Abstract
Approximately, 30 000 men die from prostate cancer (PCa) every year in the United States, mainly due to the metastasis. Thus, the key events associated with PCa metastasis are under rigorous investigation, with recent studies showing that preparation of pre-metastatic niches (PMN) in distant organs is an important step. However, the molecular basis for PMN preparation is still unclear. Hypoxia in primary tumors promotes aggressiveness; however, its precise role in metastasis is not clear. We recently reported that exosomes secreted by PCa cells under hypoxia promote stemness and invasiveness in naïve PCa cells; however, whether these extracellular vesicles also influence PMN remains unknown. In the present study, we isolated exosomes from human PCa PC3 cells under normoxic (21% O2, exosomes secreted under normoxic condition [ExoNormoxic]) and hypoxic (1% O2, exosomes secreted under hypoxic condition [ExoHypoxic]) conditions, and characterized their effect (10 μg exosomes, intraperitoneal (IP) treatment every 48 hours for 4 weeks) on key biomarkers associated with PMN in nude mice. Whole animal fluorescence imaging showed that ExoHypoxic treatment promotes matrix metalloproteinases (MMPs) activity in several putative metastatic sites. Histological studies confirmed that ExoHypoxic treatment enhanced the level of MMP2, MMP9, and extracellular matrix proteins (fibronectin and collagen) as well as increased the number of CD11b+ cells at selective PMN sites. Furthermore, proteomic profiling of exosomes by liquid chromatography/mass spectrometry identified cargo proteins in ExoNormoxic and ExoHypoxic as well as distinct canonical pathways targeted by them. These results suggest that exosomes secreted by PCa cells under hypoxia plausibly remodel distant PMN, and thus, could be a potential target to control metastatic PCa.
Keywords: exosomes, hypoxia, matrix metalloproteinases, pre-metastatic niches, proteomics
1 |. INTRODUCTION
Prostate cancer (PCa) patients with localized disease have a 5-year survival rate of almost 100% and a relatively low mortality to incidence ratio compared to other cancer types.1 However, in patients with clinically detectable metastasis, the median survival is significantly reduced, and the majority of PCa-associated deaths are attributed to tumor metastasis. In 2019 alone, ~31 620 men are estimated to die due to PCa in the United States, mostly due to metastatic spread of the disease.2 While modern medicine has become increasingly successful at diagnosing PCa at early stages, but unfortunately this has not translated into significant reduction of morbidity and mortality in metastatic cases. This clearly reflects the need to understand the mechanisms of prostate carcinogenesis more critically, specifically the key events involved in PCa metastasis. Whereas metastasis has been considered as a late event in carcinogenesis in the past, recent evidence have suggested that it could be an early event, and the preparation at distant putative metastatic sites, known as “pre-metastatic niches (PMN)” starts even earlier, which could eventually determine the success of metastasis.3–9 Accordingly, a better molecular understanding of the factors involved in PMN formation is crucial to effectively target metastasis and to reduce PCa-associated mortality.
Several factors in tumor microenvironment could influence the cancer cells’ survival, metastasis, and treatment response. Two such major biochemical factors, which could influence the tumor fate, are pH and hypoxia (low oxygen in the tumor microenvironment). Low extracellular pH in tumor microenvironment has been correlated with better survival and immune escape.10–13 The acidic condition also increases the exosomes release by the cancer cells and influences their cargo. Similar results were evident in patients with cancer in selective acidic microenvironment, presenting significantly higher levels of extracellular vesicles in their plasma as compared to both benign prostatic hypertrophy and healthy individuals.14
Hypoxia is an independent prognostic indicator of poor outcomes in prostate and other malignancies, together with higher risk for metastasis15–19; however, the precise mechanism through which hypoxic conditions within primary tumor promote metastasis is not well defined. Hypoxic conditions promote lysyl oxidase (LOX) secretion by breast tumor cells that plays a critical role in PMN preparation and metastasis.20 We have earlier reported that exosomes, extracellular vesicles of ~30 to 150 nm in size, secreted by PCa cells under hypoxic conditions are loaded with specific cytokines, growth factors, proteinases and lipids21,22; and these exosomes promote stemness, invasiveness and epithelial-to-mesenchymal transition in naïve PCa cells. Furthermore, exosomes secreted by PCa cells under hypoxic conditions promoted the cancer-associated fibroblast phenotype in prostate fibroblasts.22 These studies suggest that exosomes secreted by PCa cells under hypoxia promote remodeling of the local tumor microenvironment; however, their role in PMN preparation remains unknown.
Over a century ago, Paget23 proposed the “seed and soil” hypothesis to explain the high tendency of cancer cells to settle in specific organs.24,25 According to this hypothesis, seeds (tumor cells) could survive and grow only in conducive soil (metastatic site). In recent years, extensive studies have suggested that primary tumors prepare or tilt the future metastatic sites (ie PMN) in an effort to improve the chances of successful survival and settlement in a foreign microenvironment.3,5–9 The well characterized hallmarks of PMN include: (a) extensive extracellular matrix (ECM) remodeling including higher matrix metalloproteinases (MMPs) activity as well as accumulation of fibronectin, collagen, growth factors and chemoattractants; (b) immunosuppressive and proinflammatory milieu including an increase in the infiltration of bone marrow derived cells (BMDCs); and (c) higher vascular leakiness,.3,5,7,8,20,26–29 Key molecular players in PMN preparation include MMPs, vascular endothelial growth factors, placental growth factor, tumor necrosis factor 1 alpha (TNF1α), transforming growth factor beta (TGFβ), and LOX3,5–7,20,27; notably, hypoxia regulates the expression of several of them.20,22,30,31 We have also reported that exosomes secreted by human PCa cells under hypoxia are loaded with several of these growth factors and cytokines (TNF1α, TGFβ, and interleukin 6) along with higher MMP (2 and 9) activity.22 Based upon that, in the present study we assessed the in vivo effect of exosomes secreted by human PCa PC3 cells under hypoxia on various biomarkers associated with PMN.
2 |. METHODS
2.1 |. Cell lines and reagents
Human PCa PC3 cells were purchased from American type culture collection (Manassas, VA) and cultured in Roswell Park Memorial Institute Medium 1640 medium supplemented with 10% heat-inactivated fetal bovine serum and 100 U/mL penicillin G, and 100 μg/mL streptomycin sulfate at 37°C in a humidified 5% CO2 incubator. Media and other cell culture materials were purchased from Invitrogen Corporation (Gaithersburg, MD). Antibodies for fibronectin (sc6952) and matrix metalloproteinases (MMP9; sc6840) were from Santa Cruz Biotechnology (Dallas, TX). Antibodies for MMP2 (ab86607), CD11b (ab75476), and collagen (ab6586) were from Abcam (Cambridge, MA). MMPsense 750 dye was from Perkin Elmer. All other reagents were obtained in their commercially available highest purity grade.
2.2 |. Hypoxia exposure and exosome isolation
For normoxic (21% O2) condition, cells were cultured at 37°C in a 5% CO2 humidified environment as an adherent monolayer. Hypoxia experiments were performed in a hypoxia chamber (BioSpherix Xvivo system) at 1% O2 at 37°C in a 5% CO2 humidified environment. Cells were cultured in exosome-depleted media under either normoxia or hypoxia. Following 48 hours of incubation, conditioned media was collected and exosomes were isolated by ultracentrifugation method as reported previously.21,22 In brief, the collected cell culture media was centrifuged at 500g for 5 minutes; supernatant was again centrifuged at 2000g for 5 minutes followed by 10 000 G for 30 minutes. Supernatant was then filtered through 0.22 μm filters (Merck Millipore); and concentrated using concentrators (100 K/150 K MWCO/20 ml; Thermo Fisher Scientific). The supernatants were then ultra-centrifuged at 100 000 g for 70 minutes (L-80 Ultracentrifuge, 70.1 Ti fixed angel rotor, Beckman Colter). Finally, the pelleted exosomes were suspended in Dulbecco’s phosphate-buffered saline (DPBS). Exosomes isolated from conditioned media of PC3 cells cultured under hypoxic and normoxic conditions are mentioned as exosomes secreted under hypoxic condition (ExoHypoxic) and exosomes secreted under normoxic condition (ExoNormoxic) respectively.
2.3. |. Animal experiment, exosome labeling, and in vivo tracking
Athymic (nu/nu) male nude mice were purchased from Harlan (Indianapolis, Indiana). The treatment protocol was approved by the Institutional Animal Care and Use Committee of the University of Colorado Denver and Wake Forest Baptist Medical Center. Mice were injected intraperitoneal (IP) with phosphate buffered saline (PBS; 100 μL) or ExoHypoxic or ExoNormoxic (10 μg in total 100 uL volume of PBS) on alternate days for 4 weeks. Five mice were used for each treatment group and control. At the end of 4 weeks, MMPsense 750 dye (2 nmol/mouse; excitation: 749 nm; emission: 775 nm) was injected (intravenous) in 10 mice (2 mice in PBS group and 4 mice each in ExoNormoxic and ExoHypoxic groups) to assess MMPs activity. Mice were anaesthetized and fluorescent and X-ray images were captured by an In Vivo Multispectral FX imaging instrument (Carestream Molecular Imaging, Woodbridge, CT). MMPsense 750 is an activatable agent that is optically silent upon injection and produces fluorescent signal after cleavage by MMPs (2, 3, 7, 9, 12, and 13). Mice were killed 48 hours after MMPsense 750 injection and various organs were imaged with the In Vivo Multispectral FX imaging instrument.
In vivo tracking of exosomes was performed by labeling exosomes with ExoGlow dye (SBI biosciences, Mountain View, CA) following the vendor’s protocol with few modifications. Briefly, exosomes (400 ug) were labeled with 4 uL of ExoGlow and then ultracentrifuged at 200 000g for 2 hours at 4°C to remove the unlabelled dye. Thereafter, labelled exosomes (ExoHypoxic and ExoNormoxic) were injected IP (10 ug/100 uL) in mice and imaged at 0, 1 hour, 3, 6, and 24 hours. After 24 hours, mice were killed and various organs were imaged as described above.
2.4 |. Immunohistochemistry
Tissues were fixed in 10% buffered-formalin for 12 hours and processed conventionally. The paraffin-embedded tumor sections (5 μm-thick) were deparaffinized using xylene, and rehydrated in a graded series of ethanol with a final wash in distilled water. Antigen retrieval was performed using 10 mM citrate buffer (pH 6.0) in a decloaking chamber for 15 minutes. Endogenous peroxidase activity was blocked by immersing the sections in 3.0% H2O2 in methanol (v/v), followed by three changes in 10 mM PBS (pH 7.4). Thereafter, sections were incubated with specific primary antibody, followed by a specific biotinylated secondary antibody, and finally with conjugated horseradish peroxidase streptavidin and 3,3’-diaminobenzidine (DAB) working solution, and counterstained with hematoxylin.
2.5 |. Liquid chromatography/mass spectrometry analysis
ExoNormoxic and ExoHypoxic (10 μg each) were analyzed by liquid chromatography/mass spectrometry (LC/MS/MS) at the Proteomics and Metabolomics Shared Resource at Wake Forest Baptist Medical Center. Exosomes were lysed in radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitor cocktail. Proteins were reduced and alkylated in the presence of 10 mM dithiothreitol and 30 mM iodoacetamide, and then precipitated using cold-acetone. Protein pellet was suspended in 50 mM ammonium bicarbonate and incubated with trypsin at 1:50 trypsin-to-protein ratio at 37°C overnight. The resulting peptides were purified using a C18 desalting spin column and prepared in 5% (v/v) acetonitrile containing 1% (v/v) formic acid for LC-MS/MS analysis.
Peptides were analyzed on a LC-MS/MS system consisting of an Orbitrap Velos Pro Mass Spectrometer (Thermo Fisher Scientific, Waltham, MA) and a Dionex Ultimate-3000 nano-UPLC system (Thermo Fisher Scientific) equipped with an Acclaim PepMap 100 (C18, 5 μm, 100 Å, 100 μm × 2 cm) trap column and an Acclaim PepMap RSLC (C18, 2 μm, 100 Å, 75 μm × 50 cm) analytical column. MS spectra were acquired by top ten data dependent scans with dynamic exclusion option of 30 seconds enabled.
Spectra were searched using Sequest HT algorithm within the Proteome Discoverer v2.2 (Thermo Fisher Scientific) and UniProt human protein FASTA database. Search parameters are as follow: FT-trap instrument, parent mass error tolerance of 10 ppm, fragment mass error tolerance of 0.6 Da (monoisotopic), variable modifications of 16 Da (oxidation) on methionine and fixed modification of 57 Da (carbamidomethylation) on cysteine. Proteomics data was analyzed through the use of IPA (QIAGEN Inc, https://www.qiagenbioinformatics.com/products/ingenuitypathway-analysis). Algorithms used in IPA were described earlier.32
2.6 |. Statistical analysis
Statistical analysis was performed using SigmaStat 2.03 software (Jandel Scientific, San Rafael, CA). Data was analyzed by t test or one way analysis of variance and a statistically significant difference was considered to be at P < .05.
3 |. RESULTS AND DISCUSSION
3.1 |. ExoHypoxic promote MMPs activity in selective organs
MMPs are a cluster of at least 23 enzymes and their structure is characterized by the presence of a zinc ion at the catalytic site. Their biological activities include ECM degradation, tissue remodeling and release of various growth factors, cytokines and chemokines. Numerous studies have identified the role of MMPs in metastasis through offering cancer cells an invasive phenotype; and a positive correlation is reported in majority of studies between the high expression of MMPs and worst pathological features.33 Several studies have now also established the central role of MMPs in remodeling the microenvironment at distant organ sites before metastasis.20,27 An increase in MMPs activity is considered as one of the primary indicators of PMN preparation. MMPs play an important role in ECM remodeling, and generate chemoattractants that help in recruitment of BMDCs and cancer cells at metastatic site.5,7,20 The modification of ECM by MMPs determines the shift from a protective and homeostatic microenvironment to a growth promoting state. Hirarsuka et al27 first reported that metastatic growth of melanoma and lung cancer cells is dependent on MMP9 induction in pre-metastatic lung endothelial cells and macrophages. This study also reported a higher MMP9 expression in lung tissues from patients with tumors such as hepatocellular carcinoma, pancreatic cancer, esophageal cancer, colon cancer, cholangiocellular carcinoma, gastric cancer, melanoma, lymphoma, and ovarian cancer.27 Acuff et al34 reported that MMP9 secreted by host cells (mouse) is essential for the survival of incoming human lung carcinoma A549 cells. These studies strongly suggest that MMPs induction is one of the hallmarks of PMN preparation.
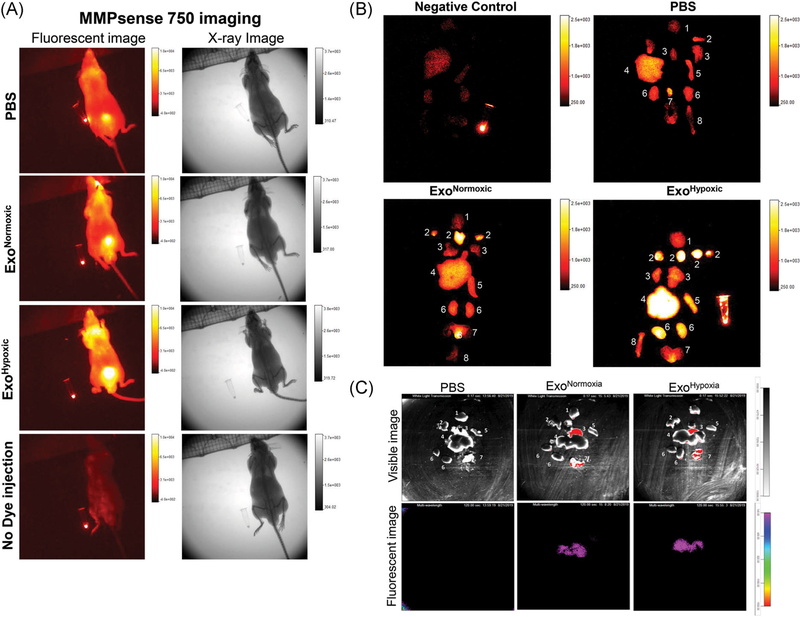
In the present study, we compared the effect of exosomes secreted by human PCa PC3 cells under normoxic (21% O2) and hypoxic (1% O2) conditions on MMPs activity. We injected ExoNormoxic and ExoHypoxic for 4 weeks (10 μg on alternate days) in male athymic nude mice and then imaged the animals for MMPs activity using MMPsense 750 dye and In Vivo Multispectral FX imaging instrument. This dye is activated by MMP2 and 9 (gelatinases), MMP3 (stromelysins), MMP7 (matrilysin), MMP12 (metalloelastase), and MMP13 (collagenase). As shown in the Figure 1A, higher signal (MMPs activity) was observed in mice injected with ExoHypoxic compared to the ExoNormoxic and PBS control. Mice without dye injection did not show any signal confirming the specificity of the dye (Figure 1A, bottom panel). Furthermore, ex vivo imaging of the organs clearly showed that ExoHypoxic treatment resulted in a significant increase in the MMPs activity in various organs as shown in Figure 1B (1. brain; 2. lymph nodes + salivary glands; 3. lung + heart; 4. liver; 5. spleen; 6. kidneys; 7. prostate + seminal vesicles + bladder; and 8. bone). More importantly, in ExoHypoxic treated mice, increased MMPs activity was observed in organs (eg liver, kidney and spleen), where PC3 cells could metastasize when injected orthotopically in the prostate of mice.35,36 These results suggest that ExoHypoxic could play an important role in the PMN preparation through increasing MMPs activity at specific organ sites.
FIGURE 1.
Human PCa ExoHypoxic promotes MMPs activity in selective organs in male athymic nude mice. A, Male athymic nude mice were injected with PBS, ExoNormoxic or ExoHypoxic (10 μg each, IP) on alternate days for 4 weeks. At the end, mice were injected with MMPsense 750 dye and imaged for fluorescence and X-ray by In Vivo Multispectral FX instrument. Representative images are shown for various groups (PBS, ExoNormoxic and ExoHypoxic). Mice without MMPsense injection served as negative control (bottom panel). In each image, dye in an eppendorf tube served as a positive control. B, Forty eight hours after MMPsense 750 dye injection, mice were killed, various organs isolated and imaged for fluorescence by In Vivo Multispectral FX imaging instrument. Organs: 1. brain; 2. lymph nodes + salivary glands; 3. lung + heart; 4. liver; 5. spleen; 6. kidneys; 7. prostate + seminal vesicles + bladder; 8. bone. Organs from animal without MMPsense dye injection served as negative control (top, left panel), while dye in an eppendorf served as positive control (in top left and bottom right images). C, Mice were injected IP with PBS or ExoGlow labelled exosomes (ExoNormoxic and ExoHypoxic) and various organs were imaged 24 hours later. Organs: 1. Brain; 2. Lung; 3. Heart; 4. Liver; 5.Spleen; 6. Kidney; 7. Prostate. ExoHypoxic, exosomes secreted under hypoxic condition; ExoNormoxic, Exosomes secreted under normoxic condition; IP, intraperitoneal; MMP, matrix metalloproteinases; PBS, phosphate buffered saline; PCa, prostate cancer [Color figure can be viewed at wileyonlinelibrary.com]
Next, we labelled exosomes with ExoGlow fluorescent dye and followed their in vivo distribution in mice. As shown in Figure 1C, 24 hours following single IP injection, both ExoNormoxic and ExoHypoxic localized in the liver tissue. We did not observe any fluorescent signal in intact animals (1-24 hours), which could be due to limited imaging depth of the fluorescent agent and/or due to weak signal (data not shown).
3.2 |. ExoHypoxic promote MMP2 and MMP9 expression in selective organs
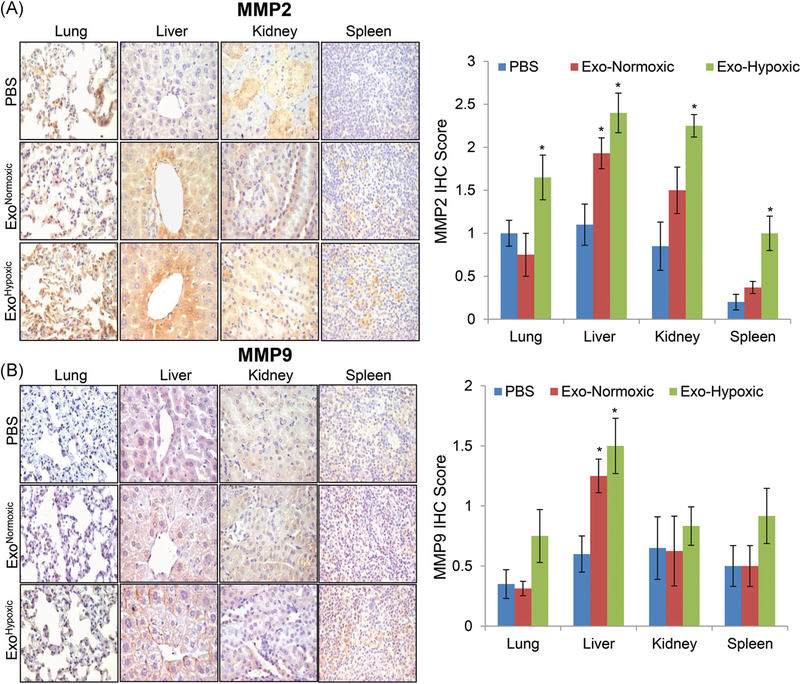
Next, we analyzed various tissues isolated from the mice treated with ExoNormoxic and ExoHypoxic (as described above) for the expression of MMP2 and MMP9. As shown in Figure 2A, there was no significant increase in MMP2 expression in various organs except liver when mice were treated with ExoNormoxic. However, in mice treatment with ExoHypoxic there was a significant increase in the MMP2 expression in lung, liver, kidney and spleen (Figure 2A). Similarly, ExoNormoxic treated mice showed higher MMP9 expression in liver tissue, while ExoHypoxic treated mice showed an increase in MMP9 expression in all organs; however, increase was statistically significant only in liver tissues.
FIGURE 2.
Human PCa ExoHypoxic promotes MMP2 and MMP9 expression in selective organs in male athymic nude mice. A,B, Male athymic nude mice were injected with PBS, ExoNormoxic or ExoHypoxic (10 μg each, IP) on alternate days for 4 weeks. At the end, mice were killed and mentioned organs were collected, processed, and analyzed for MMP2 and MMP9 expression by IHC as described in methods. Immunoreactivity for MMP2 and MMP9 was scored as 0+ (no staining), 1+ (weak staining), 2+ (moderate staining), 3+ (strong staining), 4+ (very strong staining). Data shown in the bar diagram represent mean ± SEM of immunoreactivity scores for ten randomly selected fields from 4 to 5 samples for each group. Representative images are shown at ×400. *P ≤ .05. ExoHypoxic, exosomes secreted under hypoxic condition; ExoNormoxic, Exosomes secreted under normoxic condition; IHC, immunohistochemistry; IP, intraperitoneal; MMP, matrix metalloproteinases; PBS, phosphate buffered saline; PCa, prostate cancer [Color figure can be viewed at wileyonlinelibrary.com]
The observation is in line with our previous study where we have reported that ExoHypoxic secreted by androgen receptor positive human PCa LNCaP cells have strong MMP2 and 9 activity.22 However, we need to further understand the mechanism underlying the observed effect of exosomes on MMPs activity and expression. We also need to identify the specific cell types (epithelial cells, endothelial cells, fibroblasts, or macrophages etc) affected by exosomes treatment and their contribution in increased MMPs expression and activity at PMN sites. For example, Hiratsuka et al27 identified that MMP9 is specifically induced in pre-metastatic lung endothelial cells and macrophages. Besides, exosome could increase the expression and/or activity of MMPs through transfer of their content (microRNA, proteins, and lipids) in the receipt cells. Based upon the key role of MMPs in PMN preparation, PMN should be erased using MMPs inhibitors as a part of early therapeutic intervention in patients with higher metastasis risk. Hiratsuka et al27 reported that batimastat, a MMP inhibitor could inhibit the invasion of cancer cells at pre-metastatic site. However, currently we do not have nontoxic MMPs inhibitors, which could be used for metastasis prevention without significant side effects.
3.3 |. ExoHypoxic promote matrix remodeling in selective organs
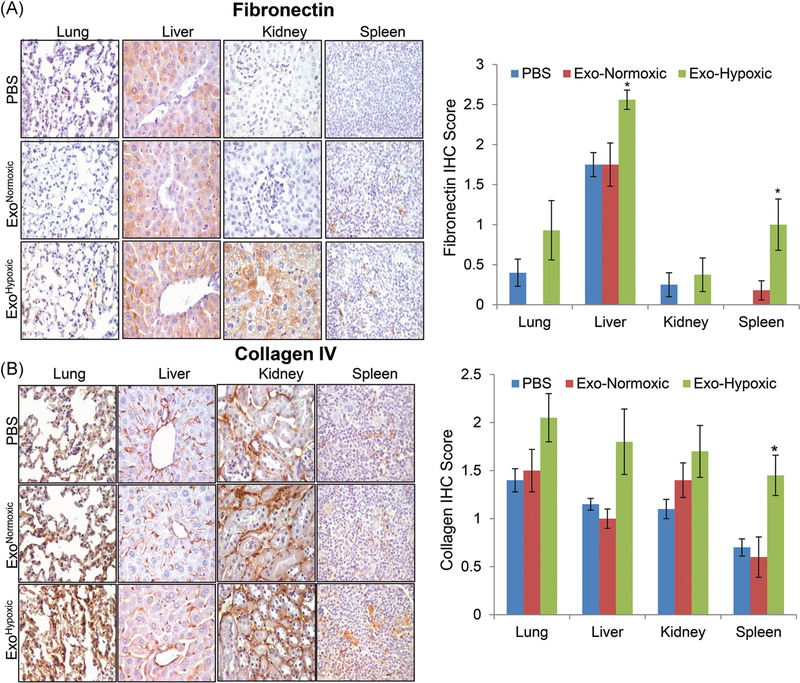
One of the central events in PMN preparation is the matrix remodeling including the accumulation of extracellular proteins and their degradation products.20 Erler et al20 showed that breast cancer cells secrete LOX under hypoxic conditions which accumulates at pre-metastatic sites crosslinking collagen IV, resulting in recruitment of CD11b+ myeloid cells. CD11b+ myeloid cells produce MMP2 that cleaves collagen IV into peptides, which serve as chemoattractant for circulating tumor cells.20 Similarly, tumor secreted factor or exosomes have also been reported to cause fibronectin accumulation at PMN, BMDCs recruitment and subsequent tumor cells settlement.5,8 Therefore, we next examined the tissues for the expression of ECM proteins namely fibronectin and collagen IV. As shown in Figure 3A, only mice treated with ExoHypoxic showed a significant increase in fibronectin expression in liver and spleen tissues. Similarly, only mice treated with ExoHypoxic showed a significant increase in collagen IV expression in spleen tissues.
FIGURE 3.
Human PCa ExoHypoxic promote matrix remodeling in selective organs in male athymic nude mice. A,B, Male athymic nude mice were injected with PBS, ExoNormoxic or ExoHypoxic (10 μg each, IP) on alternate days for 4 weeks. At the end, mice were killed and mentioned organs were collected, processed, and analyzed for fibronectin and collagen expression by IHC. Immunoreactivity for fibronectin and collagen was scored as 0+ (no staining), 1+ (weak staining), 2+ (moderate staining), 3+ (strong staining), 4+ (very strong staining). Data shown in the bar diagram represent mean ± SEM of immunoreactivity scores for ten randomly selected fields from 4 to 5 samples for each group. Representative images are shown at ×400. *P ≤ .05. ExoHypoxic, exosomes secreted under hypoxic condition; ExoNormoxic, Exosomes secreted under normoxic condition; IHC, immunohistochemistry; IP, intraperitoneal; MMP, matrix metalloproteinases; PBS, phosphate buffered saline; PCa, prostate cancer [Color figure can be viewed at wileyonlinelibrary.com]
3.4 |. Effect of exosome treatment on CD11b+ cells
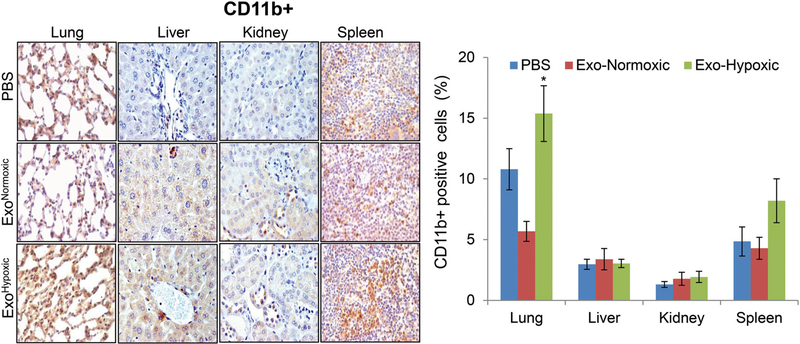
Earlier studies have reported that BMDCs play an important role in PMN preparation through ECM remodeling, and release of growth factors and chemoattractant; thus, creating an immunosuppressive and proinflammatory environment that is permissive for the subsequent invasion and growth of tumor cells.3,5–8,29,37 Among several BMDCs, myeloid lineage cells are considered most important in PMN formation as well as in subsequent metastasis.3,20,38,39 Clinical analyses have shown the accumulation of myeloid-derived suppressor CD11b+ cells at metastatic target organs but not in normal tissues.20 Accordingly, we next examined the effect of exosomes treatment on CD11b+ cells accumulation in various organs. As shown in Figure 4, we did not observe any significant change in CD11b+ cells in various organs following ExoNormoxic and ExoHypoxic treatment except in lung tissues where CD11b+ cells increased significantly following ExoHypoxic treatment. Future work will determine whether these CD11b+ cells are granulocytic (CD11b+/Ly6Cmed/Ly6G+) or monocytic (CD11b+/Ly6Chigh/Ly6G−).6 Furthermore, the effects of ExoNormoxic and ExoHypoxic treatment on the presence of other immune cells (such as neutrophils, macrophages, and NK cells etc), which are also considered important in PMN preparation,6,40,41 will be investigated.
FIGURE 4.
Effect of PCa ExoHypoxic treatment on CD11b+ cells in male athymic nude mice. Male athymic nude mice were injected with PBS, ExoNormoxic or ExoHypoxic (10 μg each, IP) on alternate days for 4 weeks. At the end, mice were killed and mentioned organs were collected, processed, and analyzed for CD11b+ cells by IHC and presented as % positive cells. Data shown in the bar diagram represent mean ± SEM of immunoreactivity scores for ten randomly selected fields from 4 to 5 samples for each group. Representative images are shown at ×400. *P ≤ .05. ExoHypoxic, exosomes secreted under hypoxic condition; ExoNormoxic, Exosomes secreted under normoxic condition; IHC, immunohistochemistry; IP, intraperitoneal; MMP, matrix metalloproteinases; PBS, phosphate buffered saline; PCa, prostate cancer [Color figure can be viewed at wileyonlinelibrary.com]
3.5 |. Characterization of proteins loaded in ExoNormoxic and ExoHypoxic by mass spectrometry
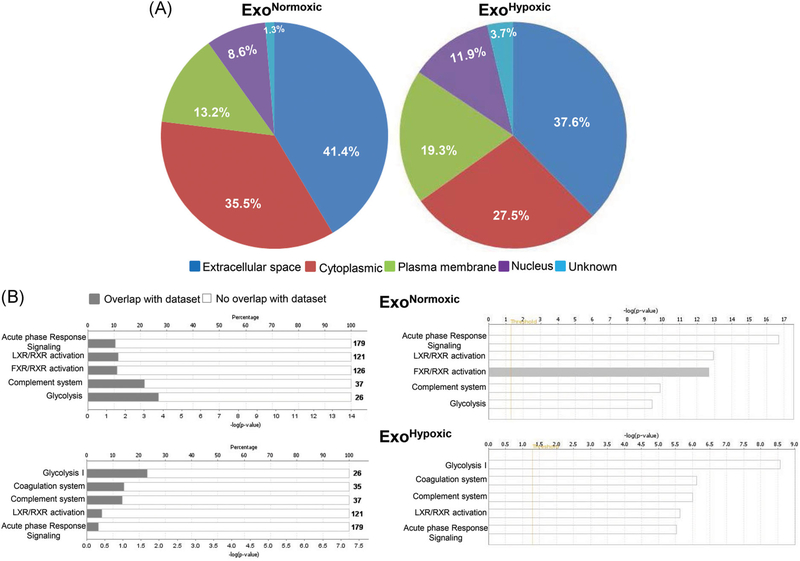
Next, to gain insight into biological effects induced by ExoNormoxic and ExoHypoxic, proteomics profiling was performed by LC/MS/MS followed by data analyses using IPA software. Details (name, cellular location, and type etc) of proteins loaded in ExoNormoxic and ExoHypoxic are provided in Tables S1 and S2, respectively. The identified proteins were categorized for their cellular localization and as shown in Figure 5A, ExoNormoxic and ExoHypoxic were loaded with proteins primarily from the extracellular space and cytoplasm, with relatively low percentages of plasma membrane and nucleus-derived proteins. Compared to ExoNormoxic, ExoHypoxic showed a greater percentage of plasma membrane and nucleus-derived proteins and a relatively lesser percentage derived from the extracellular space and cytoplasm. However, it is important to mention here that several of these proteins have multiple localizations, particularly in cancer cells.
FIGURE 5.
Characterization of proteins loaded in ExoNormoxic and ExoHypoxic by mass spectrometry. Proteomic profiling of ExoNormoxic and ExoHypoxic was performed by LC/MS/MS and data analyzed by IPA software. A, Cellular localization of proteins loaded in ExoNormoxic and ExoHypoxic are presented in pi diagrams. B, Top five canonical pathways in ExoNormoxic and ExoHypoxic are shown. In each case, percentage overlap with the dataset (left panel) as well as –log (P value; right panel) are presented. ExoHypoxic, exosomes secreted under hypoxic condition; ExoNormoxic, Exosomes secreted under normoxic condition; IPA, ingenuity pathway analysis; LC/MS/MS, liquid chromatography/mass spectrometry [Color figure can be viewed at wileyonlinelibrary.com]
Using IPA software, identified proteins were also grouped into networks of associated functions and canonical pathways. In Figure 5B, we have presented the top five canonical pathways associated with proteins loaded in ExoNormoxic and ExoHypoxic, and in each case, percentage overlap with the dataset (left panel) as well as –log (P value; right panel) are presented. The top five canonical pathways associated with ExoNormoxic were acute phase response signaling, liver X receptor/retinoid X receptor (LXR/RXR), FXR/RXR activation, complement system and glycolysis (Figure 5B, top panels). The top five canonical pathways associated with ExoHypoxic were glycolysis, coagulation system, complement system LXR/RXR, and acute phase response signaling (Figure 5B, bottom panels). Further studies are required to determine the relative abundance of key proteins loaded in ExoNormoxic and ExoHypoxic and establish their functional role in PMN preparation. Furthermore, exosomal nucleic acids (noncoding RNAs, messenger RNA, etc) and lipids also need to be characterized for their role in the PMN microenvironment.
In conclusion, results from the present study suggest the role of cancer cells’ secreted exosomes in the remodeling of microenvironment at distant organ sites. ExoHypoxic treatment increased the expression of MMPs, fibronectin, collagen IV and number of CD11b+ cells at selective PMN sites. It is evident that early detection of these PMN sites before metastasis could provide a therapeutic advantage. Blocking the early action of exosomes at PMN, such as targeting the MMPs activity, ECM remodeling etc, could be useful in preventing metastasis. Furthermore, as exosomes are accessible in biofluids and potentially have tumor specific cargo; exosomes could be useful in developing blood-based biomarkers in identifying patients at risk for aggressive metastatic disease.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the support from Dr Jingyun Lee who has performed the mass spectrometry analysis in PMSR and Dr Cristina Furdui, the Director of PMSR. This study was supported by DOD award # W81XWH-15-1-0188 (to GD), NCI RO1 grant CA102514 (to RA) and WFBCCC Proteomics and Metabolomics Shared Resource supported by NCI (P30CA012197, PI: Dr Boris Pasche).
Funding information
U.S. Department of Defense, Grant/Award Number: W81XWH-15-1-0188; National Cancer Institute, Grant/Award Numbers: CA102514, P30CA012197
Abbreviations:
- ANOVA
Analysis of variance
- ATCC
American type culture collection
- BMDCs
Bone marrow derived cells
- CAF
Cancer-associated fibroblast
- ECM
Extracellular matrix
- EMT
Epithelial-to-mesenchymal transition
- ExoHypoxic
Exosomes secreted under hypoxic condition
- ExoNormoxic
Exosomes secreted under normoxic condition
- FXR
Farnesoid X receptor
- IHC
Immunohistochemistry
- IPA
Ingenuity pathway analysis
- LC/MS/MS
Liquid chromatography/mass spectrometry
- LOX
Lysyl oxidase
- LXR
Liver X receptor
- MMPs
Matrix metalloproteinases
- NOD-SCID
Nonobese diabetic-Severe combined immunodeficiency
- PCa
Prostate cancer
- PlGF
Placental growth factor
- PMN
Pre-metastatic niches
- RXR
Retinoid X receptor
- TGFβ
Transforming growth factor beta
- TNF1α
Tumor necrosis factor 1 alpha
- VEGF
Vascular endothelial growth factors
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
REFERENCES
- 1.Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biologyof bone metastasis. Mol Cancer Ther. 2007;6:2609–2617. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 3.Sceneay J, Smyth MJ, Moller A. The pre-metastatic niche: finding common ground. Cancer Metastasis Rev. 2013;32:449–464. [DOI] [PubMed] [Google Scholar]
- 4.Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011; 71:3792–3801. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the premetastatic niche. Nature. 2005;438:820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sceneay J, Chow MT, Chen A, et al. Primary tumor hypoxia recruitsCD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72:3906–3911. [DOI] [PubMed] [Google Scholar]
- 7.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peinado H, Alečković M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. 2011;21:139–146. [DOI] [PubMed] [Google Scholar]
- 10.Gallagher FA, Kettunen MI, Day SE, et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature. 2008;453:940–943. [DOI] [PubMed] [Google Scholar]
- 11.Calcinotto A, Filipazzi P, Grioni M, et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 2012;72: 2746–2756. [DOI] [PubMed] [Google Scholar]
- 12.Azzarito T, Venturi G, Cesolini A, Fais S. Lansoprazole induces sensitivity to suboptimal doses of paclitaxel in human melanoma. Cancer Lett. 2015;356:697–703. [DOI] [PubMed] [Google Scholar]
- 13.Azzarito T, Lugini L, Spugnini EP, et al. Effect of modified alkaline supplementation on syngenic melanoma growth in CB57/BL mice. PLoS One. 2016;11:e0159763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logozzi M, Angelini DF, Iessi E, et al. Increased PSA expression on prostate cancer exosomes in in vitro condition and in cancer patients. Cancer Lett. 2017;403:318–329. [DOI] [PubMed] [Google Scholar]
- 15.Ranasinghe WKB, Xiao L, Kovac S, et al. The role of hypoxia-inducible factor 1alpha in determining the properties of castrate-resistant prostate cancers. PLoS One. 2013;8:e54251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butterworth KT, McCarthy HO, Devlin A, et al. Hypoxia selects for androgen independent LNCaP cells with a more malignant geno-and phenotype. Int J Cancer. 2008;123:760–768. [DOI] [PubMed] [Google Scholar]
- 17.Dai Y, Bae K, Siemann DW. Impact of hypoxia on the metastatic potential of human prostate cancer cells. Int J Radiat Oncol Biol Phys. 2011;81:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tatum JL. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006;82: 699–757. [DOI] [PubMed] [Google Scholar]
- 19.Deep GPG. Hypoxia-induced signaling promotes prostate cancer progression: Exosomes role as messenger of hypoxic response in tumor microenvironment. Crit Rev Oncog. 2015;20:419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erler JT, Bennewith KL, Cox TR, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlaepfer IR, Nambiar DK, Ramteke A, et al. Hypoxia induces triglycerides accumulation in prostate cancer cells and extracellular vesicles supporting growth and invasiveness following reoxygenation. Oncotarget. 2015;6:22836–22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramteke A, Ting H, Agarwal C, et al. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol Carcinog. 2015;54:554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 24.Msaouel P, Pissimissis N, Halapas A, Koutsilieris M. Mechanisms of bone metastasis in prostate cancer: clinical implications. Best Pract Res Clin Endocrinol Metab. 2008;22:341–355. [DOI] [PubMed] [Google Scholar]
- 25.Buijs JT, van der Pluijm G. Osteotropic cancers: from primary tumorto bone. Cancer Lett. 2009;273:177–193. [DOI] [PubMed] [Google Scholar]
- 26.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–1375. [DOI] [PubMed] [Google Scholar]
- 27.Hiratsuka S, Nakamura K, Iwai S, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. [DOI] [PubMed] [Google Scholar]
- 28.Joo YN, Jin H, Eun SY, Park SW, Chang KC, Kim HJ. P2Y2R activation by nucleotides released from the highly metastatic breast cancer cell contributes to pre-metastatic niche formation by mediating lysyl oxidase secretion, collagen crosslinking, and monocyte recruitment. Oncotarget. 2014;5:9322–9334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan HH, Pickup M, Pang Y, et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70:6139–6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rankin EB, Nam JM, Giaccia AJ. Hypoxia: signaling the metastatic cascade. Trends Cancer. 2016;2:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox TR, Rumney RMH, Schoof EM, et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;522:106–110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Kramer A, Green J, Pollard J Jr., Tugendreich S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics. 2014;30: 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rucci N, Sanita P, Angelucci A. Roles of metalloproteases in metastatic niche. Curr Mol Med. 2011;11:609–622. [DOI] [PubMed] [Google Scholar]
- 34.Acuff HB, Carter KJ, Fingleton B, Gorden DL, Matrisian LM. Matrixmetalloproteinase-9 from bone marrow-derived cells contributes to survival but not growth of tumor cells in the lung microenvironment. Cancer Res. 2006;66:259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cifuentes FF, Valenzuela RH, Contreras HR, Castellon EA. Development of an orthotopic model of human metastatic prostate cancer in the NOD-SCIDgamma mouse (Mus musculus) anterior prostate. Oncol Lett. 2015;10:2142–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bastide C, Bagnis C, Mannoni P, Hassoun J, Bladou F. A Nod Scidmouse model to study human prostate cancer. Prostate Cancer Prostatic Dis. 2002;5:311–315. [DOI] [PubMed] [Google Scholar]
- 37.Zoccoli A, Iuliani M, Pantano F, et al. Premetastatic niche: ready for new therapeutic interventions? Expert Opin Ther Targets. 2012; 16(suppl 2):S119–S129. [DOI] [PubMed] [Google Scholar]
- 38.Keskinov AA, Shurin MR. Myeloid regulatory cells in tumor spreading and metastasis. Immunobiology. 2014;220:236–242. [DOI] [PubMed] [Google Scholar]
- 39.Deng J, Liu Y, Lee H, et al. S1PR1-STAT3 signaling is crucial for myeloid cell colonization at future metastatic sites. Cancer Cell. 2012;21:642–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donati K, Sépult C, Rocks N, et al. Neutrophil-derived interleukin 16 in premetastatic lungs promotes breast tumor cell seeding. Cancer Growth Metastasis. 2017;10:1179064417738513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gil-Bernabé AM, Ferjančič Š, Tlalka M, et al. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood. 2012;119:3164–3175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.