Abstract
Staphylococcus aureus (S. aureus) causes a range of diseases ranging from superficial skin and soft-tissue infections to invasive and life-threatening conditions (Klevens et al., 2007; Kobayashi et al., 2015). S. aureus utilizes the Sae sensory system to adapt to neutrophil challenge. Although the roles of the SaeR response regulator and its cognate sensor kinase SaeS have been demonstrated to be critical for surviving neutrophil interaction and for causing infection, the roles for the accessory proteins SaeP and SaeQ remain incompletely defined. To characterize the functional role of these proteins during innate immune interaction, we generated isogenic deletion mutants lacking these accessory genes in USA300 (USA300ΔsaeP and USA300ΔsaeQ). S. aureus survival was increased following phagocytosis of USA300ΔsaeP compared to USA300 by neutrophils. Additionally, secreted extracellular proteins produced by USA300ΔsaeP cells caused significantly more plasma membrane damage to human neutrophils than extracellular proteins produced by USA300 cells. Deletion of saeQ resulted in a similar phenotype, but effects did not reach significance during neutrophil interaction. The enhanced cytotoxicity of USA300ΔsaeP cells toward human neutrophils correlated with an increased expression of bi-component leukocidins known to target these immune cells. A saeP and saeQ double mutant (USA300ΔsaePQ) showed a significant increase in survival following neutrophil phagocytosis that was comparable to the USA300ΔsaeP single mutant and increased the virulence of USA300 during murine bacteremia. These data provide evidence that SaeP modulates the Sae-mediated response of S. aureus against human neutrophils and suggest that saeP and saeQ together impact pathogenesis in vivo.
Keywords: sae, Staphylococcus aureus, neutrophil, gene regulation, virulence
Introduction
Staphylococcus aureus (S. aureus) is a highly-adaptable pathogen able to infect various tissues. Common manifestations of S. aureus infections range from mild skin and soft-tissue infections to invasive disease. Additionally, this pathogen has gained resistance against many anti-microbial drugs, leaving healthcare providers with few options for the treatment of infections (Chambers, 2001; Chambers and Deleo, 2009). In the past, antimicrobial-resistant S. aureus infections were mostly associated with a recent hospital stay, but the rise in community-associated infections has steadily increased since the late 1990s (Chambers, 2001; King et al., 2006). Drug-resistance combined with the lack of understanding of protective immunity to S. aureus has delayed the development of new therapeutics to treat this ubiquitous opportunistic pathogen.
The human neutrophil is essential for resolution of S. aureus infections, as individuals suffering from defects in neutrophil function are more susceptible to S. aureus infection (Lekstrom-Himes and Gallin, 2000). S. aureus has evolved many mechanisms to circumvent killing by these potent innate immune cells. The production of secreted virulence factors during pathogenesis is primarily controlled by the combined influence of two-component systems (TCSs) that sense the host environment and respond accordingly. Of these, the Sae TCS has been shown to be essential for evasion of human neutrophil killing (Voyich et al., 2009; Guerra et al., 2016). SaeR/S is immediately up-regulated following neutrophil phagocytosis and the histidine kinase, SaeS, is thought to specifically recognize neutrophil components (Voyich et al., 2005, 2009; Geiger et al., 2008; Mainiero et al., 2010; Cho et al., 2015; Zurek et al., 2015). The response regulator, SaeR, is activated following phosphorylation by SaeS and subsequently alters gene transcription by directly binding to a specific recognition sequence in the promoter region of numerous virulence genes including nuc and the bi-component leukotoxins lukF (PVL), lukGH (lukAB), and hlgBC that target human neutrophils (Nygaard et al., 2010; Olson et al., 2013; Liu et al., 2016). The upregulation of these genes facilitates S. aureus survival following neutrophil phagocytosis (Voyich et al., 2009; Flack et al., 2014). However, the sae locus also includes two accessory genes, saeP and saeQ, whose gene products are not entirely understood. Previously published in vitro studies suggest these proteins form a complex with SaeS that deactivates SaeR (Jeong et al., 2012). It has also been shown that increased expression of saeP impacts biofilm formation by increasing retention of high molecular weight DNA on the biofilm surface (Kavanaugh et al., 2019). The same study also demonstrated increases in saeP gene expression correlated with decreases in nuclease activity during biofilm development (Kavanaugh et al., 2019). Considering the importance of Sae during neutrophil interactions, we investigated the importance of saeP and saeQ during challenge with human neutrophils and in vivo using murine models of invasive disease and skin and soft-tissue infection. For these studies, we used S. aureus strain LAC, a USA300 isolate, as USA300 is the dominant clone causing community-associated methicillin resistant S. aureus (CA-MRSA) disease in the United States (David and Daum, 2010). Results demonstrate that deletion of saeP increased S. aureus cytotoxicity against neutrophils ex vivo. Moreover, the deletion of both saeP and saeQ markedly increased both nuclease expression in kidneys and overall mortality following intravenous infection.
Results
Generation of USA300 Isogenic Mutants Deficient in Either saeP or saeQ
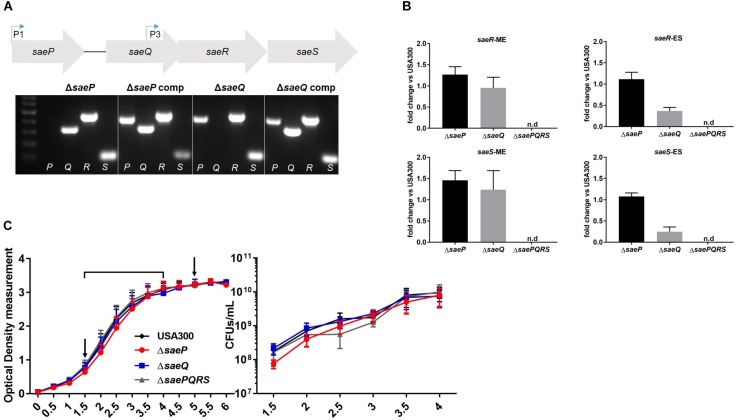
The Sae TCS is composed of four genes: saeP, saeQ, saeR, and saeS (Figure 1A). Although saeS and saeR, encoding the sensory kinase and response regulator respectively, have been demonstrated to be essential in S. aureus virulence and pathogenesis (Voyich et al., 2009; Nygaard et al., 2010, 2018; Flack et al., 2014; Liu et al., 2015, 2016; Zurek et al., 2015; Guerra et al., 2016), surprisingly little research has been performed on the two accessory genes of the Sae system, saeP and saeQ. Published data indicate that saeP encodes a lipoprotein anchored to the exterior surface of the plasma membrane, whereas saeQ encodes a transmembrane protein (Jeong et al., 2012; Kavanaugh et al., 2019). To investigate the roles of these genes, we utilized a saeP deletion mutant (USA300ΔsaeP) generated previously in Kavanaugh et al. (2019), and deleted the first third of saeQ (164 bp) in USA300 LAC using allelic replacement to create an isogenic saeQ deletion mutant (USA300ΔsaeQ) that preserves the P3 promoter (Bae and Schneewind, 2006; Jeong et al., 2011). Absence of saeP and saeQ genes were verified by PCR (Figure 1A). Importantly, deletion of saeP or saeQ did not substantially impact saeR and saeS gene expression. TaqMan® real-time RT-PCR analyses indicated a slight increase in saeR transcript levels in the USA300ΔsaeP mutant at both mid-exponential (ME) and early stationary (ES) phases of growth relative to USA300. This trend was also established for the expression of saeS in USA300ΔsaeP at ME phase. Expression of saeR and saeS were essentially unchanged in USA300ΔsaeQ relative to USA300 at ME but were decreased during ES (Figure 1B). Basal expression from the P3 promoter is likely unaffected by deletion of saeQ since the deletion ends 108 base pairs upstream of the P3 promoter (Jeong et al., 2011) and ME expression is similar to USA300. When the Sae TCS is activated, transcription from the P1 promoter increases (Novick and Jiang, 2003; Steinhuber et al., 2003). Therefore, when the system activates in ES (Flack et al., 2014), mRNA transcripts containing the saeQ deletion may have reduced stability since the mRNA secondary structure has recently been shown to be important (Marincola and Wolz, 2017). Nevertheless, this is not expected to affect the activation of SaeR/S target genes, as overexpression of saeRS does not alter the expression profile of the Sae-regulon (Mainiero et al., 2010; Liu et al., 2016). Additionally, USA300ΔsaeP and USA300ΔsaeQ strains showed no significant growth defects compared to USA300 (Figure 1C).
FIGURE 1.
Generation of an isogenic saeQ mutant. (A) (Above) Schematic of the four genes of the sae locus. Bent arrows indicate transcriptional start sites described in Novick and Jiang (2003), Steinhuber et al. (2003), and Jeong et al. (2011). (Below) Results of agarose gel electrophoresis showing USA300 mutants lacking saeP (from Kavanaugh et al., 2019), saeQ, and respective complemented strains. Letters at the bottom of the gel indicate the sae gene targeted by PCR analysis. Primer sequences are included in Table 1. (B) TaqMan® RT-PCR analysis of saeS and saeR transcript levels in USA300ΔsaeP and USA300ΔsaeQ relative to USA300 at mid-exponential phase (ME) and early stationary phase (ES). Transcript levels in samples were analyzed in triplicate and results are from two independent experiments. (C) In vitro growth of USA300, USA300ΔsaeP, USA300ΔsaeQ, and USA300ΔsaePQRS (Flack et al., 2014) measured by optical density at 600 nm (OD600) (left) and colony forming units (CFUs) (right). Timepoints are indicated by the bracket on the OD600 plot. Arrows indicate time points for RNA harvest (used in B). Data are presented as the mean ± SEM of seven independent experiments. n.d., not detected.
Deletion of saeP Increases Bacterial Survival Following Neutrophil Phagocytosis
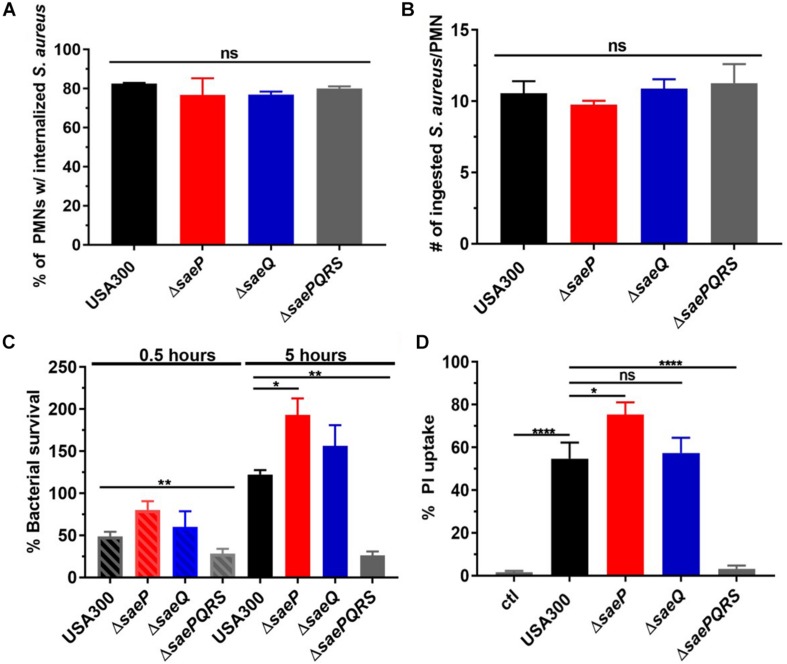
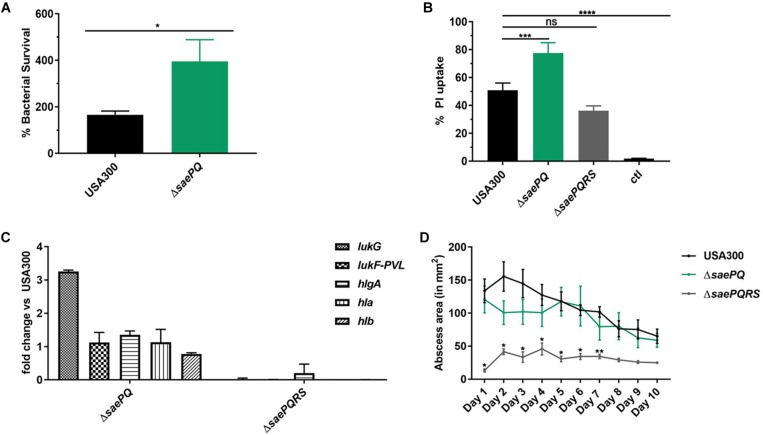
SaeS has been shown to be activated by neutrophil phagocytosis and associated components including alpha-defensin and hydrogen peroxide (Voyich et al., 2005; Geiger et al., 2008; Zurek et al., 2014). Moreover, deletion of saeR/S has been shown to significantly decrease S. aureus survival and cytolytic capacity following neutrophil phagocytosis (Voyich et al., 2009; Flack et al., 2014). However, nothing is known about how SaeP and SaeQ contribute to staphylococcal neutrophil evasion. To determine the role of these accessory proteins during interaction with human neutrophils, we initially evaluated phagocytosis and killing of USA300ΔsaeP, USA300ΔsaeQ, USA300ΔsaePQRS (Flack et al., 2014), or USA300. Importantly, there were no significant differences in the uptake of these strains by human neutrophils (Figures 2A,B), consistent with previous observations using ΔsaeR/S strains that have shown the SaeR/S system has no significant impact on neutrophil phagocytosis (Voyich et al., 2009; Guerra et al., 2016). Next, we assessed S. aureus survival after neutrophil phagocytosis. After 30 min, we measured very modest increases in the survival of the saeP and saeQ mutant strains relative to the parental wild-type strain. However, deletion of saeP significantly increased bacterial survival compared to USA300 5-h after phagocytosis. There was also a noticeable although not statistically significant increase in survival of USA300ΔsaeQ compared to USA300 at 5 h post-neutrophil exposure. We measured a significant reduction in survival of USA300ΔsaePQRS strain at both timepoints, confirming the importance of saeR/S for S. aureus survival following neutrophil phagocytosis as previously observed (Voyich et al., 2009; Figure 2C).
FIGURE 2.
Deletion of saeP significantly increases S. aureus survival and toxicity during neutrophil interaction. (A) Percent S. aureus ingested by neutrophils. Samples were collected on an Image Stream® Imaging Flow Cytometer and S. aureus internalization was analyzed using the IDEAS software® as described in Materials and Methods for three independent experiments. (B) Numbers of ingested S. aureus per PMN are shown. The average number of S. aureus detected to be ingested per PMN is ∼ 10 for all strains. Data are presented as the mean ± SEM of three independent experiments. (C) Percent survival for the indicated strains and timepoints is shown. Bacterial survival is significantly increased in USA300ΔsaeP compared to USA300 at 5 h following phagocytosis. Survival was calculated with the following equation: (CFU + PMN at timen/CFU + PMN at time0) × 100 (as in Voyich et al., 2005). Data are presented as the mean ± SEM of six independent experiments. At 0.5 h, USA300ΔsaePQRS was significantly different from USA300 **P ≤ 0.001 using one-way ANOVA with Tukey’s post-test. At 5 h, *P ≤ 0.05 and **P ≤ 0.001 relative to USA300 using one-way ANOVA and Tukey’s post-test. (D) Supernatants from USA300ΔsaeP cultures cause significantly more neutrophil plasma membrane damage compared to supernatants from USA300 cultures. S. aureus strains were grown to early stationary phase, supernatants harvested as described in Materials and Methods (diluted 1:10), and incubated with neutrophils for 1 h. Propidium iodide (PI) uptake was assessed by flow cytometry. Data are presented as the mean ± SEM of five independent experiments. *P ≤ 0.05, ****P ≤ 0.0001 using one-way ANOVA with Tukey’s post-test. ctl, neutrophil-only control. ns, not significant.
Deletion of saeP Increases Production of Neutrophil Cytolytic Factors
SaeR/S up-regulates the transcription of numerous secreted virulence factors including the bi-component leukotoxins LukG/H, PVL, and HlgB/C that specifically target and disrupt the neutrophil plasma membrane (Voyich et al., 2009; Nygaard et al., 2010; Sun et al., 2010; Ventura et al., 2010; Flack et al., 2014; Zurek et al., 2014). Since USA300ΔsaeP cells demonstrated increased survival following phagocytosis, we hypothesized that saeP might influence the production of secreted cytolytic factors (i.e., ability to permeabilize neutrophils). Indeed, neutrophils exposed to filtered supernatants taken from ES cultures of the USA300ΔsaeP mutant exhibited significantly more plasma membrane damage than neutrophils exposed to supernatants taken from cultures of USA300 as determined by propidium iodide uptake (Figure 2D). Supernatants from USA300ΔsaeQ showed no significant differences in cytolytic activity compared with USA300. Confirming previous observations with ΔsaeR/S strains, neutrophils exposed to the supernatants from USA300ΔsaePQRS cultures showed significantly reduced plasma membrane damage compared to results from exposure to culture supernatants from all other S. aureus strains tested and similar to that of neutrophils not exposed to S. aureus supernatants (Voyich et al., 2009; Flack et al., 2014). USA300ΔsaeP complemented with saeP in trans reduced the cytotoxicity of this strain to levels that paralleled USA300 (Supplementary Figure S1A).
Deletion of saeP Increases Transcript Abundance of Several Leukotoxins
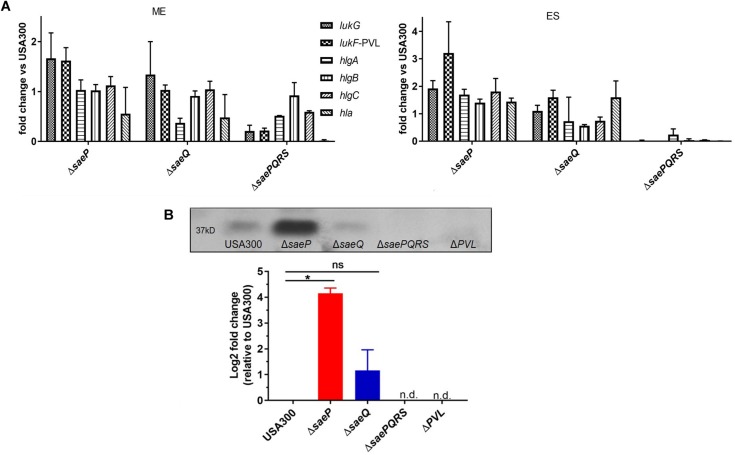
The SaeR/S system is essential for transcriptional regulation of virulence factors known to impact neutrophil function (Voyich et al., 2009; Mainiero et al., 2010; Nygaard et al., 2010; Flack et al., 2014; Zurek et al., 2014; Guerra et al., 2016). Since the USA300ΔsaeP strain demonstrated increased survival following phagocytosis by neutrophils, and supernatants from USA300ΔsaeP had increased cytolytic activity toward neutrophils, we profiled the transcript abundance of select SaeR/S-regulated virulence factors known to impact neutrophil viability. During mid-exponential phase (ME) we measured subtle increases in lukG transcript abundance in both USA300ΔsaeP and USA300ΔsaeQ mutant strains, as well as a subtle increase in lukF-PVL transcript abundance levels in USA300ΔsaeP (Figure 3A). Importantly, we measured more pronounced increases in lukG, lukF-PV, hlgA, hlgB, and hlgC transcript abundance in USA300ΔsaeP relative to USA300 during early stationary (ES) phase. Transcript abundance of all select virulence genes was reduced in the USA300ΔsaePQRS mutant in accordance with previous observations examining the influence of Sae on virulence gene transcription (Voyich et al., 2009; Nygaard et al., 2010; Flack et al., 2014; Zurek et al., 2014). Expression of saeP in trans in the USA300ΔsaeP strain reduced transcript abundance and cytotoxicity to levels at or below those measured in USA300 (Supplementary Figures S1B,C).
FIGURE 3.
USA300ΔsaeP demonstrates increased transcript abundance of several known SaeR/S-virulence factors. (A) Mean fold-change of known SaeR/S-regulated virulence genes in USA300ΔsaeP and USA300ΔsaeQ relative to USA300 is shown. Transcript abundance was measured using TaqMan® RT-PCR at mid-exponential (ME; left) and early stationary (ES; right) phases of in vitro growth. Transcripts were normalized to gyrB and calibrated to transcript abundance in USA300. Data are from at least two independent experiments. (B) The USA300ΔsaeP strain of S. aureus produces significantly more Panton Valentine Leukocidin (PVL) compared to USA300. (Top) Representative Western blot of PVL protein in supernatants from S. aureus grown overnight. (Bottom) Quantification of PVL was calculated using densitometry as described in Materials and Methods. Data shown are amounts relative to USA300 and presented as the mean ± SEM of three independent experiments. *P ≤ 0.05 One-Way ANOVA with Tukey’s post-test. n.d., not detected.
Supporting transcript analysis of lukF-PV, secreted PVL in overnight culture supernatants was significantly increased in the USA300ΔsaeP strain compared to USA300 (Figure 3B). As anticipated, PVL was essentially undetectable in culture supernatants from the ΔsaePQRS mutant, demonstrating a strong dependency on Sae for production of PVL (Figure 3B).
Deletion of saeQ Attenuates Mortality in Mice Following Intravenous Infection
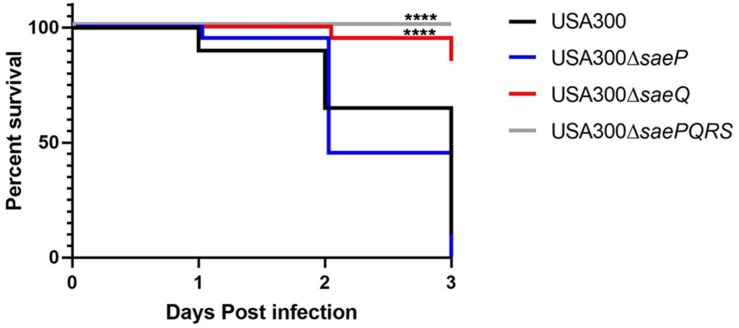
To investigate the individual roles of saeP and saeQ during staphylococcal disease, we used a well-established model of acute bacteremia (Voyich et al., 2009; Nygaard et al., 2010). Mice (groups of 10) were infected intravenously with 1 × 107 CFUs of either S. aureus USA300, USA300ΔsaeP, USA300ΔsaeQ, or USA300 ΔsaePQRS. Consistent with previous studies (Nygaard et al., 2010), ∼65% of the mice infected with USA300 died within 48 h, and on average fewer than 10% of the mice survived 72 h post-infection. Although there were no significant differences in the mortality of mice challenged with USA300ΔsaeP compared with USA300, nearly all mice infected with USA300ΔsaeQ survived 72 h post-infection (Figure 4). All mice challenged with ΔsaePQRS survived, congruent with previous studies (Voyich et al., 2009; Nygaard et al., 2010) and demonstrating the critical role of the Sae system following bloodstream infection (Figure 4).
FIGURE 4.
saeQ contributes to staphylococcal disease during bacteremia. Groups of 10 C57BL/6 mice were infected intravenously via the tail vein with 1 × 107 CFU of the indicated S. aureus strains. Survival curves are from two independent experiments ****P < 0.0001, log-rank (Mantel-Cox) test.
SaeR/S is also critical for S. aureus pathogenesis during murine skin and soft-tissue infection (SSTI) (Voyich et al., 2009; Nygaard et al., 2010, 2018). To investigate the importance of SaeP and SaeQ in SSTI, BALB/c and C57BL/6 mice were infected subcutaneously with 1 × 107 CFUs of either USA300 or our isogenic mutants, and abscess area was monitored for 10 days. While we measured a significant decrease in the abscess area of mice infected with the USA300ΔsaePQRS mutant compared to USA300, we detected no significant differences in abscess size or incidence of dermonecrosis when either saeP or saeQ were deleted (Supplementary Figures S2A,B). Taken together, although Sae TCS activity is required for full virulence in both bacteremia and SSTI, our data indicate SaeP and SaeQ are dispensable during SSTI, but SaeQ appears to be important during bacteremia.
USA300ΔsaePQ Mimics the USA300ΔsaeP Phenotype During Neutrophil Interaction, but Significantly Increases Mortality Following Intravenous Infection in Mice
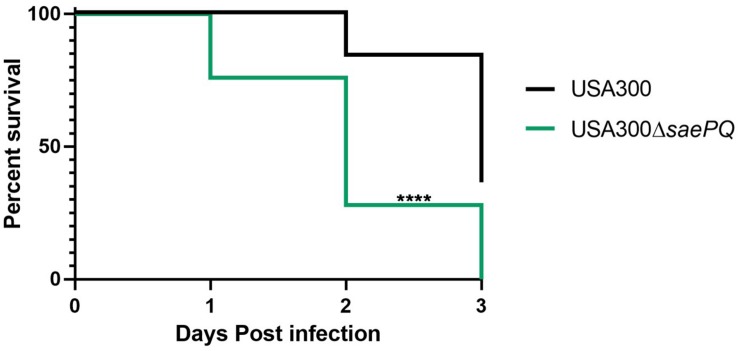
Due to our observations that USA300ΔsaeP demonstrates increased ability to survive neutrophil phagocytosis and that USA300ΔsaeQ had increased (although not significant) survival following neutrophil phagocytosis, we wondered whether a double mutant deficient in both saeP and saeQ might exhibit an enhanced phenotype in the aforementioned neutrophil assays. To test this, we first deleted the entire sae locus in LAC using allelic exchange and then introduced saeRS driven by their native P3 promoter into the chromosome at the geh locus using the single copy integration plasmid pCL55 (Liu et al., 2015). The resulting strain (hereafter referred to as USA300 ΔsaePQ) exhibited reduced expression of saeR/S during exponential growth in vitro (Supplementary Figures S3A,B). This could be due to the absence of transcriptional readthrough from the stronger P1 promoter. Regardless, the USA300 ΔsaePQ double mutant is still capable of inducing SaeR/S-mediated virulence gene expression in response to human neutrophil peptide-1 (HNP-1) exposure (Supplementary Figure S3C). Compared to USA300, USA300 ΔsaePQ exhibited a significant increase in both bacterial survival following phagocytosis as well as increased cytotoxicity toward neutrophils. This increase in virulence was consistent with observations made with USA300ΔsaeP in both neutrophil survival and plasma membrane damage (Figure 5 compared to Figure 2). We measured similar fold-changes in the expression of Sae-dependent virulence genes in USA300ΔsaePQ (also similar to those observed in USA300ΔsaeP) during ES compared to USA300 (compare lukG and lukF-PVL in Figure 5 and Figure 2). As we saw in the SSTI model following challenge with the USA300ΔsaeP or USA300ΔsaeQ mutants, mice challenged with the USA300ΔsaePQ mutant showed no significant differences in abscess area compared to USA300 (Figure 5D). However, we found that intravenous infection with the USA300ΔsaePQ double mutant led to a significant increase in mortality compared to infection with USA300 in the bacteremia model (Figure 6).
FIGURE 5.
USA300ΔsaePQ demonstrates enhanced survival following neutrophil phagocytosis. (A) USA300ΔsaePQ survives neutrophil killing significantly better than USA300 after 5 h incubation. Data are presented as the mean ± SEM of eight independent experiments. (B) PMN plasma membrane damage was significantly increased in neutrophils infected with USA300ΔsaePQ compared to infection with USA300 (neutrophil: bacteria ratio of 1:5). Propidium iodide uptake was assessed at 3 h post-infection by flow cytometry and indicate USA300ΔsaePQ produces significantly more cytolytic proteins in the supernatants than USA300. Data are presented as the mean ± SEM of five independent experiments. (C) Transcript abundance of lukG is increased in USA300ΔsaePQ relative to expression in USA300 at early stationary phase. Gene transcripts were normalized to gyrB and calibrated to the expression levels of USA300. (D) Deletion of saePQ did not significantly impact abscess area. C57BL/6 mice were infected subcutaneously with 1 × 107 CFU of S. aureus. Abscess area was measured daily and results shown are the average of five mice per group. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001 via one-way ANOVA Tukey’s post-test. Data are presented as the mean ± SEM.
FIGURE 6.
Deletion of saePQ enhances the virulence in S. aureus in a mouse model of systemic infection. Survival curves for C57BL/6 mice (n = 13/group) challenged with either 1 × 107 CFU S. aureus or USA300 or USA300ΔsaePQ via tail vein injection. Data shown are death from infection and euthanasia (for exceeding scores on the welfare rubric) during the 72 h endpoint. Data are from two independent experiments. ****P < 0.0001, log-rank (Mantel-Cox) test.
To investigate if the increased mortality in mice challenged with the USA300ΔsaePQ mutant was due to increased expression of SaeR/S-dependent virulence factors, we infected mice with either USA300 or USA300ΔsaePQ mutant carrying an integrated nuc-gfp translational fusion (Behera et al., 2019). Three days post-infection, groups of three-eight C57BL/6 mice were euthanized. Kidneys were harvested, fixed and embedded, and examined using confocal microscopy. Consistent with previous results, USA300 formed clearly-defined abscesses containing a nidus of staphylococci also referred to as a staphylococcal abscess community (SAC). These bacteria exhibit spatial regulation of nuc-gfp as we previously reported (Behera et al., 2019). That is, we detected strong expression of nuc-gfp in the core of the SAC and muted expression on the periphery of the SAC. On the other hand, the USA300ΔsaePQ mutant failed to form discrete, well-formed abscesses. Instead, widespread infiltration of the renal tissues was apparent, and the nuc-gfp reporter was highly expressed in bacteria on the periphery of the lesions (Supplementary Figure S4). Taken together, these data indicate that the saePQ mutant phenocopies a saeP mutant during human neutrophil interaction, and lack of both gene products impacts pathogenesis and virulence expression during bacteremia.
Discussion
The Sae TCS of S. aureus contributes to the expression and production of virulence and immunomodulatory factors that are essential for S. aureus neutrophil evasion and pathogenesis (Voyich et al., 2009; Nygaard et al., 2010; Sun et al., 2010; Flack et al., 2014; Zurek et al., 2014). Much is known about the molecular genetics of activation of the SaeS bifunctional kinase/phosphatase and the SaeR response regulator. However, despite its discovery over two decades ago, relatively little is known about how the auxiliary proteins SaeP and SaeQ contribute to Sae TCS activity and staphylococcal disease. Herein, we utilized saeP and saeQ single and double mutant strains to characterize the role of these accessory proteins using both ex vivo human neutrophil assays and in vivo mouse models of infection. Our results suggest that SaeP acts as a regulator of SaeR/S-dependent virulence during challenge with human neutrophils. Although no statistically significant phenotype could be established for USA300ΔsaeQ following interactions with human neutrophils, we did note an increase in the survival of mice following intravenous infection with this strain, suggesting that SaeQ contributes to SaeR/S signaling during bacteremia.
Our findings support previously-published in vitro data (Jeong et al., 2012; Kavanaugh et al., 2019) suggesting SaeP may regulate SaeR/S-dependent effectors during human neutrophil encounters. The observation that the USA300ΔsaeP strain is more cytotoxic against neutrophils and exhibits increased bacterial survival following phagocytosis suggest that the impact of SaeP on SaeR-target genes is specific and likely dependent on environmental cues. The observation that there was no additive effect of enhanced survival following neutrophil phagocytosis of USA300ΔsaePQ compared to USA300ΔsaeP also supports a specific role for saeP during interaction with human neutrophils. Future studies will continue to characterize the role of SaeQ.
S. aureus uses secreted nuclease (Nuc) along with secreted adenosine synthase (AdsA) to escape neutrophil extracellular traps (NETs) (Thammavongsa et al., 2013). Increasing Nuc expression and production in kidney tissues may increase the production of deoxyadenosine, and this could trigger caspase-3-mediated immune cell death (Thammavongsa et al., 2013). In USA300 LAC, SaeP down-regulated nuc-gfp gene expression via SaeR/S (Kavanaugh et al., 2019). Thus, the hypervirulent phenotype of the saePQ double mutant during acute bacteremia is not unexpected. However, it is unclear why the saeP and saeQ single mutants behave differently. Since we used a purified diet for the in vivo nuc-gfp mouse reporter studies, we cannot exclude the possibility that this diet amplifies mild phenotypes observed in mice challenged with the saeP and saeQ mutant strains. It would be interesting to examine the impact of SaeP and SaeQ during chronic kidney infections in mice. The observation that the saeQ mutant is highly attenuated was unexpected. Given the known protein-protein interactions between SaeQ and SaeS, it is possible that the stability and/or activity of SaeS is compromised in this mutant. Indeed, SaeQ is required for hyperactive SaeS (SaeSL18P) stability in strain Newman (Jeong et al., 2011, 2012). Clearly there is still much to learn about the function of these proteins; only a few studies investigate SaeP and/or SaeQ.
Recently, Kavanaugh et al. (2019) confirmed the cellular localization of SaeP on the cell surface as a lipoprotein, and that its C-terminal domain is facing the extracellular matrix. SaeQ is predicted to be a membrane protein with three membrane-spanning domains, and forms a complex with SaeP and SaeS in the membrane (Jeong et al., 2012). A conserved domain search revealed that the C-terminal portion of SaeP looks like a member of the DM13 superfamily of proteins. Because of its association with the DOMON domain, is thought DM13 proteins might be involved in electron transfer. SaeQ is predicted to be a member of the DoxX family of proteins similar to Bacillus subtilis putative oxidoreductases MhqP and CatD, and Escherichia coli inner membrane proteins YphA and YqjF (Iyer et al., 2007). The Sae system is responsive to cellular respiratory status but the mechanism is unclear. One model posits that inhibition of respiration by oxygen depletion or chemical disruption of the electron transport chain by reactive oxygen species or nitrosative species could lead to a block in the respiratory chain and a buildup of reduced quinones in the membrane, activating Sae activity (Mashruwala et al., 2017). It is conceivable SaePQ sense this perturbation, go inactive, and promote SaeS kinase activity. Alternatively, SaeP possesses a pI of ∼8 and is capable of binding negatively charged eDNA in acidic environments (Kavanaugh et al., 2019). It is tempting to speculate that a physical interaction with neutrophil NET DNA induces some conformational change in SaeP that hinders its ability to stimulate phosphatase activity of SaeS in the staphylococci nearest the neutrophil cuff. Either repressive mechanism could explain the apparent increased nuc-gfp expression in the periphery of lesions formed by the saePQ double mutant (Supplementary Figure S4, compare panels D vs. A). Increased nuclease expression may result in increased virulence during immune cell encounter and could explain why no discrete abscesses could be found when mice were infected with the saePQ double mutant (Supplementary Figure 4).
In vivo observations made with USA300ΔsaePQ suggest that neither saeP nor saeQ influence virulence factors that contribute to murine skin and soft-tissue abscess severity. Inasmuch as alpha-toxin (Hla) is known to play a key role in dermonecrosis caused by USA300 during murine skin and soft tissue infection (Kennedy et al., 2010), results from the skin infection model are in agreement with our gene expression data that demonstrate saeP and saeQ do not influence hla transcript abundance (Supplementary Figures S2A,B). However, the observation that USA300ΔsaePQ is hypervirulent in the bacteremia model is more difficult to explain with our current data. Potentially, different host niches have varying levels of different activating cues and levels of Sae TCS activity. Clearly, additional studies are needed to precisely determine the importance and impact of SaeP and SaeQ at these sites in vivo and to identify conditions that influence their expression and function.
Materials and Methods
Bacterial Strains and Culture
All S. aureus strains used in this study are derivatives of the clinically-relevant CA-MRSA strain USA300 (LAC) that was previously cured of the plasmid encoding erythromycin resistance (Boles et al., 2010). Unless otherwise indicated, overnight and subculture media consisted of tryptic soy broth (TSB) (EMD Millipore; Darmstadt, Germany) supplemented with 0.5% (w/v) glucose. When needed, antibiotics were included in the medium at the following concentrations: ampicillin (Amp), 50 μg ml–1; chloramphenicol (Cm), 5 μg ml–1; and erythromycin (Erm), 5 μg ml–1. Subcultures were created using 1:100 dilution of the overnight culture. For the growth curves, OD600 readings were collected every 0.5 h using a Nanodrop 2000C UV-Vis Spectrophotometer (ThermoFisher Scientific; Wilmington, DE, United States) or an Amersham Ultraspec 2100 pro UV-visible spectrophotometer and colony forming units (CFUs) were enumerated after incubation overnight at 37°C with 5% CO2 as described (Voyich et al., 2005).
Generation of Mutant Strains
Construction of the isogenic saeQ deletion mutant was performed using allelic exchange and pJB38 plasmid (Bae and Schneewind, 2006; Bose et al., 2013). The saeP mutant was constructed previously (Kavanaugh et al., 2019). To construct the saePQ strain, we first deleted the entire sae operon using pKOR1-sae (Bae and Schneewind, 2006). Next, we transduced the strain to chloramphenicol resistance, moving in the PsaeP3-saeRS construct (saeRS under the control of their native promoter, cloned into pCL55 and integrated into the geh locus, CmR). Then, we integrated the nuc-gfp reporter as described by allelic exchange (Behera et al., 2019). Briefly, DNA fragments upstream and downstream of the gene or gene fragment of interest were amplified using primers listed in Table 1, purified by agarose gel electrophoresis, then combined in a two-step overlap PCR reaction and cloned into pJB38 (Flack et al., 2014). ΔsaePQRS was made previously in Flack et al. (2014). The resulting plasmid was transformed sequentially into Escherichia coli (E. coli) strain ER2566 (New England Biolabs), then S. aureus strain RN4220, and the final background USA300 LAC (Flack, 2014). Final mutants were verified by PCR amplification of the chromosomal region of interest and DNA sequencing. Lack of saeP and saeQ in the mutant strains were verified by PCR and agarose gel electrophoresis.
TABLE 1.
Primers used to generate S. aureus mutant strains, respective complemented strains, and TaqMan® primer and probe sequences.
| Primer | Sequence | Description | References |
| Construction of USA300ΔsaeP and USA300ΔsaeQ | |||
| Forward | 5′-GTTGTTGAATTCACCTGATACATTACAGACC-3′ | 600 bp upstream of saeP | Kavanaugh et al., 2019 |
| Reverse | 5′-CAGAAATTGAGTACTAGATCTGTATTCATGCTAACTCCTCATTTC-3′ | Upstream of saeP plus overlap | Kavanaugh et al., 2019 |
| Forward | 5′-GAATACAGATCTAGTACTCAATTTCTGAGTTAAACTTTTATTTACAAC-3′ | Downstream of saeP plus overlap | Flack, 2014 |
| Reverse | 5′-GTTGTTGGTACCAAGAAACTAGCAGCATATGC-3′ | 600 bp downstream of saeP | Flack, 2014 |
| Forward | 5′-GTTGTTGAATTCCCTAACAGGTACATTCAGTTC-3′ | EcoR1, 600 bp upstream of saeQ | Flack, 2014 |
| Reverse | 5′-GCGAGTACTAGATCTCATTCTTTCTATTATTGTGTGTAATTTATAT-3′ | Upstream of saeQ plus overlap | Flack, 2014 |
| Forward | 5′-AGAATGAGATCTAGTACTCGCAAATATAGTTGCACATAC-3′ | 165 bp into saeQ plus overlap | Flack, 2014 |
| Reverse | 5′-GTTGTTGGTACCGATGGTATATGTTGTAAAGCTCTC-3′ | KpnI, 900 bp downstream of saeQ | This work |
| Forward | 5′-TAATTTAGCGCCGCCGAAGA-3′ | saeP | This work |
| Reverse | 5′-TTTTTAGCAGCTGGTGCTGT-3′ | saeP | This work |
| Forward | 5′- CTCTGTTCTTACGACCTCTAAAGTAAT-3′ | saeQ | This work |
| Reverse | 5′-GTTTAGTACCAGTCATCGCTAACA-3′ | saeQ | This work |
| Forward | 5′-GGTGGTGAATTCTTAACTTATCAAATTGAAGAAATGAGGAGTTAGC-3′ | pEPSA5-saeP-EcoR1 | This work |
| Reverse | 5′-ACCACCGGATCCAATTGATTATTTTAATTTAGCGCCGCC-3′ | pEPSA5-saeP-BamHI | This work |
| Forward | 5′-GGTGGTGAATTCTTATATAAATTACACACAATAAATAGAAAGAATGTGAACATC-3′ | pEPSA5-saeQ-EcoR1 | This work |
| Reverse | 5′-GGTGGTGGATCCTGTTCATCATCCACGATCAGTAAGT-3′ | pEPSA5-saeQ-BamHI | This work |
| Taqman® primer/probe sequences | |||
| Forward | 5′-CACCTAACAGGTACATTCAGTTCTA-3′ | saeP primer | This work |
| Reverse | 5′-GGTAGACGTATAAATCTGGACCTTT-3′ | saeP primer | This work |
| Probe | 5′-ACGGTGAAACTGTTGAAGGTAAAGCTGA-3′ | saeP probe | This work |
| Forward | 5′-CACCAGAGTGGTATAAGTGGTT-3′ | saeQ primer | This work |
| Reverse | 5′-CAAAGCCTCCAAAGAAACTAGC-3′ | saeQ primer | This work |
| Probe | 5′-TTGTTGTCCCACTCGGAGAGATTGC-3′ | saeQ probe | This work |
For complementation strains, the saeP and saeQ genes were cloned into the pEPSA5 plasmid (Forsyth et al., 2002) using restriction enzymes (EcoR1 and BamH1) and primers listed in Table 1. The resulting plasmids (pEPSA5-saePcomp and pEPSA5-saeQcomp) drive expression of the sae genes from the xylose-inducible Pxyl promoter. To induce expression, the medium was supplemented with 2% (w/v) xylose in experiments involving these strains as indicated (Forsyth et al., 2002). These plasmids were transformed into electrocompetent E. coli GM2163 (New England Biolabs), then directly into the respective mutant S. aureus strain (USA300ΔsaeP and USA300ΔsaeQ) via E. coli strain IM08B (Monk and Foster, 2012) and called pEPSA5-saePcomp and pEPSA5-saeQcomp. The resulting strains were confirmed using PCR amplification and agarose gel electrophoresis, and presence of transcript abundance verified by TaqMan RT-PCR as done previously (Voyich et al., 2009; Nygaard et al., 2010; Flack et al., 2014).
Neutrophil Isolation
Heparinized venous blood from healthy donors was collected in accordance with a protocol approved by the Institutional Review Board for Human Subjects at Montana State University. All donors provided written consent to participate in the study. Human neutrophils [polymorphonuclear leukocytes (PMNs)] were isolated under endotoxin-free conditions (<25pg ml–1) as previously described (Voyich et al., 2005, 2009). Purity (<1% PBMC contamination) and viability (<2% propidium iodide positivity) of neutrophil preparations were assessed by flow cytometry on a FACS Calibur instrument and BD Biosciences Cell Quest Pro software (version 0.3.3f1b).
Image Stream Phagocytosis Assay
Neutrophil phagocytosis was determined using a fluorescence-based flow cytometry/microscopy method described previously (Ploppa et al., 2011). Briefly, S. aureus was grown to mid-exponential phase, opsonized with 50% (vol/vol) normal human serum and labeled with 750 μL fluorescein isothiocyanate (FITC) at a final concentration of 0.002 mg mL–1. S. aureus strains were combined with neutrophils at a multiplicity of infection (MOI) of 10:1 (bacteria: neutrophils) in 96-well plates coated with human serum coated (20% v/v). Phagocytosis was synchronized by centrifugation as described (Voyich et al., 2005) and incubated at 37°C with 5% CO2 for 30 min. Cells were fixed in 2% (v/v) Periodate-Lysine-Paraformaldehyde (PLP) for 10 min at room temperature (Pieri et al., 2002). PLP was then washed away and antibodies/stains were applied: mouse anti-human CD11b antibody-PE (BD; Franklin Lakes, NJ, United States) and nuclear stain DRAQ5TM (ThermoFisher Scientific; Wilmington, DE, United States). Cells were washed and suspended in 50 μL sterile Dulbecco’s phosphate buffered saline (DPBS) and analyzed by an ImageStream®X Mark II Imaging Flow Cytometer (Millipore Sigma) the following day. Phagocytosis was analyzed using IDEAS® software (AMNIS®, Millipore Sigma, Darmstadt, Germany) where cell images were gated to include neutrophils that were both in focus and singlets. Of these cells, images fluorescing both neutrophil and S. aureus membrane dyes were analyzed using the AMNIS internalization wizard (Ploppa et al., 2011).
Bacterial Survival Assay
Bacterial survival was assessed following synchronized phagocytosis as previously described (Voyich et al., 2005). Briefly, S. aureus strains were grown to mid-exponential phase, opsonized in 50% (v/v) normal human serum, and combined with neutrophils in 96-well plates coated with 20% (v/v) human serum (MOI of 10:1) and incubated at 37°C with 5% CO2. At indicated times, 11 μL of 2% (w/v) saponin solution was added to each well and incubated for 15 min on ice. Samples were sheared using a 1 mL syringe with a blunt needle and bacteria were enumerated by dilution on tryptic soy agar (TSA) following overnight incubation at 37°C with 5% CO2.
Plasma Membrane Damage
Propidium iodide (PI) uptake was used as a measure of plasma membrane permeability to assess damage of neutrophils by secreted S. aureus proteins as described (Nygaard et al., 2012, 2018; Flack et al., 2014). Briefly, bacterial strains were cultured at 37°C for 5 h with shaking (250 RPM) in TSB. After, 1 × 109 CFUs of bacteria were collected and centrifuged for 5 min at 8,000 × g. Supernatants were sterile-filtered and diluted (as indicated in figure legends) with DPBS and exposed to neutrophils for 1 h at 37°C with 5% CO2. After incubation, cells were stained with 0.5 μL PI (1 mg mL–1 Life Technologies) and analyzed by flow cytometry on a FACS Calibur flow cytometer (BD Biosciences; Franklin Lakes, New Jersey). Neutrophil membrane damage was also assessed by flow cytometry using whole bacteria. For these experiments, neutrophils were exposed to live bacteria (MOI 5:1) and incubated for 3 h at 37°C with 5% CO2.
Transcriptional Analysis of Target Genes
TaqMan® gene expression experiments were performed as previously described (Voyich et al., 2005, 2009; Nygaard et al., 2010). Relative quantification of S. aureus target genes was determined by the change in expression of target transcripts normalized to that of the housekeeping gene [gyrase B (gyrB)] and relative to USA300 LAC transcript levels. Primer/probe sequences are described in Table 1 (Voyich et al., 2009; Nygaard et al., 2010). Where indicated, transcript abundance was also measured using SYBR Green chemistry and the absolute transcript abundance method as indicated and as described in Mlynek et al. (2018).
Western Blot Analysis
Supernatants from overnight cultures in TSB without supplemented glucose were harvested as described above, total protein was measured (Pierce BCA Protein Assay) and adjusted to 500 μg mL–1. Samples (14 μL) were resolved using 12% SDS-PAGE gels, (100 V for 45 min) and transferred onto nitrocellulose (at 10 mAmps overnight). Membranes were washed and blocked in DPBS containing 5% (w/v) milk solution for 1 h followed by incubation with rabbit anti-LukS-PV primary antibody (abcam; Cambridge, MA, United States) at a concentration of 0.6 μg mL–1 (4 h at 4°C). PVL was detected after 1 h incubation with goat anti-rabbit IgG coupled to horseradish peroxidase (at 1:10,000 dilution) (Jackson ImmunoResearch; West Grove, PA, United States) and developed using 5 mL 3,3′,5,5′-tetramethylbenzidine (TMB) substrate. Images were taken with an Gel Doc Imager (ProteinSimple; San Jose, CA, United States) and analyzed by ImageJ densitometry software (Schindelin et al., 2012).
GFP Reporter Assays With HNP-1
Bacteria were grown to exponential phase (OD600 ∼ 0.6–0.8) as described previously (Waters et al., 2016) in 250 ml DeLong flasks containing dilute Luria broth (Geiger et al., 2008) (5:1 flask:medium ratio) with vigorous shaking (280 RPM) at 37°C in a water bath. Cultures were diluted to a starting OD600 ∼ 0.1 in fresh medium and aliquoted into individual wells of a 96 well plate (cultures of 200 μl each) and incubated in a computer controlled Tecan F200 plate reader at 37°C. The optical density at 600 nm (OD600) and GFP fluorescence (485 nm excitation, 535 nm emission) values were read every 15 min after shaking (15 s, 2 mm amplitude). When OD600 values reached ∼0.4, the plate was removed, and the indicated wells were spiked with 5 μg ml–1 of the human neutrophil peptide-1 (HNP-1) or vehicle and returned to the plate reader. Data acquisition continued for an additional 12 h. Mean ± SEM relative fluorescence units (RFUs; GFP fluorescence/OD600) from three independent experiments are reported.
Mouse Infection Models
Skin and Soft-Tissue Infection (SSTI)
All studies conformed to NIH guidelines and were approved by the Institutional Animal Care and Use Committee at Montana State University. Female C57BL/6 mice (12 weeks old) were purchased from Charles River Laboratories and maintained at the Animal Resources Center at Montana State University. Female and male BALB/C mice were purchased from the Animal Resources Center at Montana State University. S. aureus strains: USA300 LAC, and isogenic mutants USA300ΔsaeP, USA300ΔsaeQ, USA300ΔsaePQRS, USA300ΔsaePQ strains were grown to mid-exponential phase, washed twice with sterile DPBS and resuspended in DPBS at a concentration of 1 × 107 cells per 50 μL. The dose was confirmed by plating serial dilutions on TSA plates For the abscess model, mice (groups of five) were shaved and inoculated with S. aureus subcutaneously into the lower back (Voyich et al., 2009; Nygaard et al., 2010). Infected area was measured using the formula: (l × w).
Bacteremia Model
Experiments were performed following a protocol approved by the Animal Care and Use Committee at Georgetown University (GUACUC). S. aureus strains were grown to exponential phase in 250 ml DeLong shake flasks (5:1 flask: medium ratio), harvested at OD600 ∼0.4–0.6, washed twice in sterile phosphate buffer saline (PBS), and resuspended to an appropriate optical density equivalent to 1 × 108 colony forming units (CFUs) ml–1. Groups of female C57BL/6 mice (6–8-weeks old, purchased from Charles River Laboratories) were infected intravenously via the tail vein with ∼1 × 107 cells in 100 μl of sterile PBS. The dose was confirmed by plating serial dilutions on TSA plates. Animals were monitored twice daily and evaluated following a GUACUC-approved scoring rubric. Infections were allowed to progress for 72 h or until humane endpoints were reached.
To analyze nuc-gfp expression in tissues, infections were performed as described for USA300 LAC and the ΔsaePQ double mutant essentially described above (and specifically in Behera et al., 2019); notably, animals were fed AIN-93 purified diet (Reeves et al., 1993). Briefly, mice were euthanized 72 h post-infection and kidneys were harvested, fixed with 10% (v/v) buffered formalin, embedded in Sub Xero clear tissue freezing medium (Mercedes Medical), and sectioned into 10 μm slices. Sections were mounted with 4′,6-diamidino-2-phenylindole (DAPI) stain and imaged using laser confocal scanning microscopy. Images were processed using ImageJ (Schindelin et al., 2012). Excitation wavelengths for the fluorescence channels are as follows: DAPI, 405 nm; GFP, 488 nm. Emitted fluorescence data were collected over the following ranges of wavelengths: DAPI, 419–481 nm, GFP, 505–551 nm.
Statistics
Statistical analyses were performed using GraphPad Prism version 8.0 (GraphPad Software, La Jolla, CA, United States) with t-tests and ANOVA as indicated. Error bars represent the standard error of the mean (SEM).
Data Availability Statement
The datasets generated for this study are available on request to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board for Human Subjects at Montana State University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MC, JV, RB, and SB contributed to the conception and design of this study. MC, RB, TE, OB, BC, WP, CF, KP, FG, TN, and JD performed the experiments and data analysis. MC, JV, and SB wrote and prepared the manuscript for submission. All authors read and approved this manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Jeannie Gripentrog for neutrophil isolations for use in our experiments, Elizabeth Palmer for previous neutrophil phagocytosis work, Justice Roberts for assistance with growth curves and Elizabeth Gritzmacher for work with the animal studies. Additionally, we acknowledge Dr. Taeok Bae for the gift of the pKOR-sae plasmid used in the Brinsmade laboratory.
Footnotes
Funding. This work was supported by the U.S. National Institutes of Health (Grants NIH-R01A1090046, 1R56AI135039-01A1, PAR98-072, U54GM115371 to JV; grants R21AI123708 and R01AI137403 to SB), as well as funds from the Montana University System Research Initiative (51040-MUSRI2015-03), the Montana State University Agriculture Experiment Station, and an equipment grant from Murdock Charitable Trust.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.00561/full#supplementary-material
Complementation of saeP and saeQ restores USA300 phenotype. Gene complementation with saeP and saeQ on the xylose-inducible pEPSA5 plasmid restores USA300 phenotype. Promoter expression induced with 2% (w/v) xylose in the medium. (A) Gene expression of saeP and saeQ is rescued in S. aureus mutant strains complemented with saeP or saeQ expressed in trans. (B) Complementation of saeP restores transcript of select S. aureus genes to levels observed in USA300 (or higher). Transcript abundance is relative to gyrB and calibrated to expression in USA300 (n = 2/gene). (C) Complementation of saeP and saeQ in trans reduces secreted cytolytic factors that target neutrophil plasma membrane damage to levels secreted by USA300. Data are presented as the mean ± SEM of four independent experiments. Stats: One-Way ANOVA with Tukey’s post-test; ns, not significant.
Deletion of saeP or saeQ did not significantly impact abscess area. Deletion of saeP or saeQ did not significantly impact abscess area. (A) BALB/C mice (5 per group) and (B) C57BL/6 mice (5 per group) were infected subcutaneously with 1 × 107 CFUs of each S. aureus strain and abscess area was subsequently monitored for 10 days. Abscess area was measured daily and results shown are the average area per strain. Representative images are from C57BL/6 mice on day 2. Graphs represent data from two biological replicates for BALB/c and one for C57BL/6. Unpaired t-test relative to USA300, ∗p-value ≤ 0.05, ∗∗p-value ≤ 0.01. Data are presented as the mean ± SEM.
The ΔsaePQ mutant has reduced saeR/S transcript levels but induces Sae-dependent genes normally. (A,B) Normalized transcript abundance is shown for USA300 and the indicated mutant strains of S. aureus grown to exponential phase using SYBR green qRT-PCR as described in Materials and Methods and as described in Mlynek et al. (2018). Data indicate the mean ± SEM from three independent experiments. Statistical analysis: ANOVA (ordinary one-way), Dunnett’s multiple comparison test, asterisks indicate the level of significance compared to the USA300 (P ≤ 0.05). For USA300ΔsaePQRS, the transcript level is below the limit of detection. (C) The indicated strains carrying a nuc-gfp reporter fusion were grown to exponential phase (OD600 ∼ 0.4) in dilute Luria broth, at which time bacteria were exposed to either vehicle (water) or HNP-1 for 12 h. The data shown are the mean relative fluorescence units (RFUs; fluorescence/OD600) ±SEM for three independent experiments performed in technical triplicate; statistical significance was assessed by paired t-test (∗P ≤ 0.05, ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001). ND, not detected: signal was below the limit of detection.
Deletion of saePQ influences lesion structure and nuc expression in infected kidneys. (A–F) Representative confocal micrographs of staphylococcal lesions in kidney produced by USA300 and USA300ΔsaePQ. Channels: nuc-sGFP (A,D), DAPI (B,E), and merge (C,F) (scale bar is 25 μm). The fluorescence image acquisition parameters used are as follows; excitation for DAPI (blue) and GFP (green) are 405 and 488 nm respectively; emission ranges are 419–481 nm (DAPI) and 505–551 nm (GFP).
References
- Bae T., Schneewind O. (2006). Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55 58–63. 10.1016/j.plasmid.2005.05.005 [DOI] [PubMed] [Google Scholar]
- Behera R. K., Mlynek K. D., Linz M. S., Brinsmade S. R. (2019). A fluorescence-based method to study bacterial gene regulation in infected tissues. J. Vis. Exp. 144:e59055. 10.3791/59055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles B. R., Thoendel M., Roth A. J., Horswill A. R. (2010). Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS One 5:e10146. 10.1371/journal.pone.0010146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose J. L., Fey P. D., Bayles K. W. (2013). Genetic tools to enhance the study of gene function and regulation in Staphylococcus aureus. Appl. Environ. Microbiol. 79 2218–2224. 10.1128/AEM.00136-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. F. (2001). The changing epidemiology of Staphylococcus aureus? Emerg. Infect. Dis. 7 178–182. 10.3201/eid0702.010204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. F., Deleo F. R. (2009). Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 7 629–641. 10.1038/nrmicro2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H., Jeong D. W., Liu Q., Yeo W. S., Vogl T., Skaar E. P., et al. (2015). Calprotectin increases the activity of the SaeRS two component system and murine mortality during Staphylococcus aureus infections. PLoS Pathog. 11:e1005026. 10.1371/journal.ppat.1005026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M. Z., Daum R. S. (2010). Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 23 616–687. 10.1128/CMR.00081-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flack C. E. (2014). Mutagenesis and structural analysis of the Staphylococcus Aureus Sae two-component system reveals the intricate nature of virulence regulation. Ph.D. thesis, University of Iowa, Iowa City, IA. [Google Scholar]
- Flack C. E., Zurek O. W., Meishery D. D., Pallister K. B., Malone C. L., Horswill A. R., et al. (2014). Differential regulation of staphylococcal virulence by the sensor kinase SaeS in response to neutrophil-derived stimuli. Proc. Natl. Acad. Sci. U.S.A. 111 E2037–E2045. 10.1073/pnas.1322125111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth R. A., Haselbeck R. J., Ohlsen K. L., Yamamoto R. T., Xu H., Trawick J. D., et al. (2002). A genome-wide strategy for the identification of essential genes in Staphylococcus aureus. Mol. Microbiol. 43 1387–1400. 10.1046/j.1365-2958.2002.02832.x [DOI] [PubMed] [Google Scholar]
- Geiger T., Goerke C., Mainiero M., Kraus D., Wolz C. (2008). The virulence regulator Sae of Staphylococcus aureus: promoter activities and response to phagocytosis-related signals. J. Bacteriol. 190 3419–3428. 10.1128/JB.01927-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra F. E., Addison C. B., de Jong N. W. M., Azzolino J., Pallister K. B., van Strijp J. A. G., et al. (2016). Staphylococcus aureus SaeR/S-regulated factors reduce human neutrophil reactive oxygen species production. J. Leukoc. Biol. 100 1–6. 10.1189/jlb.4VMAB0316-100RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer L. M., Anantharaman V., Aravind L. (2007). The DOMON domains are involved in heme and sugar recognition. Bioinformatics 23 2660–2664. 10.1093/bioinformatics/btm411 [DOI] [PubMed] [Google Scholar]
- Jeong D. W., Cho H., Jones M. B., Shatzkes K., Sun F., Ji Q., et al. (2012). The auxiliary protein complex SaePQ activates the phosphatase activity of sensor kinase SaeS in the SaeRS two-component system of Staphylococcus aureus. Mol. Microbiol. 86 331–348. 10.1111/j.1365-2958.2012.08198.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong D. W., Cho H., Lee H., Li C., Garza J., Fried M., et al. (2011). Identification of the P3 promoter and distinct roles of the two promoters of the SaeRS two-component system in Staphylococcus aureus. J. Bacteriol. 193 4672–4684. 10.1128/JB.00353-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh J. S., Flack C. E., Lister J., Ricker E. B., Ibberson C. B., Jenul C., et al. (2019). Identification of extracellular DNA-binding proteins in the biofilm matrix. mBio 10:e01137-19. 10.1128/mBio.01137-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A. D., Wardenburg J. B., Gardner D. J., Long D., Whitney A. R., Braughton K. R., et al. (2010). Targeting of Alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J. Infect. Dis. 202 1050–1058. 10.1086/656043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. D., Humphrey B. J., Wang Y. F., Kourbatova E. V., Ray S. M., Blumberg H. M. (2006). Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann. Intern. Med. 144 309–317. [DOI] [PubMed] [Google Scholar]
- Klevens R. M., Morrison M. A., Nadle J., Petit S., Gershman K., Ray S., et al. (2007). Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298 1763–1771. 10.1001/jama.298.15.1763 [DOI] [PubMed] [Google Scholar]
- Kobayashi S. D., Malachowa N., Deleo F. R. (2015). Pathogenesis of Staphylococcus aureus abscesses. Am. J. Pathol. 185 1518–1527. 10.1016/j.ajpath.2014.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekstrom-Himes J. A., Gallin J. I. (2000). Immunodeficiency diseases caused by defects in phagocytes. N. Engl. J. Med. 343 1703–1714. 10.1056/NEJM200012073432307 [DOI] [PubMed] [Google Scholar]
- Liu Q., Cho H., Yeo W. S., Bae T. (2015). The extracytoplasmic linker peptide of the sensor protein SaeS tunes the kinase activity required for staphylococcal virulence in response to host signals. PLoS Pathog. 11:e1004799. 10.1371/journal.ppat.1004799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Yeo W. S., Bae T. (2016). The SaeRS two-component system of Staphylococcus aureus. Genes 7:E81. 10.3390/genes7100081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero M., Goerke C., Geiger T., Gonser C., Herbert S., Wolz C. (2010). Differential target gene activation by the Staphylococcus aureus two-component system saeRS. J. Bacteriol. 192 613–623. 10.1128/JB.01242-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marincola G., Wolz C. (2017). Downstream element determines RNase y cleavage of the saePQRS operon in Staphylococcus aureus. Nucleic Acids Res. 45 5980–5994. 10.1093/nar/gkx296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashruwala A. A., Gries C. M., Scherr T. D., Kielian T., Boyd J. M. (2017). SaeRS is responsive to cellular respiratory status and regulates fermentative biofilm formation in Staphylococcus aureus. Infect. Immun. 85:e000157-17. 10.1128/IAI.00157-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlynek K. D., Sause W. E., Moormeier D. E., Sadykov M. R., Hill K. R., Torres V. J., et al. (2018). Nutritional regulation of the Sae two-component system by CodY in Staphylococcus aureus. J. Bacteriol. 200:e00012-18. 10.1128/JB.00012-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk I. R., Foster T. J. (2012). Genetic manipulation of staphylococci-breaking through the barrier. Front. Cell. Infect. Microbiol. 2:49. 10.3389/fcimb.2012.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Jiang D. (2003). The staphylococcal saeRS system coordinates environmental signals with agr quorum sensing. Microbiology 149 2709–2717. 10.1099/mic.0.26575-0 [DOI] [PubMed] [Google Scholar]
- Nygaard T. K., Borgogna T. R., Sward E. W., Guerra F. E., Dankoff J. G., Collins M. M., et al. (2018). Aspartic acid residue 51 of SaeR is essential for Staphylococcus aureus virulence. Front. Microbiol. 9:3085. 10.3389/FMICB.2018.03085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard T. K., Pallister K. B., DuMont A. L., DeWald M., Watkins R. L., Pallister E. Q., et al. (2012). Alpha-toxin induces programmed cell death of human T cells, B cells, and monocytes during USA300 infection. PLoS One 7:e0036532. 10.1371/journal.pone.0036532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard T. K., Pallister K. B., Ruzevich P., Griffith S., Vuong C., Voyich J. M. (2010). SaeR binds a consensus sequence within virulence gene promoters to advance USA300 pathogenesis. J. Infect. Dis. 201 241–254. 10.1086/649570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M. E., Nygaard T. K., Ackermann L., Watkins R. L., Zurek O. W., Pallister K. B., et al. (2013). Staphylococcus aureus nuclease is an SaeRS-dependent virulence factor. Infect. Immun. 81 1316–1324. 10.1128/IAI.01242-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieri L., Sassoli C., Romagnoli P., Domenici L. (2002). Use of periodate-lysine-paraformaldehyde for the fixation of multiple antigens in human skin biopsies. Eur. J. Histochem. 46 365–375. [DOI] [PubMed] [Google Scholar]
- Ploppa A., George T. C., Unertl K. E., Nohe B., Durieux M. E. (2011). ImageStream cytometry extends the analysis of phagocytosis and oxidative burst. Scand. J. Clin. Lab. Invest. 71 362–369. 10.3109/00365513.2011.572182 [DOI] [PubMed] [Google Scholar]
- Reeves P. G., Nielsen F. H., Fahey G. C. (1993). AIN-93 purified diets for laboratory rodents: final report of the American Institute of nutrition Ad Hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 123 1939–1951. 10.1093/jn/123.11.1939 [DOI] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9 676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhuber A., Goerke C., Bayer M. G., Döring G., Wolz C. (2003). Molecular architecture of the regulatory locus sae of Staphylococcus aureus and its impact on expression of virulence factors. J. Bacteriol. 185 6278–6286. 10.1128/JB.185.21.6278-6286.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F., Li C., Jeong D., Sohn C., He C., Bae T. (2010). In the Staphylococcus aureus two-component system sae, the response regulator SaeR binds to a direct repeat sequence and DNA binding requires phosphorylation by the sensor kinase SaeS. J. Bacteriol. 192 2111–2127. 10.1128/JB.01524-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thammavongsa V., Missiakas D. M., Schneewind O. (2013). Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science 342 863–866. 10.1126/science.1242255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura C. L., Malachowa N., Hammer C. H., Nardone G. A., Robinson M. A., Kobayashi S. D., et al. (2010). Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics. PLoS One 5:e11634. 10.1371/journal.pone.0011634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyich J. M., Braughton K. R., Sturdevant D. E., Whitney A. R., Saïd-Salim B., Porcella S. F., et al. (2005). Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 175 3907–3919. 10.4049/jimmunol.175.6.3907 [DOI] [PubMed] [Google Scholar]
- Voyich J. M., Vuong C., DeWald M., Nygaard T. K., Kocianova S., Griffith S., et al. (2009). The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus. J. Infect. Dis. 199 1698–1706. 10.1086/598967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters N. R., Samuels D. J., Behera R. K., Livny J., Rhee K. Y., Sadykov M. R., et al. (2016). A spectrum of CodY activities drives metabolic reorganization and virulence gene expression in Staphylococcus aureus. Mol. Microbiol. 101 495–514. 10.1111/mmi.13404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurek O. W., Nygaard T. K., Watkins R. L., Pallister K. B., Torres V. J., Horswill A. R., et al. (2014). The role of innate immunity in promoting SaeR/S-mediated virulence in i. J. Innate Immun. 6 21–30. 10.1159/000351200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurek O. W., Pallister K. B., Voyich J. M. (2015). Staphylococcus aureus inhibits neutrophil-derived IL-8 to promote cell death. J. Infect. Dis. 212 934–938. 10.1093/infdis/jiv124 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complementation of saeP and saeQ restores USA300 phenotype. Gene complementation with saeP and saeQ on the xylose-inducible pEPSA5 plasmid restores USA300 phenotype. Promoter expression induced with 2% (w/v) xylose in the medium. (A) Gene expression of saeP and saeQ is rescued in S. aureus mutant strains complemented with saeP or saeQ expressed in trans. (B) Complementation of saeP restores transcript of select S. aureus genes to levels observed in USA300 (or higher). Transcript abundance is relative to gyrB and calibrated to expression in USA300 (n = 2/gene). (C) Complementation of saeP and saeQ in trans reduces secreted cytolytic factors that target neutrophil plasma membrane damage to levels secreted by USA300. Data are presented as the mean ± SEM of four independent experiments. Stats: One-Way ANOVA with Tukey’s post-test; ns, not significant.
Deletion of saeP or saeQ did not significantly impact abscess area. Deletion of saeP or saeQ did not significantly impact abscess area. (A) BALB/C mice (5 per group) and (B) C57BL/6 mice (5 per group) were infected subcutaneously with 1 × 107 CFUs of each S. aureus strain and abscess area was subsequently monitored for 10 days. Abscess area was measured daily and results shown are the average area per strain. Representative images are from C57BL/6 mice on day 2. Graphs represent data from two biological replicates for BALB/c and one for C57BL/6. Unpaired t-test relative to USA300, ∗p-value ≤ 0.05, ∗∗p-value ≤ 0.01. Data are presented as the mean ± SEM.
The ΔsaePQ mutant has reduced saeR/S transcript levels but induces Sae-dependent genes normally. (A,B) Normalized transcript abundance is shown for USA300 and the indicated mutant strains of S. aureus grown to exponential phase using SYBR green qRT-PCR as described in Materials and Methods and as described in Mlynek et al. (2018). Data indicate the mean ± SEM from three independent experiments. Statistical analysis: ANOVA (ordinary one-way), Dunnett’s multiple comparison test, asterisks indicate the level of significance compared to the USA300 (P ≤ 0.05). For USA300ΔsaePQRS, the transcript level is below the limit of detection. (C) The indicated strains carrying a nuc-gfp reporter fusion were grown to exponential phase (OD600 ∼ 0.4) in dilute Luria broth, at which time bacteria were exposed to either vehicle (water) or HNP-1 for 12 h. The data shown are the mean relative fluorescence units (RFUs; fluorescence/OD600) ±SEM for three independent experiments performed in technical triplicate; statistical significance was assessed by paired t-test (∗P ≤ 0.05, ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001). ND, not detected: signal was below the limit of detection.
Deletion of saePQ influences lesion structure and nuc expression in infected kidneys. (A–F) Representative confocal micrographs of staphylococcal lesions in kidney produced by USA300 and USA300ΔsaePQ. Channels: nuc-sGFP (A,D), DAPI (B,E), and merge (C,F) (scale bar is 25 μm). The fluorescence image acquisition parameters used are as follows; excitation for DAPI (blue) and GFP (green) are 405 and 488 nm respectively; emission ranges are 419–481 nm (DAPI) and 505–551 nm (GFP).
Data Availability Statement
The datasets generated for this study are available on request to the corresponding authors.