.
Data from USAspending.gov were used to quantify federal spending on measure development and maintenance. We identified Measure and Instrument Development and Support contracts that were awarded to organizations by the CMS from 2008 to 2018, and estimated total spending in 2018 US dollars by adjusting total contract amounts for inflation.
Results
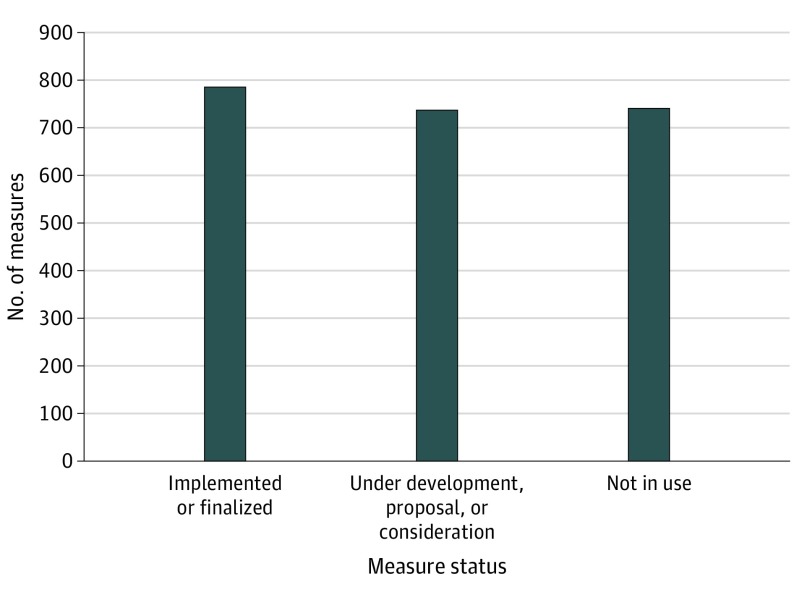
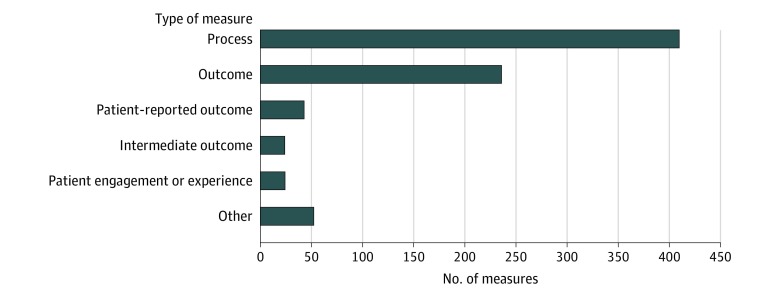
There were 2266 quality measures in the CMS Inventory Tool, of which 788 (34.8%) have been implemented or finalized for use in a CMS program and 738 (32.6%) are being developed for eventual use, have been proposed for possible use, or have been accepted for consideration by a CMS initiative (Figure 1). The remaining 740 quality measures (32.7%) had not been implemented or finalized for use, had been removed from or not accepted by a CMS program, or were no longer being developed. The 788 implemented or finalized quality measures spanned 34 CMS programs, and were most commonly process (n = 409) or outcome (n = 236) measures (Figure 2).
Figure 1. Status of Quality Measures Developed for the Centers for Medicare & Medicaid Services (CMS).
The current status of quality measures was determined based on definitions provided by the CMS Inventory Tool. Implemented or finalized indicates measures that are currently used within a CMS incentive, reimbursement, or performance program or finalized per federal rule for use in a CMS program. Under development, proposal, or consideration indicates measures that are currently being developed for eventual use in a CMS program, have been introduced in a published proposed rule for potential use in a CMS program if eventually finalized in the federal rulemaking process, or have been submitted to the prerulemaking process and accepted for consideration by a CMS program. Not in use indicates measures that were introduced in a published proposed rule but were not finalized for use in a CMS program, submitted but not accepted by a CMS program through the prerulemaking process, removed from a CMS program via federal rule and are no longer implemented, or are no longer being developed for use in a CMS program or initiative.
Figure 2. Types of Quality Measures Implemented or Finalized for Use in Centers for Medicare & Medicaid Services (CMS) Programs.
The number of quality measures that are used or finalized for use in CMS programs according to the domain of quality that they assess, as listed in the CMS Inventory Tool. Process measures are defined as an action or intervention that reflects guidelines, standards of care, or practice parameters performed during the delivery of patient care (eg, aspirin on arrival for acute myocardial infarction). Outcome measures are defined as changes in health or quality of life that result from care (eg, 30-day mortality). Patient-reported outcome measures are defined as indicators of functional status reported by a patient to their health care clinician (eg, change in functional status following total knee replacement). Intermediate outcome measures are defined as a factor or short-term result that contributes to an ultimate outcome (eg, controlling high blood pressure or hemoglobin A1c). Patient engagement or experience measures are defined as patients’ experience and satisfaction with their health care clinicians, the health care system, or both. Other includes all remaining measure categories, including access (eg, call center foreign language availability), communication and care coordination (eg, medication reconciliation for patients receiving care at dialysis facilities), composite (eg, patient safety indicator), cost or resource (eg, payment associated with a 30-day episode of care for heart failure), efficiency (eg, mammography follow-up rates), and structure (eg, use of an electronic health record).
The total inflation-adjusted amount of money awarded by the CMS between 2008 and 2018 to develop and maintain quality measures was $1 313 500 000. Thirty-five organizations received award contracts, and the top 5 organizations were awarded $872.9 million.
Discussion
Between 2008 and 2018, the CMS has invested more than $1.3 billion in quality measure development. Approximately 2300 measures have been developed, of which 788 are being used in CMS quality, reporting, and payment programs. A recent appraisal of one of these initiatives, the Quality Payment Program, revealed that only 37% of its ambulatory medicine measures were valid, highlighting the need to examine the validity of other quality measures used in CMS programs.2
In addition, the CMS currently lacks a strategy to systematically evaluate whether their quality measures improve the delivery of care and health outcomes.4 Recent evidence suggests that tying some quality measures to payment incentives may have led to unintended consequences.5,6 Therefore, the CMS should couple the implementation of measures with independent evaluations of their effects to ensure that the CMS is achieving its strategic objectives.4
Although some quality measures might not be implemented and others may be removed as clinical practice changes, the high rate at which this occurs suggests an opportunity to improve the development process. The federal government has allocated most of the $1.3 billion in funding to 5 organizations; increasing the diversity of organizations that receive award contracts could promote competition to improve the quality of developed measures.
This study likely underestimated total spending because contract information was not available for 11 organizations that received award contracts and other costs associated with measure development (eg, technology, management, or administration) may not have been captured.
Section Editor: Jody W. Zylke, MD, Deputy Editor.
References
- 1.Blumenthal D, McGinnis JM. Measuring Vital Signs: an IOM report on core metrics for health and health care progress. JAMA. 2015;313(19):1901-1902. doi: 10.1001/jama.2015.4862 [DOI] [PubMed] [Google Scholar]
- 2.MacLean CH, Kerr EA, Qaseem A. Time out: charting a path for improving performance measurement. N Engl J Med. 2018;378(19):1757-1761. doi: 10.1056/NEJMp1802595 [DOI] [PubMed] [Google Scholar]
- 3.Casalino LP, Gans D, Weber R, et al. US physician practices spend more than $15.4 billion annually to report quality measures. Health Aff (Millwood). 2016;35(3):401-406. doi: 10.1377/hlthaff.2015.1258 [DOI] [PubMed] [Google Scholar]
- 4.US Government Accountability Office Health care quality: CMS could more effectively ensure its quality measurement activities promote its objectives. Accessed December 20, 2019. https://www.gao.gov/assets/710/701512.pdf
- 5.Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C, Yeh RW. Association of the hospital readmissions reduction program with mortality among Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA. 2018;320(24):2542-2552. doi: 10.1001/jama.2018.19232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joynt Maddox KE. Financial incentives and vulnerable populations: will alternative payment models help or hurt? N Engl J Med. 2018;378(11):977-979. doi: 10.1056/NEJMp1715455 [DOI] [PubMed] [Google Scholar]