Abstract
Patellar instability is common in the second decade, and genu valgum is a risk factor for patellar instability. In skeletally immature patients, genu valgum can be gradually corrected using less-invasive, well-established, growth-modulation techniques. For skeletally immature patients with patellar instability and genu valgum, it would be desirable to address both instability and deformity. We describe our technique of physeal-respecting medial patellofemoral ligament reconstruction in skeletally immature patients using hamstring autograft and simultaneous transphyseal screw hemiepiphysiodesis to gradually correct genu valgum. The medial patellofemoral ligament reconstruction technique features posteromedial hamstring graft harvest, single patellar tunnel fixation without implant, and femoral attachment just below the distal femoral physis. The technique of growth modulation features percutaneous insertion of a single transphyseal screw through the distal medial femoral physis without interference with medial patellofemoral ligament graft placement. Our preliminary results have been encouraging with successful correction of genu valgum and satisfactory patellar stabilization without growth disturbances.
Introduction (With Video Illustration)
The risk of patellar instability is greatest in the 10- the 17-year age group, with an incidence of approximately 43 per 100,000 children per year. Several techniques have been described to address patellar instability in skeletally immature patients.1 Medial patellofemoral ligament (MPFL) reconstruction has shown promising results, but there is a 10% to 20% complication rate and an added risk for physeal injury in skeletally immature patients.2,3 Thus, MPFL reconstruction in skeletally immature patients would require technical modifications to avoid these complications.
There are several risk factors that contribute to patellar instability; increased genu valgum is one such risk factor that increases the laterally directed force and vector acting on the patella.4 Correction of increased genu valgum could be achieved by growth modulation in skeletally immature patients with at least a year of remaining growth.5,6 Our indications for distal femoral growth modulation are genu valgum >10°, mechanical axis in or outside the lateral knee compartment, and a mechanical lateral distal femoral angle <84° in a skeletally immature patient. The aim of the current report is to describe our technique of MPFL reconstruction (Video 1) and simultaneous growth modulation for correction of genu valgum in skeletally immature patients with recurrent patellar instability.
Surgical Technique
Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Preoperative Planning
Knee radiographs and magnetic resonance imaging scans are evaluated for anatomic risk factors, MPFL tear pattern, and chondral injuries (Figs 1 and 2). A full-length, hip-to-ankle, standing radiograph is obtained with the patella facing forward. Deformity assessment is done to identify the source of genu valgum (Fig 3). A left-hand radiograph is obtained to assess skeletal age (Fig 4).
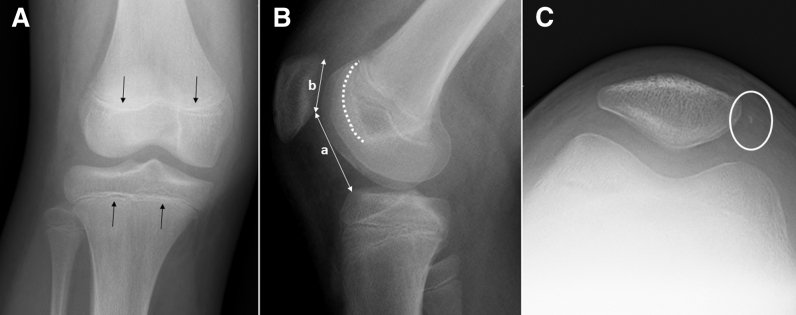
Fig 1.
Right knee radiographs including (A) anteroposterior, (B) lateral, and (C) Merchant view are obtained. The physis (black arrows) are open. Anatomic risk factors including trochlear dysplasia (white dashed line) and patellar height (a/b) are evaluated. The medial rim avulsion fracture of patella (circle) is common after patellar dislocation and does not need treatment.
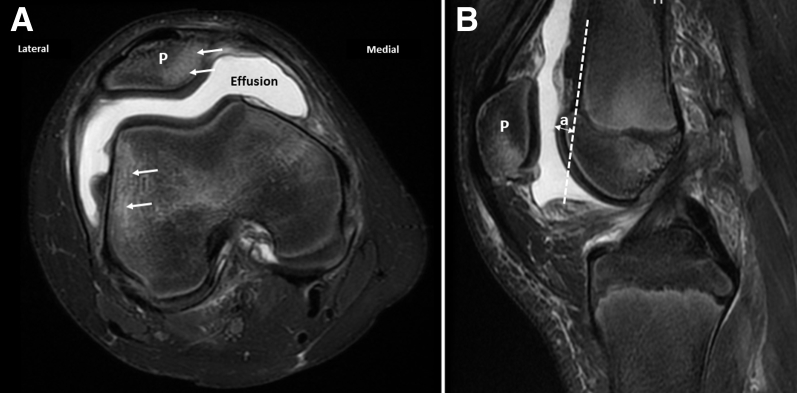
Fig 2.
Axial (A) and sagittal (B) T2-weighted magnetic resonance imaging views of the right knee show joint effusion and characteristic bruise pattern for patellar dislocation (arrows). Trochlear bump is calculated as the perpendicular distance “a” between the anterior femoral cortical line and anterior-most aspect of trochlea on mid-sagittal slice. (P, patella.)
Fig 3.
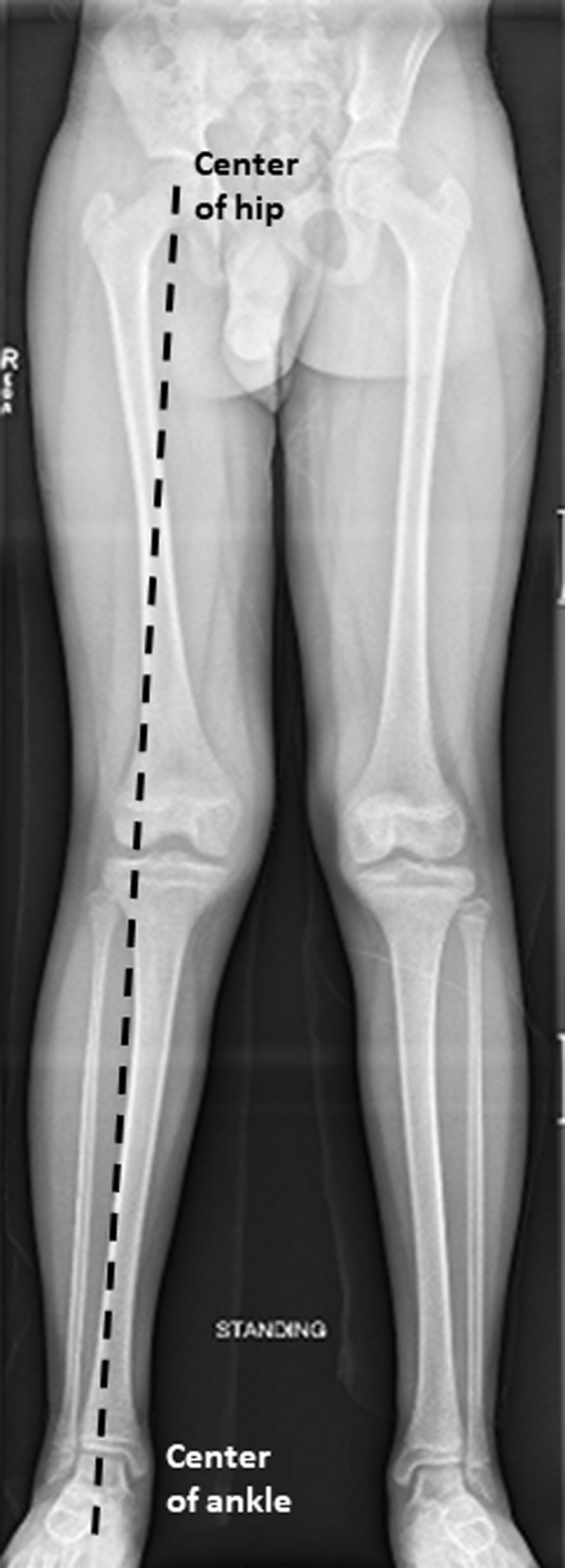
A full-length standing radiograph shows valgus alignment of the right leg with the mechanical axis (dashed line from the center of the hip to the center of the ankle) in the lateral compartment.
Fig 4.
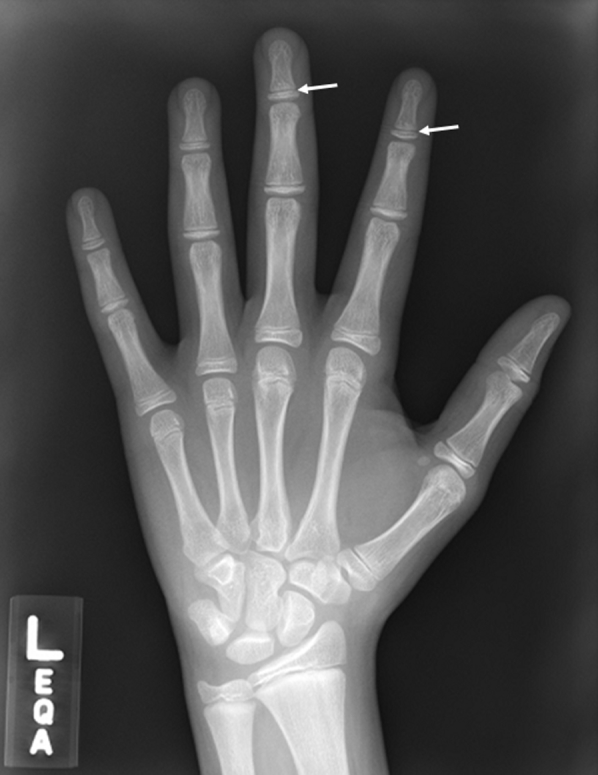
Left-hand radiograph shows open distal phalangeal physis (arrows), which indicates that the peak height velocity is not reached and patient has at least 2 years of remaining growth.
Patient Positioning
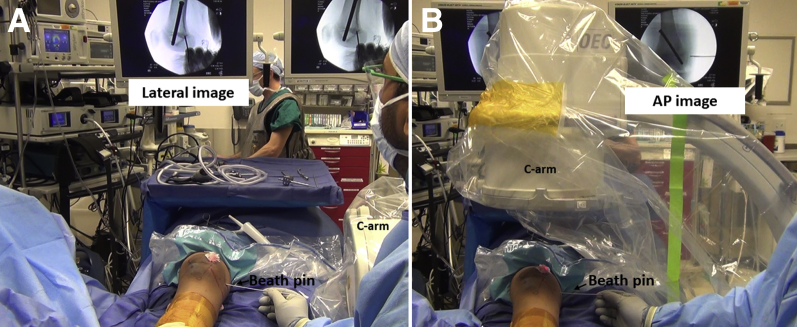
The patient is positioned supine on a radiolucent table with a tourniquet over the proximal thigh. A leg elevator (Bone Foam Inc., Corcoran, MN) under the involved leg can facilitate lateral knee radiographs (Fig 5). General anesthesia and a preoperative femoral and sciatic nerve block are performed by the anesthesiologist. An examination of the knee is performed to evaluate the medial–lateral translation of the patella in extension and 30° flexion.
Fig 5.
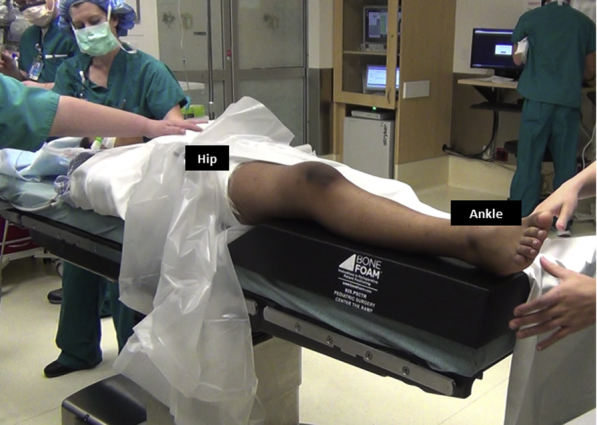
The patient is positioned supine on a radiolucent table with a well-padded tourniquet and a leg elevator under the right knee/leg to facilitate appropriate lateral radiographs.
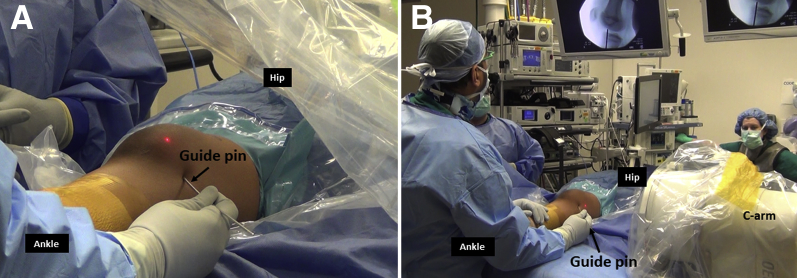
Medial Hemiepiphysiodesis
Under anteroposterior fluoroscopy, a 2.8-mm guide pin is inserted percutaneously in a retrograde direction from medial aspect of medial femoral condyle, avoiding the articular surface to intersect the distal femoral physis at its medial third–middle third junction (Figs 6 and 7, Table 1). On the lateral view, the guide pin should intersect the physis at its center and should be placed along the mid-coronal plane of the distal femur. The pin is passed to exit the lateral aspect of thigh and withdrawn from the lateral aspect of thigh until its tip is just inside the medial femoral condyle. A 1-cm longitudinal skin incision is placed around the pin and screw length is measured. The pin is overdrilled with a 4.5-mm drill bit, and a 7.0-mm fully-threaded, self-tapping, cannulated cancellous screw is inserted with at least 5 screw threads crossing the physis. Alternatively, a 6.5-mm cancellous screw can be used.
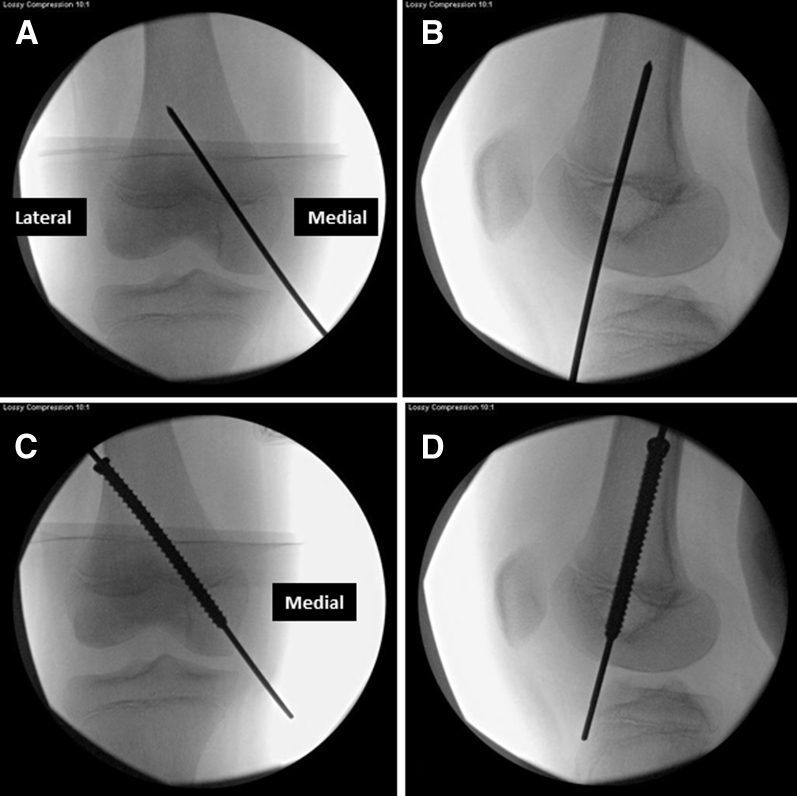
Fig 6.
Medial aspect of right knee. A 2.8-mm guide pin for transphyseal screw (arrow) is placed percutaneously in a retrograde direction from medial femoral condyle of the right knee. Its placement is checked on anteroposterior (A) and lateral (B) fluoroscopic images.
Fig 7.
Fluoroscopic images of right knee. The retrograde guide pin inserted from medial femoral condyle should intersect the distal femoral physis of the knee at its medial third–middle third junction on anteroposterior (A) view and the center of the physis on the lateral (B) view. The guide pin would exit from the lateral thigh and then it is overdrilled. A 7-mm fully threaded cannulated screw is inserted over guide pin in an antegrade direction and its position is confirmed on anteroposterior (C) and lateral (D) view.
Table 1.
Pearls and Pitfalls
| Pearls |
|
|
|
|
|
|
|
|
|
|
|
| Pitfalls |
|
|
|
|
|
|
|
MPFL, medial patellofemoral ligament.
Gracilis Tendon Harvest
With the hip in flexion and abduction and the knee in about 30° of flexion, a 2.5-cm transverse incision is placed in the medial aspect of popliteal crease over the palpable gracilis tendon. Dissection is performed toward the tendon and the fascia is opened along the tendon. A right-angle clamp is used to deliver the tendon out of the incision and it is freed of its attachments (Fig 8). An open-tendon harvester is used to detach the tendon from its tibial insertion distally and a closed-tendon harvester is used to detach the tendon proximally at its musclotendinous junction. The gracilis tendon is wrapped in a saline-soaked sponge for later use.
Fig 8.
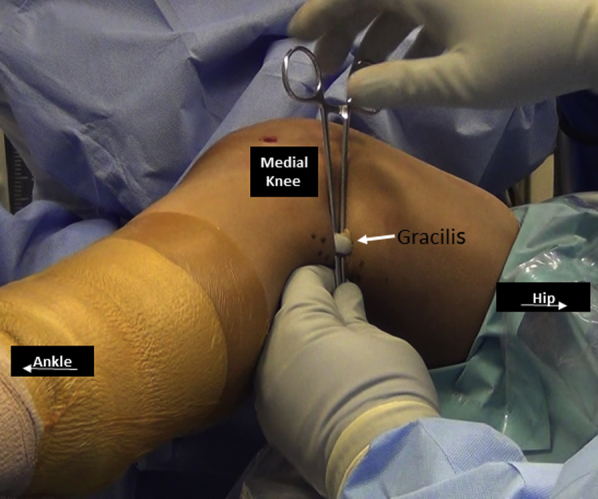
Posteromedial aspect of right knee. The gracilis tendon (arrow) is harvested through the posteromedial approach.
Knee Arthroscopy
Diagnostic knee arthroscopy is performed using standard portals (Fig 9). Patellofemoral tracking is assessed. Any intra-articular lesions are addressed at this time. Arthroscopy from superolateral portal can provide better assessment of patellofemoral tracking.
Fig 9.
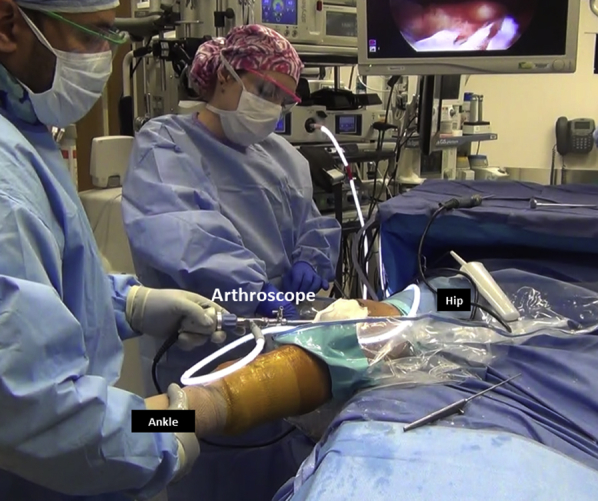
Diagnostic arthroscopy of right knee is performed with the arthroscope in the anterolateral portal. Patellofemoral tracking and chondral lesions are evaluated and treated as necessary.
Patellar Tunnel
A 3- to 4-cm para-patellar incision is made along the medial border of patella and dissection is performed down to the patella (Fig 10). The vastus medialis muscle fibers are identified (first layer). A subperiosteal dissection is performed using an electrocautery over the medial 5 mm of patella just above the widest part of patella. The plane between the medial retinaculum (second layer) and the capsule (third layer) is developed by cutting the transverse fibers of medial retinaculum.
Fig 10.
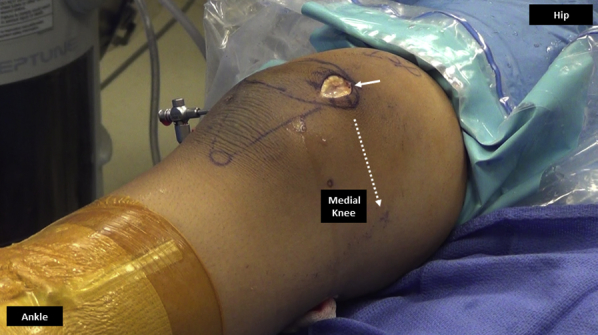
Medial aspect of right knee. A 3-cm medial parapatellar incision (solid arrow) is made along the medial border of the patella and dissection is performed medially toward the medial epicondyle (dashed arrow) to create a plane between the second and third layer of the knee.
The patellar tunnel is made just above the widest part of patella (center of superior two-thirds of patella). Two 1-cm drill holes are made using a 3.5-mm drill bit; first from medial to lateral and then from anterior to posterior to create a single 3.5-mm tunnel under a bone bridge (Fig 11). A curved curette is used to chamfer the tunnel and remove bone debris. A suture needle (0 VICRYL on OS-6 needle; Ethicon, Somerville, NJ) is passed through the tunnel and its suture end is looped to help graft passage. The thinner musclotendinous end of the gracilis tendon is passed and pulled through the patellar tunnel. Both tendon ends are whip stitched with a #2 FiberWire suture (Arthrex, Naples, FL) (Fig 12). The doubled graft is sized using a graft sizer.
Fig 11.
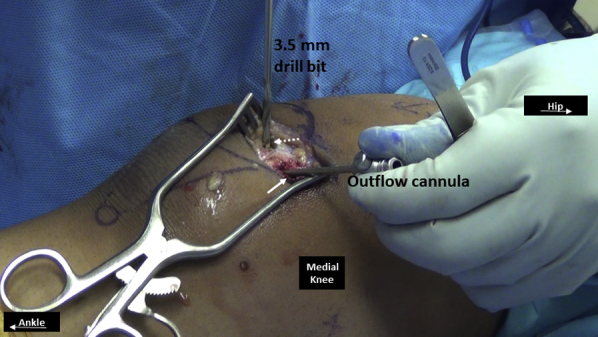
Medial side of right knee. A medial to lateral (solid arrow) and then an anterior to posterior (dashed arrow) tunnel is established using a 3.5-mm drill bit such that the tunnels converge to form a single patellar tunnel under a bone bridge. An outflow cannula or similar instrument is placed in the medial–lateral tunnel to identify its trajectory and facilitate safe anterior-posterior drilling.
Fig 12.
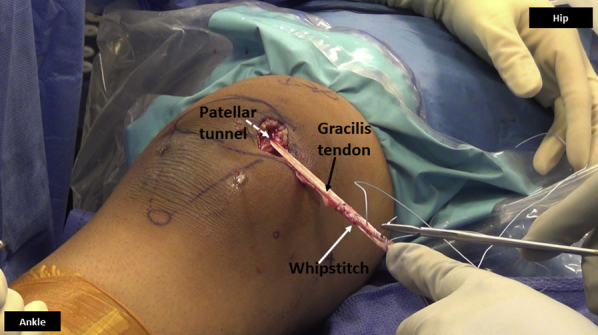
Medial side of right knee. Both ends of the gracilis tendon (black arrow) are sutured together in a whipstitch fashion (white arrow) using nonabsorbable suture, once the tendon is passed through the patellar tunnel (dashed arrow).
Femoral Tunnel
On a perfect lateral fluoroscopic view with overlap of the femoral condyles, a 2-mm Beath pin is placed percutaneously at the femoral attachment point at the level of the distal femoral physis, just anterior to the posterior femoral cortex, and inserted about 1 cm using a mallet7 (Fig 13). The position of the pin should be confirmed to be below the level of distal femoral physis on an anteroposterior view. The Beath pin is then advanced through the medial femoral epiphysis, under fluoroscopic guidance, from medial to lateral, posterior to anterior (to avoid the intercondylar notch) and inferior to the physis to exit from the lateral aspect of the knee. On the lateral view, the pin should be placed between the Blumensaat line and the transphyseal screw (Fig 14).
Fig 13.
For femoral tunnel position, a Beath pin (arrow) is placed under the guidance of lateral (A) fluoroscopic imaging of right knee. The pin is placed just anterior to the posterior femoral cortical line and at the level of the distal femoral physis. Its position is confirmed to be below the level of the distal femoral physis on an AP (B) image. (AP, anteroposterior.)
Fig 14.
Fluoroscopic image of right knee. The position of Beath pin for femoral attachment of medial patellofemoral ligament on anteroposterior (A) and lateral (B) images of knee. On the lateral view, both femoral condyles should precisely overlap posteriorly and distally (solid arrow). The pin is directed anteriorly in the triangle formed by the Blumensaat line, distal femoral physis, and the transphyseal screw. A hemostat (dashed arrow) marks the entrance of the Beath pin.
A 1-cm longitudinal incision is placed around the pin. Using a Kelly clamp and suture loop, the graft is shuttled between the second and third layer and out of the medial incision. An isometric assessment should be confirmed by wrapping the graft around the pin and putting the knee through flexion and extension to check for graft length changes (Fig 15). The Beath pin is overdrilled with an appropriate size reamer (5-6 mm) up to the opposite cortex. A nitinol guide wire for interference screw is inserted into the drilled tunnel alongside the Beath pin (Fig 16).
Fig 15.
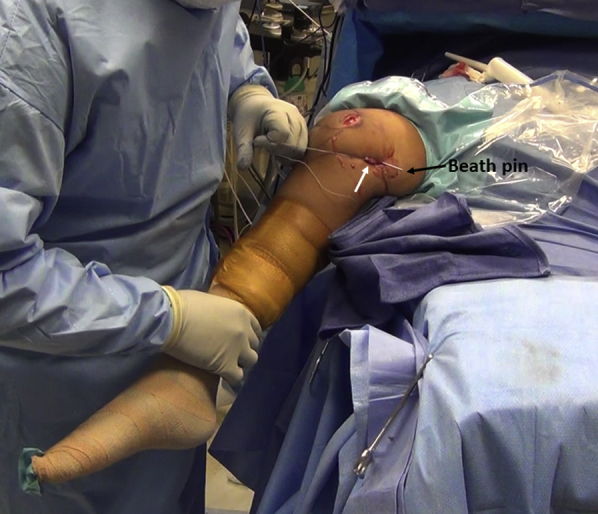
Medial side of right knee. The medial patellofemoral ligament graft (white arrow) is looped around the Beath pin (black arrow) and an isometric assessment of the graft is performed by knee flexion and extension and any graft length changes are noted.
Fig 16.
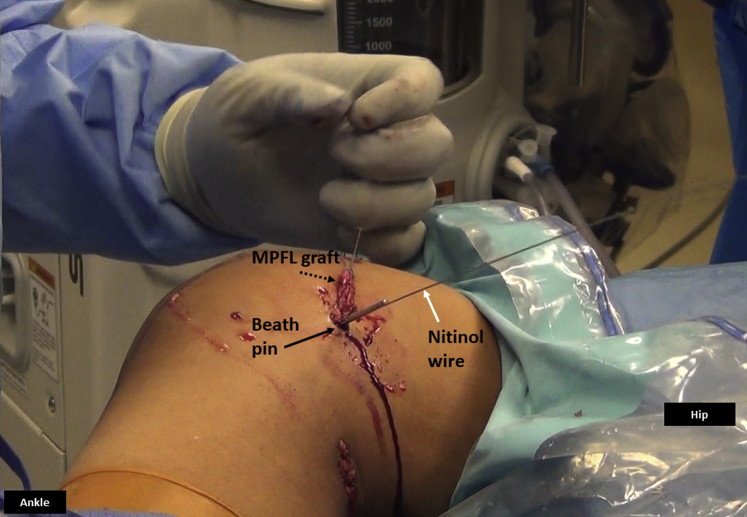
Medial side of the right knee. A nitinol wire (white arrow) is inserted in the femoral tunnel along the Beath pin (black arrow) to facilitate interference screw placement. The MPFL graft (dashed arrow) is ready to be placed in the femoral tunnel using pull-through sutures placed in the eyelet of the Beath pin. (MPFL, medial patellofemoral ligament.)
Graft Passage and Fixation
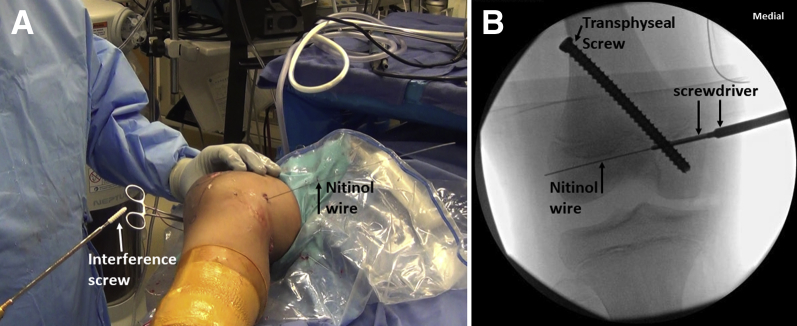
The suture ends of the graft are placed through the eyelet of the Beath pin, which is pulled out from the lateral side pulling the graft into the femoral tunnel. With the knee in about 45° flexion and the patella engaged in the trochlea, the suture ends of the graft are clamped on the lateral side of the knee against the skin (Fig 17). Patellar mobility and tracking with knee flexion and extension is confirmed. Knee arthroscopy is performed to verify the extra-articular position and functioning of the graft (Fig 18). A biocomposite interference screw (Matryx; ConMed, Utica, NY) is inserted over the nitinol wire. The size of the screw is the same as femoral tunnel size and is 25 to 30 mm in length. The complete insertion of the screw is confirmed by fluoroscopic visualization of the screwdriver tip (Fig 19).
Fig 17.
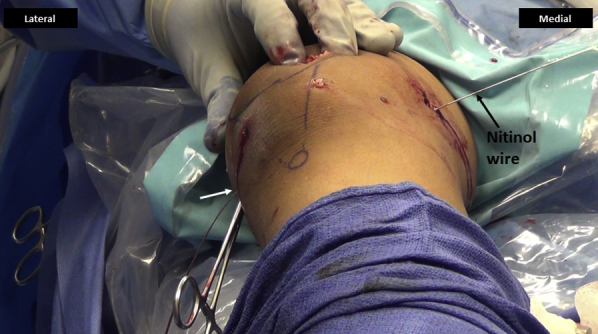
Right knee, seen from the foot end. The graft is passed in the femoral tunnel and clamped with a hemostat (white arrow) against the skin on the lateral side with the knee in about 45° of flexion. This would approximate final fixation and functioning of the graft. Nitinol wire (black arrow) is maintained in the femoral tunnel to facilitate percutaneous interference screw fixation.
Fig 18.
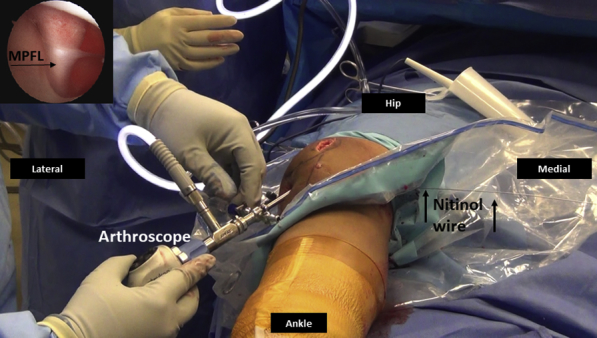
Right knee arthroscopy, viewing from anterolateral portal. The arthroscopy is performed to verify the extracapsular position and functioning of the MPFL graft. The MPFL graft is typically taut in extension (inset) and it would relax with flexion as the patella is constrained by trochlea. Nitinol wire (black arrows) is maintained in the femoral tunnel. (MPFL, medial patellofemoral ligament.)
Fig 19.
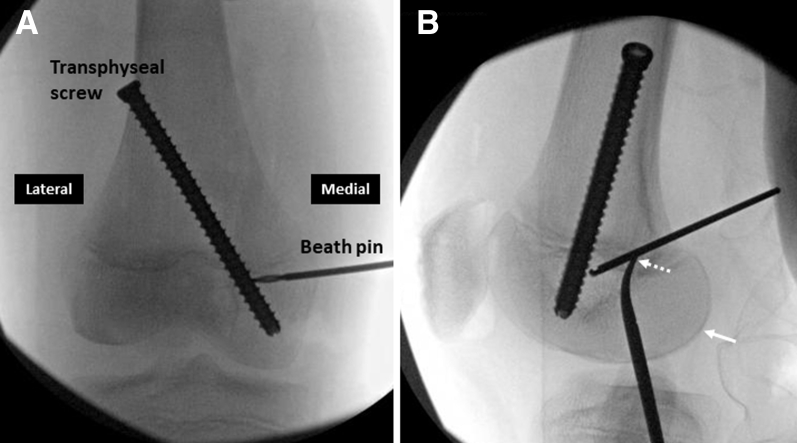
Right knee. (A) An interference screw (white arrow) is inserted over the nitinol guide (black arrow) wire and its complete insertion is confirmed by fluoroscopic visualization of the screwdriver tip (B). The relationship between the transphyseal screw and interference screw can be seen.
Closure
The closure of medial retinaculum is performed with the knee flexed to prevent overtightening of medial structures. The medial retinaculum is imbricated in a pants-over-vest fashion using 2 to 3 absorbable sutures. All 3 incisions and portals are closed in a standard fashion. A well-padded dressing is applied, along with CryoCuff (DJO, Vista, CA) and a knee immobilizer.
Postoperative Course
Physical therapy is started within 3 to 4 days once the pain is under control. The patient is allowed weight bearing as tolerated in a knee immobilizer with crutches for 3 to 4 weeks, until quadriceps function is regained. Range of motion goals are 0 to 90° at 3 weeks and 0 to 120° at 6 weeks. Strengthening is initiated after 3 months. Functional strength tests including hop tests and isokinetic dynamometer (Biodex Medical Systems, Shirley, NY) tests are performed between 4 and 6 months. Patients are released to full activities, including sports, typically at around 6 months, once all functional goals are achieved in physical therapy.
Discussion
Pediatric cadaveric studies have demonstrated that the MPFL femoral attachment is predominantly below the level of the distal femoral physis.8 The trajectory of the femoral tunnel is distal and inferior to the distal femoral physis and anterior to the intercondylar notch. This would allow a pull-out technique for the femoral guide pin and graft sutures.9 Careful fluoroscopic guidance is critical during creation of femoral tunnel. We have not encountered any growth disturbances using this MPFL reconstruction technique in 79 skeletally immature patients. The alternate technique described in the literature has been to create a short and angled socket in the medial femoral epiphysis to avoid the physis and intercondylar notch penetration.9
The advantage of implant-mediated guided growth is that it does not require accurate growth prediction, as removal of the screw after deformity correction should restore normal growth (Table 2). The advantage of transphyseal screw, as compared with a tension-band plate for growth modulation, is that the screw would not interfere with the MPFL graft placement on the medial aspect of the knee. It would also avoid knee joint irritation, stiffness, and capsular penetration that could be seen with a plate. The disadvantage of transphyseal screw is its potential to damage the physis and cause growth disturbances. The transphyseal screw is inserted first, to ensure safety of the MPFL graft and sutures. We recommend full-length radiographs every 3 to 4 months until expected correction is achieved.
Table 2.
Advantages, Disadvantages, Risks, and Limitations
|
|
|
|
|
|
|
|
|
MPFL, medial patellofemoral ligament.
The current technique of MPFL reconstruction and simultaneous growth modulation is relatively simple to use and can correct an anatomic risk factor for patellar instability. Our experience has been reported with good results and minimal complications.6 The technique can be added to the surgical armamentarium when treating recurrent patellar instability in skeletally immature patients with genu valgum.
Footnotes
The authors report that they have no conflicts of interest in the authorship and publication of this article. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
The video demonstrates the surgical technique of simultaneous MPFL reconstruction and medial hemiepiphysiodesis to address right-sided lateral patellar instability and genu valgum in a 12-year-old skeletally immature boy. Preoperative workup includes knee radiographs, full-length standing radiograph, left-hand radiograph, and magnetic resonance imaging. Patient is positioned supine on a radiolucent table with a bone foam under the involved leg. The medial hemiepiphysiodesis procedure is performed first. A 2.8-mm guide pin is inserted in a retrograde fashion from medial femoral condyle to exit the lateral aspect of thigh, such that it intersects the distal femoral physis at medial one-third, lateral two-third junction on anteroposterior view, and center of the distal femoral physis on the lateral view. A 4.5-mm drill bit, followed by a 7-mm full-threaded cancellous screw is inserted over the guide pin in an antegrade fashion. The gracilis autograft is then harvested from the posteromedial knee approach. Knee arthroscopy is performed using standard portals. Patellar tracking is evaluated and any chondral lesions are addressed. A medial incision is then placed over the patella and a single 3.5-mm tunnel is created in the patella. The gracilis graft is placed through this tunnel and both ends are whipstitched using nonabsorbable sutures. The femoral attachment point is identified using fluoroscopic images and a Beath pin is placed just distal to the distal femoral physis. The graft is placed between layer 2 and layer 3 on the medial aspect of the knee. Once isometric assessment is performed, the Beath pin is over-drilled with a 5.5-mm reamer and a nitinol wire is placed in the femoral tunnel. The graft is placed in the femoral tunnel using pull-through sutures. The knee is flexed and the sutures are clamped on the lateral aspect of the knee. Clinical and arthroscopic evaluation is performed to check the MPFL graft placement and function. Final femoral fixation is performed using 5.5-mm × 25-mm biocomposite interference screw inserted over the nitinol wire. A pants-over-vest closure is performed to imbricate the medial retinacular tissues. Layered closure is performed and well-padded dressing is applied. A knee immobilizer is placed. (MPFL, medial patellofemoral ligament.)
References
- 1.Vavken P., Wimmer M.D., Camathias C., Quidde J., Valderrabano V., Pagenstert G. Treating patella instability in skeletally immature patients. Arthroscopy. 2013;29:1410–1422. doi: 10.1016/j.arthro.2013.03.075. [DOI] [PubMed] [Google Scholar]
- 2.Seitlinger G., Moroder P., Fink C., Wierer G. Acquired femoral flexion deformity due to physeal injury during medial patellofemoral ligament reconstruction. Knee. 2017;24:680–685. doi: 10.1016/j.knee.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Parikh S.N., Nathan S.T., Wall E.J., Eismann E.A. Complications of medial patellofemoral ligament reconstruction in young patients. Am J Sports Med. 2013;41:1030–1038. doi: 10.1177/0363546513482085. [DOI] [PubMed] [Google Scholar]
- 4.Mizuno Y., Kumagai M., Mattessich S.M. Q-angle influences tibiofemoral and patellofemoral kinematics. J Orthop Res. 2001;19:834–840. doi: 10.1016/S0736-0266(01)00008-0. [DOI] [PubMed] [Google Scholar]
- 5.Métaizeau J.P., Wong-Chung J., Bertrand H., Pasquier P. Percutaneous epiphysiodesis using transphyseal screws (PETS) J Pediatr Orthop. 1998;18:363–369. [PubMed] [Google Scholar]
- 6.Parikh S.N., Redman C., Gopinathan N.R. Simultaneous treatment for patellar instability and genu valgum in skeletally immature patients: A preliminary study. J Pediatr Orthop B. 2019;28:132–138. doi: 10.1097/BPB.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 7.Huston K.L., Okoroafor U.C., Kaar S.G., Wentt C.L., Saluan P., Farrow L.D. Evaluation of the Schöttle technique in the pediatric knee. Orthop J Sports Med. 2017;5 doi: 10.1177/2325967117740078. 2325967117740078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shea K.G., Polousky J.D., Jacobs J.C., Jr. The relationship of the femoral physis and the medial patellofemoral ligament in children: A cadaveric study. J Pediatr Orthop. 2014;34:808–813. doi: 10.1097/BPO.0000000000000165. [DOI] [PubMed] [Google Scholar]
- 9.Nelitz M., Reichel H., Dornacher D., Lippacher S. Anatomical reconstruction of the medial patellofemoral ligament in children with open growth-plates. Arch Orthop Trauma Surg. 2012;132:1647–1651. doi: 10.1007/s00402-012-1593-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The video demonstrates the surgical technique of simultaneous MPFL reconstruction and medial hemiepiphysiodesis to address right-sided lateral patellar instability and genu valgum in a 12-year-old skeletally immature boy. Preoperative workup includes knee radiographs, full-length standing radiograph, left-hand radiograph, and magnetic resonance imaging. Patient is positioned supine on a radiolucent table with a bone foam under the involved leg. The medial hemiepiphysiodesis procedure is performed first. A 2.8-mm guide pin is inserted in a retrograde fashion from medial femoral condyle to exit the lateral aspect of thigh, such that it intersects the distal femoral physis at medial one-third, lateral two-third junction on anteroposterior view, and center of the distal femoral physis on the lateral view. A 4.5-mm drill bit, followed by a 7-mm full-threaded cancellous screw is inserted over the guide pin in an antegrade fashion. The gracilis autograft is then harvested from the posteromedial knee approach. Knee arthroscopy is performed using standard portals. Patellar tracking is evaluated and any chondral lesions are addressed. A medial incision is then placed over the patella and a single 3.5-mm tunnel is created in the patella. The gracilis graft is placed through this tunnel and both ends are whipstitched using nonabsorbable sutures. The femoral attachment point is identified using fluoroscopic images and a Beath pin is placed just distal to the distal femoral physis. The graft is placed between layer 2 and layer 3 on the medial aspect of the knee. Once isometric assessment is performed, the Beath pin is over-drilled with a 5.5-mm reamer and a nitinol wire is placed in the femoral tunnel. The graft is placed in the femoral tunnel using pull-through sutures. The knee is flexed and the sutures are clamped on the lateral aspect of the knee. Clinical and arthroscopic evaluation is performed to check the MPFL graft placement and function. Final femoral fixation is performed using 5.5-mm × 25-mm biocomposite interference screw inserted over the nitinol wire. A pants-over-vest closure is performed to imbricate the medial retinacular tissues. Layered closure is performed and well-padded dressing is applied. A knee immobilizer is placed. (MPFL, medial patellofemoral ligament.)