Key Points
FXII augments hemostasis in wounds of terrestrial mammals that contain soil, a silicate rich material.
FXII may be conserved in humans partly because of evolutionary advantages in minimizing blood loss.
Abstract
Bleeding is a common contributor to death and morbidity in animals and provides strong selective pressure for the coagulation system to optimize hemostasis for diverse environments. Although coagulation factor XII (FXII) is activated by nonbiologic surfaces, such as silicates, which leads to blood clotting in vitro, it is unclear whether FXII contributes to hemostasis in vivo. Humans and mice lacking FXII do not appear to bleed more from clean wounds than their counterparts with normal FXII levels. We tested the hypothesis that soil, a silicate-rich material abundant in the environment and wounds of terrestrial mammals, is a normal and potent activator of FXII and coagulation. Blood loss was compared between wild-type (WT) and FXII-knocked out (FXII−/−) mice after soil or exogenous tissue factor was applied to transected tails. The activation of FXII and other components of the coagulation and contact system was assessed with in vitro coagulation and enzyme assays. Soils were analyzed by time-of-flight secondary ionization mass spectrometry and dynamic light scattering. Soil reduced blood loss in WT mice, but not FXII−/− mice. Soil accelerated clotting of blood plasma from humans and mice in a FXII-dependent manner, but not plasma from a cetacean or a bird, which lack FXII. The procoagulant activity of 13 soils strongly correlated with the surface concentration of silicon, but only moderately correlated with the ζ potential. FXII augments coagulation in soil-contaminated wounds of terrestrial mammals, perhaps explaining why this protein has a seemingly minor role in hemostasis in clean wounds.
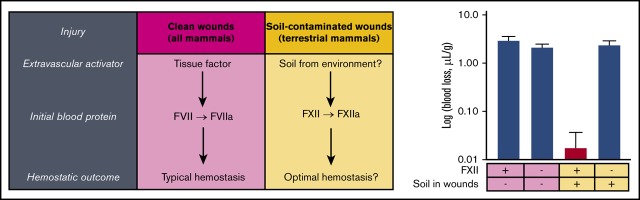
Visual Abstract
Introduction
Hemostasis is a normal response after vascular injury, leading to the formation of an insoluble plug of fibrin and blood cells that seals the wound to limit blood loss. Trauma, including injuries associated with predation, are the main cause of death of primates in the wild.1,2 Trauma is also the leading cause of death of young humans, with excessive bleeding responsible for up to 40% of mortality in trauma patients.3,4 The significant risk for death from exsanguination provided strong selective pressure to evolve an efficient coagulation system. This system became increasingly complex in the lineage of vertebrates leading to mammals,5 optimizing hemostasis for a wide range of environments.5,6
Coagulation factor XII (FXII) is a plasma protein found in the blood of most terrestrial vertebrates, appearing as a primitive ortholog just before the emergence of tetrapods.5,7,8 FXII can initiate blood clotting in vitro when blood is exposed to specific artificial9,10 and biological10-12 surfaces, which are usually negatively charged. During this process, called contact activation, single-chain zymogen FXII undergoes conformational change upon binding to surfaces, making it more susceptible to proteolytic cleavage into the 2-chain protease FXIIa, which promotes clotting via activation of coagulation factor XI (FXI).13,14 FXII has a pathophysiological role in clotting in vivo, such as by contributing to thrombus formation on medical devices that come into contact with blood.15,16 Unlike deficiencies of other coagulation factors, FXII deficiency does not appear to cause increased spontaneous or injury-related bleeding in humans or mice.17-19 The apparent lack of a bleeding phenotype suggests that FXII does not contribute to hemostasis in vivo. It is unclear what substances would activate FXII at sites of injury. However, these issues leave open the question of why a procoagulant blood protein that does not participate in hemostasis after injury has been maintained by natural selection in mammals. It is possible that FXII may only augment hemostasis after injury under certain conditions, such as when a natural activator of FXII is introduced into the wound from the environment.
Silicates such as clay and glass are among the nonbiologic substances that trigger FXII activation.9,10 Silicates are used to initiate clotting in clinical coagulation assays,10 and clay-based materials are used in hemostatic dressings to stem trauma-induced bleeding.20 Although silicates are not stored or synthesized in mammals, they are ubiquitous in the environment as major components of soil.21,22 Most terrestrial vertebrates live in regular contact with soil, allowing silicates to enter wounds on injury.23-25 We hypothesize that soils are natural activators of FXII, and that FXII contributes to hemostasis in terrestrial mammals that are in contact with soil. Although this has been suggested before,23,24 the concept may be underappreciated, as studies of bleeding in humans and laboratory animals do not typically include contact with soil. Wounds of wild terrestrial mammals are often contaminated with soil, and primates and humans sometimes rub soil in wounds to stop bleeding25,26; however, humans normally clean wounds immediately after injury to minimize infection,27 and there is typically no soil present during surgery. This hypothesis, although running counter to the general impression that FXII does not promote hemostasis in vivo, is supported by more than 60 years of research on the effects of silicates on FXII.
Materials and methods
Source of soils and mineral samples
Soils and minerals were from forests, beaches, and rivers in Bowen Island and Langley, British Columbia, Canada,28,29 and Waterville Valley, New Hampshire. Unless otherwise indicated, all samples were crushed with a mortar and pestle until they could be sifted through a sieve with 0.106-mm pores (stainless steel 100 mesh).
Mouse tail bleeding model
All procedures were approved by the University of British Columbia Animal Care Committee (protocol #A16-0176) and performed in accordance with the guidelines established by the Canadian Council on Animal Care. FXII-knocked out (FXII−/−) mice had a C56Bl/6J background.18,19 Wild-type (WT) C57Bl/6J mice were used as controls (The Jackson Laboratory). Female and male mice between 10 and 21 weeks of age were used for experiments, and groups were age and sex matched. Investigators were not blinded. Mice in Figure 1B-C were given an intraperitoneal injection of acetylsalicylic acid (ASA; 100 mg/kg, the human equivalent of high-dose [1000 mg] aspirin) 5 minutes before tail transection. Mice were anesthetized via isoflurane inhalation, and tails were transected 8 mm from the tip. Wounds received either no treatment or topical application of soils, clay, titanium, or recombinant tissue factor (12 nM, TF, Innovin, Dade Behring) by gently bringing the wound into contact with each respective material in a polypropylene tube. Tails were then immediately immersed in citrated phosphate buffered saline to collect blood and to monitor bleeding for 10 minutes. To quantify blood loss, blood-citrated phosphate buffered saline suspensions were treated with a solution that lyses red blood cells (1.5 M NH4Cl, 0.1 M NaHCO3, 0.01 M EDTA; Millipore Sigma) and were incubated at room temperature for 10 minutes while gently inverting. The absorbance of each blood-citrated phosphate buffered saline suspension was measured at 590 nm (Tecan Genios plate reader) and converted to blood loss (in microliters), using a standard curve with known amounts of mouse blood collected by intracardiac puncture. Calculated blood loss was normalized to body weight (microliters per gram). Calculating blood loss without normalization did not change the statistical significance reported of any groups.
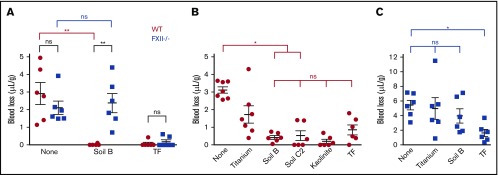
Figure 1.
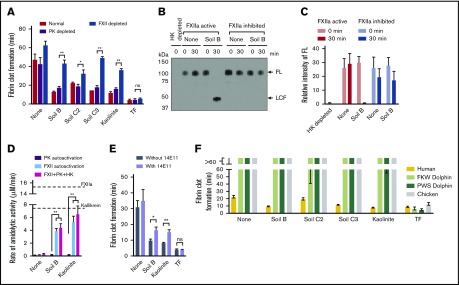
Soils are hemostatic in mouse wounds, dependent on FXII. (A) Blood loss with or without applying materials to transected tails of WT and FXII−/− mice without ASA. Total blood loss in WT (B) and FXII−/− (C) mice receiving ASA. n = 6-7. *P < .05; **P < .01. Error bars represent standard error of the mean (SEM). ns, no significant difference.
Preparing mouse platelet poor plasma
Mice were anesthetized by isoflurane inhalation, and whole blood was collected by intracardiac puncture into a syringe containing 100 μL 3.8% sodium citrate dihydrate (Millipore Sigma) and adjusted to a citrate to blood volumetric ratio of 1:9. Whole blood underwent centrifugation at 1500g for 10 minutes. The supernatant underwent centrifugation at 1500g for 10 minutes to remove residual blood cells. Platelet-poor plasma was obtained by collecting the second supernatant.
Turbidity-based plasma clotting assay
To enable clotting, a calcium-saline buffer (40 mM CaCl2 and 90 mM NaCl; Millipore Sigma) was added to citrated human normal plasma, human plasma immunodepleted of FXII, or PK (Affinity Biologicals), or dolphin (Pseudorca crassidens and Lagenorhynchus obliquidens) plasma in a 1:3 volumetric ratio. Dolphins were housed at the Vancouver Aquarium, with scheduled quarterly blood draws as part of their routine medical care. Blood plasma samples were obtained during scheduled blood draws. To inhibit FXIIa-mediated FXI activation, plasma was preincubated with 14E11 immunoglobulin G antibody (225 nM final) at 37°C for 15 minutes before adding the calcium-saline buffer. Plasma was recalcified in the absence or presence of TF (30 pM), soils (0.1 mg/mL), or clay (0.1 mg/mL). Clot formation was monitored by measuring the absorbance at 405 nm. Fibrin clot formation time was defined as the time for turbidity to reach one-half of the turbidity of a fully formed clot.
Thrombin generation in plasma
A fluorescent thrombin substrate (Boc-Asp[OBzl)-Pro-Arg-MCA, Peptide Institute Inc) was added to the calcium-saline buffer (1 µM) before the buffer was added to plasma. For mouse plasma, calcium-saline buffer (200 mM CaCl2 and 90 mM NaCl) was added in a 1:8 volumetric ratio. After addition of the calcium-saline buffer, the re-calcified human or mouse plasma was incubated at 37°C for 1 hour with soils (0.1 mg/mL), clay (0.1 mg/mL), or TF (30 pM). To inhibit FXIIa activity, plasma was preincubated with corn trypsin inhibitor (75 µg/mL; CTI, Haematological Technologies) for 15 minutes before adding calcium-saline buffer. Fluorescence (λEx/ λEm = 360/465 nm) was measured and the rate of thrombin generation was determined using a standard curve that correlated thrombin concentration to the rate of change in fluorescence.
Chromogenic assay for FXIIa and kallikrein activities
To activate plasma-FXII, soils and clay (0.1 mg/mL) were added to dilute citrated normal human plasma (1:5 in 0.9% NaCl). A chromogenic substrate for FXIIa (0.8 mM, H-D-CHA-Gly-Arg-pNA-2AcOH; 5-Diagnostic, Aniara, and BioPacific Diagnostic Inc.) was added immediately, and the absorbance at 405 nm was monitored at 37°C for 30 minutes. This substrate also has activity with kallikrein.30 Rates of change in absorbance were converted to amount of p-nitroaniline formed by using the extinction coefficient of p-nitroaniline at 405 nm (9450 M-1 cm-1 [Bachem] path length = 0.28 cm [Promega]).
Purified FXII, prekallikrein (PK), and high-molecular-weight kininogen (HK) were from Enzyme Research Laboratory. To activate purified FXII (without plasma), soil B or kaolinite (0.1 mg/mL) was incubated with FXII (100 nM) in buffer (50 mM Tris base, 150 mM NaCl at pH 7.9) at 37°C for 1 hour (100 μL reaction). PK and HK were included in some reactions (20 nM each). To activate PK, soil B or kaolinite (0.1 mg/mL) was incubated with PK and HK (50 nM each). PK and HK were also incubated with purified FXIIa (100 pM) as positive control to generate kallikrein. After a 1-hour incubation, samples were centrifuged to pellet the soil components, and an aliquot (70 μL) was transferred to a 96-well plate. Kallikrein activity was quenched by adding aprotinin (100 μg/mL), and FXIIa activity was quenched by adding CTI (100 μg/mL) followed by incubation at room temperature for 5 minutes. FXIIa or kallikrein-specific amidolytic activity was measured using the same chromogenic substrate and same method of analysis as described in plasma. The amidolytic activity of purified FXIIa (70 nM) was measured as a control.
Western blot analysis of high-molecular-weight kininogen
Diluted citrated normal plasma (1:4 in 0.9% NaCl) was supplemented with CTI (20 µg/mL) or phosphate-buffered saline, and then incubated with or without soil (0.1 mg/mL) at 37°C. After 0 and 30 minutes of incubation, aliquots were removed and mixed with a buffer containing sodium dodecyl sulfate. Samples were heated to 100°C for 10 minutes before size electrophoresis on a 4% to 15% Mini-PROTEAN TGX gel (Bio-Rad), and transferred to a nitrocellulose membrane (GE Healthcare). The membrane was blocked with Odyssey Blocking Buffer (Li-Cor) and incubated with a monoclonal rabbit anti-human primary antibody against kininogen 1 (Abcam). The membrane was washed with phosphate-buffered saline containing 0.1% Tween 20 (VWR). After incubating with the preadsorbed secondary antibody (Abcam, diluted 1/15 000), the membrane was washed, and the antibody was detected with ECL substrate (Bio-Rad). The intensities of the full-length (FL) and light chain fragment (LCF) bands were analyzed using ImageJ software.
Separating soils into high- and low-clay fractions
Uncrushed soil samples were mixed thoroughly with 10% NaCl (brine, 0.1 mg/mL) and allowed to settle. Clay particles remained in the supernatant, and low-clay (LC) particles settled to the bottom of a 50-mL falcon tube (VWR). After 10 minutes, the supernatant was decanted, centrifuged at 7650g for 30 minutes, washed with water, and centrifuged again at 7650g for 30 minutes to collect the high-clay (HC) soil fraction as a pellet. The LC soil fraction was washed with water and pelleted. Both HC and LC soil fractions were dried and crushed until they could be sifted through a 0.106 mm sieve.
Sterilizing and removing organic matter and endotoxin from soils
Soils were sterilized by autoclaving at 120°C and 13 ψ for 20 minutes. To remove organic matter (OM), soils were suspended in a sodium pyrophosphate solution (0.1 M, pH 10; Millipore Sigma) and incubated for 18 hours at room temperature with constant mixing. Solids were pelleted by centrifuging at 7650g for 30 minutes. The supernatant containing the OM was removed. The soil pellet, depleted in OM, was washed with water, dried, and crushed until it could be sifted through a 0.106-mm sieve. To remove endotoxin from soils, approximately 100 mg of each soil was heated in borosilicate glass test tubes with a Bunsen burner flame for 1 hour. Endotoxin was measured with a Pierce Chromogenic Endotoxin Quantification Kit (Thermo Fisher Scientific).
ζ potential of soil particles
Soils and minerals were suspended in water (1 mg/mL). The ζ potential of the particles was measured at 37°C, using dynamic light scattering (Zetasizer NanoZS, Malvern Panalytical).
Time-of-flight secondary ionization mass spectrometry analysis of soil surface
Data were acquired in the positive mode on a Trift V nanoTOF instrument (Physical Electronics Inc.) with 30 keV Au+ primary ion source in bunched mode. The analysis area was 400 × 400 μm2, and the total ion dose was less than 1012 ions per cm2. Charge compensation was accomplished using 10 eV electrons. The mass spectra were collected in positive mode, in the mass-to-charge range of 0 to 1850 m/z. Positive spectra were analyzed using WinCadence (version 1.18.1, Physical Electronics Inc) with mass calibration, using the H, CH3, and C2H5 peaks. The relative concentrations of Cu, Fe, Si, and Zn on the soil surface were reported as the area under the curve of the respective peaks, assuming that the matrix effect was not a significant factor.
Viscoelastic-based plasma clotting assays
Clotting of chicken plasma (BiolIVT) could not be monitored using the turbidity-based plasma clotting assay because absorbance changes were undetectable during clot formation. Instead, thromboelastography (hemostasis analyzer system, Haemonetics), a technique that measures the viscoelastic properties of plasma, was used. Citrated chicken plasma was recalcified in the presence of soils (0.1 mg/mL), clay (0.1 mg/mL), or TF (30 pM), and the time until fibrin formation began (R-time) was determined.
Statistical analysis
Data are presented as mean ± SEM. A Shapiro-Wilkes test was performed to determine whether data were normally distributed. In Figure 1, the blood losses in the same strains were compared by Kruskal-Wallis 1-way analysis of variance, followed by Dunn’s multiple comparison test. Blood losses between strains were compared using Mann-Whitney U test, because unlike the comparisons between the same strains, only pairwise comparisons were made between strains. In Figures 2-4, comparisons between multiple groups were performed with regular 1-way analysis of variance followed by Tukey’s multiple comparison test. Pairwise comparisons were performed with unpaired Student t test. Correlation was determined with Pearson’s correlation, and a best fit line is shown for statistically significant correlated observations. Analyses of variance were 1-sided tests, whereas Mann-Whitney U tests, unpaired Student t tests, and Pearson’s correlation were 2-sided tests. Statistical analyses were performed with GraphPad Prism 8 (GraphPad Software).
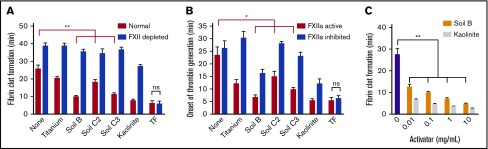
Figure 2.
Soils accelerate clotting of plasma, dependent on FXII. (A) Clot times of human normal and FXII-depleted plasma by soils and kaolinite clay. (B) Onset of thrombin generation in human plasma by soils with CTI (FXIIa inhibited) or without CTI (FXIIa active). (C) Clot times with increasing concentration of soil and kaolinite, measured by thromboelastography. n = 8-9 (A-B), n = 5 (C). *P < .01; **P < .001. Error bars represent SEM.
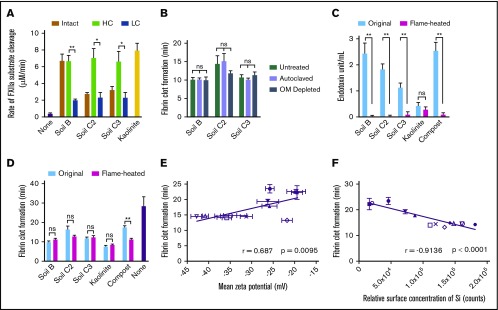
Figure 4.
Silicates are the component of soil that triggers faster clotting via activation of FXII. (A) FXII activation in plasma by intact, HC, and LC fractionated soils. (B) Clot times of plasma by sterilized (autoclaved) or OM-depleted soils. (C) Endotoxin quantification of soils after treating with a flame for 1 hour (flamed-heated). (D) Clot times of plasma by original and flame-heated soils. (E) Pearson’s correlation between clot times and ζ potential of soils, determined by dynamic light scattering. (F) Pearson’s correlation between clot times and concentration of Si on the surface of soils, determined by TOF-SIMS. Substrate cleavage and clot formation measurements were n = 5 (A, C-F) and n = 3 in triplicate (B). *P < .01; **P < .001. Error bars represent SEM.
Results
FXII augments hemostasis in soil-contaminated wounds
To determine whether soil triggers FXII activation and reduces blood loss, soils and other materials were gently applied to wounds made by transecting tails of mice. Soil significantly reduced the volume of blood loss in WT C57Bl/6 mice (Figure 1A) and accelerated onset of thrombin generation in mouse plasma in vitro (supplemental Figure 1). There was a significant difference in bleeding when soil was applied to wounds, with FXII−/− mice losing greater than 100-fold more blood than WT mice (WT = 0.02 ± 0.02 μL/g; FXII−/− = 2.37 ± 0.54 μL/g). In contrast, WT mice had similar blood loss to FXII−/− mice when no materials were applied to the wounds (WT = 2.92 ± 0.62 μL/g; FXII−/− = 2.11 ± 0.38 μL/g) or when recombinant TF, a potent physiological activator of clotting, was applied (WT = 0.07 ± 0.07 μL/g; FXII−/− = 0.18 ± 0.12 μL/g), consistent with the previous findings that FXII−/− mice with clean wounds do not appear to have a bleeding phenotype.18,19
Soil was compared with kaolinite clay, which is an aluminum silicate and a potent activator of FXII,9,10 and to fine titanium metal particles, which are a relatively weak activator of FXII31 and are expected to have a similar mechanical barrier effect as soil in the wound. To increase the bleeding challenge and enable differences in blood loss between topical treatments to be detected more easily, ASA was administered in these cohorts before tail transection. ASA inhibits platelet aggregation, and thus increased blood loss, in both WT and FXII−/− mice when TF was applied. When a soil with a high clay content was applied (45%, soil B) to WT mice receiving ASA, blood loss was approximately sevenfold less than when no materials were applied. There was no significant difference in blood loss among soils, kaolinite, or TF (Figure 1B). Blood loss after application with soil B was about fourfold less than with titanium, indicating that the soil was stemming bleeding through more than a simple mechanical effect. The blood loss after applying a soil containing less clay (2.5%, soil C2) to the wound was more variable, but was still significantly less than when no materials were applied. In contrast, soil did not decrease blood loss in FXII−/− mice compared with when no materials were applied (Figure 1C), supporting the premise that the effect of soil on reducing bleeding from wounds in WT mice was FXII-dependent.
Soils accelerate clotting of plasma from humans by directly activating FXII
Soils triggered clotting in human blood plasma in 2 complementary assays, causing faster fibrin formation (Figure 2A) and onset of thrombin generation (Figure 2B) compared with plasma without soil. Clot times with soil were significantly slower when plasma was immunodepleted of FXII (FXII depleted) or when FXIIa was inhibited by CTI (FXIIa inhibited). Clot times with TF were similar for all plasmas. Higher concentrations of soil clotted plasma faster, as measured by thromboelastography (Figure 2C).
PK, in complex with the nonenzymatic cofactor HK, binds to surfaces and is activated by surface-bound FXIIa into the protease kallikrein, which activates more FXII.32 Soil triggered clotting significantly faster in plasma immunodepleted of PK (PK depleted) than in FXII depleted plasma (Figure 3A). Soil also promoted FXIIa-dependent cleavage of FL HK, an endogenous substrate of plasma kallikrein (Figure 3B-C). Although purified PK was not autoactivated to kallikrein when incubated with soil, the activation of purified FXII by soil was amplified in the presence of PK and HK (Figure 3D). FXIIa promotes coagulation by activating FXI, a homolog of PK.7 A monoclonal immunoglobulin G antibody to FXI that specifically inhibits FXI activation by FXIIa (14E11)33 significantly prolonged clot times with soil (Figure 3E).
Figure 3.
Soils accelerate clotting of plasma from humans but not from dolphins or a bird. (A) Clot times of normal, PK-depleted, or FXII-depleted plasma by soils. (B) Western blot analysis of plasma, supplemented with or without CTI, after incubation with soil B. Full-length (FL) HK was fully converted to light chain fragment (LCF) after 30 minutes. No HK or LCF is seen in the lane with HK-depleted plasma. (C) Quantifying data in panel E, using densitometry of bands of FL and LCF. (D) Activation of purified PK and HK by soil. Dashed lines represent fully converted FXIIa and kallikrein activity after activation of PK by purified FXIIa. (E) Clot times with an antibody that inhibits FXI activation by FXIIa (14E11). (F) Clot times of plasma, comparing FXII-deficient vertebrates with humans. n = 5. *P < .01; **P < .001. Error bars represent SEM.
Soil does not accelerate clotting in plasma from dolphins or a bird
It may be less advantageous for soil to contribute to hemostasis in animals that spend less time living in and on soil. Consistent with this logic, birds and cetaceans (whales, dolphins, and porpoises) lack FXII.7 Soil did not trigger clotting in plasmas from 2 species of dolphin, nor in chickens (representing birds; Figure 3F). In the presence of soil, dolphin plasma clotted 3 times slower compared with human plasma, and chicken plasma clotted 7 times slower. In contrast, there were no significant differences in clot times between these vertebrate plasmas when coagulation was induced with TF.
Silicates are the primary procoagulant component of soil
To identify the procoagulant component of the soils, several soils were separated into HC and LC fractions, which contain higher and lower concentrations of silicates, respectively, by extracting clay particles with brine. In plasma, HC fractions activated FXII significantly faster than LC fractions (Figure 4A). Sterilizing the soil or depleting it of most of the OM did not alter clot times (Figure 4B). Removing most endotoxin from soil by heating with a flame did not alter clot times (Figure 4C-D). Many of the surfaces that activate FXII have a net negative charge.10 Divalent and transition metal ions, such as Ni2+, Cu2+, Co2+, Zn2+, and Fe3+, can enhance FXII activation.34 All 13 soils tested in the study had a negative ζ potential, indicating a net negative charge. However, their potency to accelerate clotting only moderately correlated with the magnitude of ζ potential (Figure 4E). The elemental composition at the soil surfaces was analyzed by time-of-flight secondary ionization mass spectrometry (TOF-SIMS). There was a moderate correlation between clot time and surface concentrations of Zn and Fe, whereas the correlation between clot time and surface concentration of Cu was not significant (supplemental Figure 2). Ni and Co were not detected on the surfaces of soils. The surface concentration of Si, which reflects the concentration of silicates in soil, correlated strongly with the capacity to accelerate clotting (Figure 4F). Consistent with the well-known activity of purified silicates,9,10 these results suggest that silicates in soil promote blood coagulation through direct activation of FXII.
Discussion
Although there has been speculation that natural silicates may be activators of FXII during injury, this hypothesis has not previously been tested.23,24 FXII is considered dispensable for hemostasis, even though silicates are probably common wound contaminants in terrestrial mammals.23-26 In this study, we show that soil has a potent effect on bleeding after injury when FXII is present. Soils activate FXII and trigger faster thrombin generation and clot formation in plasma from humans and mice in vitro. The hemostatic capacity of soils was evaluated in a murine tail bleeding model. In WT mice, soil-contaminated wounds had significantly reduced blood loss compared with clean wounds. Soil did not reduce blood loss in FXII−/− mice, suggesting that soil augments hemostasis in a FXII-dependent manner. The ability of soil to reduce blood loss was not the result of a simple mechanical effect, as more blood loss was observed when wounds were treated with titanium particles, a comparatively weak activator of FXII.31 The results indicate that FXII may contribute to hemostasis when wounds are in contact with soil. Similar to TF,35 the initiator of the extrinsic pathway of coagulation, the initiator of FXII activation is not normally present in blood, and only comes into contact with blood at injury sites. TF in saliva can also augment hemostasis when applied externally to wounds by licking.36 In clean wounds (those lacking soil contamination), FXII-dependent hemostasis would presumably not occur because an activator of FXII is missing. FXII-dependent hemostasis may be especially beneficial for small terrestrial mammals in their native environments, to minimize the amount of blood loss after injury.
The plasma contact system consists of FXII, FXI, PK, and HK. When blood is exposed to a surface, activation of these proteins is driven by FXIIa, making contributions to coagulation and inflammation.37 Soil can activate FXII independent of PK, but the process is enhanced in the presence of PK and HK because kallikrein participates in a positive feedback loop to activate more FXII.10 Kallikrein activates the complement system by cleaving complement factor C3 into C3a and C3b, which exerts potent antimicrobial activities.38-40 Cleavage of HK by kallikrein leads to the release of bradykinin, a short inflammatory peptide that mediates vasodilation and vascular permeability,41 neutrophil chemotaxis,42 and stimulation of peripheral nociceptors,43 and peptides with antimicrobial activities in purified systems and in plasma.44,45 Thus, soil-mediated FXII activation may contribute to host defense against infections.
Plasma from dolphins and chickens had prolonged clot times with soil compared with human plasma, consistent with absence of FXII. Although it may be advantageous for soil to contribute to hemostasis in terrestrial mammals, it is likely less so for animals such as cetaceans and birds that evolved without consistent contact with soil. Observations from phylogenetic studies support this logic. Although FXII first appeared in vertebrate evolution just before the emergence of tetrapods, the FXII gene is not found in ray-finned and cartilaginous fish, has been lost in the lineage leading to birds, and has been converted to a pseudogene incapable of protein expression in cetaceans.7,8 During vertebrate evolution, the increasing complexity of the hemostatic system in the lineage leading to mammals coincided with important changes in the circulatory system.5 Higher arterial pressure increases risk for excessive bleeding after injury, and likely provided a strong selective pressure for changes and/or additions to the core hemostatic system common to all verterbrates.5,6 A role for FXII in augmenting hemostasis in soil-contaminated wounds in terrestrial mammals may have had adaptive value, and would explain why FXII has persisted across evolution of terrestrial mammals, despite its apparently minor role in hemostasis in clean wounds.
Parts of the blood coagulation system of vertebrates may have evolved as a by-product of the innate immune system.46,47 It is likely that the FXII-driven contact system initially served a role in immunity in lower vertebrates, consistent with FXII responding to soil-based silicates in wounds. However, its contribution may have been independent of blood coagulation. FXIIa drives coagulation primarily through activation of FXI, a homolog (paralog) of PK. Although PK is found in the earliest terrestrial vertebrates, FXI arose during mammalian evolution as the result of a duplication of the PK gene.5,7 Because FXI is absent in nonmammalian vertebrates, activation of FXII by soil may have contributed to host defense against infection via FXIIa-mediated activation of PK and kallikrein-mediated cleavage of HK, rather than hemostasis. Contact activation and FXII-dependent coagulation can limit bacterial dissemination, recruit leukocytes, and promote bacterial clearance.48,49 In mammals, therefore, FXII may contribute to limiting blood loss and fighting infections. It remains to be determined whether soil-mediated activation of the contact system triggers host injury responses to prevent infection in vivo, and if there are any effects on additional downstream aspects of tissue repair.
Silicate-rich fractions of soils are the major contributor to soil-mediated FXII activation. Soils that were sterilized, depleted of OM or flame-heated triggered clotting similarly to untreated soils. This suggests that the procoagulant response mediated by the soils tested was not a result of live microbes, microbial proteases, endotoxin, or other OM. All soils tested had a net negative charge, although the magnitude of the charge only moderately correlated with clotting times. Despite previous reports of divalent and transition metals enhancing FXII activation on certain surfaces,34 the rate of soil-induced clotting only moderately correlated with the surface concentration of Fe and Zn. Instead, the procoagulant capacity of each soil could be strongly predicted by the surface concentration of Si, reflecting the amount of silicates present in the soil. This is consistent with the well-known ability of purified silicates to activate FXII.9,10
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank J. Kim (Interfacial Analysis & Reactivity Laboratory, University of British Columbia) for help with TOF-SIMS. The authors thank the animals used in this study.
This work was funded by the Canadian Institutes of Health Research (FDN-148370, MSH-130166), the Natural Sciences and Engineering Research Council (RGPIN 2018-04918), the Michael Smith Foundation for Health Research (16498), the Canadian Foundation for Innovation (31928), and the BC Knowledge Development Fund.
Footnotes
For original data, please contact Christian J. Kastrup at ckastrup@msl.ubc.ca
Authorship
Contribution: L.J.J. designed and performed most experiments, analyzed and interpreted data, and wrote the paper; N.M., S.K.N., and E.N.P.P helped perform experiments, analyzed and interpreted data, and edited the paper; M.H. collected the dolphin plasma and interpreted data; D.G. provided FXII−/− mice and 14E11 antibody, helped design experiments, interpreted data, and wrote the paper; L.M.L. helped design experiments and provided soil and clay samples; and C.J.K. designed experiments, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christian J. Kastrup, University of British Columbia, 2185 East Mall Room 211, Vancouver, BC V6T 1Z4, Canada; e-mail: ckastrup@msl.ubc.ca.
References
- 1.Semple S, Cowlishaw G, Bennett PM. Immune system evolution among anthropoid primates: parasites, injuries and predators. Proc Biol Sci. 2002;269(1495):1031-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terio KA, Kinsel MJ, Raphael J, et al. Pathologic lesions in chimpanzees (Pan trogylodytes schweinfurthii) from Gombe National Park, Tanzania, 2004-2010. J Zoo Wildl Med. 2011;42(4):597-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3-S11. [DOI] [PubMed] [Google Scholar]
- 4.Curry N, Hopewell S, Dorée C, Hyde C, Brohi K, Stanworth S. The acute management of trauma hemorrhage: a systematic review of randomized controlled trials. Crit Care. 2011;15(2):R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doolittle RF. Step-by-step evolution of vertebrate blood coagulation. Cold Spring Harb Symp Quant Biol. 2009;74(0):35-40. [DOI] [PubMed] [Google Scholar]
- 6.Fiusa MM, Carvalho-Filho MA, Annichino-Bizzacchi JM, De Paula EV. Causes and consequences of coagulation activation in sepsis: an evolutionary medicine perspective. BMC Med. 2015;13(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ponczek MB, Gailani D, Doolittle RF. Evolution of the contact phase of vertebrate blood coagulation. J Thromb Haemost. 2008;6(11):1876-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chana-Muñoz A, Jendroszek A, Sønnichsen M, et al. Origin and diversification of the plasminogen activation system among chordates. BMC Evol Biol. 2019;19(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margolis J. Initiation of blood coagulation by glass and related surfaces. J Physiol. 1957;137(1):95-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tillman BF, Gruber A, McCarty OJT, Gailani D. Plasma contact factors as therapeutic targets. Blood Rev. 2018;32(6):433-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller F, Mutch NJ, Schenk WA, et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139(6):1143-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Meijden PE, Munnix IC, Auger JM, et al. Dual role of collagen in factor XII-dependent thrombus formation. Blood. 2009;114(4):881-890. [DOI] [PubMed] [Google Scholar]
- 13.Griffin JH. Role of surface in surface-dependent activation of Hageman factor (blood coagulation factor XII). Proc Natl Acad Sci USA. 1978;75(4):1998-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanov I, Matafonov A, Sun MF, et al. Proteolytic properties of single-chain factor XII: a mechanism for triggering contact activation. Blood. 2017;129(11):1527-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yau JW, Stafford AR, Liao P, Fredenburgh JC, Roberts R, Weitz JI. Mechanism of catheter thrombosis: comparison of the antithrombotic activities of fondaparinux, enoxaparin, and heparin in vitro and in vivo. Blood. 2011;118(25):6667-6674. [DOI] [PubMed] [Google Scholar]
- 16.Yau JW, Liao P, Fredenburgh JC, et al. Selective depletion of factor XI or factor XII with antisense oligonucleotides attenuates catheter thrombosis in rabbits. Blood. 2014;123(13):2102-2107. [DOI] [PubMed] [Google Scholar]
- 17.Ramot B, Singer K, Heller P, Zimmerman HJ. Hageman factor (HF) deficiency. Blood. 1956;11(8):745-752. [PubMed] [Google Scholar]
- 18.Pauer HU, Renné T, Hemmerlein B, et al. Targeted deletion of murine coagulation factor XII gene-a model for contact phase activation in vivo. Thromb Haemost. 2004;92(3):503-508. [DOI] [PubMed] [Google Scholar]
- 19.Renné T, Pozgajová M, Grüner S, et al. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202(2):271-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker SE, Sawvel AM, Zheng N, Stucky GD. Controlling bioprocesses with inorganic surfaces: layered clay hemostatic agents. Chem Mater. 2007;19(18):4390-4392. [Google Scholar]
- 21.Amundson R, Berhe AA, Hopmans JW, Olson C, Sztein AE, Sparks DL. Soil science. Soil and human security in the 21st century. Science. 2015;348(6235):1261071. [DOI] [PubMed] [Google Scholar]
- 22.Ito A, Wagai R. Global distribution of clay-size minerals on land surface for biogeochemical and climatological studies. Sci Data. 2017;4(1):170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooley BC. The dirty side of the intrinsic pathway of coagulation. Thromb Res. 2016;145:159-160. [DOI] [PubMed] [Google Scholar]
- 24.Zhu S, Herbig BA, Yu X, Chen J, Diamond SL. Contact pathway function during human whole blood clotting on procoagulant surfaces. Front Med (Lausanne). 2018;5:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Springer DA, Phillippi-Falkenstein K, Smith G. Retrospective analysis of wound characteristics and tetanus development in captive macaques. J Zoo Wildl Med. 2009;40(1):95-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhattacharya S. Wound healing through the ages. Indian J Plast Surg. 2012;45(2):177-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jelinek G, Kelly AM, Brown A. Textbook of Adult Emergency Medicine. 4th ed Edinburgh, United Kingdom: Churchill Livingstone; 2014. [Google Scholar]
- 28.Luttmerding HA. Technical data: soil profile descriptions and analytical data Kelowna, BC, Canada: British Columbia Ministry of Environment; 1981. [Google Scholar]
- 29.Sheldrick BH, Wang C. Data quality report and compilation of data for ECSS reference soil samples. CLBR, contribution no. 94-46 Ottawa: Agriculture and Agri-Food Canada; 1995. [Google Scholar]
- 30.Puy C, Tucker EI, Wong ZC, et al. Factor XII promotes blood coagulation independent of factor XI in the presence of long-chain polyphosphates. J Thromb Haemost. 2013;11(7):1341-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arvidsson S, Askendal A, Tengvall P. Blood plasma contact activation on silicon, titanium and aluminium. Biomaterials. 2007;28(7):1346-1354. [DOI] [PubMed] [Google Scholar]
- 32.Wiggins RC, Bouma BN, Cochrane CG, Griffin JH. Role of high-molecular-weight kininogen in surface-binding and activation of coagulation factor XI and prekallikrein. Proc Natl Acad Sci USA. 1977;74(10):4636-4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng Q, Tucker EI, Pine MS, et al. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood. 2010;116(19):3981-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mutch NJ, Waters EK, Morrissey JH. Immobilized transition metal ions stimulate contact activation and drive factor XII-mediated coagulation. J Thromb Haemost. 2012;10(10):2108-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butenas S, Bouchard BA, Brummel-Ziedins KE, Parhami-Seren B, Mann KG. Tissue factor activity in whole blood. Blood. 2005;105(7):2764-2770. [DOI] [PubMed] [Google Scholar]
- 36.Berckmans RJ, Sturk A, van Tienen LM, Schaap MC, Nieuwland R. Cell-derived vesicles exposing coagulant tissue factor in saliva. Blood. 2011;117(11):3172-3180. [DOI] [PubMed] [Google Scholar]
- 37.Long AT, Kenne E, Jung R, Fuchs TA, Renné T. Contact system revisited: an interface between inflammation, coagulation, and innate immunity. J Thromb Haemost. 2016;14(3):427-437. [DOI] [PubMed] [Google Scholar]
- 38.Nordahl EA, Rydengård V, Nyberg P, et al. Activation of the complement system generates antibacterial peptides. Proc Natl Acad Sci USA. 2004;101(48):16879-16884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sonesson A, Ringstad L, Nordahl EA, Malmsten M, Mörgelin M, Schmidtchen A. Antifungal activity of C3a and C3a-derived peptides against Candida. Biochim Biophys Acta. 2007;1768(2):346-353. [DOI] [PubMed] [Google Scholar]
- 40.Yuste J, Sen A, Truedsson L, et al. Impaired opsonization with C3b and phagocytosis of Streptococcus pneumoniae in sera from subjects with defects in the classical complement pathway. Infect Immun. 2008;76(8):3761-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marceau F, Regoli D. Bradykinin receptor ligands: therapeutic perspectives. Nat Rev Drug Discov. 2004;3(10):845-852. [DOI] [PubMed] [Google Scholar]
- 42.Ehrenfeld P, Millan C, Matus CE, et al. Activation of kinin B1 receptors induces chemotaxis of human neutrophils. J Leukoc Biol. 2006;80(1):117-124. [DOI] [PubMed] [Google Scholar]
- 43.Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 2010;120(11):3760-3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nordahl EA, Rydengård V, Mörgelin M, Schmidtchen A. Domain 5 of high molecular weight kininogen is antibacterial. J Biol Chem. 2005;280(41):34832-34839. [DOI] [PubMed] [Google Scholar]
- 45.Frick IM, Akesson P, Herwald H, et al. The contact system–a novel branch of innate immunity generating antibacterial peptides. EMBO J. 2006;25(23):5569-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delvaeye M, Conway EM. Coagulation and innate immune responses: can we view them separately? Blood. 2009;114(12):2367-2374. [DOI] [PubMed] [Google Scholar]
- 47.Krem MM, Di Cera E. Evolution of enzyme cascades from embryonic development to blood coagulation. Trends Biochem Sci. 2002;27(2):67-74. [DOI] [PubMed] [Google Scholar]
- 48.Herwald H, Mörgelin M, Olsén A, et al. Activation of the contact-phase system on bacterial surfaces–a clue to serious complications in infectious diseases. Nat Med. 1998;4(3):298-302. [DOI] [PubMed] [Google Scholar]
- 49.Berends ET, Kuipers A, Ravesloot MM, Urbanus RT, Rooijakkers SH. Bacteria under stress by complement and coagulation. FEMS Microbiol Rev. 2014;38(6):1146-1171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.