Key Points
In adult ITP, a platelet count threshold was recognized for mucosal and organ bleeding but not for skin bleeding.
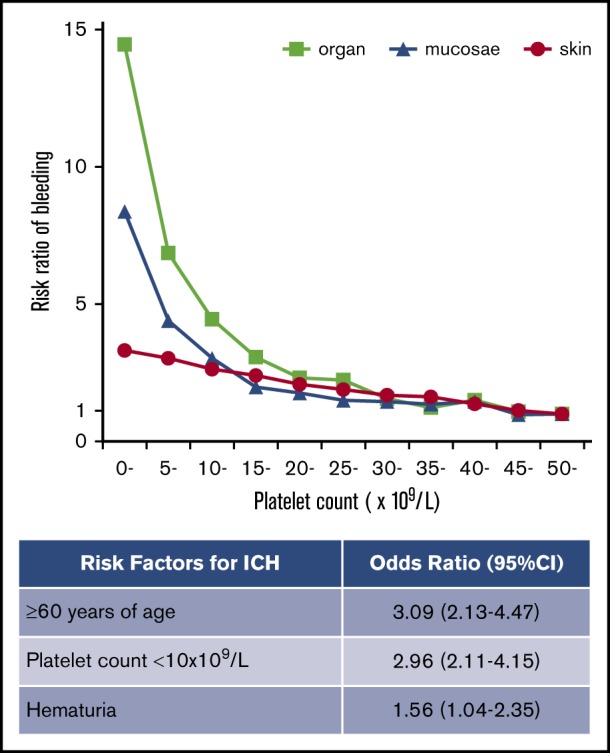
Risk factors for ICH in adult ITP were age ≥60 years, platelet count <10 × 109/L, and presence of hematuria.
Abstract
Bleeding manifestations in primary immune thrombocytopenia (ITP) range from skin petechiae to life-threatening intracranial hemorrhage (ICH). However, the relation between these various bleeding manifestations and the platelet count in ITP remains poorly characterized. Using a nationwide database of patients with ITP during the years 2005 to 2014 (10 years) in Japan, we analyzed 19 415 adult patients newly diagnosed with ITP, including 222 with ICH. The frequency of skin purpura was 64.8%, and this increased linearly with thrombocytopenia without a specific platelet count threshold. In contrast, mucosal bleeding (epistaxis and gingival bleeding) and organ bleeding (melena, hematuria, and ICH) increased exponentially with thrombocytopenia at a platelet count threshold of 10 to 15 × 109/L. Age showed a much weaker correlation than platelet count with skin and mucosal bleeding. However, the incidence of organ bleeding increased exponentially above 60 years of age. Multivariate analysis showed that the presence of mucosal bleeding was a risk factor for occurrence of melena and hematuria but not for ICH. The frequency of ICH was 1.1% and risk factors for ICH were age ≥60 years (odds ratio [OR], 3.09; 95% confidence interval [CI], 2.13-4.47; P < .001), platelet count <10 × 109/L (OR, 2.96; 95% CI, 2.11-4.15; P < .001), and the presence of hematuria (OR, 1.56; 95% CI, 1.04-2.35; P = .033). The relation between ICH and platelet count varied with age. This large-scale analysis of risk factors for bleeding in ITP has revealed distinct characteristics of skin, mucosal, and organ bleeding in adult patients with newly diagnosed ITP, thus indicating those who are at a high risk of severe organ bleeding.
Visual Abstract
Introduction
Primary immune thrombocytopenia (ITP) is an autoimmune disorder characterized by isolated thrombocytopenia and varying degrees of bleeding manifestations.1 Many patients with ITP have mild bleeding symptoms, including skin petechiae and purpura, and some have mucosal bleeding that includes epistaxis and gingival bleeding for which medical intervention may be required. Conversely, a small minority of patients experience severe organ bleeding, including intracerebral hemorrhage (ICH) and gastrointestinal bleeding, which have a large impact on morbidity and mortality.2-4
It is generally believed that more profound thrombocytopenia results in more severe bleeding. A previous study of 54 pediatric patients with ITP showed that the severity of skin and oral bleeding was correlated with platelet count, whereas that of epistaxis and organ bleeding was not.5 A recent study of 302 adult patients with ITP found that the frequency of mucosal bleeding or any bleeding increased significantly below a platelet count of 20 × 109/L, whereas organ bleeding, including ICH, gastrointestinal bleeding, and macroscopic hematuria, was not significantly correlated with platelet count.6 These studies suggested an association of platelet count with mild bleeding manifestations but not with severe organ bleeding. One concern about this suggestion is that the rarity of severe organ bleeding may prevent sufficient statistical analysis. The largest study to have analyzed risk factors for ICH was reported by Psaila et al.7 In that study, 40 patients with ICH and 80 matched ITP control subjects were analyzed, and a platelet count <10 to 20 × 109/L, bleeding symptoms beyond petechiae and ecchymosis, hematuria, and head trauma were identified as risk factors for ICH. In the largest study of ICH in adult patients with ITP reported to date, multivariate analysis of 27 patients identified occurrence of any life-threatening bleeding and nonresponse to steroid as risk factors for ICH.8 Surprisingly, no previous studies of adult ITP have reported a significant correlation between platelet count and ICH.6,8-11 Because the incidence of ICH is reportedly 1.4% and 0.4% in adults and children with ITP, respectively,12 more precise analysis of this rare event requires much larger cohorts.
In the current study, we used a nationwide database of patients in Japan with ITP and analyzed 19 415 adult patients with ITP, including 222 with ICH. This is the largest study to have analyzed risk factors for bleeding in ITP. Our aims were to characterize skin, mucosal, and organ bleeding, and to identify risk factors for severe bleeding events, including ICH.
Materials and methods
Database
In Japan, primary ITP is designated as one of the intractable diseases for which treatment is subsidized by the government. When physicians diagnose primary ITP, they apply to regional public agencies for medical aid. Experts in hematology check the application form to exclude any cases that might be misdiagnosed as other thrombocytopenic diseases. The data on patients with primary ITP are then collected by the Ministry of Health, Labor, and Welfare of Japan and used for registration in the national database of ITP patients. This database includes details of patient characteristics, bleeding manifestations, and laboratory tests.13 Use of the database for the present study was approved by the Ministry of Health, Labor, and Welfare of Japan, and our analytical procedure was approved by the Ethics Committee of Ehime University Hospital.
Study population
The database comprises 2 categories of patients: newly diagnosed and yearly renewed. For newly diagnosed patients, a close relation between platelet count and bleeding manifestations is evident in the database, as platelet count and bleeding manifestations are recorded simultaneously at diagnosis. Conversely, in the database for yearly renewed patients, the lowest platelet count and any bleeding manifestations observed within 1 year are reported, implying no temporal relationship between the platelet count and bleeding manifestations. Therefore, for this study, we analyzed the database for newly diagnosed patients during the years 2005 to 2014 (the most recent 10-year period currently available).
There were few pediatric patients in the database because separate medical aid is available for pediatric patients with ITP in Japan, and many pediatricians prefer to use this child-directed aid. This study therefore analyzed adult patients only (ie, ≥18 years of age).
Bleeding manifestations
Bleeding manifestations listed in the database were purpura, gingival bleeding, epistaxis, hypermenorrhea, hematuria, melena, and ICH. The severity of each type of bleeding was not graded on a scale. Purpura includes skin bleeding such as petechiae and ecchymosis. Hypermenorrhea was not evaluated in this study because it was limited to young female subjects, and its pathogenesis was diverse. Hematuria and melena usually refer to macroscopic bleeding, but patients with microscopic hematuria and positivity in the stool occult blood test were included. Diagnosis of ICH was left to the physician’s discretion, and detailed information about ICH was not recorded.
In addition to analysis of individual bleeding manifestation, we categorized bleeding manifestations as 3 types: skin, mucosal, and organ bleeding. Skin bleeding indicated purpura; mucosal bleeding included gingival bleeding and epistaxis; and organ bleeding included hematuria, melena, and ICH.
Statistical analyses
Platelet counts were categorized by 5 × 109/L, and those ≥50 × 109/L were grouped. Ages were categorized every 5 years, and those aged <30 years and those aged ≥80 years were grouped. Categorical variables, including sex and bleeding symptoms, were expressed as percentages and compared by using Pearson’s χ2 test. All tests were 2-sided, and a P value of .05 was considered to be significant. Significant independent predictors of bleeding events were identified by using logistic regression analysis. In this analysis, the following predictors were included as explanatory variables: sex, age, platelet count, purpura, gingival bleeding, epistaxis, hematuria, melena, and ICH. Age was categorized as <60 years and ≥60 years because the number of patients aged over and under 60 years was almost equal. Platelet count was categorized as <10 and ≥10 × 109/L based on the general practice of previous studies. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. All statistical analyses were performed by using SPSS version 22.0 (IBM Japan, Tokyo, Japan).
Results
Frequency of bleeding manifestations
During the 10-year period, 21 811 patients with newly diagnosed ITP were registered in the database. After excluding 1364 patients aged <18 years and 1032 with missing demographic or laboratory data, 19 415 evaluable patients were analyzed. The patients with any missing data accounted for 4.7% of the total and were not biased by age, sex, or region compared with patients analyzed.
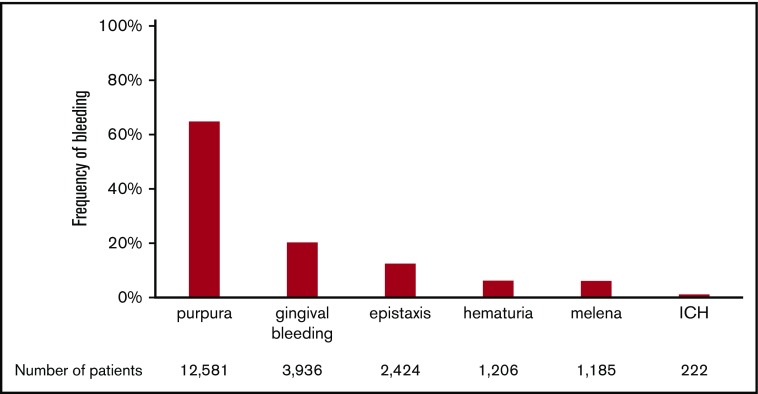
The frequencies of the various bleeding manifestations are shown in Figure 1. The most frequent bleeding manifestation was purpura, which was observed in 12 581 (64.80%) of 19 415 newly diagnosed patients. The next most frequent manifestation was gingival bleeding (20.27%), followed by epistaxis (12.49%), hematuria (6.21%), melena (6.10%), and ICH (1.14%) The incidence of ICH was within the range analyzed in a systematic review (1.4%; 95% CI, 0.9-2.1).12
Figure 1.
Frequency of bleeding manifestations in adult ITP. The percentage of patients with each bleeding manifestation is shown. Patients with multiple bleeding manifestations are included in each bleeding manifestation column. The total number of patients in each column is also shown at the bottom of the figure.
Relation between platelet count and bleeding manifestations
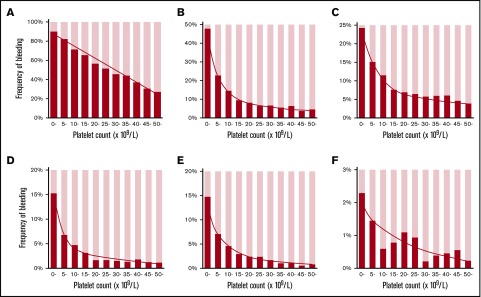
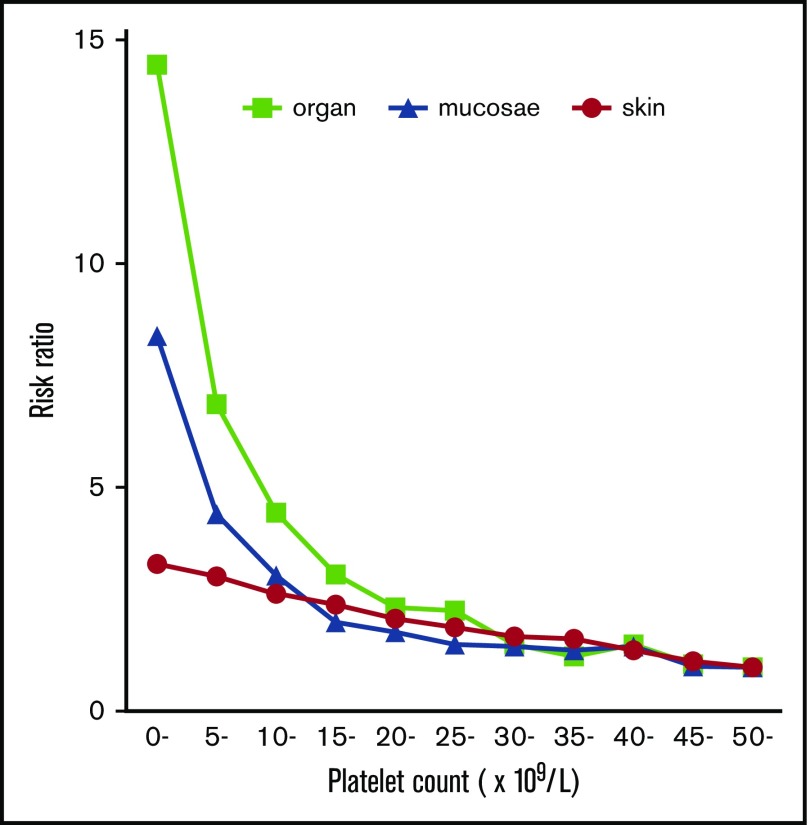
The frequency of purpura increased linearly with thrombocytopenia, exhibiting a strong negative correlation with platelet count (Figure 2A). No threshold for appearance of purpura was seen at platelet counts of ∼50 × 109/L. In contrast to purpura, the frequency of gingival bleeding and epistaxis had no linear relation with platelet count but increased steeply at platelet counts <15 × 109/L, which seemed to represent a threshold (Figure 2B-C). Similarly, the frequency of hematuria and melena increased exponentially at the same platelet count threshold (Figure 2D-E). ICH increased steeply below platelet counts of 10 × 109/L, although the correlation with platelet count was somewhat irregular (Figure 2F). When bleeding manifestations were categorized as skin, mucosal, and organ bleeding, and compared with the bleeding frequency at a platelet count >50 × 109/L, skin bleeding increased linearly by a factor of 3.31, whereas mucosal and organ bleeding increased exponentially by a factor of 8.38 and 14.46, respectively (Figure 3).
Figure 2.
Relation between platelet count and bleeding manifestations. The frequency of bleeding manifestations is expressed as the percentage of patients with the indicated bleeding manifestation for every 5000 platelet counts. Skin purpura (A); gingival bleeding (B); epistaxis (C); hematuria (D); melena (E); ICH (F). Patients with a platelet count ≥50 × 109/L were grouped together. The linear and curved line in each graph represents the overall change in the bleeding frequency. Note that each graph has a different y-axis scale.
Figure 3.
Risk ratio of skin, mucosal, and organ bleeding according to platelet count. The bleeding risk for patients with a platelet count ≥50 × 109/L was set to 1.0, and the relative risk ratio for every 5000 platelets was calculated.
Relation between age and bleeding manifestations
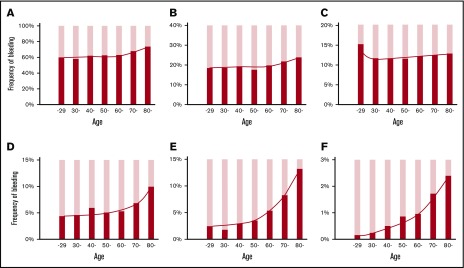
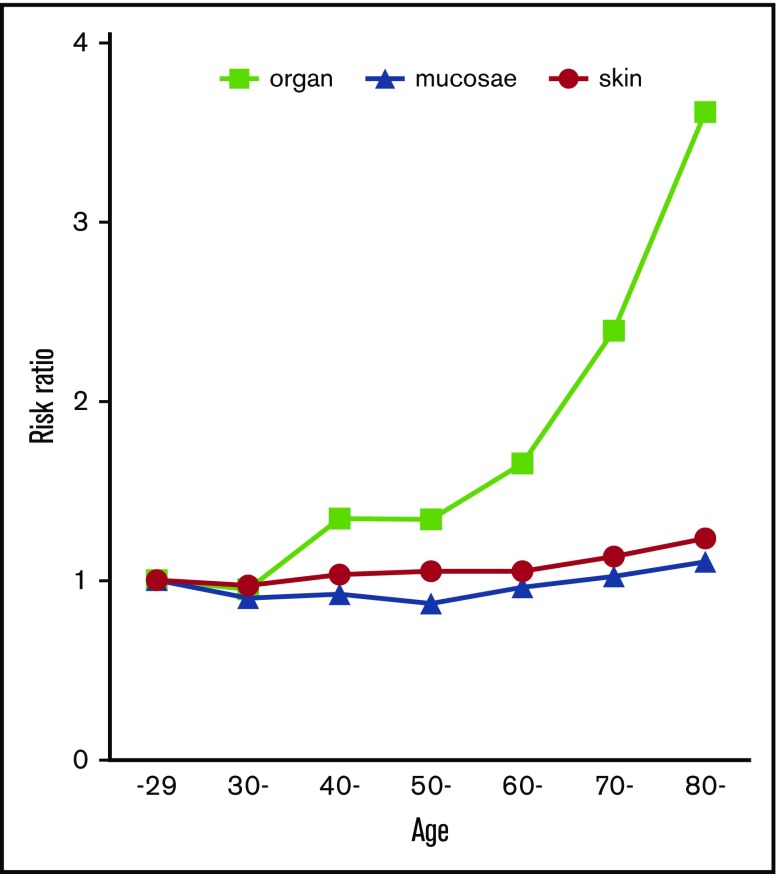
The frequency of purpura increased with age, but its increase was modest compared with the correlation to platelet count (Figure 4A). Gingival bleeding also increased modestly with age (Figure 4B). The frequency of epistaxis was independent of age (mean ages of patients with vs without epistaxis, 59.4 ± 20.1 years vs 59.7 ± 19.2 years; P = .471) (Figure 4C). However, hematuria, melena, and ICH increased with age and were especially frequent at age >70 years (Figure 4D-F). When bleeding manifestations were categorized as skin, mucosal, and organ bleeding and compared with the bleeding frequency at age <30 years (the youngest population), skin and mucosal bleeding increased modestly by a factor of 1.09 to 1.23; organ bleeding increased steeply by a factor of 3.62 at ≥80 years of age (Figure 5).
Figure 4.
Relation between age and bleeding manifestations. The frequency of patients with bleeding manifestations is expressed as the percentage of patients with the indicated bleeding manifestation for every 10 years of age. Skin purpura (A); gingival bleeding (B); epistaxis (C); hematuria (D); melena (E); ICH (F). Patients aged ≤29 years and ≥80 years were combined into one column, respectively. The linear and curved line in each graph represents the overall change in the bleeding frequency. Note that each graph has a different y-axis scale.
Figure 5.
Risk ratio of skin, mucosal, and organ bleeding according to age. The bleeding risk for patients at ≤29 years of age was set to 1.0, and the relative risk ratio for every 10 years of age was calculated. Patients aged ≥80 years were calculated together.
Risk factors for bleeding manifestations
Multivariate analysis showed that a platelet count <10 × 109/L was associated with all bleeding manifestations examined in this study (Table 1). Age ≥60 years was associated with purpura, melena, and ICH, whereas it was not associated with gingival bleeding, epistaxis, or hematuria. Melena was associated with gingival bleeding and epistaxis, whereas ICH was not, implying that mucosal bleeding is a risk factor for melena but not for ICH. The independent risk factors for ICH were age ≥60 years (OR, 3.09; 95% CI, 2.13-4.47; P < .001), a platelet count of <10 × 109/L (OR, 2.96; 95% CI, 2.11-4.15; P < .001), and the presence of hematuria (OR, 1.56; 95% CI, 1.04-2.35; P = .033). Paradoxically, ICH was negatively associated with purpura (OR, 0.63; 95% CI, 0.46-0.87; P = .005).
Table 1.
Multivariate analysis of risk factors for bleeding
| Predictor | Purpura | Gingival bleeding | Epistaxis | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Male | 0.67 (0.62-0.71) | <.001 | 1.06 (0.98-1.15) | .145 | 1.46 (1.33-1.60) | <.001 |
| ≥60 y of age | 1.27 (1.19-1.36) | <.001 | 0.98 (0.90-1.07) | .685 | 0.81 (0.74-0.90) | <.001 |
| Platelet count <10 × 109/L | 5.07 (4.69-5.48) | <.001 | 4.17 (3.81-4.57) | <.001 | 2.29 (2.05-2.55) | <.001 |
| Purpura | 3.22 (2.87-3.61) | <.001 | 1.34 (1.19-1.50) | <.001 | ||
| Gingival bleeding | 3.15 (2.80-3.53) | <.001 | 2.32 (2.10-2.56) | <.001 | ||
| Epistaxis | 1.28 (1.13-1.44) | <.001 | 2.37 (2.14-2.61) | <.001 | ||
| Hematuria | 2.05 (1.68-2.50) | <.001 | 2.36 (2.07-2.69) | <.001 | 1.61 (1.40-1.86) | <.001 |
| Melena | 1.04 (0.88-1.23) | .674 | 1.41 (1.23-1.62) | <.001 | 1.37 (1.17-1.59) | <.001 |
| ICH | 0.62 (0.44-0.86) | <.001 | 1.27 (0.92-1.74) | .144 | 1.21 (0.85-1.73) | .285 |
| Hematuria | Melena | ICH | ||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Male | 1.17 (1.03-1.33) | .015 | 1.31 (1.16-1.48) | <.001 | 0.98 (0.74-1.28) | .858 |
| ≥60 y of age | 1.10 (0.96-1.26) | .179 | 2.63 (2.25-3.08) | <.001 | 3.09 (2.13-4.47) | <.001 |
| Platelet count <10 × 109/L | 2.93 (2.49-3.46) | <.001 | 4.15 (3.51-4.91) | <.001 | 2.96 (2.11-4.15) | <.001 |
| Purpura | 2.08 (1.71-2.53) | <.001 | 1.09 (0.92-1.28) | .342 | 0.63 (0.46-0.87) | .005 |
| Gingival bleeding | 2.36 (2.07-2.69) | <.001 | 1.40 (1.22-1.61) | <.001 | 1.27 (0.93-1.74) | .129 |
| Epistaxis | 1.66 (1.44-1.91) | <.001 | 1.38 (1.19-1.61) | <.001 | 1.22 (0.86-1.73) | .270 |
| Hematuria | 2.47 (2.09-2.91) | <.001 | 1.56 (1.04-2.35) | .033 | ||
| Melena | 2.44 (2.06-2.88) | <.001 | 0.99 (0.64-1.54) | .965 | ||
| ICH | 1.52 (1.00-2.30) | .048 | 0.97(0.62-1.52) | .896 | ||
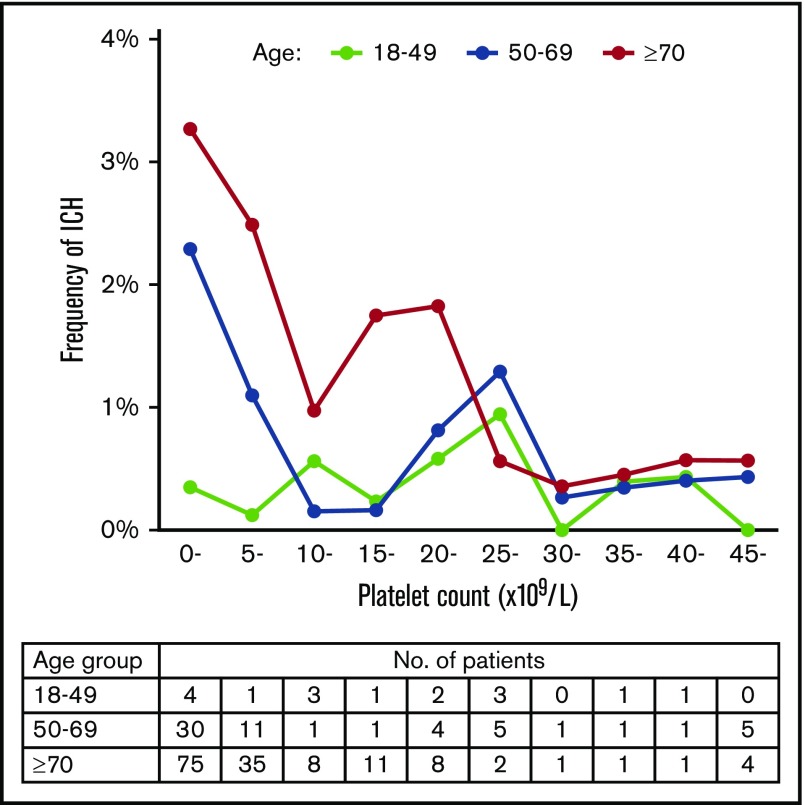
We further examined whether the association of platelet count with ICH varies with age (Figure 6). Patients with ITP were divided into 3 age groups: 18 to 49, 50 to 69, and ≥70 years. Each of these groups had approximately the same number of patients with ITP. In the 18- to 49-year-old age group, there was no clear relation between platelet count and the frequency of ICH. Multivariate analysis showed that a platelet count <10 × 109/L was not associated with ICH in this age group (OR, 0.96; 95% CI, 0.35-2.65; P = .936). In the 50- to 69-year-old and ≥70 year age groups, however, ICH increased steeply below platelet counts of 10 × 109/L, which was identified as a risk factor for ICH as well as the result with patients at all ages (OR, 3.00; 95% CI, 1.66-5.42; P < .001 for 50- to 69-year-old age group and OR, 2.47; 95% CI, 1.65-3.71, P < .001 for the ≥70-year-old age group). In the group aged ≥70 years, ICH apparently increased below a higher platelet count (25 × 109/L), although ICH did not continuously increase below a platelet count of 25 × 109/L.
Figure 6.
Relation between platelet count and frequency of ICH in different age groups. The frequency of ICH is expressed as the percentage of patients with ICH for every 5000 platelet counts. Patients with a platelet count ≥45 × 109/L were grouped together. The table below the figure shows the number of patients with ICH segmented according to age group and platelet count.
Discussion
The correlation between bleeding and platelet count in ITP has been examined in previous studies that showed increased bleeding below a platelet count of 20 × 109/L.5,6,11,14,15 The current results are consistent with this finding and reveal a distinct feature in which the incidence of skin bleeding increased linearly with thrombocytopenia within a platelet count range of 0 to 50 × 109/L, whereas mucosal and organ bleeding increased exponentially below a platelet count of 10 to 15 × 109/L. One possible explanation for this difference is that skin bleeding, including petechiae, is easy to observe, whereas mucosal and organ bleeding do not have overt manifestations at the mucosal petechiae stage and only become evident when gross bleeding occurs; the result leads to late and explosive onset of mucosal and organ bleeding at lower platelet counts. If this is the case, then a similar relation between bleeding and thrombocytopenia should be observed in patients with thrombocytopenic conditions other than ITP. Interestingly, a previous study of thrombocytopenic patients with leukemia, lymphoma, and bone marrow transplantation showed a linear increase in minor bleeding and a modest exponential increase in major bleeding, although the authors did not refer to this difference.16 Another possibility is that a lower platelet count may be sufficient for hemostasis in mucosal tissue. However, any difference in the mechanism of hemostasis between skin and mucosa remains to be characterized.
The correlation between bleeding and age in ITP has also been examined in previous studies, which showed an increased incidence of bleeding with age.3,11,14,15,17,18 Our current study showed that skin and mucosal bleeding were much more weakly correlated with age than with platelet count, whereas the incidence of organ bleeding increased steeply above 60 to 70 years of age. This result supports previous studies that showed only a slight age-related difference in any (mostly skin) and mucosal bleeding6 and a marked increase in organ (ICH and gastrointestinal) bleeding with age.18 Our study provides evidence that organ bleeding clearly increases with age, whereas skin and mucosal bleeding do not.
Mucosal bleeding is considered to be more dangerous than skin bleeding and is often cited as an indicator that treatment of ITP is required, although no such statistical relation has been verified.19 The current study showed that mucosal bleeding was an independent risk factor for melena and hematuria. This finding substantiates the clinical significance of mucosal bleeding, often referred to as “wet purpura,” that is associated with more severe bleeding. In this study, however, there was no association between mucosal bleeding and ICH. This negative result is consistent with previous small-scale studies showing no significant association of mucosal bleeding with ICH.7,8 It should be noted that in clinical practice, ICH occurs independently of the presence of mucosal bleeding. However, a representative mucosal bleeding is oral mucosal blood blisters, and its association with ICH was not examined in this study. A previous study reported the association of oral cavity bleeding with ICH.20 Our study analyzed only 2 types of mucosal bleeding: gingival bleeding and epistaxis. Hematuria can be considered as a type of mucosal bleeding, although it was classified as organ bleeding in the current study. Our result that hematuria was associated with ICH may represent a link between mucosal bleeding and ICH. We therefore cannot rule out the possibility of the general link between ICH and mucosal bleeding, although our study clarified the relation between ICH and individual bleeding manifestations such as gingival bleeding, epistaxis, and hematuria.
Previous studies have indicated that risk factors for ICH in adult patients with ITP include age >60 years,17 exposure to anticoagulant drugs,6 a history of visceral bleeding or any life-threatening bleeding,8 and the presence of hypertension and diabetes.10 To identify independent risk factors for ICH in ITP, multivariate analyses have been performed in 3 studies involving 10 patients with ICH,8 27 with ICH,10 and 21 with severe bleeding, including 4 with ICH.6 Those studies showed that neither age nor platelet count was a risk factor. However, several retrospective studies found that the majority of ITP patients with ICH were aged >60 years and had platelet counts <10 to 20 × 109/L, suggesting that age and platelet count are risk factors for ICH.11,17,18,21 In the current study, the multivariate analysis of 222 patients with ICH identified age ≥60 years and a platelet count <10 × 109/L as independent risk factors for ICH. However, in analyzing risk factors according to age, a platelet count <10 × 109/L was not a risk factor for ICH in young patients aged 18 to 49 years. Conversely, in elderly patients aged ≥70 years, the frequency of ICH increased below a platelet count of 25 × 109/L. These results suggest that risk assessment of ICH in ITP should consider an age-dependent platelet count threshold. However, our study may not have enough statistical power to determine this threshold because the number of patients with ICH segmented according to age and platelet count was small in this subanalysis. Larger studies are needed to confirm our results on an age-dependent platelet count threshold for ICH.
We identified hematuria as another risk factor for ICH in adult patients with ITP. Interestingly, hematuria was also reportedly associated with ICH in a study of pediatric patients with ITP.7 In addition, hematuria was identified as a risk factor for ICH in patients with aplastic anemia.22 These findings suggest that hematuria may be an indicator of imminent ICH in thrombocytopenic patients.
Another notable finding of our multivariate analysis is that the absence of purpura was associated with ICH. Our study did not include patients who developed ICH during the time-course of chronic ITP but only patients with ICH at the diagnosis of ITP who may have had less time for appearance of purpura due to sudden onset of ICH after thrombocytopenia. If this is the case, our patient selection may be involved in the negative association of purpura with ICH. Interestingly, a previous study of ICH in pediatric patients with ITP also found a negative association of petechiae with ICH.7 This earlier study included patients with ICH both at diagnosis and during the time-course of ITP. Although we have no clear explanation for this paradoxical result at present, future studies will verify if this negative association has a clinical implication.
The current study had several limitations. First, because the study population comprised patients with newly diagnosed ITP only, it was not possible to evaluate the relations among various bleeding manifestations over the long-term course of the disease. We also could not assess the effect of ITP treatment and comorbidities on bleeding manifestations because the database for newly diagnosed ITP was focused on the laboratory findings and bleeding manifestations at diagnosis. Second, the severity of each bleeding manifestation was not graded on a scale. Several tools for grading the severity of bleeding in ITP have been used, although a standardized tool is not available.5,20,23 The international working group on ITP recently developed a bleeding scale classified into 3 categories: skin, visible mucosae, and organ, with gradation of severity (the SMOG system).24 Although the usefulness of the SMOG system in clinical studies remains to be determined, our study has provided information on the incidence and risk factors for skin, mucosal, and organ bleeding. Third, our study did not analyze some of the classical risk factors for ICH such as hypertension, diabetes mellitus, dyslipidemia, and the use of anticoagulant drugs. These factors were not included in multivariate analysis because our database did not have such information. Fourth, this study involved Japanese patients only, precluding any generalized conclusions, although no ethnic differences in the frequency of bleeding manifestations in ITP have been reported.
In conclusion, the current study yielded information on patients with ITP that could be potentially valuable for treatment decision-making. Our finding that mucosal and organ bleeding increased exponentially below a platelet count of ∼15 × 109/L justifies a platelet threshold of <20 or 30 × 109/L as an indicator of the need for treatment of newly diagnosed patients in the recent guidelines for ITP.25,26 Identification of risk factors for ICH in ITP has particularly significant clinical implications because early treatment of ITP for such high-risk patients would help to avoid any devastating effect of ICH on prognosis. Further studies are warranted to examine the benefits of treatments based on risk factors in patients with newly diagnosed ITP.
Acknowledgment
This study was supported by a Grant-in-Aid for scientific research on blood coagulation abnormalities from the Ministry of Health, Labour, and Welfare of Japan (H29-Nanchitou-General-012).
Authorship
Contribution: T.H. designed the project and wrote the manuscript; N.S. performed statistical analysis and wrote the manuscript; Y.K. contributed to the design and direction of research; M.K., K.F., H.K., and T.T. offered helpful discussion and critically reviewed the manuscript; M.M. and Y.T. supervised the study and critically reviewed the manuscript; and all authors are members of the ITP team in the blood coagulation abnormalities research group of the Ministry of Health, Labour, and Welfare of Japan.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Takaaki Hato, Department of Blood Transfusion and Cell Therapy, Ehime University Hospital, 454 Shitsukawa, Toon, Ehime 791-0295, Japan; e-mail: takahato@m.ehime-u.ac.jp.
References
- 1.Arnold DM. Bleeding complications in immune thrombocytopenia. Hematology Am Soc Hematol Educ Program. 2015;2015:237-242. [DOI] [PubMed] [Google Scholar]
- 2.Schoonen WM, Kucera G, Coalson J, et al. Epidemiology of immune thrombocytopenic purpura in the General Practice Research Database. Br J Haematol. 2009;145(2):235-244. [DOI] [PubMed] [Google Scholar]
- 3.Cohen YC, Djulbegovic B, Shamai-Lubovitz O, Mozes B. The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch Intern Med. 2000;160(11):1630-1638. [DOI] [PubMed] [Google Scholar]
- 4.Frederiksen H, Maegbaek ML, Nørgaard M. Twenty-year mortality of adult patients with primary immune thrombocytopenia: a Danish population-based cohort study. Br J Haematol. 2014;166(2):260-267. [DOI] [PubMed] [Google Scholar]
- 5.Buchanan GR, Adix L. Grading of hemorrhage in children with idiopathic thrombocytopenic purpura. J Pediatr. 2002;141(5):683-688. [DOI] [PubMed] [Google Scholar]
- 6.Piel-Julian ML, Mahévas M, Germain J, et al. ; CARMEN investigators group . Risk factors for bleeding, including platelet count threshold, in newly diagnosed immune thrombocytopenia adults. J Thromb Haemost. 2018;16(9):1830-1842. [DOI] [PubMed] [Google Scholar]
- 7.Psaila B, Petrovic A, Page LK, Menell J, Schonholz M, Bussel JB. Intracranial hemorrhage (ICH) in children with immune thrombocytopenia (ITP): study of 40 cases. Blood. 2009;114(23):4777-4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melboucy-Belkhir S, Khellaf M, Augier A, et al. Risk factors associated with intracranial hemorrhage in adults with immune thrombocytopenia: a study of 27 cases. Am J Hematol. 2016;91(12):E499-E501. [DOI] [PubMed] [Google Scholar]
- 9.Nørgaard M, Jensen AO, Engebjerg MC, et al. Long-term clinical outcomes of patients with primary chronic immune thrombocytopenia: a Danish population-based cohort study. Blood. 2011;117(13):3514-3520. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor R, Pati HP, Mahapatra M, Monga A. Presence of essential hypertension or diabetes mellitus is a predictor of intracranial bleeding in elderly patients: a study of 108 patients with isolated thrombocytopenia from a single reference center. Turk J Haematol. 2015;32(2):158-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuda H, Tsuji T, Tsuji M, Yamasaki H. Life-threatening bleeding episodes in primary immune thrombocytopenia: a single-center retrospective study of 169 inpatients. Ann Hematol. 2017;96(11):1915-1920. [DOI] [PubMed] [Google Scholar]
- 12.Neunert C, Noroozi N, Norman G, et al. Severe bleeding events in adults and children with primary immune thrombocytopenia: a systematic review. J Thromb Haemost. 2015;13(3):457-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurata Y, Fujimura K, Kuwana M, Tomiyama Y, Murata M. Epidemiology of primary immune thrombocytopenia in children and adults in Japan: a population-based study and literature review. Int J Hematol. 2011;93(3):329-335. [DOI] [PubMed] [Google Scholar]
- 14.Neylon AJ, Saunders PW, Howard MR, Proctor SJ, Taylor PR; Northern Region Haematology Group . Clinically significant newly presenting autoimmune thrombocytopenic purpura in adults: a prospective study of a population-based cohort of 245 patients. Br J Haematol. 2003;122(6):966-974. [DOI] [PubMed] [Google Scholar]
- 15.Zhou H, Fu R, Wang H, et al. Immune thrombocytopenia in the elderly: clinical course in 525 patients from a single center in China. Ann Hematol. 2013;92(1):79-87. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence JB, Yomtovian RA, Dillman C, et al. Reliability of automated platelet counts: comparison with manual method and utility for prediction of clinical bleeding. Am J Hematol. 1995;48(4):244-250. [DOI] [PubMed] [Google Scholar]
- 17.Cortelazzo S, Finazzi G, Buelli M, Molteni A, Viero P, Barbui T. High risk of severe bleeding in aged patients with chronic idiopathic thrombocytopenic purpura. Blood. 1991;77(1):31-33. [PubMed] [Google Scholar]
- 18.Moulis G, Palmaro A, Montastruc JL, Godeau B, Lapeyre-Mestre M, Sailler L. Epidemiology of incident immune thrombocytopenia: a nationwide population-based study in France. Blood. 2014;124(22):3308-3315. [DOI] [PubMed] [Google Scholar]
- 19.Neunert CE. Individualized treatment for immune thrombocytopenia: predicting bleeding risk. Semin Hematol. 2013;50(suppl 1):S55-S57. [DOI] [PubMed] [Google Scholar]
- 20.Page LK, Psaila B, Provan D, et al. The immune thrombocytopenic purpura (ITP) bleeding score: assessment of bleeding in patients with ITP. Br J Haematol. 2007;138(2):245-248. [DOI] [PubMed] [Google Scholar]
- 21.Zhou F, Xu Y, Zhang Z, Wu X, Jin R. Severe hemorrhage in Chinese children with immune thrombocytopenia. J Pediatr Hematol Oncol. 2015;37(3):e158-e161. [DOI] [PubMed] [Google Scholar]
- 22.Owattanapanich W, Auewarakul CU. Intracranial hemorrhage in patients with hematologic disorders: prevalence and predictive factors. J Med Assoc Thai. 2016;99(1):15-24. [PubMed] [Google Scholar]
- 23.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207-214. [DOI] [PubMed] [Google Scholar]
- 24.Rodeghiero F, Michel M, Gernsheimer T, et al. Standardization of bleeding assessment in immune thrombocytopenia: report from the International Working Group. Blood. 2013;121(14):2596-2606. [DOI] [PubMed] [Google Scholar]
- 25.Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia [published correction appears in Blood Adv. 2020;4(2):252]. Blood Adv. 2019;3(23):3829-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]