Highlights
-
•
Aluminum based adjuvants (ABAs) decrease viability in astrocytes and macrophages.
-
•
Size of aluminum particles influence viability to some extent.
-
•
Reactive oxygen species formation is consequent to loss of cell viability.
-
•
After ABA exposure, cytokine production is distinct based on cell type evaluated.
Keywords: Aluminum-based adjuvants (ABAs), Macrophages, Astrocytes, Reactive oxygen species (ROS), Proinflammatory cytokines
Abstract
Aluminum-based adjuvants (ABAs) are used in human vaccines to enhance the magnitude of protective immune responses elicited against specific pathogens. One hypothesis is that stress signals released by aluminum-exposed necrotic cells play a role in modulating an immune response that contributes to the adjuvant’s effectiveness. We hypothesized that aluminum adjuvant-induced necrosis would be similar irrespective of cellular origin or composition of the adjuvant. To test this hypothesis, human macrophages derived from peripheral monocytic cell line (THP-1) and cells derived from the human brain (primary astrocytes) were evaluated. Three commercially available formulations of ABAs (Alhydrogel, Imject alum, and Adju-Phos) were examined. Alum was also used as a reference. Cell viability, reactive oxygen species formation, and production of tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) were quantified. Cells were exposed to different concentrations (10−100 μg/mL) of the adjuvants for 24 h or 72 h. The two FDA approved adjuvants (Alhydrogel and Adju-Phos) decreased cell viability in both cell types. At the 72 h time point, the decrease in viability was accompanied with increased ROS formation. The size of the aluminum agglomerates was not relatable to the changes observed. After exposure to ABAs, astrocytes and macrophages presented a distinct profile of cytokine secretion which may relate to the function and unique characteristics of each cell type. These variations indicate that aluminum adjuvants may have differing capability of activating cells of different origin and thus their utility in specific vaccine design should be carefully assessed for optimum efficacy.
1. Introduction
Vaccination, an important medical procedure initiated in infancy, provides immunological protection from a variety of infectious diseases that are encountered in the environment. This procedure defends the individual against a myriad of pathogens without the necessity of experiencing the infectious disease sequela. Many vaccines are composed of highly purified antigens and require the addition of an adjuvant to provide an adequate immune response. Aluminum-based adjuvants (ABA) have been used since 1932 and are presently used in more than 30 licensed vaccines (Di Pasquale et al., 2015). The types of adjuvants that are available for use in human vaccines include aluminum hydroxide gel (Alhydrogel) and an aluminum phosphate gel (Adju-Phos). Another adjuvant, Imject Alum, is available for research and manufacturing but not clinical use (Instructions Imject Alum). Alum is a water soluble aluminum salt composed of aluminum potassium sulfate. It is a reagent that forms the basis of preparation of aluminum-containing adjuvants (Hem et al., 2007). In this study, we evaluated all four of these aluminum containing salts to determine how the cellular response is affected by dose and composition of ABAs or alum.
Aluminum adjuvants are present in vaccines against hepatitis A, hepatitis B, diphtheria-tetanus-pertussis (DTaP), Haemophilus influenzae type b (Hib), human papillomavirus (HPV) and pneumococcus infectious agents (Center for Disease Control, CDC, 2016). Based on the manufacturer, the type and aluminum content of vaccines licensed for use in the United States, varies. For instance, the Dtap vaccine manufactured by GSK (tradename Kinrix) contains ≤0.6 mg of aluminum present as aluminum hydroxide while Dtap vaccine manufactured by Sanofi-Pasteur (tradename Daptacel) contains 0.33 mg of aluminum present as aluminum phosphate (HogenEsch et al., 2018). It is thought that the amount of aluminum found in these vaccines poses a low risk of harm compared to its benefits (Mitkus et al., 2011). In the present study, we used 10−100 μg/mL concentrations which are in the range used by other investigators to assess the in vitro response to ABAs (Ulanova et al., 2001; Mold et al., et al., 2016; Vrieling et al., 2020). The mechanism by which ABAs strengthen vaccine efficiency is likely to encompass multiple pathways (Shi et al., 2019) that lead to a mainly type-2 (humoral) mediated immunity (Kuroda et al., 2013).
A mechanism by which ABAs appear to enhance the efficacy of vaccines is by inducing cytotoxicity and the subsequent release of damage associated molecular patterns (DAMPs) that activate cellular immune response to the coexisting antigen in the vaccine (Kono and Rock, 2008; Marichal et al., 2011). Previous research demonstrated differences in the physiochemical properties of Alhydrogel, Imject Alum, and Adju-Phos in regards to their solubility, uptake, and viability using human monocytic (THP-1) cells (Mold et al., 2016). In the present study we further evaluated the response of THP-1 cells that were differentiated into macrophages, as well as human astrocytes, exposed to ABAs or alum. We hypothesized that irrespective of adjuvant composition, or tissue derivation of cells, ABAs dose-dependently would cause the same degree of necrosis that then leads to proinflammatory cytokine release. Tissue resident macrophages would be encountered almost immediately after administration of ABA-containing vaccines. Astrocytes on the other hand would be protected by the blood brain barrier. However, it has been proposed that aluminum in vaccines may persist in cells of the immune system (such as macrophages) and that these cells can travel to distal sites such as the brain and indirectly impact these tissues (Gherardi et al., 2019). Astrocytes are immune-competent resident brain cells that play an important role in homeostatic regulation of the brain. We had previously shown that prolonged exposure to low levels of aluminum, present in the drinking water, enhances oxidative and inflammatory markers specifically in mouse brains (Campbell et al., 2004; Becaria et al., 2006). Furthermore, the proinflammatory effect of aluminum was confined to cells of glial origin (Campbell et al., 1999, 2002). In the present study, we aimed to also investigate the potential of aggregated forms of aluminum, such as that present in ABAs, to cause an oxidative or inflammatory response in brain-derived (astrocytes) in comparison to peripherally-derived (macrophages) immune-competent cells.
Our results suggest that although cell viability was decreased in both cell types, accompanied with an increased rate of ROS formation after longer exposures, there was a specific profile of cytokine secretion in response to ABA exposure. Furthermore, the composition of the ABAs distinctively influenced the cellular response. These results underscore the importance of careful evaluation of each ABA in specific vaccine design to promote the greatest efficacy while avoiding potential adverse effects.
2. Methods
2.1. Preparation of aluminum adjuvants
Alhydrogel® adjuvant 2% (catalog #: vac-alu-250, CAS number 21645-51-2) composed of aluminum hydroxide gel (aluminum content: 10 mg/mL) and Adju-Phos® (catalog #: vac-phos-250, CAS number 7784-30-7) containing aluminum phosphate gel (aluminum content: 0.5 %), were obtained from InvivoGen (http://www.invivogen.com). Imject® Alum (catalog #: 77161) containing an aqueous solution of aluminum hydroxide (40 mg/mL) and magnesium hydroxide (40 mg/mL) was aquired from ThermoFisher (Waltham, MA). Aluminum potassium sulfate dodecahydrate (alum), (product #: 237086-100 G) was from Sigma Aldrich.
2.2. Particle analysis
Particle size of the aluminum agglomerates suspended in the media were analyzed using the NanoBrook 90Plus particle size analyzer. The effective diameter (nm) and dispersity index of the aluminum nanoparticles, in the prepared solutions, were assessed (n = 3). The suspended dilutions were read at 24 h and again at 72 h to determine how the size of Al gel clusters changed over the duration of cell incubations.
2.3. Exposure
2.3.1. Macrophages
The human monocytic cell line (THP-1) was purchased from ATCC (Manassas, VA) and maintained at 37 °C in 5% CO2. The cells were grown in RPMI-1640 medium (ATCC) containing 0.05 mM 2-mercaptoethanol and 10 % fetal bovine serum. THP-1 cells were seeded (10,000 cells/well) in a 96-well plate. Cells were incubated with 12-O-tetradecanoylphorbol-13-acetate (TPA) for 48 h to induce differentiation to macrophages (Tsuchiya et al., 1982). Cells were then incubated for 24 h or 72 h in the absence or presence of the prepared aluminum adjuvants (10−100 μg/mL).
2.3.2. Astrocytes
Primary human astrocytes (HA, Catalog #1800) were purchased from ScienCell Research Laboratories (Carlsbad, CA) and maintained at 37 °C in 5% CO2. They were grown in a proprietary medium obtained from ScienCell (Catalog #1831) consisting of basal medium, fetal bovine serum, growth supplement, and penicillin/streptomysin solution. Cells were grown in poly-L-lysine coated 96-well plates at a density of 10,000 cells/well. Once seeded, cells were incubated for 48 h at 37 °C in 5% CO2. Cells were then incubated for 24 h or 72 h with the prepared aluminum adjuvants (10−100 μg/mL).
2.4. Viability assay
The cellTiter-Glo luminescent cell viability assay by Promega (Madison, WI) was used to evaluate cell viability at the conclusion of the last kinetic reading for ROS formation. The protocol provided by the manufacturer was followed. Briefly, 50 μL of CellTiter-Glo® reagent was added to each well. This was incubated for 10 min at room temperature and luminescence was measured. The assay quantifies the amount of ATP generated in each corresponding well. It can be assumed that the number of viable cells is directly proportional to the ATP generated.
2.5. Reactive oxygen species (ROS) formation
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) was used to measure non-specific ROS formation after ABA exposure. This dye rapidly diffuses into cells and is hydrolyzed by intracellular esterases to DCFH and subsequently oxidized to form the highly fluorescent dichlorofluorescein (DCF). A DCF standard curve was used to quantify ROS formation. BioTek Synergy HT Microplate Reader was used to measure the fluorescence generated kinetically for 1 h at 10 min intervals at an excitation of 485 nm and emission of 528 nm. Results shown are for amount of DCF generated in 30 min and normalized based on viable cells in corresponding well.
2.6. IL-6 & TNF-α levels
Formation of IL-6 (analytical sensitivity: <2 pg/mL) and TNF-α (analytical sensitivity: <0.09 pg/mL) was quantitated using supernatant removed from the treated cells. ELISA kits purchased from Invitrogen (Carlsbad, CA) were used to measure Human IL-6 or TNF-α in 96 well plates coated with antibodies for the corresponding cytokine in accordance with the manufacturer’s protocol. The absorbance was recorded at 450 nm using a BioTek Synergy HT Microplate Reader.
2.7. Statistical analysis
Statistical analysis was conducted using GraphPad Prism v7.0. Statistical significance was determined using one-way ANOVA followed by Dunnett’s post-hoc test. Values were considered significant at p ≤ 0.05. Error bars in figures represent standard error of the mean.
3. Results
3.1. Particle analysis
In both astrocyte and macrophage media, and at both time points, Adju-Phos particles were the largest-sized and alum the smallest based on effective diameter. The dose of the adjuvant did not substantially change the size of the particles except for Adju-Phos where the higher concentrations tended to coalesce into larger particles (24 h: 10 μg/mL at 2166 ± 72 nm versus 50 μg/mL at 3810 ± 53 nm; 72 h: 10 μg/mL at 2180 ± 45 nm versus 50 μg/mL at 3518 ± 120 nm). This tendency was most pronounced in the macrophage medium. Alum particles tend to be smaller in the macrophage medium compared to the astrocyte medium (astrocyte medium, 24 h: 50 μg/mL at 844 ± 64 nm versus macrophage medium, 24 h: 50 μg/mL at 422 ± 4 nm). The reason for these dissimilarities between the macrophage and astrocyte media could be related to the composition of the solutions (see methods section above). The dispersity index for the aluminum salts were relatively uniform and small (in the range of 0.073 ± 0.001−0.295 ± 0.076) indicating that the solutions were relatively homogenous. Incubating the prepared aluminum salts at 37 °C for 72 h did not appear to alter the particle size or the dispersity index significantly, indicating that the adjuvants are stable at the time points assessed (Table 1 ).
Table 1.
Physicochemical characteristics of aluminum adjuvants.
| Astrocyte Medium (24 h) |
Astrocyte Medium (72 h) |
|||
|---|---|---|---|---|
| Aluminum adjuvant | Effective Diameter (nm) | Dispersity Index | Effective Diameter (nm) | Dispersity Index |
| Alhydrogel (10 μg/mL) | 1048 ± 4 | 0.177 ± 0.048 | 1047 ± 11 | 0.150 ± 0.027 |
| Alhydrogel (50 μg/mL) | 1188 ± 4 | 0.073 ± 0.001 | 1189 ± 16 | 0.158 ± 0.015 |
| IMJECT (10 μg/mL) | 1346 ± 17 | 0.136 ± 0.065 | 1286 ± 67 | 0.179 ± 0.016 |
| IMJECT (50 μg/mL) | 1394 ± 69 | 0.295 ± 0.076 | 1412 ± 53 | 0.180 ± 0.066 |
| ALUM (10 μg/mL) | 672 ± 26 | 0.21 ± 0.004 | 658 ± 6 | 0.247 ± 0.024 |
| ALUM (50 μg/mL) | 844 ± 64 | 0.23 ± 0.023 | 563 ± 6 | 0.271 ± 0.006 |
| Adju-Phos (10 μg/mL) | 2114 ± 42 | 0.129 ± 0.042 | 1745 ± 20 | 0.151 ± 0.69 |
| Adju-Phos (50 μg/mL) | 2474 ± 44 | 0.147 ± 0.045 | 2583 ± 80 | 0.127 ± 0.48 |
| Macrophage Medium (24 h) | Macrophage Medium (72 h) | |||
|---|---|---|---|---|
| Aluminum adjuvant | Effective Diameter (nm) | Dispersity Index | Effective Diameter (nm) | Dispersity Index |
| Alhydrogel (10 μg/mL) | 1057 ± 11 | 0.235 ± 0.006 | 1332 ± 15 | 0.107 ± 0.041 |
| Alhydrogel (50 μg/mL) | 1198 ± 22 | 0.167 ± 0.071 | 1152 ± 16 | 0.151 ± 0.033 |
| IMJECT (10 μg/mL) | 1288 ± 42 | 0.185 ± 0.042 | 1222 ± 74 | 0.174 ± 0.025 |
| IMJECT (50 μg/mL) | 1382 ± 58 | 0.142 ± 0.030 | 1555 ± 10 | 0.126 ± 0.062 |
| ALUM (10 μg/mL) | 435 ± 4 | 0.250 ± 0.013 | 404 ± 5 | 0.213 ± 0.017 |
| ALUM (50 μg/mL) | 422 ± 4 | 0.283 ± 0.015 | 413 ± 4 | 0.286 ± 0.017 |
| Adju-Phos (10 μg/mL) | 2166 ± 72 | 0.143 ± 0.046 | 2180 ± 45 | 0.128 ± 0.055 |
| Adju-Phos (50 μg/mL) | 3810 ± 53 | 0.178 ± 0.171 | 3518 ± 120 | 0.259 ± 0.014 |
The effective diameter and dispersity index of aluminum adjuvants or alum (10 μg/mL or 50 μg/mL) in astrocyte or macrophage media. The values were determined after a 24 h or 72 h incubation (n = 3).
3.2. Cell viability
3.2.1. Macrophages
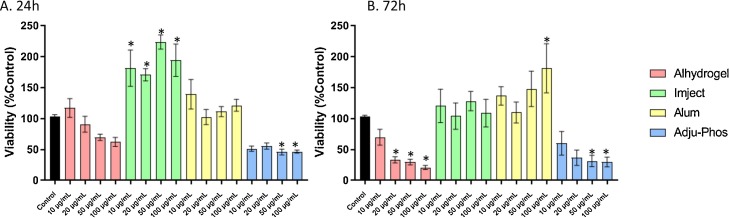
After a 24 h exposure to ABAs, macrophages exposed to Imject had increased levels of ATP compared to control. This may reflect increased mitochondrial activity. In contrast, Adju-Phos-exposed cells showed severely decreased ATP levels which may reflect cell death. These effects were not dose-dependent. At the 72 h time point, exposure to both Alhydrogel and Adju-Phos caused a decrease in ATP content. The effects at this time point were dose-dependent. At 72 h exposure, no significant change was evident in macrophages exposed to Imject. At the highest concentration of alum, there was an increase in ATP levels (Fig. 1 ).
Fig. 1.
Cell viability in THP-1 derived macrophages treated with ABAs or alum for 24 h (A) or 72 h (B). Viability is calculated as percent control from arbitrary units of luminescence. The data presented are a combination of two sets of experiments conducted on separate dates (n = 8 technical replicates). Error bars represent the standard error of the mean (SEM). *p ≤ 0.05 compared to control group.
3.2.2. Astrocytes
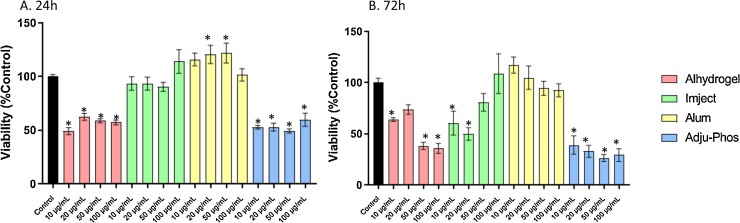
After 24 h or 72 h exposure to Alhydrogel or Adju-Phos, there was a decrease in cell viability. Imject did not affect cell viability at 24 h but after 72 h exposure, caused cell death at the lower concentrations tested. Exposure to alum at 24 h caused an increase after exposure to 20 μg/mL or 50 μg/mL but this was not evident at the 72 h time point (Fig. 2 ).
Fig. 2.
Cell viability in human primary astrocytes treated with ABAs or alum for 24 h (A) or 72 h (B). Viability is calculated as percent control from arbitrary units of luminescence. The data presented are a combination of two sets of experiments conducted on separate dates (n = 8 technical replicates). Error bars represent the standard error of the mean (SEM). *p ≤ 0.05 compared to control group.
3.3. Reactive oxygen species (ROS) formation
3.3.1. Macrophages
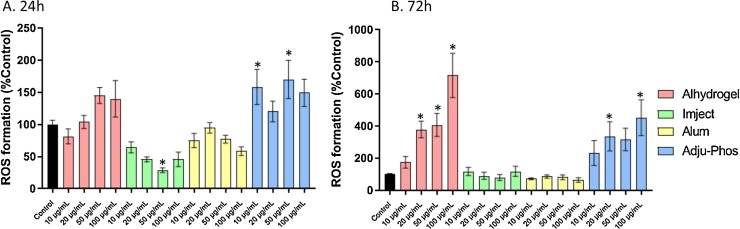
After a 72 h exposure to Alhydrogel or Adju-Phos, there was a dose-dependent increase in ROS formation. This may have been related to the observed decrease in viability. Imject and alum did not alter ROS formation (Fig. 3 ).
Fig. 3.
Reactive oxygen species formation in THP-1 derived macrophages treated with ABAs or alum for 24 h (A) or 72 h (B). ROS was calculated as rate of DCF formation in 30 min and converted to %Control. The data presented are a combination of two sets of experiments conducted on separate dates (n = 8 technical replicates). Error bars represent the standard error of the mean (SEM). *p ≤ 0.05 compared to control group.
3.3.2. Astrocytes
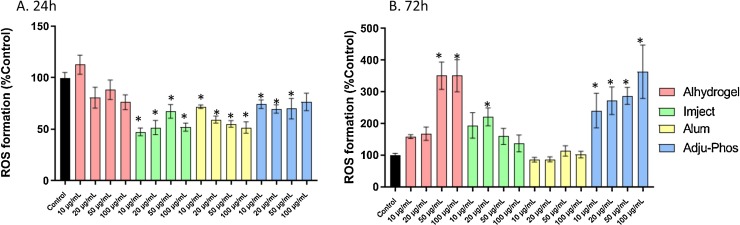
After a 24 h exposure, there was generally a decline in ROS formation after treatment with Imject, alum, or Adju-Phos. Exposure to Alhydrogel caused no such depression. At the longer 72 h time point, exposure to Alhydrogel (50 μg/mL & 100 μg/mL) or Adju-Phos (all concentrations), resulted in a consistent increase in ROS formation. This was concurrent with and perhaps causal to the decreased cell viability observed at this time point. There was an increase in ROS formation only at the 20 μg/mL exposure to Imject implying an overall lower level of toxicity of this adjuvant (Fig. 4 ).
Fig. 4.
Reactive oxygen species formation in human primary astrocytes treated with ABAs or alum for 24 h (A) or 72 h (B). ROS was calculated as rate of DCF formation in 30 min and converted to %Control. The data presented are a combination of two sets of experiments conducted on separate dates (n = 8 technical replicates). Error bars represent the standard error of the mean (SEM). *p ≤ 0.05 compared to control group.
3.4. Proinflammatory cytokine levels
3.4.1. TNF-α
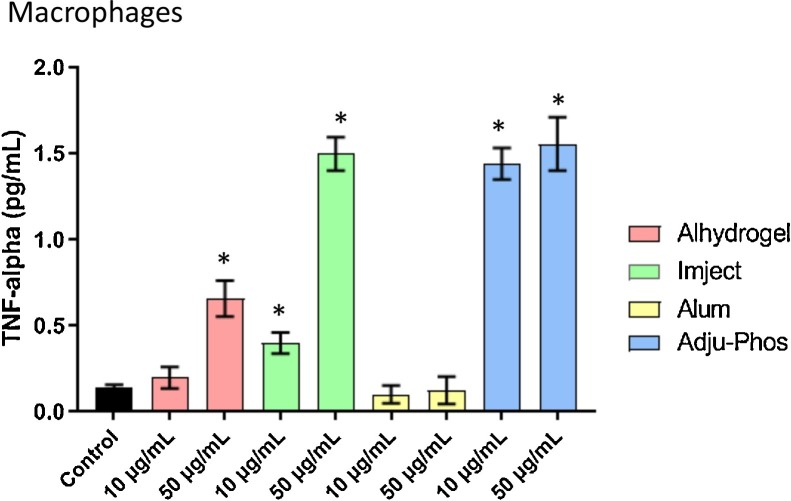
There was a dose dependent increase in the levels of this cytokine in macrophages treated with Alhydrogel or Imject. The levels of TNF-α were increased after exposure to both 10 and 50 μg/mL of Adju-Phos (Fig. 5 ). There were no detectable levels of this cytokine in the supernatant of the astrocytes treated with any of the aluminum adjuvants or alum.
Fig. 5.
Secreted levels of TNF-α in collected media from macrophages treated with ABAs or alum (10 μg/mL or 50 μg/mL) for 24 h. Astrocytes did not produce detectable levels of this cytokine. The data presented is n = 4 technical replicates conducted in duplicates. Error bars represent the standard error of the mean (SEM). *p ≤ 0.05 compared to control group.
3.4.2. IL-6
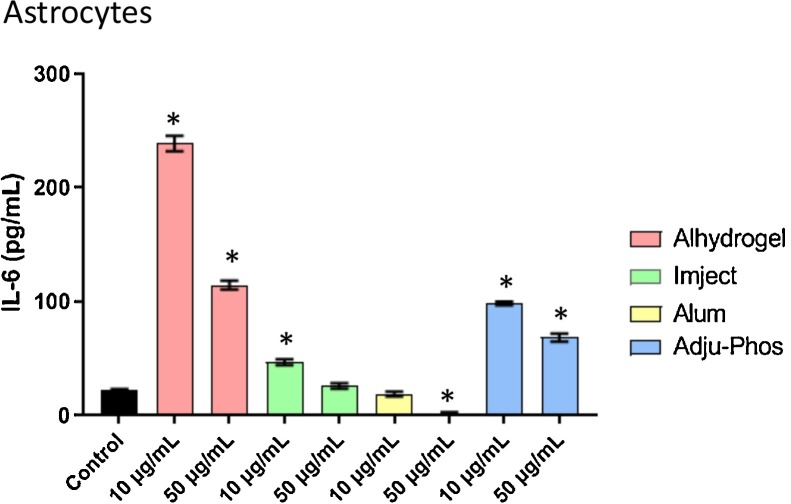
In macrophages, this cytokine was not detected in the supernatant of control or ABA treated (10 μg/mL or 50 μg/mL) samples. In contrast, the levels of this cytokine were markedly increased in supernatants from astrocytes after exposure to all three ABAs tested (Alhydrogel < Adju-Phos < Imject). However, this increase was non-linear and most pronounced at lower concentrations (Fig. 6 ).
Fig. 6.
Secreted levels of IL-6 in collected media from astrocytes treated with ABAs or alum (10 μg/mL or 50 μg/mL) for 24 h. Macrophages did not produce dettectable levels of the cytokine. The data presented is n = 4 technical replicates conducted in duplicates. Error bars represent the standard error of the mean (SEM). *p ≤ 0.05 compared to control group.
4. Discussion
Global vaccination has reduced the burden of infectious diseases and has led to the eradication of smallpox (Greenwood, 2014). ABAs enhance vaccine effectiveness by potentiating immune responses and are widely used because they are cost-effective and in general have a good safety profile. Because of this safety profile, aluminum adjuvants are often considered as the “gold standard” against which newly developed adjuvants should be compared (HogenEsch et al., 2018). Studies have shown that there are various mechanisms by which ABAs contribute to immunogenicity of vaccines. One of the best studied of these is the role aluminum salts play in adsorption of the inoculated antigen. ABAs have the capability of turning the soluble antigens into colloids which can then more effectively be internalized, decrease peptide degradation, and enhance MHCII expression by antigen-presenting cells (Ghimire et al., 2012).
Irrespective of antigen binding, ABAs themselves are colloidal and as such are capable of orchestrating a cellular immune response regardless of the presence of an immunogen (Vrieling et al., 2020). Aluminum hydroxide gel (Alhydrogel) is crystalline, but the degree of the structure is based on manufacturing setting and composition of the buffers in which it is diluted. Aluminum phosphate gel (Adju-Phos) is non-crystalline due to disruption of the crystallization process by phosphate (HogenEsch et al., 2018). Because the composition of the media may have influenced the size of the aluminum particles, we evaluated the effective diameter of the aluminum adjuvants in both astrocyte and macrophage media for 24 h & 72 h. Incubation of alhydrogel in both astrocyte and macrophage medium for 24 h resulted in formation of particles in the 1048–1198 nm range at both concentrations tested. Adju-phos particles were larger (2114–3810 nm) and this was concentration-dependent mainly in the macrophage medium. Imject particles were approximately 1288–1394 nm in diameter while alum particles were the smallest (range of 422–844 nm) particularly in the macrophage medium assessed. With increasing incubation time (72 h), the size of the aluminum particles did not significantly alter.
After 24 h, there was pronounced cytotoxicity observed only after exposure to Adju-Phos and this may be related to the larger size of the particles. This is in agreement with a study showing that in THP-1 cells, Adju-Phos displayed much greater cytotoxicity compared to Alhydrogel (Mold et al., 2016). Neither alum nor Imject (neither of which are used clinically) caused decrease in cell viability in the macrophages. The degree to which this finding might influence the effectiveness of Imject as an adjuvant should be further investigated. Both Alhydrogel and Adju-Phos caused a pronounced loss of cell viability in macrophages derived from THP-1 cells after a 72 h exposure. No clear relationship between particle size and toxicity was observed at this later time point, and thus additional physicochemical properties may contribute to loss of cell viability. Cell viability in astrocytes was also decreased to the same extent after exposure to either Alhydrogel or Adju-Phos after 24 h. Comparing exposure to the two clinically relevant ABAs, the loss of viability in astrocytes after 72 h was more pronounced after exposure to Adju-Phos, especially at the lower concentrations. Overall, susceptibility of astrocytes did not appear to be dose-dependent as the lower concentrations of Adju-Phos (10 & 20 μg/mL) were just as capable of reducing viability as the larger doses (50 & 100 μg/mL). After the 72 h exposure, both Alhydrogel and Adju-phos caused enhancement of ROS formation (especially at the higher doses tested) in both THP-1 derived macrophages and astrocytes. However, an enhancement of general ROS formation was not observed at the 24 h time point in the astrocytes. Thus the loss of viability does not appear to be associated with oxidative stress, but the cell death appears to contribute to subsequent enhancement of oxidative events as judged by an increase in general ROS formation.
Aluminum adjuvants have been shown to cause cellular necrosis and the subsequent production of uric acid, which acts as a threat signal, functions to activate the Nlrp3 inflammasome (Eisenbarth et al., 2008). The Nlrp3 inflammasome plays an important role in augmenting the inflammatory reaction (Baroja-Mazo et al., 2014) which is a mechanism by which ABAs modulate cellular behavior in an immune response. DNA released from ABA-exposed dying cells can also be perceived as a damage associated molecular pattern (DAMP) that can modify immunity initiated by aluminum containing vaccines (Marichal et al., 2011). A study on the kinetics of the inflammatory response induced by ABAs demonstrated that after vaccination, neutrophils are recruited to the area followed by macrophages (Lu and HogenEsch, 2013). Macrophages engulf aluminum hydroxide adjuvants, which remain inside the cells as crystal particles, and enhance antigen-presentation by the cells (Rimaniol et al., 2004).
While the changes in viability and ROS formation share similarities in both macrophages and astrocytes, ABA induced cytokine responses of the two cell types differ sharply. Macrophages exposed to all three of the aluminum adjuvants show a dose-dependent increase in TNF-α. On the other hand, astrocytes exposed to the ABAs released significant amounts of IL-6 in a nonmonotonic dose response pattern. We have previously reported that exposure to aluminum sulfate (500μM) for 48 h resulted in upregulation of oxidative events in glioma but not neuroblastoma cells (Campbell et al., 1999), indicating that depending on the type of brain-derived cell evaluated, aluminum may cause a specific response. After a 6d exposure to the same concentration of aluminum sulfate, we observed that a human glioblastoma cell line released TNF-α but not IL-6 in response to the Al salt (Campbell et al., 2002). Thus the glioblastoma cell line appears to have a cytokine response resembling that of macrophages rather than that of primary human astrocytes. Cytokine release in response to aluminum salts may underlie the immunomodulatory role of aluminum adjuvants and may be closely related to the characteristics and function of the type of cell evaluated. To what degree these differences in cytokine profile may dictate the safety and efficacy of ABAs needs to be further investigated.
Aluminum is a strong phosphate binder and was used for the treatment of hyperphosphatemia in dialysis patients before concerns regarding its connection to neurological abnormalities led to cessation of its widespread usage (Mudge et al., 2011). Chronic aluminum intake from the environment has been considered a potential co-factor of several neurological diseases, including Alzheimer’s disease (Campbell et al., 2001; Campbell, 2002; Bondy and Campbell, 2017). Chronic exposure to aluminum in the drinking water at levels found in some residential water supplies, led to an increase in oxidative and inflammatory events that appear specific to the brain (Campbell et al., 2004; Becaria et al., 2006). Therefore, concerns have been raised regarding the safety of aluminum adjuvants in individuals who may be predisposed to adverse neurological consequences.
Behavioral abnormalities were detected in mice exposed to clinically relevant doses of aluminum hydroxide or the human papillomavirus vaccine, Gardasil, which contains aluminum as an adjuvant (Inbar et al., 2016). Altered behavior and an increase in microglial density was observed in mice exposed to Alhydrogel (Crépeaux et al., 2017). In this study, the lowest concentration of aluminum caused the largest effect. The biphasic effect on IL-6 release that we observed in human astrocytes exposed to ABAs is concordant with this observation and may in part explain the reason why low concentrations of aluminum salts may exert a more selectively neurological response. Macrophagic myofasciitis (MMF) is a rare muscle disease that has been linked to ABA containing vaccines (Gherardi and Authier, 2012). This rare condition has also been associated with cognitive abnormalities (Aoun Sebaiti et al., 2018). Elevations of specific cytokines have been suggested as markers for MMF (Cadusseau et al., 2014). Therefore, while the use of ABAs may provide a very important and useful tool in eliciting immunogenicity in vaccines, there may be a select group of individuals who may be predisposed to adverse consequences of aluminum-containing vaccines. For example, individuals with autoimmune diseases may over-react to the immunogenicity of ABAs. The increase in immune activity would in turn worsen these conditions.
5. Conclusion
The recent COVID-19 pandemic is a strong reminder that there are genetic variations that dictate individual response to the same pathogen. In severe COVID-19 cases, there is a cytokine storm that leads to lung damage and eventual death (Shi et al., 2020). A similar genetic predisposition to mount an inappropriately strong immune response to aluminum particles may predispose a subpopulation of individuals to potential adverse health outcomes. While differing cell types may be equally vulnerable to Al salts, the consequences of astrocyte injury may be worse than macrophage damage. This may be reflected by reports that the brain is distinctively vulnerable to low concentrations of aluminum salts. Therefore, in individuals with underlying neurological conditions, the risk/benefits of ABA containing vaccines should be evaluated before administration.
Funding source
This study was supported by intramural and student funds provided by Western University of Health Sciences.
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- Aoun Sebaiti M., Kauv P., Charles-Nelson A., Van Der Gucht A., Blanc-Durand P., Itti E., Gherardi R.K., Bachoud-Levi A.C., Authier F.J. Cognitive dysfunction associated with aluminum hydroxide-induced macrophagic myofasciitis: a reappraisal of neuropsychological profile. J. Inorg. Biochem. 2018;181:132–138. doi: 10.1016/j.jinorgbio.2017.09.019. [DOI] [PubMed] [Google Scholar]
- Baroja-Mazo A., Martín-Sánchez F., Gomez A.I., Martínez C.M., Amores-Iniesta J., Compan V., Barberà-Cremades M., Yagüe J., Ruiz-Ortiz E., Antón J., Buján S., Couillin I., Brough D., Arostegui J.I., Pelegrín P. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat. Immunol. 2014;15 doi: 10.1038/ni.2919. 738- [DOI] [PubMed] [Google Scholar]
- Becaria A., Lahiri D.K., Bondy S.C., Chen D., Hamadeh A., Li H., Taylor R., Campbell A. Aluminum and copper in drinking water enhance inflammatory or oxidative events specifically in the brain. J. Neuroimmunol. 2006;176:16–23. doi: 10.1016/j.jneuroim.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Bondy S.C., Campbell A. Aluminum and neurodegenerative diseases. In: Aschner M., Costa L.G., editors. Vol. 1. 2017. pp. 135–156. (Environmental Factors in Neurodegenerative Diseases. Advances in Neurotoxicology). [Google Scholar]
- Cadusseau J., Ragunathan-Thangarajah N., Surenaud M., Hue S., Authier F.J., Gherardi R.K. Selective elevation of circulating CCL2/MCP1 levels in patients with longstanding post-vaccinal macrophagic myofasciitis and ASIA. Curr. Med. Chem. 2014;21:511–517. doi: 10.2174/09298673113206660287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. The potential role of aluminum in Alzheimer’s disease. Nephrol. Dial. Transplant. 2002;17:1–4. doi: 10.1093/ndt/17.suppl_2.17. [DOI] [PubMed] [Google Scholar]
- Campbell A., Prasad K.N., Bondy S.C. Aluminum induced oxidative events in cell lines: glioma are more responsive than neuroblastoma. Free Radic. Biol. Med. 1999;26:1166–1171. doi: 10.1016/s0891-5849(98)00308-6. [DOI] [PubMed] [Google Scholar]
- Campbell A., Sayre L.M., Smith M.A., Bondy S.C., Perry G. Mechanisms by which metals promote events connected to neurodegenerative diseases. Brain Res. Bull. 2001;55:125–132. doi: 10.1016/s0361-9230(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Campbell A., Yang E.Y., Tsai-Turton M., Bondy S.C. Pro-inflammatory effects of aluminum in human glioblastoma cells. Brain Res. 2002;933:60–65. doi: 10.1016/s0006-8993(02)02305-3. [DOI] [PubMed] [Google Scholar]
- Campbell A., Becaria A., Lahiri D.K., Sharman K., Bondy S.C. Chronic exposure to aluminum in drinking water increases inflammatory parameters selectively in the brain. J. Neurosci. Res. 2004;75:565–572. doi: 10.1002/jnr.10877. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; 2016. Vaccine Safety.https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html 12 Sept. 2016. Web. Accessed: 13 July 2017; from: [Google Scholar]
- Crépeaux G., Eidi H., David M.O., Baba-Amer Y., Tzavara E., Giros B., Authier F.J., Exley C.6, Shaw C.A., Cadusseau J., Gherardi R.K. Non-linear dose-response of aluminium hydroxide adjuvant particles: selective low dose neurotoxicity. Toxicology. 2017;375:48–57. doi: 10.1016/j.tox.2016.11.018. [DOI] [PubMed] [Google Scholar]
- Di Pasquale A., Preiss S., Tavares Da Silva F., Garcon N. Vaccine Adjuvants: from 1920 to 2015 and beyond. Vaccines. 2015;3:320–343. doi: 10.3390/vaccines3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth S.C., Colegio O.R., O’Connor W., Sutterwala F.S., Flavell R.A. Crucial role for the Nalp3 inflammasome in the immunomodulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardi R.K., Authier F.J. Macrophagic myofasciitis: characterization and pathophysiology. Lupus. 2012;21:184–189. doi: 10.1177/0961203311429557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardi R.K., Crépeaux G., Authier F.J. Myalgia and chronic fatigue syndrome following immunization:macrophagic myofasciitis and animal studies support linkage to aluminumadjuvant persistency and diffusion in the immune system. Autoimmunity Rev. 2019;18:691–705. doi: 10.1016/j.autrev.2019.05.006. [DOI] [PubMed] [Google Scholar]
- Ghimire T.R., Benson R.A., Garside P., Brewer J.M. Alum increases antigen uptake, reduces antigen degradation and sustains antigen presentation by DCs in vitro. Immunol. Lett. 2012;147:55–62. doi: 10.1016/j.imlet.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. The contribution of vaccination to global health: past, present and future. Philos. Trans. Biol. Sci. 2014;369:20130433. doi: 10.1098/rstb.2013.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hem S.L., Johnston C.T., HogenEsch H. Imject Alum is not aluminum hydroxide adjuvant or aluminum phosphate adjuvant. Vaccine. 2007;25(27):4985–4986. doi: 10.1016/j.vaccine.2007.04.078. [DOI] [PubMed] [Google Scholar]
- HogenEsch H., O’Hagan D.T., Fox C.B. Optimizing the utilization of aluminum adjuvants in vaccines: you might just get what you want. Npj Vaccines. 2018;3(51) doi: 10.1038/s41541-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imject Alum Instructions, Pierce Rockford Il. https://assets.thermofisher.com/TFS-Assets/LSG/manuals/MAN0011195_Imject_Alum_UG.pdf.
- Inbar R., Weiss R., Tomljenovic L., Arango M.T., Deri Y., Shaw C.A., Chapman J., Blank M., Shoenfeld Y. Behavioral abnormalities in female mice following administration of aluminum adjuvants and the human papillomavirus (HPV) vaccine Gardasil. Immunol. Res. 2016;65:136–149. doi: 10.1007/s12026-016-8826-6. [DOI] [PubMed] [Google Scholar]
- Kono H., Rock K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda E., Coban C., Ishii K.J. Particulate adjuvant and innate immunity: past acheivements, present findings, and future propspects. Int. Rev. Immunol. 2013;32:209–220. doi: 10.3109/08830185.2013.773326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F., Hogenesch H. Kinetics of the inflammatory response following intramuscular injection of aluminum adjuvant. Vaccine. 2013;31:3979–3986. doi: 10.1016/j.vaccine.2013.05.107. [DOI] [PubMed] [Google Scholar]
- Marichal T., Ohata K., Bedoret D., Mesnil C., Sabatel C., Kobiyama K., Lekeux P., Coban C., Akira S., Ishii K.J., Bureau F., Desmet C.J. DNA released from dying host cells mediates aluminum adjuvant activity. Nat. Med. 2011;17:996–1003. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- Mitkus R.J., King D.B., Hess M.A., Forshee R.A., Walderhaug M.O. Updated aluminum pharmacokinetics following infant exposures through diet and vaccination. Vaccine. 2011;29:9538–9543. doi: 10.1016/j.vaccine.2011.09.124. [DOI] [PubMed] [Google Scholar]
- Mold M., Shardlow E., Exley C. Insight into the cellular fate and toxicity of aluminium adjuvants used in clinically approved human vaccinations. Sci. Rep. 2016;6:31578. doi: 10.1038/srep31578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge D.W., Johnson D.W., Hawley C.M., Campbell S.B., Isbel N.M., van Eps C.L., Petrie J.J.B. Do aluminium-based phosphate binders continue to have a role in contemporary nephrology practice? BMC Nephrol. 2011;12:20. doi: 10.1186/1471-2369-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimaniol A.C., Gras G., Verdier F., Capel F., Grigoriev V.B., Porcheray F., Sauzeat E., Fournier J.G., Clayette P., Siegrist C.A., Dormont D. Aluminum hydroxide adjuvant induces macrophage differentiation towards a specialized antigen-presenting cell type. Vaccine. 2004;13:3127–3135. doi: 10.1016/j.vaccine.2004.01.061. [DOI] [PubMed] [Google Scholar]
- Shi S., Zhu H., Xia X., Liang Z., Ma X., Sun B. Vaccine adjuvants: understanding the structure and mechanisms of adjuvanticity. Vaccine. 2019;37:3167–3178. doi: 10.1016/j.vaccine.2019.04.055. [DOI] [PubMed] [Google Scholar]
- Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., Bucci E., Piacentini M., Ippolito G., Melino G. COVID-19 Infection: the perspectives on immune responses. Cell Death Differ. 2020 doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya S., Kobayashi Y., Goto Y., Okumura H., Nakae S., Konno T., Tada K. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 1982;42:1530–1536. [PubMed] [Google Scholar]
- Ulanova M., Tarkowski A., Hahn-Zoric M., Hanson L.A. The common vaccine adjuvant aluminum hydroxide up-regulates accessory properties of human monocytes via an Interleukin-4-dependent mechanism. Infect. Immun. 2001;69:1151–1159. doi: 10.1128/IAI.69.2.1151-1159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieling H., Kooijman S., de Ridder J.W., Thies-Weesie D.M.E., Soema P.C., Jiskoot W., van Riet E., Heck A.J.R., Philipse A.P., Kersten G.F.A., Meiring H.D., Pennings J.L., Metz B. Activation of human monocytes by colloidal aluminum salts. J. Pharm. Sci. 2020;109:750–760. doi: 10.1016/j.xphs.2019.08.014. [DOI] [PubMed] [Google Scholar]