Abstract
Growth factors (GFs) play a crucial role in directing stem cell behavior and transmitting information between different cell populations for tissue regeneration. However, their utility as therapeutics is limited by their short half-life within the physiological microenvironment and significant side effects caused by off-target effects or improper dosage. “Smart” materials that can not only sustain therapeutic delivery over a treatment period but also facilitate on-demand release upon activation are attracting significant interest in the field of GF delivery for tissue engineering. Three properties are essential in engineering these “smart” materials: (1) the cargo vehicle protects the encapsulated therapeutic; (2) release is targeted to the site of injury; (3) cargo release can be modulated by disease-specific stimuli. The aim of this review is to summarize the current research on stimuli-responsive materials utilized as intelligent vehicles for controlled GF delivery; we focus on five main subfields of tissue engineering: skin, bone and cartilage, muscle, blood vessel, and nerve. Challenges in achieving such “smart” materials and perspectives on future applications of stimuli-responsive GF delivery for tissue regeneration are also discussed.
Keywords: stimuli-responsive delivery, growth factor, tissue engineering, regenerative medicine
Graphical Abstract

“Smart” materials that can achieve on demand release of therapeutics in response to biological stimuli on the disease sites attract growing interest in the field of tissue engineering. The aim of this review focuses on summarizing recent advances in stimuli-responsive growth factor release strategies to injured tissue regeneration with improved efficacy and mitigated side effect.
1. Introduction
Tissue engineering is a rapidly developing field aiming to regenerate or replace defective or diseased tissues through the application of biomaterials, cells, or bioactive molecules.[1] For tissue regeneration, cellular function and behavior is modulated by interactions between a cell and its environment; other cells play an important role as communication occurs through either direct cell-cell contact or the secretion of small molecules, such as hormones and mediators. Growth factors (GFs) play an important role in the transmission of information between different cell populations. Within organ systems, these proteins control and regulate bioactivities of different cells, including regulation of cell proliferation, migration, and differentiation.[2, 3] GFs initiate their functions by binding to specific receptors which triggers a signaling cascade by promoting the secretion of mediators and further inducing the differentiation of the target cells.[4] Although different cell types produce the same GFs, identical proteins can elicit different cellular responses based on the concentration and receiving cell type.
Despite their important role in normal physiological functions, a number of limitations exist in applying GFs to tissue engineering. GFs suffer from short biological half-lives because they can be easily degraded or deactivated within physiological microenvironment.[3] Moreover, GF function is concentration dependent and inappropriate dosage or release kinetics of GF could result in distinct differentiation of stem cells and undesired side effects.[3] For example, bone morphogenic proteins (BMPs) are able to promote the osteogenic and chondrogenic differentiation of stem cells at different local concentrations.[5, 6] However, overdose of BMP-2 may cause uncontrolled bone formation, soft tissue inflammation, and even carcinogenesis.[7] To overcome such issues, controlled and sustained GF release systems have been extensively studied.[3, 4, 8] One of the primary approaches is to encapsulate GFs within polymeric matrix as to protect them from degradation. Additionally, GF release kinetics can be controlled by adjusting the degradation rate of the polymeric matrix or facilitating physical and chemical interactions between GFs and the matrix.
Apart from sustained release, “smart” materials with the ability to sense signals within the physiological environment and react correspondingly are gaining attention for applications in drug delivery, tissue engineering, and biomedical devices.[9–15] As reported in previous studies, tissue injuries lead to site-dependent environmental changes and abnormal cellular activity. Multiple factors, including enzymes [16–36], reduction and oxidation (redox) reactions [37–44], hypoxia [32, 45–49], pH [47, 50–54], temperature [55–57] and the other characteristics [42, 58–60], become dysregulated at the onset of injury or during the progression of the healing (Figure 1). These hallmarks can be monitored to serve as a reference to evaluate the extent of injury, stage of wound healing, and necessity for treatment. For example, in the healing process for chronic wounds, the microenvironment is turned acidic early on to prevent bacterial infection and the pH returns to normal levels over time as therapeutic interventions are used and the wound heals.[61, 62] However, the pH of burn wound fluid is slightly basic and decreases with therapy and healing.[51] While these changes in pH already enhance the wound healing potential, they can also be exploited by “smart” materials as potential stimuli for on-demand drug release.
Figure 1.

A) Summary of the specific hallmarks and cellular activities associated with typical injuries and disorders of the human body. These hallmarks have the potential to be utilized as specific inducers in responsive or targeted drug delivery; B) Mechanisms for stimuli-responsive “smart” materials in GFs delivery.
Stimuli-responsive GF delivery systems have been designed to tailor therapeutic release profiles based on the need of the injury. Microenvironmental triggers, such as enzymes, pH, redox gradients, and temperature can be used to passively tune drug release while external inducers such as ultrasound, magnetic fields, and light can be used to promote on-demand release of bioactive molecules. In response to these inducers, “smart” materials can adjust therapeutic release by changing their physical or chemical properties like degradation, swelling/shrinking, sol-gel transition, hydrophobicity shift, and competitive binding (Figure 1B). This responsiveness facilitates on-demand release when triggered preventing improper and off-targeting release leading to serious side effects.
In this review, we summarize the stimuli-responsive GF release strategies that can be tuned to respond to the dynamic microenvironment of injured tissues in five main fields of tissue engineering: (i) skin; (ii) bone and cartilage; (iii) skeletal and cardiac muscle; (iv) blood vessel and (v) nerve. The strategies covered in this review are summarized in Table 1. We will also discuss our perspectives on the future design requirements for stimuli-responsive GF delivery in tissue regeneration.
Table 1.
the summarize of the stimuli-responsive growth factor delivery strategies covered in this review.
| Tissues | Stimuli | Materials | Growth Factor | Application | Reference |
|---|---|---|---|---|---|
| Skin | Enzyme | PCL fiber containing MMP cleavage site | EGF | HaCaT cells | [74] |
| pH | alginate/CaCO3 composite microparticles | bFGF | Full thickness wound model and Mouse fibroblasts (NIH-3T3) | [78] | |
| poly (N-isopropylacrylamide-co-acrylic acid) hydrogel | VEGF and EGF | Full thickness wound model and HaCaT cells | [61] | ||
| Temperature | PNIPAm hydrogels encapsulate chitosan inverse opal particles | FGF | Anti-bacteria and rats infectious wound | [81] | |
| Poly (polyethylene glycol citrate-co-N-isopropylacrylamide) (PPCN) | SDF-1 | Human dermal fibroblasts, human epithelial keratinocytes and diabetic wound model | [82] | ||
| Glycidyl methacrylated chitooligosaccharide, di-acrylated Pluronic | rhEGF | Dorsal burn wounds models | [83] | ||
| PNIPAm, alginate, silver nanoparticles (AgNPs) | / | Anti-bacteria, full-thickness excision skin wound healing model | [84] | ||
| Redox | polymer poly-(1,4-phenyleneacetone dimethylene thioketal) with thioketal bonds | SDF-1 | BMSCs and full-thickness skin defects | [89] | |
| Monoolein cubosome | EGF | Artificial skin and CCD-986sk cell | [92] | ||
| Bone and cartilage | Enzyme | branched poly(ethylene glycol) (4armPEG) with MMPs cleavage peptide crosslinker | BMP-2 | Rat calvarial defects | [97] |
| Nanocapsule with MPC monomer and MMP cleavable peptide crosslinker | BMP-2 | MSCs and tibial injury | [98] | ||
| CS-PEG hybrid hydrogel | BMP-2 | BMSCs | [99] | ||
| MaHA hydrogel with MMP-sensitive peptides | BMP-2 and SDF-1 | Calvarial defects model | [100] | ||
| PEGDA hydrogels with HNE-sensitive linkers | BMP-2 | / | [101] | ||
| pH | MSF with N,O-Carboxymethyl chitosan (NOCC) | BMP-2 | BMSCs | [103] | |
| mesoporous silica nanoparticles with pH sensitive chitosan | BMP-2 | BMSCs | [104] | ||
| MSF-TAA-Fe3O4 material | BMP-2 | BMSCs | [105] | ||
| Temperature | RGD-grafted NiPAM/NASI | BMP-2 | C2C12 cells | [106] | |
| PLGA-PEG-PLGA copolymers | 125I-BMP-2 | / | [107] | ||
| alginate | BMP-2 and PRGF | calvaria critical bone defect in OP female rats | [108] | ||
| Redox | PEO and PEG-PCL nanogel with -S-S- bond | BMP-2 | BMSCs and mandibular bone defect | [111] | |
| 4-armed PEG-SH | BMP-2 | C2C12 cells and ectopic bone formation | [112] | ||
| Ultrasound | PEGylated liposome | BMP-2 | NIH/3T3, RAW264.7, C2C12 cells, Ectopic Bone Formation | [113] | |
| Magnetic | Magnetic liposomes with Fe3O4 | BMP-2 | C2C12 and femoral critical bone defect | [114] | |
| Magnetic liposomes with Fe3O4 | TGF-β1 | articular cartilage defect model | [115] | ||
| Multi-layered capsule | TGF- β3 | Chondrogenic differentiation of Adipose Stem Cells in vitro | [116] | ||
| Skeletal and cardiac muscle | Enzyme | GSH‐modified collagen hydrogel (collagen‐GSH) | bFGF | Balb/c 3T3 cell line, HUVECs, rat myocardial infarction model | [130] |
| starPEG-heparin hydrogels | SDF-1α | myeloid EPC | [131] | ||
| PEG | VEGF, HGF | Rat acute myocardial infarction model | [125] | ||
| PH and Temperature | Collagen | VEGF, FGF and PLGF | HUVECs | [123] | |
| P(NIPAm-co-PAA-co-BA) copolymer and PAA | bFGF | Rat infarcted myocardium model | [134] | ||
| Calcium ion | Alginate | IGF-1, VEGF | C3H/6J mice, clonally derived primary myoblasts | [138] | |
| Alginate | IGF-1, VEGF | Hindlimb ischemia model | [129] | ||
| Blood vessel | Enzyme | Nanocapsule with plasmin sensitive linker | PDGF, VEGF | Stroke, diabetic wound | [147] |
| PEG hydrogel with degradable peptide sequenced linker | VEGF | Vascularization | [148] | ||
| pH and Temperature | P(NIPAm-co-PAA-co-BA) microspheres | FGF | Ischemic limb | [52] | |
| Collagen coated PNIPAm nanoparticles | VEGF | hBMSCs | [150] | ||
| polyurea conjugated PNIPAm | VEGF | Vascularization | [151] | ||
| NIPAm and PAA synthesized p(NIPAm-co-PAA) hydrogels | bFGF | Vascularization | [149] | ||
| VEGF receptor | Heparin modified PEG | VEGF | porcine aortic endothelial cells (PAE KDR) | [152] | |
| Mechanical | Alginate | VEGF | Vascularization | [153] | |
| Ultrasound | Perfluorocarbon containing fibrin gel | / | / | [154] | |
| Nerve | Enzyme | Fibrin gel | neurotrophin-3, PDGF | / | [158] |
| Heparin associated with fibrin gel | bFGF | Dorsal root ganglia neurite extension | [159] | ||
| diblock copolypeptide hydrogel depot | NGF | forebrain cholinergic neurons | [162] | ||
| beta hairpin peptide-based hydrogel (MAX8) | NGF, BDNF | PC12 cell neurite-like extension | [163] | ||
| pH | 2-methacryloyloxyethyl phosphorylcholine Nanocapsule with pH sensitive crosslinker | NGF | PC12 cell, rhesus macaque and mouse model | [165] | |
| Temperature | Agarose hydrogel | BDNF | Spinal cord defect | [166] | |
| Methylcellulose and hyaluronan | / | Spinal cord injury | [167] | ||
| laminin-1 (LN) conjugated methylcellulose | / | Cortical neurons | [168] |
2. Stimuli-responsive growth factor delivery systems in tissue regeneration
2.1. Skin
Injuries to the skin are extremely common and suffered by a large population worldwide. Among these injuries are chronic wounds that present unique challenges for healing because of the multi-stage healing process and dynamic wound microenvironment.[63] Chronic wounds arise from impaired regeneration mechanisms in the wound healing cascade (hemostasis, inflammation, migration, proliferation and remodeling).[64] These wounds are often incurable, remaining open for more than 12 weeks, leading to increased rates in disability, amputation and mortality.[65] Various GFs play important roles in regulating the wound healing process.[66] Therefore, exogenous GF therapy is one approach in regulating the wound healing process. Platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), transforming growth factor-β (TGF-β), and insulin-like growth factor (IGF) have all been applied in the topical management of chronic wounds.[67, 68] However, exogenously administered GFs suffer from rapid degradation within the wound site, leading to only transient therapeutic concentrations of GFs thus reducing their therapeutic efficacy.[2] To address these challenges, traditional GF delivery formulations require high doses and/or long periods of repeated administration which may lead to serious side effects [69] as well as high costs [70]. In order to improve upon delivery, wound-responsive GF delivery systems have the potential to both protect encapsulated GFs and tune release by delivering the right dosage at the right time.[71] Chronic wounds exhibit a hostile microenvironment with an excess of free radicals, digestive enzymes, and abnormal wound pH – all of which could function as stimuli for “smart” release (Figure 1). Here, we summarize some of the research efforts on controlled release of GFs using stimuli-responsive systems for skin regeneration.
2.1.1. Enzyme
Within chronic wound environments, various proteolytic enzymes, such as matrix metalloproteinases (MMPs), collagenases, elastases, and serine proteases, are up-regulated.[72, 73] Their increased prevalence can be exploited to deliver GFs through enzyme-triggered cleavage sites. For example, Kim et al. covalently conjugated genetically engineered EGF to coextruded polymer fiber patches using an MMP cleavage sequence. The genetically engineered EGF was rapidly released upon exposure to MMP-9, an MMP that is up-regulated in the early stage of wound healing environment. The EGF-loaded, MMP-responsive fiber significantly promoted proliferation and migration of human keratinocytes in the presence of the biological trigger MMP-9.[74]
2.1.2. pH
The wound healing process is commonly accompanied by pH changes due to bacterial infection, inflammation and oxygenation.[75] These chemical changes in the environment have been exploited by pH-responsive materials to tune delivery. Generally, pH responsive materials contain ionizable groups or acid-cleavable bonds that are capable of releasing bioactive cargos in response to environmental pH changes.[76] Immediately after injury, the local pH of the wound is temporarily increased to 7 by exudation of blood and other body fluids. During the healing process, the pH of the wound decreases to 4–5.[71] In addition, bacterial infection also leads to the production of ammonia which will raise the pH of the wounds.[77] Because pH is an indicator of wound state, many studies have focused on developing GF delivery systems that can respond to specific pH levels. Banerjee et al. fabricated a pH-sensitive poly (N-isopropylacrylamide-co-acrylic acid) (poly(NIPAm-co-AAc)) hydrogel to deliver VEGF and EGF. The pH-dependent changes of the material’s physical properties, such as network structure, swelling, and solute mobility, contributed to the sustained release of GFs within the pH range of a wound (6.7–7.9). In vivo study further revealed that this GF-loaded, pH-responsive hydrogel facilitated a better healing response compared to delivery systems without the stimuli-responsive mechanism.[61] Shi et al. developed uniform alginate/CaCO3 composite microparticles (~430 μm) with pH-sensitivity using microfluidic technology. Compared with the pure alginate particles, the addition of CaCO3 effectively improved the antacid ability of the microparticles and reduced the initial burst release. Based on this technique, bFGF-loaded composite microparticles achieved a slow and sustained release of bFGF and significantly promoted cell proliferation and migration rate in vitro.[78]
2.1.3. Temperature
Injured skin is often accompanied by increased tissue metabolism and immune response, such as inflammatory cytokine induced vasodilation, which leads to changes in temperature in the local area.[28] However, the mild local temperature changes in the wound relative to normal skin is not sufficient to create a sensitive, wound environment-specific temperature responsive drug delivery system. Current temperature responsive drug delivery systems are primarily responsive to physiological temperatures (around 37˚C), where materials that exhibit lower critical solution temperature (LCST) within 30 to 40˚C are the most frequently used.[71, 79] Temperature change may lead to both physical and chemical changes in materials, which can be leveraged for drug delivery purposes.[80] Poly(N-isopropylacrylamide) (PNIPAm)-based polymers with LCST of ~32˚C are often used for temperature-response drug delivery. When the temperature reaches above the critical temperature, the material changes from hydrophilic to hydrophobic, pushing hydrophilic drugs out of the carrier. Chen et al. encapsulated FGF-loaded PNIPAm hydrogels into the nanopores of chitosan inverse opal particles (Figure 2). During the release process, the characteristic reflection peaks and structural colors of the composite inverse opal particles can be blue shifted, facilitating real-time release monitoring based on reflection images and spectra. To investigate the release profile, fluorescein isothiocyanate labelled bovine serum albumin (FITC-BSA) was loaded in these particles and exposed to several temperature cycles (45 ˚C for 5 min and room temperature for another 15 min). They found that 60% of BSA could be released within one cycle with 5% of PNIPAM, whereas BSA release did not reach 40% after 5 cycles with 25% PNIPAM. The higher concentration of PNIPAM might have stronger intermolecular hydrogen bonds that could cause lower response to temperature, it is therefore promising to achieve personalized drug release profiles for different clinical needs by varying the hydrogel concentration. In a rat infectious wound model, the composite microcarriers demonstrated the ability to increase angiogenesis, collagen deposition, and granulation tissue formation to promote wound healing.[81] Furthermore, the critical temperature of PNIPAm can be increased to over 37˚C by grafting other monomers onto the chain. Zhu et al. synthesized Poly (polyethylene glycol citrate-co-N-isopropylacrylamide) (PPCN) via sequential polycondensation and free radical polymerization reactions for sustained release of SDF-1. In a diabetic mouse splinted full-thickness excisional wound model, the PPCN+SDF-1 group showed the shortest wound healing time, accelerated granulation tissue formation and epithelial maturation, and the highest perfusion vascular density.[82] Other temperature-responsive GF delivery systems are based on Pluronic acid. Choi et al. mixed glycidyl methacrylated-chitooligosaccharide, diacrylated Pluronic, and EGF. The mixture is photo-cross-linkable to form a hydrogel. When the temperature rises above the LCST, the poly(oxypropylene) (PPO) segments of Pluronic become hydrophobic and intermolecular association of Pluronic increases. In dorsal burn wound models, the hydrogel increased the concentration of EGF in wound beds and maintained keratinocytic differentiation.[83] Inspired by embryonic wound contraction, Blacklow et al. designed another temperature-triggered contractible material, which may become a new paradigm for wound management.[84] The tough, adhesive hydrogel containing a thermo-responsive component, PNIPAm, adhered to the skin to actively contract the wound as the hydrogel contracted at approximately 32˚C. This temperature-responsive technology has the potential to go beyond physical contraction and deliver drugs, including GFs, for skin regeneration.
Figure 2.

Temperature responsive chitosan inverse opal particles designed as FGF delivery vector in wound healing.[81] A) Schematic illustration of the fabrication and responsive drug release of the temperature responsive chitosan inverse opal particles. B, C) SEM images of the silica colloidal crystal beads (SCCB) templates. D) Microstructure of chitosan hybrid SCCB. E, F) SEM images of inner microstructures of inverse opal particles with interconnected pores. G) Surface of temperature-responsive hydrogel filled chitosan inverse opal particles. H) Schematic and reflection images and spectra of biomass inverse opal particles at 37 °C. The reflection peak of this systems was blue shifted during the release, which can be detected to monitor the drug delivery process. I) H&E stained histologic images and evaluation on the tissue thickness of each group. Copyright 2018, American Chemical Society.
2.1.4. Redox
Reactive oxygen species (ROS) are abundant in the wound environment and play a key role in the wound healing process.[85] During inflammation, phagocytes, mainly neutrophils, consume most of the oxygen [86] and produce bactericidal ROS [87]. However, bacterial tolerance to ROS leads to excessive concentrations that detriment normal wound healing.[88] One of the most common methods used to harness excess ROS for wound healing is the implementation of oxidation-responsive sulfur-based materials. Tang et al. synthesized stromal cell-derived factor-1 (SDF-1) capsuled nanoparticles (NPs) based on an ROS-responsive polymer poly-(1,4-phenyleneacetone dimethylene thioketal). ROS-reactive thioketal bonds in the polymer can be cleaved in the presence of ROS to release encapsulated SDF-1, promoting the chemotaxis of bone marrow mesenchymal stem cells (BMSCs) toward the wound and inducing vascularization in the wound for healing.[89] Other materials, such as ferrocene-containing materials, boronic ester groups, phenylboronic acid (PBA) derivatives, are also widely studied as emerging ROS responsive materials.[90, 91] On the other hand, the secretion of endogenous antioxidants, such as low-molecular weight antioxidant glutathione (GSH), plays an important role in the regulation of the redox balance in site of the injury. In this context, reduction-responsive materials can be designed to deliver drugs within a wound site. For example, Kim et al. fabricated a reduction-responsive monoolein cubosome with cysteamine-crosslinked alginate inside its inner channels to deliver EGF. The cysteine’s disulfide bond with an amine terminal group interacts with the carboxyl group of alginates through electrostatic interaction to form networks hindering the release of EGF. In an environment with excessive GSH, the disulfide bond of the cysteine is broken, leading to the release of EGF.[92]
2.2. Bone and cartilage
Ailments of the bone and cartilage, such as defects, osteomyelitis, osteoporosis, arthritis, osteoarthritis and bone cancer, are prevalent conditions that have severe consequences on quality of life. As life expectancy continues to rise, so do incidences of age-related skeletal diseases.[36, 93] The regeneration and remodeling of bone and cartilage are achieved by complex interactions between cells, GFs and extracellular matrix (ECM) to induce and promote the proliferation, differentiation and migration of stem cells. Both laboratory and clinical studies have indicated that GFs like BMPs, IGFs and TGF-β can stimulate the proliferation and differentiation of osteoblasts to enhance cartilage regeneration.[94, 95] Although therapeutic BMP-2 and BMP-7 have been approved by the FDA for clinical applications, researchers are still working to improve the delivery to achieve better therapeutic efficacy and fewer side effects by sustaining suitable concentrations in situ. Previous studies have indicated that the elevated presence of ROS, hypoxia, acidity and MMPs are associated with skeletal and cartilage diseases (Figure 1).[32, 34–36, 44, 54] To achieve controlled, stimuli-responsive GF delivery in situ, several systems with components responding to the above disease-state specific features and other external stimuli were carried out. Here, we summarize these stimuli-responsive GF delivery approaches for bone and cartilage diseases.
2.2.1. Enzyme
Enzymes have been reported to play an important role in the bioactivities of the skeletal system with bone disorders. For example, MMPs are digestive enzymes that play a crucial function in ECM remodeling. More than half of the MMP proteins are expressed by bone and cartilage cells under pathological conditions, such as rheumatoid arthritis, osteoarthritis, and osteoporosis.[96] As a result, several systems mimicking enzyme-mediated matrix turnover have been developed to control GF delivery for tissue regeneration. Lutolf et al. engineered a branched poly (ethylene glycol) (4-arm PEG)-based MMP-responsive delivery system to release BMP-2 for bone regeneration. The hydrogel contained a combination of pendant oligopeptide ligands (RGDSP) for cell adhesion and MMP cleavable linkers among the PEG chains. These bioactive networks could undergo MMP-induced proteolytic degradation to release BMP-2 and promote bone regeneration in a rat calvarial defect model.[97] Qi et al. reported BMP-2 nanocapsules used to enhance BMP-2 delivery to fracture sites. The nanocapsules were synthesized via in situ polymerization of 2-(methacryloyloxy)ethyl phosphorylcholine (MPC) monomers with MMP cleavable linker on the surface of BMP-2. The MPC shell stabilizes the GF and prolongs the degradation time in the blood. Enriched MMP environments, such as the site of a fracture, will promote the release of BMP-2 by the MMP (mainly MMP-2 and MMP-9)-induced degradation of the linker. As a result, the formed nanocapsules sustained local GF concentration and reduced systemic side effects in rat tibial injury model.[98] Anjum et al. demonstrated a modularly assembled, transglutaminase-crosslinked poly(ethylene glycol) (TG-PEG) and MMP Lysine-peptide grafted chondroitin sulfate (CS) precursor (CS-MMP-Lys) hybrid hydrogel platform (CS-PEG) to tune BMP-2 binding and release to induce bone regeneration.[99] Holloway et al. developed a material that concurrently delivered SDF-1α and BMP-2 through a hydrogel composed of a maleimide-modified hyaluronic acid (MaHA) combined with both cell-adhesive peptides (RGD, pendent) and MMP-sensitive peptides (VPMS↓MRGG, cross-linker). Crosslinking occurred between the maleimide functional groups on HA and the thiols within the cysteine residues on the peptides. MMP-induced SDF-1α and BMP-2 co-delivery showed better therapeutic efficacy compared to same concentrations of SDF-1α and BMP-2 delivered independently.[100] Human neutrophil elastase (HNE) is a serine protease secreted by neutrophils, the first cells recruited to inflammatory sites. Aimetti et al. introduced a novel HNE-responsive hydrogel-based drug delivery system consisting of HNE-sensitive linkers photopolymerized to PEG diacrylate (PEGDA) hydrogels as pendent groups.[101] The hydrogels facilitated precise control over delivery kinetics and has potential applications in cellular responsive delivery systems for inflammations.
2.2.2. pH
Bone formation and resorption are balanced processes in a healthy skeletal system and bone disorders generally stem from an imbalance between them. It has been shown that bone resorption creates a positive feedback loop - creating an acidic microenvironment that leads to further reduction in pH to promote more resorption.[102] The pH of bone tissue can also be altered by inflammatory bone diseases which generate a local acidic microenvironment due to the enrichment of immune cells. Because of these naturally occurring conditions, pH-responsive systems for protein and drug delivery can be engineered. Gan et al. reported a novel pH-responsive protein delivery system based on mesocellular silica foam (MCF). MCF is composed of ultra-large spherical pores (>20 nm) interconnected by smaller windows and as a result has high surface area relative to volume.[103] BMP-2 was encapsulated in the ultra-large pores and covered with N,O-Carboxymethyl chitosan (NOCC), a water-soluble chitosan derivative with pH-sensitive behavior. Within normal physiological environments, the release of BMP-2 was inhibited by the coverage of NOCC. However, at the site of injury the BMP-2 could be released as NOCC was dispersed in the acidic environment. In another study, Gan et al. used a similar pH-responsive chitosan-functionalized mesoporous silica nanoparticle systems to co-deliver BMP-2 and dexamethasone for bone regeneration.[104] In addition, responsive Fe3O4 NPs were combined with mesoporous silica scaffolds for BMP-2 delivery.[105] pH-responsive release was achieved due to the interactions between Fe3O4 NPs and the silica scaffolds. Under physiological condition (pH~7.4), the protein was entrapped in the pore of the silica matrices by Fe3O4 NPs due to the intact 7-(hydroxymethyl)-2,4,6-Triphenyl-1,3,5-Triazaadamantane (TAA) linker. In acidic conditions (pH~5.0) however, BMP-2 rapidly diffused from the system as the shielding mechanism of the protein was disabled. Additionally, Fe3O4 NPs can respond to magnetic fields and move accordingly; non-invasive magnetic control over the drug delivery site is another potentially beneficial drug delivery tool.
2.2.3. Temperature
Healthy patients have thermoregulatory mechanisms that work incessantly to maintain stable body temperature. However, some pathophysiological scenarios, such as systemic inflammation, osteomyelitis and osteoarthritis, are characterized by elevated temperatures relative to healthy tissue.[36] In these cases, thermo-responsive drug delivery systems can use the temperature differences to control drug release. The critical temperature of different LCST materials is the key parameter for tailoring the temperature-mediated release profile. In addition to artificially-produce regions of high temperature in the body, external heaters can also be used to induce thermo-responsive delivery. Smith et al. conjugated peptides containing arginine–glycine–aspartic acid (RGD) sequences to PNIPAm polymers via amine-reactive N-acryloxysuccinimide (NASI) groups.[106] The authors showed that the RGD-grafted NiPAM/NASI BMP-2 delivery material induced more adhesion and osteoblastic activity of cells. Beyond PNIPAm, researchers have reported the use of PLGA-PEG-PLGA copolymers as thermo-responsive delivery vehicles of BMP-2 for bone regeneration.[107] Viscoelastic properties, particularly the temperature-responsive behavior of PLGA-PEG-PLGA, had a significant dependence on the structural features the copolymer. Both the sol-gel transition temperature and maximum viscosity temperature of the copolymer were optimized to be at 35˚C. Segredo-Morales et al. presented a novel injectable thermo-responsive hydrogel loaded with microspheres of 17β-estradiol, BMP-2 and plasma rich in growth factors (PRGF). Local application of the GF rich gel was able to induce bone regeneration in osteoporotic female rats.[108] The hydrogel system is composed of a poloxamine (T-1307) reinforced with calcium chloride crosslinked alginate. PRGF and BMP-2 were encapsulated within PLGA microspheres. 17β-estradiol was loaded into poly(D,L-lactide) (PLA-S) microspheres to function as bone-active substances in the defect. The impaired bone repair process in osteoporotic rats was improved by sustained supply of BMP-2, PRGF and 17β-estradiol using this temperature responsive delivery system.
2.2.4. Redox
Inflammation is commonly accompanied with augmented production of ROS, which function as a systemic defense mechanism against microbial pathogens and other foreign bodies. Similarly, exceptionally upregulated ROS levels were also detected in the cases of arthritis and osteoarthritis.[36] This kind of unbalanced redox environment can cause irreversible damage to biomolecules and tissue.[109, 110] Recently, redox-responsive systems have been extensively studied due to their ability to not only mitigate ROS stress but also to trigger the release of therapeutics. Gong et al. engineered novel, redox-responsive, core-shell structured fibers for controlled release of BMP-2 for mandibular bone defect regeneration (Figure 3).[111] BMP-2 was encapsulated in the core region with Poly(ethylene oxide) (PEO), and the outer shell was composed of poly(ε-caprolactone) (PCL) and redox-responsive nanogels (cross-linked 6-arm PEG-polycaprolactone/6-arm PEG-polycaprolactone-sulfhydryl nanogels, c-6A PEG-PCL/6A PEG-PCL-SH NGs). The degradation of nanogels was controlled by GSH levels resulting in the formation of nanochannels inside the nanogel and increasing shell permeability and drug release from the inner region. After 48 h of incubation, about 13%, 55%, and 90% of BMP-2 was released in the presence of 10 μM, 0.1 mM, and 1 mM GSH, respectively, while only 3% BMP-2 was released in the absence of GSH. In addition, stepwise responsiveness was observed with the increase of GSH concentration (Figure 3F). Yang et al. synthesized a 4-arm mercapto-PEG (PEG-SH) and disulfide-bridged hydrogel loaded with BMP-2 using gentle oxidation.[112] The redox-response was achieved by the cleavage of disulfide bonds under the reducing action of thiol-carrying molecules such as reductive GSH, which is also secreted in excess in an abnormal ROS microenvironment.
Figure 3.

ROS responsive nanofiber for BMP-2 delivery with core-shell structure designed for bone regeneration.[111] A) Schematic of the fabrication of the nanogel-in-nanofiber device and the ROS responsive delivery via ROS-induced degradation of the nanogel and the nanochannels formed to promote the diffusion rate of BMP-2 loaded in the core of nanofiber. B) TEM images of nanogel. (scale bar of the inset: 30 nm) C, D) TEM images of nanogel-in-fiber structure. E) SEM images of core-shell structure nanofiber with nanogel. F) Release profile of ROS responsive nanofiber in different concentration of GSH. Stepwise responsiveness was observed with the increasing concentration of GSH. G) The differences on diameters of nanofiber without nanogel, nanofiber with nanogel and nanofiber with nanogel in GSH condition. H) Evaluation of apparent repair area, mean gray value and bone repair level, respectively, in critical-sized rat mandible defect model. Copyright 2018, American Chemical Society.
2.2.5. External stimuli
Apart from the internal triggers caused by specific diseases, some external stimuli, such as ultrasound, magnetic field and external heat application, can also be applied to induce the delivery of GFs for bone regeneration. These strategies have the potential to achieve targeted delivery with controlled dosage at desired time points. Crasto et al. introduced a PEGylated liposome-BMP-2 nanocomplex that releases BMP-2 in response to exposure to nonthermogenic clinical diagnostic ultrasound.[113] Their results showed that diagnostic ultrasound could disrupt the bi-layered membrane of the liposomes and release the cargo from within while the membranes of nearby cells remained unaffected by the same exposure. This observation also suggests that liposomes may be ideal carriers for sono-disruptable release of therapeutic payloads in vivo. Matsuo et al. reported the magnetic liposome with nano-Fe3O4 to target delivery of BMP-2.[114] They also applied similar magnetic liposomes as vehicles to deliver TGF-β for cartilage regeneration.[115] Correia et al. engineered a multi-layered, magnetically-responsive, liquid-filled capsule with an isolated environment inside in which encapsulated stem cells underwent self-regulated chondrogenesis.[116] They demonstrated that this magnetic field-responsive multilayered capsule, containing COL-1 and TGF-β3-modified PLLA microspheres and adipose stem cells, provided both injectability and in situ fixation, important parameters in the development of technology to regenerate cartilage.
2.3. Cardiac and skeletal muscle
Muscle regeneration is an intricate process involving satellite cell differentiation and new myofiber formation.[117] Impaired healing ability, triggered by aging, localized ischemia or severe damage, can cause disability of the patient, reducing the quality of their daily life. Given that millions of people are haunted with muscular diseases in the United States alone, it is imperative that innovative strategies are developed to assist with skeletal muscle repair.[118–120] Similar to musculoskeletal diseases, dysfunction of cardiac muscle is troublesome as well.[121] Myocardial infarction (MI), as well as its consequent process of heart failure, is one major type of cardiovascular diseases.[122] Resulting from the obstruction of the flow of oxygenated blood to the heart, MI can lead to localized myocardial hypoxia and, subsequently, apoptosis or necrosis.[123] Considering the limited regeneration ability of myocardial cells, cell delivery, stem cells in particular, has emerged as a prospective approach to treat MI-induced scarring of the cardiac muscle.[121, 124] However, unsolved problems such as limited cell survival in the ischemic area and toxicity caused by cell diffusion restrict the efficacy of clinical treatments, resulting in a poor prognosis for MI patients.[2, 125] In this case, regulating the cellular microenvironment to accelerate endogenous cardiac stem or progenitor cell regeneration is a promising method for myocardial remodulation. GFs, because of their vital roles in the regulation of cellular signaling pathways, have been capturing extensive attention for decades. Various GFs including bFGF, VEGF, PDGF and hepatocyte growth factor (HGF) are involved in the regeneration process at different points across the stages of repair.[126] A variety of unique features in the regenerative muscle microenvironment are deployed as stimuli. The context of tissue ischemia, for example, leads to hypoxia and ROS generation, accompanied by lactic acid accumulation and increase in protease levels.[21, 47, 127] Additionally, some researchers make use of the biodegradability of hydrogels such as alginate, chitosan, PEG and fibrin for optimal release kinetics in physical conditions.[128, 129] Below is a detailed summary of several types of stimuli-responsive GF delivery strategies for the treatment of muscular disease.
2.3.1. Enzyme
It is notable that a large volume of cell apoptosis and necrosis in muscular diseases can lead to a rise of protease in ECM. A sudden increase of MMPs, especially MMP-2, is observed in the process of MI. MMPs can decompose various proteins that maintain myocardial cell connections and interactions. In that case, the condition of ischemia is worsened as a result of vascular cell damage. Delivering GFs to promote cell proliferation and angiogenesis can counteract the negative impact of MMPs. Under this hypothesis, Fan et al. designed an MMP-2/9 cleavable hydrogel for on-demand bFGF release (Figure 4). The smart hydrogel function was realized by linking bFGF, GSH S-transferase (GST) and MMP-2/9 cleavable peptide PLGLAG (TIMP) together. In this way, bFGF will only be released in the MMP rich regions of the MI. TIMP, as a substrate of MMPs, can also serve to alleviate MMP-triggered tissue deconstruction. As shown in Figure 4B, in vitro release of bFGF was increased for the GST-TIMP-bFGF group in the presence of MMP-2 while it was much lower in the absence of MMP-2 or after MMP-2 inhibition, which indicated that the release of bFGF could specifically respond to MMP-2. The in vivo release of bFGF from GST-TIMP-bFGF increased from 3 to 5 days, which is consistent with the upregulation of MMP-2 after MI (Figure 4C). Because the release of bFGF is correlated with MMP levels, the material facilitated the specific, on-demand release of bFGF that prevented side effects of excessive GFs. Additionally, fused peptides were loaded in collagen modified with GSH, which elevated binding affinity of the GST-tagged peptides. It was shown that an MMP-responsive hydrogel alleviated MI-induced vascular wall thinning and promoted the proliferation of vascular cells.[130] It is quite convenient to link hydrogels with protease cleavable linkers for responsive delivery. Similar MMP-responsive features were adopted in SDF-1α delivery. Prokoph and colleagues incorporated an MMP-cleavable site in their starPEG-heparin hydrogels, which alone can promote angiogenesis in MI. Such MMP-triggered SDF-1α delivery attracted the migration and infiltration of endothelial progenitor cells (EPCs) in MI part from bone marrow. It was reported that the sustained release of SDF-1α recruited EPCs homing to the infarct region, hinting at the MMP responsive delivery efficacy of the starPEG-heparin hydrogel.[131] Interestingly, besides GFs, MMP-responsive drug delivery systems can also be applied for myostatin inhibitor delivery for sarcopenia, a reduction of muscle mass and contractility because of aging and treatments. By combining myostatin inhibitors with protease cleavable linkers, Braun et al. successfully downregulated the NF-κB pathway in the site of skeletal myopathy, where the MMP level increased.[132]
Figure 4.

MMP-2/9 cleavable hydrogel that achieved on-demand bFGF release for myocardial infarction.[130] A) Schematic illustration of the preparation and the process of the responsive release from the MMP responsive injectable hydrogel. B) bFGF release triggered by MMP-2 at different time points. C) bFGF release profile at different time points in vivo. D) SEM images of MMP responsive injectable hydrogel. E) This system alleviated ventricular wall thinning and decreased the MMP-2 and MMP-9 expression levels in a myocardial infarction model. Copyright 2019, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Collagenase is another potential trigger for hydrogel-embedded GF release. Salimath et al. developed a PEG-based gel fused with collagenase substrate peptide sequence. In this way, they co-delivered VEGF, HGF and progenitor cells together in a MI rat model. The protease-responsive GF delivery system remedied the loss of vessels by inducing migration and proliferation of vascular endothelial cells.[125] Traditional chemical conjugations such as the use of EDC and NHS are non-specific, which raises the risk of side-effects and impedes a reproducible result in clinical use. The target specificity of enzymes endows protease-responsive delivery with spatiotemporal precision.[133] Protease-targeted GF delivery, without a strict requirement in the physiochemistry properties of delivering materials, are assumed to have a wide application in responsive GF delivery.
2.3.2. PH and Temperature
Organ ischemia is always correlated with lactic acidosis due to a switch in cellular metabolism pathway driven by localized hypoxia, nitrite decrease and toxin accumulation. Compared with normal physiological conditions (pH 7.4–7.6), pH at the site of the ischemic myocardium drops to 6–7. Combing pH features with physiological temperature range as dual stimuli provides an alternative way for GF responsive delivery. Temperature-dependent gelation characteristics make it possible for injection and in situ solidification. In order to realize pH-regulated GF delivery, it is essential to fabricate high-precision pH-responsive biomaterials for delivering GFs. Garbern et al. developed an injectable hydrogel system which can transform from liquid to solid responding to a pH change from 7.4 to 6.8 at 37°C. Such pH and temperature responsive hydrogel systems are copolymers of NIPAm, butyl acrylate (BA) (p(NIPAm-co-PAA-co-BA)) and propylacrylic acid (PAA). After loading bFGF in the hydrogel system, hydrogel was injected to the ischemic part of a rabbit’s heart. It was found that bFGF was retained longer within the hydrogel system, which remedied local blood flow significantly by mediating the formation of new vessels.[134] Analogously, many acid-labile hydrogels can be utilized for pH-responsive GF delivery. Ran and coworkers developed a multi-block copolymer (MBCP), composed of Pluronic and di-(ethylene glycol) divinyl ether, that swells and degrades in acidic environment. Though pH and temperature sensitive gels were used to deliver plasmid DNA for muscle reprogramming, it also provided us with a platform for pH-responsive delivery of GFs. By developing the sol-gel vested with a temperature dependent gelation feature, liquid gel can be easily injected at room temperature and formed as solid, preventing unnecessary diffusion of GFs.[135] Hadjipanayi et al. took advantage of the concentration-dependent gelation trait of collagen for temperature-dependent GF delivery. In vitro cultured cell-derived GFs such as VEGF, FGF and PLGF were collected and embedded in collagen gel for injection. Despite the simple design, collagen-based temperature sensitive gels have a wide array of applications in the localized GF injection for muscle regeneration.[123]
2.3.3. Calcium ion
Sustained supply of GFs at muscle injury sites enabling a long-term solution for prolonged regenerative activity will never fail to fascinate researchers. Various biodegradable hydrogels, known for their biocompatibility and biodegradability, are utilized for long-term GF delivery.[136] Alginate, due to its calcium ion-dependent crosslinking and degradation, is an ideal hydrogel considering that the physiological calcium concentration is optimal for its slow decomposition.[128] Based on this property, researchers can design systems using calcium ion-responsive materials to sustain release of GFs for muscle regeneration. Monney et al. compared alginate-mediated IGF-1 and VEGF delivery with bolus delivery for muscle regeneration. They found that, surprisingly, bolus delivery had little positive effect on vascularization and muscle generation, both of which are hallmarks of therapeutic IGF-1 and VEGF.[129] Since alginate is a mixture extracted from algae, the degradation rate and shape-maintaining ability can be tuned by adjusting the molecular weight, mannuronic and guluronic ratio (M/G ratio), crosslinking time and Calcium solution concentration for crosslinking.[137] A shape-memory alginate delivery system was reported for IGF-1 and VEGF therapy as well. That unique trait makes it possible for compressing alginate since the shape can be restored after contacting the moist microenvironment in patient’s body.[138] Apart from combined delivery, BMP-2, PDGF and VEGF were all tested in such an alginate delivery system. Plus, by fusing RGD peptides, they enhanced HGF and FGF- secreting cell adhesion on alginate, broadening the application of the Ca2+ -dependent biodegradable scaffold for GF delivery.[139]
2.4. Blood vessel
Adequate nutrient supply from blood vessels is essential for supporting normal tissue function. Three key processes of vascular network development include vasculogenesis, angiogenesis, and arteriogenesis. During these processes, various GFs (VEGF, FGF, PDGF, TGF) are synergized with microenvironmental cues (hypoxia and shear stress) to develop a mature vascular system.[140–142] Abnormal blood supply to an organ leads to pathological conditions such as ischemia and reperfusion. Thus, reestablishment of vascular function is an essential first step in both tissue regeneration and reducing ischemic conditions. Sustained delivery of GFs in materials responsive to the pathological state of the tissue is highly desired as it maximizes therapeutic efficacy and mitigates associated cytotoxicity. In the vascular system, several features within pathological sites can serve as stimuli for “smart” GF delivery. Limited blood supply and imbalanced metabolic activities can exacerbate tissue hypoxia. Moreover, profound inflammatory responses elevate the production of ROS and ATP from multiple cell types.[143] Such inflammatory conditions are also associated with acidic extracellular pH and overexpression of multiple enzymes including MMPs.[144] As a result, these internal disease-state specific features can function as triggers for local delivery of angiogenic GFs to stimulate vascular network development only when it is needed.[145] Another method of responsive delivery is to take advantage of normal physiological conditions such as temperature. Temperature can induce changes in gelation allowing the material to be retained within the targeted areas while further sustaining encapsulated GF delivery. Furthermore, external stimuli, including mechanical stress, light and ultrasound, provide another method of spatiotemporally controlling GF delivery. Here we summarize some of the research efforts in each category.
2.4.1. Enzyme
Wen et al. developed VEGF nanocapsules based on in situ radical polymerization. The encapsulated VEGF can be protected and selectively released under specific enzymatic triggers by incorporating protease-degradable crosslinkers. Upregulated expression of MMPs and serine proteases, such as plasmin, within the injured site can induce VEGF release and boost blood vessel formation.[146] Zhu et al. also designed a smart system for the delivery of multiple GFs with spatiotemporal control via enzymatic stimulation. By changing the chirality of amino acids within certain enzyme-specific sequences, proteolytic kinetics can altered without impacting enzyme specificity. Sequential delivery of VEGF and PDGF was demonstrated and can be applied to multi-GF delivery systems that require sequential administration.[147] Similarly, Van Hove et al. tethered a VEGF-mimicking peptide to a PEG hydrogel backbone using MMP degradable linkers. The sequence of linker can be varied to adjust the MMP degradation kinetics and achieve temporal control over the therapeutic release profile.[148]
2.4.2. pH and temperature
Anaerobic cellular metabolism within sites of ischemia leads to the production of a mildly acidic microenvironment. Joshi et al. leveraged pH as an indicator of ischemic presence and recovery to stimulate on demand release of FGF-2. FGF-2 was loaded into microspheres made of temperature-responsive NIPAm and pH-responsive acrylic acid. Temperature responsiveness facilitated the gelation and retention of a solid form after injection. pH-responsiveness also promoted rapid gel formation and, more importantly, sustained GF release in the mildly acidic ischemic environment. After recovering to physiological pH, the polymer was eliminated by dissolution.[52] Garbern et al. assembled NIPAm and PAA to synthesize p(NIPAm-co-PAA) hydrogels using the reversible addition fragmentation chain transfer (RAFT) polymerization technique.[149] When exposed to physiological pH (7.4), the polymer did not form gels and was soluble at 50 °C. Conversely, when exposed to a lower pH (≤5.5), the polymer exhibited a temperature-dependent sol-gel phase transition and physically gelled at 37 °C. The loaded bFGF can remain bioactive even after being stored in the hydrogel for up to 40 hours at 37 °C. The VEGF loaded p(NIPAm-co-PAA) hydrogels also showed a pH-dependent sustained drug release. This hydrogel can be designed to undergo gelation at a certain pH in vivo and gradually dissolve to distribute the drug until the pH of the injury site returns to a normal level. Adibfar et al. delivered VEGF with thermo-responsive PNIPAm NPs for angiogenic purposes. VEGF can be associated with PNIPAm chains to form NPs at 4 °C and elevated temperature encountered later spurred the release of bioactive agents. Further decoration of NPs with collagen slowed the release.[150] Lee et al. demonstrated the use of a sulfonated thermal reversible gel based on polyurea-conjugated PNIPAm for VEGF delivery. Sulfonation aimed to mimic heparin in promoting electrostatic binding with VEGF while temperature responsiveness facilitated its use as a minimally invasive injection with high interstitial retention.[151]
2.4.3. Cell receptor
GFs induce a cellular response by the binding to specific receptors on the surface of a target cell. This specificity provides a unique opportunity to localize GF delivery by targeting specific cell receptors. Yamaguchi et al. proposed a self-assembled hydrogel based on heparin-modified star polymer PEG. VEGF has heparin binding domain that can crosslink the hydrogel among heparin-modified PEG polymer (Figure 5). This hydrogel formation mechanism facilitated stimuli-responsive release of GFs through hydrogel erosion near cells presenting the VEGF receptor (vascular endothelial cells). As shown in Figure 5C, the hydrogel demonstrated an accumulated release of up to 30% over 10 days in PBS while the presence of VEGFR-2 can trigger the release of 80% total VEGF during the same period.[152]
Figure 5.

A cell receptor-responsive system was designed for sustained and targeting VEGF delivery.[152] A) Schematic of the preparation of the star copolymer hydrogel and the release progress with receptor-mediated erosion. B) The mechanical property of the VEGF-crosslinked hydrogel. C) VEGF release profile with or without the VEGFR-2 receptor. D) Proliferation of porcine aortic endothelial cells with blank hydrogel and hydrogel with different concentrations of VEGF. E, F) Live/dead assay of porcine aortic endothelial cells with blank hydrogel and hydrogel crosslinked with VEGF. Copyright 2018, American Chemical Society.
2.4.4. Mechanical:
Beyond the changes in the chemical environment, the physiological environment is a mechanically dynamic environment as well. Most drug delivery systems are designed to operate under static conditions; therefore, utilizing mechanical signals for inducing GF release could potentially guide tissue regeneration. Lee et al. designed mechanically responsive alginate hydrogel systems for vascularization purposes. A certain degree of reversible binding between VEGF and a polysaccharide hydrogel matrix could benefit from mechanical control. VEGF-loaded hydrogels with cyclic mechanical triggers displayed a significant increase in vascularization compared to a control not subjected to mechanical inducers.[153]
2.4.5. Ultrasound:
Tissue regeneration normally involves multiple stages with different GFs sequentially playing crucial roles. Moncion et al. utilized an acoustically responsive scaffold to achieve sequential delivery of GFs by simply tuning the acoustic pressure. The perfluorocarbon emulsion phase can evaporate to release the encapsulated GFs upon induction by ultrasound.[154]
2.5. Neural system
Neurotrophins (NTs) play crucial roles in regulating neural function and development. Nerve growth factor (NGF) is the first major GF to be characterized in this category in addition to three other common factors found in mammals: brain-derived neurotrophic factor (BDNF), NT-3, and NT-4.[155] Internal sources of NTs come from sympathetic and sensory target organs and are transported into the terminals of nerves for further signal transduction. In the case of an injury to the nervous system, an associated inflammatory response is elicited leading to macrophage recruitment. These macrophages secrete cytokines capable of inducing synthesis of NTs in Schwann cells and fibroblasts neighboring the injured nerve. The physiological response of producing various NTs is essential for the maintenance of healthy neural development and regeneration. Apart from endogenous maintenance and protection mechanisms of NT generation, external supplement and delivery of such protein-based therapeutics may provide another method of treating pathological conditions in which endogenous levels of NTs are insufficient for functionality or repair.[156] Because of the existence of the blood-brain barrier (BBB), it is generally more challenging for delivery of protein-based therapeutics across the BBB to the central nervous system (CNS) in contrast to the peripheral nervous system (PNS). As a result, therapeutics are often delivered systemically with delivery strategies integrating mechanisms for both targeting and bio-responsiveness to enhance therapeutic efficacy and minimize adverse reactions.[157] Alternatively, surgical procedures, while invasive, have the benefit of local implantation which avoids the need for GFs crossing the BBB and allows more efficient and direct delivery of various NTs to the disease site with mitigated systemic cytotoxicity. Biomaterial scaffolds can be engineered to respond to the physiological and pathological stimuli of the CNS to better control the release of therapeutics. Some neural injury specific conditions, such as disruption of ionic balance of K+, Na+, and Ca2+ and increased release of glutamate extracellularly, are seldom applied as stimuli for GF delivery. The most commonly used stimuli are the inflammation-associated increase of ROS and the elevated expression of MMPs.[26, 42] External control mechanisms that either facilitate the delivery of encapsulated drugs encapsulation or promote GF release can also be beneficial.
2.5.1. Enzyme
Physiological environments contain various endogenous enzymes that can be used to assist in situ gelation or enzyme-responsive release of cargo. Various naturally-derived materials are good candidates as scaffolds for neurotrophic GFs because of their innate enzymatic responsiveness. Johnson et al. utilized fibrin gel for controlled release of NT-3 and PDGF. A prepolymer solution of fibrinogen can be polymerized into fibrin gel by thrombin in the lesion site. This gel could ensure local therapeutic delivery as electrostatic interactions between the factors and fibrin help slow the release of encapsulated factors.[158] To improve the release kinetics, Sakiyama-Elbert et al. incorporated heparin into a fibrin matrix to slow the diffusion-based release of NGF (as NGF and heparin have weak affinity for one another). The degradation of the scaffold and release of these GFs can rely on enzymatic factors such as heparinase and plasmin to tune drug delivery.[159]
Multidomain peptides are a unique class of carriers for delivery of biologics. Additionally, these materials have superior biocompatibility and controlled biodegradability. Addition of specific cleavage peptide sequences corresponding to various enzymes, such as trypsin, neutrophil elastase, papain and MMP, can tune the release profile based on physiological stimuli.[160, 161] Song et al. achieved sustained delivery of NGF with diblock copolypeptide hydrogel depot. The engineered copolypeptides can self-assemble to form hydrogels that persist up to 4 weeks within the mouse forebrain.[162] Lindsey et al. utilized a beta hairpin peptide-based hydrogel (MAX8) to perform injectable neurotrophic GF delivery. MAX8 contains peptide sequences that can undergo gelation under physiological pH, temperature, and salt concentration. The physical crosslinking mechanism enables its shear thinning property under high stress while simultaneously protecting the encapsulated GFs.[163]
2.5.2. pH
Systemic delivery of neurotrophic factors presents challenges including rapid clearance by serum proteins, uptake by macrophages and crossing the BBB to the disease site.[164] Nanomedicine provides possibilities for enhanced circulation half-life and penetration of these factors. Xu et al. developed a nano-capsule system with pH-sensitive properties for NGF delivery that is capable of penetrating the BBB mediated by the endogenous neurotransmitter transport system (Figure 6). In situ polymerization around NGF provides a protective shell around the GF to facilitate prolonged circulation. Additionally, the monomers used in polymerization include choline and an acetylcholine analog that further facilitates penetrating the BBB. The crosslinker used is pH-sensitive PLA diacrylate, which can be hydrolyzed under nerve physiological conditions to free the encapsulated NGF to function on site. As shown in Figure 6C, in vitro release of n(NGF) under different pH conditions (6.0 and 7.4) validated pH sensitive property of the nanocapsules where n(NGF) can be continuously released for days but with a higher rate under acidic pH=6.0.[165]
Figure 6.
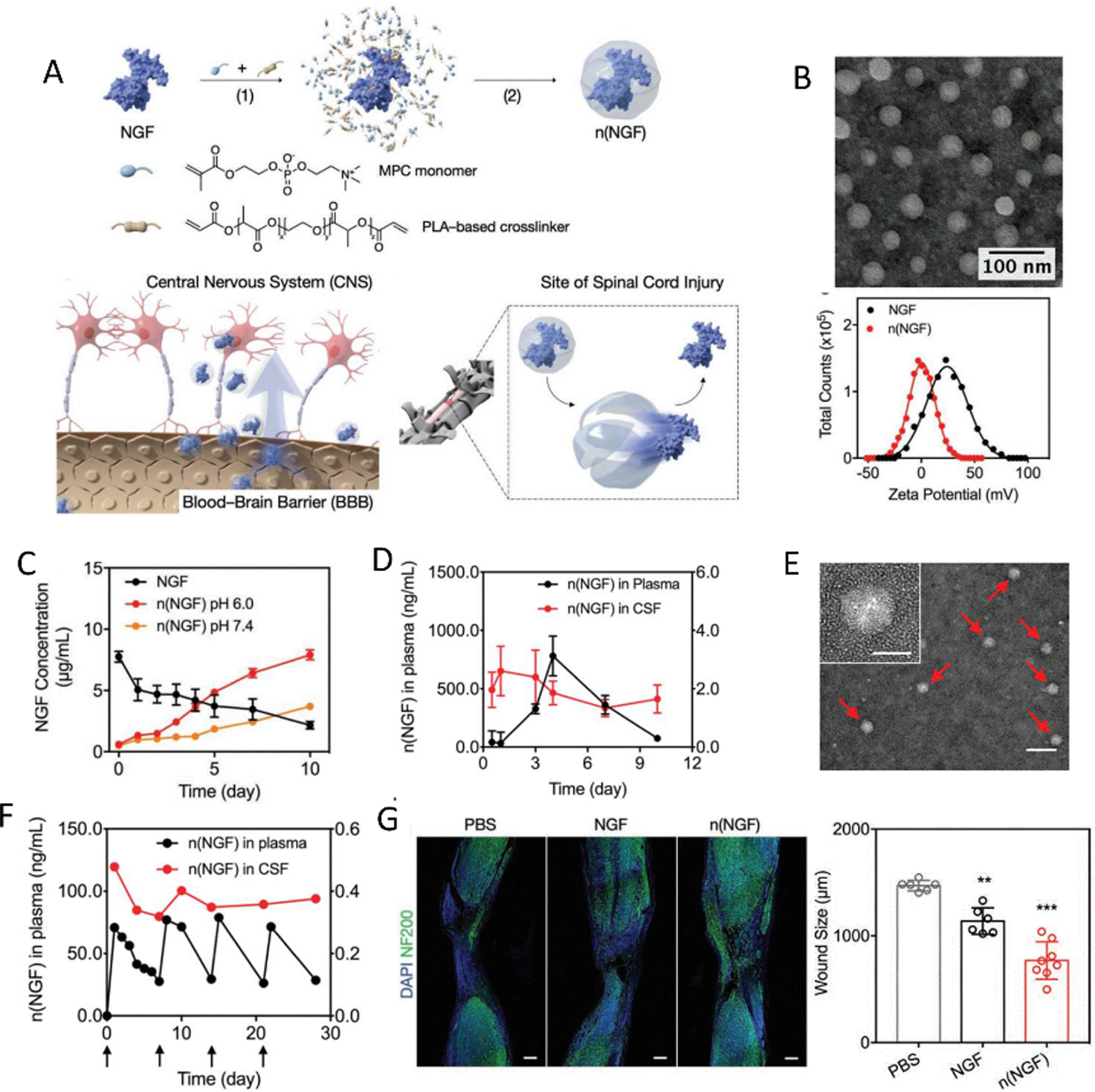
An efficient pH responsive growth factor nanocapsule that has the potential to cross the BBB was applied for neural regeneration in CNS.[165] A) Schematic for the preparation and application of this responsive delivery system. B) TEM images and zeta potential of NGF nanocapsule. C) The release profile of NGF nanocapsule in different pH environment. D) Pharmacokinetics of NGF nanocapsule in plasma and cerebrospinal fluid at different time points after intravenous injection. E) TEM images of NGF nanocapsule in cerebrospinal fluid one day after the injection. F) Pharmacokinetics of NGF nanocapsule in the plasma and CSF of rhesus macaque after multiple intravenous injections at different time points. G) Fluorescence images and analysis of mice spinal cord after injury and different treatments. (Neurofilaments are stained with NF200). Copyright 2019, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
2.5.3. Temperature
The temperature responsive mechanisms for these materials are generally used to facilitate facile injection at lower temperatures and rapid gelation at the physiological temperature of 37˚C. Temperature-responsive gelation of NTs encapsulated in a hydrogel can localize therapeutics within targeted area and minimize the waste of GFs. Jain et al. utilized agarose hydrogels as thermo-responsive carriers for the delivery of BDNF after spinal cord injury. A customized cooling system with cooled nitrogen gas sped up the gelling of agarose and ensure the conformity to the cavity of spinal cord cavity. In addition to the favorable thermal properties of agarose, the material offers other advantages in terms of biocompatibility after implantation, tunable porosity and mechanical properties for axon penetration, and sustained release of neuroprotective factors.[166] Gupta et al. engineered a fast gelling GF delivery system for the injured spinal cord based on a blend of hyaluronan and methylcellulose. Methylcellulose displays inverse thermal gelling behavior in which elevated temperature induces gelation via the formation of hydrophobic junctions. Hyaluronan possesses long polymer chains and random coil entanglements, which provide shear thinning properties for injection. The resulting combined hydrogel system is superior in terms of fast gelation, limited cell adhesion, and biocompatibility.[167] Stabenfeldt et al. designed a bioactive thermal reversible hydrogel system for neural tissue engineering based on laminin-1 (LN) conjugated methylcellulose. The presence of functional LN ligands within a hydrogel matrix promotes neuron attachment and adhesion.[168]
1. Conclusion and perspective
Stimuli-responsive delivery of GFs has been extensively studied in the past two decades in order to maximize the therapeutic effects of the proteins. Incorporation of GFs into materials offers several benefits; the primary advantage is GF encapsulation to protect the molecules from denaturation or clearance within the harsh physiological environment.[3] Moreover, diverse “smart” materials take advantage of stimuli-responsive mechanisms based on indicators of a disease state or the natural physiological properties of the tissue; these materials allow the delivery of the proper dosage with excellent spatiotemporal specificity.[9] These materials are able to keep the local concentration of GFs within the site of the disease, avoiding unnecessary side effects caused by burst or off-target release. Moreover, regeneration of tissues is a complicated process and it has been proven that synergy of dual or multiple GFs simultaneously or sequentially could be beneficial for tissue regeneration.[169, 170] In this context, “smart” delivery systems for dual or multiple GFs are under development for better therapeutic efficacy.[61, 147] For such purposes, materials with different degradability or response to different triggers can be developed. Designing delivery systems with multi-layer structures can be one strategy for programed release of GFs. In this regard, identification of distinct biological triggers and development of a variety of stimuli-responsive materials are both crucial to accomplish this goal.
Challenges still exist in the application of such “smart” materials for tissue engineering. For many of the triggers described above, stimuli may be not unique to the disease states, which could cause the undesired release of GFs. For instance, arthritis leads to an increase in the secretion of MMPs, but other causes of systemic inflammation may also promote the secretion of MMPs. Therefore, MMP-responsive delivery of GF systems for cartilage regeneration may be unable to specifically target the arthritic cartilage and release the GF in other inflamed areas unintentionally. To address this issue, specific stimuli associated unique to specific diseases should be identified and utilized as a key design parameter. Another strategy can be applied to improve delivery specificity via a multi-inducer system. Diseases usually induce an array of changes within the microenvironment, including enzyme, pH, ROS and temperature. The design of more sophisticated, logic-gated responsive carriers could enable better control in the recognition of targets and avoidance of off-target side effects. These dual/multi-stimuli responsive systems should be further investigated to improve the specificity of targeting and controlled delivery in GF applications.
Although there are many superior properties of stimuli-responsive GF-delivering materials for tissue regeneration, few stimuli-responsive systems have reached clinical stages.[9, 36] Generally, commercialization of the products relies on the clinical safety, therapeutic efficacy and feasibility of scale-up. Considering these three factors, we provide some basic guidance related to the key parameters of the materials to maximize their chance at approval. First, the materials used should be highly biocompatible and avoid cytotoxicity from the material itself. In addition, versatile stimuli responsive systems may require complicated chemistry; while this isn’t inherently problematic, the selection of uncommon reagents may reduce the robustness of scale up, increase the risks of cytotoxicity, and mitigate the responsiveness of the material. Furthermore, the delivery of the materials should be considered. Minimally invasive procedures help avoid surgical complications and decrease the severity of inflammation, both important factors to be considered in the design of the “smart” delivery systems.[171] Commercialization of these technologies relies on a balance of these factors with regulatory opinions and administration procedures and must be considered carefully to expand a materials’ applications.
Another important trend to consider is the push to achieve responsiveness and “intelligence” in a personalized and patient-specific manner.[36, 172] The hallmarks of disease can vary significantly amongst individuals and this provides additional challenges for the development and implementation of these systems. The application of precise, stimuli-responsive systems must be guided by both the shared and distinguished features among patients. Comprehensive biophysiological characterization on the disease hallmarks of each patient should also be studied. The development of stimuli responsive delivery systems for GFs poses unique challenges including the presence of interpatient variability and non-specific disease indicators; however, significant progress has been made in the recent past that has taken aim at surmounting these challenges to improve the delivery of GFs as therapeutics.
Acknowledgement
M.Q., X.J., and X.Z. contributed equally to this work. This work has been supported by the National Institutes of Health fund to A.K. (5R01AR057837, 1R01EB021857, 1R01AR066193, 1R01GM126831, 1R01AR073135). X.J., Q.W., and L.R. acknowledge the support from China Scholarship Council. X.J. acknowledge the support from National Natural Science Foundation of China (No. 81703940) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (2019YSHL019).
Biographies

Moyuan Qu is a Ph.D. student majoring in dentistry in West China School of Stomatology, Sichuan University. Now, he works with Prof. Ali Khademhosseini in the Center for Minimally Invasive Therapeutics at University of California-Los Angeles (UCLA) as a visiting graduate researcher. His research interests focus on tissue engineering scaffolds and drug delivery systems.

Wujin Sun is a postdoctoral scholar working with Prof. Ali Khademhosseini in the Center for Minimally Invasive Therapeutics at University of California-Los Angeles (UCLA). He completed his Ph.D. studies in the Joint Department of Biomedical Engineering at the University of North Carolina at Chapel Hill, and North Carolina State University. He is interested in integrating biomaterial engineering and cell engineering for healthcare applications.

Ali Khademhosseini is the Levi Knight Professor of Bioengineering, Chemical Engineering and Radiology at the University of California-Los Angeles (UCLA). He is the Founding Director of the Center for Minimally Invasive Therapeutics at UCLA. Previously, he was a professor of Medicine at Harvard Medical School. He is recognized as a leader in combining microengineering and nanoengineering approaches with advanced biomaterials for regenerative medicine applications.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Moyuan Qu, Department of Bioengineering, California NanoSystems Institute and Center for Minimally Invasive Therapeutics (C-MIT) University of California, Los Angeles, Los Angeles, CA 90095, USA; State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, China.
Xing Jiang, Department of Bioengineering, California NanoSystems Institute and Center for Minimally Invasive Therapeutics (C-MIT) University of California, Los Angeles, Los Angeles, CA 90095, USA; School of Nursing, Nanjing University of Chinese Medicine, Nanjing 210023, China.
Xingwu Zhou, Department of Bioengineering, California NanoSystems Institute and Center for Minimally Invasive Therapeutics (C-MIT) University of California, Los Angeles, Los Angeles, CA 90095, USA; Department of Chemical and Biomolecular Engineering, University of California-Los Angeles, Los Angeles, CA 90095, USA.
Canran Wang, Department of Bioengineering, California NanoSystems Institute and Center for Minimally Invasive Therapeutics (C-MIT) University of California, Los Angeles, Los Angeles, CA 90095, USA; Department of Biochemistry and Molecular Cell Biology, Shanghai Key Laboratory for Tumor Microenvironment and Inflammation, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China.
Qingzhi Wu, Department of Bioengineering, California NanoSystems Institute and Center for Minimally Invasive Therapeutics (C-MIT) University of California, Los Angeles, Los Angeles, CA 90095, USA; State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070, China.
Li Ren, Department of Bioengineering, California NanoSystems Institute and Center for Minimally Invasive Therapeutics (C-MIT) University of California, Los Angeles, Los Angeles, CA 90095, USA; School of Life Sciences, Northwestern Polytechnical University, Xi’an 710072, China.
Jixiang Zhu, Department of Bioengineering, California NanoSystems Institute and Center for Minimally Invasive Therapeutics (C-MIT) University of California, Los Angeles, Los Angeles, CA 90095, USA; Department of Biomedical Engineering, School of Basic Medical Sciences, Guangzhou Medical University, Guangzhou 511436, China.
Songsong Zhu, State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, China.
Peyton Tebon, Department of Bioengineering, California NanoSystems Institute and Center for Minimally Invasive Therapeutics (C-MIT) University of California, Los Angeles, Los Angeles, CA 90095, USA.
Wujin Sun, Department of Bioengineering, California NanoSystems Institute and Center for Minimally Invasive Therapeutics (C-MIT) University of California, Los Angeles, Los Angeles, CA 90095, USA.
Ali Khademhosseini, Department of Bioengineering, California NanoSystems Institute and Center for Minimally Invasive Therapeutics (C-MIT) University of California, Los Angeles, Los Angeles, CA 90095, USA; Department of Chemical and Biomolecular Engineering, University of California-Los Angeles, Los Angeles, CA 90095, USA; Jonsson Comprehensive Cancer Center, Department of Radiology University of California-Los Angeles, Los Angeles, CA 90095, USA.
Reference
- [1].Khademhosseini A, Langer R, Nat. Protoc 2016, 11, 1775. [DOI] [PubMed] [Google Scholar]
- [2].Lee K, Silva EA, Mooney DJ, J. R. Soc. Interface 2011, 8, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tayalia P, Mooney DJ, Adv. Mater 2009, 21, 3269. [DOI] [PubMed] [Google Scholar]
- [4].Aguilar LMC, Silva SM, Moulton SE, J. Control. Release 2019, 306, 40. [DOI] [PubMed] [Google Scholar]
- [5].Zhou N, Li Q, Lin X, Hu N, Liao JY, Lin LB, Zhao C, Hu ZM, Liang X, Xu W, Chen H, Huang W, Cell Tissue Res. 2016, 366, 101. [DOI] [PubMed] [Google Scholar]
- [6].Boerckel JD, Kolambkar YM, Dupont KM, Uhrig BA, Phelps EA, Stevens HY, Garcia AJ, Guldberg RE, Biomaterials 2011, 32, 5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tian H, Zhao J, Brochmann EJ, Wang JC, Murray SS, Cytokine Growth Factor Rev. 2017, 34, 73. [DOI] [PubMed] [Google Scholar]
- [8].Vo TN, Kasper FK, Mikos AG, Adv. Drug Deliv. Rev 2012, 64, 1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lu Y, Aimetti AA, Langer R, Gu Z, Nat. Rev. Mater 2017, 2, 16075. [Google Scholar]
- [10].Mura S, Nicolas J, Couvreur P, Nat. Mater 2013, 12, 991. [DOI] [PubMed] [Google Scholar]
- [11].Sun W, Hu Q, Ji W, Wright G, Gu Z, Physiol. Rev 2016, 97, 189. [Google Scholar]
- [12].Wang J, Yu J, Zhang Y, Zhang X, Kahkoska AR, Chen G, Wang Z, Sun W, Cai L, Chen Z, Qian C, Shen Q, Khademhosseini A, Buse JB, Gu Z, Sci. Adv 2019, 5, eaaw4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ahadian S, Khademhosseini A, Regen. Biomater 2018, 5, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang J, Wang Z, Yu J, Kahkoska AR, Buse JB, Gu Z, Adv. Mater 2019, e1902004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ruan H, Hu Q, Wen D, Chen Q, Chen G, Lu Y, Wang J, Cheng H, Lu W, Gu Z, Adv. Mater 2019, 31, e1806957. [DOI] [PubMed] [Google Scholar]
- [16].Reiss MJ, Han YP, Garcia E, Goldberg M, Yu H, Garner WL, Surgery 2010, 147, 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Muller M, Trocme C, Lardy B, Morel F, Halimi S, Benhamou PY, Diabetic Med. 2008, 25, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Young PK, Grinnell F, J. Invest. Dermatol 1994, 103, 660. [DOI] [PubMed] [Google Scholar]
- [19].Hastbacka J, Freden F, Hult M, Bergquist M, Wilkman E, Vuola J, Sorsa T, Tervahartiala T, Huss F, PLoS One 2015, 10, e0125918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Latifa K, Sondess S, Hajer G, Manel BH, Souhir K, Nadia B, Abir J, Salima F, Abdelhedi M, Sci. Rep 2016, 6, 29371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].DeLeon-Pennell KY, Meschiari CA, Jung M, Lindsey ML, Prog. Molec. Biol. Transl. Sci 2017, 147, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Baird MF, Graham SM, Baker JS, Bickerstaff GF, J. Nutr. Metab 2012, 2012, 960363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Martinez-Amat A, Boulaiz H, Prados J, Marchal JA, Padial Puche P, Caba O, Rodriguez-Serrano F, Aranega A, Br. J. Sports Med 2005, 39, 830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Callegari GA, Novaes JS, Neto GR, Dias I, Garrido ND, Dani C, J. Hum. Kinet 2017, 58, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shiomi T, Lemaitre V, D’Armiento J, Okada Y, Pathol. Int 2010, 60, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Veeravalli KK, Dasari VR, Tsung AJ, Dinh DH, Gujrati M, Fassett D, Rao JS, Neurobiol. Dis 2009, 36, 200. [DOI] [PubMed] [Google Scholar]
- [27].Roth D, Piekarek M, Paulsson M, Christ H, Bloch W, Krieg T, Davidson JM, Eming SA, Am. J. Pathol 2006, 168, 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Power G, Moore Z, O’Connor T, J. Wound Care 2017, 26, 381. [DOI] [PubMed] [Google Scholar]
- [29].Turner CT, Lim D, Granville DJ, Matrix Biol. 2019, 75–76, 126. [DOI] [PubMed] [Google Scholar]
- [30].Bowler PG, Ann. Med 2002, 34, 419. [DOI] [PubMed] [Google Scholar]
- [31].Moor AN, Vachon DJ, Gould LJ, Wound Repair Regen. 2009, 17, 832. [DOI] [PubMed] [Google Scholar]
- [32].Konisti S, Kiriakidis S, Paleolog EM, Nat. Rev. Rheumatol 2012, 8, 153. [DOI] [PubMed] [Google Scholar]
- [33].Salminen-Mankonen HJ, Morko J, Vuorio E, Curr. Drug Targets 2007, 8, 315. [DOI] [PubMed] [Google Scholar]
- [34].Green MJ, Gough AK, Devlin J, Smith J, Astin P, Taylor D, Emery P, Rheumatology (Oxford) 2003, 42, 83. [DOI] [PubMed] [Google Scholar]
- [35].Itagaki T, Honma T, Takahashi I, Echigo S, Sasano Y, Anat. Rec. (Hoboken) 2008, 291, 1038. [DOI] [PubMed] [Google Scholar]
- [36].Lavrador P, Gaspar VM, Mano JF, Control J. Release 2018, 273, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kunkemoeller B, Kyriakides TR, Antioxid. Redox Signal 2017, 27, 823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Parihar A, Parihar MS, Milner S, Bhat S, Burns 2008, 34, 6. [DOI] [PubMed] [Google Scholar]
- [39].Donato-Trancoso A, Monte-Alto-Costa A, Romana-Souza B, J. Dermatol. Sci 2016, 83, 60. [DOI] [PubMed] [Google Scholar]
- [40].Barbieri E, Sestili P, J. Signal Transduct 2012, 2012, 982794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Iadecola C, Anrather J, Nat. Med 2011, 17, 796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Silva NA, Sousa N, Reis RL, Salgado AJ, Prog. Neurobiol 2014, 114, 25. [DOI] [PubMed] [Google Scholar]
- [43].Ueno C, Hunt TK, Hopf HW, Plast. Reconstr. Surg 2006, 117, 59S. [DOI] [PubMed] [Google Scholar]
- [44].Griffiths HR, Redox Rep 2005, 10, 273. [DOI] [PubMed] [Google Scholar]
- [45].Catrina SB, Zheng X, Diabetes-Metab. Res. Rev 2016, 32 Suppl 1, 179. [DOI] [PubMed] [Google Scholar]
- [46].Bonham CA, Rodrigues M, Galvez M, Trotsyuk A, Stern-Buchbinder Z, Inayathullah M, Rajadas J, Gurtner GC, Wound Repair Regen. 2018, 26, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA, Proc. Natl. Acad. Sci. U. S. A 2002, 99, 12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Eltzschig HK, Carmeliet P, N. Engl. J. Med 2011, 364, 656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Xing D, Liu L, Marti GP, Zhang X, Reinblatt M, Milner SM, Harmon JW, Wound Repair Regen. 2011, 19, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].McArdle C, Lagan KM, McDowell DA, Curr. Diabetes Rev 2014, 10, 177. [DOI] [PubMed] [Google Scholar]
- [51].Sharpe JR, Booth S, Jubin K, Jordan NR, Lawrence-Watt DJ, Dheansa BS, J. Burn Care Res 2013, 34, e201. [DOI] [PubMed] [Google Scholar]
- [52].Joshi RV, Nelson CE, Poole KM, Skala MC, Duvall CL, Acta Biomater. 2013, 9, 6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ono S, Imai R, Ida Y, Shibata D, Komiya T, Matsumura H, Burns 2015, 41, 820. [DOI] [PubMed] [Google Scholar]
- [54].Chakkalakal DA, Mashoof AA, Novak J, Strates BS, McGuire MH, J. Biomed. Mater. Res 1994, 28, 1439. [DOI] [PubMed] [Google Scholar]
- [55].Martinez-Jimenez MA, Aguilar-Garcia J, Valdes-Rodriguez R, Metlich-Medlich MA, Dietsch LJP, Gaitan-Gaona FI, Kolosovas-Machuca ES, Gonzalez FJ, Sanchez-Aguilar JM, Plast. Reconstr. Surg 2013, 132, 1015e. [DOI] [PubMed] [Google Scholar]
- [56].Armstrong DG, Lavery LA, J. Foot Ankle Surg 1996, 35, 335. [DOI] [PubMed] [Google Scholar]
- [57].Fierheller M, Sibbald RG, Adv. Skin Wound Care 2010, 23, 369. [DOI] [PubMed] [Google Scholar]
- [58].Chen GY, Nunez G, Nat. Rev. Immunol 2010, 10, 826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Uttara B, Singh AV, Zamboni P, Mahajan RT, Curr. Neuropharmacol 2009, 7, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Cotman CW, Anderson AJ, Ann.NY Acad.Sci 2000, 924, 112. [DOI] [PubMed] [Google Scholar]
- [61].Banerjee I, Mishra D, Das T, Maiti TK, J. Biomater. Sci.-Polym. Ed 2012, 23, 111. [DOI] [PubMed] [Google Scholar]
- [62].Schneider LA, Korber A, Grabbe S, Dissemond J, Arch. Dermatol. Res 2007, 298, 413. [DOI] [PubMed] [Google Scholar]
- [63].Dickinson LE, Gerecht S, Front. Physiol 2016, 7, 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Blakytny R, Jude E, Diabetic Med. 2006, 23, 594. [DOI] [PubMed] [Google Scholar]
- [65].Whittam AJ, Maan ZN, Duscher D, Wong VW, Barrera JA, Januszyk M, Gurtner GC, Adv. Wound Care (New Rochelle) 2016, 5, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M, Wound Repair Regen. 2008, 16, 585. [DOI] [PubMed] [Google Scholar]
- [67].Marti-Carvajal AJ, Gluud C, Nicola S, Simancas-Racines D, Reveiz L, Oliva P, Cedeno-Taborda J, Cochrane Database Syst Rev. 2015, CD008548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Werner S, Grose R, Physiol. Rev 2003, 83, 835. [DOI] [PubMed] [Google Scholar]
- [69].Halper J, Vet. Pathol 2010, 47, 77. [DOI] [PubMed] [Google Scholar]
- [70].Briquez PS, Hubbell JA, Martino MM, Adv. Wound Care (New Rochelle) 2015, 4, 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Saghazadeh S, Rinoldi C, Schot M, Kashaf SS, Sharifi F, Jalilian E, Nuutila K, Giatsidis G, Mostafalu P, Derakhshandeh H, Yue K, Swieszkowski W, Memic A, Tamayol A, Khademhosseini A, Adv. Drug Deliv. Rev 2018, 127, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].McCarty SM, Percival SL, Adv. Wound Care (New Rochelle) 2013, 2, 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wolcott RD, Rhoads DD, Dowd SE, J. Wound Care 2008, 17, 333. [DOI] [PubMed] [Google Scholar]
- [74].Kim SE, Lee PW, Pokorski JK, ACS Macro Lett. 2017, 6, 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ninan N, Forget A, Shastri VP, Voelcker NH, Blencowe A, ACS Appl. Mater. Interfaces 2016, 8, 28511. [DOI] [PubMed] [Google Scholar]
- [76].Hoffman AS, Adv. Drug Deliv. Rev 2013, 65, 10. [DOI] [PubMed] [Google Scholar]
- [77].Leveen HH, Falk G, Borek B, Diaz C, Lynfield Y, Wynkoop BJ, Mabunda GA, Rubricius JL, Christoudias GC, Ann. Surg 1973, 178, 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Shi M, Zhang H, Song T, Liu X, Gao Y, Zhou J, Li Y, ACS Appl. Mater. Interfaces 2019, 11, 22730. [DOI] [PubMed] [Google Scholar]
- [79].Francesko A, Petkova P, Tzanov T, Curr. Med. Chem 2018, 25, 5782. [DOI] [PubMed] [Google Scholar]
- [80].Klouda L, Eur. J. Pharm. Biopharm 2015, 97, 338. [DOI] [PubMed] [Google Scholar]
- [81].Chen C, Liu Y, Wang H, Chen G, Wu X, Ren J, Zhang H, Zhao Y, ACS Nano 2018, 12, 10493. [DOI] [PubMed] [Google Scholar]
- [82].Zhu Y, Hoshi R, Chen S, Yi J, Duan C, Galiano RD, Zhang HF, Ameer GA, Control J. Release 2016, 238, 114. [DOI] [PubMed] [Google Scholar]
- [83].Choi JS, Yoo HS, J. Biomed. Mater. Res. Part A 2010, 95, 564. [DOI] [PubMed] [Google Scholar]
- [84].Blacklow SO, Li J, Freedman BR, Zeidi M, Chen C, Mooney DJ, Sci. Adv 2019, 5, eaaw3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Dunnill C, Patton T, Brennan J, Barrett J, Dryden M, Cooke J, Leaper D, Georgopoulos NT, Int. Wound J 2017, 14, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Allen DB, Maguire JJ, Mahdavian M, Wicke C, Marcocci L, Scheuenstuhl H, Chang M, Le AX, Hopf HW, Hunt TK, Arch. Surg 1997, 132, 991. [DOI] [PubMed] [Google Scholar]
- [87].Desmet CM, Preat V, Gallez B, Adv. Drug Deliv. Rev 2018, 129, 262. [DOI] [PubMed] [Google Scholar]
- [88].Aderibigbe BA, Buyana B, Pharmaceutics 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Tang T, Jiang H, Yu Y, He F, Ji SZ, Liu YY, Wang ZS, Xiao SC, Tang C, Wang GY, Xia ZF, Int. J. Nanomed 2015, 10, 6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Ma Y, Dong WF, Hempenius MA, Mohwald H, Vancso GJ, Nat. Mater 2006, 5, 724. [DOI] [PubMed] [Google Scholar]
- [91].Broaders KE, Grandhe S, Frechet JM, J. Am. Chem. Soc 2011, 133, 756. [DOI] [PubMed] [Google Scholar]
- [92].Kim TH, Yoo DS, Kim J-C, Biotechnol. Bioprocess Eng 2019, 24, 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Chen R, Wang J, Liu C, Adv. Funct. Mater 2016, 26, 8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Mohan S, Baylink DJ, Clin. Orthop. Rel. Res 1991, 30. [PubMed] [Google Scholar]
- [95].Trippel SB, J. Rheumatol. Suppl 1995, 43, 129. [PubMed] [Google Scholar]
- [96].Paiva KB, Granjeiro JM, Arch. Biochem. Biophys 2014, 561, 74. [DOI] [PubMed] [Google Scholar]
- [97].Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, Hubbell JA, Nat. Biotechnol 2003, 21, 513. [DOI] [PubMed] [Google Scholar]
- [98].Qi H, Yang L, Li X, Sun X, Zhao J, Hou X, Li Z, Yuan X, Cui Z, Yang X, Biomater. Sci 2019, 7, 1675. [DOI] [PubMed] [Google Scholar]
- [99].Anjum F, Lienemann PS, Metzger S, Biernaskie J, Kallos MS, Ehrbar M, Biomaterials 2016, 87, 104. [DOI] [PubMed] [Google Scholar]
- [100].Holloway JL, Ma H, Rai R, Hankenson KD, Burdick JA, Macromol. Biosci 2015, 15, 1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Aimetti AA, Tibbitt MW, Anseth KS, Biomacromolecules 2009, 10, 1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Arnett TR, Dempster DW, Endocrinology 1986, 119, 119. [DOI] [PubMed] [Google Scholar]
- [103].Gan Q, Zhu J, Yuan Y, Liu H, Zhu Y, Liu C, J. Mat. Chem. B 2015, 3, 2281. [DOI] [PubMed] [Google Scholar]
- [104].Gan Q, Zhu J, Yuan Y, Liu H, Qian J, Li Y, Liu C, J. Mat. Chem. B 2015, 3, 2056. [DOI] [PubMed] [Google Scholar]
- [105].Gan Q, Zhu J, Yuan Y, Liu C, J. Nanosci. Nanotechnol 2016, 16, 5470. [DOI] [PubMed] [Google Scholar]
- [106].Smith E, Yang J, McGann L, Sebald W, Uludag H, Biomaterials 2005, 26, 7329. [DOI] [PubMed] [Google Scholar]
- [107].Santoveña A, Monzón C, Alvarez-Lorenzo C, del Rosario C, Delgado A, Evora C, Concheiro A, Llabrés M, Fariña JB, J. Pharm. Sci 2017, 106, 3353. [DOI] [PubMed] [Google Scholar]
- [108].Segredo-Morales E, García-García P, Reyes R, Pérez-Herrero E, Delgado A, Évora C, Int. J. Pharm 2018, 543, 160. [DOI] [PubMed] [Google Scholar]
- [109].Lavrovsky Y, Chatterjee B, Clark RA, Roy AK, Exp. Gerontol 2000, 35, 521. [DOI] [PubMed] [Google Scholar]
- [110].Filippin LI, Vercelino R, Marroni NP, Xavier RM, Clin. Exp. Immunol 2008, 152, 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Gong T, Liu T, Zhang L, Ye W, Guo X, Wang L, Quan L, Pan C, ACS Biomater. Sci. Eng 2017, 4, 240. [DOI] [PubMed] [Google Scholar]
- [112].Yang F, Wang J, Cao L, Chen R, Tang L, Liu C, J. Mat. Chem. B 2014, 2, 295. [DOI] [PubMed] [Google Scholar]
- [113].Crasto GJ, Kartner N, Reznik N, Spatafora MV, Chen H, Williams R, Burns PN, Clokie C, Manolson MF, Peel SA, Control J. Release 2016, 243, 99. [DOI] [PubMed] [Google Scholar]
- [114].Matsuo T, Sugita T, Kubo T, Yasunaga Y, Ochi M, Murakami T, J. Biomed. Mater. Res. Part A 2003, 66, 747. [DOI] [PubMed] [Google Scholar]
- [115].Tanaka H, Sugita T, Yasunaga Y, Shimose S, Deie M, Kubo T, Murakami T, Ochi M, J. Biomed. Mater. Res. Part A 2005, 73, 255. [DOI] [PubMed] [Google Scholar]
- [116].Correia CR, Gil S, Reis RL, Mano JF, Adv. Healthc. Mater 2016, 5, 1346. [DOI] [PubMed] [Google Scholar]
- [117].Collins, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE, Cell 2005, 122, 289. [DOI] [PubMed] [Google Scholar]
- [118].Lee DE, Bareja A, Bartlett DB, White JP, Cells 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Baghdadi MB, Tajbakhsh S, Dev. Biol 2018, 433, 200. [DOI] [PubMed] [Google Scholar]
- [120].Nakayama KH, Shayan M, Huang NF, Adv. Healthc. Mater 2019, 8, e1801168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Ferrini A, Stevens MM, Sattler S, Rosenthal N, Front. Cardiovasc. Med 2019, 6, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zhang BH, Guo CX, Wang HX, Lu LQ, Wang YJ, Zhang LK, Du FH, Zeng XJ, Heart Vessels 2014, 29, 679. [DOI] [PubMed] [Google Scholar]
- [123].Hadjipanayi E, Cheema U, Hopfner U, Bauer A, Machens HG, Schilling AF, Control J. Release 2012, 161, 852. [DOI] [PubMed] [Google Scholar]
- [124].Pedron S, van Lierop S, Horstman P, Penterman R, Broer DJ, Peeters E, Adv. Funct. Mater 2011, 21, 1624. [Google Scholar]
- [125].Salimath AS, Phelps EA, Boopathy AV, Che PL, Brown M, Garcia AJ, Davis ME, PLoS One 2012, 7, e50980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Prabhath A, Vernekar VN, Sanchez E, Laurencin CT, Int. J. Pharm 2018, 544, 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Moris D, Spartalis M, Spartalis E, Karachaliou GS, Karaolanis GI, Tsourouflis G, Tsilimigras DI, Tzatzaki E, Theocharis S, Ann. Transl. Med 2017, 5, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Subbiah R, Guldberg RE, Adv. Healthc. Mater 2019, 8, e1801000. [DOI] [PubMed] [Google Scholar]
- [129].Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, Vandenburgh HH, Mooney DJ, Proc. Natl. Acad. Sci. U. S. A 2010, 107, 3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Fan C, Shi J, Zhuang Y, Zhang L, Huang L, Yang W, Chen B, Chen Y, Xiao Z, Shen H, Zhao Y, Dai J, Adv. Mater 2019, 31, e1902900. [DOI] [PubMed] [Google Scholar]
- [131].Prokoph S, Chavakis E, Levental KR, Zieris A, Freudenberg U, Dimmeler S, Werner C, Biomaterials 2012, 33, 4792. [DOI] [PubMed] [Google Scholar]
- [132].Braun AC, Gutmann M, Ebert R, Jakob F, Gieseler H, Luhmann T, Meinel L, Pharm. Res 2017, 34, 58. [DOI] [PubMed] [Google Scholar]
- [133].Pompe T, Salchert K, Alberti K, Zandstra P, Werner C, Nat. Protoc 2010, 5, 1042. [DOI] [PubMed] [Google Scholar]
- [134].Garbern JC, Minami E, Stayton PS, Murry CE, Biomaterials 2011, 32, 2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Namgung R, Nam S, Kim SK, Son S, Singha K, Kwon JS, Ahn Y, Jeong MH, Park IK, Garripelli VK, Jo S, Kim WJ, Biomaterials 2009, 30, 5225. [DOI] [PubMed] [Google Scholar]
- [136].Silva AKA, Richard C, Bessodes M, Scherman D, Merten O-W, Biomacromolecules 2008, 10, 9. [DOI] [PubMed] [Google Scholar]
- [137].Ramos PE, Silva P, Alario MM, Pastrana LM, Teixeira JA, Cerqueira MA, Vicente AA, Food Hydrocolloids 2018, 77, 8. [Google Scholar]
- [138].Wang L, Cao L, Shansky J, Wang Z, Mooney D, Vandenburgh H, Mol. Ther 2014, 22, 1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Wang L, Shansky J, Borselli C, Mooney D, Vandenburgh H, Tissue Eng. Part A 2012, 18, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Carmeliet P, Nat. Med 2000, 6, 389. [DOI] [PubMed] [Google Scholar]
- [141].Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC, Nature 1997, 386, 403. [DOI] [PubMed] [Google Scholar]
- [142].Schaper W, Ito WD, Circ.Res 1996, 79, 911. [DOI] [PubMed] [Google Scholar]
- [143].Eltzschig HK, Eckle T, Nat. Med 2011, 17, 1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Parks WC, Wilson CL, Lopez-Boado YS, Nat. Rev. Immunol 2004, 4, 617. [DOI] [PubMed] [Google Scholar]
- [145].Banfi A, Holnthoner W, Martino MM, Yla-Herttuala S, Front. Bioeng. Biotechnol 2018, 6, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Wen J, Anderson SM, Du J, Yan M, Wang J, Shen M, Lu Y, Segura T, Adv. Mater 2011, 23, 4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Zhu S, Nih L, Carmichael ST, Lu Y, Segura T, Adv. Mater 2015, 27, 3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Van Hove AH, Antonienko E, Burke K, Brown E 3rd, Benoit DS, Adv. Healthc. Mater 2015, 4, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Garbern JC, Hoffman AS, Stayton PS, Biomacromolecules 2010, 11, 1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Adibfar A, Amoabediny G, Baghaban Eslaminejad M, Mohamadi J, Bagheri F, Zandieh Doulabi B, Mater. Sci. Eng. C-Mater. Biol. Appl 2018, 93, 790. [DOI] [PubMed] [Google Scholar]
- [151].Lee DJ, Rocker AJ, Bardill JR, Shandas R, Park D, J. Biomed. Mater. Res. Part A 2018, 106, 3053. [DOI] [PubMed] [Google Scholar]
- [152].Yamaguchi N, Zhang L, Chae BS, Palla CS, Furst EM, Kiick KL, J. Am. Chem. Soc 2007, 129, 3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Lee KY, Peters MC, Anderson KW, Mooney DJ, Nature 2000, 408, 998. [DOI] [PubMed] [Google Scholar]
- [154].Moncion A, Lin M, Kripfgans OD, Franceschi RT, Putnam AJ, Fabiilli ML, Ultrasound Med. Biol 2018, 44, 2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Huang EJ, Reichardt LF, Annu. Rev. Neurosci 2001, 24, 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Aloe L, Rocco ML, Bianchi P, Manni L, J. Transl. Med 2012, 10, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Zhong Y, Bellamkonda RV, Soc JR. Interface 2008, 5, 957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Johnson PJ, Tatara A, Shiu A, Sakiyama-Elbert SE, Cell Transplant. 2010, 19, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Sakiyama-Elbert SE, Hubbell JA, Control J. Release 2000, 65, 389. [DOI] [PubMed] [Google Scholar]
- [160].Li Y, Wang F, Cui H, Bioeng. Transl. Med 2016, 1, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Galler KM, Aulisa L, Regan KR, D’Souza RN, Hartgerink JD, J. Am. Chem. Soc 2010, 132, 3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Song B, Song J, Zhang S, Anderson MA, Ao Y, Yang CY, Deming TJ, Sofroniew MV, Biomaterials 2012, 33, 9105. [DOI] [PubMed] [Google Scholar]
- [163].Lindsey S, Piatt JH, Worthington P, Sonmez C, Satheye S, Schneider JP, Pochan DJ, Langhans SA, Biomacromolecules 2015, 16, 2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Friden PM, Walus LR, Watson P, Doctrow SR, Kozarich JW, Backman C, Bergman H, Hoffer B, Bloom F, Granholm AC, Science 1993, 259, 373. [DOI] [PubMed] [Google Scholar]
- [165].Xu D, Wu D, Qin M, Nih LR, Liu C, Cao Z, Ren J, Chen X, He Z, Yu W, Guan J, Duan S, Liu F, Liu X, Li J, Harley D, Xu B, Hou L, Chen ISY, Wen J, Chen W, Pourtaheri S, Lu Y, Adv. Mater 2019, 31, e1900727. [DOI] [PubMed] [Google Scholar]
- [166].Jain A, Kim YT, McKeon RJ, Bellamkonda RV, Biomaterials 2006, 27, 497. [DOI] [PubMed] [Google Scholar]
- [167].Gupta D, Tator CH, Shoichet MS, Biomaterials 2006, 27, 2370. [DOI] [PubMed] [Google Scholar]
- [168].Stabenfeldt SE, Garcia AJ, LaPlaca MC, J. Biomed. Mater. Res. Part A 2006, 77, 718. [DOI] [PubMed] [Google Scholar]
- [169].Chen FM, Zhang M, Wu ZF, Biomaterials 2010, 31, 6279. [DOI] [PubMed] [Google Scholar]
- [170].Richardson TP, Peters MC, Ennett AB, Mooney DJ, Nat. Biotechnol 2001, 19, 1029. [DOI] [PubMed] [Google Scholar]
- [171].Ashammakhi N, Ahadian S, Darabi MA, El Tahchi M, Lee J, Suthiwanich K, Sheikhi A, Dokmeci MR, Oklu R, Khademhosseini A, Adv. Mater 2019, 31, e1804041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Sun W, Lee J, Zhang S, Benyshek C, Dokmeci MR, Khademhosseini A, Adv. Sci 2019, 6, 1801039. [DOI] [PMC free article] [PubMed] [Google Scholar]


