Graphical abstract
Keywords: NSAIDs, COX, COX-2, Viral infections, COVID-19, Literature review
Abstract
Taking anti-inflammatory drugs, including non-steroidal (NSAIDs), during Covid-19 infection, how much is risky? The French Minister of Health, who has raised an alarm on a possible risk deriving from the use of ibuprofen for the control of fever and other symptoms during the disease, opened the debate a few days ago.
In this paper we examine available evidence from preclinical and clinical studies that had analysed the role of COX in the inflammatory process and the effects of NSAIDs in patients with infections. Most of the published studies that suggested not protective effects of NSAIDs were mainly performed in vitro or on animals. Therefore, their meaning in humans is to be considered with great caution. Based also on data suggesting protective effects of NSAIDs, we concluded that currently there is no evidence suggesting a correlation between NSAIDs and a worsening of infections. Further studies will be certainly needed to better define the role of NSAIDs and particularly COX2 inhibitors in patients with infections. In the meantime, we must wait for results of the revision started by the PRAC on May 2019 on the association ibuprofen/ketoprofen and worsening of infections. Since nowadays no scientific evidence establishes a correlation between NSAIDS and worsening of COVID-19, patients should be advice against any NSAIDs self-medication when COVID-19 like symptoms are present.
1. Summary
NSAIDs act through the inhibition of endoperoxide synthesis enzymes, also known as cyclooxygenase (COX) enzymes that catalyze the two-step conversion of arachidonic acid into thromboxane, prostaglandins, and prostacyclins [1]. Two types of COX are currently recognized: COX-1, which is constitutively expressed in the body and it is involved in homeostatic functions, including those related to gastrointestinal mucosa lining, kidney function, and platelet aggregation; COX-2, which is expressed during an inflammatory response where mitogens and cytokines are produced. COX-2 is responsible for the production of prostanoids. These lipid mediators are involved in processes that lead to vasodilation, increased vascular permeability and leukocyte chemotaxis [2]. Based on their selectivity in the inhibition of COX, NSAIDs are defined nonselective, when they inhibit both COX-1 and COX-2, and COX-2 selective.
NSAIDs are extensively used for the treatment of pain and inflammation, including in patients with chronic inflammatory disorders such as rheumatoid arthritis and osteoarthritis [3]. Even though NSAIDs represent one of the most used drug classes worldwide, their chronic use carries a well-known risk of gastrointestinal complications [4]. NSAIDs are associated with cardiovascular and renal adverse effects as well [5,6].
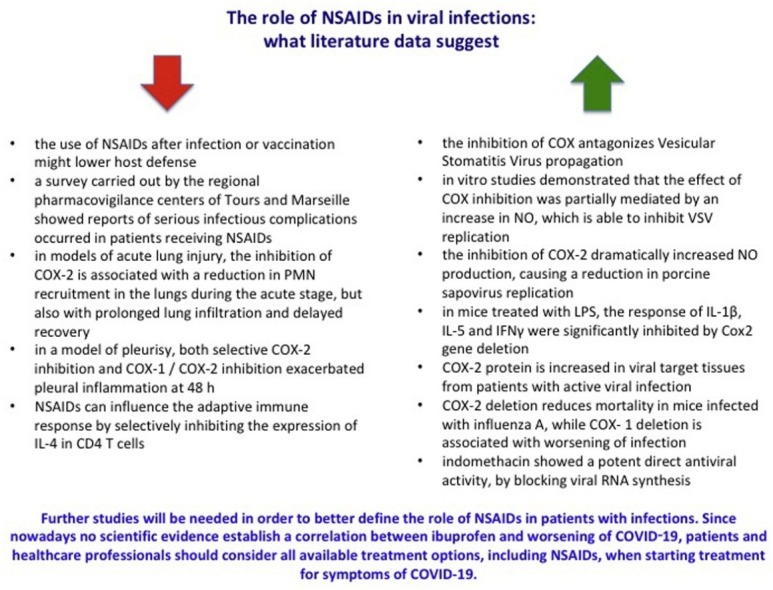
In 2009 a group of researchers from the University of Rochester warned that the use of NSAIDs might lower host defence following infection or vaccination [7]. On April 2019 the French regulatory agency (ANSM) issued a statement related to the results of a survey carried out by the regional pharmacovigilance centres of Tours and Marseille. They reported serious infectious complications that occurred in patients receiving NSAIDs, mainly ibuprofen and ketoprofen, used in the treatment of fever and pain. The analysis identified 337 serious cases (which led to hospitalization or death) of infectious complications with ibuprofen and 49 serious cases with ketoprofen (including cases of dermohypoderma, necrotizing fasciitis, sepsis, pneumonia complicated by an abscess, pleurisy, empyema). These events were observed after very short treatment periods (2–3 days), even when taking NSAIDs was combined with antibiotic therapy [8]. However, the statement of ANSM was based on a study [9] with many biases (the delay of treatment of bacterial infections leading to increased complications, the use of ibuprofene/ketoprofen in patients with serious symptoms, more severe cases were exposed to ibuprofen/ketoprofen due to confounding by indication) that may have affected the exposure-outcomes of the research. In addition, on March 16th 2020, the French Minister of Health has recommended the use of paracetamol instead of ibuprofen or oral cortisone for the treatment of fever in patients with Covid-19. Furthermore, French Authorities announced that NSAIDs may worsen clinical conditions of patients with COVID-19 based on the evaluation of 4 patients affected by the disease, with no comorbidity, for whom the only identified risk factor was NSAID treatment. For these reasons, these data cannot be considered as definitive.
During the inflammatory process, NSAIDs have demonstrated to alter adherence, degranulation and phagocytosis and reactive oxygen species (ROS) production by polymorphonuclear neutrophils (PMN). In vivo these drugs seem to reduce the recruitment of PMNs and modify their intrinsic functions. Furthermore, in models of acute pleural effusion, the treatment with ibuprofen, indomethacin and flurbiprofen have significantly reduced the volume of exudate and the migration of leukocytes. NSAIDs, including ibuprofen, inhibit TNFα-induced NFκB transcriptional activity, further contributing to reducing the local release of pro-inflammatory cytokines, including IL-8 [[10], [11], [12], [13], [14]]. Besides the effects mediated by cyclooxygenases, literature data suggested the key role of COX-2 in the resolution of inflammation. Leukotrienes and prostaglandins stimulate the local release of lipoxins, in particular PMNs, which can interact with specific receptors on leukocytes. This leads to an inhibition of inflammation mediated by PMNs and improving the phagocytosis of PMN by macrophages [[15], [16], [17], [18]].
A literature review of both preclinical and clinical studies on this topic was carried out. We have analyzed the available current relevant literature on the role of cyclooxygenases in inflammatory conditions underlying infections, providing suggestions for the role of NSAIDs in these conditions.
Considering preclinical studies, some of them [[19], [20], [21], [22]] suggested a not protective role of COX/NSAIDs during infections. For instance, in models of acute lung injury, the inhibition of COX-2 was associated with a reduction in the recruitment of PMNs in the lungs as well as with a prolonged lung infiltration and delayed recovery [19]. Similarly, in a model of pleurisy, either selective COX-2 or COX-1/COX-2 inhibition limited the volume of exudate and the recruitment of inflammatory cells within the pleura at 2 h, but exacerbated pleural inflammation at 48 h [20]. NSAIDs can influence the adaptive immune response by inhibiting the expression of IL-4 in CD4 T cells [21] and compromise the early production of IFN-γ by innate immune cells that represents an effective strategy for defence against viruses [22].
On the other hand, further preclinical studies, carried out both in vitro or on animals, found opposite results. Chen N et al. evaluated in vitro and in vivo the effects of aspirin, indomethacin and celecoxib on the replication of Vesicular Stomatitis Virus (VSV) induced encephalitis. According to their results, the inhibition of COX reduces the VSV propagation. In vitro studies have demonstrated that the effect of COX inhibition could be mediated by an increase in Nitric Oxide (NO), which inhibits VSV replication. Indeed, when NO production was inhibited, no differences in viral titer between treated and control cells were detected [23]. The protective effects of NSAIDs were also reported by a study of Alfajaro MM et al. who reported that the inhibition of COX-2 considerably increased the production of NO, causing a reduction in porcine sapovirus (pathogen responsible of severe acute gastroenteritis) replication, suggesting possible new targets for the treatment of sapovirus infection [24]. Reassuring results were also obtained in a further study that used mice deficient in COX isoforms to investigate the role of prostanoids in the modulation of the inflammatory response to bacterial and viral pathogen-associated molecular patterns (PAMPs). The results showed that in mice treated with LPS, the response of interleukins and interferons were significantly inhibited by the deletion of Cox2 gene. On the other hand, the deletion of Cox1gene did not alter the cytokine response to LPS [25]. These results are supported by other studies that showed how COX-2 protein is increased in tissues from patients with viral infection [26,27] and that COX-2 and COX-1 deletion are associated with a reduction in mortality in mice infected with influenza A and with worsening of infection, respectively [28]. Amici et al. evaluated the effect of indomethacin on the replication of coronavirus responsible for the development of severe acute respiratory syndrome (SARS). Specifically, the authors analyzed in vivo the virus titers in infected dogs treated with indomethacin (1 mg/kg body weight). Indomethacin showed a potent direct antiviral activity by blocking viral RNA synthesis. No effects on coronavirus binding or entry into host cells were observed. Since the antiviral activity of indomethacin occurred at concentrations higher than those needed for COX inhibition, the authors suggested that the effect was COX-independent. To support this, they highlighted that aspirin did not affect coronavirus replication up to millimolar concentrations. However, the possible mechanism was not investigated [29]. On the other hand, the results of a recent study seem to suggest that protein kinase R (PKR) might represent a target for the antiviral activity of indomethacin. The phosphorylation of the eukaryotic initiation factor-2 α-subunit, which derived from the activation of PKR by indomethacin, could be a key element in the antiviral activity of the drug [30]. As regards to viral infections, studies on celecoxib appear interesting. In a mouse infection model, the combined treatment with the polymerase basic protein 2 (PB2) oligonucleotides and celecoxib was associated to a significant reduction in the viral load, an improvement in lung lesions and animal survival compared to PB2 oligonucleotides alone [31]. Similarly, in a mouse model infected with CK1 or H5N1 influenza viruses, the combined treatment with celecoxib and zanamivir ameliorated lung inflammation and significantly improved the survival rate compared to zanamivir (p < 0.05) or celecoxib alone (p < 0.05) [32].
Nowadays few clinical studies are available on this topic. Among those that reported a not protective effect of NSAIDs, we found a retrospective study carried out in pediatric patients (age: 28 days-15 years) admitted to two French hospitals and diagnosed with community-acquired pneumonia from 1995 to 2003. The results showed that ibuprofen was the only therapy administered before hospitalization to be associated independently with complicated pneumonia [33]. Similarly, the results of a case-control study, carried out in a pediatric population with an acute viral infection between 2006 and 2009, reported an increased risk of empyema associated with exposure to NSAIDs and a reduction in the risk with the use of antibiotics. Authors concluded that NSAIDs should not be considered as first-line treatment during acute viral infections in children [8]. A case series published in 2015 reported the cases of two patients with H1N1 flu and a history of prolonged NSAID abuse (ibuprofen). Both patients had a respiratory failure that required access to an intensive care unit [34]. A further paper published on BMJ in 2009 [35] reported that NSAIDs might worsen flu symptoms and increase the risk of multi-organ failure. Other studies revealed that the use of NSAIDs was associated with a worst course of skin and soft tissues bacterial and viral infections [[36], [37], [38], [39], [40]]. Based on these data, Voirot et al. [41] proposed two hypotheses that could justify the association between the use of NSAIDs and the risk of complications in patients with pneumonia. The first is a temporal hypothesis, according to which NSAIDs may prevent the timely recognition of pneumonia, leading to a delay in the diagnosis thereby promoting a more invasive disease, with a higher frequency of pleural emphysema and bacteremia and delay the start of appropriate therapy. The second is an immunological hypothesis, according to which NSAIDs may reduce the recruitment of innate immune cells and modify the intrinsic functions of PMNs, resulting in lower bacterial clearance and promoting a more serious form of pneumonia [41]. On the other hand, a study carried out by Langhendries JP et al. suggests that the repeated exposure of infants to acetaminophen and ibuprofen may induce immune deviations [42]. We could then think that a patient with Covid-19 infection who is taken NSAIDs is a cause of concern. But research published in 2012 suggested that NSAIDs have an immune-enhancing impact and that improve the efficacy of anti-cancer immunotherapies [43]. Since clinical controlled studies are lacking, their meaning in humans is to be considered with great caution. Even though several studies have investigated the protective effects of NSAIDs in viral infection, the role of inflammation in regulating virus replication and survival is still not completely understood. However, it is known that patients with up-regulated COX-2 levels and inflammatory conditions have high incidences of Epstein Barr Virus associated malignancies indicating a possible role of COX-2 in virus-mediated tumorigenesis [44]. Finally, recently published preliminary results from a randomized controlled trial, carried out on 120 inpatients with influenza A (H3N2), showed that the reduction in mortality and cytokine levels was higher for the combination of celecoxib-oseltamivir compared to oseltamivir alone, without increasing adverse effects [45].
In conclusion, inflammation represents a physiologic response to tissue damage due to several factors, such as pathogen infection, chemical irritation, and injury. Gradually the inflammation process advances various types of cells are activated and attracted to the inflammation site through a signalling network involving a large number of mediators such as growth factors, cytokines, and chemokines. All recruited cells at the inflammatory site participate to the defence response but their excess or longer endurance induces tissue damage favouring the worsening of the disease regardless of the cause [46,47]. COX-2 is critical in the inflammatory response process and to be involved in the pathogenesis of influenza virus infection [48]. The same applies to COVID-19 disease for which the induction of a pro-inflammatory cytokine storm is similar to other highly pathogenic human invasive virus [49].
Regarding to this, it has been demonstrated that lack of COX-1 induces to the increased cellular influx, while the lack of COX-2 mitigates the recruitment of inflammatory cells. In the light of the above results, it is interesting to investigate the distinct roles of COX-1 and COX-2 in different diseases and preclinical models. Usually, NSAIDs are used in clinical practice for flu-like symptoms control during influenza viral infection. By applying the findings of previous studies to a clinical setting, the use of NSAIDs with predominantly COX-1 inhibiting activity may induce more severe inflammation phenomena compared with the treatment based on the administration of COX-2 in strengthened inhibitors, such as celecoxib or etoricoxib, during influenza infection which may improve the clinical course.
Ultimately, COX-1 and COX-2 have essential role but conflicting effects on the host immune response to influenza viral infection, likely mediated via impaired production of PGs and Lts following infection. The deficiency of the inhibition of COX-1 induces a strengthened inflammatory response and earlier release of proinflammatory cytokines. Vice versa, deficiency/inhibition of COX-2 results in decreased inflammation and proinflammatory cytokine release, which in turn lead to reduced morbidity and improved survival.
Even though COX-2 selective inhibitors may have benefits in patients with viral infections, probably including also COVID-19, according to FitzGerald GA, there is no evidence of benefit or risk for the use of NSAIDs in patients with Covid-19 infection [50] and further studies will be certainly needed to better define this association. In the meantime, as highlighted by the EMA on March 18th 2020, we must wait for results of the revision started by the PRAC on May 2019 on the association ibuprofen/ketoprofen and worsening of infections. Since nowadays no scientific evidence establishes a correlation between NSAIDS and the worsening of COVID-19, patients should be advised against any NSAIDs self-medication when COVID-19 like symptoms begins. Lastly, the EMA also highlighted the need for epidemiological studies to provide adequate evidence on any effect of NSAIDs on disease prognosis for COVID-19. Consequently, NSAIDs should be used with extreme caution, and only under medical control [51].
Declaration of Competing Interest
There are no conflicts to declare.
Acknowledgements
We are grateful for the help and support of the Italian Society of Pharmacology (SIF) which includes the following members: Prof Liberato Berrino, Dr Marzia Del Re, Prof Renato Bernardini, Prof Cristiano Chiamulera, Prof Antonio D’Avolio, Prof Gianluca Trifirò, Prof Luca Pani, Prof Emilio Clementi, Prof Romano Danesi, Prof Giuseppe Cirino, Prof Alessandro Mugelli, Prof Giambattista Bonanno, Prof Nicoletta Brunello, Prof Annamaria De Luca, Prof Patrizia Hrelia, Prof Marco Pistis, Prof Carla Ghelardini, Prof Maurizio Taglialatela
References
- 1.Vane J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 1971;231(June (25)):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 2.Chaiamnuay S., Allison J.J., Curtis J.R. Risks versus benefits of cyclooxygenase-2-selective nonsteroidal antiinflammatory drugs. Am. J. Health Syst. Pharm. 2006;63(October (19)):1837–1851. doi: 10.2146/ajhp050519. [DOI] [PubMed] [Google Scholar]
- 3.Cappell M.S., Schein J.R. Diagnosis and treatment of nonsteroidal anti-inflammatory drug-associated upper gastrointestinal toxicity. Gastroenterol. Clin. North Am. 2000;29(March (1)):97–124. doi: 10.1016/s0889-8553(05)70109-6. vi. Review. PubMed PMID: 10752019. [DOI] [PubMed] [Google Scholar]
- 4.Rafaniello C., Ferrajolo C., Sullo M.G., Sessa M., Sportiello L., Balzano A., Manguso F., Aiezza M.L., Rossi F., Scarpignato C., Capuano A. Risk of gastrointestinal mplications associated to NSAIDs, low-dose aspirin and their combinations: results of a pharmacovigilance reporting system. Pharmacol. Res. 2016;104(February):108–114. doi: 10.1016/j.phrs.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 5.Wongrakpanich S., Wongrakpanich A., Melhado K., Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9(1):143–150. doi: 10.14336/AD.2017.0306. Published 2018 Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coxib and traditional NSAID Trialists’ (CNT) Collaboration, Bhala N., Emberson J., Merhi A., Abramson S., Arber N., Baron J.A., Bombardier C., Cannon C., Farkouh M.E., FitzGerald G.A., Goss P., Halls H., Hawk E., Hawkey C., Hennekens C., Hochberg M., Holland L.E., Kearney P.M., Laine L., Lanas A., Lance P., Laupacis A., Oates J., Patrono C., Schnitzer T.J., Solomon S., Tugwell P., Wilson K., Wittes J., Baigent C. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(August (9894)):769–779. doi: 10.1016/S0140-6736(13)60900-9. Epub 2013 May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bancos S., Bernard M.P., Topham D.J., Phipps R.P. Ibuprofen and other widely used non-steroidal anti-inflammatory drugs inhibit antibody production in human cells. Cell. Immunol. 2009;258(1):18–28. doi: 10.1016/j.cellimm.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.https://ansm.sante.fr/S-informer/Points-d-information-Points-d-information/Anti-inflammatoires-non-steroidiens-AINS-et-complications-infectieuses-graves-Point-d-Information.
- 9.Le Bourgeois M., Ferroni A., Leruez-Ville M., Varon E., Thumerelle C., Brémont F., Fayon M.J., Delacourt C., Ligier C., Watier L., Guillemot D., Children, Antibiotics, Nonsteroidal Anti-inflammatory Drugs and Childhood Empyema (ChANCE) Study Group Nonsteroidal anti-inflammatory drug without antibiotics for acute viral infection increases the empyema risk in children: a matched case-control study. J. Pediatr. 2016;175(August):47–53. doi: 10.1016/j.jpeds.2016.05.025. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan H.B., Edelson H.S., Korchak H.M., Given W.P., Abramson S., Weissmann G. Effects of non-steroidal anti-inflammatory agents on human neutrophil functions in vitro and in vivo. Biochem. Pharmacol. 1984;33:371–378. doi: 10.1016/0006-2952(84)90228-4. [DOI] [PubMed] [Google Scholar]
- 11.Ip M., Lomas D.A., Shaw J., Burnett D., Stockley R.A. Effect of non-steroidal anti-inflammatory drugs on neutrophil chemotaxis–an in vitro and in vivo study. Br. J. Rheumatol. 1990;29:363–367. doi: 10.1093/rheumatology/29.5.363. [DOI] [PubMed] [Google Scholar]
- 12.Perianin A., Roch-Arveiller M., Giroud J.P., Hakim J. In vivo interaction of nonsteroidal anti-inflammatory drugs on the locomotion of neutrophils elicited by acute non-specific inflammations in the rat–effect of indomethacin, ibuprofen and flurbiprofen. Biochem. Pharmacol. 1984;33:2239–2243. doi: 10.1016/0006-2952(84)90661-0. [DOI] [PubMed] [Google Scholar]
- 13.Day R.O., Graham G.G. Non-steroidal anti-inflammatory drugs (NSAIDs) BMJ. 2013;11(June (346)):f3195. doi: 10.1136/bmj.f3195. Erratum in: BMJ. 2013;347:f4310. PubMed PMID: 23757736. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh R., Alajbegovic A., Gomes A.V. NSAIDs and cardiovascular diseases: role of reactive oxygen species. Oxid. Med. Cell. Longev. 2015;2015 doi: 10.1155/2015/536962. Epub 2015 Sep 20. Review. PubMed PMID: 26457127; PubMed Central PMCID: PMC4592725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dauletbaev N., Lam J., Eklove D., Iskandar M., Lands L.C. Ibuprofen modulates NF-kB activity but not IL-8 production in cystic fibrosis respiratory epithelial cells. Respir. Int. Rev. Thorac. Dis. 2010;79:234–242. doi: 10.1159/000255342. [DOI] [PubMed] [Google Scholar]
- 16.Rocca J., Manin S., Hulin A., Aissat A., Verbecq-Morlot W., Prulière-Escabasse V., Wohlhuter-Haddad A., Epaud R., Fanen P., Tarze A. New use for an old drug: COX-independent anti-inflammatory effects of sulindac in models of cystic fibrosis. Br. J. Pharmacol. 2016;173:1728–1741. doi: 10.1111/bph.13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy B.D., Clish C.B., Schmidt B., Gronert K., Serhan C.N. Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 18.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukunaga K., Kohli P., Bonnans C., Fredenburgh L.E., Levy B.D. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J. Immunol. 1950;2005(174):5033–5039. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- 20.Gilroy D.W., Colville-Nash P.R., Willis D., Chivers J., Paul-Clark M.J., Willoughby D.A. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 21.Cianferoni A., Schroeder J., Kim J. Selective inhibition of interleukin-4 gene expression in human T cells by aspirin. Blood. 2001;97:1742–1749. doi: 10.1182/blood.V97.6.1742. [DOI] [PubMed] [Google Scholar]
- 22.Inaoka M., Kimishima M., Takahashi R. Non-steroidal anti-inflammatory drugs selectively inhibit cytokine production by NK cells and γδ T cells. Exp Dermatol. 2006;15:981–990. doi: 10.1111/j.1600-0625.2006.00505.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen N., Warner J.L., Reiss C.S. NSAID treatment suppresses VSV propagation in mouse CNS. Virology. 2000;276(October (1)):44–51. doi: 10.1006/viro.2000.0562. [DOI] [PubMed] [Google Scholar]
- 24.Alfajaro M.M., Choi J.S., Kim D.S., Seo J.Y., Kim J.Y., Park J.G., Soliman M., Baek Y.B., Cho E.H., Kwon J., Kwon H.J., Park S.J., Lee W.S., Kang M.I., Hosmillo M., Goodfellow I., Cho K.O. Activation of COX-2/PGE2 promotes sapovirus replication via the inhibition of nitric oxide production. J. Virol. 2017;91(January (3)) doi: 10.1128/JVI.01656-16. pii: e01656-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirkby N.S., Zaiss A.K., Wright W.R. Differential COX-2 induction by viral and bacterial PAMPs: consequences for cytokine and interferon responses and implications for anti-viral COX-2 directed therapies. Biochem. Biophys. Res. Commun. 2013;438(2):249–256. doi: 10.1016/j.bbrc.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzgerald D.W., Bezak K., Ocheretina O., Riviere C., Wright T.C., Milne G.L., Zhou X.K., Du B., Subbaramaiah K., Byrt E., Goodwin M.L., Rafii A., Dannenberg A.J. The effect of HIV and HPV coinfection on cervical COX-2 expression and systemic prostaglandin E2 levels. Cancer Prev. Res. 2012;5:34–40. doi: 10.1158/1940-6207.CAPR-11-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nunez O., Fernandez-Martinez A., Majano P.L., Apolinario A., Gomez-Gonzalo M., Benedicto I., Lopez-Cabrera M., Bosca L., Clemente G., Garcia-Monzon C., Martin-Sanz P. Increased intrahepatic cyclooxygenase 2, matrix metalloproteinase 2, and matrix metalloproteinase 9 expression is associated with progressive liver disease in chronic hepatitis C virus infection: role of viral core and NS5A proteins. Gut. 2004;53:1665–1672. doi: 10.1136/gut.2003.038364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carey M.A., Bradbury J.A., Seubert J.M., Langenbach R., Zeldin D.C., Germolec D.R. Contrasting effects of cyclooxygenase-1 (COX-1) and COX-2 deficiency on the host response to influenza A viral infection. J. Immunol. 2005;175:6878–6884. doi: 10.4049/jimmunol.175.10.6878. [DOI] [PubMed] [Google Scholar]
- 29.Amici C., Di Caro A., Ciucci A., Chiappa L., Castilletti C., Martella V., Decaro N., Buonavoglia C., Capobianchi M.R., Santoro M.G. Indomethacin has a potent antiviral activity against SARS coronavirus. Antiviral Ther. 2006;11(8):1021–1030. PubMed PMID: 17302372. [PubMed] [Google Scholar]
- 30.Amici C., La Frazia S., Brunelli C., Balsamo M., Angelini M., Santoro M.G. Inhibition of viral protein translation by indomethacin in vesicular stomatitis virus infection: role of eIF2α kinase PKR. Cell. Microbiol. 2015;17(September (9)):1391–1404. doi: 10.1111/cmi.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin Y., Zhang G., Hu Y., Ding M., Li Y., Cao S., Xue J., Sun L.Q., Wang M. Inhibition of highly pathogenic avian H5N1 influenza virus propagation by RNA oligonucleotides targeting the PB2 gene in combination with celecoxib. J. Gene Med. 2011;13(April (4)):243–249. doi: 10.1002/jgm.1558. [DOI] [PubMed] [Google Scholar]
- 32.Li C., Li C., Zhang A.J.X., To K.K.W., Lee A.C.Y. Avian influenza a H7N9 virus induces severe pneumonia in mice without prior adaptation and responds to a combination of zanamivir and COX-2 inhibitor. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0107966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.François P., Desrumaux A., Cans C., Pin I., Pavese P., Labarère J. Prevalence and risk factors of suppurative complications in children with pneumonia. Acta Paediatr. 2010;99(June (6)):861–866. doi: 10.1111/j.1651-2227.2010.01734.x. [DOI] [PubMed] [Google Scholar]
- 34.Prower E., Hasnain O., Oscier C. H1N1 pneumonitis associated with long-term non-steroidal anti-inflammatory drug abuse. BMJ Case Rep. 2015 doi: 10.1136/bcr-2014-205237. 2015:bcr2014205237. Published 2015 Apr 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hama R. A/H1N1 flu. NSAIDs and flu. BMJ. 2009;338:b2345. doi: 10.1136/bmj.b2345. [DOI] [PubMed] [Google Scholar]
- 36.Brun-Buisson C.J., Saada M., Trunet P., Rapin M., Roujeau J.C., Revuz J. Haemolytic streptococcal gangrene and non-steroidal anti-inflammatory drugs. Br. Med. J. Clin. Res. Ed. 1985;290:1786. doi: 10.1136/bmj.290.6484.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aronoff D.M., Bloch K.C. Assessing the relationship between the use of nonsteroidal antiinflammatory drugs and necrotizing fasciitis caused by group A streptococcus. Medicine (Baltimore) 2003;82:225–235. doi: 10.1097/01.md.0000085060.63483.bb. [DOI] [PubMed] [Google Scholar]
- 38.Souyri C., Olivier P., Grolleau S., Lapeyre-Mestre M. French Network of Pharmacovigilance Centres Severe necrotizing soft-tissue infections and nonsteroidal anti-inflammatory drugs. Clin. Exp. Dermatol. 2008;33:249–255. doi: 10.1111/j.1365-2230.2007.02652.x. [DOI] [PubMed] [Google Scholar]
- 39.Avril M.F., Peyramond D. Érysipèle et fasciite nécrosante: prise en charge Texte court. Méd. Mal. Infect. 2000;30:239–240. doi: 10.1016/S0399-077X(00)89136-2. [DOI] [Google Scholar]
- 40.Mikaeloff Y., Kezouh A., Suissa S. Nonsteroidal anti-inflammatory drug use and the risk of severe skin and soft tissue complications in patients with varicella or zoster disease. Br. J. Clin. Pharmacol. 2008;65:203–209. doi: 10.1111/j.1365-2125.2007.02997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voiriot G., Philippot Q., Elabbadi A., Elbim C., Chalumeau M., Fartoukh M. Risks related to the use of non-steroidal anti-inflammatory drugs in community-acquired pneumonia in adult and pediatric patients. J. Clin. Med. 2019;8(6):786. doi: 10.3390/jcm8060786. Published 2019 Jun 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langhendries J.P., Allegaert K., Van Den Anker J.N., Veyckemans F., Smets F. Possible effects of repeated exposure to ibuprofen and acetaminophen on the intestinal immune response in young infants. Med. Hypotheses. 2016;87(February):90–96. doi: 10.1016/j.mehy.2015.11.012. Epub 2015 Nov 23. [DOI] [PubMed] [Google Scholar]
- 43.Hussain M., Javeed A., Ashraf M., Al-Zaubai N., Stewart A., Mukhtar M.M. Non-steroidal anti-inflammatory drugs, tumour immunity and immunotherapy. Pharmacol. Res. 2012;66(July (1)):7–18. doi: 10.1016/j.phrs.2012.02.003. Epub 2012 Feb 21. Review. PubMed PMID: 22449788. [DOI] [PubMed] [Google Scholar]
- 44.Gandhi J., Khera L., Gaur N., Paul C., Kaul R. Role of modulator of inflammation cyclooxygenase-2 in gammaherpesvirus mediated tumorigenesis. Front. Microbiol. 2017;8:538. doi: 10.3389/fmicb.2017.00538. Published 2017 Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hung I.F., Wang To K.K., Chan J., Chan K.H., Yuen K.Y., ECCMID . 2019. O0815 Treatment of Severe Influenza a Infection with Celecoxib.www.escmid.org [Google Scholar]
- 46.Mocarski E.S., Jr. Virus self-improvement through inflammation: no pain, no gain. Proc. Nat. Acad. Sci. U. S. A. 2002;99:3362–3364. doi: 10.1073/pnas.072075899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Julkunen I., Melen K., Nyqvist M., Pirhonen J., Sareneva T., Matikainen S. Inflammatory responses in influenza A virus infection. Vaccine. 2000;19(Suppl. 1):S32–7. doi: 10.1016/s0264-410x(00)00275-9. [DOI] [PubMed] [Google Scholar]
- 48.Lee S.M., Cheung C.Y., Nicholls J.M. Hyperinduction of cyclooxygenase-2- mediated proinflammatory cascade: a mechanism for the pathogenesis of avian influenza H5N1 infection. J. Infect. Dis. 2008;198:525–535. doi: 10.1086/590499. [DOI] [PubMed] [Google Scholar]
- 49.Fung S.Y., Yuen K.S., Ye Z.W., Chan C.P., Jin D.Y. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg. Microbes Infect. 2020;9(December (1)):558–570. doi: 10.1080/22221751.2020.1736644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.FitzGerald Garret A. 2020. Misguided Drug Advice for COVID-19.https://science.sciencemag.org/content/early/2020/03/19/science.abb8034 PUBLISHED ONLINE20 MAR, Available at. [DOI] [PubMed] [Google Scholar]
- 51.2020. EMA Gives Advice on the Use of Non-Steroidal Anti-Inflammatories for COVID-19.https://www.ema.europa.eu/en/news/ema-gives-advice-use-non-steroidal-anti-inflammatories-covid-19 Available at. [Google Scholar]


