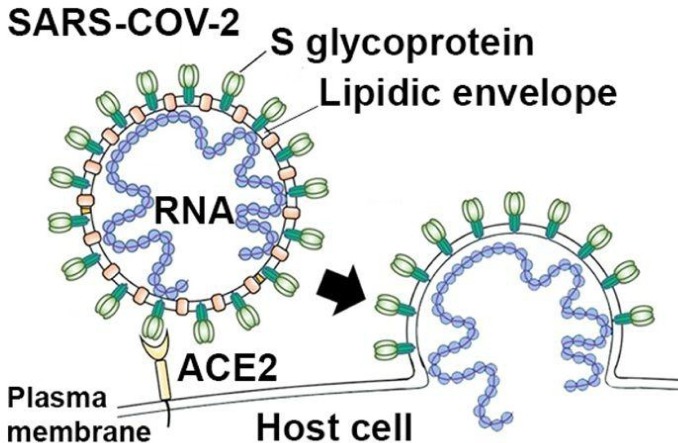
Graphical abstract
Coronavirus SARS-CoV-2 enters host cells via ligation of its spike protein (S glycoprotein) with host cell ACE2 receptor that is primed by TMPRSS2 protease. ACE2- and TMPRSS2-mediated cell entry can be blocked by experimental and established drugs. Virus replication and assembly can be inhibited by antiviral drugs targeting viral RNA-dependent RNA polymerase (RdRp) and main protease (3Clpro).
Keywords: COVID-19, SARS-CoV-2, Drugs, Camostat, Chloroquine, Remdesivir, Lopinavir, Ritonavir, Favipiravir, Arbidol, Phytochemicals
Abstract
Outbreak and pandemic of coronavirus SARS-CoV-2 in 2019/2020 will challenge global health for the future. Because a vaccine against the virus will not be available in the near future, we herein try to offer a pharmacological strategy to combat the virus. There exists a number of candidate drugs that may inhibit infection with and replication of SARS-CoV-2. Such drugs comprise inhibitors of TMPRSS2 serine protease and inhibitors of angiotensin-converting enzyme 2 (ACE2). Blockade of ACE2, the host cell receptor for the S protein of SARS-CoV-2 and inhibition of TMPRSS2, which is required for S protein priming may prevent cell entry of SARS-CoV-2. Further, chloroquine and hydroxychloroquine, and off-label antiviral drugs, such as the nucleotide analogue remdesivir, HIV protease inhibitors lopinavir and ritonavir, broad-spectrum antiviral drugs arbidol and favipiravir as well as antiviral phytochemicals available to date may limit spread of SARS-CoV-2 and morbidity and mortality of COVID-19 pandemic.
1. Introduction
The outbreak of coronavirus SARS-CoV-2 in Wuhan, China in December 2019, the cause of Corona Virus Disease of 2019 (COVID-19), represents a pandemic threat to global health [1,2]. The WHO declared COVID-19 as a pandemic on March 11th 2020. The outbreak has spread to more than 185 countries with more than 3,200,000 confirmed cases, more than 230,000 confirmed deaths and more than 1,000,000 total recoveries worldwide as of May 1st 2 2020 [3]. Hundreds of millions of lives have been affected as a result of mandatory isolations/quarantines. This pandemic has the potential to overwhelm national healthcare systems, and have major consequences on global economy if SARS-CoV-2 spread and virulence is not contained, or effective treatments are not developed.Coronaviruses are grouped into alpha, beta, gamma, and delta classes. Coronaviruses can infect both humans and animals. The source of the beta coronavirus SARS-CoV-2 is believed to be bats, which carry the virus with no signs of disease [4]. Beta coronaviruses caused earlier outbreaks of severe acute respiratory syndromes (SARS), including SARS-CoV (2002/2003 in Guangdong, China) and Middle East Respiratory Syndrome virus MERS-CoV (2012 in Saudi Arabia) [5]. Beta coronaviruses are pathogenic for humans and have a single stranded RNA genome, encapsulated by a membrane envelope [6]. The coronavirus crown-like (“corona”) morphology is created by transmembrane spike glycoproteins (S proteins) that form homotrimers protruding from the viral surface [7]. The S proteins of SARS-CoV and SARS-CoV-2 display structural homology and conserved ectodomains, so earlier strategies employed to prevent binding of SARS-CoV to its host cell receptor angiotensin-converting enzyme 2 (ACE2) may be relevant, since SARS-CoV-2 also employs ACE2 for cell entry [8,9]. ACE2, an exopeptidase expressed on epithelial cells of the respiratory tract, may constitute a pharmacological target to limit cell entry of SARS-CoV-2. The established antimalarial drugs chloroquine and hydroxychloroquine have been shown to inhibit terminal phosphorylation of ACE2 and to elevate the pH in endosomes, respectively. Chloroquine and hydroxychloroquine constitute candidate drugs against SARS-CoV-2 infection and COVID-19 disease, and are now investigated for their therapeutic efficacy in international clinical trials with COVID-19 patients (i. e. SOLIDARITY Trial). The glycosylated S protein of SARS-CoV-2 is highly immunogenic to the host immune system, and murine polyclonal antibodies against SARS-Co-V S protein potently inhibit SARS-CoV-2 S-mediated cell entry, indicating that cross-neutralizing antibodies targeting conserved S epitopes can be elicited upon vaccination [9]. Similar to the earlier SARS and MERS beta coronaviruses, SARS-CoV-2 primarily infects alveolar epithelial cell of the lung, leading to a severe bilateral peripheral pneumonia with ground glass opacity in CT images (COVID-19 disease), with a mortality rate of 2 % to 5 % [10]. SARS-CoV-2 also can contribute to multiple organ failure, affecting heart, liver, kidney, central nervous system and gastrointestinal tract [11]. Epidemiology thus far suggests that SARS-CoV-2 is more contagious than SARS-CoV or MERS-CoV [12]. Multiple mechanisms now identified in the infective and replication processes of SARS-CoV-2 offer targets for pharmacological interventions. Infection of pneumocytes, macrophages and pulmonary mast cells requires viral S protein. This invasion process which involves attachment of S protein to the ACE2 receptor is facilitated by host cell derived serine protease TMPRSS2 [8]. Agents that inhibit TMPRSS2, such as camostat mesilate, may be useful in blocking viral host cell entry. After host cell entry, the viral single-stranded positive RNA is released for replication of virus RNA and translation of virus polyproteins that are finally cleaved into mature effector proteins by virus proteases [13]. The S protein interaction with ACE2 on host cell cytoplasmic membrane initiates viral infection. Strategies capable of disrupting S protein interaction with ACE2 could be of significant therapeutic value, because the binding affinity of SARS-CoV-2 S protein to ACE2 is 10−20-fold higher than for the S protein of SARS-CoV which may contribute to the higher contagiousness of SARS-CoV-2 as compared to SARS-CoV [12]. Although SARS-CoV and SARS-CoV-2 have only 79 % genomic sequence similarity, they share a highly conserved receptor binding domain for their S proteins [1]. There is also potential for targeting other highly conserved proteins associated with SARS-CoV and SARS-Co-V-2, including RdRp and 3Clpro (also termed Mpro), which share over 95 % similarity between the two viruses, despite only 79 % genomic sequence sharing. RdRp is an RNA-dependent RNA polymerase required for replicating the viral genome within the host cell. 3Clpro and Plpro are both viral proteases which break down viral polyprotein into functional units within host cells that are finally assembled into new viruses. The 3Clpro sequences between the two viruses are 96 % similar, the Plpro sequence identity is 83 %, and their active sites show a high degree of conservation [14]. Drugs that have recently been shown to target MERS-CoV in mice [15], and to inhibit Ebola virus RdRp and SARS-CoV-2 proteases in humans, such as remdesivir and ritonavir/lopinavir, also constitute candidate drugs against SARS-CoV-2 and are now investigated for their therapeutic efficacy in COVID-19 patients in 2 international clinical trials (SOLIDARITY Trial and DisCoVeRy Trial). Finally, certain phytochemicals and natural products with high antiviral activity should be considered for treatment of SARS-CoV-2 infection and COVID-19 disease.
2. Inhibitors of cell entry of SARS-CoV-2
2.1. Inhibitors of TMPRSS2 serine protease
Results from previous studies reveal that diverse viruses, including Ebola virus, SARS-coronavirus (SARS-CoV), MERS-coronavirus (MERS-CoV) and influenza virus employ host cell proteases for activation of their envelope glycoproteins [[16], [17], [18]]. Cleavage and activation of the spike protein (S protein) of SARS-CoV that is required for membrane fusion and host cell entry is mediated by transmembrane protease/serine subfamily member 2 (TMPRSS2), an airway and alveolar cell serine protease [[19], [20], [21]]. Pöhlmann and coworkers recently demonstrated that SARS-CoV-2 also employs TMPRSS2 for SARS-CoV-2 S protein priming and S protein-driven cell entry [8]. Using camostat mesilate, a clinically proven and commercial serine protease inhibitor that partially blocks infection by SARS-CoV and HCoV-NL63 in HeLa cell expressing ACE2 and TMPRSS2 [22], it was shown that inhibition of TMPRSS2 in human lung Calu-3 cells by camostat mesilate significantly reduced infection with SARS-CoV-2 [8].
2.1.1. Camostat mesilate (Foipan™)
Camostat, (FOY-305), [N,N-dimethylcarbamoylmethyl 4-(4-guanidinobenzoyloxy)-phenylacetate] methanesulfate and camostat mesilate (Foipan™), alternatively termed camostat mesylate, (NI-03), (CAS number: 59721−28-7), constitute synthetic serine protease inhibitors that were developed decades ago for the treatment of oral squamous cell carcinoma [23,24], dystrophic epidermolysis [25], exocrine pancreatic enzyme inhibition [26,27], and chronic pancreatitis [[28], [29], [30], [31]]. Camostat mesilate (NI-03) is manufactured as an oral drug by Nichi-Iko Pharmaceutical Co., Ltd., and Ono Pharmaceutical, Japan, with a three times daily dose recommendation of 100 mg–300 mg [30,31]. In a clinical trial investigating camostat mesilate against dyspepsia associated with non-alcoholic mild pancreatic disease, 95 patients received 200 mg camostat mesilate three times daily for 2 weeks and showed only mild, but no severe adverse effects [28], indicating that camostat mesilate is a well-tolerated drug.
2.1.2. Nafamostat mesilate (Buipel™)
Nafamostat mesilate (Buipel™), (6-amidino-2-naphthyl-4-guanidino benzoate-dimethanesulfonate) (FUT-175), (CAS number: 81525−10-2), is a clinical proven and synthetic serine protease inhibitor approved in Japan for the treatment of acute pancreatitis, disseminated intravascular coagulation and for anticoagulation in extracorporeal circulation [[32], [33], [34]]. In a screening approach of about 1100 drugs approved by the FDA, nafamostat mesilate has been identified to inhibit MERS-CoV S protein-mediated viral membrane fusion with TMPRSS2-expressing lung Calu-3 host cells by inhibiting TMPRSS2 protease activity [35]. Since the S proteins of MERS-Cov and SARS-CoV-2 share considerable amino acid sequence homology [1,9], nafamostat mesilate may also inhibit cell entry of SARS-CoV-2. In cell culture experiments with simian Vero E6 cells infected with SARS-CoV-2, nafamostat mesilate was shown to be inhibitive against SARS-CoV-2 infection at EC50 of 22.50 μM [36], suggesting that nafamostat mesilate is able to prevent SARS-CoV-2 infection. In a multicenter, randomized, open-label, phase 2 trial in 19 patients with severe acute pancreatitis, nafamostat mesilate was administered intravenously at a daily dose of 240 mg for 5 days without severe adverse effects [34].
2.2. Inhibitors of angiotensin-converting enzyme 2 (ACE2) and antimalarial/parasiticide drugs
SARS-CoV and related coronaviruses directly interact via their S proteins with angiotensin-converting enzyme 2 (ACE2), a host cell exopeptidase and metallocarboxypeptidase that catalyses the conversion of angiotensin I to the nonapeptide angiotensin and the conversion of angiotensin II to angiotensin 1–7, to initiate S protein-mediated cell entry [[37], [38], [39]]. It was demonstrated recently that also SARS-CoV-2 uses ACE2 as a receptor for S protein-driven host cell entry [8,9]. Therefore, ACE2 constitute a molecular target to inhibit cell entry of SARS-CoV-2. Unfortunately, ACE inhibitors as standard drugs for the treatment of hypertension and chronic heart failure fail to inhibit ACE2 [40], but a number of other drugs and compounds have been shown to inhibit ACE2.
2.2.1. Chloroquine phosphate and hydroxychloroquine
Chloroquine phosphate (Resochin™) and its derivative hydroxychloroquine (Quensyl™, Plaquenil™, Hydroquin™, Dolquine™, Quinoric™) have been used for decades for the prophylaxis and treatment of malaria and for the treatment of chronic Q fever and various autoimmune diseases [41], and have recently been demonstrated as potential broad-spectrum antiviral drugs [42,43]. Chloroquine phosphate inhibits terminal phosphorylation of ACE2, and hydroxychloroquine elevates the pH in endosomes which are involved in virus cell entry [44,45], both mechanisms constitute relevant antiviral mechanisms of chloroquine and hydroxychloroquine. In vivo, hydroxychloroquine is metabolized into chloroquine. Chloroquine phosphate has previously been shown to inhibit SARS-CoV infection and spread in vitro [44,46], and results from very recent studies reveal that chloroquine phosphate and, more effectively, hydroxychloroquine also inhibit replication of SARS-CoV-2 in simian Vero cells [46,47]. By using a physiologically-based pharmacokinetic model for chloroquine phosphate and hydroxychloroquine in human lung fluid, it was demonstrated that the concentrations of hydroxychloroquine recommended for treatment of SARS-CoV-2 infection comprise an oral loading dose of 400 mg twice daily at day 1, followed by an oral maintenance dose of 200 mg twice daily for 4 days [47]. These results were deduced from in vitro data obtained from SARS-CoV-2-infected Vero cells treated with hydroxychloroquine [47]. A recent pilot trial conducted in more than 10 hospitals in Wuhan, Jingzhou, Guangzhou, Bejing, Shanghai, Chongqing and Ningbo, China, with more than 100 patients with COVID-19 disease demonstrated that treatment with chloroquine phosphate is superior to control treatment in inhibiting the exacerbation of pneumonia, improving lung imaging findings, promoting laboratory virus-negative conversion, and shortening the course of COVID-19 disease [48]. Chloroquine phosphate should be administered as an oral daily dose of 250 mg until clinical convalescence [49]. Thus, in view of these results and the urgent clinical demand regarding SARS-CoV-2/COVID-19 pandemia, chloroquine phosphate should be recommended to treat COVID-19 associated pneumonia in larger populations [48]. A recent open-label non-randomized clinical trial conducted in March 2020 in France with 20 COVID-19 patients treated with daily 600 mg hydroxychloroquine for 6 days demonstrated at day 6 a negative viral load (negative nasopharyngeal PCR) in 57 % of the hydroxychloroquine-treated patients, as compared to negative viral load in 12.5 % of untreated COVID-19 patients (control group, n = 16) [50]. In a randomized clinical trial conducted in February 2020 in Wuhan, China, sixty two COVID-19 patients were randomized to receive either daily 400 mg hydroxychloroquine for 5 days (n = 31) or no pharmacological treatment (n = 31) [51]. Improvement and absorption of pneumonia as analyzed in chest CT at day 6 was observed in 80.6 % of the hydroxychloroquine-treated patients vs. 54.8 % in the untreated patients [51]. The results from these small studies therefore strongly suggest that hydroxychloroquine has therapeutic efficacy in COVID-19 disease. Thus, a considerable number of clinical trials investigating therapeutic efficacy of chloroquine phosphate and hydroxychloroquine in patients with SARS-CoV-2 infection and COVID-19 disease have been initiated in China, Great Britain, Spain and Thailand [[52], [53], [54], [55], [56]].
2.2.2. Cepharanthine/selamectin/mefloquine hydrochloride
The triple combination of cepharanthine (an anti-inflammatory alkaloid from Stephania cepharantha Hayata), (CAS number: 48,104,902), selamectin (an avermectin isolated from Streptomyces avermitilis and used as an anti-helminthic and parasiticide drug in veterinary medicine), (CAS number. 220119−17-5), and mefloquine hydrochloride (Lariam™, used for the prophylaxis and treatment of malaria) [[57], [58], [59]] has recently been shown to inhibit infection of simian Vero E6 cells with pangolin coronavirus GX_P2V/2017/Guangxi (GX_P2V), whose S protein shares 92.2 % amino acid identity with that of SARS-CoV-2 [60]. Further, it was demonstrated that GX_P2V also uses ACE2 as the receptor for viral cell entry [60]. Two libraries of 2406 clinically approved drugs were screened for their ability to inhibit cytopathic effects on Vero E6 cells by GX_P2V, and only the combination of cepharanthine, selamectin and mefloquine hydrochloride was identified as candidate drug combination against SARS-CoV-2 infection [60].
2.2.3. Experimental inhibitors of ACE2
Shortly after the identification of the angiotensin-converting enzyme 2 (ACE2), a metallocarboxypeptidase that mediates various cardiovascular and renal functions, peptide inhibitors of the enzyme were developed by selection of constrained peptide libraries displayed on phage [61]. The most potent inhibitor, termed DX600, with the amino acid sequence of Ac-GDYSHCSPLRYYPWWKCTYPDPEGGG-NH2 had a Ki of 2.8 nm and an IC50 of 10.1 μM [61]. Subsequent experimental studies in mice and in human cell lines revealed that DX600 is a potent ACE2 inhibitor specific for only human ACE2 [62,63]. Other small-peptide and tripeptide inhibitors have been developed for potent and selective inhibition of human ACE2 and inhibition of SARS-CoV cell entry in vitro [[64], [65], [66]]. More recently, synthetic small-molecule inhibitors of human ACE2, including MLN-4760 (CAS number: 305335−31-3) [63,67], N-(2-aminoethyl)-1 aziridine-ethanamine [68] and the TNF-α converting enzyme (TACE) small-molecule inhibitor TAPI-2 that blocks SARS-CoV S protein-induced shedding of ACE2 [69,70] have been developed for experimental inhibition of SARS-CoV cell entry. Moreover, the phytochemical nicotianamine (CAS number: 34441−14-0), a metal chelator ubiquitously present in higher plants [71], was identified in high concentrations in soybean, and was shown as a potent inhibitor of human ACE2 with an IC50 of 84 nM [72]. Because dietary phytochemicals as naturally occurring compounds display a wide safety profile and less pharmacological side effects [73], nicotianamine constitutes a candidate drug for ACE2 inhibition and thus blockade of SARS-CoV-2 cell entry. Finally, a recent study demonstrates that a clinical-grade soluble recombinant human ACE2 protein (hrsACE2) inhibits attachment of SARS-CoV-2 to simian Vero-E 6 cells, and inhibits infection of engineered human capillary organoids and kidney organoids by SARS-CoV-2 isolated from a nasopharyngeal sample of a patient with confirmed COVID-19 disease [74], suggesting that hrsACE2 can block host cell entry of SARS-CoV-2 and early stages of SARS-CoV-2 infections.
3. Inhibitors of replication, membrane fusion and assembly of SARS-CoV-2
3.1. Remdesivir
Remdesivir (GS-5734), (CAS number: 1809249−37-3), is a novel small-molecule adenine nucleotide analogue antiviral drug that has shown efficacy against Ebola virus in rhesus monkeys. Once-daily intravenous administration of 10 mg kg(-1) remdesivir for 12 days resulted in profound suppression of Ebola virus replication and protected 100 % of Ebola virus-infected animals against lethal disease [75]. Remdesivir displays antiviral activity against other single stranded RNA viruses, including filoviruses, pneumoviruses, paramyxoviruses, and the coronaviruses MERS-CoV and SARS-CoV [[76], [77], [78]]. Remdesivir is a prodrug that is metabolized into its active form GS-441524, an adenine nucleotide analogue that interferes with the activity of viral RNA-dependent RNA polymerase (RdRp) and that promotes evasion of proofreading by viral exoribonuclease, leading to inhibition of viral RNA synthesis [78]. Remdesivir acts early in infection, and decreases viral RNA levels in a dose-dependent manner that parallels impairment of viral load in vitro [78]. These and related mechanisms of action of remdesivir have been demonstrated in vitro for SARS-CoV [78], Ebola virus [79] and MERS-CoV [80]. A recent study demonstrates in cell culture experiments with simian Vero E6 cells infected with SARS-CoV-2 that remdesivir is inhibitive against SARS-CoV-2 infection at EC90 of 1.76 μM, a concentration achieved in vivo in nonhuman primate models [36]. It was further shown that remdesivir efficiently inhibited SARS-CoV-2 infection of human liver cancer Huh-7 cells, which are sensitive to SARS-CoV-2 infection [36]. The prophylactic and therapeutic efficacy of remdesivir have been shown recently in a nonhuman primate model (rhesus monkeys) of MERS-CoV infection [81]. Prophylactic treatment of rhesus monkeys with remdesivir initiated 24 h prior to inoculation of MERS-CoV completely prevented virus-induced disease, inhibited virus replication in respiratory tissues, and prevented the occurrence of lung lesions [81]. Remdesivir treatment of rhesus monkeys 12 h after inoculation of MERS-CoV also provided a significant clinical benefit, with reduction in clinical signs, reduced virus replication in respiratory tissues, and decreased occurrence and severity of lung lesions [81]. From a randomized, controlled trial of Ebola virus therapeutics that was conducted in response to the Ebola virus outbreak in the Democratic Republic of Congo in August 2018, there are human safety data available for remdesivir. In a subgroup of 175 patients treated with remdesivir (loading dose of 200 mg on day 1, followed by a maintenance dose of 100 mg for 9−13 days), 9 patients experienced serious adverse events [82], indicating that remdesivir is a relatively safe drug. A recent clinical trial with remdesivir for a compassionate use to 53 COVID-19 patients receiving oxygen support or mechanical ventilation due to an oxygen saturation of 94 % or less demonstrated that intravenous treatment with 200 mg remdesivir at day 1, followed by 100 mg daily for 9 days, resulted in clinical improvement in 36 of the 53 patients (68 %) [83]. However, the mortality rate was 18 % among patients receiving invasive ventilation and 5% among patients not receiving invasive ventilation [83], suggesting that remdesivir constitutes a therapeutic option for COVID-19 patients not receiving invasive ventilation. Based on the experimental and clinical results described above, clinical trials with remdesivir in COVID-19 patients have been initiated recently, in China [84,85], the USA, Republic of Korea and Singapore [86], in the USA, Hong Kong, Republic of Korea, Singapore and Taiwan [87,88], in the USA [89], and in France [90,91].
3.2. Lopinavir/ritonavir (Kaletra™)
Lopinavir (ABT-378) is a highly potent inhibitor of the human immunodeficiency virus (HIV) protease essential for intracellular HIV assembly that was developed in 1998 to circumvent HIV resistance towards the protease inhibitor ritonavir (ABT-538), caused by mutation of valine at position 82 (Val 82) in the active site of HIV protease in response to ritonavir therapy [92]. Because the metabolism of lopinavir is strongly inhibited by ritonavir, concomitant oral administration of lopinavir and ritonavir exceeded the in vitro antiviral EC50 of lopinavir by >50-fold after 8 h in rat, dog, and monkey plasma [92]. Coadministration of 400 mg lopinavir with 50 mg ritonavir enhanced in healthy human volunteers the area under the concentration curve of lopinavir in plasma by 77-fold over that observed after dosing with lopinavir alone, and mean concentrations of lopinavir exceeded the EC50 for >24 h [92]. Therefore, the combination of lopinavir and ritonavir (Kaletra™) has been established as an effective oral drug for the treatment of HIV-infected individuals when used in combination with other antiretroviral agents [93,94].An initial study in 2003 demonstrated that lopinavir at 4 μg/mL inhibited the cytopathic effect in a plaque reduction assay with fetal rhesus kidney-4-cells infected with SARS-CoV (HKU-39,849 isolate) [95]. In this study, newly diagnosed SARS patients infected with SARS-CoV were treated with the combination of lopinavir (400 mg)/ritonavir (100 mg) orally every 12 h for 14 days. At day 21, SARS patients treated with lopinavir/ritonavir had a milder disease course in terms of diarrhea, recurrence of fever, worsening of chest radiographs and reduction of viral load, compared to a historical control group [95]. In a nonhuman primate model of common marmosets infected with MERS-CoV, lopinavir/ritonavir-treated animals displayed an improved clinical outcome compared to untreated animals, with improved weight loss, lung imaging and pathological findings, and lower mean viral loads in necropsied lung and extrapulmonary tissues [96]. In response to these findings, an ongoing randomized control trial (MIRACLE Trial) was initiated to determine the therapeutic efficacy of lopinavir/ritonavir combined with interferon β-1b in patients infected with MERS-CoV [97]. In a recent randomized, controlled, open-label trial involving 199 hospitalized adult patients in Wuhan/China with confirmed SARS-CoV-2 infection and COVID-19 pneumonia, oral administration of twice daily 400 mg lopinavir and 100 mg ritonavir for 14 days revealed rather disappointing results, compared to the control group (100 patients) receiving standard-care treatment [98]. Treatment with lopinavir/ritonavir was not associated with a difference from standard care in the time to clinical improvement, and mortality at 28 days was similar in the lopinavir/ritonavir group and the standard-care group [98]. Moreover, treatment with lopinavir/ritonavir treatment did not reduce viral RNA loads or duration of viral RNA detectability as compared with standard supportive care. SARS-CoV-2 RNA was still detected in 40.7 % of the patients in the lopinavir/ritonavir group at the end of the trial at day 28 [98]. However, the numbers of lopinavir/ritonavir recipients who had serious complications (acute kidney injury and secondary infections) or requiring noninvasive or invasive mechanical ventilation for respiratory failure were fewer than in those not receiving lopinavir/ritonavir treatment [98]. These results and observations require additional studies to determine whether treatment with lopinavir/ritonavir given at a certain disease stage can reduce some complications in COVID-19 patients [98]. Finally, clinical trials with lopinavir/ritonavir in COVID-19 patients have been initiated recently in China [[99], [100], [101], [102]], Hong Kong [103], Republic of Korea [104], and in Europe (DisCoVeRy Trial), investigating remdesivir, lopinavir/ritonavir, and lopinavir/ritonavir plus interferon β-1a [91].
3.3. Umifenovir (Arbidol™)
Umifenovir (Arbidol™), (ethyl-6-bromo-4-[(dimethylamino)methyl]-5-hydroxy-1-methyl-2 [(phenylthio)methyl]-indole-3-carboxylate hydrochloride monohydrate), (CAS number: 131707−25-0), is a small indole-derivate molecule manufactured by JSC Pharmstandard, Russia [105]. Umifenovir prevents viral host cell entry by inhibition of membrane fusion of viral envelope and host cell cytoplasmic membrane via inhibition of clathrin-mediated endocytosis, thereby preventing virus infection [[106], [107], [108]]. Umifnovir is licensed in Russia and China for oral prophylaxis and treatment of infections with influenza A and B viruses and other respiratory viruses [105], and has been demonstrated to inhibit in vitro infection with globally prevalent pathogenic viruses, including hepatitis C virus, hepatitis B virus, Ebola virus, Lassa virus, human herpesvirus 8, poliovirus, and vesicular stomatitis virus [109,110], ultimately defining umifenovir as a broad-spectrum antiviral drug. In a clinical pilot trial conducted in January 2020 in Wuhan, China, 36 patients with COVID-19 disease received 400 mg umifenovir three times a day for 9 days; 31 untreated COVID-19 patients served as a control group [111]. In this trial, treatment with umifenovir showed a tendency to reduce viral load determined by RT-PCR, and decreased mortality (0 % vs. 16 %), as compared to the control group [111]. In a single-center, retrospective cohort study conducted in February 2020 in Guangdong, China, 16 patients with COVID-19 disease received orally 200 mg umifenovir every 8 h plus lopinavir (400 mg)/ritonavir (100 mg) every 12 h for 5–21 days; seventeen COVID-19 patients received lopinavir (400 mg)/ritonavir (100 mg) every 12 h and served as a control group [112]. After 14 days of treatment, detection of SARS−COV-2 by RT-PCR was negative in 94 % of the umifenovir-treated patients vs. 53 % in the control group, and the chest computed tomography scans were improving for 69 % of the umifenovir-treated patients vs. 29 % in the control group [112]. In view of these promising clinical results, clinical trials with umifenovir alone or in combination with lopinavir/ritonavir, chloroquine phosphate or carrimycin have been recently initiated in China [[113], [114], [115], [116]].
3.4. Favipiravir (Avigan™)
Favipiravir (Avigan™), (T-705), (6-fluoro-3-hydroxy-2-pyrazinecarboxamide), (CAS number: 259793−96-9), is an oral pyrazinecarboxamide derivative and guanine analogue developed by Toyama Chemical, Japan that selectively and potently inhibits the RNA-dependent RNA polymerase (RdRp) of RNA viruses and induces lethal RNA transversion mutations, thereby producing a nonviable virus phenotype [[117], [118], [119]]. Favipiravir inhibits replication of a large number of RNA viruses, including influenza A virus, flavi-, alpha-, filo-, bunya-, arena- and noroviruses as well as West Nile virus, yellow fever virus, foot-and-mouth-disease virus, Ebola virus and Lassa virus [120,121]. In a historically controlled, single-arm proof-of-concept trial conducted in response to the Ebola virus outbreak in September 2014 in Guinea, ninety nine adults with confirmed positive semi-quantitative Ebola virus RT-PCR (results expressed in “cycle threshold” [Ct]) were treated with 6000 mg favipiravir at day 1, followed by 2400 mg favipiravir at days 2−10 [122]. Patients were randomized by their Ct 20-value, and Ct 20 was adjusted to a RNA viral load of 7.7 log10 viral genome copies/mL. Mortality at day 14 of patients in the Ct ≥ 20-group (n = 55) was 20 %, whereas mortality in the CT < 20-group (n = 44) was 91 % [122], suggesting that favipiravir is active in Ebola patients with high viral load. In response to this trial in Ebola patients, clinical trials with favipiravir combined with a monoclonal antibody against the human interleukin-6 receptor, tocilizumab [123] or favipiravir in combination with chloroquine phosphate and the viral neuramidase inhibitor oseltamivir [124] have been initiated recently in China.
3.5. Inhibitors of SARS-CoV-2 3Clpro protease
3Clpro (also termed Mpro) constitutes the main protease of beta coronaviruses that is essential for processing of polyproteins translated from the viral RNA [125]. Recently, the X-ray structures of the unligated SARS-CoV-2 3Clpro and its complex with α-ketoamides designed as specific inhibitors of 3Clpro were reported [126]. Two pyridine-containing α-ketoamides, designated 13a and 13b, displayed favorable pharmacokinetic properties in mice and were detected at sufficient concentrations in lung tissue and broncheo-alveolar lavage fluid within 4 hours–24 hours after subcutaneous administration, demonstrating lung tropism of the compounds [126]. Besides subcutaneous administration, inhalation of nebulized 13b by mice resulted in high and long-lasting (24 h) concentrations in lung tissue, without any adverse effects [126], pointing out a role of pyridine-containing α-ketoamides in COVID-19 therapy. In a recent study that employed combined structure-assisted drug design, virtual drug screening and high-throughput screening, a mechanism-based inhibitor of 3Clpro, termed N3, was identified by computer-aided drug design [127]. N3, a Michael acceptor inhibitor that can inhibit the 3Clpros of SARS-CoV and MERS-CoV, was shown to form a covalent bond with and to be an irreversible inhibitor of SARS-CoV-2 3Clpro [127]. Further, in a high-throughput screening approach for identifying inhibitors of SARS-CoV-2 3Clpro, ebselen, an organoselenium compound with anti- inflammatory, anti-oxidant and cytoprotective properties, was identified [127]. In a plaque-reduction assay with simian Vero cells infected with SARS-CoV-2, N3 and ebselen displayed antiviral and cell protection efficacy at EC50 values of 16.77 μM and 4.67 μM, respectively [127], ultimately demonstrating their antiviral potential against SARS-CoV-2.
4. Phytochemicals and natural products targeting coronaviruses
Natural products can inhibit various steps in viral infection and replication, and many of them have broad-spectrum antiviral effects, the mechanisms of which have not been fully characterized. They also can function as immunomodulators, suppressing inflammatory reaction responsible for the major morbidity and mortality of SARS-CoV-2 infection. Phytochemicals, especially flavonoids, which are widely distributed in food plants and botanicals, have been shown to interfere with NLRP3 inflammasome signaling [128]. The respiratory distress syndrome associated with SARS coronaviruses develops in part due to viral activation of the NLRP3 inflammasome within activated macrophages and T helper-1 lymphocytes, which causes increased production of inflammatory cytokines [129]. Several flavonoids that interfere with activation of the NLRP3 inflammasome may modulate inflammatory response to SARS beta coronaviruses: luteolin [130], myricetin [131], apigenin [132], quercetin [133] kaempferol [134], baicalin [135], and wogonoside [136]. These flavonoids have been shown to be active against a wide variety of viruses, via multiple mechanisms [137,138], and are available as nutraceutical supplements at a daily dose ranging from 100 mg to 500 mg. Emodin (6-methyl-1,3,8-trihydroxyanthraquinone) (CAS number: 518−82-1) is an anthraquinone compound found in various Chinese herbs and is also produced by many species of fungi, including members of the genera Aspergillus, Pyrenochaeta, and Pestalotiopsis. Emodin has been shown to inhibit the interaction of SARS-CoV S protein with its receptor ACE2 in a dose-dependent manner [139]. Resveratrol (trans-3,5,4′-trihydroxystilbene) (CAS number: 501−36-0) is a stilbenoid and a natural polyphenol that is found in high concentrations in the skins of red wine grapes (Vitis vinifera), in red wine and in sprouted peanuts (Arachis hypogaea). Resveratrol has been demonstrated in vitro to inhibit MERS-CoV infection and to prolong cellular survival after virus infection. Further, expression of MERS-CoV nucleocapsid protein essential for virus replication as well as MERS-CoV-induced host cell apoptosis are inhibited by resveratrol [140], suggesting that resveratrol may also be effective against SARS-CoV-2 infection.
5. Discussion and conclusion
The emergence of the novel beta coronavirus SARS-CoV-2 from Wuhan, Hubei province, China in December 2019 rapidly led to a pandemic involving more than 3,200,000 infected persons and more than 230,000 deaths in 185 countries as of May 1st 2020. SARS-CoV-2 infection can cause a severe bilateral pneumonia that may progress to multi-organ failure and death (COVID-19 disease), with a mortality rate of 2 % to 5 % [10], though this may be inflated, due to inadequate knowledge of the true number of cases, from countries which only test individuals with strong symptoms. Many cases appear to be mild or even asymptomatic, so the true number of cases remains unknown. Since no vaccine will be available for large populations until the end of 2020, it is mandatory to identify approved off-label and experimental drugs against SARS-CoV-2 infection and COVID-19 disease. Such drugs may constitute inhibitors of TMPRSS2 and ACE2, established antimalarial drugs, antiviral drugs inhibiting viral RdRp, proteases and virus/host cell membrane fusion as well as phytochemicals with antiviral activity, as reviewed herein. Besides preventive strategies against SARS-CoV-2 infection practiced in many countries, such as quarantine of confirmed infected individuals, contact tracing by smartphone, protection of individuals at high risk of infection, nationwide limited curfew, and the urgent development and rapid provision of a vaccine, the WHO and the European Union recently and urgently initiated clinical trials to test remdesivir, chloroquine and hydroxychloroquine, lopinavir/ritonavir, and lopinavir/ritonavir plus interferon β-1a in COVID-19 patients worldwide in the SOLIDARITY Trial [141] and in the DisCoVeRy Trial [91]. Based on the experiences with infections by other viruses, such as HIV and influenza virus, post-exposure prophylaxis with candidate drugs against SARS-CoV-2 infection may be effective in preventing disease after potential exposure to the virus or positive test results, and in reducing the risk of secondary virus spread and COVID-19 disease. Candidate drugs for post-exposure prophylaxis of SARS-CoV-2 infection could be clinically proven drugs such as camostat mesilate which prevents virus host cell entry by inhibiting TMPRSS2 [8], and chloroquine phosphate which inhibits terminal phosphorylation of ACE2, or hydroxychloroquine which is metabolized in vivo to chloroquine [44]. For the treatment of ordinary and severe COVID-19 pneumonia, and to lower the mortality rate of COVID-19 disease, the antiviral drugs remdesivir, favipiravir, umifenovir, and lopinavir/ritonavir plus interferon β-1a should be administered, in particular after the consideration of (preliminary) results from the recent ongoing clinical trials SOLIDARITY and DisCoVeRy [141,91] that can be expected soon. Moreover, administration of the antiviral candidate drugs shortly after symptom onset may reduce infectiousness to other individuals by diminishing viral shedding in the respiratory secretions of SARS-CoV-2 infected patients that usually peaks at 5–6 days after symptom onset and lasts up to 14 days [142] (albeit shorter time courses of nasopharyngeal viral shedding, peak: day 4, end: day 7, have been reported in mild cases of COVID-19 [143]), and prophylactic treatment of contacts could reduce their risk of becoming infected. Further, due to their wide safety profile, less pharmacological side effects and easy availability, phytochemicals, such as flavonoids, emodin and resveratrol with postulated therapeutic efficacy against SARS-CoV-2 infection and COVID-19 disease may be used by large populations for pre- and post-exposure prophylaxis of SARS-CoV-2 infection and in COVID-19 disease. Interestingly, a broad-spectrum antiviral drug, the ribonucleoside analogue β-d-N4-hydroxycytidine (NHC, EIDD-1931), with antiviral activity against SARS-CoV and MERS-CoV in mice and against SARS-CoV-2 in primary human airway epithelial cells has been recently developed for the treatment of newly emerging coronavirus infections of the future [144].Finally, in the urgent case of the accelerating COVID-19 pandemic, implementation of pharmacological antiviral prophylaxis and treatment of large populations has several requirements regarding high efficacy, drug safety, high availability, and economy.
Authors´ contributions
CN and DLM conceived the original idea and wrote large parts of the manuscript. AS and US wrote section 3 and prepared Table 1 and Fig. 1 . SL finished the manuscript.
Table 1.
Off-label drugs against SARS-CoV-2 and COVID-19 disease.
| Drug | Class | Target | Dosage | Reference |
|---|---|---|---|---|
| Camostat mesilate (FoipanTM) | Serine protease inhibitor | TMPRSS2 | 200 mg three times daily, for 2 weeks, peroral | [8,28] |
| Nafamostat mesilate (BuipelTM) | Serine protease inhibitor | TMPRSS2 | 240 mg daily, for 5 days, peroral | [34,35,36] |
| Chloroquine phosphate (ResochinTM) | Antimalarial drug | ACE2 | 250 mg daily until clinical convalescence, peroral | [48,49] |
| Hydroxychloroquine (QuensylTM, PlaquenilTM, HydroquinTM, DolquineTM, QuinoricTM) | Antimalarial drug | Endosome, pH elevation | 400 mg loading dose twice daily at day 1, 200 mg twice daily for 4 days, or 600 mg for 6 days, or 400 mg for 5 days, peroral | [47,49,50,51] |
| Remdesivir | Antiviral drug | RdRp | 200 mg loading dose at day 1, 100 mg for 9−13 days, peroral or intravenous | [36,77,82,83] |
| Lopinavir/ritonavir (KaletraTM) | Antiviral drug | Viral proteases | 400 mg lopinavir and 100 mg ritonavir twice daily, for 14 days, peroral | [95,96,98] |
| Umifenovir (ArbidolTM) | Antiviral drug | Membrane fusion, clathrin-mediated endocytosis | 400 mg three times daily, for 9 days, peroral | [111,112] |
| Favipiravir (AviganTM) | Antiviral drug | RdRp | 6000 mg loading dose at day 1, 2, 400 mg for days 2−10, peroral | [122] |
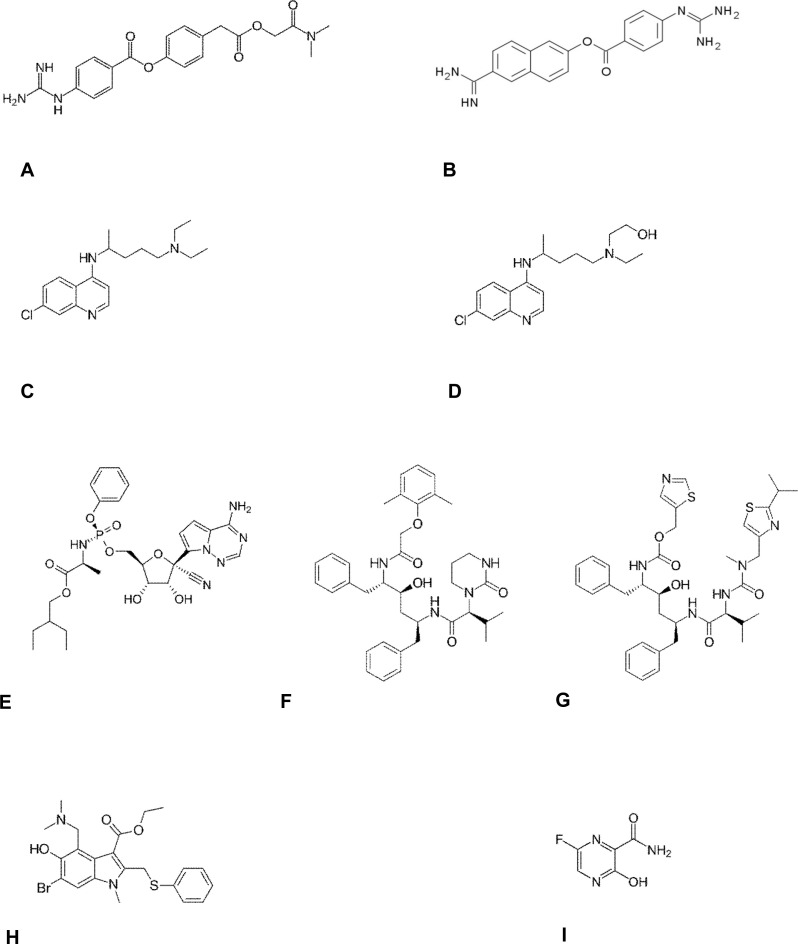
Fig. 1.
Structural formulas of candidate drugs against SARS-CoV-2 and COVID-19.
(A) camostat, (B) nafamostat, (C) chloroquine, (D) hydroxychloroquine, (E) remdesivir (F) lopinavir, (G) ritonavir, (H) umifenovir, (I) favipiravir
Declaration of Competing Interest
None.
Acknowledgments
We are grateful to all of the colleagues who have given critical comments on this work.
References
- 1.Lu R., Zhao X., Li J., Niu P., Wang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2019;383(8):727–733. doi: 10.1056/NEJMoa2001017. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.https://coronavirus.jhu.edu/map.html Johns Hopkins University, USA, (2020).
- 4.Li C., Yang Y., Ren L. Genetic evolution analysis of 2019 novel coronavirus and coronavirus from other species. Infect. Genet. Evol. 2020;82:104285. doi: 10.1016/j.meegid.2020.104285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anthony S.J., Johnson C.K., Greig D.J., Kramer S., Che X., Wells H. Global patterns in coronavirus diversity. Virus Evol. 2017;3(1):vex012. doi: 10.1093/ve/vex012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tortorici M.A., Veesler D. Structural insights into coronavirus entry. Adv. Virus Res. 2019;105:93–116. doi: 10.1016/bs.aivir.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease receptor. Cell. 2020;(March 4) doi: 10.1016/j.cell.2020.02.052. pii: S0092-8674(20)30229-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;(March 6) doi: 10.1016/j.cell.2020.02.058. pii: S0092-8674(20)30262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Y.C., Chen C.S., Chan Y.J. The outbreak of COVID-19: an overview, J. Chin. Med. Assoc. 2020;83(2):217–220. doi: 10.1097/JCMA.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang B., Bragazzi N.L., Li Q., Tang S., Xiao Y., Wu J. An updated estimation of the risk of transmission of the novel coronavirus (2019-nCov) Infect. Dis. Model. 2020;5:248–255. doi: 10.1016/j.idm.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Báez-Santos Y.M., St John S.E., Mesecar A.D. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morse J.S., Lalonde T., Xu S., Liu W.R. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem. 2020;21(5):730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11(1):222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertram S., Heurich A., Lavender H., Gierer S., Danisch S., Perin P. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS One. 2012;7(4):e:35876. doi: 10.1371/journal.pone.0035876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y., Vedantham P., Lu K., Agudelo J., Carrion R., Nunneley J.W. Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res. 2015;116:76–84. doi: 10.1016/j.antiviral.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaya M., Shimotai Y., Hatachi Y., Lusamba Kalonji N., Tando Y., Kitajima Y. The serine protease inhibitor camostat inhibits influenza virus replication and cytokine production in primary cultures of human tracheal epithelial cells. Pulm. Pharmacol. Ther. 2015;33:66–74. doi: 10.1016/j.pupt.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuyama S., Nagata N., Shirato K., Kawase M., Takeda M., Tagushi F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J. Virol. 2010;84(24) doi: 10.1128/JVI.01542-10. 12685-12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shulla A., Heald-Sargent T., Subramanya G., Zhao J., Perlman S., Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011;85(2):873–882. doi: 10.1128/JVI.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glowacka I., Bertram S., Müller M.A., Allen P., Soilleux E., Pfefferle S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011;85(9):4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawase M., Shirato K., van der Hoek L., Taguchi F., Matsuyama S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J. Virol. 2012;86(12):6537–6545. doi: 10.1128/JVI.00094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohkoshi M., Fujji S. Effect of the synthetic protease inhibitor [N,N-dimethylcarbamoyl-methyl 4-(4-guanidinobenzoyloxy)-phenylacetate] methanesulfate on carcinogenesis by 3-methylcholanthrene in mouse skin. J. Natl. Cancer Inst. 1983;71(5):1053–1057. [PubMed] [Google Scholar]
- 24.Ohkoshi M., Oka T. Clinical experience with a protease inhibitor [N,N-dimethylcarbamoylmethyl 4-(4-guanidinobenzoyloxy)-phenylacetate] methanesulfate for prevention of recurrence of carcinoma of the mouth and in treatment of terminal carcinoma. J. Maxillofac. Surg. 1984;12(4):148–152. doi: 10.1016/s0301-0503(84)80235-0. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda S., Manabe M., Muramatsu T., Takamori K., Ogawa H. Protease inhibitor therapy for recessive dystrophic epidermolysis bullosa. In vitro effect and clinical trial with camostat mesylate. J. Am. Acad. Dermatol. 1988;18(16):1246–1252. doi: 10.1016/s0190-9622(88)70130-9. [DOI] [PubMed] [Google Scholar]
- 26.Göke B., Stöckmann F., Müller R., Lankisch P.G., Creutzfeld W. Effect of a specific serine protease inhibitor on the rat pancreas: systemic administration of camostate and exocrine pancreatic secretion. Digestion. 1984;30(3):171–178. doi: 10.1159/000199102. [DOI] [PubMed] [Google Scholar]
- 27.Adler G., Müllenhoff A., Koop I., Bozkurt T., Göke B., Beglinger C. Stimulation of pancreatic secretion in man by a protease inhibitor (camostate) Eur. J. Clin. Invest. 2020;18(1) doi: 10.1111/j.1365-2362.1988.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 28.Sai J.K., Suyama M., Kubokawa Y., Matsumura Y., Inami K., Watanabe S. Efficacy of camostat mesilate against dyspepsia associated with non-alcoholic mild pancreatic disease. J. Gastroenterol. 2010;45(3):335–341. doi: 10.1007/s00535-009-0148-1. 1988 98-104. [DOI] [PubMed] [Google Scholar]
- 29.Yamawaki H., Futagami S., Kaneto K., Agawa S., Higuchi K., Murakami M. Camostat mesilate, pancrelipase, and rabeprazole combination therapy improves epigastric pain in early chronic pancreatitis and functional dyspepsia with pancreatic enzyme abnormalities. Digestion. 2019;99(4):283–292. doi: 10.1159/000492813. [DOI] [PubMed] [Google Scholar]
- 30.Ramsey M.L., Nuttall J., Hart P.A. A phase 1/2 trial to evaluate the pharmacokinetics, safety, and efficacy of NI-03 in patients with Chronic pancreatitis: study protocol for a randomized controlled trial on the assessment of camostat treatment in chronic pancreatitis (TACTIC) Trials. 2019;20(1):501. doi: 10.1186/s13063-019-3606-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NCT02693093, ClinicalTrials.gov, (2016), Feb 26.
- 32.Iwako M., Ino Y., Motoyoshi A., Ozeki M., Sato T., Kumuri M. Pharmacological studies of FUT-175, nafamostat mesilate. V. Effects on the pancreatic enzymes and experimental acute pancreatitis in rats. Jpn. J. Pharmacol. 1986;41(2):155–162. doi: 10.1254/jjp.41.155. [DOI] [PubMed] [Google Scholar]
- 33.Hiraishi M., Yamazaki Z., Ichikawa K., Kanai F., Idezuki Y., Onishi K. Plasma collection using nafamostat mesilate and dipyridamole as an anticoagulant. Int. J. Artif. Organs. 1988;11(3):212–216. [PubMed] [Google Scholar]
- 34.Hirota M., Shimosegawa T., Kitamura K., Takeda K., Takeyama Y., Mayumi T. Continuous regional arterial infusion versus intravenous administration of the protease inhibitor nafamostat mesilate for predicted severe acute pancreatitis: a multicenter, randomized, open-label, phase 2 trial. J. Gastroenterol. 2020;55(3):342–352. doi: 10.1007/s00535-019-01644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto M., Matsuyama S., Li X., Takeda M., Kawaguchi Y., Inoue J.I. Identification of nafamostat as a potent inhibitor of middle east respiratory syndrome coronavirus S protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay. Antimicrob. Agents Chemother. 2016;60(11):6532–6539. doi: 10.1128/AAC.01043-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronoavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ge X.Y., Li J.L., Xang X.L., Chumura A.A., Zhu G., Epstein J.H. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503(7477):535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song W., Gui M., Wang X., Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathol. 2018;14(8) doi: 10.1371/journal.ppat.1007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner A.J., Tipnis S.R., Guy J.L., Rice G., Hooper N.M. ACEH/ACE2 is a novel mammalian metallocarboxypeptidase and a homologue of angiotensin-converting enzyme insensitive to ACE inhibitors. Can. J. Physiol. Pharmacol. 2002;80(4):346–353. doi: 10.1139/y02-021. [DOI] [PubMed] [Google Scholar]
- 41.Ben-Zvi I., Kivity S., Langevitz P., Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin. Rev. Allergy Immunol. 2012;42(2):145–153. doi: 10.1007/s12016-010-8243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savarino A., Di Trani L., Donatelli I., Cauda R., Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect. Dis. 2006;6(2):67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan Y., Zou Z., Sun Y., Li X., Xu K.F., Wei Y. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013;32(2):300–302. doi: 10.1038/cr.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2(August 22):69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Bari M.A.A. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol. Res. Perspect. 2017;5(1) doi: 10.1002/prp2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keyaerts E., Vijge L., Maes P., Neyts J., Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem. Biophys. Res. Commun. 2004;323(1):264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao X., Ye F., Zhang M., Cui C., Huang B., Nui P. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;(March 9) doi: 10.1093/cid/ciaa237. pii: ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14(1) doi: 10.5582/bst.2020.01047. [53] [DOI] [PubMed] [Google Scholar]
- 49.Stahlmann R., Lode H. Medication for COVID-19 – an overview of approaches currently under study. Arztebl. 2020;117(13):213–219. doi: 10.3238/arztebl.2020.0213. (2020) 72-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a tratment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;(March 20):105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRXiv. 2020;(March 31) doi: 10.1101/2020.03.22.20040758. [DOI] [Google Scholar]
- 52.Zhang Q., Wang Y., Qi C., Shen L., Li J. Clinical trial analysis of 2019-nCoV therapy registered in China. J. Med. Virol. 2020;(February 28) doi: 10.1002/jmv.25733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care. 2020;(March 10) doi: 10.1016/j.jcrc.2020.03.005. pii: S0883-9441(20)30390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan K.W., Wong V.T., Tang S.C.W. COVID-19: An update on the epidemiological, clinical, preventive and therapeutic evidence and guidelines of integrative Chinese-Western medicine for the management of 2019 novel coronavirus disease. Am. J. Chin. Med. (Gard City N Y) 2020;(March 13):1–26. doi: 10.1142/S0192415X20500378. [DOI] [PubMed] [Google Scholar]
- 55.NCT04303507, ClinicalTrials.gov, (2020), Mar 11.
- 56.NCT04303299, ClinicalTrials.gov, (2020), Mar 11.
- 57.Bailly C. Cepharanthine: An update of its mode of action, pharmacological properties and medical applications. Phytomedicine. 2019;62(September):152956. doi: 10.1016/j.phymed.2019.152956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khoja S., Huynh N., Warnecke A.M.P., Asatryan L., Jakowec M.-W., Davies D.L. Preclinical evaluation of avermectins as novel therapeutic agents for alcohol use disorders. Psychopharmacology (Berl.) 2018;235(6):1697–1709. doi: 10.1007/s00213-018-4869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tickell-Painter M., Maayan N., Saunders R., Pace C., Sinclair D. Mefloquine for preventing malaria during travel to endemic areas. Cochrane Database Syst. Rev. 2017;10(October 30) doi: 10.1002/14651858.CD006491.pub4. CD006491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan H.H., Wang L.Q., Liu W.L., An X.P., Liu Z.D., He X.Q. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus (2019-nCoV) related coronavirus model. Chin. Med. J. 2020;(March 6) doi: 10.1097/CM9.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang L., Sexton D.J., Skogerson K., Devlin M., Smith R., Sanyal I. Novel peptide inhibitors of angiotensin-converting enzyme 2. J. Biol. Chem. 2003;278(18):15532–15540. doi: 10.1074/jbc.M212934200. [DOI] [PubMed] [Google Scholar]
- 62.Pedersen K.B., Sriramula S., Chhabra K.H., Xia H., Lazartigues E. Species-specific inhibitor sensitivity of angiotensin-converting enzyme 2 (ACE2) and its implication for ACE2 activity assays. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301(5):R1293–R1299. doi: 10.1152/ajpregu.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ye M., Wysocki J., Gonzalez-Pacheco F.R., Salem M., Evora K., Garcia-Halpin L. Murine recombinant angiotensin-converting enzyme 2: effect on angiotensin II-dependent hypertension and distinctive angiotensin-converting enzyme 2 inhibitor characteristics on rodent and human angiotensin-converting enzyme 2. Hypertension. 2012;60(3):730–740. doi: 10.1161/HYPERTENSIONAHA.112.198622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guy J.L., Jackson R.M., Jensen H.A., Hooper N.M., Turner A.J. Identification of critical active-site residues in angiotensin-converting enzyme 2 (ACE2) by site-directed mautagenesis. FEBS J. 2005;272(14):3512–3520. doi: 10.1111/j.1742-4658.2005.04756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han D.P., Penn-Nicholson A., Cho M.W. Identification of critical determinants on ACE2 for SARS-CoV entry and development of a potent entry inhibitor. Virology. 2006;350(1):15–25. doi: 10.1016/j.virol.2006.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mores A., Matziari M., Beau F., Cuniasse P., Yiotakis A., Dive V. Development of potent and selective phosphinic peptide inhibitors of angiotensin-converting enzyme 2, J. Med. Chem. 2008;51(7):2216–2226. doi: 10.1021/jm701275z. [DOI] [PubMed] [Google Scholar]
- 67.Trask A.J., Groban L., Westwood B.M., Varagic J., Ganten D., Gallagher P.E. Inhibition of angiotensin-converting enzyme 2 exacerbates cardiac hypertrophy and fibrosis in Ren-2 hypertensive rats. Am. J. Hypertens. 2010;23(6):687–693. doi: 10.1038/ajh.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huentelmann M.J., Zubcevic J., Hernandez Prada J.A., Xiao X., Dimitrov D.S., Raizada M.K. Structure-based discovery of a novel angiotensin-converting enzyme 2 inhibitor. Hypertension. 2004;44(6):903–906. doi: 10.1161/01.HYP.0000146120.29648.36. [DOI] [PubMed] [Google Scholar]
- 69.Haga S., Nagata N., Okamura T., Yamamoto N., Sata T., Yamamoto N. TACE antagonists blocking ACE2 shedding caused by the spike protein of SARS-CoV are candidate antiviral compounds. Antiviral Res. 2010;85(3):551–555. doi: 10.1016/j.antiviral.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mohler K.M., Sleath P.R., Fitzner J.N., Ceretti D.P., Anderson M., Kerwar S.S. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature. 1994;370(6486):218–220. doi: 10.1038/370218a0. [DOI] [PubMed] [Google Scholar]
- 71.Takahashi M., Terada Y., Nakai I., Nakanishi H., Yoshimura E., Mori S. Role of nicotianamide in the intracellular delivery of metals and plant reproductive development. Plant Cell. 2003;15(6):1263–1280. doi: 10.1105/tpc.010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takahashi S., Yoshiya T., Yoshizawa-Kumagaye K., Sugiyama T. Nicotianamide is a novel angiotensin-converting enzyme 2 inhibitor in soybean. Biomed. Res. 2015;36(3):219–224. doi: 10.2220/biomedres.36.219. [DOI] [PubMed] [Google Scholar]
- 73.Naujokat C., McKee D.L. The "big five" phytochemicals targeting cancer stem cells: curcumin, EGCG, sulforaphane, resveratrol and genistein. Curr. Med. Chem. 2020;(February 27) doi: 10.2174/0929867327666200228110738. [DOI] [PubMed] [Google Scholar]
- 74.Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M. Inhibition of SARS-CoV2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020 doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531(7594):381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lo M.K., Jordan R., Arvey A., Sudhamsu J., Shrivastava-Ranjan P., Hotard A.L. GS-5734 and its parent nucleoside analog inhibit filo-, pneumo-, and paramyxoviruses. Sci. Rep. 2017;7(1):43395. doi: 10.1038/srep43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9(396):eaal3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9(2):e00221–18. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. Mechanisms of inhibition of Ebola virus RNA-dependent RNA polymerase by remdesivir. Viruses. 2019;11(4):326. doi: 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from middle east respiratory syndrome coronavirus. J. Biol. Chem. 2020;(February 24) doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the 345 rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. U.S.A. 2020;(February 13) doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mulangu S., Dodd L.E., Davey R.T., Thsihani Mbaya O., Proschan M., Mukadi D. A randomized, controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A. Compassionate use of remdesivir for patients with severe COVID-19. N. Engl. J. Med. 2020;(April 10) doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.NCT04252664, ClinicalTrials.gov, (2020), Feb 5.
- 85.NCT04257656, ClinicalTrials.gov, (2020), Feb 6.
- 86.NCT04280705, ClinicalTrials.gov, (2020), Feb 21.
- 87.NCT04292730, ClinicalTrials.gov, (2020), Mar 3.
- 88.NCT04292899, ClinicalTrials.gov, (2020), Mar 3.
- 89.NCT04302766, ClinicalTrials.gov, (2020), Mar 10.
- 90.NCT04314817, ClinicalTrials.gov, (2020), Mar 19.
- 91.NCT04315948, ClinicalTrials.gov, (2020), Mar 20.
- 92.Sham H.L., Kempf D.J., Molla A., Marsh K.C., Kumar G.N., Chen C.M. ABT-378, a highly potent inhibitor of the human immunodeficiency virus protease. Antimicrob. Agents Chemother. 1998;42(12):3218–3224. doi: 10.1128/aac.42.12.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Benson C.A., Deeks S.G., Brun S.C., Gulick R.M., Eron J.J., Kessler H.A. Safety and antiviral activity at 48 weeks of lopinavir/ritonavir plus nevirapine and 2 nucleoside reverse-transcriptase inhibitors in human immunodeficiency virus type 1-infected protease inhibitor-experienced patients. J. Infect. Dis. 2002;185(5):599–607. doi: 10.1086/339014. [DOI] [PubMed] [Google Scholar]
- 94.Corbett A.H., Lim M.L., Kashuba A.D. Kaletra (lopinavir/ritonavir) Ann. Pharmacother. 2002;36(7-8):1193–1203. doi: 10.1345/aph.1A363. [DOI] [PubMed] [Google Scholar]
- 95.Chu C.M., Cheng V.C.C., Hung I.F.N., Wong M.M.L., Chan K.H., Chan K.S. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chan J.F., Yao Y., Yeung M.L., Deng W., Bao L., Jia L. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 2015;212(12):1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arabi Y.M., Asiri A.Y., Assiri A.M., Aziz Jokhdar H.A., Alothman A., Balkhy H.H. Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-β1b (MIRACLE trial): statistical analysis plan for a recursive two-stage group sequential randomized controlled trial. Trials. 2020;21(1):8. doi: 10.1186/s13063-019-3846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;(March 18) doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.NCT04255017, ClinicalTrials.gov, (2020), Feb 5.
- 100.NCT04261907, ClinicalTrials.gov, (2020), Feb 10.
- 101.NCT04286503, ClinicalTrials.gov, (2020), Feb 27.
- 102.NCT04295551, ClinicalTrials.gov, (2020), Mar 4.
- 103.NCT04276688, ClinicalTrials.gov, (2020), Feb 19.
- 104.NCT04307693, ClinicalTrials.gov, (2020), Mar 13.
- 105.Blaising J., Polyak S.J., Pécheur E.I. Arbidol as a broad sprctrum antivital: un update. Antiviral Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leneva I.A., Russell R.J., Boriskin Y.S., Hay A.J. Characteristics of arbidol-resistant mutants of influenza virus: implications for the mechanism of anti-influenza action of arbidol. Antiviral Res. 2009;81(2):132–140. doi: 10.1016/j.antiviral.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 107.Boriskin Y.S., Pécheur E.I., Polyak S.J. Arbidol: a broad-spectrum antiviral that inhibits acute and chronic HCV infection. Virol. J. 2006;3:56. doi: 10.1186/1743-422X-3-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Blaising J., Lévy P.L., Polyak S.J., Stanifer M., Boulant S., Pécheur E.I. Arbidol inhibits viral entry by interfering with clathrin-dependent trafficking. Antiviral Res. 2013;100(1):215–219. doi: 10.1016/j.antiviral.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 109.Pécheur E.I., Borisevich V., Halfmann P., Morrey J.D., Smee D.F., Prichard M. The synthetic antiviral drug arbidol inhibits globally prevalent pathogenic viruses. J. Virol. 2016;90(6):3086–3092. doi: 10.1128/JVI.02077-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hulseberg C.E., Fénéant L., Szymanska de Wijs K.M., Kessler N.P., Nelson E.A., Shoemaker C.J. Arbidol and other low-molecular-weight drugs that inhibit Lassa and Ebola viruses. J. Virol. 2019;93(8):e02185–18. doi: 10.1128/JVI.02185-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020;(March 16) doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H. Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study. J. Infect. 2020;(March 11) doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.NCT04255017, ClinicalTrials.gov, (2020), Feb 5.
- 114.NCT04260594, ClinicalTrials.gov, (2020), Feb 7.
- 115.NCT04273763, ClinicalTrials.gov, (2020), Feb 18.
- 116.NCT04286503, ClinicalTrials.gov, (2020), Feb 27.
- 117.Furuta Y., Takahashi K., Fukuda Y., Kuno M., Kamiyama T., Kozaki K. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob. Agents Chemother. 2002;46(4):977–981. doi: 10.1128/AAC.46.4.977-981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Furuta Y., Komeno T., Nakamura T., (T-705) Favipiravir. A broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jin Z., Smith L.K., Rajwanshi V.K., Kim B., Deval J. The ambiguous base-pairing and high substrate efficiency of T-705 (favipiravir) ribofuranosyl 5’-triphosphate towards influenza A virus polymerase. PLoS One. 2014;8(7) doi: 10.1371/journal.pone.0068347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Delang L., Abdelnabi R., Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral Res. 2018;153:85–94. doi: 10.1016/j.antiviral.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 121.Furuta Y., Takahashi K., Shiraki K., Sakamoto K., Smee D.F., Barnard D.L. T-705 (favipiravir) and related compounds: novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res. 2009;82(3):95–102. doi: 10.1016/j.antiviral.2009.02.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sissoko D., Laouenan C., Folkesson E., M´Lebing A.B., Beavogui A.H., Baize S. Experimental treatment with favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med. 2016;13(3) doi: 10.1371/journal.pmed.1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.NCT04310228, ClinicalTrials.gov, (2020), Mar 17.
- 124.NCT04303299, ClinicalTrials.gov, (2020), Mar 11.
- 125.Anand K., Ziebuhr J., Wadhwani P., Mesters J.R., Hilgenfeld R. Coronavirus main protease (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300(5626):1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- 126.Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;(March 20) doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y. Structure of Mpro from COVID-19 virus and discovery of its inhibitors. Nature. 2020;(April 9) doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 128.Lim H., Min D.S., Park H., Kim H.P. Flavonoids interfere with NLRP3 inflammasome activation. Toxicol. Appl. Pharmacol. 2018;355:93–102. doi: 10.1016/j.taap.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 129.Chen I.Y., Moriyama M., Chang M.F., Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol. 2019;10(50) doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang B.C., Li Z., Xu W., Xiang C.H., Ma Y.F. Luteolin alleviates NLRP3 inflammasome activation and directs macrophage polarization in lipopolysaccharide-stimulated RAW264.7 cells. Am. J. Transl. Res. 2018;10(1):265–273. [PMC free article] [PubMed] [Google Scholar]
- 131.Chen H., Lin H., Xie S., Huang B., Qian Y., Chen K. Myricetin inhibits NLRP3 inflammasome activation via reduction of ROS-dependent ubiquitination of ASC and promotion of ROS-independent NLRP3 ubiquitination. Toxicol. Appl. Pharmacol. 2019;365:19–29. doi: 10.1016/j.taap.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 132.Yamagata K., Hashiguchi K., Yamamoto H., Tagami M. Dietary apigenin reduces induction of LOX-1 and NLRP3 expression, leukocyte adhesion, and acetylated low-density lipoprotein uptake in human endothelial cells exposed to trimethylamine-N-oxide. J. Cardiovasc. Pharmacol. 2019;74(6):558–565. doi: 10.1097/FJC.0000000000000747. [DOI] [PubMed] [Google Scholar]
- 133.Choe J.Y., Kim S.K. Quercetin and ascorbic acid suppress fructose-induced NLRP3 inflammasome activation by blocking intracellular shuttling of TXNIP in human macrophage cell lines. Inflammation. 2017;40(3):980–994. doi: 10.1007/s10753-017-0542-4. [DOI] [PubMed] [Google Scholar]
- 134.Lim H., Min D.S., Park H., Kim H.P. Flavonoids interfere with NLRP3 inflammasome activation. Toxicol. Appl. Pharmacol. 2018;355:93–102. doi: 10.1016/j.taap.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 135.Fu S., Xu L., Li S., Qiu Y., Liu Y., Wu Z. Baicalin suppresses NLRP3 inflammasome and nuclear factor-kappa B (NF-κB) signaling during haemophilus parasuis infection. Vet. Res. 2016;47(1):80. doi: 10.1186/s13567-016-0359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun Y., Zhao Y., Yao J., Zhao L., Wu Z., Wang Y. Wogonoside protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NF-κB and NLRP3 inflammasome activation. Biochem. Pharmacol. 2015;94(2):142–154. doi: 10.1016/j.bcp.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 137.Dai W., Bi J., Li F., Wang S., Huang X., Meng X. Antiviral efficacy of lavonoids against enterovirus 71 infection in vitro and in newborn mice. Viruses. 2019;11(7):625. doi: 10.3390/v11070625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Moghaddam E., Teoh B.T., Sam S.S., Lani R., Hassandarvish P., Chik Z. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci. Rep. 2014;4:5452. doi: 10.1038/srep05452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ho T.Y., Wu S.L., Chen J.C., Li C.C., Hsiang C.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. 2007;74(2):92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lin S.C., Ho C.T., Chuo W.H., Li S., Wang T.T., Lin C.C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017;17(1):144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kupferschmidt K., Cohen J. WHO launches global megatrial of the four most promising coronavirus treatments. Science. 2020;(March 2) https://www.sciencemag.org/news/2020/03/who-launches-global-megatrial-four-most-promising-coronavirus-treatments [Google Scholar]
- 142.Mitja O., Clotet B. Use of antiviral drugs to reduce COVID-19 transmission. Lancet Glob. Health. 2020;(March 19) doi: 10.1016/S2214-109X(20)30114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wölfel R., Corman V.C., Guggemos W., Seilmaier M., Zange S., Müller M.A. Virological assessment of hospitalized patients with COVID-19. Nature. 2020;(April 1) doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 144.Sheahan T.P., Sims A.C., Zhou S., Graham R.L., Pruijssers A.J., Agostini M.L. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020;(April 4) doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]