Abstract
Purpose
Combining immunotherapeutic agents with different mechanisms of action may enhance efficacy. We describe the safety and efficacy of talimogene laherparepvec (T-VEC; an oncolytic virus) in combination with ipilimumab (a cytotoxic T-lymphocyte–associated antigen 4 checkpoint inhibitor) in patients with advanced melanoma.
Methods
In this open-label, multicenter, phase Ib trial of T-VEC in combination with ipilimumab, T-VEC was administered intratumorally in week 1 (106 plaque-forming units/mL), then in week 4 and every 2 weeks thereafter (108 plaque-forming units/mL). Ipilimumab (3 mg/kg) was administered intravenously every 3 weeks for four infusions, beginning in week 6. The primary end point was incidence of dose-limiting toxicities. Secondary end points were objective response rate by immune-related response criteria and safety.
Results
Median duration of treatment with T-VEC was 13.3 weeks (range, 2.0 to 95.4 weeks). Median follow-up time for survival analysis was 20.0 months (1.0 to 25.4 months). Nineteen patients were included in the safety analysis. No dose-limiting toxicities occurred, and no new safety signals were detected. Grade 3/4 treatment-related adverse events (AEs) were seen in 26.3% of patients; 15.8% had AEs attributed to T-VEC, and 21.1% had AEs attributed to ipilimumab. The objective response rate was 50%, and 44% of patients had a durable response lasting ≥ 6 months. Eighteen-month progression-free survival was 50%; 18-month overall survival was 67%.
Conclusion
T-VEC with ipilimumab had a tolerable safety profile, and the combination appeared to have greater efficacy than either T-VEC or ipilimumab monotherapy.
INTRODUCTION
Tumor immunotherapy has become an established treatment of metastatic melanoma and is being increasingly applied to other cancer types.1,2 A hallmark of tumors likely to respond to immunotherapy is a lymphocyte-predominant tumor microenvironment.3-5 To date, immunotherapy designed to promote lymphocyte accumulation within established tumors, activate lymphocyte function and cytotoxicity, and prevent T-cell suppression has shown the most promise.6 This includes the T-cell checkpoint inhibitors cytotoxic T-lymphocyte antigen-4 (CTLA-4)7-10 and programmed death receptor-1,11 which help activate antitumor T cells that may have been inactivated or exhausted and may help recruit lymphocytes to the tumor microenvironment.4
Ipilimumab, a CTLA-4 inhibitor, and anti-programmed death receptor-1 antibodies nivolumab and pembrolizumab have demonstrated significant efficacy and tolerable toxicity in patients with unresectable and metastatic melanoma.12-14 Ipilimumab in combination with nivolumab improved objective response rate (ORR) compared with ipilimumab monotherapy (57.6% for ipilimumab plus nivolumab, 43.7% for nivolumab alone, and 19.0% for ipilimumab alone). Progression-free survival (PFS) also improved with combination therapy (11.5 months for ipilimumab plus nivolumab, 6.9 months for nivolumab alone, and 2.9 months for ipilimumab alone). However, significantly more treatment-related grade 3 or 4 adverse events (AEs), including immune-related AEs, have been seen with ipilimumab plus nivolumab (55%) compared with monotherapy with ipilimumab (27%) or nivolumab (16%).13
Talimogene laherparepvec is an oncolytic immunotherapy derived from herpes simplex virus 1 that was designed to replicate selectively in tumor cells, resulting in lytic cell death and release of tumor-derived antigens and granulocyte-macrophage colony-stimulating factor (GM-CSF). GM-CSF can activate T cells, induce dendritic cell maturation, and potentiate a systemic, T-cell-mediated antitumor immune response.15-20 In clinical trials, subcutaneous GM-CSF plus ipilimumab versus ipilimumab alone yielded an overall survival (OS) benefit (17.5 v 12.7 months) but not a PFS benefit.21
The efficacy and toxicity of talimogene laherparepvec in advanced melanoma was evaluated in a randomized phase III trial comparing intratumoral talimogene laherparepvec with subcutaneous GM-CSF. With talimogene laherparepvec, the primary end point of durable response rate (DRR; continuous response lasting ≥ 6 months) was significantly higher (16% v 2%; odds ratio, 8.9; P < .001), ORR improved (26% v 6%), and OS improved numerically but not statistically by 4.4 months (hazard ratio, 0.79; 95% CI, 0.62 to 1.00; P = .051).22 Tumor regression was seen in tumors both injected and not injected with talimogene laherparepvec. The incidence of grade 3/4 talimogene laherparepvec-related AEs was 11%.
The oncolytic properties of talimogene laherparepvec result in release of tumor-derived antigens, local production of GM-CSF, and cross-priming of CD8+ T-cell responses by dendritic cells.15-20 Ipilimumab has shown activity in enhancing T-cell recruitment and preventing exhaustion of activated T cells.4 Thus, we postulated that combining these two immunotherapies with different mechanisms of action could result in enhanced efficacy. We report the results of a phase Ib study (NCT01740297) to determine safety, tolerability, and efficacy of talimogene laherparepvec plus ipilimumab, as assessed by the incidence of dose-limiting toxicities (DLTs) in patients with advanced melanoma.
METHODS
Patients
Key inclusion criteria were age ≥ 18 years; diagnosis of histologically confirmed stage IIIB-IVM1c melanoma that was not suitable for surgical resection; injectable cutaneous, subcutaneous, or nodal lesion; no prior systemic therapy except prior adjuvant therapy ≥ 6 months from last therapy; Eastern Cooperative Oncology Group performance status 0 or 1; and adequate hematologic, hepatic, renal, and coagulation function.
Key exclusion criteria were primary uveal or mucosal melanoma; history or evidence of CNS metastases; symptomatic autoimmune disease; evidence of clinically significant immunosuppression; chronic use of immune-suppressing agents; active herpetic skin lesions; long-term use of systemic antiherpetic agents (eg, acyclovir); known HIV disease; or known acute or chronic hepatitis B or hepatitis C infection.
The study was conducted in accordance with the provisions of the Declaration of Helsinki and with Good Clinical Practice guidelines as defined by the International Conference on Harmonization. The study was approved by the institutional review board at each site, and all patients provided written informed consent.
Study Design
This was an open-label, phase Ib/II study of talimogene laherparepvec plus ipilimumab. A Dose-Level Review Team, consisting of ≥ 1 clinician, safety representative, and biostatistician from the sponsor study team and ≥ 1 investigator who recruited patients into the phase Ib part of the study, monitored and reviewed safety data to evaluate possible drug effects and DLTs.
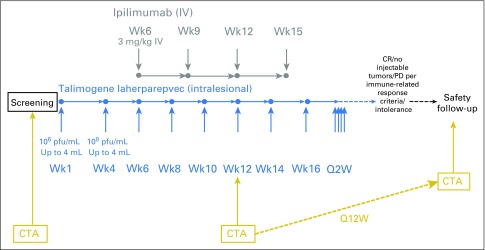
The phase Ib portion is reported here and was conducted at five US sites. The study design and the dosing diagram are shown in Appendix Figure A1, online only. Talimogene laherparepvec was initially administered as monotherapy. On day 1 of week 1, talimogene laherparepvec was administered by intralesional injection into injectable cutaneous, subcutaneous, and superficial lymph node tumors at a dose of 106 plaque-forming units (pfu)/mL. On day 1 of week 4 and every 2 weeks thereafter, talimogene laherparepvec was administered intratumorally at a dose of 108 pfu/mL. Ipilimumab administration began on day 1 of week 6 (ie, at the time of the third talimogene laherparepvec dose). Ipilimumab was administered intravenously at a dose of 3 mg/kg every 3 weeks for four infusions. When talimogene laherparepvec and ipilimumab were administered on the same day, talimogene laherparepvec was administered first.
Talimogene laherparepvec was continued until complete response (CR), all injectable tumors disappeared, progressive disease (PD) per immune-related response criteria (irRC), or drug intolerance. The safety follow-up visit occurred approximately 30 days after the last day of talimogene laherparepvec or 60 days after the last day of ipilimumab, whichever was later. Patients were followed for survival for approximately 24 months after the end of enrollment.
The primary end point was incidence of DLTs, defined as any treatment-related nonlaboratory grade ≥ 4 AE, grade ≥ 4 immune-mediated dermatitis or endocrinopathy, and grade ≥ 3 immune-mediated AE of any other type (eg, pneumonitis, pancreatitis, nephritis, uveitis, vasculitis, etc). Talimogene laherparepvec plus ipilimumab was deemed safe if the incidence of DLTs was < 33% during the DLT evaluation period (the 6 weeks after first administration of ipilimumab) in the first six to nine patients. Secondary end points were safety and ORR by irRC. Exploratory end points were time to response, duration of response, best overall response, DRR, disease control rate, PFS, OS, and T-cell subset characterization.
Assessments
All AEs were categorized on the basis of Common Terminology Criteria for Adverse Events (version 3). Tumors were assessed by the investigator at baseline, week 12, and every 12 weeks thereafter or as clinically indicated. Disease progression before response is often seen with ipilimumab23 and talimogene laherparepvec.22 To account for this, we used irRC,23 which requires a subsequent tumor assessment at least 4 weeks later to confirm a response and allows for continued treatment, despite growth of existing lesions or appearance of new lesions until progression is confirmed.
Statistical Analysis
DLTs were evaluated among patients who had received ≥ 1 dose of talimogene laherparepvec and ipilimumab and had the opportunity to receive treatment ≥ 6 weeks from the initial ipilimumab dose. Safety was evaluated among all patients who received ≥ 1 dose of talimogene laherparepvec or ipilimumab. Efficacy end points were evaluated among all patients who received both talimogene laherparepvec and ipilimumab. Exact binomial two-sided 95% CIs are provided for ORR, and Kaplan-Meier estimates are provided for PFS and OS. Post hoc analyses were conducted to examine responses at any time in injected and uninjected lesions.
T-Cell Subset Characterization
T-cell subsets were characterized to determine whether treatment with talimogene laherparepvec increased the number of effector cells with an activated phenotype in peripheral blood. Blood was collected for T-cell characterization before treatment, at weeks 1, 4, 6, 9, and 15, and at the safety follow-up. Whole blood was analyzed by standard flow cytometry methods for T-cell subset counts (CD45, CD3, CD4, CD8, BD Trucount) and expression of HLA-DR and inducible T-cell costimulator (ICOS) on T-cell subsets (CD45, CD3, CD4; see Appendix Table A1, online only, for flow panel design). P values evaluating differences in T-cell subsets between time points were calculated using a linear mixed-effects model of change from baseline in an immunophenotyping parameter, with baseline value included as a continuous factor. Results in fluorescence intensity or cells/µL units were log10 transformed before statistical analysis. The false discovery rate was controlled at 5% when generating hypotheses from the full set of T-cell subsets using the Benjamini-Hochberg method.24 Associations with response were tested for baseline and change from baseline during treatment. Flow cytometry end points were modeled as continuous variables. Analysis of variance was used for best change in lesion size from baseline, and logistic models were used for objective responses (CR or partial response [PR]).
RESULTS
Patients
In the phase Ib portion, 21 patients were screened and 19 were enrolled across five US sites from February 2013 to July 2013. Reported analyses include data through May 22, 2015. All 19 patients were included in the safety analysis. One patient received only one dose of talimogene laherparepvec before withdrawing consent and enrolling in hospice care. Thus, the efficacy analysis included 18 patients who received both talimogene laherparepvec and ipilimumab. Median duration of treatment with talimogene laherparepvec was 13.3 weeks (range, 2.0 to 95.4 weeks). Median follow-up time for survival analysis was 20.0 months (1.0 to 25.4 months). Baseline patient demographics are listed in Table 1. Median age was 61 (29 to 84) years; 42% of patients had stage IIIB-IVM1a disease, and 58% had IVM1b/c disease; 74% of patients had an Eastern Cooperative Oncology Group performance status of 0; and 42% of patients had wild-type BRAF status.
Table 1.
Demographic and Disease Characteristics
| Characteristic | No. (%) N = 19 |
|---|---|
| Sex | |
| Male | 8 (42) |
| Female | 11 (58) |
| Median age, years (min, max) | 61 (29, 84) |
| ECOG PS | |
| 0 | 14 (74) |
| 1 | 5 (26) |
| Disease stage | |
| IIIB | 1 (5) |
| IIIC | 3 (16) |
| IVM1a | 4 (21) |
| IVM1b | 5 (26) |
| IVM1c | 6 (32) |
| Baseline LDH | |
| ≤ ULN | 15 (79) |
| > ULN | 1 (5) |
| Unknown/missing | 3 (16) |
| BRAF mutation status | |
| Mutant | 11 (58) |
| Wild-type | 8 (42) |
| HSV-1 status | |
| Negative | 5 (26) |
| Positive | 13 (68) |
| Missing | 1 (5) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; HSV-1, herpes simplex virus 1; LDH, lactate dehydrogenase; ULN, upper limit of normal.
Safety
Talimogene laherparepvec and ipilimumab were given in combination without any dose reductions from their full therapeutic doses (talimogene laherparepvec: 108 pfu/mL; ipilimumab: 3 mg/kg). No DLTs were reported during the initial DLT evaluation period (primary end point) or throughout the phase Ib study.
Five patients (26.3%) reported grade ≥ 3 treatment-related AEs, with three patients (15.8%) reporting grade ≥ 3 AEs attributable to talimogene laherparepvec and four patients (21.1%) reporting grade ≥ 3 AEs attributable to ipilimumab (Table 2). Nausea was the only grade ≥ 3 treatment-related AE reported in more than one patient. The only grade 4 treatment-related AEs reported were increased lipase and increased amylase, both attributed to ipilimumab. There were no treatment-related grade 5 AEs.
Table 2.
Summary of Adverse Events
| Adverse Event | Total | Attributed to Talimogene Laherparepvec | Attributed to Ipilimumab | |||
|---|---|---|---|---|---|---|
| Any Grade No. (%) | Grade 3/4 No. (%) | Any Grade No. (%) | Grade 3/4 No. (%) | Any Grade No. (%) | Grade 3/4 No. (%) | |
| Any treatment-related AE | 18 (94.7) | 5 (26.3) | 17 (89.5) | 3 (15.8) | 15 (78.9) | 4 (21.1) |
| Treatment-related AEs > 10% by preferred term | ||||||
| Chills | 11 (57.9) | 0 | 11 (57.9) | 0 | 6 (31.6) | 0 |
| Pyrexia | 11 (57.9) | 1 (5.3) | 11 (57.9) | 1 (5.3) | 7 (36.8) | 0 |
| Fatigue | 9 (47.4) | 1 (5.3) | 6 (31.6) | 1 (5.3) | 8 (42.1) | 1 (5.3) |
| Diarrhea | 8 (42.1) | 1 (5.3) | 6 (31.6) | 1 (5.3) | 6 (31.6) | 1 (5.3) |
| Pruritus | 8 (42.1) | 0 | 6 (31.6) | 0 | 4 (21.1) | 0 |
| Rash | 8 (42.1) | 0 | 6 (31.6) | 0 | 4 (21.1) | 0 |
| Headache | 7 (36.8) | 0 | 7 (36.8) | 0 | 6 (31.6) | 0 |
| Nausea | 7 (36.8) | 2 (10.5) | 7 (36.8) | 1 (5.3) | 5 (26.3) | 2 (10.5) |
| Influenza-like illness | 3 (15.8) | 1 (5.3) | 3 (15.8) | 1 (5.3) | 1 (5.3) | 1 (5.3) |
| Pain | 3 (15.8) | 0 | 3 (15.8) | 0 | 2 (10.5) | 0 |
| Vision blurred | 3 (15.8) | 0 | 2 (10.5) | 0 | 2 (10.5) | 0 |
| ALT increased | 2 (10.5) | 0 | 1 (5.3) | 0 | 1 (5.3) | 0 |
| Back pain | 2 (10.5) | 0 | 2 (10.5) | 0 | 1 (5.3) | 0 |
| Dehydration | 2 (10.5) | 1 (5.3) | 1 (5.3) | 1 (5.3) | 2 (10.5) | 1 (5.3) |
| Erythema | 2 (10.5) | 0 | 1 (5.3) | 0 | 1 (5.3) | 0 |
| Hypokalemia | 2 (10.5) | 0 | 2 (10.5) | 0 | 0 | 0 |
| Injection site reaction | 2 (10.5) | 0 | 2 (10.5) | 0 | 0 | 0 |
| Night sweats | 2 (10.5) | 0 | 2 (10.5) | 0 | 1 (5.3) | 0 |
| Rash, erythematous | 2 (10.5) | 0 | 1 (5.3) | 0 | 1 (5.3) | 0 |
| Vomiting | 2 (10.5) | 1 (5.3) | 2 (10.5) | 1 (5.3) | 2 (10.5) | 1 (5.3) |
| Vulvovaginal mycotic infection | 2 (10.5) | 0 | 1 (5.3) | 0 | 1 (5.3) | 0 |
NOTE. AEs of any grade reported in > 10% of patients are shown. AEs were collected during treatment or up to 30 days after the last talimogene laherparepvec administration or 60 days after the last ipilimumab administration, whichever was later. All treatment-related grade 3/4 events were grade 3 events except for two grade 4 treatment-related events of elevated amylase and lipase attributed to ipilimumab occurring in a single patient. One grade 5 AE of metastasis to the central nervous system was reported as unrelated to study treatment.
Abbreviation: AE, adverse event.
All patients reported at least one AE (either related or unrelated to treatment), with grade ≥ 3 AEs reported in six patients (31.6%). No AEs led to discontinuation of talimogene laherparepvec or ipilimumab. One death occurred during the treatment period due to CNS metastasis. This death was not considered treatment related.
Efficacy
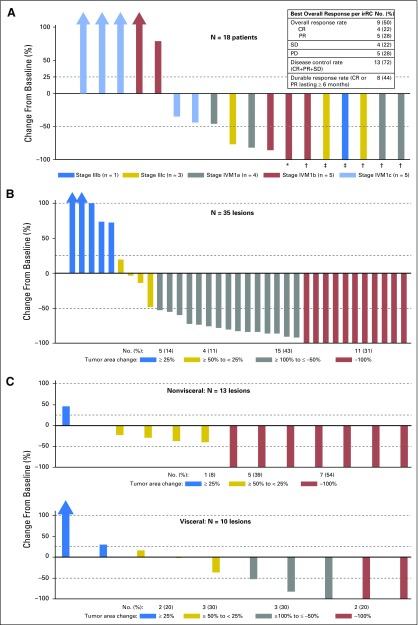
The best overall response at the patient level and lesion level is shown in Figure 1. ORR using irRC was 50% (95% CI, 26.0 to 74.0). Four patients (22%) had a confirmed CR. All were still in CR 1 year later. Five (28%) had a confirmed PR, and four (22%) had stable disease (SD). Of all confirmed responders, only one did not have a response lasting ≥ 6 months; DRR was 44% (Fig 1A). All eight patients with stage IIIB-IVM1a melanoma without visceral metastases were able to achieve a best overall response of SD or better (Fig 1A).
Fig 1.
Maximal change in tumor burden from baseline is shown. Changes in tumor burden are presented at the patient level (A) and at the lesion level (injected lesions in panel B and uninjected lesions in panel C). CR, complete response; irRC, immune-related response criteria; PD, progressive disease; PR, partial response; SD, stable disease. *Patient had a 99.8% reduction in tumor burden. †Patients had a confirmed complete response. ‡Patients had 100% reduction in tumor burden, but response was unconfirmed.
Responses were seen in both injected (Fig 1B) and uninjected lesions (Fig 1C). Among the 35 measurable lesions that were directly injected with talimogene laherparepvec, 26 (74%) regressed ≥ 50%, and 11 (31%) regressed completely. Among 23 uninjected measurable lesions, 12 (52%) regressed ≥ 50%, and nine (39%) regressed completely. Both visceral and uninjected nonvisceral lesions regressed: seven of 13 (54%) measurable nonvisceral lesions and five of 10 (50%) measurable visceral lesions regressed ≥ 50% (Figs 1C; Appendix Fig A2).
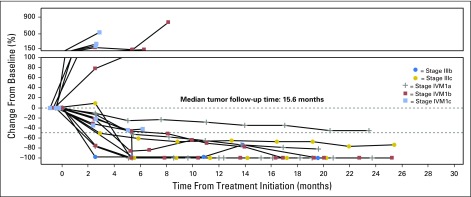
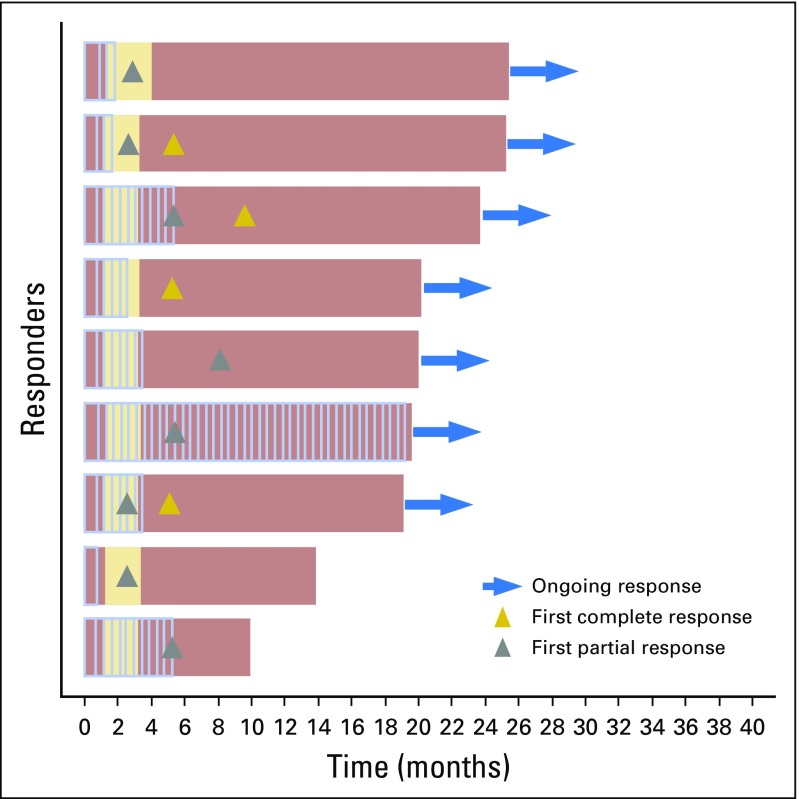
Confirmed responses were observed among patients with stage IIIB-IVM1a (six of eight), as well as stage IVM1b/c melanoma (three of 10; Fig 2). Among nine confirmed responders, median time to response was 5.3 months (range, 2.6 to 8.1 months; Fig 3).
Fig 2.
Changes in tumor burden by disease stage.
Fig 3.
Timing and duration of responses relative to administration of talimogene laherparepvec and ipilimumab. Data are shown for confirmed responders. Administration of talimogene laherparepvec is indicated by light blue vertical lines; the interval from the first to last ipilimumab administration is indicated in yellow; and the bar ends at the first progressive disease if there is progressive disease, otherwise, at the last tumor assessment.
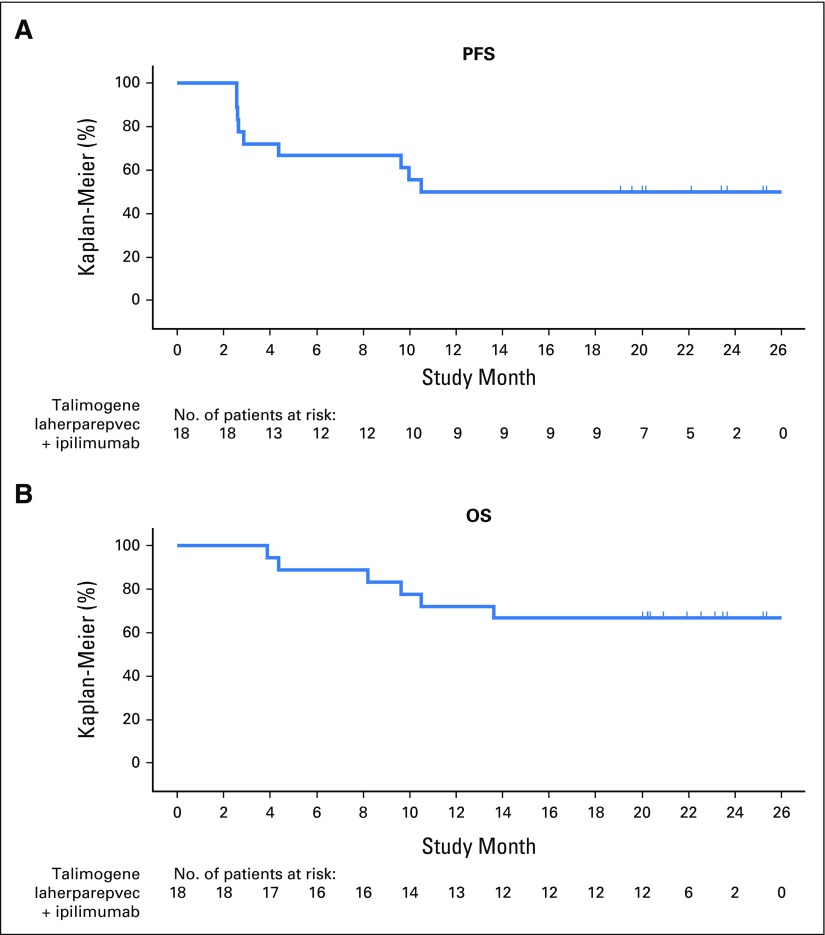
Kaplan-Meier estimates of median PFS (Fig 4A) and median OS (Fig 4B) were not reached. Probabilities of PFS at 12 and 18 months were both 50%. Probability of survival was 72% at 12 months and 67% at 18 months.
Fig 4.
Kaplan-Meier analysis of (A) progression-free survival (PFS) and (B) overall survival (OS).
T-Cell Subset Characterization
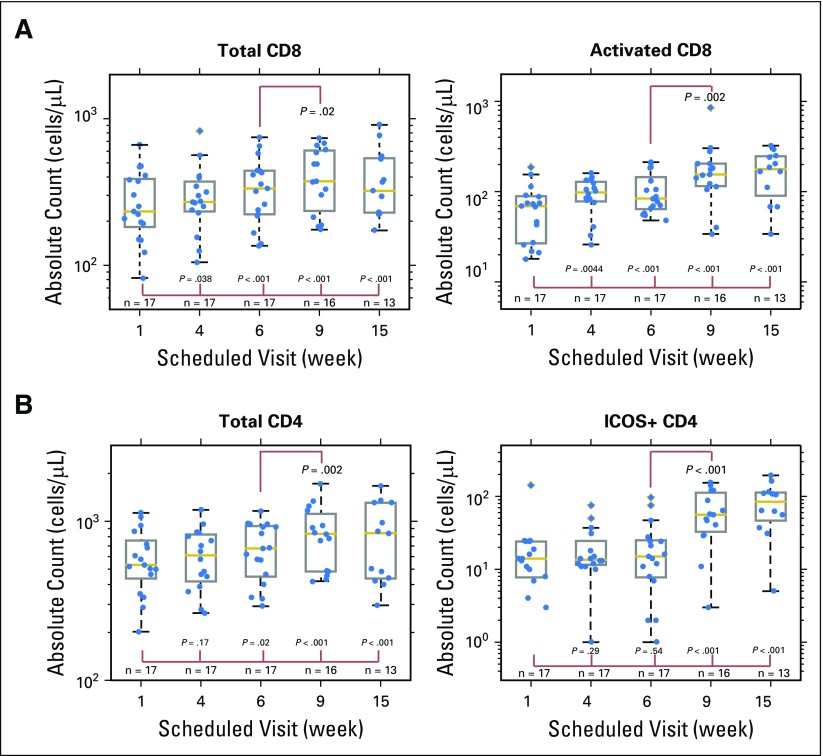
Total CD8+ T cells and activated CD8+ T cells (CD3+, CD4-, HLA-DR+)25-28 in peripheral blood significantly increased from baseline by 1.15-fold and 1.51-fold, respectively (Appendix Fig A3A). Changes in activated CD8+ T cells were significant beginning at the first available postbaseline sampling time point (4 weeks), and higher levels of total and activated CD8+ T cells were observed at week 6 just before the start of ipilimumab in combination with continued talimogene laherparepvec treatments (increases of 1.28-fold and 1.65-fold, respectively). See Appendix Table A2 for other flow parameters with significant changes.
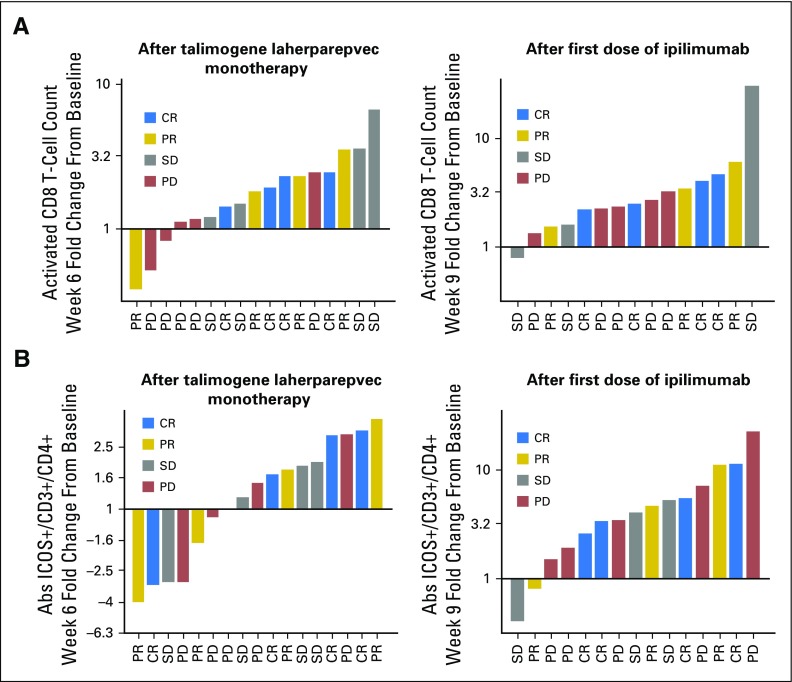
Increases in activated CD8+ T cells seemed to correspond with patient response. At week 6, after receiving two doses of talimogene laherparepvec, four of five patients with the smallest increases in activated CD8+ T cells had PD (Appendix Fig A4A). After ipilimumab was given, there seemed to be less differentiation by increase in activated CD8+ T-cell count between patients with disease control versus PD.
CD4+ T cells expressing ICOS, an activation marker upregulated by CTLA-4 blockade, significantly increased from baseline at weeks 9 and 15 after ipilimumab was given, consistent with previous reports.29 In contrast to activated CD8+ T cells, no increase in CD4+ T cells expressing ICOS was seen during the talimogene laherparepvec monotherapy period (Appendix Figs A3B and A4B).
DISCUSSION
In this phase Ib open-label study of talimogene laherparepvec plus ipilimumab, no DLTs were observed, and both drugs could be safely combined at their respective monotherapy doses for melanoma treatment. No new emerging toxicities were observed, and the incidence of grade 3/4 AEs (26.3%) was similar to single-agent ipilimumab. Durable CRs were observed in four of 18 patients (22%). Of four patients with confirmed CRs, all were still in CR beyond 1 year. ORR by irRC was 50%, and 44% of patients had a response lasting ≥ 6 months. Historically, initial responses with ipilimumab and other checkpoint inhibitors have correlated with improved survival.12,13 Although preliminary, PFS and OS data for talimogene laherparepvec in combination with ipilimumab to date are encouraging.
ORR and DRR results seen with talimogene laherparepvec plus ipilimumab are also encouraging; however, caution should be used when comparing these results with historical data. In the phase III pivotal trial evaluating ipilimumab versus glycoprotein 100 in previously treated patients with metastatic melanoma, ORR for ipilimumab alone was 10.9%; the incidence of ipilimumab-related grade 3/4 AEs was 22.9%; 71.4% had stage IVM1c disease; and 37.6% had elevated lactate dehydrogenase (LDH).12 In the phase III trial of ipilimumab plus dacarbazine versus dacarbazine in previously untreated patients, ORR for ipilimumab plus dacarbazine was 15.2%; 56.0% of patients had stage IVM1c disease, and 40.3% had elevated LDH.14 In the phase III pivotal trial evaluating talimogene laherparepvec versus subcutaneous GM-CSF in first-line advanced melanoma, ORR for talimogene laherparepvec was 26.4%; the incidence of talimogene laherparepvec-related grade 3/4 AEs was 11%; 22.0% had stage IVM1c disease, and 4.4% had elevated LDH.22 Although the clinical activity observed for talimogene laherparepvec plus ipilimumab in our phase Ib study was nominally higher than these historical data, our study only included 19 patients whose characteristics may not be directly comparable with those included in the larger monotherapy trials. Thus, although promising, results must be further confirmed in the ongoing randomized phase II trial (NCT01740297).
If talimogene laherparepvec contributes to a systemic antitumor immune response, expansion of activated effector cells may correlate with patient response. Patients experiencing disease control (confirmed CR, PR, or SD) seemed to have greater increases in activated CD8+ T cells in response to talimogene laherparepvec monotherapy than did patients with PD. This differentiation was no longer apparent after ipilimumab administration. Although this correlation will need to be validated, it suggests that activated CD8+ T cells stimulated by talimogene laherparepvec may be more specific for mediating an antitumor response than those stimulated by ipilimumab, which may activate additional nontumor-specific CD8+ T cells that do not contribute to response. In agreement with previous ipilimumab studies, we observed increases in CD4+, ICOS+ T cells, which has been associated with therapeutic response in monotherapy trials.26 Further research is ongoing to determine antigen specificity of T-cell responses after talimogene laherparepvec administration and whether treatment expands emergence of a neoantigen-specific T-cell repertoire, as has been previously reported for ipilimumab and was associated with improved clinical outcomes.30,31
An ongoing, randomized phase II trial on the basis of this study comparing talimogene laherparepvec plus ipilimumab with ipilimumab alone in patients with advanced melanoma is under way (NCT01740297). A phase Ib/III trial of talimogene laherparepvec plus pembrolizumab in melanoma (NCT02263508) is in progress; in an early efficacy analysis, the combination appeared tolerable, and 56% of 16 evaluable patients had at least an unconfirmed response.32 Thus, the combination of talimogene laherparepvec with immune checkpoint inhibitors may represent potential new treatment options for patients with regionally or distantly metastatic melanoma with or without visceral disease that is injectable and cannot be adequately addressed by surgery.
Acknowledgment
We thank the patients, friends, families, caregivers, study staff, and investigators for their participation in this study. We also thank Michael Wolf, Wei Wang, Daisy Wang, and Yang Zhang (Amgen) for biostatistical support. We thank Jennifer Gansert, David Taft, Gary Means, and Jessica Stern (Amgen), who participated in data analysis and preparation. Medical writing support was provided by Kerri Hebard-Massey (Amgen).
Appendix
Fig A1.
Dosing and assessment schedule. CR, complete response; CTA, clinical tumor assessment; PD, progressive disease; Q2W, once every 2 weeks; Q12W, once every 12 weeks.
Fig A2.
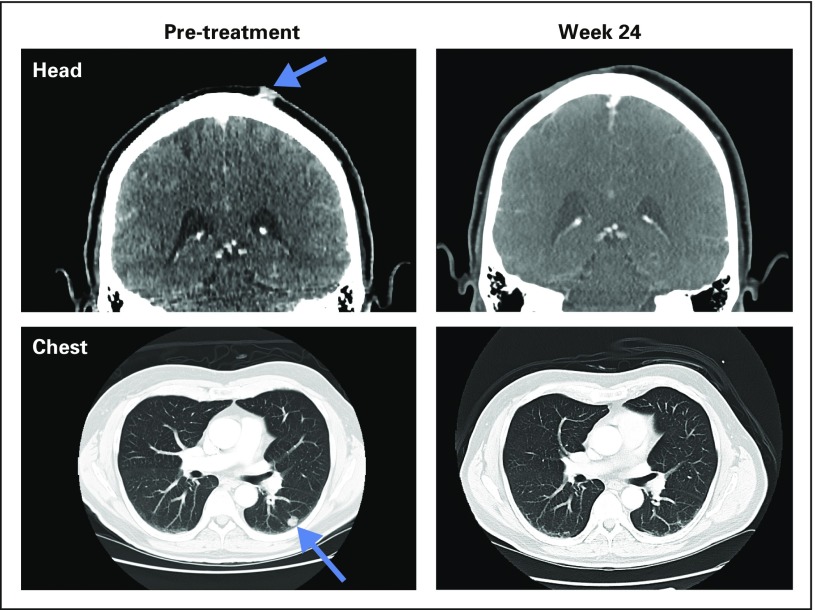
Complete response in patient with stage IVM1b melanoma treated with ipilimumab + talimogene laherparepvec. The initial right scalp lesion was resected in 2010. Recurrence was in scalp, left lower lobe of lung, and left hilar node in 2013 (CT scans on left). Complete response was documented at 24 weeks after initial treatment (CT scans on right). The patient is currently without recurrent disease at over 2 years.
Fig A3.
Changes in subsets of T cells after treatment. Samples were collected at baseline (week 1), after talimogene laherparepvec was given as monotherapy (weeks 4 and 6), and after talimogene laherparepvec was given in combination with ipilimumab (weeks 9 and 15). Data points are overlaid on the box plots. Each box plot shows the range between 25th percentile (q1) and 75th percentile (q3) as a gray box, with a yellow line showing the 50th percentile. The whiskers on each box are q3 ± 1.5 × (q3 − q1). (A) Total and activated CD8+ T cells. Activated CD8+ T cells are defined as HLA-DR+CD3+CD4− cells. (B) Total and ICOS+ CD4+ T cells. ICOS, inducible T-cell costimulator.
Fig A4.
Changes in subsets of T-cells according to confirmed best response. (A) Changes in activated CD8+ T-cell count after administration of talimogene laherparepvec and talimogene laherparepvec + ipilimumab. (B) Changes in ICOS+ CD4+ T-cell count after administration of talimogene laherparepvec and talimogene laherparepvec + ipilimumab. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Table A1.
Design of Flow Panel to Characterize T-Cell Subsets
| FITC | PE | PerCP | APC | |
|---|---|---|---|---|
| TBNK 1* | CD3 | CD8 | CD45 | CD4 |
| TBNK 2* | CD3 | CD16/56 | CD45 | CD19 |
| Control | CD3 | MsIgG | CD45 | CD4 |
| HLA-DR† | CD3 | HLA-DR | CD45 | CD4 |
| Treg† | CD127 | CD25 | CD45 | CD4 |
| ICOS† | CD3 | ICOS | CD45 | CD4 |
Abbreviations: APC, allophycocyanin; FITC, fluorescein isothiocyanate; ICOS, inducible T-cell costimulator; PE, R-phycoerythrin; PerCP, peridinin chlorophyll protein complex.
Established TBNK antibody cocktails (BD Biosciences, San Jose, CA) for use in Trucount assays.
Reportables include relative cell counts, percentages, and PE molecules of equivalent soluble fluorochrome.
Table A2.
Immunophenotyping Parameters With Significant Changes
| Comparison | Fold Change | P | Mean at Baseline (Week 1) |
|---|---|---|---|
| Week 4* v Week 1 | |||
| Abs HLA-DR+/CD3+/CD4− | 1.51 | .0044 | 1.76 |
| MESF HLA-DR+ (CD3+/CD4−) | 1.26 | .018 | 3.71 |
| %CD3−/CD19+ | −1.21 | .0048 | 13.74 |
| Week 6* v Week 1 | |||
| Abs HLA-DR+/CD3+/CD4− | 1.65 | < .001 | 1.76 |
| MESF HLA-DR+ (CD3+/CD4−) | 1.28 | .012 | 3.71 |
| Abs CD3+/CD8+ | 1.28 | < .001 | 2.40 |
| Abs CD3+ | 1.19 | .0017 | 2.92 |
| Abs lymphs | 1.19 | .0011 | 3.08 |
| MESF CD25+ (CD4+) | −1.09 | .0032 | 3.63 |
| MESF CD25 (CD4+) | −1.19 | .015 | 3.33 |
| Week 9† v Week 6 | |||
| Abs ICOS+/CD3+/CD4+ | 3.18 | < .001 | 1.12 |
| %ICOS+ (CD3+/CD4+) | 2.69 | .0011 | 2.74 |
| %ICOS+ (CD3+/CD4−) | 2.64 | .0070 | 0.64 |
| Abs ICOS+/CD3+/CD4− | 2.23 | < .001 | 0.46 |
| Abs HLA-DR+/CD3+/CD4+ | 2.20 | < .001 | 1.75 |
| %HLA-DR+ (CD3+/CD4+) | 1.81 | < .001 | 9.01 |
| Abs HLA-DR+/CD3+/CD4− | 1.65 | .0016 | 1.76 |
| MESF HLA-DR+ (CD3+/CD4−) | 1.56 | < .001 | 3.71 |
| Abs CD25hi/CD127low/CD4+ | 1.53 | < .001 | 1.77 |
| %HLA-DR+ (CD3+/CD4−) | 1.46 | .0025 | 20.10 |
| Abs CD3+/CD4+ | 1.23 | .0020 | 2.74 |
| Abs CD3+ | 1.22 | .0014 | 2.92 |
| %CD25hi/CD127low (CD4+) | 1.21 | < .001 | 8.78 |
| Abs lymphs | 1.16 | .0034 | 3.08 |
| %CD3+ | 1.05 | .0136 | 70.04 |
| MESF ICOS (CD3+/CD4−) | −1.30 | .015 | 2.11 |
Abbreviations: Abs, absolute; HLA, human leukocyte antigen; ICOS, inducible T-cell costimulator; MESF, molecules of equivalent soluble fluorochrome.
Week 4 and week 6 data points were acquired during monotherapy with talimogene laherparepvec.
Week 9 data points were acquired after the first dose of ipilimumab.
Footnotes
Support provided by Amgen.
Presented in part at the Society for Immunotherapy of Cancer Annual Meeting, National Harbor, MD, November 8-10, 2013; American Society of Clinical Oncology Annual Meeting, Chicago, IL, May 30-June 3, 2014; European Society for Medical Oncology 2014 Congress, Madrid, Spain, September 26-30, 2014; Society for Melanoma Research 2014 International Congress, Zurich, Switzerland, November 13-16, 2014; and the American Society of Clinical Oncology Annual Meeting, Chicago, IL, May 29-June 2, 2015.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01740297.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Administrative support: Igor Puzanov, Mohammed M. Milhem, David Minor, Omid Hamid, Lisa Chen, Abraham Anderson, Jeffrey Chou, Howard L. Kaufman, Robert H.I. Andtbacka
Provision of study materials or patients: Igor Puzanov, Mohammed M. Milhem, David Minor, Omid Hamid, Howard L. Kaufman, Robert H.I. Andtbacka
Collection and assembly of data: Igor Puzanov, Mohammed M. Milhem, David Minor, Omid Hamid, Howard L. Kaufman, Robert H.I. Andtbacka
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Talimogene Laherparepvec in Combination With Ipilimumab in Previously Untreated, Unresectable Stage IIIB-IV Melanoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Igor Puzanov
Consulting or Advisory Role: Amgen, Genentech
Travel, Accommodations, Expenses: Amgen, Merck
Mohammed M. Milhem
Consulting or Advisory Role: EMD Serono
Travel, Accommodations, Expenses: EMD Serono
David Minor
Stock or Other Ownership: Bristol-Myers Squibb (I)
Honoraria: Bristol-Myers Squibb, Merck
Consulting or Advisory Role: Bristol-Myers Squibb, Novartis
Speakers' Bureau: Bristol-Myers Squibb, Merck
Omid Hamid
Consulting or Advisory Role: Amgen, Novartis, Roche, Bristol-Myers Squibb
Speakers' Bureau: Bristol-Myers Squibb, Genentech, Novartis
Ai Li
Employment: Amgen, Puma Biotechnology
Stock or Other Ownership: Amgen, Puma Biotechnology
Travel, Accommodations, Expenses: Amgen, Puma Biotechnology
Lisa Chen
Employment: Amgen
Stock or Other Ownership: Amgen
Michael Chastain
Employment: Amgen
Stock or Other Ownership: Amgen
Kevin S. Gorski
Employment: Amgen
Stock or Other Ownership: Amgen
Abraham Anderson
Employment: Amgen
Stock or Other Ownership: Amgen
Patents, Royalties, Other Intellectual Property: Biomarker-related patents owned by Amgen
Travel, Accommodations, Expenses: Amgen
Jeffrey Chou
Employment: Amgen, Pfizer
Stock or Other Ownership: Amgen
Howard L. Kaufman
Honoraria: Amgen, EMD Serono, Merck, Prometheus, Sanofi Pasteur
Consulting or Advisory Role: Amgen, Merck, Merck Serono, Prometheus
Speakers' Bureau: Merck
Research Funding: Bristol-Myers Squibb/Medarex
Travel, Accommodations, Expenses: EMD SeronoSanofi
Robert H.I. Andtbacka
Honoraria: Amgen, Schering Plough
Consulting or Advisory Role: Amgen, Schering Plough
Research Funding: Amgen (Inst), Viralytics (Inst), Takara Bio (Inst)
REFERENCES
- 1.Hanahan D, Weinberg RA: Hallmarks of cancer: The next generation Cell 144:646–674,2011 [DOI] [PubMed] [Google Scholar]
- 2.Raval RR Sharabi AB Walker AJ, etal: Tumor immunology and cancer immunotherapy: Summary of the 2013 SITC primer J Immunother Cancer 2:14,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. doi: 10.1038/cr.2015.3. Hannani D, Vétizou M, Enot D, et al: Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res 25:208-224, 2015 [Erratum: Cell Res 25:399-400, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohlhapp FJ Broucek JR Hughes T, etal: NK cells and CD8+ T cells cooperate to improve therapeutic responses in melanoma treated with interleukin-2 (IL-2) and CTLA-4 blockade J Immunother Cancer 3:18,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Elsas A, Hurwitz AA, Allison JP: Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation J Exp Med 190:355–366,1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pardoll DM: The blockade of immune checkpoints in cancer immunotherapy Nat Rev Cancer 12:252–264,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarhini AA Edington H Butterfield LH, etal: Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab PLoS One 9:e87705,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong L Kwek SS O’Brien S, etal: Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF Cancer Res 69:609–615,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber J: Ipilimumab: Controversies in its development, utility and autoimmune adverse events Cancer Immunol Immunother 58:823–830,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapadia D, Fong L: CTLA-4 blockade: Autoimmunity as treatment J Clin Oncol 23:8926–8928,2005 [DOI] [PubMed] [Google Scholar]
- 11.McDermott DF, Atkins MB: PD-1 as a potential target in cancer therapy Cancer Med 2:662–673,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodi FS O’Day SJ McDermott DF, etal: Improved survival with ipilimumab in patients with metastatic melanoma N Engl J Med 363:711–723,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larkin J Chiarion-Sileni V Gonzalez R, etal: Combined nivolumab and ipilimumab or monotherapy in untreated melanoma N Engl J Med 373:23–34,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robert C Thomas L Bondarenko I, etal: Ipilimumab plus dacarbazine for previously untreated metastatic melanoma N Engl J Med 364:2517–2526,2011 [DOI] [PubMed] [Google Scholar]
- 15.Liu BL Robinson M Han ZQ, etal: ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties Gene Ther 10:292–303,2003 [DOI] [PubMed] [Google Scholar]
- 16.Conti L, Gessani S: GM-CSF in the generation of dendritic cells from human blood monocyte precursors: Recent advances Immunobiology 213:859–870,2008 [DOI] [PubMed] [Google Scholar]
- 17.Demir G, Klein HO, Tuzuner N: Low dose daily rhGM-CSF application activates monocytes and dendritic cells in vivo Leuk Res 27:1105–1108,2003 [DOI] [PubMed] [Google Scholar]
- 18.Lonial S: Immunomodulation: The role of hematopoietic cytokines Support Cancer Ther 1:80–88,2004 [DOI] [PubMed] [Google Scholar]
- 19.Kaufman HL Ruby CE Hughes T, etal: Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma J Immunother Cancer 2:11,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufman HL Kim DW DeRaffele G, etal: Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma Ann Surg Oncol 17:718–730,2010 [DOI] [PubMed] [Google Scholar]
- 21.Hodi FS Lee S McDermott DF, etal: Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: A randomized clinical trial JAMA 312:1744–1753,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andtbacka RH Kaufman HL Collichio F, etal: Talimogene laherparepvec improves durable response rate in patients with advanced melanoma J Clin Oncol 33:2780–2788,2015 [DOI] [PubMed] [Google Scholar]
- 23.Wolchok JD Hoos A O’Day S, etal: Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria Clin Cancer Res 15:7412–7420,2009 [DOI] [PubMed] [Google Scholar]
- 24.Benjamini Y, Hochberg Y: Controlling the false discovery rate: A practical and powerful approach to multiple testing JR Stat Soc 57:289–300,1995 [Google Scholar]
- 25.Attia P Phan GQ Maker AV, etal: Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4 J Clin Oncol 23:6043–6053,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carthon BC Wolchok JD Yuan J, etal: Preoperative CTLA-4 blockade: Tolerability and immune monitoring in the setting of a presurgical clinical trial Clin Cancer Res 16:2861–2871,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maker AV Phan GQ Attia P, etal: Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: A phase I/II study Ann Surg Oncol 12:1005–1016,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phan GQ Yang JC Sherry RM, etal: Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma Proc Natl Acad Sci USA 100:8372–8377,2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber JS Hamid O Chasalow SD, etal: Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma J Immunother 35:89–97,2012 [DOI] [PubMed] [Google Scholar]
- 30.Snyder A, Wolchok JD, Chan TA: Genetic basis for clinical response to CTLA-4 blockade N Engl J Med 372:783,2015 [DOI] [PubMed] [Google Scholar]
- 31.Van Allen EM Miao D Schilling B, etal: Genomic correlates of response to CTLA-4 blockade in metastatic melanoma Science 350:207–211,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Long GV, Drummer R, Ribas A, et al: Primary analysis of MASTERKEY-265 phase 1b study of talimogene laherparepvec (T-VEC) and pembrolizumab (pembro) for unresectable stage IIIB-IV melanoma. Presented at Society for Melanoma Congress, San Francisco, CA, Nov 16-21, 2015. [Google Scholar]