Abstract
Purpose:
To identify predictors of hypothyroidism after chemoradiation for Hodgkin lymphoma (HL) and to compare outcomes after intensity-modulated radiation therapy RT (IMRT) with those after 3-dimensional conformal RT (3D-CRT).
Patients and Methods:
Ninety patients given involved-site IMRT in 2009–2014 were evaluated for treatment-induced hypothyroidism, defined as elevated thyroid-stimulating hormone (TSH) or decreased free thyroxine (fT4) levels or both. Receiver operating characteristic curve analysis identified individuals at low vs. high risk based on dosimetric variables. Dosimetric cutoff points were verified with an external dataset of 50 patients given 3D-CRT.
Results:
Most patients given IMRT (75 [83%]) had stage II HL and the median prescribed dose was 30.6 Gy; in the 3D-CRT group 32 (64%) had stage II HL and the median prescribed dose was 32.0 Gy. No differences were found in proportions of patients with bilateral (P=0.982) or unilateral neck involvement (P=0.074) between either group. Hypothyroidism rates were marginally higher in the IMRT group, with estimated 3-year rates of freedom from hypothyroidism of 56.1% for the 3D-CRT group and 40% for the IMRT group (P=0.057). Univariate analysis showed that smaller thyroid volume and higher thyroid dose were associated with hypothyroidism in both groups (P<0.05). In the IMRT group, V25 and the absolute volume of thyroid spared from 25 Gy (VS25Gy) were the strongest predictors of hypothyroidism (P=0.001 and P<0.001). Cutoff points of 63.5% (V25) and 2.2 mL (VS25Gy) classified patients as high-risk (80%−82%) or low-risk (37%−44%) (P<0.001). Use of a thyroid avoidance structure reduced the incidence of hypothyroidism (P<0.05) in the IMRT group.
Conclusions:
The percentage of thyroid receiving 25 Gy and the volume of thyroid spared from 25 Gy predicted risk of hypothyroidism after either IMRT or 3D-CRT for HL. IMRT may confer a higher risk than 3D-CRT unless a treatment avoidance structure is used during planning.
Keywords: involved-site radiotherapy, hypothyroidism, dosimetric variables, thyroid avoidance structure, IMRT, 3D-CRT
Summary
In this comprehensive evaluation of risk factors associated with hypothyroidism after IMRT and involved-site RT for HL, identified variables included thyroid V25 and V30 and both absolute thyroid volume (cut point 11.2 mL) and volume spared from ≥25 Gy (≥2.2 mL). We further recommend contouring the thyroid and using thyroid avoidance structures in treatment planning when they do not compromise target coverage.
INTRODUCTION
Hodgkin lymphoma (HL) survivors often face long-term sequelae of therapy. Hypothyroidism, although well-documented among patients with head and neck squamous cell carcinoma (HNSCC) who receive chemoradiation, is not well characterized among patients with HL treated with chemotherapy followed by involved-site radiation therapy (RT) [1]. Treatment-related hypothyroidism typically occurs within 2 years of completing RT, but can appear more than a decade later [2, 3]. Lifelong daily oral thyroid hormone replacement coupled with intermittent serum studies to ensure optimal dosing is required, which can cost more than $4000/year if ≥3 dose changes are required [4]. Long-term complications of untreated hypothyroidism can include cardiac and cognitive dysfunction and depression [5].
Among patients with HNSCC, hypothyroidism after RT is associated with doses of 40–50 Gy to the thyroid [6, 7]. Patients with HL generally are prescribed lower RT doses (20–30 Gy), but hypothyroidism rates are still considerable [2]. In one study of 53 patients given involved-field 3-dimensional conformal radiation therapy (3D-CRT) after chemotherapy for HL [8], 22 (41%) had developed hypothyroidism. The volume of thyroid receiving at least 30 Gy (V30) was identified as the strongest predictor.
In the current study, we sought to determine the incidence of hypothyroidism among HL patients treated with doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD) followed by involved-site RT delivered with intensity-modulated RT (IMRT) planning techniques. We examined clinical and dosimetric factors for associations with hypothyroidism and compared and validated our findings in an independent group treated with 3D-CRT [8].
MATERIALS AND METHODS
Patients and treatments
After Institutional review board approval, we retrospectively reviewed the records of 90 patients with stage I-IV HL treated with IMRT to the mediastinum, with or without the neck, at [xxx institution] from 2009 through 2014 who had at least 18 months’ follow-up after RT. Patients lacking pretreatment thyroid studies or with history of pretreatment thyroid dysfunction were excluded. Patients had received frontline systemic therapy with ABVD; those with relapsed or refractory disease treated with salvage chemotherapy, stem-cell transplantation, or consolidative RT were also included. IMRT was administered with anteroposterior/posteroanterior weighted “butterfly” 6-MV photons [9]. After 2012, deep-inspiration breathhold was generally used. Patients were treated on a 10–15° incline board to reduce cardiac and breast exposure [10]. The involved-site approach was used for target delineation since 2008, before publication of the International Lymphoma Radiation Oncology Group guidelines [1]. The volume of thyroid that was not included in the target volume was contoured and used as an avoidance structure for IMRT treatment planning, at the treating physician’s discretion.
Patients given 3D-CRT (the validation cohort) are described elsewhere [8]. Briefly, 50 HL patients with dosimetric information and thyroid function tests available before and after treatment at the Department of Radiotherapy of (xxx institution) were included. Patients had received systemic therapy followed by consolidative RT from 2001 through 2009. RT was given as involved-field therapy with 6- to 20-MV photons and anteroposterior/posteroanterior fields [11].
Serum thyroid-stimulating hormone (TSH) and free thyroxine (fT4), was tested at follow-up visits. Hypothyroidism was defined as a TSH value above the institutional normal range ([institution 1] 0.27 – 4.2 mU/mL; [institution 2] 0.41–4.30 μU/mL) or an fT4 value lower than the institutional normal range ([institution 1] 0.93 – 1.7 ng/dL; [institution 2] 0.75 −1.7 ng/dL).
Dosimetric analysis
The thyroid was contoured by one physician blinded to thyroid function for each cohort. Thyroid variables included the total thyroid volume (mL), minimum dose (Dmin), the mean dose (Dmean), the maximum dose (Dmax), the near-maximum dose (D2%), and the percentage of thyroid gland volume exceeding X Gy (Vx) from 5 to 30 Gy in increments of 5 Gy. The corresponding absolute volume of spared thyroid gland (Vsx) was also calculated.
Statistical analysis
Summary statistics were provided with frequency and percentage for categorical variables, and mean with standard deviation (SD), for continuous variables. Continuous variables were compared between two patient groups (e.g. the 3D-CRT and IMRT groups) by a two-sample t test if the data were normally distributed; otherwise a Wilcoxon rank sum test was used (e.g., with and without hypothyroidism). Fisher’s exact test was used to evaluate associations between two categorical variables. Multivariate logistic regression was used to identify factors associated with the development of hypothyroidism. Variables with P≤0.25 on the univariate analyses were considered in a saturated model. We then applied backward elimination to remove variables from the model until all remaining variables were statistically significant. Regarding the collinearity in model fitting, we examined the strength of association between two variables with Pearson correlation analyses and used t tests to determine if the correlation coefficient (r) was significantly different from zero. Receiver operating characteristic (ROC) curve analyses were used to identify threshold values for grouping patients into high- or low-risk groups. The Youden index was used to identify the cut-off point with the optimized sensitivity and specificity. Kaplan-Meier analysis was used to estimate freedom from hypothyroidism over time after RT, with comparisons between groups made with the log-rank test (Mantel-Cox). Reverse Kaplan-Meier method was used to calculate median follow up times. All statistical evaluations were two-sided. P values of ≤0.05 were considered statistically significant. Statistical analysis was carried out using SAS version 9.4 (SAS Institute, Cary, NC) and SPSS version 24 (SPSS Inc., Chicago, IL).
RESULTS
Of the 90 patients in the IMRT group, the median age at diagnosis was 31 years (range 19–78). Seventeen patients (19%) received salvage chemotherapy for relapsed and refractory disease. The median prescribed dose was 30.6 Gy (range 20–45.15 Gy), in median 1.8-Gy fractions (range 1.75–2.3 Gy). Of the 50 people in the 3D-CRT group, the median age at diagnosis was 28.5 years (range 14–70), the median prescribed dose was 32.0 Gy (range 30–36 Gy), and fraction sizes were generally 1.5–1.8 Gy. No differences were found in the proportion of patients with bilateral (P=0.982) or unilateral neck involvement (P=0.074) in either group.
Median follow-up were 41.1 months for the IMRT group (95% confidence interval [CI] 29.1–53.1) and 32.0 months (95% CI 18.9–45.1) for the 3D-CRT group (P=0.069). Fifty-nine patients in the IMRT group (66%) developed hypothyroidism (median time to onset 13 months, range 1.5 – 48.8 months), and 20 patients in the 3D-CRT group (40%) developed hypothyroidism (median time to onset 19.5 months, range 3 – 82 months). Ann Arbor stage, number of chemotherapy cycles, neck involvement, and total thyroid volume were associated with hypothyroidism in the IMRT group; disease stage and neck involvement were associated with hypothyroidism in the 3D-CRT group. Total thyroid volume was associated with hypothyroidism in the IMRT group (P=0.01) but not in the 3D-CRT group (P=0.2).
Numerous dosimetric variables were associated with the hypothyroidism in both groups (Table 2). Of the 90 patients in the IMRT group, 12 had a thyroid avoidance structure used during treatment planning, which was associated with lower rates of hypothyroidism (33% [4 of 12] with avoidance vs. 70% [55 of 78] without, P=0.02).
Table 2.
Dosimetric characteristics of patients with and without treatment related hypothyroidism (HT) treated with IMRT and 3D conformal
| IMRT | 3D | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | All Patients | No HT(n=31) | With HT (n=59) | P Value | All Patients | No HT (n=30) | With HT (n=20) | P Value | P (IMRT vs 3DCRT, all patients) |
| Prescription dose | 31.5 [4.4] | 31.8 [5.2] | 31.4 [4.0] | 0.748 | 31.4 [1.7] | 30.9 [1.4] | 32.3 [1.8] | 0.012 | 0.90 |
| V30, % | 66.3 [34.9] | 49.1 [36.7] | 75.3 [30.4] | <0.001 | 55.3 [40.9] | 37.6 [36.2] | 81.8 [33.1] | 0.0004 | 0.09 |
| VS30Gy, mL | 4.15 [4.8] | 6.99 [5.9] | 2.66 [3.4] | <0.001 | 7.83 [8.6] | 11.3 [8.9] | 2.5 [4.5] | <0.001 | 0.001 |
| V25, % | 72.5 [33.4] | 55.2 [35.9] | 81.6 [28.4] | <0.001 | 70.24 [36.6] | 58.9 [35.6] | 87.3 [31.7] | 0.0046 | 0.71 |
| VS25Gy, mL | 3.43 [4.6] | 6.19 [5.6] | 1.98 [3.1] | <0.001 | 5.45 [7.8] | 8.01 [8.7] | 1.62 [4.1] | 0.004 | 0.055 |
| V20, % | 78.7 [27.6] | 64.2 [32.9] | 86.4 [21] | 0.001 | 71.9 [35.6] | 61.2 [35.3] | 87.8 [30.4] | 0.0038 | 0.21 |
| VS20Gy, mL | 2.64 [3.9] | 4.95 [5.2] | 1.43 [2.2] | <0.001 | 5.18 [7.7] | 7.61 [8.6] | 1.54 [3.8] | 0.005 | 0.010 |
| V15, % | 81.9 [25.6] | 68.3 [32.2] | 89.1 [17.8] | <0.001 | 73.2 [35.0] | 63.1 [35.2] | 88.3 [29.5] | 0.0029 | 0.09 |
| VS15Gy, mL | 2.26 [3.6] | 4.40 [5.0] | 1.13 [1.8] | <0.001 | 4.95 [7.5] | 7.28 [8.6] | 1.47 [3.6] | 0.006 | 0.005 |
| V10, % | 84.8 [23.4] | 72.7 [30.4] | 91.1 [15.7] | <0.001 | 73.8 [34.5] | 63.8 [34.9] | 88.8 [28.5] | 0.0029 | 0.03 |
| VSlOGy, mL | 1.91 [3.3] | 3.83 [4.7] | 0.90 [1.6] | <0.001 | 4.84 [7.4] | 7.14 [8.5] | 1.39 [3.4] | 0.006 | 0.002 |
| V5, % | 89.2 [20.1] | 79.1 [27.5] | 94.4 [12.1] | <0.001 | 76.3 [32.6] | 67.5 [33.3] | 89.5 [27.4] | 0.0034 | 0.005 |
| VS5Gy, mL | 1.40 [2.9] | 3.01 [4.2] | 0.55 [1.2] | <0.001 | 4.19 [6.3] | 6.13 [7.1] | 1.28 [3.2] | 0.006 | <0.001 |
| Mean thyroid dose, Gy | 26.2 [8.24] | 22.2 [10.0] | 28.3 [6.2] | 0.002 | 23.9 [10.3] | 20.7 [9.6] | 28.8 [9.6] | 0.0011 | 0.16 |
| Min thyroid dose, Gy | 14.0 [12.5] | 9.1 [11.5] | 16.5 [12.4] | 0.008 | 15.6 [13.5] | 10.3 [12.2] | 23.7 [11.3] | 0.0010 | 0.47 |
| Max thyroid dose, Gy | 33.5 [4.2] | 33.2 [5.4] | 33.6 [3.4] | 0.395 | 30.3 [8.0] | 29.3 [8.2] | 31.8 [7.5] | 0.0477 | 0.003 |
| Thyroid D2, Gy | 32.6 [4.4] | 31.9 [6.0] | 32.9 [3.3] | 0.157 | 29.1 [8.9] | 27.9 [9.6] | 30.8 [7.6] | 0.0826 | 0.002 |
| Total thyroid volume, mL | 11.9 [3.7] | 13.4 [3.7] | 11.2 [3.5] | 0.01 | 16.1 [8.3] | 17.6 [9.5] | 13.8 [5.5] | 0.20 | <0.001 |
| Thyroid avoidance for planning, no. (%) | 12 (13.3%) | 8 (25.8%) | 4 (6.8%) | 0.020 | |||||
Abbreviations: Vx, percentage of thyroid gland volume receiving ≥ X Gy; VSx, absolute volumes of spared thyroid gland; D2, maximum dose to 2% of the structure.
All values are means [SD] unless otherwise noted.
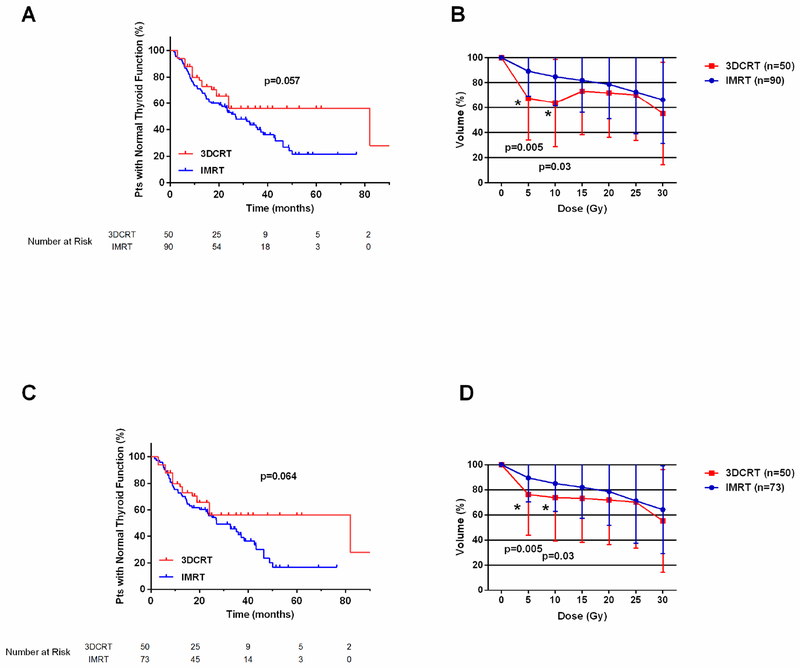
Rates of hypothyroidism were marginally higher in the IMRT group than in the 3D-CRT group: estimated 3-year rates of freedom from hypothyroidism were 56.1% for the 3D-CRT group and 40% in the IMRT group (P=0.057 Fig. 1A). In comparing the thyroid volume doses for patients in both groups, we found no statistically significant differences in mean V15, V20, V25, or V30 values in either group. However, the mean V5 (P=0.005) and mean V10 doses (P=0.03) were higher among patients treated with IMRT (Fig. 1B). When this analysis was repeated excluding the 17 patients who received IMRT for relapsed or refractory HL, again the rates of hypothyroidism may have been higher among the remaining 73 patients (P=0.064, Fig. 1C), and the mean V5 (P=0.005) and V10 (P=0.03) were higher as well (Fig. 1D). Comparison of the Vsx values in both groups was notable for the IMRT group having smaller volumes across all variables (Table 2, Supplementary Fig. S1).
Fig. 1.
(A) Freedom from hypothyroidism and (B) mean thyroid volume doses among patients with HL treated with IMRT (n=90) or 3D-CRT (n=50). (C) Freedom from hypothyroidism and (D) mean thyroid volume doses among patients treated with IMRT (n=73) or 3D-CRT (n=50) who had achieved a complete response to frontline chemotherapy.
On ROC analysis of the dosimetric variables in the IMRT cohort, the area under the curve (AUC) was significantly different from 0.5 for all variables tested, and the difference was greatest for variables based on 25 Gy (V25, AUC=0.719; VS25 Gy, AUC=0.737; Table 3). A threshold of 63.5% for V25 successfully classified patients into high-risk (80% [48 of 60]) and low-risk (37% [11 of 30]) groups for the development of hypothyroidism (P<0.001, odds ratio [OR] 6.9). The cumulative incidence of hypothyroidism was lower among patients who had at least 2.2 mL of the thyroid spared from 25 Gy (43.6% vs. 82.4% for those with spared volumes <2.2 mL, P<0.001, OR 6.0).
Table 3.
Receiver Operating Characteristic analysis for the IMRT cohort (n=90)
| Variable | AUC (95% CI) | P Value | Cutoff | Sensitivity (95% Cl) | Specificity (95% Cl) | Incid HT Low-Risk | Incid HT High-Risk | P Value | Odds Ratio (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| V30 | 0.693 (0.572–0.814) | 0.003 | 62.0% | 74.6% (61.6–85.0) | 61.3% (42.2–78.2) | 78.6% | 44.1% | 0.001 | 4.6 (1.8–11.8) |
| VS30Gy | 0.716 (0.597–0.836) | 0.001 | 2.7 mL | 66.1% (52.6–77.9) | 74.2% (55.4–88.1) | 83.0% | 46.5% | <0.001 | 5.6 (2.1–14.8) |
| V25 | 0.719 (0.604–0.834) | 0.001 | 63.5% | 81.4% (69.1-90-3) | 61.3% (42.2–78.2) | 80.0% | 36.7% | <0.001 | 6.9 (2.6–18.3) |
| VS25Gy | 0.737 (0.622–0.851) | <0.001 | 2.2 mL | 71.2% (57.9–82.2) | 71.0% (52.0–85.8) | 82.4% | 43.6% | <0.001 | 6.0 (2.3–15.8) |
| V20 | 0.711 (0.595–0.827) | 0.001 | 86.0% | 69.5% (56.1–80.8) | 71.0% (52.0–85.8) | 82.0% | 45.0% | <0.001 | 5.6 (2.1–14.4) |
| VS20Gy | 0.724 (0.608–0.840) | 0.001 | 1.5 mL | 69.5% (56.1–80.8) | 67.7 (48.6–83.3) | 80.4% | 46.2% | 0.001 | 4.78 (1.9–12.2) |
| V15 | 0.706 (0.589–0.823) | 0.001 | 92.5% | 67.8% (54.4–79.4) | 71.0% (52.0–85.8) | 81.6% | 46.3% | 0.001 | 5.1 (2.0–13.3) |
| VS15Gy | 0.720 (0.603–0.836) | 0.001 | 0.97 mL | 69.5% (56.1–80.8) | 67.7% (48.6–83.3) | 80.4% | 46.2% | 0.001 | 4.8 (1.9–12.2) |
| V10 | 0.700 (0.582–0.818) | 0.002 | 89.0% | 74.6% (61.6–85.0) | 64.5% (45.4–80.8) | 80.0% | 42.9% | 0.001 | 5.3 (2.1–13.7) |
| VSlOGy | 0.712 (0.595–0.830) | 0.001 | 0.69 mL | 71.2% (57.9–82.2) | 67.7% (48.6–83.3) | 80.8% | 44.7% | 0.001 | 5.2 (2.0–13.3) |
| V5 | 0.679 (0.556–0.803) | 0.005 | 93.0% | 79.7% (67.2–89.0) | 54.8% (36.0–72.7) | 77.0% | 41.4% | 0.002 | 4.8 (1.8–12.3) |
| VS5Gy | 0.687 (0.563–0.810) | 0.004 | 0.97 mL | 83.1% (71.0–91.6) | 54.8% (36.0–72.7) | 77.8% | 37.0% | 0.001 | 6.0 (2.2–15.9) |
| Mean | 0.711 (0.597–0.825) | 0.001 | 29.4 Gy | 64.4% (50.9–76.5) | 71.0% (52.0–85.8) | 80.9% | 48.8% | 0.002 | 4.4 (1.7–11.3) |
| Thyroid vol | 0.673 (0.555–0.792) | 0.007 | 11.2 cm3 | 57.6% (44.1–70.4) | 77.4% (58.9–90.4) | 82.9% | 51.0% | 0.002 | 4.7 (1.7–12.5) |
Abbreviations: CI, confidence interval; Incid HT, incidence of hypothyroidism
From Fisher’s exact test
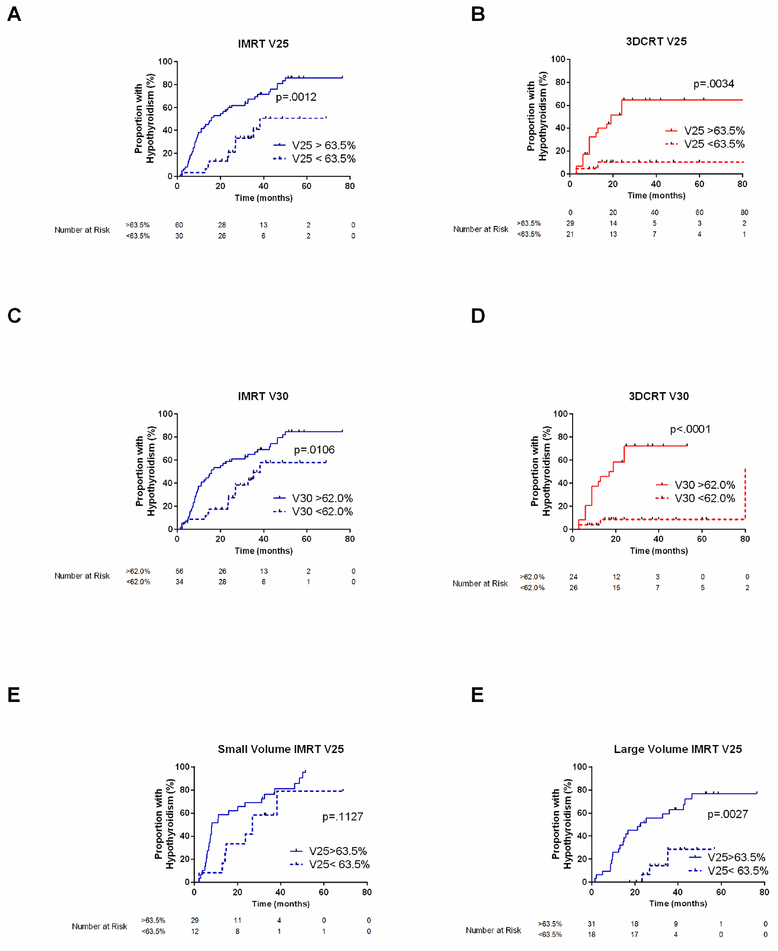
Because more accurate software was used to extract the dosimetric variables for this analysis than was the case for the previous report on the 3D CRT group [8], we repeated the ROC analysis on the 3D-CRT dataset (Table 4). The cut points evaluating the absolute volumes spared from various dose levels were significant in the 3D-CRT dataset (VS30Gy-VS5Gy), but for dose levels ≤25 Gy, the cut points were all less than 0.5 mL. Of all dosimetric variables, however, the AUC was highest for the 30-Gy variables V30 (AUC=0.817) and VS30Gy (AUC=0.843). The threshold identified for V30, 62.5%, was identical to the previously published V30 cut point. In the IMRT cohort, the AUC for V30 was 0.693 and the threshold identified was 62.0%, almost identical to the cut point derived from the 3D-CRT group. Figure 2A–D depicts hypothyroidism in the IMRT and 3D-CRT cohorts stratified by the V30 and V25 cut points identified in the IMRT cohort.
Table 4.
Receiver operating characteristics curve analysis for the 3DCRT cohort (n=50)
| -----------------------------------Analysis based on 3DCRT derived cutpoints----------------------------------------------------------- | ----------------------- Analysis based on IMRT derived cutpoints----------------------- | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | AUC (95% CI) | P Value | Cut point | Sensitivity, % (95% CI) | Specificity, % (95% CI) | HT Inc H-R, % | HT Inc L-R, % | P Value | OR (95% CI) | Cutpoint | Sensitivity,% (95% CI) | Specificity, % (95% Cl) | HT Inc H-R, % | HT Inc L-R, % | P Value | OR (95% CI) |
| V30, % | 0.817 (0.688–0.946) | <0.001 | 62.5 | 85.0 (62.1–96.8) | 76.7 (57.7–90.1) | 70.8 | 11.5 | <0.001 | 18.6 (4.2–82.7) | 62.0 | 85.0 (62.1–96.8) | 76.7 (57.7–90.1) | 70.8 | 11.5 | <0.001 | 18.6 (4.2–82.7) |
| VS30Gy, mL | 0.843 (0.729–0.956) | <0.001 | 7.2 | 85.0 (62.1–96.8) | 73.3 (54.1–87.7) | 68.0 | 12.0 | <0.001 | 15.6 (3.5–67.8) | 2.7 | 75.0 (50.9–91.3) | 76.7 (57.7–90.1) | 68.2 | 17.9 | <0.001 | 9.9 (2.6–36.9) |
| V25, % | 0.740 (0.591–0.889) | 0.004 | 98.06 | 85.0 (62.1–96.8) | 73.3 (54.1–87.7) | 68.0 68.0% | 12.0 12.0% | <0.001 | 15.6 (3.6–67.8) | 63.5 | 85.0 (62.1–96.8) | 60.0 (40.6–77.3) | 58.6 | 14.3 | 0.003 | 8.5 (2.0–35.5) |
| VS25Gy, mL | 0.762 (0.625–0.898) | 0.002 | 0.26 | 85.0 (62.1–96.8) | 73.3 (54.1–87.7) | 68.0 | 12.0 | <0.001 | 15.6 (3.6–67.8) | 2.2 | 85.0 (62.1–96.8) | 63.3 (43.9–80.1) | 60.7 | 13.6 | 0.001 | 9.8 (2.3–41.1) |
| V20, % | 0.744 (0.596–0.892) | 0.004 | 99.0 | 85.0 (62.1–96.8) | 70.0 (50.6–85.3) | 65.4 | 12.5 | <0.001 | 13.2 (3.1–56.6) | 86.0 | 85.0 (62.1–96.8) | 63.3 (43.9–80.1) | 60.7 | 13.6 | 0.001 | 9.8 (2.3–41.1) |
| VS20Gy, mL | 0.768 (0.633–0.904) | 0.001 | 0.07 | 1.5 | ||||||||||||
| V15, % | 0.745 (0.597–0.893) | 0.004 | 99.7 | 85.0 (62.1–96.8) | 70.0 (50.6–85.3) | 65.4 | 12.5 | <0.001 | 13.2 (3.1–56.6) | 92.5 | 85.0 (62.1–96.8) | 63.3 (43.9–80.1) | 60.7 | 13.6 | 0.001 | 9.8 (2.3–41.1) |
| VS15Gy, mL | 0.768 (0.632–0.903) | 0.001 | 0.05 | 0.97 | ||||||||||||
| V10, % | 0.746 (0.598–0.894) | 0.003 | 100.0 | 85.0 (62.1–96.8) | 70.0 (50.6–85.3) | 65.4 | 12.5 | <0.001 | 13.2 (3.1–56.6) | 89.0 | 85.0 (62.1–96.8) | 60.0 (40.6–77.3) | 58.6 | 14.3 | 0.003 | 8.5 (2.0–35.5) |
| VSlOGy, mL | 0.768 (0.632–0.903) | 0.001 | 0.01 | 0.69 | ||||||||||||
| V5, % | 0.738 (0.592–0.885) | 0.005 | 99.2 | 85.0 (62.1–96.8) | 66.7 (47.2–82.7) | 63.0 | 13.0 | <0.001 | 11.3 (2.7–48.0) | 93.0 | 85.0 (62.1–96.8) | 60.0 (40.6–77.3) | 58.6 | 14.3 | 0.003 | 8.5 (2.0–35.5) |
| VS5Gy, mL | 0.752 (0.614–0.890) | 0.003 | 0.07 | 0.97 | ||||||||||||
| Mean thyroid dose, Gy | 0.793 (0.648–0.938) | <0.001 | 30.0 | 85.0 (62.1–96.8) | 80.0 (61.4–92.3) | 73.9 | 11.1 | <0.001 | 22.7 (5.0–103.5) | 29.4 | 85.0 (62.1–96.8) | 63.3 (43.9–80.1) | 60.7 | 13.6 | 0.001 | 9.8 (2.3–41.1) |
Abbreviations: HT Inc H-R, incidence of hypothyroidism high risk group; HT Inc L-R, incidence of hypothyroidism low risk group
Analyses were not done for the volume spared 20 Gy and lower (VS20Gy, VS15Gy, VS10Gy and VS5Gy) because the thresholds identified were small and therefore of limited clinical utility.
Fig. 2.
Incidence of hypothyroidism stratified by dichotomized dosimetric variables. (A and C) Patients treated with IMRT and (B and D) patients treated with 3D-CRT stratified according to cut points identified by ROC curves in the IMRT group: V25=63.5% and V30=62%. (E) Patients given IMRT with thyroid volumes <11.2 mL or (F) ≥11.2 mL stratified by V25=63.5%.
Baseline thyroid volume was found to be associated with hypothyroidism, with patients with smaller thyroids at higher risk. ROC analysis identified a threshold of 11.2 mL for the total thyroid volume associated with hypothyroidism in the IMRT group (Table 3). A total of 82.9% of patients with thyroids smaller than 11.2 mL developed hypothyroidism versus 51.0% of those with larger thyroids (P=0.002). The volume-based dosimetric cut points identified in the entire IMRT group did not effectively distinguish risk of hypothyroidism for patients with small thyroid volumes (Fig. 2E, F). Of the 12 patients in the IMRT group without neck involvement, 5 developed hypothyroidism, and all 5 had thyroids smaller than 11.2 mL (median 8.6 mL, range 5.7–10.6).
Validation
We then evaluated the statistical significance of the dosimetric cut points identified from the IMRT dataset on the 3D-CRT cohort (Table 4). All dichotomized variables identified from the IMRT dataset significantly distinguished 3D-CRT patients at high versus low risk of hypothyroidism. When we evaluated the sensitivity and specificity of both sets of cut points on the 3D-CRT dataset, the results were similar (Table 4).
Multivariate analysis
To identify candidate factors for multivariate analysis, we examined the correlation between statistically significant clinical factors identified in the IMRT cohort with Pearson correlation analysis. Ann Arbor stage was associated with number of ABVD cycles (r=0.363, P=0.0005) as well as neck involvement (r=0.35, P=0.0007). Therefore, we chose only 1 of those clinical variables (neck involvement) for model inclusion. The dosimetric variables were also highly correlated with each other, so we included only 1 dichotomized dosimetric Vx variable in the model at a time. We did not use the absolute volume variables (Vsx) in the model because the thresholds identified in the 3D-CRT group were small (most <0.5 mL) and thus of limited clinical utility. The final multivariate model included thyroid volume (OR 0.83, 95% CI 0.72–0.96, P=0.014) and V25 (OR 7.46, 95% CI 2.64–21.28, P=0.002). When we tested this model on the 3D-CRT group, V25 retained significance (OR 7.3, 95% CI 1.17–21.25, P=0.007) but thyroid volume did not (OR 0.95, 95% CI 0.85–1.07, P=0.405).
DISCUSSION
Here we evaluated thyroid function after IMRT and involved-site targeting in a group of 90 patients with mediastinal HL with or without neck involvement. Even after IMRT, more than 65% of patients developed hypothyroidism. Small thyroid volumes and several dosimetric variables were associated with hypothyroidism, the most influential of which were the proportion of the thyroid receiving 25 Gy (V25) or as an alternative the absolute volume of thyroid that was spared from 25 Gy (VS25Gy). Cumulative incidence of hypothyroidism for patients with a V25 >63.5% was 80% and for patients with spared volumes <2.2 mL was 82.4%. Patients whose treatment plans included use of a thyroid avoidance structure had a lower risk of hypothyroidism after IMRT. When compared with a validation group of 3DCRT patients, those treated with IMRT had marginally higher rates of hypothyroidism. The dosimetric variables identified in the IMRT population were robust and successfully distinguished patients according to risk of hypothyroidism in both groups.
Information on dosimetric factors and the development of hypothyroidism comes largely from studies of patients with HNSCC, for whom the prescribed doses often exceed 60 Gy; thus it has been difficult to discern the effect of lower RT doses on the development of hypothyroidism [7]. Although a systematic meta-analysis of radiation-induced hypothyroidism found considerable variation in the dose-response of the thyroid across studies, many studies suggested a 50% risk of hypothyroidism at thyroid doses of 45 Gy [12].
In the current study of HL patients, the incidence of hypothyroidism was slightly higher after IMRT compared to 3D-CRT. This is an important finding given that the IMRT patients in this study were treated with contemporary involved-site RT, as opposed to the larger, involved-field approach used for the 3D-CRT group. Other studies of HNSCC patients have also found higher rates of hypothyroidism after IMRT as opposed to 3D-CRT. In one such study of HNSCC patients the hypothyroidism rate in the IMRT group was higher (IMRT, 51.1% vs 3D-CRT, 27.3%, P=0.021) [13]. The authors attributed this difference to a higher dose per fraction in the IMRT group; differences in lower-dose variables were not reported. In the current study, the fraction sizes were generally lower in the 3D-CRT group. When we evaluated the absolute volumes of thyroid that were not exposed to various radiation doses, the IMRT group had smaller volumes throughout. Moreover, proportions of thyroid exposed to lower doses (V5 and V10) were higher in the IMRT group. This finding is hypothesis-generating and suggests that the larger thyroid volumes exposed to 5 Gy and 10 Gy could have contributed to the increased rates of hypothyroidism in patients given IMRT. With regard to planning for IMRT versus 3D-CRT, increased low doses have led to the recognition that new dosimetric variables may be relevant for toxicity. Among HL patients treated with IMRT, V5 was a significant predictor of radiation pneumonitis [14, 15]. A simple and cost-effective strategy to reduce doses to the thyroid gland for patients given IMRT is use of thyroid avoidance structures, which may translate into reduced long-term endocrine toxicity.
Reducing low doses to the thyroid gland also has implications for secondary malignancy. Among childhood cancer survivors, the risk of thyroid cancer seems to increase as RT doses approach 30 Gy, with a subsequent decrease in secondary thyroid cancer risk for doses >30 Gy [16], thought to be due to death of thyroid follicular cells that can no longer undergo future malignant transformation. Limiting low doses to the thyroid therefore has other potential benefits in reducing long-term radiation-related morbidity beyond hypothyroidism.
The only patient-related factor identified as associated with hypothyroidism in the IMRT group was thyroid volume. Although not observed in this analysis of the 3D-CRT cohort, this is likely due to low number of events, as more sophisticated multivariate logistic regression methods with resampling techniques did indeed demonstrate an association between thyroid volume and hypothyroidism in the 3D-CRT dataset [17]. In a study of HNSCC patients treated with IMRT, rates of RT-related hypothyroidism were high among patients with thyroid volumes smaller than 8 cm3 [18]. The doses received by patients with smaller thyroids were so considerable that the authors excluded that group when they attempted to identify dosimetric thresholds. Indeed, in our IMRT group, the dosimetric thresholds did not distinguish between high-and low-risk patients among those with thyroid volumes <11.2 mL, suggesting that for such patients the thyroid volume goals used to evaluate IMRT plans should be more stringent to reduce the incidence of hypothyroidism in this high-risk cohort.
In the current study, small spared thyroid volumes were associated with increased risk of hypothyroidism after IMRT. In the same study of HNSCC patients given IMRT, those with at least 3 mL of thyroid gland spared from ≥45 Gy had significantly lower rates of hypothyroidism than those with smaller spared volumes [18]. This principle of protecting an absolute volume of thyroid from injury to limit hypothyroidism was also demonstrated in surgical series of patients undergoing hemithyroidectomy. One prospective study of 150 such patients involved estimating the volume of the non-excised lobe [19]. Patients with a thyroid remnant <3.2 mL had a threefold greater risk of hypothyroidism than those with larger remnant volumes (P<0.001). In our study, the most significant absolute volume constraint was the volume of thyroid spared from 25 Gy, with a high-risk threshold of 2.2 mL.
Use of a thyroid avoidance structure is a simple way to reduce thyroid exposure when target volumes permit it. Other strategies have been explored to preserve thyroid function, including TSH suppression during RT with oral thyroxine [20]. More invasive strategies include free thyroid transfer to the forearm among patients with advanced HNSCC [21].
The observation that the radiation dose threshold for hypothyroidism seems to be lower for HL patients than for HNSCC patients is interesting, especially because age in general has not been shown to be a risk factor for RT-induced hypothyroidism [12]. ABVD chemotherapy alone does not seem to cause thyroid dysfunction, so it is unlikely that systemic therapy is responsible for rendering HL patients more sensitive to radiation-related hypothyroidism [22]. Vogelius and colleagues appreciated an apparent increased risk of hypothyroidism among HL patients at lower mean thyroid doses than head and neck cancer patients in a literature-based meta-analysis of 4 studies involving 1027 patients. Those authors hypothesized that the HL population were not truly at an increased risk of hypothyroidism at lower doses than HNSCC patients, but that these results could be explained by attention to survivorship and long-term toxicity, and therefore thyroid dysfunction may have been evaluated earlier and more often for HL survivors [12]. This would imply that HNSCC patients may have the same dose thresholds for hypothyroidism after RT, but perhaps that hypothyroidism in this patient population is underdiagnosed.
Another plausible explanation for the lower dose threshold found in our study of HL patients compared with previous reports among HNSCC patients is that we defined hypothyroidism based on serum abnormalities (subclinical hypothyroidism) and not on receipt of thyroid replacement therapy. Although this could be regarded as a potential shortcoming, this serum-based definition is a more sensitive and unbiased approach for capturing all patients who may eventually require thyroid hormone replacement. The decision to initiate hormone therapy is often influenced by clinical judgment and symptomatology, even if fT4 is normal. In a study of 461 pediatric patients who had increased TSH but normal fT4 levels after HL therapy, the TSH normalized in only 2% of patients, illustrating the significance of subclinical hypothyroidism [2].
Besides the retrospective nature of our study, an additional limitation is that two different physicians contoured the thyroid gland in the two patient groups. However given the characteristic hyper-dense appearance of the thyroid (from iodine content) on non-contrast CT simulation, large variations in thyroid delineation between physicians may be minimal.
In conclusion, the current report provides a comprehensive evaluation of risk factors associated with hypothyroidism after IMRT and involved-site RT for HL. We identified V25 as an important dosimetric factor associated with hypothyroidism, and we validated the robustness of V30 as a vital dosimetric element for patients treated with 3D-CRT or IMRT. When treating HL, the doses to the thyroid should be kept as low as possible, although additional dosimetric guidelines can be used to treatment direct planning, e.g., maintaining the V30 at <62%, V25 <63.5%, V10<90%, and V5 <93.0%. The thyroid should always be contoured and a thyroid avoidance structure used if that does not compromise target coverage. At least 2.2 mL of gland should be spared from ≥25 Gy. Patients with small thyroids (<11.2 mL) probably require stricter dosimetric criteria.
Supplementary Material
Supplementary Figure S1. Volume of thyroid gland spared among all patients treated with intensity-modulated radiation therapy (IMRT; n=90) or 3-dimensional conformal radiation therapy (3D-CRT; n=50).
Table 1.
Patient and treatment characteristics according to treatment-related hypothyroidism after IMRT or 3D conformal radiation therapy
| IMRT | 3D-CRT (Validation Cohort) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | All Patients No. (%) | No HT No. (%) | With HT No. (%) | P Value | All Patients No. (%) | No HT No. (%) | With HT No. (%) | P Value |
| All patients | 90 | 31 | 59 | 50 | 30 | 20 | ||
| Female | 55 (61) | 18 (58) | 37 (63) | 0.82 | 25 (50) | 12 (40) | 13 (65) | 0.148 |
| Disease stage | ||||||||
| 1 | 6 (7) | 5 (16) | 1 (2) | 0.04 | 7 (14) | 7 (23) | 0 (0) | 0.018 |
| 2 | 75 (83) | 25 (81) | 50 (85) | 32 (64) | 14 (47) | 18 (90) | ||
| 3 | 7 (8) | 1 (3) | 6 (10) | 7 (14) | 6 (20) | 1 (5) | ||
| 4 | 2(2) | 0 | 2 (3) | 3 (6) | 2 (7) | 1 (5) | ||
| Unknown | 1 (2) | 1 (3) | ||||||
| B symptoms | 39 (43) | 15 (48) | 24 (41) | 0.51 | 22 (44) | 14 (47) | 8 (40) | 0.565 |
| Smoker | 17 (19) | 5 (16) | 12 (20) | 0.78 | 9 (18) | 4 (13) | 5 (25) | 0.454 |
| Bulky (>10) | 39 (43) | 11 (35.5) | 28 (47.5) | 0.37 | 6 (12) | 3 (10) | 3 (15) | 0.672 |
| No. chemo cycles* | ||||||||
| ≤4 | 35 (48) | 16 (64) | 19 (40) | 0.054 | 7 (14) | 7 (23) | 0 | 0.123 |
| >4 | 38 (52) | 9 (36) | 29 (60) | 42 (84) | 23 (77) | 19 (95) | ||
| unknown | 1 (2) | 1 (5) | ||||||
| Salvage chemo | 17 (19) | 6 (19) | 11 (19) | 1.00 | — | |||
| SCT | 16 (18) | 5 (16) | 11 (19) | 1.00 | — | |||
| Neck Involvement | ||||||||
| Not involved | 12 (13) | 7 (23) | 5 (8.5) | 0.04 | 14 (28) | 11 (37) | 3 (15) | 0.005 |
| Unilateral | 31 (34) | 13 (42) | 18 (30.5) | 10 (20) | 9 (30) | 1 (5) | ||
| Bilateral | 47 (52) | 11 (35.5) | 36 (61) | 26 (52) | 10 (33) | 16 (80) | ||
| Total thyroid volume, mL, mean [SD] | 11.9 [3.7] | 13.4 [3.7] | 11.2 [3.5] | 0.01 | 16.1 [8.3] | 17.6 [9.5] | 13.8 [5.5] | 0.20 |
| Follow-up time, mo, median [95% CI] | 41.1 [29.0–53.1] | 32.0 [18.9–45.1] | 0.069 | |||||
| Median time to HT [95% CI] | 26.9 [17.7–36.2] | 82.0 [0.54–163.9] | 0.056 | |||||
Abbreviations: HT, hyperthyroidism; SCT, stem cell transplantation; mo, months.
Among the IMRT subset, this variable was analyzed only for patients who did not receive salvage chemotherapy (n=73).
Funding:
Supported in part by Cancer Center Support (Core) Grant CA016672 from the National Cancer Institute, National Institutes of Health, to The University of Texas MD Anderson Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None.
References
- 1.Specht L, Yahalom J, Illidge T et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the international lymphoma radiation oncology group (ILROG). Int J Radiat Oncol Biol Phys 2014; 89: 854–862. [DOI] [PubMed] [Google Scholar]
- 2.Metzger ML, Hudson MM, Somes GW et al. White race as a risk factor for hypothyroidism after treatment for pediatric Hodgkin’s lymphoma. J Clin Oncol 2006; 24: 1516–1521. [DOI] [PubMed] [Google Scholar]
- 3.Smith GL, Smith BD, Garden AS et al. Hypothyroidism in older patients with head and neck cancer after treatment with radiation: a population-based study. Head Neck 2009; 31: 1031–1038. [DOI] [PubMed] [Google Scholar]
- 4.Ernst FR, Barr P, Elmor R et al. The Economic Impact of Levothyroxine Dose Adjustments: the CONTROL HE Study. Clin Drug Investig 2017; 37: 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter Y, Sippel RS, Chen H. Hypothyroidism after a cancer diagnosis: etiology, diagnosis, complications, and management. Oncologist 2014; 19: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim MY, Yu T, Wu HG. Dose-volumetric parameters for predicting hypothyroidism after radiotherapy for head and neck cancer. Jpn J Clin Oncol 2014; 44: 331–337. [DOI] [PubMed] [Google Scholar]
- 7.Sachdev S, Refaat T, Bacchus ID et al. Thyroid V50 Highly Predictive of Hypothyroidism in Head-and-Neck Cancer Patients Treated With Intensity-modulated Radiotherapy (IMRT). Am J Clin Oncol 2017; 40: 413–417. [DOI] [PubMed] [Google Scholar]
- 8.Cella L, Conson M, Caterino M, et al. Thyroid V30 predicts radiation-induced hypothyroidism in patients treated with sequential chemo-radiotherapy for Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys 2012;82:1802–1808. [DOI] [PubMed] [Google Scholar]
- 9.Voong KR, McSpadden K, Pinnix CC et al. Dosimetric advantages of a “butterfly” technique for intensity-modulated radiation therapy for young female patients with mediastinal Hodgkin’s lymphoma. Radiat Oncol 2014; 9: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dabaja BS, Rebueno NC, Mazloom A et al. Radiation for Hodgkin’s lymphoma in young female patients: a new technique to avoid the breasts and decrease the dose to the heart. Int J Radiat Oncol Biol Phys 2011; 79: 503–507. [DOI] [PubMed] [Google Scholar]
- 11.Yahalom J, Mauch P. The involved field is back: issues in delineating the radiation field in Hodgkin’s disease. Ann Oncol 2002; 13 Suppl 1: 79–83. [DOI] [PubMed] [Google Scholar]
- 12.Vogelius IR, Bentzen SM, Maraldo MV et al. Risk factors for radiation-induced hypothyroidism: a literature-based meta-analysis. Cancer 2011; 117: 5250–5260. [DOI] [PubMed] [Google Scholar]
- 13.Murthy V, Narang K, Ghosh-Laskar S et al. Hypothyroidism after 3-dimensional conformal radiotherapy and intensity-modulated radiotherapy for head and neck cancers: prospective data from 2 randomized controlled trials. Head Neck 2014; 36: 1573–1580. [DOI] [PubMed] [Google Scholar]
- 14.Pinnix CC, Smith GL, Milgrom S, et al. Predictors of radiation pneumonitis in patients receiving intensity modulated radiation ther-apy for hodgkin and non-hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2015;92:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cella L, D’Avino V, Palma G et al. Modeling the risk of radiation-induced lung fibrosis: Irradiated heart tissue is as important as irradiated lung. Radiother Oncol 2015; 117: 36–43. [DOI] [PubMed] [Google Scholar]
- 16.Sigurdson AJ, Ronckers CM, Mertens AC et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): a nested case-control study. Lancet 2005; 365: 2014–2023. [DOI] [PubMed] [Google Scholar]
- 17.Cella L, Liuzzi R, Conson M, et al. Development of multivariate NTCP models for radiation-induced hypothyroidism: A comparative analysis. Radiat Oncol 2012;7:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chyan A, Chen J, Shugard E et al. Dosimetric predictors of hypothyroidism in oropharyngeal cancer patients treated with intensity-modulated radiation therapy. Radiat Oncol 2014; 9: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang BH, Wong CKH, Wong KP et al. Effect of Thyroid Remnant Volume on the Risk of Hypothyroidism After Hemithyroidectomy: A Prospective Study. Ann Surg Oncol 2017; 24: 1525–1532. [DOI] [PubMed] [Google Scholar]
- 20.Massimino M, Gandola L, Collini P et al. Thyroid-stimulating hormone suppression for protection against hypothyroidism due to craniospinal irradiation for childhood medulloblastoma/primitive neuroectodermal tumor. Int J Radiat Oncol Biol Phys 2007; 69: 404–410. [DOI] [PubMed] [Google Scholar]
- 21.Harris J, Barber B, Almarzouki H et al. Free thyroid transfer: Short-term results of a novel procedure to prevent post-radiation hypothyroidism. Head Neck 2017; 39: 1234–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bethge W, Guggenberger D, Bamberg M et al. Thyroid toxicity of treatment for Hodgkin’s disease. Ann Hematol 2000; 79: 114–118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Volume of thyroid gland spared among all patients treated with intensity-modulated radiation therapy (IMRT; n=90) or 3-dimensional conformal radiation therapy (3D-CRT; n=50).