Abstract
Drought is one of the most prominent limiting factors that negatively affect crop productivity by manipulating its physiological pathway. One hundred twenty diverse bread wheat genotypes were used in a pot experiment to explore the relationship among their fifteen physio-biochemical traits (PBT) by using multivariate analysis, heatmapping and stress tolerance index (STI) for grain yield as a marker trait to identify high yielding genotype with maximum stress tolerance capability. Increased proline and sugar accumulation were observed from control to moisture deficient environments by 159% and 122%, respectively. Moreover, leaf membrane stability index (LMSI), leaf relative water content (LRWC), relative dry weight (RDW), chlorophyll content, leaf surface area (LSA), Leaf succulence (LS), canopy temperature depression (CTD), relative excised leaf water loss (RELWL) and leaf osmotic potential (LOP) showed significantly decreasing trend in drought stress treatment as compared to well-watered plants by −21%, −21%, −34%, −22%, −38%, −37%, −46%, −18% and −35% respectively. Additionally, principal component analysis and genotype by trait biplot analysis showed that initial 7 principal components (PC1 to PC7) represented 77.27% and 79.02% of total cumulative variation under control and drought stress respectively. Genotypic-Phenotypic correlation revealed that most of the attributes were higher in case of genotypic correlation component (rg) as compared to the phenotypic correlation component (rp) indicating more genetic association between traits. The darker and lighter colour scale produced by heatmap exhibited contrasting nature of genotypes, as positive side with higher values represented drought resistance while values on the negative side with lower values showed susceptible performance of genotypes. Our results concluded that the studied PBT associated with STI for grain yield are the main factors which may contribute in improved productivity of wheat crop and if these traits show appropriate performance under stress condition the crop will show the more productive returns under changing climate.
Keywords: Drought, Wheat, Physio-biochemical traits, Stress tolerance index, Heatmap, Correlation coefficient
Graphical abstract
Highlights
-
•
Physio-biochemical traits as an indicator of wheat drought stress tolerance
-
•
Multivariate statistical analysis for the selection of drought resistance genotypes
-
•
Genotypic-Phenotypic correlation indicated more genetic association between traits.
-
•
Heatmap positive side represents drought resistance while negative side showed susceptible performance of genotypes.
1. Introduction
Climate change is a prevalent concern that has imposed several challenging ventures on contemporary agriculture. The rising temperature and varying precipitation caused by climate change have been widely reported to increase the frequency and severity of drought throughout the world (Ahmed et al., 2012a, Ahmed et al., 2012b; Hui-Mean et al., 2018). Recent work depicted the extensive impacts of climate change on crop production not just unanimously declared its negative impact on worldwide food production but also predicted the more severe influence on crop production in future (Khan et al., 2020; Asseng et al., 2019; Ramirez-Cabral et al., 2017; Pravalie et al., 2020). Eighty percent of the global agricultural rainfed farming land that produces about 70% of the world's staple foods is under threat due to variability in rain under changing climate (Sharma et al., 2010; van Ogtrop et al., 2014). Ahmed et al., 2019a, Ahmed et al., 2019b, Ahmed et al., 2019c reported that 17–70% yield losses in cereals are caused by water shortage. Wheat is a widely cultivated staple food is more exposed to water deficit conditions that can result in 50–90% yield reduction compared to its irrigated potential (Awan et al., 2017). It provides livelihood security to most of the people in Pakistan and is under threat due to multiple stress factors (Liu et al., 2019). Drought is one of the most important abiotic factor that reduces the yield under rainfed environment (Forouzani and Karami, 2011; Liu and Hwang, 2015). It could be more challenging for the cereals cultivating regions of South Asia that comprises arid and semi-arid climates e.g. Pakistan. Around 25% of the Pakistan cultivated land under is rainfed and it is expected that in future yields of the important crops like wheat, corn, rice, pearl millet and mustard will decrease in this region. These yield reductions, especially in staple crops, could be worse for the agriculture-based economies like Pakistan where wheat contains the largest share of the total cultivated farm area, contributing 2.6% in GDP and accounts for 8.9% value-added in agriculture (Mahmood et al., 2019). Since frequency and occurrence of drought spells are increasing day by day due to the climate change. The drought tolerance mechanism is complex and controlled by many traits. Identification of traits that are involved in drought stress tolerance could help the breeders to develop drought tolerant genotypes.
Wheat is one of the most important cereal as a food source for >50% of the world's population (McKevith, 2004). As a consequence of burgeoning population, its inclining demand is expected to reach up to 40%. The yield of wheat is compromised due to various abiotic and biotic factors, and drought is among the prevalent constraints (Abbas et al., 2005). Drought, an accumulated, recurring environmental hazard is believed to be caused by precipitation deficit and excess evapotranspiration for longer spells (Touma et al., 2015; Zhang et al., 2019). Its frequency and severity are expected to become more severe in coming days due to global warming (Farooq et al., 2009; Wang et al., 2019). Wheat sensitivity to drought stress has been reported by the various researchers (Wang et al., 2019) and it can cause up to 90% yield loss depending upon the growth stage, genotype, and intensity and duration of drought spell. Drought stress results in inhibition of photosynthesis that have been associated with decrease in chlorophyll content, cell membrane stability, causing loss of membrane permeability and damage to the various physiological and biochemical functions that eventually affect the growth of plant (Ahmed et al., 2019a, Ahmed et al., 2019b, Ahmed et al., 2019c; Batool et al., 2013; Ma et al., 2017). However, the response of plants regarding leaf water relations, stomatal regulations, photosynthesis and other regulatory processes could differ among the species and varieties (Wang et al., 2019). Therefore, attaining drought tolerance to cope with the declining water resources and to lessen the damaging effects on crop growth, utilization of available variability of wheat genotypes under normal and drought conditions through PBT can be very useful.
Plant PBT traits are considered an important selection tool for improvement against drought stress due to their relationship with the adaption mechanisms of plants under stressful conditions (Sallam et al., 2019). Drought tolerant plants tend to maintain high water content and accumulate osmo-regulators like soluble sugars and proline to cope with the prevailing stress conditions (Abid, 2016). Wheat plants can change their phenotype and the partitioning of dry matter in response to drought stress, e.g. smaller plants, smaller leaf area, increased root biomass or less green leaf area, thereby reducing injury under drought stress (Richards et al., 2010). Wheat genotypes with higher values of stress tolerance index (STI) are generally recognized as drought tolerated genotypes (Mohammadijoo et al., 2015). Moreover, Nouraein et al. (2013) also elaborated that STI has more benefits for the selection of appropriate crop cultivars in both stress and non-stress climatic variabilities. Since, Pakistan being a hub of wheat origin, have plentiful germplasm of wheat (Anwaar et al., 2019), the assessment of drought impacts and wheat response to drought was studied using a large number of genotypes to observe the PBT response of wheat germplasm exposed to optimum and drought stress regimes. It was hypothesized that the PBT under study would respond differently under the two different circumstances, and their relative performance in this perspective will prove useful for the future breeding programs related to the improvement of wheat tolerance against moisture stress. In this context, the core intention of the present study was to explore the impacts of drought stress on bread wheat PBT. The study aims to perform a comprehensive statistical assessment of the association among various PBT under optimum and moisture deficient regimes which will suggest the basis of drought tolerance for dry-land cropping in rainfed and semiarid areas of the world.
2. Materials and methods
2.1. Experimental site
The experiment was conducted at experimental site of Wheat Wide Crosses, National Agriculture Research Center (NARC), Islamabad, Pakistan (latitude of 33°37′N; longitude of 73°5′E; altitude 1770 ft), with day/night average temperature of 30 ±8 °C and 15 ±5 °C, respectively. The average total rainfall and relative humidity of the site were 261.23 mm and 45–64% respectively, during the growing period of the crop given in the Supplementary material (Table S1).
2.2. Genetic material
Germplasm of one hundred and twenty (120) diverse wheat (Triticum aestivum L.) genotypes were collected from various sources, including Plant Genetics Resource Institute, National Agricultural Research Centre Islamabad (PGRI-NARC), Wheat Program, National Agricultural Research Centre Islamabad (WP-NARC), Barani Agricultural Research Institute (BARI), Chakwal and Directorate of Agriculture Research (Cereal Crops) Agriculture Research Institute, Quetta (Supplementary material, Table S2). This material was composed of different approved Triticum aestivum cultivars and advanced lines.
2.3. Experimental description
Two-way factorial randomized complete design (CRD) with three replications was used in the current study. The first factor was genotypes and the second factor represented the induction of drought treatment. The plants were grown in plastic pots having an approximate dimension of 28″ of top diameter, 21″ of base diameter and 8″depth, in which 8 kg of sandy loam soil was used during the wheat growing season of 2017–18. Six seeds were sown in each pot, and optimum agronomical and cultural practices including weeding and hoeing were pursued throughout the growing period of the crop.
Physiological and biochemical traits (PBT) with grain yield as a marker trait of all collected wheat genotypes were studied by arranging pots in two environments i.e. Normal environment (E1) as control and water stress environment (E2). Both sets of plants were sown on 1st November 2017 and harvested at physiological maturity on 20th April 2018. The control set of plants were kept in the open environment under ordinary conditions while the second arrangement of drought treatment of pots was set aside under rain shelter and plants were exposed to a dry spell cycle at pre-anthesis (95 ±10 Days after sowing) stage according to BBCH (Biologische Bundesanstalt, Bundessortenamt und CHemische Industrie) Code No. 56–57 based on cereal code system of Zadoks (Lancashire et al., 1991; Zadoks et al., 1974), where 60–70% of inflorescence emerged. The control set of pots were watered normally to maintain a well-watered level as per optimum basis whenever required. The drought was induced by withholding supply of water for about 8–12 days up-till the symptoms of drought in the form of temporary leaf wilting or leaf rolling started (Gusmao et al., 2012). Once, plants reached pre-anthesis stages; irrigation was intermittent for 8–12 days for stressed arrangements of pots as reported by Bajji et al. (2001) while control set of plants were watered normally.
2.4. Soil physiochemical properties and fertilizer
The soil was analyzed before the sowing of the crop. The soil texture was loamy (Silt % = 0.35, Sand % = 0.31 and Clay % = 0.34) with observed electrical conductivity (EC; d Sm−1) of 0.30, pH: 7.31, available phosphorus (P): 16.24 mg kg−1, available potassium (K): 218.38 mg kg−1, and soil saturation of 35%. Similarly measured nitrogen (N) percentage was 0.042 with NO3 −1-N content (mg kg−1) of 7.80. Soil organic carbon percentage was 0.85 with bulk density of 1.30 g cm−3. Soil drain upper limit (mm mm−1) was 0.38 with lower limit value of 0.10 mm mm−1. The soil was sieved by using 1.0 cm soil sieve and blended with urea fertilizer of N/P/K (5.0/3.0/2.0 g pot−1) along with di-ammonium sulfate, potassium phosphate and zinc sulfate for every pot.
2.5. Quantification of physio-biochemical traits
Fully appeared flag leaves were excised from every replication in both the treatments at late morning between 11.00 and 12.00 am-noon and utilized for the measurement of physiological and biochemical traits, which includes: (i) Proline content (PC) (ii) Total soluble sugar content (Sugar) (iii) Leaf membrane stability index (LMSI) (iv) Leaf relative water content (LRWC) (v) Relative dry weight (RDW) (vi) Relative saturation deficit (RSD) (vii) Water saturation deficit (WSD) (viii) Relative excised leaf water loss (RELWL) (ix) Leaf osmotic potential (LOP) (x) Chlorophyll content (SPAD) (xi) Leaf surface area (LSA) (xii) Leaf succulence (LS) (xiii) Canopy temperature (CnpTemp) (xiv) Canopy temperature depression (CTD) and (xv) Epicuticle wax content (EWC).
Proline content (mg g−1 FW) in leaves was measured by the technique of Bates et al. (1973), and Sugar (mg g−1 FW) was measured according to the procedure given by Dubois et al. (1951) with some modification as described by Johnson et al. (1966).
LMSI (%) for control treatment and stressed set of pots was estimated by the technique proposed by Sairam et al. (1997).
| (1) |
where C1 and C2 are the conductivity before and after autoclaving, respectively for control and stressed treatments.
The leaf water-related traits including LRWC (%), RSD (%), WSD, RDW and RELWL were computed as described earlier (Barrs and Weatherley, 1962; Turner, 1986; Sangakkara et al., 1996; Ali and Awan, 2009; Clarke, 1992).
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
where FW, DW, TW and W4h were fresh weight, dry weight, turgid weight and weight after 4 h in grams respectively of the incubation period.
Leaf Osmotic Potential (LOP; Osmol Kg−1) was measured by rehydration method on Osmometer-030 (Gonotec) Cryoscopy Osmometer as proposed by Blum (1989).
| (7) |
where C is the concentration (Osmometer reading), R is Gas Factor (0.008314), and T is laboratory temperature in Kelvin, i.e. 298 K used for all genotypes in both environments (E1 and E2) for the calculation of LOP only.
Chlorophyll content was determined with the “Konica Minolta SPAD-502” by taking three averages of five flag leaves per pot (i.e. 3 × 5 leaves) as reported by Babar et al. (2006). Canopy temperature (°C) and CTD (°C) was measured from each pot by using a Handheld Infrared Thermometer (Model AG-42.telatemp crop, Fullerton, CA) on bright and clear sunny days between 1 pm to 3 pm with an approximate distance of half to one-meter from the upper edge of the pot and about 50 (±5) cm above the canopy with an fairly accurate angle of 30°–60° from straight giving a canopy view of 10 cm × 25 cm (Ayeneh et al., 2002).
| (8) |
where the ambient temperature of the open experimental area was observed with Handheld Thermometer.
Leaf surface area (cm2) was measured manually by taking leaf length and width at a maximum broader portion of all leaves of whole five guarded plants and subtracting the results with wheat leaf area Co-factor, i.e., 0.83 (Xiong et al., 2006). Leaf succulence (LS) was calculated as proposed by Allen Jr et al. (1991). Epicuticle wax content (μg m−2) was measured by the method proposed by Fernandes et al. (1964).
| (9) |
| (10) |
Moreover, Grain yield per plant was recorded as a marker trait from all the spikes of a plant from each pot for all the test genotypes. This was further used to calculate stress tolerance index (STI) according to the formula given by Fernandez (1992).
| (11) |
where Yp is grain yield of a test genotype under non-stressed condition (E 1), Ys is grain yield of a test genotype under drought-stressed condition (E 2) and MYp is mean yield of all test genotypes under non-stressed condition (Supplementary material, Table S3).
2.6. Statistical data analysis
The data was analyzed and evaluated by “IBM Statistical Program for Social Science (SPSS Version 22)” software with Analysis of Variance (ANOVA) under General Linear Model (GLM) according to Steel et al. (1997). One hundred and twenty diverse bread wheat genotypes and water treatment (two levels in the pot experiment) were all set as Fixed Factors, and all of the physiological traits under study were individually placed as Dependent Variables.
A model was selected to analyze genotypes, water treatments, and the interaction between genotypes and Genotype × W-Treatment. Furthermore, if the significant differences were observed, the posthoc test was carried out. Tukey's Honestly Significant Difference (HSD) test was used as a posthoc test to locate the significant differences in the interaction effects; P < 0.05 was considered statistically significant. Principal component analysis (PCA) and Heatmap was performed by using R-Studio software to categorize various physiological plant traits which depict the distinctness among the wheat genotypes, while genetic and phenotypic correlation study was done to study correlation patterns for each trait at genotypic and phenotypic level as phenotypic correlation reflects genotypic correlation which is inherited and represents nature of association between two traits. For this purpose, software Multi Environment Trail Analysis with R for Windows (META-R Version-6.03-CIMMYT) was utilized (R Development Core Team, 2020).
3. Results
3.1. Proficiency of physiological mechanics and stress tolerance index for grain yield
Drought treatment significantly influenced the rate and efficiency of all of the PBT by causing serious damages to various physiological pathways. The obtained results from CRD Two Factorial Analysis of Variance (ANOVA) signposted that there was a significant variance between the genotypes with regard to the studied traits at both of the moisture treatments. All of the PBT showed a significantly different response at pre-anthesis stage independently, except canopy temperature and canopy temperature depression, which responded non-significantly in terms of genotypes and Genotype × Water treatments (W-Treatments) interaction but revealed significant shadow with respect to water treatments effects (Table 1 ).
Table 1.
Mean squares values for the physiological and biochemical traits (PBT) through General Linear Model Analysis of Variance (GLM-ANOVA).
| Variables | Corrected model |
Intercept |
Genotype |
W-Treatment |
Genotype × W-Treatment |
Error |
|---|---|---|---|---|---|---|
| (d.f.: 239) | (d.f.: 1) | (d.f.: 119) | (d.f.: 1) | (d.f.: 119) | (d.f.: 480) | |
| Proline content (mg g−1 FW) | 0.784 | 875.981 | 0.075⁎⁎ | 171.366⁎⁎ | 0.059⁎⁎ | 0.002 |
| Sugar (mg g−1 FW) | 2.457 | 3678.440 | 0.282⁎⁎ | 525.941⁎⁎ | 0.233⁎⁎ | 0.002 |
| Leaf membrane stability index (%) | 237.220 | 3,244,996.203 | 97.953⁎⁎ | 43,242.311⁎⁎ | 15.099⁎⁎ | 1.074 |
| Leaf relative water content (%) | 299.669 | 3,377,718.743 | 125.751⁎⁎ | 48,803.972⁎⁎ | 65.988⁎⁎ | 6.001 |
| Relative saturation deficit (%) | 217.406 | 406,887.217 | 74.297⁎⁎ | 38,732.828⁎⁎ | 36.856⁎⁎ | 3.612 |
| Water saturation deficit (%) | 299.669 | 714,742.529 | 125.751⁎⁎ | 48,803.972⁎⁎ | 65.988⁎⁎ | 6.001 |
| Relative dry weight | 0.021 | 88.147 | 0.008⁎⁎ | 3.607⁎⁎ | 0.005⁎⁎ | 0.001 |
| Leaf osmotic potential (-MPA) | 0.306 | 2690.183 | 0.108⁎⁎ | 59.546⁎⁎ | 0.006⁎⁎ | 0.000 |
| Chlorophyll content (SPAD) | 129.471 | 1,248,150.685 | 87.121⁎⁎ | 19,377.388⁎⁎ | 10.073⁎⁎ | 1.019 |
| Canopy temperature (°C) | 0.953 | 425,673.903 | 0.222ns | 187.189⁎⁎ | 0.119ns | 0.318 |
| Canopy temperature depression (°C) | 0.953 | 2044.515 | 0.222ns | 187.189⁎⁎ | 0.119ns | 0.318 |
| Relative excised leaf water loss (g−1DW hr−1) | 0.044 | 290.210 | 0.049⁎⁎ | 2.770⁎⁎ | 0.015⁎⁎ | 0.000 |
| Epicuticle wax content (μg m−2) | 13,431.939 | 25,364,537.515 | 3355.399⁎⁎ | 2,427,748.189⁎⁎ | 3220.108⁎⁎ | 4.184 |
| Leaf surface area (cm2) | 95.896 | 281,700.877 | 39.867⁎ | 15,720.551⁎⁎ | 20.625ns | 30.568 |
| Leaf succulence (mg m−2) | 31.364 | 98,430.473 | 15.677⁎⁎ | 5131.651⁎⁎ | 4.191⁎⁎ | 0.622 |
Note: W: Water, FW: Fresh weight, DW: Dry weight, and NS: non-significant.
Significant P < 0.01.
Significant P < 0.05.
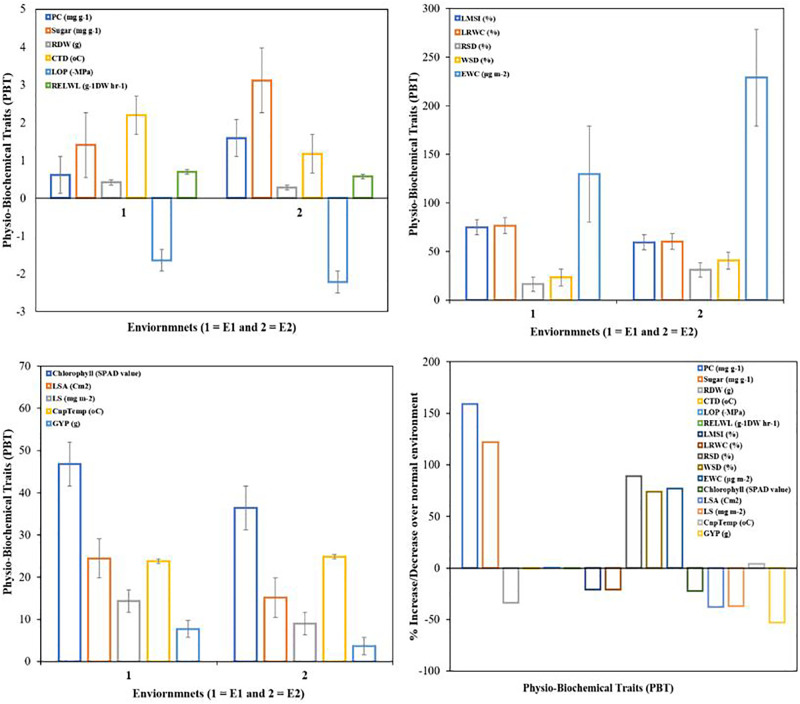
Significant Genotype × W-Treatments interaction is a signal in the modified behavior of breeding germplasm under the umbrella of varied moisture states. Both of the water treatments exhibited significantly different results, as increases in proline and sugar accumulation were observed from control to moisture deficient environments by 159% and 122%, respectively (Fig. 1 ). Moreover, LMSI%, LRWC%, RDW, chlorophyll content, LSA, LS, CTD, RELWL % and LOP showed significantly decreasing trend in drought stress treatment as compared to well-watered plants by −21%, −21%, −34%, −22%, −38%, −37%, −46%, −18% and −35% respectively as shown in Fig. 1. Furthermore, RSD %, WSD %, EWC and CnpTemp depicted higher significant values in moisture lacking states than moisture sufficient situation by 89%, 74%, 77% and 4% correspondingly caused by drought stress.
Fig. 1.
Mean performance of different parameters in control and drought stress (N = 120).
Similarly, a descending trend in performance of different leaf related parameters have been detected in drought stress treatment as compared to the well-watered conditions as drought severely affected leaf water potential of leaf related traits by altering their metabolic activities in leaf tissues and caused dehydration. These traits involve LMSI, LRWC, RDW, chlorophyll content, LSA, LSC, CTD and RELWL. The Analysis of Variance has depicted the significant and non-significant values of some traits showing the response of control conditions as well as the stress conditions based on mean performance of PBT (Table 2 ).
Table 2.
Mean performance of the physiological-biochemical traits (PBT) and stress tolerance index (STI) for grain yield of wheat genotypes under normal (E1) and drought stress (E2).
| Genotypes | Condition | PC | Sugar | LMSI | LRWC | RDW | RSD | WSD | Chlorophyll | LSA | LS | CnpTemp | CTD | LOP | RELWL | EWC | GYP | STI | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NARC-2009 | Normal (E1) | 0.758 | ±0.008 | 1.679 | ±0.012 | 85.41 | ±0.060 | 88.51 | ±1.553 | 0.540 | ±0.004 | 7.453 | ±0.991 | 11.486 | ±1.553 | 53.3 | ±0.578 | 20.4 | ±0.626 | 17.892 | ±0.083 | 23.0 | ±0.122 | 3.03 | ±0.122 | −1.375 | ±0.006 | 0.88 | ±0.009 | 148.73 | ±0.225 | 9.19 | ±0.123 | 0.836 |
| Stress (E2) | 1.956 | ±0.006 | 3.726 | ±0.037 | 72.71 | ±0.473 | 78.22 | ±1.385 | 0.338 | ±0.006 | 16.276 | ±1.208 | 21.780 | ±1.385 | 43.3 | ±0.273 | 11.6 | ±0.251 | 12.537 | ±0.218 | 24.1 | ±0.100 | 1.90 | ±0.100 | −2.015 | ±0.006 | 0.71 | ±0.010 | 314.23 | ±0.838 | 5.38 | ±0.124 | ||
| Pakistan-2013 | Normal (E1) | 0.745 | ±0.004 | 1.658 | ±0.008 | 81.71 | ±0.105 | 86.21 | ±0.645 | 0.525 | ±0.004 | 9.043 | ±0.442 | 13.789 | ±0.645 | 51.3 | ±0.639 | 20.6 | ±0.708 | 17.197 | ±0.097 | 23.4 | ±0.186 | 2.63 | ±0.186 | −1.393 | ±0.003 | 0.87 | ±0.004 | 144.70 | ±1.012 | 8.91 | ±0.092 | 0.758 |
| Stress (E2) | 1.924 | ±0.006 | 3.712 | ±0.032 | 68.80 | ±1.234 | 77.03 | ±1.077 | 0.322 | ±0.005 | 17.367 | ±0.942 | 22.966 | ±1.077 | 42.1 | ±0.088 | 11.9 | ±0.232 | 11.480 | ±0.169 | 24.7 | ±0.145 | 1.27 | ±0.145 | −2.024 | ±0.006 | 0.70 | ±0.003 | 312.14 | ±1.243 | 5.03 | ±0.079 | ||
| Borlaug-2016 | Normal (E1) | 0.484 | ±0.011 | 1.150 | ±0.008 | 69.79 | ±0.168 | 62.75 | ±0.381 | 0.368 | ±0.009 | 27.244 | ±0.433 | 37.252 | ±0.381 | 37.5 | ±0.186 | 21.8 | ±1.333 | 11.210 | ±0.426 | 24.0 | ±0.131 | 2.00 | ±0.131 | −1.926 | ±0.006 | 0.48 | ±0.006 | 108.51 | ±0.515 | 7.11 | ±0.153 | 0.361 |
| Stress (E2) | 1.214 | ±0.008 | 2.451 | ±0.024 | 46.88 | ±0.498 | 57.24 | ±0.826 | 0.236 | ±0.009 | 34.583 | ±0.619 | 42.759 | ±0.826 | 28.8 | ±0.273 | 13.5 | ±0.171 | 6.153 | ±0.023 | 25.1 | ±0.061 | 0.93 | ±0.061 | −2.415 | ±0.004 | 0.33 | ±0.004 | 176.05 | ±0.65 | 3.00 | ±0.07 | ||
| FSD-2008 | Normal (E1) | 0.636 | ±0.008 | 1.571 | ±0.008 | 69.07 | ±0.150 | 82.28 | ±0.626 | 0.475 | ±0.024 | 12.000 | ±0.232 | 17.716 | ±0.626 | 50.8 | ±0.252 | 21.4 | ±0.268 | 16.799 | ±0.421 | 23.9 | ±0.208 | 2.10 | ±0.208 | −1.754 | ±0.006 | 0.74 | ±0.012 | 145.33 | ±1.62 | 7.59 | ±0.297 | 0.404 |
| Stress (E2) | 1.549 | ±0.008 | 2.642 | ±0.046 | 56.35 | ±0.395 | 59.93 | ±0.205 | 0.274 | ±0.002 | 31.451 | ±0.115 | 40.065 | ±0.205 | 39.9 | ±0.418 | 12.4 | ±1.284 | 8.859 | ±0.316 | 24.2 | ±0.150 | 1.83 | ±0.150 | −2.269 | ±0.007 | 0.59 | ±0.012 | 218.15 | ±0.809 | 3.13 | ±0.334 | ||
| Galaxy-2013 | Normal (E1) | 0.632 | ±0.006 | 1.241 | ±0.035 | 75.26 | ±0.228 | 83.17 | ±0.514 | 0.376 | ±0.024 | 12.229 | ±0.254 | 16.828 | ±0.514 | 49.8 | ±0.12 | 25.8 | ±1.025 | 16.560 | ±0.626 | 24.0 | ±0.120 | 2.03 | ±0.120 | −1.591 | ±0.008 | 0.71 | ±0.007 | 110.62 | ±1.239 | 7.66 | ±0.167 | 0.446 |
| Stress (E2) | 1.651 | ±0.020 | 2.578 | ±0.016 | 57.47 | ±0.254 | 59.11 | ±0.764 | 0.278 | ±0.007 | 32.004 | ±0.764 | 40.893 | ±0.764 | 37.2 | ±2.413 | 11.8 | ±0.733 | 11.315 | ±0.254 | 24.4 | ±0.343 | 1.57 | ±0.343 | −2.117 | ±0.012 | 0.67 | ±0.011 | 266.92 | ±0.936 | 3.44 | ±0.274 | ||
| NR-457 | Normal (E1) | 0.659 | ±0.006 | 1.608 | ±0.013 | 74.16 | ±0.199 | 75.89 | ±2.091 | 0.411 | ±0.019 | 17.058 | ±1.247 | 24.113 | ±2.091 | 49.9 | ±1.901 | 21.5 | ±0.654 | 11.420 | ±0.278 | 23.7 | ±0.023 | 2.33 | ±0.023 | −1.686 | ±0.010 | 0.80 | ±0.006 | 124.17 | ±1.141 | 7.48 | ±0.375 | 0.438 |
| Stress (E2) | 1.560 | ±0.057 | 3.339 | ±0.043 | 60.95 | ±0.386 | 58.89 | ±1.368 | 0.281 | ±0.022 | 32.117 | ±1.864 | 41.114 | ±2.138 | 34.6 | ±1.419 | 17.4 | ±0.487 | 9.360 | ±0.193 | 25.0 | ±0.213 | 0.97 | ±0.213 | −2.143 | ±0.006 | 0.46 | ±0.013 | 296.03 | ±1.151 | 3.46 | ±0.153 | ||
| NR-499 | Normal (E1) | 0.756 | ±0.012 | 1.635 | ±0.01 | 82.69 | ±0.163 | 82.21 | ±0.169 | 0.524 | ±0.006 | 11.674 | ±0.153 | 17.790 | ±0.169 | 50.7 | ±0.291 | 20.5 | ±0.565 | 16.627 | ±0.044 | 23.5 | ±0.328 | 2.53 | ±0.328 | −1.401 | ±0.003 | 0.85 | ±0.008 | 149.52 | ±0.311 | 8.71 | ±0.066 | 0.721 |
| Stress (E2) | 1.868 | ±0.012 | 3.671 | ±0.012 | 70.38 | ±0.162 | 74.97 | ±0.791 | 0.301 | ±0.019 | 19.252 | ±1.014 | 25.032 | ±0.791 | 41.9 | ±0.737 | 12.3 | ±0.249 | 11.967 | ±0.141 | 24.5 | ±0.186 | 1.47 | ±0.186 | −2.019 | ±0.005 | 0.69 | ±0.008 | 314.88 | ±0.104 | 4.90 | ±0.113 | ||
| NR-528 | Normal (E1) | 0.605 | ±0.008 | 1.249 | ±0.011 | 81.49 | ±0.130 | 84.98 | ±0.773 | 0.388 | ±0.001 | 10.818 | ±0.565 | 15.018 | ±0.773 | 36.3 | ±0.384 | 20.7 | ±1.021 | 13.069 | ±0.704 | 23.6 | ±0.203 | 2.37 | ±0.203 | −1.782 | ±0.009 | 0.68 | ±0.001 | 140.13 | ±0.709 | 7.54 | ±0.293 | 0.443 |
| Stress (E2) | 1.676 | ±0.019 | 3.193 | ±0.032 | 63.31 | ±0.122 | 56.57 | ±0.708 | 0.306 | ±0.003 | 33.246 | ±0.593 | 43.428 | ±0.708 | 29.3 | ±0.436 | 13.4 | ±0.825 | 9.371 | ±0.047 | 25.0 | ±0.231 | 1.00 | ±0.231 | −2.379 | ±0.006 | 0.50 | ±0.040 | 280.45 | ±0.751 | 3.46 | ±0.297 | ||
| NR-448 | Normal (E1) | 0.507 | ±0.009 | 1.221 | ±0.01 | 75.11 | ±0.129 | 70.88 | ±1.143 | 0.429 | ±0.009 | 20.387 | ±0.92 | 29.123 | ±1.143 | 49.1 | ±0.797 | 24.9 | ±0.725 | 17.566 | ±0.768 | 24.0 | ±0.379 | 2.00 | ±0.379 | −1.520 | ±0.007 | 0.69 | ±0.016 | 145.58 | ±1.62 | 7.57 | ±0.317 | 0.426 |
| Stress (E2) | 1.821 | ±0.021 | 3.466 | ±0.016 | 60.71 | ±0.161 | 57.67 | ±0.877 | 0.237 | ±0.013 | 34.211 | ±0.44 | 42.328 | ±0.877 | 38.9 | ±0.12 | 12.9 | ±0.516 | 11.097 | ±0.457 | 24.9 | ±0.047 | 1.07 | ±0.047 | −2.122 | ±0.006 | 0.57 | ±0.015 | 177.59 | ±0.574 | 3.35 | ±0.288 | ||
| NR-514 | Normal (E1) | 0.469 | ±0.008 | 1.143 | ±0.002 | 68.07 | ±0.299 | 63.16 | ±0.939 | 0.367 | ±0.002 | 26.948 | ±0.731 | 36.835 | ±0.939 | 37.0 | ±0.115 | 34.0 | ±0.464 | 11.193 | ±0.351 | 23.7 | ±0.246 | 2.27 | ±0.246 | −1.953 | ±0.006 | 0.46 | ±0.002 | 110.28 | ±0.816 | 7.26 | ±0.306 | 0.347 |
| Stress (E2) | 1.189 | ±0.010 | 2.346 | ±0.016 | 45.11 | ±0.099 | 55.75 | ±0.87 | 0.233 | ±0.001 | 35.898 | ±0.698 | 44.247 | ±0.87 | 28.7 | ±0.133 | 18.4 | ±0.187 | 6.107 | ±0.155 | 25.4 | ±0.136 | 0.57 | ±0.136 | −2.443 | ±0.004 | 0.34 | ±0.002 | 175.05 | ±0.37 | 2.82 | ±0.106 | ||
| NR-516 | Normal (E1) | 0.458 | ±0.004 | 1.127 | ±0.004 | 67.14 | ±0.237 | 62.24 | ±0.262 | 0.340 | ±0.006 | 28.189 | ±0.166 | 37.760 | ±0.262 | 35.4 | ±0.379 | 24.5 | ±0.5 | 10.813 | ±0.461 | 24.2 | ±0.104 | 1.80 | ±0.104 | −1.975 | ±0.002 | 0.45 | ±0.003 | 107.19 | ±0.121 | 6.88 | ±0.205 | 0.298 |
| Stress (E2) | 1.121 | ±0.012 | 2.314 | ±0.005 | 44.23 | ±0.270 | 54.52 | ±0.286 | 0.219 | ±0.004 | 37.311 | ±0.143 | 45.476 | ±0.286 | 27.6 | ±0.186 | 15.8 | ±0.292 | 5.813 | ±0.221 | 25.8 | ±0.049 | 0.20 | ±0.049 | −2.448 | ±0.002 | 0.31 | ±0.005 | 174.74 | ±0.301 | 2.56 | ±0.176 | ||
| HSD critical value for comparison | 0.1610 | 0.1751 | 3.9100 | 9.2430 | 0.0994 | 7.1723 | 9.2432 | 3.8096 | 20.8620 | 2.9760 | 2.1274 | 2.1274 | 0.0663 | 0.0837 | 7.7183 | 2.0609 | ||||||||||||||||||
| Coefficient of variation (%) | 3.88 | 2.05 | 1.54 | 3.58 | 7.52 | 8.00 | 7.77 | 2.42 | 27.95 | 6.75 | 2.0.32 | 33.46 | 0.91 | 3.50 | 1.09 | 9.64 | ||||||||||||||||||
Note: Here PC: Proline content, Sugar: Total soluble sugar content, LMSI: Leaf membrane stability index, LRWC: Leaf relative water content, RDW: Relative dry weight, RSD: Relative saturation deficit, WSD: Water saturation deficit, RELWL: Relative excised leaf water loss, LOP: Leaf osmotic potential, LSA: Leaf surface area, LS: Leaf succulence, CnpTemp: Canopy temperature, CTD: Canopy temperature depression, EWC: Epicuticle wax content, GY: Grain Yield Plant−1 (g) and STI: stress tolerance index for Grain Yield. The values with ±0.000 indicate Standard Error beside mean potential of PBTs.
3.2. Grain yield potential dynamics under normal (E1) and drought (E2) conditions
The overall mean grain yield per plant of one hundred and twenty bread wheat diverse genotypes under normal (E1) and drought (E2) was 7.693 (g) and 3.641(g) correspondingly. Drought stress caused 53% reduction in grain yield from normal environment (Fig. 1). The maximum grain yield plant−1 (g) was observed for wheat genotype NARC-2009 (9.19 and 5.38 under E1 and E2 environments respectively) followed by Pakistan-2013 (8.91 and 3.00 under E1 and E2 environments respectively), while minimum grain yield plant−1 (g) was recorded for NR-516 (6.88 and 2.56 under E1 and E2 respectively) followed by NR-514 (7.26 and 2.82 under E1 and E2 water regimes respectively). The mean STI value for grain yield was 0.475 with 46.8% of the test genotypes having above average STI. The highest STI value for grain yield plant−1 was obtained for NARC-2009 followed by Pakistan-2013 and NR-499, i.e. 0.836, 0.758 and 0.721 respectively (Table 2). On the contrasting side, wheat genotypes NR-516 showed lowest STI for grain yield plant−1 followed by NR-514 and Borlaug-2016, i.e. 0.298, 0.347 and 0.361 respectively and these genotypes exhibited comparatively lower yield under both moisture zones as well (Table 2).
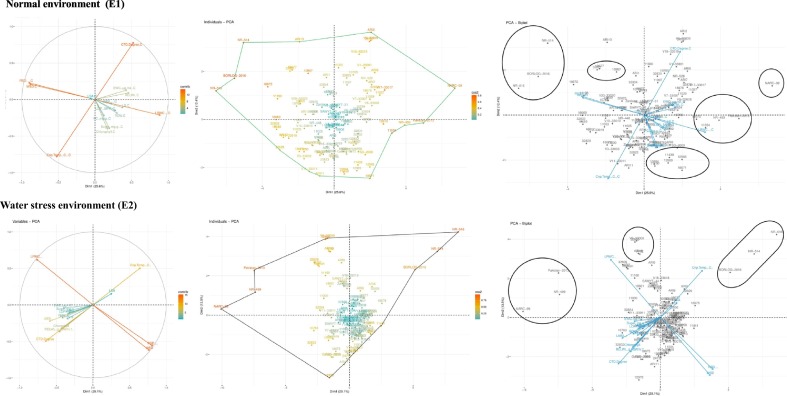
3.3. Principal component analysis (PCA) for physio-biochemical traits
The total of the eigenvalues is generally equivalent to the quantity of the traits under study; subsequently, in the water stress regime and control treatment, the first factor holds the information contained in 2.09% and 1.96% respectively of the original trait quantities. From the moisture deficient treatment, seven important components were fundamental, donating 79.02% of the total variation as depicted in Table 3 . The first six important components were the most influential with an aggregate commitment to the all-out variation of 73.01%. Physiological attributed traits including RSD, WSD and CnpTemp had high positive loading into the first Principal component pursued by LRWC and CnpTemp in second component's segment, whereas CTD and LRWC had high negative stacking into the first principal component pursued by CTD, RSD and WSD in second Principal component of stress treatment. Out of the total reserved principal components, PC1, PC2 and PC3 with individual estimations of 29.10%, 13.51% and 9.17% correspondingly contributed more to the all-out diversity as given in Table 3. Thus, seven vital principal components were imperative under ideal water conditions, representing 77.27% of the total generated variation, of which 71.10% was represented by the initial six components. Just LRWC had high positive loading into the first principal component trailed by CTD in the second one. However, WSD and RSD had high negative stacking into the first Principal component, after that Chlorophyll content and CnpTemp into the second principal component under optimum water routine. The PC1, PC2 and PC3 with individual valuations of 25.61%, 12.40% and 10.68% respectively contributed more to the absolute variation out of total attainable principal components engaged as depicted in Table 3. The positive and negative loading demonstrates the availability of positive and negative association inclines between the components along with measured variables. In this way, the previously mentioned characters which load high positively or negatively contributed more to the assorted variation.
Table 3.
Rotated component matrix of physiological-biochemical traits (PBT) of 120 wheat genotypes assessed under two environments i.e. optimum conditions (E1) and water stressed (E2).
| Traits | Stress region (E2) |
Traits | Control region (E1) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | ||
| RSD | 0.390 | −0.394 | −0.097 | −0.040 | −0.005 | 0.041 | 0.056 | LRWC | 0.455 | −0.156 | 0.288 | −0.012 | 0.101 | −0.111 | −0.012 |
| WSD | 0.369 | −0.439 | −0.036 | −0.026 | 0.061 | −0.041 | 0.052 | CTD | 0.268 | 0.575 | −0.147 | −0.154 | −0.109 | −0.087 | 0.002 |
| CnpTemp | 0.310 | 0.356 | −0.305 | 0.166 | 0.371 | −0.064 | 0.038 | RELWL | 0.238 | 0.069 | −0.301 | 0.125 | 0.205 | −0.019 | 0.437 |
| LSA | 0.124 | 0.111 | −0.085 | 0.413 | −0.540 | −0.176 | 0.472 | EWC | 0.221 | 0.096 | −0.134 | 0.435 | −0.108 | 0.074 | −0.313 |
| LS | −0.157 | −0.076 | −0.510 | −0.405 | −0.060 | −0.042 | −0.270 | LS | 0.195 | −0.081 | 0.032 | 0.107 | −0.273 | 0.768 | 0.159 |
| LOP | −0.168 | −0.088 | −0.381 | −0.030 | −0.088 | −0.711 | −0.096 | RDW | 0.157 | −0.138 | −0.260 | −0.370 | −0.155 | 0.236 | −0.337 |
| PC | −0.171 | −0.136 | −0.420 | 0.246 | −0.237 | 0.337 | −0.221 | LOP | 0.146 | −0.112 | −0.198 | 0.375 | −0.469 | 0.027 | −0.003 |
| Sugar | −0.178 | −0.062 | −0.081 | −0.388 | 0.298 | 0.158 | 0.451 | LMSI | 0.118 | −0.040 | −0.345 | 0.028 | 0.594 | 0.293 | 0.296 |
| EWC | −0.195 | −0.030 | −0.152 | −0.222 | −0.057 | −0.070 | 0.635 | Sugar | 0.102 | −0.294 | −0.356 | −0.220 | −0.199 | −0.257 | 0.118 |
| RDW | −0.203 | −0.125 | 0.317 | 0.066 | 0.338 | −0.419 | −0.032 | Chlorophyll | 0.089 | −0.300 | −0.341 | 0.017 | −0.238 | −0.332 | 0.260 |
| Chlorophyll | −0.207 | −0.227 | 0.099 | 0.392 | 0.178 | −0.163 | −0.036 | PC | 0.065 | −0.166 | −0.302 | −0.377 | 0.188 | 0.124 | −0.459 |
| RELWL | −0.222 | −0.260 | −0.216 | 0.303 | 0.204 | 0.021 | 0.099 | LSA | −0.037 | 0.012 | 0.251 | −0.513 | −0.305 | 0.157 | 0.435 |
| LMSI | −0.286 | −0.158 | −0.152 | 0.321 | 0.279 | 0.320 | 0.120 | CnpTemp | −0.268 | −0.578 | 0.136 | 0.156 | 0.110 | 0.087 | 0.008 |
| CTD | −0.313 | −0.353 | 0.310 | −0.148 | −0.372 | 0.066 | −0.063 | WSD | −0.455 | 0.156 | −0.288 | 0.012 | −0.100 | 0.111 | 0.012 |
| LRWC | −0.369 | 0.439 | 0.037 | 0.026 | −0.061 | 0.041 | −0.052 | RSD | −0.463 | 0.170 | −0.249 | 0.057 | −0.079 | 0.078 | 0.051 |
| Eigenvalue (%) | 4.366 | 2.027 | 1.376 | 1.174 | 1.049 | 0.959 | 0.902 | Eigenvalue (%) | 3.8411 | 1.86 | 1.6027 | 1.3038 | 1.0822 | 0.9744 | 0.9267 |
| Standard deviation | 2.09 | 1.42 | 1.17 | 1.08 | 1.02 | 0.98 | 0.95 | Standard deviation | 1.96 | 1.36 | 1.27 | 1.14 | 1.04 | 0.99 | 0.96 |
| Proportion of total individual variance (%) | 29.10 | 13.51 | 9.17 | 7.83 | 6.99 | 6.40 | 6.01 | Proportion of total individual variance (%) | 25.61 | 12.40 | 10.68 | 8.69 | 7.22 | 6.50 | 6.18 |
| Cumulative variance proportion (%) | 29.10 | 42.62 | 51.79 | 59.62 | 66.61 | 73.01 | 79.02 | Cumulative variance proportion (%) | 25.61 | 38.01 | 48.69 | 57.38 | 64.60 | 71.10 | 77.27 |
Note: Here PC: Proline content, Sugar: Total soluble sugar content, LMSI: Leaf membrane stability index, LRWC: Leaf relative water content, RDW: Relative dry weight, RSD: Relative saturation deficit, WSD: Water saturation deficit, RELWL: Relative excised leaf water loss, LOP: Leaf osmotic potential, LSA: Leaf surface area, LS: Leaf succulence, CnpTemp: Canopy temperature, CTD: Canopy temperature depression and EWC: Epicuticle wax content.
Typically, it is standard to pick one variable from these recognized gatherings of principle components, henceforth, from stress treatment for the primary gathering RSD is the best choice, which had the biggest stacking from component one (Table 3).
3.4. Principal component genotype by trait (GT) biplot association for physio-biochemical traits
The connections between the different traits and wheat genotypes with particular principal components are additionally represented by the main principal components biplots in Fig. 2 for the normal and water stressed environments. The GT biplot of the mean execution of the wheat genotypes under stress treatment illustrated the 42.62% of the total variation of the institutionalized information. The vast majority of the genotypes were dispersed in the positive side of the first principal component. Over the 120 verified wheat genotypes the variable RSD was positively allied with WSD on PC1 and adversely connected with LRWC on PC2, and thrice of these traits were found with higher magnitude because of their longest vector length among all of the traits, hence contributed most in overall variation (Fig. 2). The length and angle of principal vectors (variables) represents variance and co-variance respectively. A maximum number of variables depicted less magnitude with nearly smaller or medium vector length as RSD, WSD, LRWC, CnpTemp and CTD with longer vector length indicating comparatively higher variance than rest of the variables during stress phase. All PBT with smaller or closer vector angle with each other possessed positive association between them. EWC, Sugar content, LOP, RDW and PC were found exceptionally positive corresponded with one another, however, with less contribution. LSA exhibited a negative correlation with all of the traits except CnpTemp with relatively smaller vector length. Despite the fact that the low magnitude Chlorophyll content was positively associated with RELWL and longer arm of CTD.
Fig. 2.
Genotype by trait (GT) vector biplot under normal (above panel) and water stress environments (lower panel).
The distance among genotype and the biplot origin is an exclusive proportion of the genotype, i.e., how it contrasts from an “average” genotype. In this manner, genotypes Pakistan-2013, NARC-2009, NR-499, Borlaug-2016, NR-514, NR-516, 12970 and V9-33009 with long vectors are those that have extreme values for one or more traits as shown in Table 3. Such genotypes may or may not be superior, but they may be useful as parents for some useful drought-responsive physiological traits.
Under optimum moisture level, the genotypes and the majority of the physiological traits were additionally progressively focused on the positive side of the first principal component. Among all wheat genotypes RSD, WSD, LRWC, CnpTemp, and CTD were most contributing characteristics on both of the principal components with the longest vector length as contributed higher proportion of variance. The total soluble sugar content was positively associated with chlorophyll on PC1 yet with low magnitude. Furthermore, proline content was likewise connected with these two traits with least vector span. CnpTemp is highly negative corresponded with CTD on extremes of inverse shafts and essentially contributed to overall diversity. The most reduced magnitude was exhibited by LSA at extremely near to the origin towards PC2 under normal moisture phase (Fig. 2). The wheat genotypes NR-516, Borlaug-2016, NR-514, ARI-13, ARI-2, NARC-2009, Pakistan-2013, NR-499, 18671, ARI-11, 11004, V11-33011 and 32828 might be ideal or most exceedingly worst in terms of the executed performance under well-watered condition (Fig. 2).
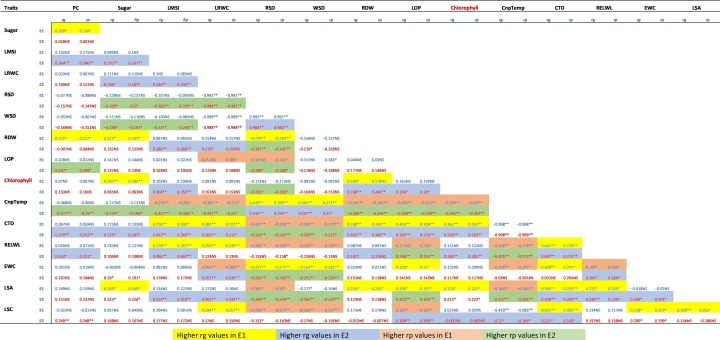
3.5. Genetic and phenotypic correlation coefficients
In the present study, the correlation analysis between leaf related traits were carried out under the two environmental conditions namely optimum water condition (E1) and water deficit condition (E2). Genotypic and phenotypic correlations between all possible studied trait pairs are presented in Table 4 . In terms of genotypic correlation, 61 physio-biochemical trait associations (rg) were higher than their phenotypic correlation (rp) coefficients. Proline content (PC) exhibited greater genotypic correlation (rg) with LMSI, CTD and ELWL under moisture deficient condition while under control condition it has higher rg values with sugar content and RDW. The greater genotypic paired association was found for sugar with LMSI, LRWCl and CTD under drought regime whereas under optimum moisture state it showed efficient genotypic association with RDW, chlorophyll and LSA. Similarly, LMSI exhibited a higher degree of rg with LRWC, RDW, chlorophyll, CTD, ELWL and LSA under drought stress while it also has greater rg values with CTD and ELWL under normal irrigating pots. LRWC possess higher rg values with RDW, CTD, EWC and LSA under drought condition while with CTD, ELWL and LSC under normal moisture regime. In the case of RSD, it has greater rg numbers with WSD and CnpTemp under moisture deficient phase while it has greater rg values with RDW, CnpTemp and EWC under optimum environment. WSD possess larger rg values in a stress environment only with CnpTemp while under normal climate, it has an efficient genotypic association with CnpTemp and EWC. The greater genotypic association of RDW was found with chlorophyll content under stress condition and with chlorophyll, CTD and LSA under normal water regimes. The larger rg values were also observed for LOP with chlorophyll, CTD and LSC under moisture lacking states and with CTD, EWC and LSA under optimum condition. Chlorophyll content has a higher genetic correlation with CTD and RELWL under drought conditions and with CTD and LSA under normal irrigated pots. In case of canopy temperature, the greater rg value was observed only with LSC under moisture deficient environment. The paired higher genetic correlation than the phenotypic correlation for the trait CTD with RELWL, LSA and LSC under drought, while CTD also showed higher rg values with ELWL, EWC, LSA and LSC under optimum condition. RELWL demonstrated greater genotypic paired association with EWC and LSA under stress whereas under optimum irrigation it associated with LSA only. The higher degree of rg was also obtained for EWC with LSA and LSA under moisture stress and optimum level, respectively.
Table 4.
Genotypic-Phenotypic correlation coefficient matrix of physiological-biochemical traits (PBT) of 120 wheat genotypes assessed under two environments i.e. optimum conditions (E1) and water stressed (E2).
Note: Here rp: Phenotypic correlation, rg: genotypic correlation, PC: Proline content, Sugar: Total soluble sugar content, LMSI: Leaf membrane stability index, LRWC: Leaf relative water content, RDW: Relative dry weight, RSD: Relative saturation deficit, WSD: Water saturation deficit, RELWL: Relative excised leaf water loss, LOP: Leaf osmotic potential, SPAD: Chlorophyll content, LSA: Leaf surface area, LS: Leaf succulence, CnpTemp: Canopy temperature, CTD: Canopy temperature depression and EWC: Epicuticle wax content. ** Significant P<0.01. * Significant P<0.05. and NS: non-significant.
On the other hand, 47 phenotypic associations (rp) were higher than their corresponding genotypic correlation (rg) coefficients. These trait pairs of the higher magnitude of rp than rg were; Proline content (PC) with LOP and CnpTemp under stress condition; Sugar with RSD, WSd and CnpT under drought; LMSI with RSD, WSD and CnpTemp under stress environment and under normal water supply it has greater rp value with CnpTemp.
The higher phenotypic paired association was found for LRWC with RSD and CnpTemp under stress while with LOP, CnpTemp and EWC under optimum moisture environment. RSD showed higher rp value with RDW, LOP, chlorophyll, CTD, EWC and LSA under stress environment, while also has higher rp values with LOP, CTD, ELWL, LSA, and LSC under the optimum level of moisture. In the case of WSD, the higher rp values were obtained with CTD, EWC and LSA under stress situation while with CTD, ELWL and LSC under normal irrigation. RDW is traits that have greater rp value under both E1 and E2 environments only with canopy temperature (CnpTemp). The higher phenotypic paired association of LOP was recorded with CnpTemp, ELWL, and LSA under both moisture regimes. Moreover, chlorophyll content showed higher rp association only with canopy temperature under both water environments; while CnpTemp exhibited a greater rate of phenotypic correlation with ELWL and LSA under moisture stress phase and with ELWL, EWC and LSA under optimum environment. Lastly, the higher rate of phenotypic paired association than genotypic correlation was obtained for ELWL only with EWC under normal irrigated pots.
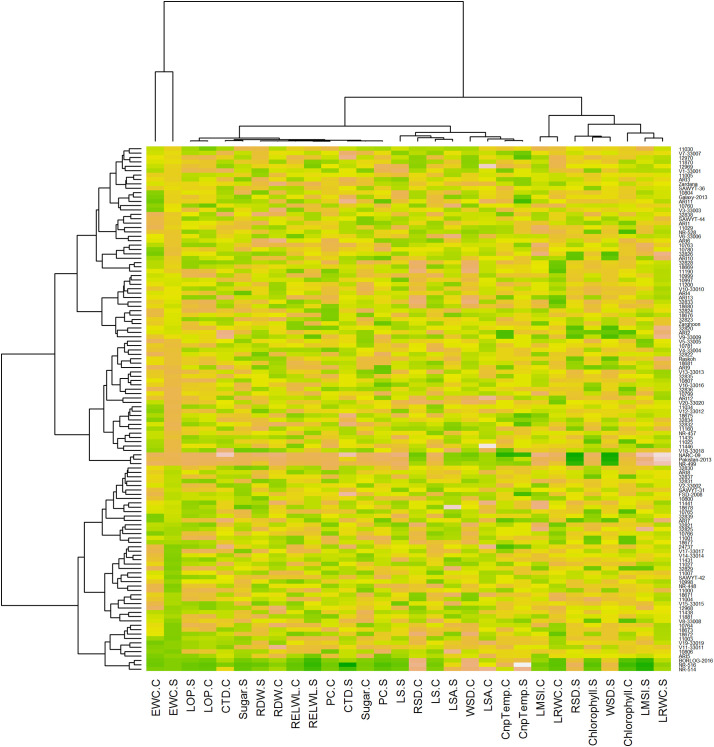
3.6. Heatmapping of physio-biochemical traits of bread wheat
Heat map indicated the relative performance of the genotypes in both conditions for which the data was recorded. According to colour scale the positive darker scale represents drought resistant genotypes while negative darker strips exhibit susceptible ones (Fig. 4). Similarly, as colour intensity goes down, the genotypes show moderate performance on the both ranges, i.e., positive and negative. It was noted that EWC values were higher in stress conditions than normal for most of the genotypes, which is a sign of plant response under moisture deficient regime. LOP showed much variation among the genotypes both in normal and stress conditions, but no significant changes were found between the two conditions for the same genotype. CTD was showing contrasting effect in different genotypes, but one prominent feature was that all the genotypes were performing above the average line in case of normal conditions but in stress condition, some reduction was observed in all the genotypes for CTD due to hotter canopy temperature. Sugar content was found to behave in a strange way as it was changing with genotype and also with the environment. It was in the normal range for most of the genotypes in control but goes on higher due to stress conditions. It clearly indicates that sugar content is dependent largely on the growing conditions and this stress enhances the sugar accumulation in leaves as shown in (Fig. 3).
Fig. 3.
Heat map analysis of physiological and biochemical traits.
Relative dry weight and relative excised leaf water loss were found to behave in an abnormal way as they didn't seem to be correlated with the growing conditions as they mainly differ by genotype. In wheat genotypes, these were higher in control but shown to be reduced under moisture deficient regimes. Proline content (PC) and LS were depicted to have medium to higher values for most of the genotypes. These two traits were seen to be continuously varying by changing the genotype. Proline content was found to be largely affected by drought conditions as it was noted to be increased for each genotype when compared in both of the conditions where it increased in stress conditions. LS was observed to have medium to moderate prominent differences in performance by some genotypes in two different conditions. Although varied by genotypes but seemed to be affected by stress applied. RSD values were depicted to be greatly affected by drought stress as in every genotype, its value increased by a certain level under stress phase. All the genotypes were observed to be average performers in case of LSA and WSD, as their values varied by genotypes but in a continuous range. Most of the genotypes were noted to be lying along with the mean value. Moisture stress affected LSA and WSD to a greater extent.
Canopy temperature in control conditions was noticed to be moderate limit except for 8 genotypes where it reached to the upper extremity. But in stress conditions, most of the genotypes were found to show incline in their CnpTemp. All upper extremes in culture were then recorded around the average range in stress mode. But the point to ponder was that 6 such genotypes exhibited less maximum limit in stress while there was a lower range in number. Chlorophyll content of leaves was seen not to be affected by growing conditions it was depicted that it express in terms of the genetic potential of the genotype to express without any interference of the environmental conditions as shown in (Fig. 4). LMSI was depicted to be decreasing by a smaller level in case of stress as compared to normal conditions. As this trait maintained the cellular membrane, so it seemed to be helpful for plant to cope with the stress affects. LRWC was noted higher in control conditions, but its values lowered down for the same genotype when drought stress is applied to them.
Three genotypes were observed to show peculiar behavior in stress conditions, namely NARC-09, Pakistan-2010 and NR-499 as these genotypes possessed higher positive or negative values than other genotypes in most of the PBT. These genotypes were found to be less affected by stress phase when data was recorded in stress condition as compared to other genotypes which suggest these genotypes to be resistant to drought as overall trend of their performance in the form of coloured gradient strips is clearly distinctly depicted by Fig. 3 . When other genotypes were analyzed for aforesaid parameters, three genotypes, namely NR-514, NR-516 and Borlaug-2016, were found to perform in contradiction to resistant genotypes, as these three wheat genotypes possessed lower positive or negative values in majority of the traits and drastically influenced by severe drought so these were referred to as susceptible genotypes as clearly shown by performance trend in Fig. 3. It is clear from heatmap that these genotypes were showing to attain lesser value in stress as compared to normal conditions. As proline content, LMSI and LRWC are important parameters for determining the drought tolerance ability of a genotype along with the other traits, therefore, in most of the genotype's proline content was found to be higher in stress and some were accumulating less proline in leaves under drought, but drought tolerant genotypes were behaving the almost same accumulation of proline in both the conditions. LMSI was found to be negatively correlated with the tolerance to drought as it is clear from the picture that darker the corresponding band lesser will be the ability to tolerate on the positive colour region, so the susceptible referred strains had more LMSI value under stress than control. Some other genotypes were also found to behave differently in control and stress but analysis cannot be made based only upon the performance in terms of LMSI. Resistant to drought is also linked to a lower value of LRWC in stress than control, but genotype with higher values in stress showed dehydration tolerance capability In present condition, both the resistant and susceptible mentioned genotypes were found to have lower LRWC value in stress as compared to normal but when the whole picture is analyzed it is concluded that among all the tested genotypes NARC-09, Pakistan-2013 and NR-499 are resistant to drought due to their stable physiological metabolism but NR-514, NR-516 and Borlaug-2016 are highly susceptible to drought stress as illustrated in (Fig. 3). These complementary genotypes can be further employed in the wheat breeding program by using various copulating designs to achieve maximum drought tolerance in filial generations of the crop, which may directly contribute to enhanced productivity.
4. Discussion
The productivity of wheat has been compromised due the various abiotic stress constraints including the drought, so developing drought tolerant wheat cultivars is the foremost goal of wheat breeders. Major factors responsible for the drought are the low rainfall and the erratic changes in the precipitation. For this reason, the identification of drought resistance responsible factors, their performance under moisture stress regimes is the pinpoint objectives of plant researchers (Toker et al., 2007). Under managed moisture deficient condition the screening of the various physio-morphic attributes of the wheat genotype provide an efficient way to the plant breeder that the improved performance of these traits can be used to select material in order to develop the moisture stress wheat genotypes (Mwadzingeni et al., 2016).
Selection criteria for enhanced grain yield under both moisture conditions permit test genotypes to sustain their rankings for improved production since similar genotypes will be relied upon to perform well in either circumstance. The remarked sustainability of higher grain yield under moisture deficient and ideal conditions in certain genotypes e.g. NARC-2009, Pakistan-2013 and NR-499 in present study supports the outcomes of Foulkes et al. (2007). They concluded that screening of traits for drought resistance would have value in future breeding programs aimed at improving yields. Bread wheat genotypes with improved yield and productivity under both optimum and moisture stress environments demonstrated higher values of STI which further validate the authenticity of this tolerance index in selecting for high yield under either climatic conditions (Fernandez, 1992). Although, because of extreme drought stress enforced on the wheat genotypes bringing about normal yield reduction of about 53% in this study as contrasted with 26% reported under moderate stress applied by Foulkes et al. (2007).
Plants have evolved complex physiological and biochemical adaptations to adjust and adapt to variety of stresses including drought (Osakabe et al., 2014). Similarly, water stress leads to denaturalization of enzymes and damage in the structure of protein. Plant metabolic reactions are mainly controlled by protein. To protect such proteins and metabolic enzymes, plant accumulates a certain higher number of osmo-protectants. For this reason, under stress condition, high proline content and sugar accumulation maintained the structure of the protein, permits osmotic adjustment and act as protective agents for the enzymes. Similar results of higher proline and total soluble sugar accumulation in plants under stress environments as compared to normal were reported by Kavi Kishor and Sreenivasulu (2014). Since moisture stress leads to extreme scarcity of water in the root zone. Therefore, for the survival, plant will slow down the loss of water from leaves surface by adapting some resistance strategies which involve the descending trend in some physiological traits including LMSI%, LRWC%, RDW, chlorophyll content, LSA, LS, CTD °C and RELWL %.; hence by this adaptation, the plant can minimize the water loss for survival (Ali and Awan, 2009). However, under the moisture lacking states some of the plant focused parameters depicted the ascending trend in the percentages as compared to the sufficient moisture situation, which includes RSD, WSD and CnpTemp.
Osmotic regulation is important to cope with water deficit conditions, maintaining turgor pressure in cell and physiological processes to postpone the prevailing dehydration (Chen and Jiang, 2010). Proline and sugars are among the well-known organic osmolyte and accumulation of proline under stress conditions have been observed in many plants' species (Ahmed et al., 2017). Consistent with all these findings, our results showed the highly significant positive genetic correlation and significant positive phenotypic correlation of the proline and sugar contents with leaf membrane stability index under the limited water environment (E2), implying that both these osmo-regulators can maintain the structure of cell membrane under stress, helping plant to survive the stressful conditions. Chen et al. (2017) reported the accumulation of proline and sugar contents in wheat under the drought stress, with comparatively higher accumulation in the susceptible wheat cultivar. Therefore, it can be inferred that proline content (PC) can be proved as a great physiological marker when comparing the different wheat genotypes under drought stress.
Canopy temperature (CnpTemp) has been a good indicator of plant water status as it can be used as a non-destructive and contact-free method to estimate the changes in stomatal conductance (Jackson et al., 1981). The depression between the air temperature and CnpTemp is referred to as canopy temperature depression. The increase in water transpiration can cause a decrease in plant surface temperature and vice versa, under drought condition (Maes and Steppe, 2012). Similar relation of CnpTemp and canopy temperature depression can be observed in our results through the highly significant negative correlation in both the E1 and E2. Previous reports showed that it has a wide utilization under drought (Yan and Fregeau-Reid, 2008), heat stress (Ayeneh et al., 2002) and for irrigation scheduling (Gontia and Tiwari, 2008). Several authors have studied the significant genotypic effects of canopy temperature depression and reported a positive significant correlation with grain yield in wheat and many other plant species (Blum, 1989). In present studies, the significant positive correlation between proline content and canopy temperature depression under water limited conditions suggests that a higher canopy depression value is dependent on higher levels of osmo-protectants in plants, that ultimately results in protection of plants under stress. The significant positive rg values of canopy temperature depression with LMSI, LRWC and EWC in E2 and, with Chlorophyll in both E1 and E2 and highly significant higher rg and rp values with CnpTemp, suggests that transpiration from the leaves causes increase in CTD which is dependent on healthy leaf traits, i.e. stability of leaf membrane, high relative water content and high pigment formation which can only be achieved by the tolerant varieties under drought stress as shown in Table 4. Consistent with our results, drought-affected plants were reported to have higher CnpTemp than normal wheat plants (Siddique et al., 2000). Therefore, a selection criterion including CnpTemp and CTD can also help breeders while selecting for a drought-tolerant genotype.
The biotic and abiotic stresses often lead to the leakage of electrolytes from cell membrane, disrupting its stability, making it more permeable and cause disorders in cell functions (Passioura, 2006). Therefore, the stability of the cell membrane under stress may indirectly indicate the stability of plants under the stress conditions. The stability of cell membrane is an efficient physiological parameter (Sullivan, 1972) and has been utilized by various researchers for screening against drought and heat stress-tolerant genotypes in wheat (Farooq et al., 2011), rice (Farooq et al., 2009) and cotton (Ali and Awan, 2009). The drought-tolerant plants possess stable membrane and can maintain its integrity. In present study, we also found a positive significant rg values between leaf membrane stability index (LMSI) and osmo-protectants, i.e. proline and sugars, supported by the highly significant positive correlation with RELWL, canopy temperature depression, chlorophyll content and significant positive correlation with LSA, as shown in Table 4 which inferring that a stable cell membrane can maintain effective cellular activities to sustain amount of photosynthetic pigments, making it possible for the leaves to tolerate drought stress. All these results endorse the previous reports about the positive association between cell membrane stability and survival of plants, in drought-tolerant wheat varieties under the stress (Chen et al., 2017) as indicated in Table 4.
Genotypic-Phenotypic correlation revealed that 61 trait pairs association showed rg values higher than the corresponding rp, revealing that PBT association were under genetic control and their genetic variance plays the main role in their expression (Bhattacharyya et al., 2010). Contrastingly, 47 trait pairs showed higher rp values than rg, indicating that environmental influence can alter PBT expression at the phenotypic level (Sinclair, 2011; Li et al., 2020; Kebede et al., 2019; Al Khateeb et al., 2017). Leaf related traits and their architecture are important factors for the adaptation of crop to changing environment (Alqudah et al., 2016). The relative water content of leaf is a reliable indicator of water condition of leaf cells and shows an important correlation with stress tolerance. The strong association between LRWC and drought tolerance has been reported previously (Moshelion et al., 2015). The positive and significant correlation of the LRWC with LMSI and CTD, positive and highly significant rg value with LSA and highly significant negative values with RSD and WSD under limited water conditions (E2) in our study suggests that a high LRWC ensures the stability of membrane as well as the cooler canopies to maintain leaf anatomy, sustaining leaf functions, ultimately protecting the plants against drought stress (Table 4). Our results are similar with the previous findings of Almeselmani et al. (2012) where they reported that physiological traits could enable plants to survive and adapt under water stress environments thus maintaining growth and productivity. Hence, they recommended that these traits should be used in breeding programs for selection of tolerant genotypes.
The components of water potential are generally considered as reliable parameters to know about the water status of plant tissue (Schonfeld et al., 1988). The leaves with high tissue water retention and better osmotic adjustments have been reported to perform better under water deficit condition due to delayed leaf dehydration rates (França et al., 2000). Our results showed the significant negative correlation between leaf osmotic potential (LOP) and CnpTemp, significant positive correlation with CnpTemp and LS in both E1 and E2. This suggest that better osmotic adjustment by the cells can keep a good canopy environment for plant that can help plant in retaining their turgidity to sustain photosynthetic activities and leaf water status under the optimum as well as drought conditions. Also, the positive rp values between LOP, LSA and ELWL shows the association between osmotic adjustments and leaf architecture and water retention capacities, but this association is more under the influence of the environment. Similarly, in present study plant accumulates more sugar content under stress condition so sub-molecule of sugar in the form of ATP or carbohydrates supply energy to leaf membrane for their stabilization as leaf is most prominent plant organ that play crucial role to cope moisture deficiency by modifying several leaf related pathways. Furthermore, higher sugar accumulation may also serve to maintain leaf canopies cooler during intense drought spells; therefore, sugar has greater genotypic paired association with LMSI, LRWC and CTD. On the other hand under optimum environment, when leaves were fully hydrated and normal accumulation of sugar content were supplying sufficient amount of energy to leaves and normal chlorophyll content was derived by leaf surfaces that is why sugar had strong genetic paired association with these traits including Chlorophyll and LSA. Moreover, RDW of leaves gathered enormous quantity of sugar under normal condition, so it has greater paired association with sugar content. Nehe et al. (2020) quantified the genetic variability in Indian spring wheat cultivars and identified traits for the improved grain yield and grain protein content. Similar to our findings Senapati and Semenov (2019) concluded that in order to to accelerate breeding, wheat ideotypes designing based on the key traits could be powerful tool for the wheat improvement and closing the yield gap and to explore the crop yield potential.
5. Conclusion
This study explores the impact of drought stress which drastically impacted the PBT and grain yield as a marker trait of all bread wheat genotypes. Analysis of Variance signposted that there was a significant variance between all of the genotypes with regard to the studied PBT at both of the moisture regimes. Among all PBT proline and sugar content were found to be largely affected and therefore accumulates in tissues as a respond mechanism with enormous quantities (159% and 122% respectively) under stress condition, but these two might not deliver as a healthy in-direct selection marker or predictor when quantified at a single point of time. On the basis of Genotypic-Phenotypic correlation revealed that 61 trait pairs association showed rg values higher than the corresponding rp, revealing that their association was under genetic control and their genetic variance plays the main role in their expression. Contrastingly, 47 traits pairs showed higher rp values than rg, indicating that environmental influence can alter their expression at the phenotypic level. Thus, genotypic correlations are more crucial for breeding procedures of crop improvement. Moreover, heatmapping showed higher EWC values in stress conditions than normal for most of the genotypes, which is a sign of plant response under moisture deficient regime. Similarly, proline content was found to be largely affected by drought conditions as it was noted to be increased for each genotype when compared in both of the conditions where it increased in stress conditions.
Our findings revealed high dependency of wheat grain yield on favorable climatic conditions as drought stress negatively impacted wheat kernel yield. Higher stress tolerance index for improved grain yield under stress condition is a good selection marker to identify high yielding germplasm at dry land farming. However, genotype, which had proficient physiological metabolism to retain moisture content during the stress phase, were seen as tolerant and produced improved grain yield with higher STI, as this reveals the potential of wheat genotypes to exploit improved climatic conditions. On the basis of studied PBT and STI for grain yield, we consider some genotypes as drought tolerant with better yield potential and some genotypes as susceptible to moisture stress with low productivity. According to outcome of mean performance and various statistical tools genotype NARC-09 was found as a drought-tolerant wheat genotype trailed by Pakistan-2013 and NR-499 because these performed well in most of the PBT and gave comparatively higher yield under both water regimes, whereas wheat advance line NR-516 was declared as drought-sensitive pursued by NR-514 and Borlaug-2016 due to their deprived physiological and grain producing capability under both moisture treatments.
Therefore, we wish to portray consideration to the dire need of tending to the negative effects of environmental changes by evolving procedures for lessening climate dependency that can assist farming industry to tackle current scenario of climate change. Further investigations are vital, including progressively assorted genotypes during different years at various agro-ecological zones to approve the potential intensity of the referenced molecular markers for drought-stressed bread wheat, which will ultimately lead towards crop improvement by release of advance wheat cultivars to cope changing climate in the upcoming years.
Funding
This work was funded by the scholarship awarded by the Agriculture Research Institute (ARI), Quetta, Agriculture and Cooperatives Department, Government of Balochistan, -Pakistan for carried out Ph.D. research program. Moreover, the entire study was supported by Department of Plant Breeding and Genetics, PMAS-Arid Agriculture University, Rawalpindi and National Agriculture Research Centre (NARC), Islamabad-Pakistan.
CRediT authorship contribution statement
Kashif Ahmed: Funding acquisition, Investigation, Formal analysis, Writing - original draft, Writing - review & editing. Ghulam Shabbir: Conceptualization, Methodology. Mukhtar Ahmed: Conceptualization, Methodology, Writing - original draft, Writing - review & editing. Kausar Nawaz Shah: Conceptualization, Methodology.
Declaration of competing interest
All authors declare no conflict of interest.
Acknowledgments
We thank Agriculture Research Institute, Quetta; Department of Plant Breeding and Genetics, PMAS-Arid Agriculture University, Rawalpindi and National Agriculture Research Centre (NARC), Islamabad-Pakistan for supporting this research. The authors are thankful to all valuable reviewers who putted lot of efforts to improve this article. Furthermore, authors dedicate this work to the COVID-19 patients, front line doctors, paramedic staff and all others who are directly or indirectly fighting against this disease.
Editor: Fernando A.L. Pacheco
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2020.139082.
Appendix A. Supplementary data
Supplementary tables
References
- Abbas M., Sheikh A.D., Sabir H.M., Nighat S. Factors responsible for low wheat productivity in Central Punjab. Pak. J. Agr. Sci. 2005;42:3–4. [Google Scholar]
- Abid M. Improved tolerance to post-anthesis drought stress by pre-drought priming at vegetative stages in drought-tolerant and-sensitive wheat cultivars. Plant Physiol. Biochem. 2016;106:218–227. doi: 10.1016/j.plaphy.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Ahmed M., Hassan F.U., Aslam M., Aslam M.A. Physiological attributes based resilience of wheat to climate change. Int. J. Agric. Biol. 2012;14:407–412. [Google Scholar]
- Ahmed M., Hassan F., Asif M. Physiological response of bread wheat (Triticum aestivum L.) to high temperature and moisture stresses. Aust. J. Crop. Sci. 2012;6:749. [Google Scholar]
- Ahmed M., Hassan F.U., Qadir G., Shaheen F.A., Aslam M.A. Response of proline accumulation in bread wheat (Triticum aestivum L.) under rainfed conditions. J. Agric. Meteorol. 2017;73(4):147–155. doi: 10.2480/agrmet.D-14-00047. [DOI] [Google Scholar]
- Ahmed M., Aslam M.A., Hassan F., Hayat R., Ahmad S. Biochemical, physiological and agronomic response of wheat to changing climate of rainfed areas of Pakistan. Pak. J. Bot. 2019;51:535–551. doi: 10.30848/pjb2019-2(10). [DOI] [Google Scholar]
- Ahmed H.G.M.D., Sajjad M., Li M., Azmat M.A., Rizwan M., Maqsood R.H., Khan S.H. Selection criteria for drought-tolerant bread wheat genotypes at seedling stage. Sustainability. 2019;11(9):2584. doi: 10.3390/su11092584. [DOI] [Google Scholar]
- Ahmed K., Shahid S., Chung E.S., Wang X.J., Harun S.B. Climate change uncertainties in seasonal drought severity-area-frequency curves: case of arid region of Pakistan. J. Hydrol. 2019;570:473–485. doi: 10.1016/j.jhydrol.2019.01.019. [DOI] [Google Scholar]
- Al Khateeb W., Al Shalabi A.A., Schroeder D., Musallam I. Phenotypic and molecular variation in drought tolerance of Jordanian durum wheat (Triticum durum Desf.) landraces. Physiol. Mol. Biol. Plants. 2017;23:311–319. doi: 10.1007/s12298-017-0434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M.A., Awan S.I. Inheritance pattern of seed and lint traits in cotton (Gossypium hirsutum) Int. J. Agric. Biol. 2009;11(1):44–48. [Google Scholar]
- Allen L.H., Jr., Bisbal E.C., Boote K.J., Jones P.H. Soybean dry matter allocation under sub-ambient and super-ambient levels of carbon dioxide. Agron. J. 1991;83(5):875–883. doi: 10.2134/agronj1991.00021962008300050020x. [DOI] [Google Scholar]
- Almeselmani M., Saud A.A.R., Al-Zubi K., Hareri F., Al-Nassan M., Ammar M.A., Al-Sael H.A. Physiological attributes associated to water deficit tolerance of Syrian durum wheat varieties. Exp. Agric. Hort. 2012;8:21–41. (Article ID:1929-0861-2012-08) [Google Scholar]
- Alqudah A.M., Koppolu R., Wolde G.M., Graner A., Schnurbusch T. The genetic architecture of barley plant stature. Front. Genet. 2016;7:117. doi: 10.3389/fgene.2016.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwaar H.A., Perveen R., Mansha M.Z., Abid M., Sarwar Z.M., Aatif H.M.…Rizwan M. Assessment of grain yield indices in response to drought stress in wheat (Triticum aestivum L.) Saudi J. Biol. Sci. 2019 doi: 10.1016/j.sjbs.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseng S., Martre P., Maiorano A., Rötter R.P., O’Leary G.J., Fitzgerald G.J., Reynolds M.P. Climate change impact and adaptation for wheat protein. Glob. Chang. Biol. 2019;25:155–173. doi: 10.1111/gcb.14481. [DOI] [PubMed] [Google Scholar]
- Awan K.A., Ali J., Akmal M. Yield comparison of potential wheat varieties by delay sowing as rainfed crop for peshawar climate. Sarhad J. Agric. 2017;33(3):480–488. [Google Scholar]
- Ayeneh A., Ginkel M., Reynolds M.P., Ammar K. Comparison of leaf, spike, peduncle, and canopy temperature depression in wheat under heat stress. Field Crops Res. 2002;79:173184. doi: 10.1016/s0378-4290(02)00138-7. [DOI] [Google Scholar]
- Babar M.A., Reynolds M.P., Van Ginkel M., Klatt A.R., Raun W.R., Stone M.L. Spectral reflectance to estimate genetic variation for in-season biomass, leaf chlorophyll, and canopy temperature in wheat. Crop Sci. 2006;46:1046–1057. doi: 10.2135/cropsci2005.0211. [DOI] [Google Scholar]
- Bajji M., Lutts S., Kinet J.M. Water deficit effects on solute contribution to osmotic adjustment as a function of leaf ageing in three durum wheat (Triticum durum Desf.) cultivars performing differently in arid conditions. Plant Sci. 2001;160:669–681. doi: 10.1016/S0168-9452(00)00443-X. [DOI] [PubMed] [Google Scholar]
- Barrs H.D., Weatherley P.E. A re-examination of the relative turgidity technique for estimating water deficit in leaves. Aust. J. Biol. Sci. 1962;15:413–428. doi: 10.1071/bi9620413. [DOI] [Google Scholar]
- Bates L.S., Waldren R.P., Teare I.D. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Batool A., Noorka I.R., Afzal M., Syed A.H. Estimation of heterosis, heterobeltiosis and potence ratio over environments among pre and post Green Revolution Spring wheat in Pakistan. J. Basic Appl. Sci. 2013;9:36–43. doi: 10.6000/1927-5129.2013.09.07. [DOI] [Google Scholar]
- Bhattacharyya R., Roy B., Kabi M.C., Basu A.K. Character association and path analysis of seed yield and its attributes in rice as affected by bio-inoculums under tropical environment. Trop. Agr. Res. Ext. 2010;10:23–28. doi: 10.4038/tare.v10i0.1867. [DOI] [Google Scholar]
- Blum A. Osmotic adjustment and growth of barley genotypes under drought stress. Crop Sci. 1989;29(1):230–233. doi: 10.2135/cropsci1989.0011183X002900010052x. [DOI] [Google Scholar]
- Chen H., Jiang J.-G. Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environ. Rev. 2010;18:309–319. doi: 10.1139/A10-014. [DOI] [Google Scholar]
- Chen K., Horton R.M., Bader D.A., Lesk C., Jiang L., Jones B., Zhou L., Chen J.Bi., Kinney P.L. Impact of climate change on heat-related mortality in Jiangsu Province, China. Environ. Pollut. 2017;224:317–325. doi: 10.1016/j.envpol.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J.M. Phenological variability: effect on determination of leaf water loss in wheat. Crop Sci. 1992;32:1457–1459. doi: 10.2135/cropsci1992.0011183X003200060029x. [DOI] [Google Scholar]
- Dubois M., Gilles K.A., Hammiltron J.K., Robers P.A., Smith F. A colorimetric method for the determination of sugars. Nature. 1951;168:167–168. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- Farooq M., Basra S.M.A., Wahid A., Ahmad N., Saleem B.A. Improving the drought tolerance in rice (Oryza sativa L.) by exogenous application of salicylic acid. J. Agron. Crop Sci. 2009;195(4):237–246. doi: 10.1111/j.1439-037X.2009.00365.x. [DOI] [Google Scholar]
- Farooq M., Bramley H., Palta J.A., Siddique K.H. Heat stress in wheat during reproductive and grain-filling phases. Crit. Rev. Plant Sci. 2011;30(6):491–507. doi: 10.1080/07352689.2011.615687. [DOI] [Google Scholar]
- Fernandes A.S., Baker E.A., Martin J.T. Studies on plant cuticle: VI. The isolation and fractionation of cuticular waxes. Ann. Appl. Biol. 1964;53(1):43–58. doi: 10.1111/j.1744-7348.1964.tb03779.x. [DOI] [Google Scholar]
- Fernandez G.C. Proceeding of the International Symposium on Adaptation of Vegetables and Other Food Crops in Temperature and Water Stress, Aug. 13–16, Shanhua, Taiwan, 1992. 1992. Effective selection criteria for assessing plant stress tolerance; pp. 257–270.https://ci.nii.ac.jp/naid/10029135114/ [Google Scholar]
- Forouzani M., Karami E. Agricultural water poverty index and sustainability. Agron. Sustain. Dev. 2011;31(2):415–431. doi: 10.1051/agro/2010026. [DOI] [Google Scholar]
- Foulkes M., Sylvester-Bradley R., Weightman R., Snape J. Identifying physiological traits associated with improved drought resistance in winter wheat. Field Crops Res. 2007;103:11–24. doi: 10.1016/j.fcr.2007.04.007. [DOI] [Google Scholar]
- França M.G.C., Thi A.T.P., Pimentel C., Rossiello R.O.P., Zuily-Fodil Y., Laffray D. Differences in growth and water relations among Phaseolus vulgaris cultivars in response to induced drought stress. Environ. Exp. Bot. 2000;43(3):227–237. doi: 10.1016/S0098-8472(99)00060-X. [DOI] [PubMed] [Google Scholar]
- Gontia N.K., Tiwari K.N. Development of crop water stress index of wheat crop for scheduling irrigation using infrared thermometry. Agric. Water Manag. 2008;95(10):1144–1152. doi: 10.1016/j.agwat.2008.04.017. [DOI] [Google Scholar]
- Gusmao M., Siddique K.H.M., Flower K., Nesbitt H., Veneklaas E.J. Water deficit during the reproductive period of grass pea (Lathyrus sativus L.) reduced grain yield but maintained seed size. J. Agron. Crop Sci. 2012;198:430–441. doi: 10.1111/j.1439-037X.2012.00513.x. [DOI] [Google Scholar]
- Hui-Mean F., Yusop Z., Yusof F. Drought analysis and water resource availability using standardised precipitation evapotranspiration index. Atmos. Res. 2018;201:102–115. doi: 10.1016/j.atmosres.2017.10.014. [DOI] [Google Scholar]
- Jackson, R.D., Idso, S.B., Reginato, R.J., Pinter Jr, P.J., 1981. Canopy temperature as a crop water stress indicator. Water Resour. Res., 17(4), pp. 1133–1138. doi: 10.1029/WR017i004p01133. [DOI]
- Johnson R.P., Balwani T.L., Johnson L.J., Mcclure K.E., Denority B.A. Corn plant maturity. II. Effect on invitro cellular digestibility and soluble carbohydrates content. J. Anim. Sci. 1966;25:617. doi: 10.2527/jas1966.253617x. [DOI] [Google Scholar]
- Kavi Kishor P.B., Sreenivasulu N. Proline homeostasis. Plant Cell Environ. 2014;37:300–311. doi: 10.1111/pce.12157. [DOI] [PubMed] [Google Scholar]
- Kebede A., Kang M.S., Bekele E. Chapter five-advances in mechanisms of drought tolerance in crops, with emphasis on barley. In: Sparks D.L., editor. Advances in Agron. Vol. 156. Academic Press; 2019. pp. 265–314. [Google Scholar]
- Khan M.A., Tahir A., Khurshid N., Ahmed M., Boughanmi H. Economic effects of climate change-induced loss of agricultural production by 2050: a case study of Pakistan. Sustainability. 2020;12(3):1216. doi: 10.3390/su12031216. [DOI] [Google Scholar]
- Lancashire P.D., Bleiholder H., Boom T.V.D., Langelüddeke P., Stauss R., WEBER E., Witzenberger A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991;119(3):561–601. doi: 10.1111/j.1744-7348.1991.tb04895.x. [DOI] [Google Scholar]
- Li P., Fan Y., Yin S., Wang Y., Wang H., Xu Y. Multi-environment QTL mapping of crown root traits in a maize RIL population. The Crop J. 2020 doi: 10.1016/j.cj.2019.12.006. [DOI] [Google Scholar]
- Liu Y., Hwang Y. Improving drought predictability in Arkansas using the ensemble PDSI forecast technique. Stoch. Env. Res. Risk A. 2015;29(1):79–91. doi: 10.1007/s00477-014-0930-3. [DOI] [Google Scholar]
- Liu B., Martre P., Ewert F., Porter J.R., Challinor A.J., Müller C., Ahmed M. Global wheat production with 1.5 and 2.0 °C above pre-industrial warming. Glob. Chang. Biol. 2019;25(4):1428–1444. doi: 10.1111/gcb.14542. [DOI] [PubMed] [Google Scholar]
- Ma J., Li R., Wang H., Li D., Wang X., Zhang Y.…Li Y. Transcriptomics analyses reveal wheat responses to drought stress during reproductive stages under field conditions. Front. Plant Sci. 2017;8:592. doi: 10.3389/fpls.2017.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes W.H., Steppe K. Estimating evapotranspiration and drought stress with ground-based thermal remote sensing in agriculture: a review. J. Exp. Bot. 2012;63(13):4671–4712. doi: 10.1093/jxb/ers165. [DOI] [PubMed] [Google Scholar]
- Mahmood N., Arshad M., Kächele H., Ma H., Ullah A., Müller K. Wheat yield response to input and socioeconomic factors under changing climate: evidence from rainfed environments of Pakistan. Sci. Total Environ. 2019;688:1275–1285. doi: 10.1016/j.scitotenv.2019.06.266. [DOI] [PubMed] [Google Scholar]
- McKevith B. Nutritional aspects of cereals. Nutr. Bull. 2004;29(2):111–142. [Google Scholar]
- Mohammadijoo S., Mirasi A., Saeidi-aboeshaghi R., Amiri M. Evaluation of bread wheat (Triticum aestivum L.) genotypes based on resistance indices under field conditions. Int. J. Biosci. 2015;6:331–337. doi: 10.12692/ijb/6.2.331-337. [DOI] [Google Scholar]
- Moshelion M., Halperin O., Wallach R., Oren R.A.M., Way D.A. Role of aquaporins in determining transpiration and photosynthesis in water-stressed plants: crop water-use efficiency, growth and yield. Plant Cell Environ. 2015;38(9):1785–1793. doi: 10.1111/pce.12410. [DOI] [PubMed] [Google Scholar]
- Mwadzingeni L., Shimelis H., Dube E., Laing M.D., Tsilo T.J. Breeding wheat for drought tolerance: progress and technologies. J. Integr. Agr. 2016;15(5):935–943. doi: 10.1016/S2095-3119(15)61102-9. [DOI] [Google Scholar]
- Nehe A.S., Misra S., Murchie E.H., Chinnathambi K., Tyagi B., Foulkes M.J. Nitrogen partitioning and remobilization in relation to leaf senescence, grain yield and protein concentration in Indian wheat cultivars. Field Crops Res. 2020;251:107778. doi: 10.1016/j.fcr.2020.107778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouraein M., Mohammadi S.A., Aharizad S., Moghaddam M., Sadeghzadeh B. Evaluation of drought tolerance indices in wheat recombinant inbred line population. Ann. Biol. Res. 2013;4(3):113–122. http://scholarsresearchlibrary.com/archive.html Scholars Research Library. [Google Scholar]
- Osakabe Y., Osakabe K., Shinozaki K., Tran L.S. Response of plants to water stress. Front. Plant Sci. 2014;5:86. doi: 10.3389/fpls.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passioura J. The drought environment: physical, biological and agricultural perspectives. J. Exp. Bot. 2006;58(2):113–117. doi: 10.1093/jxb/erl212. [DOI] [PubMed] [Google Scholar]
- Pravalie R., Sîrodoev I., Patriche C., Roșca B., Piticar A., Bandoc G.…Mănoiu V. The impact of climate change on agricultural productivity in Romania. A country-scale assessment based on the relationship between climatic water balance and maize yields in recent decades. Agric. Syst. 2020;179:102767. doi: 10.1016/j.agsy.2019.102767. [DOI] [Google Scholar]
- R Development Core Team R: a language and environment for statistical computing. 2020. http://www.R-project.org
- Ramirez-Cabral N.Y., Kumar L., Shabani F. Global alterations in areas of suitability for maize production from climate change and using a mechanistic species distribution model (CLIMEX) Sci. Rep. 2017;7(1):1–13. doi: 10.1038/s41598-017-05804-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R.A., Rebetzke G.J., Watt M., Condon A.T., Spielmeyer W., Dolferus R. Breeding for improved water productivity in temperate cereals: phenotyping, quantitative trait loci, markers and the selection environment. Funct. Plant Biol. 2010;37:85–97. doi: 10.1071/FP09219. [DOI] [Google Scholar]
- Sairam R.K., Deshmukh P.S., Shukla D.S. IARI; New Delhi: 1997. Increased Antioxidant Enzyme Activity in Response to Drought and Temperature Stress Related With Stress Tolerance in Wheat Genotypes. Abstract: National Seminar (ISSP) p. 69. [Google Scholar]
- Sallam A., Alqudah A.M., Dawood M.F., Baenziger P.S., Börner A. Drought stress tolerance in wheat and barley: advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 2019;20(13):3137. doi: 10.3390/ijms20133137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangakkara H.R., Hartwig H.A., Nosberger J. Response of root branching and shoot water potential of Phaeseolus valgaris L. to soil moisture and fertilizer potassium. J. Agron. Crop Sci. 1996;177:165–173. doi: 10.1111/j.1439-037X.1996.tb00234.x. [DOI] [Google Scholar]
- Schonfeld M.A., Johnson R.C., Carver B.F., Mornhinweg D.W. Water relations in winter wheat as drought resistance indicators. Crop Sci. 1988;28(3):526–531. doi: 10.2135/cropsci1988.0011183X002800030021x. [DOI] [Google Scholar]
- Senapati N., Semenov M.A. Assessing yield gap in high productive countries by designing wheat ideotypes. Sci. Rep. 2019;9:5516. doi: 10.1038/s41598-019-40981-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma B.R., Rao K.V., Vittal K.P.R., Ramakrishna Y.S., Amarasinghe U. Estimating the potential of rainfed agriculture in India: prospects for water productivity improvements. Agric. Water Manag. 2010;97(1):23–30. [Google Scholar]
- Siddique M.R.B., Hamid A.I.M.S., Islam M.S. Drought stress effects on water relations of wheat. Bot. Bull. Acad. Sinica. 2000:41. [Google Scholar]
- Sinclair T.R. Challenges in breeding for yield increase for drought. Trends in Plant Sci. 2011;16:289–293. doi: 10.1016/j.tplants.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Steel R.G., Torrie J.H., Dickey D.A. McGraw-Hill; 1997. Principles and Procedures of Statistics: A Biological Approach. [DOI] [Google Scholar]
- Sullivan C.Y. Oxford & IBH Pub. Co; 1972. Mechanisms of Heat and Drought Resistance in Grain Sorghum and Methods of Measurement. Sorghum in Seventies. [Google Scholar]
- Toker C., Canci H., Yildirim T. Evaluation of perennial wild Cicer species for drought resistance. Genet. Resour. Crop. Evol. 2007;54:1781–1786. doi: 10.1007/s10722-006-9197-y. [DOI] [Google Scholar]
- Touma D., Ashfaq M., Nayak M.A., Kao S.C., Diffenbaugh N.S. A multi-model and multi-index evaluation of drought characteristics in the 21st century. J. Hydrol. 2015;526:196–207. doi: 10.1016/j.jhydrol.2014.12.011. [DOI] [Google Scholar]
- Turner N.C. Crop water deficits: a decade of progress. Adv. Agron. 1986;39:1–51. doi: 10.1016/S0065-2113(08)60464-2. [DOI] [Google Scholar]
- van Ogtrop F., Ahmad M., Moeller C. Principal components of sea surface temperatures as predictors of seasonal rainfall in rainfed wheat growing areas of Pakistan. Meteorol. Appl. 2014;21(2):431–443. doi: 10.1002/met.1429. [DOI] [Google Scholar]
- Wang X., Mao Z., Zhang J., Hemat M., Huang M., Cai J., Jiang D. Osmolyte accumulation plays important roles in the drought priming induced tolerance to post-anthesis drought stress in winter wheat (Triticum aestivum L.) Environ. Exp. Bot. 2019;166 doi: 10.1016/j.envexpbot.2019.103804. [DOI] [Google Scholar]
- Xiong Y.C., Li F.M., Zhang T. Performance of wheat crops with different chromosome ploidy: root-sourced signals, drought tolerance and yield performance. Planta. 2006;224(3):710–718. doi: 10.1007/s00425-006-0252-x. [DOI] [PubMed] [Google Scholar]
- Yan W., Fregeau-Reid J. Breeding line selection based on multiple traits. Crop Sci. 2008;48(2):417–423. doi: 10.2135/cropsci2007.05.0254. [DOI] [Google Scholar]
- Zadoks J.C., Chang T.T., Konzak C.F. A decimal code for the growth stages of cereals. Weed Res. 1974;14(6):415–421. doi: 10.1111/j.1365-3180.1974.tb01084.x. [DOI] [Google Scholar]
- Zhang S., Wu Y., Sivakumar B., Mu X., Zhao F., Sun P., Han J. Climate change-induced drought evolution over the past 50 years in the southern Chinese Loess Plateau. Environ. Model. Softw. 2019;122 doi: 10.1016/j.envsoft.2019.104519. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables