Patients presenting with recurrence of anginal symptoms after saphenous vein bypass surgery pose an increasingly frequent challenge. In general, approximately 40% to 50% of saphenous vein graft (SVG) will become diseased or occluded within the first ten years after surgery.[1] Because adverse clinical outcomes, such as high restenosis rates and distal embolization, have been reported, percutaneous coronary intervention (PCI) for SVG is not recommended as a first-line strategy.[2] Distal embolization occurs in 2%–17% of patients despite advances in therapy, including the utilization of embolic protection device (EPD), which may lead to increased mortality at both short follow-up and midterm follow-up.[3] However, these patients often associated with inappropriate anatomic characteristics of native vessel intervention including calcification, tortuosity, abundant plaque burden, and complex chronic total occlusion (CTO) lesions. Moreover, repeat coronary bypass surgery is an option but is technically more demanding and is associated with a higher mortality. Therefore, it can sometimes result in SVG stenosis being the only intervention option. This is why the recanalization of SVG lesions, especially totally occluded lesions, remains one of the most challenging procedures in interventional cardiology.
Excimer laser device was introduced three decades ago and first received Food and Drug Administration (FDA) approval in 1992. In previous studies, excimer laser coronary atherectomy (ELCA) has shown to be an effective PCI treatment for complex coronary lesions, including CTO, calcified lesions, stent restenosis, balloon-crossing failures, rich thrombus in acute coronary syndrome and diseased SVG. Recently, excimer laser therapy has shown to be an efficient and safe therapy for SVG disease in patients who routinely use EPD.[4] However, data on the use of excimer laser therapy for SVG lesions without the use of EPD, especially in patients in whom EPD cannot be used, are limited. The optimal therapy for SVG lesions is an evolving issue and various interventional technologies have been applied to these lesions including excimer laser angioplasty.
Here, we present three cases of totally occluded SVG treated by ELCA without the application of EPD and were finally treated with drug-eluting stent (DES). Coronary dissection and no-reflow phenomena were not observed during the procedure, and there were no occurrence of major adverse cardiovascular events during hospitalization.
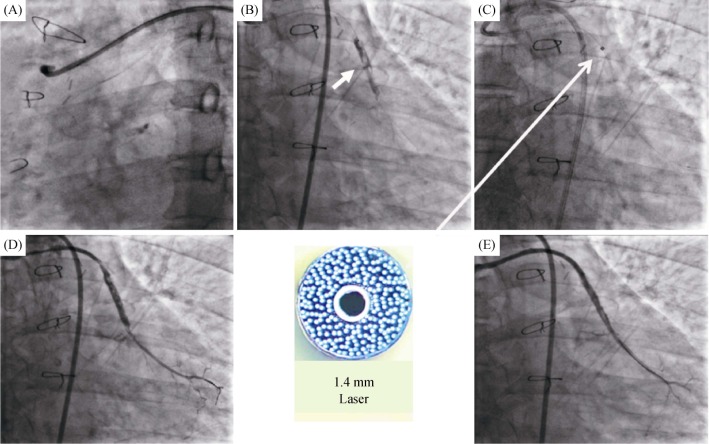
Case 1. A 57-year-old man with a history of hypertension was admitted to our hospital due to sustained chest pain for 12 h, he was diagnosed with non-ST elevation myocardial infarction (NSTEMI). Cardiac troponin T levels were as high as 4.18 ng/mL (Reference: 0.00–0.014 ng/mL) during hospitalization. An electrocardiogram suggested T wave inversions of the II, III, and aVF leads, and an echocardiographic examination showed a normal left ventricular ejection fraction and hypokinesis in the inferolateral wall. The patient underwent coronary artery bypass graft (CABG) surgery in 2007. Coronary angiography revealed acute total occlusion of the ostial SVG to the obtuse marginal (OM) branch, the left internal mammary artery (LIMA) and right coronary artery (RCA) graft were unobstructed. The blockage of the native left coronary artery (LCX) was without suitable collateralization to supply its distal bed and was considered a difficult revascularization (Figure 1A). There was angiographic evidence that the SVG to the OM branch was the culprit for the current myocardial infarction. The PCI was performed using a 7Fr JR3.5 (Judkins Right) guiding catheter with a right femoral approach. Intravenous heparin was administered to maintain an activated clotting time of > 350 s. The preferred Finecross130 cm microcatheter + Fielder XT-R guidewire (Asahi, Tokyo, Japan) were positioned into the distal segment of SVG. The true lumen position was detected by blood aspiration and dye injection through the microcatheter. The tip injection showed the SVG contained a substantial thrombus in the proximal middle of the SVG (Figure 1B).
Figure 1. Example use of the excimer laser coronary atherectomy in the SVG to the OM branch.
(A): Coronary angiogram showing the total occlusion of the SVG to the OM branch; (B): dye injection revealed a heavy thrombus burden (arrow); (C): the 1.4 mm laser catheter was inserted and the laser ablation was performed (arrow); and (D & E): the final angiogram. OM: obtuse marginal; SVG: saphenous vein graft.
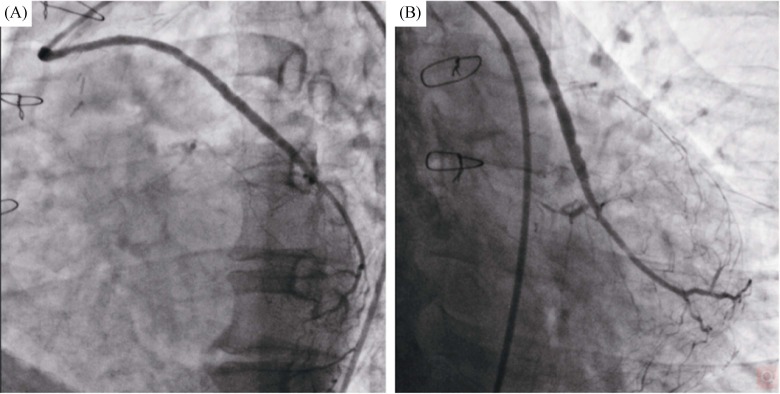
Then, the 1.4 mm laser catheter (Spectranetics CVX-300, Colorado Springs, CO, USA) was recommended for the initial fluence was 45 MJ/mm2, and the repetition rate was 25 Hz (Figure 1C). The fluence and repetition rates were increased at the discretion of the operator when the laser catheter could not be advanced, and the final repetition rates were up to 60 MJ/mm2, which can obtain optimal ablation. After predilation with the 2.0 mm × 15 mm balloon (Ryujin, Terumo, Tokyo, Japan) in the proximal SVG, two DESs (2.5 mm × 36 mm & 3.3 mm × 33 mm) (Xience Xpedition, Abbott Vascular, Santa Clara, USA) were successfully implanted. A gentle angiogram showed blood flow TIMI grade 2 or 3 was achieved. However, a residual thrombus in the middle SVG was found in Figure 1D. Repeat laser ablation 20 times with the fluence and repetition rates of 60 MJ/mm2 and 40 Hz, one additional stent (Xience Xpedition, 2.75 mm × 28 mm) was deployed in the middle of the lesion. After postdilation with the 3.0 mm × 12 mm noncompliant balloon (Quantum Maverick, Boston Scientific, Natick, USA). Repeat angiography showed blood flow TIMI grade 3, and coronary dissection and perforation were not founded (Figure 1E). There were no occurrence of re-elevation of troponin and major adverse cardiovascular events during hospitalization. At the 6-month follow-up, no recurrent angina or stenosis of the great artery was documented (Figure 2).
Figure 2. The 6-month follow-up angiography of the SVG to the OM branch.
(A): The left anterior oblique 45° view of the SVG to the OM branch angiogram; and (B): the caudal view of the SVG to the OM branch angiogram. OM: obtuse marginal; SVG: saphenous vein graft.
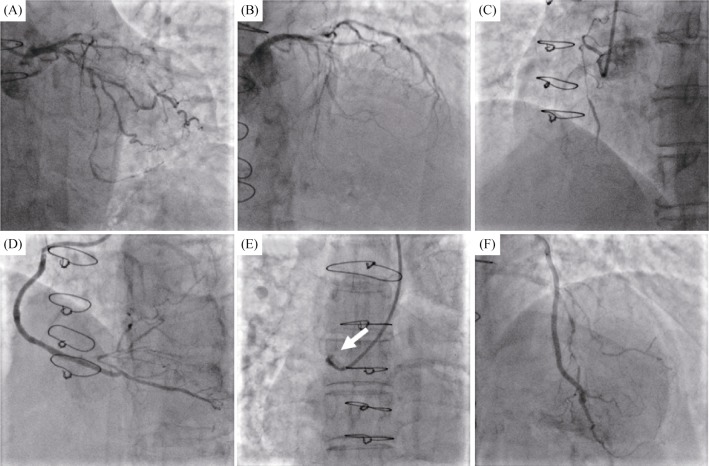
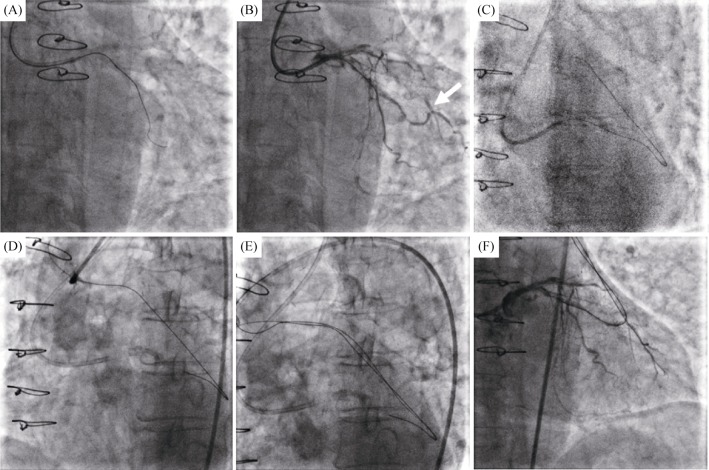
Case 2. A 76-year-old female patient with a history of CABG and coronary risk factors, including hypertension, diabetes mellitus and dyslipidemia, was admitted to our department due to the exacerbation of angina. No abnormalities were found during laboratory examinations or on echocardiography at admission. Coronary angiography was shown in Figure 3, 90% stenosis with calcification in the proximal LCX, the proximal left anterior descending (LAD) and the ostial RCA were occluded. The SVG to RCA and the LIMA to LAD were unobstructed, but the SVG to the OM branch graft was completely occluded. Thus, an attempt to dredge the native LCX seemed reasonable. The PCI was conducted with a right femoral approach using a 6Fr EBU3.75 guiding catheter. The Corsair135 mm microcatheter with Fielder XT-R guidewire was positioned the distal LCX. Unfortunately, the microcatheter and small balloons (1.0 mm × 10 mm & 1.25 mm × 15 mm) failed to pass through the severe LCX stenosis (Figure 4A). A dedicated attempt with rotational atherectomy was planned because of severe calcification, but the rotation-wire insertion was unsuccessful. The patient continued to present aggravated angina symptoms. Careful assessment of the patient's angiography revealed that competitive retrograde blood flow seemed to have developed from the OM branch to the graft anastomosis (Figure 4B). The retrograde approach to reopening the SVG was attempted. Interestingly, the retrograde guidewire easily cross the occluded SVG into the ascending aorta (Figures 4C & 4D). Then the antegrade guidewire (Fielder XT-R) was inserted into the SVG CTO body (Figure 4E). Simultaneously, a gentle tip injection positioned the guidewire in the true lumen and a large amount of thrombus were founded in the middle of SVG (Figures 4F & 5A).
Figure 3. Preoperative coronary angiography.
(A): The 90% stenosis with calcification in the proximal segment of the left circumflex artery; (B): the proximal segment of the LAD was total occlusion; (C): the ostial RCA were completely occluded; (D): the SVG to RCA was unobstructed; (E): the SVG to the OM branch was totally occluded (arrow); and (F): the left internal mammary artery to LAD was unobstructed. LAD: left anterior descending; OM: obtuse marginal; RCA: right coronary artery; SVG: saphenous vein graft.
Figure 4. The SVG to the OM branch revascularization procedure.
(A): Microcatheter and small balloons failed to cross the left circumflex artery lesion; (B): competitive retrograde blood flow seemed to have developed from the OM branch to the graft anastomosis (arrow); (C & D): the retrograde guidewire passed through the occlusion segment and entered into the ascending aorta; (E): the antegrade guidewire crossed the lesions to the distal OM branch under the direction of the retrograde wire; and (F): angiogram positioned the antegrade guidewire in the true lumen. OM: obtuse marginal; SVG: saphenous vein graft.
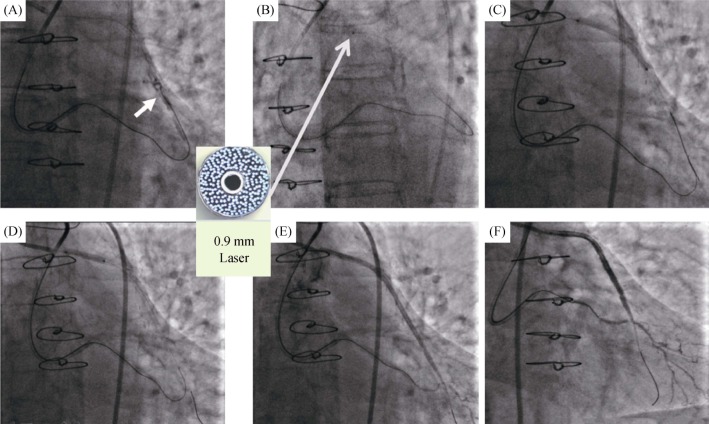
Figure 5. Example use of the excimer laser coronary atherectomy in the SVG to the OM branch.
(A): Tip injection showed a large amount of thrombus in the middle of SVG (arrow); (B): ablation from the proximal to the distal SVG was performed with the 0.9 mm laser catheter (arrow); (C): two drug-eluting stents (2.75 mm × 36 mm & 3.0 mm × 33 mm) were implanted from the distal to the ostial degenerated SVG; (D): post dilation with the 3.0 mm × 12 mm noncompliant balloon; and (E & F): the final angiogram result after laser facilitated percutaneous coronary intervention. OM: obtuse marginal; SVG: saphenous vein graft.
Then, the 0.9 mm laser catheter was used in the procedure with a specific fluence and the repetition rate were 60 MJ/mm2 and 60 Hz (Figure 5B). After predilation with the 2.0 mm × 15 mm balloon (Ryujin, Terumo, Tokyo, Japan) in the ostial stenosis to make sure the laser catheter could contact with the lesions. Two DESs (2.75 mm × 36 mm & 3.0 mm × 33 mm) were deployed from the distal to the ostial stenosis and post dilated by the 3.0 mm × 12 mm noncompliant balloon (Figures 5C & 5D). A successful angiographic result with blood flow TIMI grade 3 was achieved (Figures 5E & 5F). There were no electrocardiographic changes and the occurrence of adverse events during the periprocedural period.
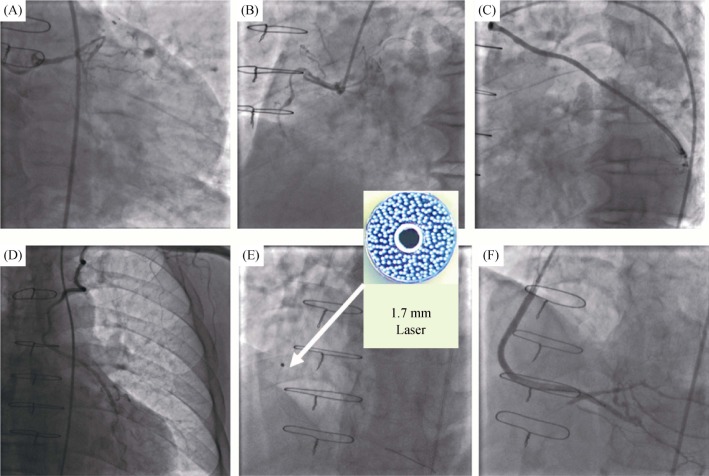
Case 3. A 71-year-old female with a history of CABG was admitted to our hospital. The patient was hospitalized due to unstable angina and underwent a coronary angiogram, which revealed severe, three-vessel coronary artery disease in Figure 6. The LIMA to LAD and the graft to the first OM branch were unobstructed. The graft to the posterior descending artery (PDA) was totally occluded (Figures 6A–D). An antegrade attempt to recanalize the RCA CTO has failed in other hospital since last year. A retrograde approach was not attempted due to the absence of proper collaterals to distally fill the CTO. The patient continued to exhibit symptoms of effort angina. As a last strategy, we proceeded to recanalize the SVG to PDA. The Amplatz Left 1.0 (AL1.0, Cordis, USA) guiding catheter was engaged. The Fielder XT-R (Asahi, Tokyo, Japan) guidewire cross the occluded lesions. The 1.7 mm excimer laser catheter was used starting with the repetition rate of 40 Hz and the fluence of 40 MJ/mm2, and finally increasing to the fluence of 60 MJ/mm2 (Figure 6E). Two DESs (Xience Xpedition, 3.0 mm × 38 mm & 3.5 mm × 38 mm) were deployed and followed by the 3.5 mm × 12 mm noncompliant balloon (Quantum Maverick, Boston Scientific, Natick, USA) inflation with good angiographic results (Figure 6F). The patient did not have any chest pain and cardiac enzymes remained negative after post-procedure. The patient was discharged after three days.
Figure 6. Example use of the excimer laser coronary atherectomy in the SVG to the PDA.
(A–D): The coronary angiogram demonstrate the LIMA to LAD and the SVG to the OM branch were unobstructed, and the SVG to the PDA was occluded; (E): the 1.7 mm laser catheter through the SVG lesions and ablation (arrow); and (F): the good angiographic results after two stents were deployed. LAD: left anterior descending; LIMA: left internal mammary artery; OM: obtuse marginal; PDA: posterior descending artery; SVG: saphenous vein graft.
SVG is associated with a high incidence of platelet thrombi and lipid-laden plaques, which have been implicated in distal microvascular spasms, no-reflow phenomenon, and distal embolization, thus leading to increased rates of periprocedural myocardial infarction. Despite the use of EPD, the incidences of distal embolization and the no-reflow phenomenon remain high. A recent study showed that the distal embolization rate associated with these procedures was approximately 11%, and creatinine kinase levels were significantly elevated by 43%.[5] However, not all SVG lesions are suitable for EPD, approximately 33%–57% of SVG lesions may not permit the application of EPD because of their special location or tortuosity, an abundant plaque burden or the lack of a landing zone.[6] Furthermore, distal embolization may occur during attempted placement of the EPD (“the first-pass effect”) prior to implementing any definitive therapy for the diseased SVG.[7],[8] It's obvious that EPD already have some limitations, and the optimal therapy for SVG lesions was unknown. The PCI of SVG is technically feasible if distal embolization and no-reflow phenomenon can be limited. In this scenario, the excimer laser can reduce distal embolization and may potentially become an exciting armamentarium of the interventional cardiologist.
Excimer laser device recently became available in China although excimer laser utilization as an adjunctive device for PCI has introduced three decades ago in the United State. Its mechanics are based on ultraviolet energy (308 nm), which is capable of disintegrating a thrombus and ablating an underlying plaque. However, the result of the previous studies on excimer laser as an adjunctive treatment for degenerate coronary graft lesions is not so encouraging. Giugliano GR, et al.[9] conducted the COronary graft Results following Atherectomy with Laser (CORAL) trial, which included 98 patients with degenerated SVG recruited from 18 centers, to evaluate the safety and efficacy of excimer laser therapy. The primary endpoint occurred in 18/98 (18.4%) patients. Major procedural complications included the no-reflow phenomenon (n = 5) and major dissection (n = 1). There is no perforations occurred. However, this trial also showed that laser facilitated angioplasty of SVG did not convey a significant advantage over conventional treatment in the absence of EPD. Moreover, Ebersole D, et al.[10] established that excimer laser therapy is associated with a high success rate (98.2%) in acute SVG occlusions. Al-Lamee R, et al.[11] enrolled 34 patients with SVG CTO and combined excimer laser with EPD therapy, but they achieved only a 68% success rate. The EPD was used in 78% of the patients. It is unknown whether these relatively low procedural success rates are mainly due to the implanted EPD (“the first-pass effect”). In our excimer laser therapy cases without the application of EPD, the overall success rate was 100%. Theoretical analysis suggests that laser ablation of a degenerated SVG lesion should reduce embolic complications and potentially obviate the need for the EPD, but the potential application of such a technique needs to be tested in randomized controlled trials or large registries.
This article presents three cases of totally occluded SVG, which successfully treated with excimer laser therapy without the use of the EPD. In the first case, the patient suffered from NSTEMI, and the SVG to the OM branch was considered to be the culprit vessel. The presence of a thrombus increases the risk of embolization; thus, we decided to conduct the PCI in the SVG to the OM branch after considering all technical aspects. We consistently believed that laser ablation would rapidly remove the loose plaques and thrombus to limit this risk. The attempt to perform the PCI in the native LCX was failed in the second patient. The LCX had complex anatomies for revascularization, including calcification and tortuosity; additionally, the strategy of stenting was not an attractive choice. In this situation, recanalization of the SVG CTO was performed with an excimer laser as an adjuvant therapy for plaque debulking. The third patient was SVG CTO, and when many attempts to reopen the native RCA CTO failed, we successfully revascularized the SVG CTO with the ELCA treatment. Despite the considerable success of the excimer laser in three case, there is still no evidence to confirm that similar or improved results, compared between excimer lasers treatment and other therapeutic approach. Meanwhile, imaging-guided ELCA might be provided more useful information, especially to evaluate SVG morphology before or after excimer laser ablation. Furthermore, SVG stenting is associated with high rates of restenosis or occluded although our study has not observed. Whether excimer laser combine with a drug-coat balloon might improve these outcomes, it needs to be texted in the future.
The current European Society of Cardiology (ESC) guidelines outline the recommendations for EPD from class I to class II.[12] Another registry showed that EPD are used in 21% of contemporary SVG PCI.[8] Therefore, based on these studies, the excimer laser facilitated angioplasty of SVG appears to confer a significant advantage over conventional strategy, even in the absence of EPD. The three such cases presented in our study are excellent examples. However, several factors involved in SVG PCI are worthy of emphasis. Firstly, a low penetration force and polymer jacket guidewire (Fielder XT/Pilot 50 series) are preferred, and step-by-step guidewire advancement should be contralaterally verified to increase the chance of tracking the vessel and reducing the risk of perforation. Minimize the “First-pass effect” including attempted placement of guidewire and microcatheter before excimer laser therapy. Secondly, choosing the appropriate laser catheter is more crucial for the successful operation, and forceful pushing must be avoided and slow advancement (< 0.5–1 mm/s) should be performed. Continuous flushing of saline and removal of contrast during laser emission are mandatory. At initial suggested the fluence was 25 MJ/mm2 and the final fluence need up to the 60–90 MJ/mm2, yield maximal plaque ablation. Last but not least, the principle of the procedure should be strictly observed. The patience and learning curve of the operator are also important.
In conclusion, the revascularization of totally occluded SVG of patients is feasible as an optional strategy, and the use of the excimer laser as an adjuvant therapy can reduce embolization and achieve an optimal angiographic result, thus potentially obviating the need for EPD in the future.
Acknowledgments
All authors had no conflicts of interest to disclose.
References
- 1.Niccoli G, Belloni F, Cosentino N, et al. Case-control registry of excimer laser coronary angioplasty versus distal protection devices in patients with acute coronary syndromes due to saphenous vein graft disease. Am J Cardiol. 2013;112:1586–1591. doi: 10.1016/j.amjcard.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Brilakis ES, O'Donnell CI, Penny W, et al. Percutaneous coronary intervention in native coronary arteries versus bypass grafts in patients with prior coronary artery bypass graft surgery: insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking program. JACC Cardiovasc Interv. 2016;9:884–893. doi: 10.1016/j.jcin.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 3.Brennan JM, Al-Hejily W, Dai D, et al. Three-year outcomes associated with embolic protection in saphenous vein graft intervention: results in 49325 senior patients in the Medicare-linked National Cardiovascular Data Registry Cath PCI Registry. Circ Cardiovasc Interv. 2015;8:e001403. doi: 10.1161/CIRCINTERVENTIONS.114.001403. [DOI] [PubMed] [Google Scholar]
- 4.Fracassi F, Roberto M, Niccoli G. Current interventional coronary applications of excimer laser. Expert Rev Med Devices. 2013;10:541–549. doi: 10.1586/17434440.2013.811846. [DOI] [PubMed] [Google Scholar]
- 5.Ebersole D, Dahm JB, Das T, et al. Excimer laser revascularization of saphenous vein grafts in acute myocardial infarction. J Invasive Cardiol. 2004;16:177–180. [PubMed] [Google Scholar]
- 6.Topaz O, Lippincott R, Bellendir J, et al. “Optimally spaced” excimer laser coronary catheters: performance analysis. J Clin Laser Med Surg. 2001;19:9–14. doi: 10.1089/104454701750066884. [DOI] [PubMed] [Google Scholar]
- 7.Mohandes M, Rojas S, Torres M, et al. Percutaneous coronary intervention of chronically occluded saphenous vein grafts using excimer laser atherectomy as an adjuvant therapy. Cardiovasc Revasc Med. 2017;18:2–6. doi: 10.1016/j.carrev.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Nishino M, Mori N, Takiuchi S, et al. Indications and outcomes of excimer laser coronary atherectomy: efficacy and safety for thrombotic lesions-The ULTRAMAN registry. J Cardiol. 2017;69:314–319. doi: 10.1016/j.jjcc.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Giugliano GR, Falcone MW, Mego D, et al. A prospective multicenter registry of laser therapy for degenerated saphenous vein graft stenosis: the COronary graft Results following Atherectomy with Laser (CORAL) trial. Cardiovasc Revasc Med. 2012;13:84–89. doi: 10.1016/j.carrev.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Ebersole D, Dahm JB, Das T, et al. Excimer laser revascularization of saphenous vein grafts in acute myocardial infarction. J Invasive Cardiol. 2004;16:177–180. [PubMed] [Google Scholar]
- 11.Al-Lamee R, Ielasi A, Latib A, et al. Clinical and angiographic outcomes after percutaneous recanalization of chronic total saphenous vein graft occlusion using modern techniques. Am J Cardiol. 2010;106:1721–1727. doi: 10.1016/j.amjcard.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. EuroIntervention. 2019;14:1435–1534. doi: 10.4244/EIJY19M01_01. [DOI] [PubMed] [Google Scholar]