Physicians in the intensive care unit (ICU), in charge of patients with severe acute respiratory distress syndrome (ARDS) due to Coronavirus (SARS-CoV-2), face a difficult dilemma: to improve gas exchange, oxygen transport and tissue oxygenation using mechanical ventilation (MV), or to limit ventilator-induced lung injury (VILI) associated with prolonged MV. In addition, while MV often requires deep sedation with or without neuromuscular blocking agents (NMBA) to tolerate MV and limit the risk of VILI, deep and/or prolonged sedation is associated with several complications, e.g. delirium, withdrawal syndromes, propofol -related infusion syndrome (PRIS), haemodynamic instability, ICU acquired muscle weakness, and difficult MV weaning leading to a sustained utilisation of ICU resources [1], [2]. As the Coronavirus pandemic is associated with a shortage of sedatives and NMBA drugs in several countries including France, it is critical to discuss the role of sedation in this particular context.
Using deep sedation with or without NMBA in patients with ARDS aims at improving pulmonary compliance and suppressing ventilatory drive to facilitate the adaptation of patient to the ventilator and the tolerance of hypercapnia due to the protective ventilation with low tidal volume. However, two features characterise the COVID-related ARDS [3]: the pulmonary compliance is initially normal or even high in the absence of bacterial infection, and ventilatory drive may be altered, i.e. patients can have severe hypoxemia with no tachypnoea and/or dyspnoea. Using deep and prolonged sedation (for 2 weeks) with NMBA could not be necessarily required for all patients with COVID-related ARDS. Recent guidelines [collaborators, 2010, #3385] have highlighted the need for an individual management of sedation–analgesia, adjustable over time, reaching the lightest level of sedation, i.e.: a Richmond Agitation Sedation Scale (RASS) score between −2 and +1, and giving priority to the pain control, i.e. a Behavioural Pain Scale (BPS) score below 5 at rest and during nociceptive procedures [4].
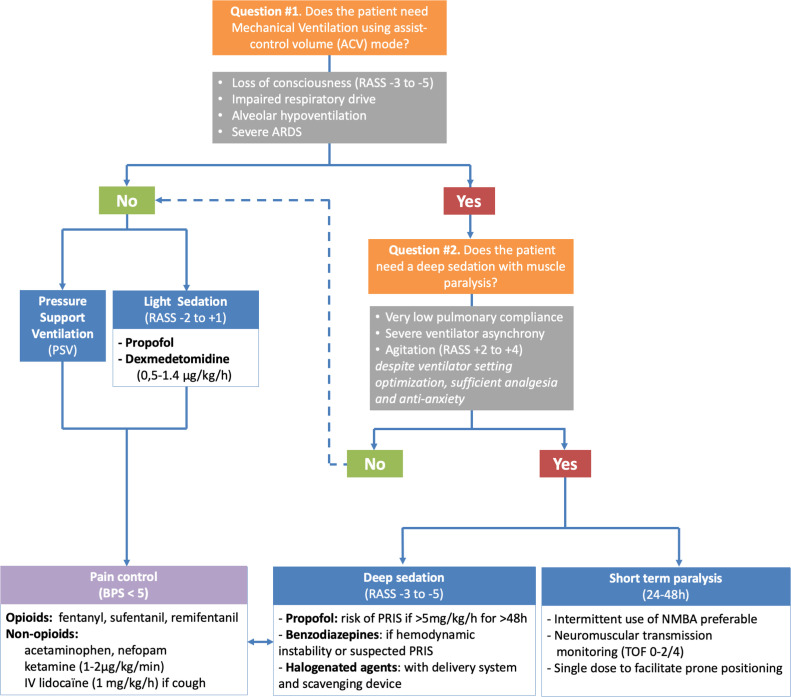
In intubated and mechanically ventilated patients with COVID-19, there are two inseparable questions to consider every day (Fig. 1 ), found below.
Fig. 1.
Algorithm to optimising the use of sedatives, opioids, and neuromuscular blocking agents in mechanically ventilated patients for COVID-19. ARDS: Acute Respiratory Distress Syndrome; BPS: Behavioural Pain Scale; RASS: Richmond Agitation Sedation Scale; NMBA: Neuromuscular Blocking Agents; TOF: Train of Four.
1. Does the patient need MV using assist-control volume (ACV) mode?
Yes, in the case of loss of consciousness (RASS −3 to −5), alteration of ventilatory drive, alveolar hypoventilation or severe ARDS. Continuous sedation–analgesia is thus necessary (cf. infra).
In other clinical situations, the first-line choice of ventilator mode should be pressure support ventilation (PSV) for patients with preserved consciousness and ventilatory drive [5]. More comfortable than ACV, PSV allows the patient to determine inspiratory and expiratory times. PSV delivers a decelerating inspiratory flow which is not limited if the patient needs high inspiratory flow. In this situation, a deep sedation level is not required. In association with non-opioids, opioids should be considered in case of severe pain and/or persistent dyspnoea despite the optimisation of ventilator settings such as minimal inspiratory trigger without auto-triggering, inspiratory slope more or less steep, PSV level between 5 and 15 cmH2O, and adjusted expiratory cycling.
2. Does the patient need a deep sedation with muscle paralysis (NMBA)?
Yes, in the case of impaired pulmonary compliance, severe ventilator asynchrony, incoercible cough or sustained agitation (RASS +2 to +4) despite optimisation of ventilator settings, analgesia, and sedation/anti-anxiety. Therapeutic modalities should include:
-
•
the use of short-term muscle relaxation (24–48 h), if possible, through intermittent rather than continuous infusion of NMBA for economic issues. The effective dosing of NMBA (cisatracurium, atracurium, rocuronium) must be monitored using a neuromuscular transmission monitor to reach train of four (TOF) scan of 0–2, then reassessing the need for NMBA. A transient muscle paralysis may be needed during prone positioning;
-
•
among sedative agents, propofol is the first-line agent due to its shorter time to wake up compared to benzodiazepines and to the impossibility to reach a deep sedation using dexmedetomidin. Of note is the risk of PRIS, especially at dose exceeding 5 mg/kg/h for more than 48 h. Benzodiazepines (midazolam, diazepam, lorazepam) are options to consider if the haemodynamic status is worsening with propofol, or if PRIS is suspected. Halogenated agents (sevoflurane, isoflurane) might be an option because they can induce a deep sedation without tachyphylaxis; they require, however, team training and a delivery method combined with a scavenging device;
-
•
among analgesics, fentanyl and sufentanil are the first-line opioids. The continuous infusion of remifentanil is possible although it requires a progressive de-escalation to prevent withdrawal syndrome. Low-dose ketamine (1–2 mcg/kg/min) can be considered as a co-analgesic [2]. Intravenous lidocaine (1 mg/kg/h) may be used to reduce incoercible cough.
In other clinical situations, a light level of sedation (RASS between −2 and +1) can be achieved with propofol or a continuous infusion of dexmedetomidin (0.5 to 1.4 mcg/kg/h, with no bolus), in association with opioid and non-opioid analgesics (acetaminophen, nefopam) [1], [2].
Alteration of the ventilatory drive, ventilator asynchrony, frequent cough, and withdrawal syndrome can hamper the liberation from ventilator and create a vicious cycle with the reuse of deep sedation and NMBA. In order to limit an excessive use of these drugs during a period at risk of shortage, and to prevent prolonged MV in a context of limited available ICU resources, other drugs such as anti-psychotics, gamma-hydroxybutyric acid (GHB), alpha-2 agonists (clonidine, dexmedetomidin) should be considered in association with current recommended drugs, as well as other ventilator modes such as pressure modes (PVC, APRV and PSV). The ultimate challenge for the anaesthesiologist/intensivist is to adapt the ventilator to the patient, not the reverse.
Funding
Support for this study was provided solely via institutional and/or departmental sources.
Disclosure of interest
JFP declares having received personal fees from Orion Pharma and Integra Lifesciences. GC declares having received personal fees from Orion Pharma and Aspen Medical. EF declares having received personal fees from Drager Medical, GE Healthcare, Edwards Lifesciences, Orion Pharma, Fresenius Kabi, Baxter, and Fisher & Paykel Healthcare. LV declares having received personal fees from Air liquid and Baxter. SJ declares having received personal fees from Drager Medical, Medtronic, Baxter, Fresenius Medical and Fisher & Paykel Healthcare. JMC declares having received personal fees from Drager Medical, GE Healthcare, Sedana Medical, Baxter, Amomed, Fisher & Paykel Healthcare, Orion Pharma, Philips Medical, and Fresenius Medical Care.
References
- 1.Chanques G., Drouot X., Payen J.F. 2008–2018: ten years of gradual changes in the sedation guidelines for critically ill patients. Anaesth Crit Care Pain Med. 2018;37:509–511. doi: 10.1016/j.accpm.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Devlin J.W., Skrobik Y., Gelinas C. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 3.Gattinoni L., Chiumello D., Caironi P. COVID-19 pneumonia: different respiratory treatment for different phenotypes? Intensive Care Med. 2020 doi: 10.1007/s00134-020-06033-2. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Payen J.F., Bosson J.L., Chanques G., Mantz J., Labarere J., Investigators D. Pain assessment is associated with decreased duration of mechanical ventilation in the intensive care unit: a post hoc analysis of the DOLOREA study. Anesthesiology. 2009;111:1308–1316. doi: 10.1097/ALN.0b013e3181c0d4f0. [DOI] [PubMed] [Google Scholar]
- 5.Chanques G., Conseil M., Roger C. Immediate interruption of sedation compared with usual sedation care in critically ill postoperative patients (SOS-Ventilation): a randomised, parallel-group clinical trial. Lancet Respir Med. 2017;5:795–805. doi: 10.1016/S2213-2600(17)30304-1. [DOI] [PubMed] [Google Scholar]