Abstract
BACKGROUND
Surgical resection is the primary treatment for nonfunctional (NF) pituitary adenomas, but gross-total resection is difficult to achieve in all cases. NF adenomas overexpress folate receptor alpha (FRα).
OBJECTIVE
To test the hypothesis that we could target FRα for highly sensitive and specific intraoperative detection of NF adenomas using near-infrared (NIR) imaging.
METHODS
Fourteen patients with NF pituitary adenoma were infused with the folate analog NIR dye OTL38 preoperatively. NIR fluorescence signal-to-background ratio (SBR) was recorded for each tumor during resection of the adenomas. Extent of surgery was not modified based on the presence or absence of fluorescence. Immunohistochemistry was performed to assess FRα expression in all specimens. Magnetic resonance imaging (MRI) was performed postoperatively to assess residual neoplasm.
RESULTS
Nine adenomas overexpressed FRα and fluoresced with a NIR SBR of 3.2 ± 0.52, whereas the 5 non-FRα-overexpressing adenomas fluoresced with an SBR of 1.5 ± 0.21. Linear regression demonstrated a significant correlation between intraoperative SBR and the FRα expression (P-value < .001). Analysis of 14 margin samples revealed that the surgeon's impression of the tissue had 83% sensitivity, 100% specificity, 100% positive predictive value, and 89% negative predictive value, while NIR fluorescence had 100% for all values. NIR fluorescence accurately predicted postoperative MRI results in 78% of FRα-overexpressing patients.
CONCLUSION
Preoperative injection of folate-tagged NIR dye provides strong signal and visualization of NF pituitary adenomas. It is 100% sensitive and specific for detecting margin neoplasm and can predict postoperative MRI findings. Our results suggest that NIR fluorescence may be superior to white-light visualization alone and may improve resection rates in NF pituitary adenomas.
Keywords: Fluorescence, Imaging, Intraoperative, Near-infrared, Nonfunctional, Pituitary-adenoma
ABBREVIATIONS
- CI
confidence interval
- FRα
folate receptor alpha
- GTR
gross total resection
- iMRI
intraoperative magnetic resonance imaging
- NF
nonfunctional
- NIR
near-infrared
- NPV
negative predictive value
- PPV
positive predictive value
- SBR
signal-to-background ratio
Surgical resection via the transsphenoidal approach remains the primary treatment for many pituitary adenomas, which account for approximately 10% of intracranial tumors.1-3 Achieving complete resection, however, can be difficult and the tumor recurrence rate can be as high as 20% after surgery, often requiring retreatment.4-6 Current strategies to improve surgical resection include use of intraoperative magnetic resonance imaging (iMRI), but this is not currently recommended by the Congress of Neurological Surgeons due to its high false positive rate, cost, and limited availability.7-10 There thus remains a need for an intraoperative imaging technique that is simple to perform and easy to interpret.
Our group has previously demonstrated that near-infrared (NIR) fluorescent dyes can be used to visualize intracranial tumors intraoperatively with high sensitivity.11-14 Many
nonfunctional (NF) pituitary adenomas overexpress folate receptor alpha (FRα) compared to normal pituitary gland or surrounding intracranial structures.15-17 This makes FRα an attractive target for specific labeling of NF pituitary adenomas.18 OTL38 (On Target Laboratories, West Lafayette, Indiana) is a folate analog conjugated to a cyanine dye that specifically binds folate receptors. In a previous study, we explored the technical aspects of using folate-receptor-targeted intraoperative NIR imaging for a variety of pituitary adenoma histologic subtypes.19 In this study focusing on NF adenomas, we demonstrate that NIR imaging with OTL38 permits sensitive and specific detection of neoplastic tissue compared to visible light alone and accurately predicts the presence and location of residual tumor.
METHODS
Study Design
The study institution's Institutional Review Board approved of this prospective cohort study. Inclusion criteria were age over 18 and diagnosis of pituitary tumor with no symptoms of thyroid-stimulating hormone (TSH), growth hormone (GH), or adrenocorticotropic hormone (ACTH) overproduction. Main exclusion criteria were pregnancy and history of allergy to folic acid. All patients underwent preoperative magnetic resonance imaging (MRI) of the brain/sella (Table 1). Patients were made aware that the extent of their surgery would not be substantially changed by enrolling in the study. Informed consent was obtained from all patients.
TABLE 1.
Clinical and Pathological Characteristics of Study Subjects
| Patient ID | Sex, age | Pathology | FRα H-score | Preoperative biochemical status | Clinical features at presentation | Visual field deficit (preop/postop) | Prior treatment | Maximum tumor dimension (mm) | Cavernous sinus invasion Knosp Grade | Residual tumor location | Postoperative complications/hormonal changes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OTL5 | F, 67 | Null-cell | 0 | Mild hyperprolactinemia hypogonadotropic hypogonadism | Incidental MRI finding | No/No | No | 20 | 1 | None | None/euhormonal |
| OTL7 | M, 80 | Null-cell | 200 | Euhormonal | Bitemporal hemianopsia | Yes/No | No | 17 | 0 | None | None/euhormonal |
| OTL12 | M, 45 | Null-cell | 270 | Hypogonadotropic hypogonadism | Right temporal hemianopsia headaches | Yes/Yes | No | 28 | 2 | Left sella | None/resolving hypogonadism |
| OTL16 | F, 53 | Null-cell | 5 | Euhormonal | Incidental MRI finding | No/No | No | 15 | 0 | None | Transient SIADH/Euhormonal |
| OTL21 | F, 46 | Null-cell | 260 | Euhormonal | Headache | Yes/Yes | Transsphenoidal resection, 8 and 3 yr prior | 20 | 4 | Left CS | None/Euhormonal |
| OTL26 | M, 71 | Null-cell | 200 | Hypothyroid, Hypogonadotropic hypogonadism | Incidental MRI finding | No/No | No | 27 | 1 | Anterior to sella | None/persistent hypothyroidism and hypogonadism |
| OTL29 | M, 75 | Null-cell | 280 | Panhypopituitarism | Memory issues | No/No | No | 20 | 1 | None | None/persistent hypothyroidism and hypogonadism |
| OTL30 | M, 75 | Null-cell | 260 | Panhypopituitarism | Bitemporal hemianopsia | Yes/Yes | No | 21 | 1 | None | None/persistent hypopituitarism |
| OTL31 | M, 61 | Null-cell | 250 | Euhormonal | Incidental MRI finding | No/No | No | 20 | 2 | None | None/euhormonal |
| OTL1 | F, 39 | LH, FSH, Rare TSH | 225 | Elevated αSU Mild hyperprolactinemia | Seizure headache | No/No | No | 55 | 3 | Left CS | Transient DI/euhormonal |
| OTL22 | F, 78 | LH, FSH | 240 | Euhormonal | Bitemporal hemianopsia diplopia | Yes/Yes | No | 50 | 4 | Right CS | Transient SIADH/euhormonal |
| OTL2 | F, 67 | GH | 100 | Hypogonadotropic hypogonadism | Bitemporal hemianopsia | Yes/No | Transsphenoidal resection, 6 yr prior | 37 | 3 | None | Transient SIADH/euhormonal |
| OTL23 | F, 29 | GH | 0 | Euhormonal | Incidental MRI finding | No/No | No | 16 | 1 | Right CS | None/euhormonal |
| OTL24 | M, 24 | GH, PRL | 0 | Mild hyperprolactinemia Hypothyroidism Hypogonadotropic hypogonadism | Headaches Blurred vision | Yes/No | No | 35 | 2 | None | None/hypogonadotropic hypogonadism |
αSU = alpha subunit; CS = cavernous sinus; DI = diabetes insipidus; FSH = follicular stimulating hormone; GH = growth hormone; LH = luteinizing hormone; MRI = magnetic resonance imaging; PRL = prolactin; SIADH = syndrome of inappropriate antidiuretic hormone
OTL38 Administration
Patients were instructed to discontinue folate supplements or multivitamins that included folate 48 h prior to surgery, in order to reduce potential interactions with OTL38. Patients were brought to the infusion suite 2 to 4 h prior to surgery, given precautionary 25 mg intravenous diphenhydramine and then infused with 0.025 mg/kg of OTL38 over 1 h. Patient vital signs were monitored throughout the infusion and for 30 min afterwards before being checked in for surgery.
NIR Imaging System
In the operating room, NIR signal was measured using the FDA-approved VisionSense IridiumTM camera system (VisionSense, Philadelphia, Pennsylvania) equipped with a 4-mm endoscope. The excitation source is a laser tuned to 785 nm and the sensor is filtered for emission in the 800 to 835 nm range. This system was recently compared to other NIR visualization systems, and its sensor gain and sensitivity are considered the best among the commercially available systems.20
Surgical Approach and Study Procedure
The endonasal endoscopic transsphenoid approach was performed by otorhinolaryngologists using standard endoscopic technique.21,22 Upon exposing the sella, the attending neurosurgeon used the VisionSense endoscope to record NIR images. The dura was initially kept intact in order to visualize NIR signal through the dura. After tumor exposure, NIR fluorescence within the tumor was visualized (Figures 1 and 2), and specimens were taken from the tumor mass for histopathologic diagnosis. Surgery then proceeded with conventional equipment, without the use of NIR fluorescence.
FIGURE 1.
Subject 31 with FRα H-score of 250 demonstrates strong NIR fluorescence and GTR is achieved by removing fluorescent specimen. A and B, Sagittal and coronal preoperative MRI demonstrating a 20-mm sellar mass with a Knosp grade 2 invasion of the cavernous sinus. C and D, Upon initial exposure after dura opening, the tumor is visualized with white light in C, and it can be seen in real time with NIR overlay with an SBR of 3.1 in D. E and F, White light alone does not show convincing residual E, but the NIR overlay view demonstrates a piece of inferior dura with positive NIR signal with an SBR of 2.5 (F, white circle). This sample was believed to be dura only with no neoplasm and was taken in entirety as a margin sample by the senior surgeon (J.Y.K.L.). However, pathology revealed that portions of the specimen were neoplastic. G and H, Day 1 postoperative sagittal and coronal MRI does not demonstrate any evidence of residual neoplasm.
FIGURE 2.
Subject 26 with FRα H-score of 200 demonstrates strong NIR fluorescence, with residual neoplasm. A and B, Sagittal and coronal preoperative MRI demonstrating a 27-mm sellar mass with a Knosp grade 2 invasion of the cavernous sinus. C and D, Upon initial exposure after the dura has been opened, the tumor can be seen with the white light view in C, and it can be seen in real time with NIR overlay directly on top of the white light view in D, with an SBR of 3.9. E and F, White light does not show convincing residual E, but the NIR overlay view shows strong residual fluorescence with an SBR of 3.4 protruding on the left anterior side of the sella. This margin specimen was not biopsied by the senior surgeon (M.S.G.). G and H, Two-month postoperative sagittal and coronal MRI demonstrate evidence of residual tumor in the anterior part of the sella (white arrows), consistent with the fluorescent area seen in F.
After the attending neurosurgeon was satisfied that a complete resection had been achieved, NIR imaging was used to identify areas of residual fluorescence (Figures 1E and 1F; Figures 2E and 2F). The senior surgeon biopsied areas that he felt were appropriate and specimens were coded (yes/no for NIR fluorescence and for tumor based on surgeon's impression) before being sent to pathology. Otorhinolaryngology and neurosurgery jointly performed the closure.
Patients were admitted to the intensive care unit after surgery. A postoperative MRI was ordered for the first postoperative day and then after 2 and/or 6 mo.
Immunohistochemistry for FRα
The main tumor specimens from each patient were chemically fixed and embedded in paraffin blocks at 5-μm thickness. Murine monoclonal antibodies against folate receptors (1:20 dilution of NCL-L-FRalpha; Leica Biosystems, Wetzlar, Germany) were used for FRα immunohistochemistry. In order to quantify FRα expression in each sample, H-scores were calculated by a single neuropathologist. The H-score ranges from 0 to 300, with 300 implying strong staining in all cells and 0 implying no staining in any cell.23,24
NIR Image Analysis for signal-to-background ratio
The NIR imaging data were analyzed using the VisionSense software (VSPlayer v1.8.05.01; VisionSense) and ImageJ (https://imagej.nih.gov/ij/; National Institute of Health, Bethesda, Maryland) to calculate the signal-to-background ratio (SBR), as described in our previous paper.19 It was previously noted that the distance between the endoscope and the tissue impacts NIR false-positive rates. Since the VisionSense IridiumTM is not able to measure the distance to the fluorescence, the medial opticocarotid recess (MOCR), which is a useful landmark in the transsphenoid approach, was used as the substitute measurement for distance estimation.25-27 All initial images were taken at a distance where MOCR spanned less than one-half of the screen, as our prior work demonstrated this to be the ideal distance to minimize false positives.19
Data Analysis
Statistical comparison with linear regression, t-tests, and nonparametric tests were performed using STATA 10TM (StataCorp LLC, College Station, Texas). Two-by-two contingency tables were constructed to calculate the sensitivity/specificity/positive predictive value/negative predictive value and receiver operating characteristic (ROC) analysis.
RESULTS
Clinical Data
A total of 14 patients with NF pituitary adenomas were enrolled between October 2015 and March 2017 (7 males, mean age 57.8 with range 24-80). No patient had any serum or clinical evidence of TSH, GH, or ACTH overproduction prior to surgery (Table 1).
All patients tolerated OTL38 injections without adverse event and no permanent surgical complications were identified.
Folate Receptor Immunohistochemistry
Standard postoperative immunohistochemistry staining revealed 9 null-cell adenomas, 2 silent gonadotroph adenomas, and 3 silent somatotroph adenomas (Table 1). Further immunohistochemistry for FRα was performed on paraffin-embedded tissue sections using the protocol described in Methods (Figure 3; Table 1).
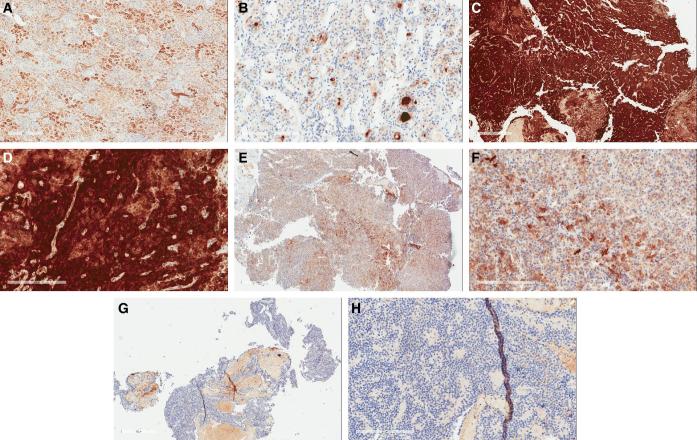
FIGURE 3.
Immunohistochemistry demonstrates level of FRα expression in pituitary adenomas. A, Kidney as positive control with H-score of 190 (scale bar = 600 μm). B, Normal adenohypophysis with H-score 34 (scale bar = 200 μm). C and D, Low and high power view (scale bars = 600, 200 μm respectively) of a null-cell adenoma subject ID 12 with high H-score of 270. E and F, Low and high power view (scale bars = 600, 200 μm respectively) of somatotroph adenoma of subject ID 2 with intermediate H-score of 100. G and H, Low and high power view (scale bars = 600, 200 μm respectively) of a null-cell adenoma of subject ID 5 with low H-score of 0. The red areas in panels G and H are red blood cells and folate staining was only measured in the blue areas, where the pituitary cells are present.
Non-neoplastic pituitary tissue from 11 specimens demonstrated FRα H-scores of 50 ± 40, with a median value of 34. Nine adenomas—7 of the 9 null-cell adenomas and both gonadotroph adenomas—demonstrated significant FRα overexpression (H-score > 150). All 3 somatotroph adenomas and 2 null-cell adenomas demonstrated FRα H-score < 100.
Intraoperative NIR Fluorescence
Prior to dura opening, the 14 adenomas fluoresced with an average SBR of 1.8 ± 0.40. After dura opening, the average SBR increased to 2.6 ± 0.92. However, the data showed a bimodal distribution, with 5 adenomas demonstrating SBRs less than 2 (mean 1.5 ± 0.18) and 9 adenomas demonstrating SBRs greater than 2.5 (mean 3.2 ± 0.52; Figure 4).
FIGURE 4.

Intraoperative NIR fluorescence SBR strongly correlates to FRα expression levels. The 9 adenomas with FRα H-scores ≥ 200 demonstrate mean SBR of 3.2 ± 0.52, while the 5 adenomas with FRα H-scores ≤ 100 demonstrate mean SBR of 1.5 ± 0.18 (P-value < .001). At the time of surgery, only the NIR SBR is known. Thus, being able to predict FRα expression levels during surgery is crucial. This linear regression analysis (95% CI demonstrated in shaded area) demonstrates significant correlation between intraoperative SBR and FRα expression (P-value < .001). Each 1-point increase in SBR correlates to approximately 108-point change in FRα H-score.
Correlation of FRα Expression and SBR
Our previous study had suggested a positive correlation between intraoperative SBR and the adenoma's FRα expression.19 A postoperative linear regression in this study showed significant correlation between SBR and FRα H-score (Figure 4, P-value < .001). For each 100-point increase in H-score, the SBR increased by 0.69. Conversely, each 1-point change in SBR predicted a 108-point increase in H-score, up to a maximum of 300.
In this cohort of patients, no adenomas demonstrated FRα H-score between 100 and 200, suggesting that NF pituitary adenomas express either very low levels of FRα or very high levels. Furthermore, as noted above, normal pituitary adenohypophysis expressed FRα with H-scores of 50 ± 40. Thus, we decided to classify adenomas with FRα H-scores > 150 (2.5 standard deviations higher than the mean for non-neoplastic tissue) as those that overexpress FRα. In our model, an H-score of 150 corresponds to an intraoperative SBR of 2.5. The cut-off of 2.5 is an arbitrary choice, and ideally greater numbers of patients and data points would be needed to choose a more scientific cut-off point.
Inter-rater Reliability for Fluorescence Assessment
In order to determine the inter-rater reliability of intraoperative determination of high SBR vs low SBR, we conducted a post hoc analysis of video data. Videos from 8 of the 14 patients were selected for imaging analysis (4 with high FRα H-scores, 4 with low H-scores). Three blinded researchers from our group independently calculated the SBR after brief training by an experienced member of the team. Between the 3 researchers, the Pearson's Correlation values were calculated at 0.972, 0.966, and 0.955, all indicating strong inter-rater reliability. Furthermore, using the intraoperative SBR cut-off of 2.5 to predict whether the tumor overexpressed FRα or not, all 3 blinded researchers correctly distinguished the four FRα overexpressing adenomas from the 4 that did not. Thus, Cohen's Kappa was calculated to be 1.0, suggesting an almost-perfect correlation.28
NIR to Detect Margins
After resecting the adenoma, the attending surgeon inspected the margins for potential residual neoplasm. A total of 14 margin samples were collected from 9 patients in this cohort of 14 patients. On final pathologic analysis, 6 specimens were positive for adenoma. At the time of surgery, the specimens were coded as positive or negative for neoplasm based on white-light appearance and texture; NIR fluorescence SBR was also used to code the specimens as positive (SBR > 2.5) or negative for fluorescence (Table 2).
TABLE 2.
Test Characteristics of White-light and NIR Light in Visualization of Margin Samples
| White light | Sensitivity | Specificity | PPV | NPV | ROC area | ||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Point estimate | 83% | 100% | 100% | 89% | 0.944 | ||
| Tumor on pathology | Yes | 5 | 1 | 95% CI | 36%-99% | 60%-100% | 46%-100% | 51%-99% | 0.836-1.0 |
| No | 0 | 8 | |||||||
| Total N | 14 | ||||||||
| Near-infrared light | Sensitivity | Specificity | PPV | NPV | ROC area | ||||
| Yes | No | Point Estimate | 100% | 100% | 100% | 100% | 1.0 | ||
| Tumor on pathology | Yes | 6 | 0 | 95% CI | 54%-100% | 63%-100% | 54%-100% | 63%-100% | 1.0-1.0 |
| No | 0 | 8 | |||||||
| Total N | 14 | ||||||||
CI = confidence interval; NPV = negative predictive value; PPV = positive predictive value; ROC = receiver operating characteristic
Postoperative analysis demonstrated that the surgeon's impression of the tissue without NIR fluorescence was 83% sensitive (95% confidence interval [CI], 36%-99%) and 100% specific (95% CI, 60%-100%), with a positive predictive value (PPV) of 100% (95% CI, 46%-100%), negative predictive value (NPV) of 89% (95% CI, 51%-99%) and an ROC area of 0.944 (95% CI, 0.836-1.0). One specimen, in subject 31 with FRα H-score of 250, was thought to be dura without neoplasm infiltrates, but was sampled as it was demonstrating NIR fluorescence with SBR of 2.5 (Figures 1E and 1F). Pathology later revealed that the specimen contained neoplastic tissue. Thus, NIR fluorescence demonstrated 100% for all values with an ROC area of 1.0.
Operative Outcomes
Gross total resection (GTR) was seen on postoperative MRI in 8 of 14 patients (57%); of these, 2 adenomas had Knosp grade 0 cavernous sinus invasion, 3 had Knosp grade 1, 2 had Knosp grade 2, and 1 had Knosp grade 3. Six of 14 patients showed residual neoplasm on postoperative MRI, with all of them having cavernous sinus invasion on preoperative MRI (2 Knosp grade 1, 1 Knosp grade 2, 1 Knosp grade 3, and 2 Knosp grade 4). Of the 7 patients with Knosp grade 0 or 1 cavernous sinus invasion, GTR rate was 85.7%. To date, 1 out of the 6 patients (subject 21) with residual neoplasm on postoperative MRI has needed further intervention; the patient had a history of 2 transsphenoidal procedures prior to the most recent surgery and was treated with Gamma Knife stereotactic radiosurgery (Elekta AB, Stockholm, Sweden) 1 yr postsurgery (Figure 5).
FIGURE 5.
Limitations of OTL38 and linear endoscope in detecting residual neoplasm in the cavernous sinus. NIR imaging still requires direct line of sight which cannot always be accomplished with the VisionSense zero-degree endoscope. A and B, Preoperative sagittal and coronal MRI in subject 21 with FRα H-score of 260 demonstrate a 20-mm adenoma with a Knosp grade 4 invasion of the left cavernous sinus. C and D, Upon initial exposure after dura opening, the tumor can be seen with both white light alone C and the NIR-overlay view with a NIR SBR of 3.4 D. E and F, Upon completion of surgery using white light, the NIR camera is introduced to assess for margins. The NIR-overlay view shows mild areas of NIR fluorescence (SBR = 2.5) in the left inferior sella F. This area was not biopsied by the senior surgeon (M.S.G.), but it corresponds to the 2-mo follow-up sagittal MRI showing sellar residual (G, white arrow). The same view, however, does not show the left cavernous sinus neoplasm surrounding the internal carotid artery, seen in the 2-mo follow-up coronal MRI (H, white arrow). Thus, a major limitation of the current technique is the limited views around corners into the cavernous sinus with the zero-degree endoscope. The patient required follow-up Gamma Knife stereotactic surgery (Elekta AB) 1 yr after the surgery.
NIR Imaging Prediction of Postoperative MRI
Since the extent of surgery was not changed based on the presence or absence of NIR fluorescence, improvement of degree of resection was not the goal of this study. Instead, we chose to determine whether NIR imaging could predict the presence and location of residual tumor seen on postoperative MRI. In the 9 patients with FRα-overexpressing adenomas, 5 demonstrated residual neoplasm on postoperative MRI, and NIR imaging demonstrated 80% sensitivity (95% CI, 30%-99%), 100% specificity (95% CI, 40%-100%), 100% PPV (95% CI, 40%-100%), and 80% NPV (95% CI, 30%-99%) in predicting the presence and anatomic location of the residual tumors in these 5 patients. For instance, subject 26 had a postoperative MRI that demonstrated residual neoplasm in the anterior side of the sella (Figures 2G and 2H). This corresponded perfectly with the area of fluorescence in the left anterior sella seen after resection (Figures 2E and 2F). Overall, NIR fluorescence predicted postoperative MRI results with 100% sensitivity in patients with less than Knosp grade 4 cavernous sinus invasion (Table 3, columns 4 and 5). As will be discussed further below, visualization of residual neoplasm was limited in Knosp grade 4 adenomas by the lack of ability to visualize tissue behind intact bone.
TABLE 3.
Summary of Preoperative and Postoperative MRI Status and Postresection NIR Fluorescence Status
| Cavernous sinus invasion | GTR (n) | GTR (%) | FRα-overexpressing | Residual neoplasm predicted by NIR | Clinically significant tumor |
|---|---|---|---|---|---|
| Knosp grade (n) | adenomas (n) | in FRα-overexpressing patients (%)a | recurrenceb (%) | ||
| 0 (2) | 2 | 100 | 1 | 100 | 0 |
| 1 (5) | 4 | 80 | 3 | 100 | 0 |
| 2 (3) | 1 | 33 | 2 | 100 | 0 |
| 3 (2) | 1 | 50 | 1 | 100 | 0 |
| 4 (2) | 0 | 0 | 2 | 0 | 50% |
FRα = folate receptor alpha; GTR = gross total resection; MRI = magnetic resonance imaging.
aPostoperative MRI from day 1, 2-mo, and 6- to 12-mo follow-up visits were used. If postoperative day 1 MRI was inconclusive regarding residual neoplasm, a later-date MRI was used.
bDefined as recurrence needing repeat treatment or causing new symptoms at 1-yr follow-up.
DISCUSSION
GTR of pituitary adenomas remains a major surgical challenge, with current published GTR rates of approximately 50% to 70%.10 A frequently studied tool to facilitate GTR is iMRI, which has demonstrated improved GTR rates, but also results in significant false positives.7,29 On the other hand, NIR imaging of targeted fluorophores can offer a highly specific, real-time detection modality for surgeons.30
Folate is an essential vitamin for biosynthesis, with multiple transport modalities across cell membranes.31-33 Pioneering work by Evans and Oyesiku established that FRα is overexpressed in NF pituitary adenomas, especially in null-cell and gonadotroph adenomas; FRα-overexpression has also been reported in ovarian, lung, and breast carcinomas.16,17,34-38 Previous studies have further demonstrated the feasibility of targeting FRα-overexpressing carcinomas using folate ligands.39-42
Based on these prior studies, we hypothesized that intraoperative NIR imaging with OTL38, a folate analog linked to a NIR cyanine dye, could reliably distinguish neoplastic pituitary tissue from benign tissue. In our first publication on this topic, we evaluated the technical aspects that influence intraoperative NIR imaging in 15 pituitary adenomas, 3 of which overexpressed FRα. In this study, we restricted our study population to only NF adenomas, adding 9 new patients—with 6 FRα overexpressing adenomas—in addition to 5 from our prior study. Here, we demonstrate highly sensitive and specific detection of NF adenomas using OTL38, with the potential for clinical benefit.
Rationale for NIR Imaging
Much of prior experiences with fluorescent-guided neurosurgery utilize visible-light fluorophores, such as 5-ALA or EC17.42,43 Yet, we chose to use the folate-conjugated NIR fluorophore OTL38 for 2 reasons. First, there is minimal autofluorescence in the NIR spectrum in central nervous system tissues. In addition, NIR light penetrates tissue better due to longer wavelengths. With the superior NIR imaging capabilities of the VisionSense IridiumTM—demonstrated in 2 independent studies—neoplastic tissue that would normally be undetectable, such as those behind the dura, can be easily visualized.11-14,20
Validity of FRα as a Target for Optical Contrast
In this study, 9 of 14 patients (64%) with NF adenomas demonstrated FRα-overexpression (H-score > 150) based on immunohistochemistry (Table 1), consistent with prior reports.17,38 The NIR SBR for the 5 adenomas with low FRα expression was 1.5 ± 0.18 and the SBR for the 9 adenomas with high FRα expression was 3.2 ± 0.52 (P-value < .001). Linear-regression model shows that a cut-off SBR of ≥2.5 could be used to distinguish FRα-overexpressing adenomas (Figure 4). As described in our prior publication, holding the endoscope away from the target, such that the distance between the MOCRs occupy less than 50% of the imaging field, allows the surgeon to accurately calculate the NIR SBR for the adenoma. This is important because the FRα expression of each adenoma is not known prior to surgery and OTL38 should theoretically be used only in FRα-overexpressing adenomas; in patients with predicted FRα overexpression, the margins can be inspected for residual adenoma after standard resection. Of note, we demonstrate in this paper that 3 blinded researchers were able to independently calculate the SBRs and predict FRα-expression levels with very high inter-rater reliability, suggesting that our methods should be reproducible.
Clinical Benefit
GTR of NF pituitary adenomas is often limited by intrinsic factors, such as invasion of the cavernous sinus (Knosp grade 1-4 adenomas).44 In the intraoperative MRI suite, cytoreduction could be improved by taking the time to obtain an MRI scan and then by performing further surgical resections.8,29 Similarly, real-time intraoperative molecular imaging with an optical contrast agent such as OTL38 may be able to replicate the findings of intraoperative MRI in the subset of pituitary adenomas that overexpress FRα, without the associated cost or equipment.
Previously, we suggested that intraoperative NIR fluorescence offered sensitive detection of pituitary adenomas.19 This study, using a larger patient population, demonstrated further benefit. In the 14 margin specimens sampled, both NIR fluorescence and the surgeons’ impression of the tissue under white light demonstrated 100% specificity in distinguishing tumor from nontumor. However, NIR fluorescence was more sensitive (100% vs 83%), as 1 specimen was not appreciated to be neoplastic during the surgery (Figure 1). Overall, the area under the ROC curve was greater for NIR fluorescence (1.0 vs 0.944), suggesting that NIR fluorescence offers more accurate tumor detection (Table 2).
Furthermore, NIR fluorescence was able to predict postoperative MRI results in all FRα-overexpressing adenomas with less than Knosp grade 4 cavernous sinus invasion, suggesting that NIR fluorescence can reliably detect residual neoplasm after resection by conventional methods. It is possible that had we resected all the fluorescent areas during the surgery, no residual neoplasm would have been detected on postoperative MRI in those patients. Indeed, in subject 31, a fluorescent piece of dura was sampled, leaving no fluorescence behind; final pathology revealed neoplastic tissue in the sample and follow-up imaging to date has not demonstrated recurrence of the tumor (Figure 1). Thus, we propose that targeted intraoperative NIR fluorescence offers the same benefits as intraoperative MRI devices at a fraction of the added time and cost and could contribute to increasing GTR in pituitary adenoma patients.
Limitations
Our NIR imaging technique has some technical limitations. As described in our previous paper, there is a learning curve to intraoperative NIR signal analysis.19 For instance, limiting false positives by maintaining distance between the endoscope and tissue is important, yet is not always easy if the surgeon has not opened the sphenoid sinus wide enough to visualize these anatomic structures.
More importantly, in some cases, residual neoplasm can be difficult to visualize with our technique. In general, residual neoplasms surrounding the cavernous sinus are either deep or hidden behind intact sphenoid bone and are not visualized easily with the endoscope (Figure 5). In subject 21, the tumor was easily visualized with NIR fluorescence prior to resection (Figures 5C and 5D). After resection, a small area of fluorescence was visualized, which corresponded to one of the 2 residual neoplasms seen on follow-up MRI (Figures 5E-5H). However, the residual neoplasm surrounding the left cavernous sinus was not visualized with NIR fluorescence. We suspect that the VisionSense zero-degree endoscope could not properly visualize this area due to obstruction by the intact sella bone. We expect that having angled endoscopes, currently not available with VisionSense, will improve our detection rate.
Furthermore, because OTL38 is a folate analog that binds FRα, it cannot be used to detect adenomas with low FRα expression. For instance, postoperative MRI in subject 24 demonstrated suprasellar residual neoplasm, which was not visualized with NIR imaging, as the adenoma had an FRα H-score of 0, indicating no expression of FRα (Table 1). As previous studies by Evans et al17,38 and our prior paper demonstrated,19 hormone-secreting functional pituitary adenomas do not overexpress FRα, limiting the use of OTL38 to NF adenomas. Furthermore, some NF adenomas do not overexpress FRα, as seen in our patient population; real-time NIR imaging using an SBR cut-off of 2.5 distinguishes the overexpressing adenomas from nonoverexpressing adenomas to more appropriately guide the use of intraoperative fluorescence in such cases.
A major limitation of this technique is that only 9 of 14 nonfunctioning adenoma patients overexpressed FRα. Hence, this technique may not be of help in a significant proportion of patients who undergo pituitary surgery. We do believe, however, that the initial views of the adenoma at the appropriate distance can help the surgeon to determine whether the tumor overexpresses FRα, and then interpretations of residual NIR signal would have to be based on the initial NIR signal strength. Future work would be needed to corroborate this approach.
Future Direction
We demonstrate that folate-receptor-targeted intraoperative NIR imaging offers reliable detection of neoplasm, with the potential to enhance GTR rates. We propose that studies in which the extent of surgery is modified based on fluorescent data are now feasible, allowing surgeons to directly evaluate whether NIR imaging improves GTR rates and patient outcomes.
CONCLUSION
Intraoperative NIR imaging using the folate-linked NIR fluorophore OTL38 can discriminate tissues with high and low FRα expressions. In the FRα-overexpressing NF adenomas, NIR imaging of margins provide higher sensitivity compared to white light alone. In addition, NIR imaging can predict postoperative MRI findings and detect residual neoplasm within direct line of sight of the endoscope. These results suggest that intraoperative NIR visualization of folate receptors has the potential to improve resection rates and patient outcomes in pituitary adenoma surgeries.
Disclosures
This work was supported in part by the National Institutes of Health R01 CA193556 (SS), and the Institute for Translational Medicine and Therapeutics of the Perelman School of Medicine at the University of Pennsylvania (J.Y.K.L.). In addition, research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000003 (J.K.Y.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr Lee owns stock options in VisionSense. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Notes
A poster of the abstract from this work was presented at the World Molecular Imaging Congress 2017, sponsored by the World Molecular Imaging Society and held in Philadelphia, Pennsylvania from September 13 to 16, 2017.
Operative Neurosurgery Speaks! Audio abstracts available for this article at www.operativeneurosurgery-online.com.
COMMENT
I appreciate the authors’ efforts in the field of intraoperative visualization of nonfunctional pituitary adenoma. Although I find that this study was based on small-series data, the signal-to-background ratio (SBR) 2.5 as the cut point for FR overexpressing adenomas was not well defined, and some nonfunctional adenomas do not overexpress FR. However, this new technique using folate receptor NIR optical imaging for intraoperative visualization for nonfunctional pituitary adenomas is novelty enough. It may improve the surgical safety and quality.
Jinn-Rung Kuo
Tainan, Taiwan
REFERENCES
- 1. Ezzat S, Asa SL, Couldwell WTet al.. The prevalence of pituitary adenomas. Cancer. 2004;101(3):613-619. [DOI] [PubMed] [Google Scholar]
- 2. Greenman Y, Cooper O, Yaish Iet al.. Treatment of clinically nonfunctioning pituitary adenomas with dopamine agonists. Eur J Endocrinol. 2016;175(1):63-72. [DOI] [PubMed] [Google Scholar]
- 3. Lee JYK, Bohman L-E, Bergsneider M. Contemporary neurosurgical techniques for pituitary tumor resection. J Neurooncol. 2014;117(3):437-444. [DOI] [PubMed] [Google Scholar]
- 4. Losa M, Mortini P, Barzaghi Ret al.. Early results of surgery in patients with nonfunctioning pituitary adenoma and analysis of the risk of tumor recurrence. J Neurosurg. 2008;108(3):525-532. [DOI] [PubMed] [Google Scholar]
- 5. Thawani J, Ramayya A, Pisapia J, Abdullah K, Lee J, Grady M. Operative strategies to minimize complications following resection of pituitary macroadenomas. J Neurol Surg Part B Skull Base. 2017;78(2):184-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sheehan J, Lee C-C, Bodach Met al.. Congress of neurological surgeons systematic review and evidence-based guideline for the management of patients with residual or recurrent nonfunctioning pituitary adenomas. Neurosurgery. 2016;79(4):E539-E540. [DOI] [PubMed] [Google Scholar]
- 7. Berkmann S, Schlaffer S, Buchfelder M. Tumor shrinkage after transsphenoidal surgery for nonfunctioning pituitary adenoma. J Neurosurg. 2013;119(6):1447-1452. [DOI] [PubMed] [Google Scholar]
- 8. Berkmann S, Schlaffer S, Nimsky C, Fahlbusch R, Buchfelder M. Follow-up and long-term outcome of nonfunctioning pituitary adenoma operated by transsphenoidal surgery with intraoperative high-field magnetic resonance imaging. Acta Neurochir. 2014;156(12):2233-2243. [DOI] [PubMed] [Google Scholar]
- 9. Faje A, Tritos NA, Swearingen B, Klibanski A. Neuroendocrine disorders: pituitary imaging. Handb Clin Neurol. 2016;136:2233-2243. [DOI] [PubMed] [Google Scholar]
- 10. Kuo JS, Barkhoudarian G, Farrell CJet al.. Congress of neurological surgeons systematic review and evidence-based guideline on surgical techniques and technologies for the management of patients with nonfunctioning pituitary adenomas. Neurosurgery. 2016;79(4):E536-E538. [DOI] [PubMed] [Google Scholar]
- 11. Lee JYK, Pierce JT, Zeh Ret al.. Intraoperative near-infrared optical contrast can localize brain metastases. World Neurosurg. 2017;106:120-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cho SS, Zeh R, Pierce JT, Salinas R, Singhal S, Lee JYK. Comparison of near-infrared imaging camera systems for intracranial tumor detection. Mol Imaging Biol. 2017. Available at:https://www.ncbi.nlm.nih.gov/pubmed/28741043. Accessed August 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee JYK, Pierce JT, Thawani JPet al.. Near-infrared fluorescent image-guided surgery for intracranial meningioma. J Neurosurg. 2018;128(2):380-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee JYK, Thawani JP, Pierce Jet al.. Intraoperative near-infrared optical imaging can localize gadolinium-enhancing gliomas during surgery. Neurosurg. 2016;79(6):856-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Larysz D, Żebracka-Gala J, Rudnik Aet al.. Original article expression of genes FOLR1, BAG1 and LAPTM4B in functioning and non-functioning pituitary adenomas. Folia Neuropathol. 2012;3(3):277-286. [DOI] [PubMed] [Google Scholar]
- 16. Evans C-O, Yao C, Laborde D, Oyesiku NM. Folate Receptor expression in pituitary adenomas cellular and molecular analysis. Vitam Horm. 2008;79:235-266. [DOI] [PubMed] [Google Scholar]
- 17. Evans C-O, Reddy P, Brat DJet al.. Differential expression of folate receptor in pituitary adenomas. Cancer Res. 2003;63(14):4218-4224. [PubMed] [Google Scholar]
- 18. Sega EI, Low PS. Tumor detection using folate receptor-targeted imaging agents. Cancer Metastasis Rev. 2008;27(4):655-664. [DOI] [PubMed] [Google Scholar]
- 19. Lee JYK, Cho SS, Zeh Ret al.. Folate receptor overexpression can be visualized in real time during pituitary adenoma endoscopic transsphenoidal surgery with near-infrared imaging. J Neurosurg. 2017;1-14. Available at: https://www.ncbi.nlm.nih.gov/pubmed/28841122. Accessed September 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DSouza AV, Lin H, Henderson ER, Samkoe KS, Pogue BW. Review of fluorescence guided surgery systems: identification of key performance capabilities beyond indocyanine green imaging. J Biomed Opt. 2016;21(8):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O’Malley BW, Grady MS, Gabel BCet al.. Comparison of endoscopic and microscopic removal of pituitary adenomas: single-surgeon experience and the learning curve. Neurosurg Focus. 2008;25(6):E10. [DOI] [PubMed] [Google Scholar]
- 22. Elhadi AM, Hardesty DA, Zaidi HAet al.. Evaluation of surgical freedom for microscopic and endoscopic transsphenoidal approaches to the sella. Neurosurgery. 2015;11(Suppl 2):69-78-9. [DOI] [PubMed] [Google Scholar]
- 23. Budwit-Novotny DA, McCarty KS, Cox EBet al.. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46(10):5419-5425. [PubMed] [Google Scholar]
- 24. McCarty KS, Szabo E, Flowers JLet al.. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Res. 1986;46(8 Suppl):4244s-4248s. [PubMed] [Google Scholar]
- 25. Kikuchi R, Toda M, Wakahara S, Fujiwara H, Jinzaki M, Yoshida K. Analysis of the medial opticocarotid recess in patients with pituitary macroadenoma using three-dimensional images. World Neurosurg. 2016;93:139-143. [DOI] [PubMed] [Google Scholar]
- 26. Labib MA, Prevedello DM, Fernandez-Miranda JCet al.. The medial opticocarotid recess: an anatomic study of an endoscopic “key landmark” for the ventral cranial base. Oper Neurosurg. 2013;72:ons 66-ons 76. [DOI] [PubMed] [Google Scholar]
- 27. Yang Y, Zhan G, Liao Jet al.. Morphological characteristics of the sphenoid sinus and endoscopic localization of the cavernous sinus. J Craniofac Surg. 2015;26(6):1983-1987. [DOI] [PubMed] [Google Scholar]
- 28. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159. [PubMed] [Google Scholar]
- 29. Berkmann S, Schlaffer S, Nimsky C, Fahlbusch R, Buchfelder M. Intraoperative high-field MRI for transsphenoidal reoperations of nonfunctioning pituitary adenoma. J Neurosurg. 2014;121(November):1-10. [DOI] [PubMed] [Google Scholar]
- 30. Zhang RR, Schroeder AB, Grudzinski JJet al.. Beyond the margins: real-time detection of cancer using targeted fluorophores. Nat Rev Clin Oncol. 2017;14(6):347-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matherly LH, Goldman DI. Membrane transport of folates. Vitam Horm. 2003;66:403-456. [DOI] [PubMed] [Google Scholar]
- 32. Zhao R, Goldman ID. The proton-coupled folate transporter: physiological and pharmacological roles. Curr Opin Pharmacol. 2013;13(6):875-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao R, Diop-Bove N, Visentin M, Goldman ID. Mechanisms of membrane transport of folates into cells and across epithelia. Annu Rev Nutr. 2011;31(1):177-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boogerd LSF, Boonstra MC, Beck A-Jet al.. Concordance of folate receptor-α expression between biopsy, primary tumor and metastasis in breast cancer and lung cancer patients. Oncotarget. 2016;7(14):17442-17454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ledermann JA, Canevari S, Thigpen T. Targeting the folate receptor: diagnostic and therapeutic approaches to personalize cancer treatments. Ann Oncol. 2015;26(10):2034-2043. [DOI] [PubMed] [Google Scholar]
- 36. Toffoli G, Cernigoi C, Russo A, Gallo A, Bagnoli M, Boiocchi M. Overexpression of folate binding protein in ovarian cancers. Int J Cancer. 1997;74(2):193-198. [DOI] [PubMed] [Google Scholar]
- 37. Wang Z, Shi H, Guo J, Li C. A current review of folate receptor alpha as potential tumor target in non-small-cell lung cancer. Drug Des Devel Ther. 2015;9:4989-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Evans C-O, Young AN, Brown MRet al.. Novel patterns of gene expression in pituitary adenomas identified by complementary deoxyribonucleic acid microarrays and quantitative reverse transcription-polymerase chain reaction. J Clin Endocrinol Metab. 2001;86(7):3097-3107. [DOI] [PubMed] [Google Scholar]
- 39. Yamada Y, Nakatani H, Yanaihara H, Omote M. Phase I clinical trial of 99mTc-etarfolatide, an imaging agent for folate receptor in healthy Japanese adults. Ann Nucl Med. 2015;29(9):792-798. [DOI] [PubMed] [Google Scholar]
- 40. Sega EI, Low PS. Tumor detection using folate receptor-targeted imaging agents. Cancer Metastasis Rev 2008;27(4):655-664 [DOI] [PubMed] [Google Scholar]
- 41. Hoogstins CES, Tummers QRJG, Gaarenstroom KNet al.. A novel tumor-specific agent for intraoperative near-infrared fluorescence imaging: A translational study in healthy volunteers and patients with ovarian cancer. Clinical Cancer Res. 2016;22(12):2929-2938. [DOI] [PubMed] [Google Scholar]
- 42. van Dam GM, Themelis G, Crane LMet al.. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-? targeting: first in-human results. Nat Med. 2011;17(10):1315-1319. [DOI] [PubMed] [Google Scholar]
- 43. Stummer W, Pichlmeier U, Meinel Tet al.. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392-401. [DOI] [PubMed] [Google Scholar]
- 44. Paluzzi A, Fernandez-Miranda JC, Tonya Stefko S, Challinor S, Snyderman CH, Gardner PA. Endoscopic endonasal approach for pituitary adenomas: a series of 555 patients. Pituitary. 2014;17(4):307-319. [DOI] [PubMed] [Google Scholar]