Abstract
Background and Aims
Recently, smooth muscle hypertrophy has been suggested to be a contributor to small bowel lesions secondary to Crohn’s disease [CD], in addition to inflammation and fibrosis. Here, we assess the value of magnetic resonance imaging [MRI] for the characterisation of histopathological tissue composition of small bowel CD, including inflammation, fibrosis, and smooth muscle hypertrophy.
Methods
A total of 35 consecutive patients [male/female 17/18, mean age 33 years] with ileal CD, who underwent small bowel resection and a preoperative contrast-enhanced MRI examination within 1 month before surgery, were retrospectively included. Image assessment included qualitative [pattern/degree of enhancement, presence of ulcerations/fistulas/abscesses] and quantitative parameters [wall thickness on T2/T1-weighted images [WI], enhancement ratios, apparent diffusion coefficient [ADC], Clermont and Magnetic Resonance Index of Activity [MaRIA] scores). MRI parameters were compared with histopathological findings including active inflammation, collagen deposition, and muscle hypertrophy using chi square/Fisher or Mann-Whitney tests and univariate/multivariate logistic/linear regression analyses.
Results
Forty ileal segments were analysed in 35 patients. Layered pattern at early-post-contrast phase was more prevalent (odds ratio [OR] = 8; p = 0.008), ADC was significantly lower [OR = 0.005; p = 0.022], and MaRIA score was significantly higher [OR = 1.125; p = 0.022] in inflammation grades 2–3 compared with grade 1. Wall thickness on T2WI was significantly increased [OR = 1.688; p = 0.043], and fistulas [OR = 14.5; p = 0.017] were more prevalent in segments with disproportionately increased muscle hypertrophy versus those with disproportionately increased fibrosis. MaRIA/Clermont scores, wall thickness on T1WI and T2WI, and ADC were all significantly correlated with degree of muscular hypertrophy.
Conclusions
MRI predicts the degree of inflammation, and can distinguish prominent muscle hypertrophy from prominent fibrosis in ileal CD with reasonable accuracy (area under receiver operating characteristic curve [AUROC] > 0.7).
Keywords: Imaging, biomarkers, pathology
1. Introduction
Crohn’s disease [CD] is a chronic inflammatory bowel disease that can involve any location within the gastrointestinal tract but most frequently the distal small bowel [terminal ileum].1 It is characterised by transmural inflammation with remitting and relapsing episodes. Pathologically, involvement by CD is manifested by inflammation and fibrosis which may co-exist in stenotic lesions.2,3 Assessment of bowel tissue composition in patients with CD, including the degree of inflammatory activity and fibrosis content particularly in stenotic lesions, is extremely important for directing appropriate management, as surgery may be the only option in cases of prominent fibrosis.4 Several pathology studies have reported that smooth muscle hypertrophy is a contributor to strictures in CD, sometimes described to be more predominant than fibrosis.5–10 It is noteworthy that in some studies, the presence of smooth muscle hypertrophy was included in the histopathological fibrosis score in CD.11,12
Imaging plays an important role at initial diagnosis, for assessing response to therapy, and for diagnosing complications in CD.13–15 Diffusion-weighted imaging [DWI] is a magnetic resonance imaging [MRI] technique that quantifies the degree of Brownian water motion in tissues.16 It is established that highly cellular tissues such as neoplasms or inflammation/infection demonstrate restricted diffusion.16 The apparent diffusion coefficient [ADC] computed from DWI decreases in tissues with restricted diffusion such as in active inflammatory bowel disease.17–20 There are a limited number of single-centre studies that have compared CT11 or MRI with/without DWI with/without FDG-PET with surgical pathological findings in CD.12,21–27 Wall thickness, pattern of enhancement [layered] and T2 signal intensity have been described as sensitive to inflammation.21,23,24,27 The degree of wall enhancement has variable performance depending on the post-contrast phases and the studies.21,23,24 The assessment of bowel wall fibrosis with MRI seems to be less accurate. Zappa et al. showed that wall thickness and hyperintensity on T2-weighted imaging [T2WI], as well as the presence of fistulous disease, are correlated with histopathological fibrosis score.23 More recently, Rimola et al. showed that MRI is accurate for detecting the presence of severe fibrosis in CD lesions on the basis of delayed pattern of enhancement,12 and Catalano et al. showed that ADC is not useful in detecting bowel wall fibrosis.25 To our knowledge, there are no published data correlating MRI with small bowel smooth muscle hypertrophy in CD.
The objective of our study was to assess the value of MRI including DWI for the characterisation of histopathological tissue composition of small bowel CD, particularly including smooth muscle hypertrophy, as well as inflammation and fibrosis.
2. Methods
This retrospective Health Insurance Portability and Accountability Act [HIPAA]-compliant study was approved by our Institutional Review Board, and informed consent was waived.
2.1. Patients
A computerised search of our imaging database from March 2013 to October 2014 was conducted by the study coordinator, an abdominal radiologist with 5 years of experience in body MRI [MW], to identify patients who underwent MRI and surgery. Our inclusion criteria were: patients with CD involving the small bowel [proved by endoscopy and histological analysis at the time of the diagnosis], who underwent small bowel resection, with pre-surgical MRI including DWI within 1 month before surgery. The flowchart of the study is presented in Fig. 1. The final population included 35 patients with ileal CD [male/female 17/18, mean age 33 years]. The mean delay between MRI and surgery was 10.6 days [range, 1–30 days]. The indications for surgery, the administration of an anti-tumour necrosis factor [anti-TNF] treatment at the time of the surgery, and previous small bowel surgery were recorded.
Figure 1.
Flow chart of the study population [CD, Crohn’s disease].
Eleven of the 35 patients [31%] had previous small bowel surgery. Clinical indications for the current surgery were: disease refractory to medical therapy [n = 6, 17%]; stricturing disease [n = 12, 34%]; and penetrating complications [n = 17, 49%]; 21 [60%] of patients were on anti-TNF treatment at time of surgery.
2.2. MRI technique
Patients were asked to fast for 6 h before the examination. The MRI examinations were performed using clinical 1.5 or 3T systems [Avanto/Aera/Skyra, Siemens Healthineers, Germany; Optima/Discovery, General Electrics]. Thirty patients [86%] were imaged at 1.5T and five patients at 3.0T [14%]. To achieve adequate small bowel distension, three bottles of Volumen [EZ-E-M, Westbury, NY] were given orally, beginning 45 min before the MRI, and one bottle of water was given once the patient was in the scanner. To reduce bowel peristalsis, 1 mg of glucagon was administered intramuscularly before the beginning of the examination [except in diabetic patients].
For all sequences, we used parallel imaging factor 2 with a rectangular field of view of 300 x 400 mm, which was adjusted for patient’s body size. The protocol [Table 1] included axial and coronal T2-weighted single-shot fast spin echo [SS-FSE] without fat suppression, 2D and 3D axial T1-weighed in-phase and out-of-phase, axial T2-weighted fast spin echo with fat suppression, axial diffusion-weighted [DWI], and axial and coronal T1-weighted gradient echo with fat suppression images, all covering the abdomen and pelvis. After injection of gadoterate meglumine [Dotarem, Guerbet, France], axial fat-suppressed T1-weighted gradient echo [VIBE or LAVA] images at 25 s [early phase], and axial and coronal images at 60 s [parenchymal phase] and 180 s [delayed phase], were acquired. For DWI, we used free-breathing axial fat-suppressed single-shot echoplanar imaging sequence with 3 b values: 50, 500, and 1000 s/mm2 or 50, 400, and 800 s/mm2. ADC maps were generated by fitting a linear model to the logarithmic signal data at the different b-values, using a custom-written script in MATLAB R2015a [MathWorks Inc, Natick, MA, USA].
Table 1.
MR enterography protocol obtained on different MRI systems.
| Parameter | T2 SS-FSE | T2 SS-FSE | T2 FSE with fat suppression | 2D and 3D T1 in/out of phase | DWI | 3D T1WI | 3D T1WI |
|---|---|---|---|---|---|---|---|
| Acquisition plane | Axial | Coronal | Axial | Axial | Axial | Axial* | Coronal** |
| Repetition time [range, ms] | 580-1300 | 582-1300 | 3000-5484 | 3.8-209 | 4000-8000 | 2.90-4.33 | 2.84-4.97 |
| Echo time [range, ms] | 82-228 | 80-91 | 83-116 | 1.23-2.39 | 69.6-94 | 1.24-2.12 | 1.3-2.48 |
| Flip angle | 90 | 90 | 90 | 10-80 | 90 | 10-15 | 10-15 |
| Matrix [range] | 256*256/ 512*512 |
256*256/ 512*512 |
256*256/ 512*512 |
288*288/ 512*512 |
256*256/ 391*384 | 256*256/ 512*512 |
256*256/ 512*416 |
| Field of view [max]*** | 400*400 | 540*370 | 400*400 | 380*380 | 380*360 | 380*380 | 520*340 |
| Slice thickness [mm] | 4-6 | 4-7.2 | 6-7 | 3-7 | 6-7 | 2-5 | 2.8-5 |
| Number of averages | 1 | 1 | 1 | 1 | 1/2/2-4 | 1 | 1 |
| Parallel imaging factor | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| b-Values | - | - | - | - | 50, 400, 800, or 50,500,1000 | - | - |
| Maximum acquisition time | 1:40 min | 1:38 min | 3:48 min | 56 s | 4:23 min | 36 s | 34 s |
SS-FSE, single-shot fast spin echo; FSE, fast spin echo; DWI, diffusion-weighted imaging; T1WI, T1-weighted imaging.
* Pre- and post-contrast injection, obtained at early phase [25 s], parenchymal phase [60 s], and delayed phase [180 s].
**Post-contrast acquisition obtained after 180 s.
***Adapted to patient size.
2.3. Image analysis
Two abdominal radiologists (observer 1 [BT] and observer 2 [MC], with 15 and 10 years of experience in Body MRI, respectively) reviewed images in consensus, blinded to clinical data, on a picture archiving and communication system [PACS] workstation. The readers knew that patients underwent surgery. For all involved ileal segments, they assessed: the length of the involved small bowel segment; the presence of ulceration[s], fistula[s], or abscess[es]; the presence of perivascular inflammatory infiltration of the mesentery [comb sign]; and the presence of upstream dilation [assessing for stenotic lesions], using both T2W images and post-contrast T1W images. The degree of enhancement T1W images on the parenchymal phase was scored as absent, mild, moderate, or marked, and the pattern of enhancement on the early, parenchymal, and delayed post-contrast phases was scored as layered [both mucosa-submucosa and serosa enhancing, with a central band of relatively reduced enhancement], mucosal [enhancement of the superficial layer only], or homogeneous [three layers of bowel wall]12
2.4. Quantitative image analysis
A third radiologist, an abdominal radiologist with 5 years of experience in Body MRI (observer 3 [CB]) performed the quantitative analysis using Osirix software [v 5.5.2, Geneva, Switzerland], for all involved ileal segments identified during the qualitative analysis by observers 1 and 2, who saved images with arrows targeting the segment to be analysed.
Bowel thickness [including both layers] was measured on T1W post-contrast and T2W images. Bowel wall signal intensity was measured on pre- and post-contrast T1W images, using a freehand-drawn region of interest [ROI], as large as possible, including both bowel wall layers. In case of stratification of the enhancement, the ROI included only the enhancing part. The enhancement ratio [ER] for each phase was computed using the following formula [WSI: wall signal intensity]:
An approximately 5-cm2 ROI was also drawn in the background noise to measure standard deviation [SD] of noise, and compute the relative contrast enhancement [RCE], using the following formula27:
A freehand ROI was drawn on the ADC map to measure mean ADC for each involved bowel segment, including the largest part possible of the involved segment. If multiple ROIs were drawn on different slices, the ADC values of the ROIs were averaged. Two sets of b-values were used [50, 500, and 1000 s/mm2 or 50, 400, and 800 s/mm2] but were very close, with the assumption that ADC differences were small. As the slice thickness was different between DWI and T1W imaging, the ROI were drawn separately on each sequence.
Finally, the MaRIA [Magnetic Resonance Index of Activity] and the Clermont scores were computed, using the following formulas19,26,27:
For MaRIA score computation, the wall thickness was measured on post-contrast T1W images. For oedema and ulceration, the score was multiplied by 1 in case of presence, and by 0 in case of absence, as described by Rimola et al.27
2.5. Histopathological analysis
Histopathological analysis was performed retrospectively by a specialised gastrointestinal pathologist [HMK], on resected small bowel specimens. For each resection specimen, one section from the area of the ileum most involved by CD was selected. The most involved section was either derived from the pathology report, as a slide corresponding to the area designated as ‘stricture’, or an area of the bowel that appeared most thickened on examination of the slides. Sections involving fistulas were avoided, due to mural distortion by abscess component.
Active inflammation was assessed on haematoxylin and eosin staining [H&E] slides using a 3-point scale, based on the depth of neutrophil infiltrates: 1, none or in mucosa only; 2, submucosa; and 3, muscularis propria/subserosa/serosa. Bowel wall oedema [fluid within the interstitial spaces of the tissue] was assessed using a 2-point scale: 1, no oedema or minimal oedema; and 2, obvious oedema. Active inflammation and wall oedema were assessed separately because they are not always associated.
Immunostaining of smooth muscle actin [SMA] for muscular hypertrophy and Sirius red special staining for collagen deposition were performed.28,29 Sirius red was chosen over Masson trichrome, because it has been shown that Sirius red is more specific and reliable for collagen staining than Masson trichrome.30 Stained slides were digitally scanned and analysed using HALO morphometry software [v1.1, Indica Labs] by another pathologist [XZ]. Intramural areas of muscle and collagen were calculated for each affected full-thickness area. The area was normalised by dividing by the length of the section (mean of inner and outer measurements in each layer of wall [normalizsd SMA and normalized Sirius red]). The ratio between normalised SMA and normalised Sirius red was calculated [ratio <1: disproportionately increased fibrosis; ratio > 1: disproportionately increased muscular hypertrophy].
2.6. Imaging-pathology correlation
The study coordinator collected all the data and was responsible for the correlation between MRI and pathological findings. Pathological analysis was matched with MR images, based on surgical and pathology reports and MRI analysis, to ensure that the same bowel segments were analysed in case of patients with multifocal bowel involvement, according to location and length of involvement.
2.7. Statistical analysis
Quantitative variables are described as mean ± standard deviation [SD]. Qualitative variables are described as number of cases [percentage of cases]. MRI parameters were compared between affected and non-affected bowel segments stratified by histopathological inflammation score, edema score, and the ratio between normalised SMA and normalised Sirius red, using the chi square or the Fisher exact test for qualitative variables and the Mann-Whitney test for quantitative variables. Logistic regression univariate and multivariate analyses were also performed to compare the ability of MRI parameters, to predict inflammation/oedema and fibrosis/muscular hypertrophy. Linear regression analysis was performed between quantitative MRI parameters and histopathological scores. For significant quantitative parameters, a receiver operating characteristic curve analysis [ROC] was also performed. The tests were always two-sided, with a level of significance set at p <0.05. All analyses were performed using SPSS software [version 17.0, SPSS Inc., Chicago, IL].
3. Results
3.1. Histopathological findings
Forty ileal segments were analysed in the 35 included patients; 35 segments [87.5%] had a stricture on pathological examination. Inflammation was classified as grade 1 in 15 segments [37.5%], grade 2 in eight segments [20%], and grade 3 in 17 segments [42.5%]. Oedema was classified as grade 1 and grade 2 in 20 [50%] and 20 [50%] bowel segments, respectively.
Quantitative tissue morphometry showed that the smooth muscle component [normalised SMA area] and the fibrosis component [normalised Sirius red area] were both prominently increased in the diseased sections. The mean ± SD normalised SMA area was 1570 ± 666 mm2 in involved bowel wall and 767 ± 409 mm2 in uninvolved bowel wall. The normalised Sirius red area was 1160 ± 554 mm2 in involved bowel wall and 764 ± 429 mm2 in uninvolved bowel wall. However, the proportions of the muscle and fibrosis components were heterogeneous among the 40 segments. The mean ratio between normalised SMA and normalised Sirius red was 1.56 ± 0.74, with 31 [77%] segments with a ratio >1 [prominent smooth muscle hypertrophy] and nine [23%] segments with a ratio <1 [prominent fibrosis]. Six [15%] bowel segments showed inflammation grade 1 and prominent fibrosis, nine [22.5%] segments inflammation grade 1 and prominent muscle hypertrophy, three [7.5%] segments inflammation grade 2–3 and prominent fibrosis, and the majority [22 segments, 55%] showed inflammation grade 2–3 and prominent muscle hypertrophy.
3.2. MRI-pathology correlation
3.1.1. MRI findings according to the degree of inflammation
The length of the involved bowel was significantly greaterer in segments with grade 3 inflammation; however, the association was not significant and was weak [OR = 1.17]. A layered/mucosal only pattern of enhancement at 25 s and 60 s, and a marked degree of enhancement at 60 s, were more frequent in segments with higher inflammation grade. The layered/mucosal only pattern of enhancement at 25 s had the strongest association with inflammation [OR = 8; p = 0.008]. A higher percentage of ulcerations, fistulas, abscesses and comb signs were found in cases of inflammation [grades 2/3], without reaching significance [Tables 2–3, Figs 2–3]:
Table 2.
Imaging parameters stratified by histopathological inflammation grade [in 35 patients with CD and 40 ileal segments analysed].
| Parameter | Grade 1 [n*** = 15] | Grade 2/3 [n*** = 25] | p |
|---|---|---|---|
| Length of involved bowel | 5.93 ± 3.99 | 9.32 ± 5.79 | 0.035 |
| Pattern of enhancement at 25 s* | 0.01 | ||
| • Homogeneous | 10 [67%] | 4 [20%] | |
| • Layered/mucosal only | 5 [33%] | 16 [80%] | |
| Pattern of enhancement at 60 s | 0.05 | ||
| • Homogeneous | 11 [73%] | 10 [40%] | |
| • Layered/mucosal only | 4 [27%] | 15 [60%] | |
| Pattern of enhancement at 180 s** | 1 | ||
| • Homogeneous | 11 [79%] | 19 [79%] | |
| • Layered/mucosal only | 3 [21%] | 5 [21%] | |
| Degree of enhancement at 60 s | 0.024 | ||
| • Mild-moderate enhancement | 7 [47%] | 3 [12%] | |
| • Marked enhancement | 8 [53%] | 22 [88%] | |
| Ulceration | 6 [40%] | 18 [72%] | 0.09 |
| Fistula | 5 [33%] | 16 [64%] | 0.1 |
| Abscess | 3 [20%] | 9 [36%] | 0.47 |
| Comb sign | 2 [13%] | 7 [32%] | 0.44 |
| Upstream dilation | 10 [40%] | 15 [60%] | 0.749 |
| Enhancement ratio at 25 s* | 162.1 ± 98.4 | 184.9 ± 71.9 | 0.4 |
| Enhancement ratio at 60 s | 176.4 ± 67.1 | 200.4 ± 63.5 | 0.222 |
| Enhancement ratio at 180 s** | 173.2 ± 61.2 | 188.9 ± 55.1 | 0.455 |
| ADC* | 1.28 ± 0.27 | 1.07 ± 0.14 | 0.028 |
| Wall thickness on T1W post-contrast | 6.86 ± 2.65 | 9.22 ± 3.63 | 0.018 |
| Wall thickness on T2WI | 6.79 ± 2.22 | 7.79 ± 2.03 | 0.148 |
| MaRIa score | 23.3 ± 8.0 | 31.0 ± 13.5 | 0.013 |
| Clermont score | 28.5 ± 7.2 | 33.2 ± 8.2 | 0.115 |
Significant p-values are bolded. Quantitative data are presented as mean ± standard deviation. Qualitative data are presented as number of cases [percentage of cases].
*Not available in five patients.
**Not available in two patients.
***Number of bowel segments.
Table 3.
Univariate and multivariate logistic regression analyses between imaging parameters and inflammation grade [in 35 patients with CD and 40 ileal segments analysed].
| Parameter | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR [CI 95%] | p | OR [CI 95%] | p | |
| Length of involved bowel | 1.17 [0.99-1.39] | 0.068 | ||
| Pattern of enhancement at 25 s* | 8.000 [1.73-37.09] | 0.008 | 3.901 [0.221-68.94] | 0.353 |
| Pattern of enhancement at 60 s | 4.125 [1.02-16.67] | 0.047 | 3.195 [0.13-79.54] | 0.514 |
| Pattern of enhancement at 180 s** | 0.965 [0.192-4.84] | 0.965 | ||
| Degree of enhancement at 60 s | 6.417 [1.33-3.03] | 0.021 | 23.511 [1.22-453.71] | 0.037 |
| Ulceration | 3.857 [1-14.92] | 0.05 | ||
| Fistula | 3.556 [0.92-13.70] | 0.065 | ||
| Abscess | 2.250 [0.59-10.14] | 0.291 | ||
| Comb sign | 2.528 [0.45-14.20] | 0.292 | ||
| Upstream dilation | 1.312 [0.36-4.78] | 0.680 | ||
| Enhancement ratio at 25 s* | 1.003 [0.99-1.01] | 0.421 | ||
| Enhancement ratio at 60 s | 1.006 [1.00-1.02] | 0.260 | ||
| Enhancement ratio at 180 s** | 1.005 [0.99-1.02] | 0.397 | ||
| ADC* | 0.005 [0-0.478] | 0.022 | 0.001 [0-1.33] | 0.060 |
| Wall thickness on T1W post-contrast | 1.439 [1.04-2] | 0.030 | 1.048 [0.66-1.67] | 0.844 |
| Wall thickness on T2WI | 1.289 [0.91-1.83] | 0.157 | ||
| MaRIa score | 1.125 [1.02-1.25] | 0.022 | ||
| Clermont score | 1.096 [0.98-1.23] | 0.111 | ||
Significant p-values are bolded.
OR, odds ratio; CI 95%, 95% confidence intervals.
*Not available in five patients.
**Not available in two patients
Figure 2.
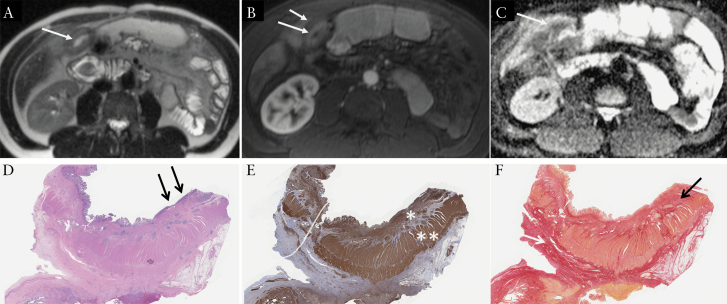
A 45-yearold male patient with ileal Crohn’s disease who underwent small bowel resection for bowel stricture. MRI demonstrates: A] thickened ileal wall [at 10.5 mm] on T2-weighted image [arrows]; B] layered pattern of enhancement [enhancement of the mucosa only [long arrow], with submucosal oedema [short arrow]] on early post-contrast T1-weighted image; C] corresponding low ADC [at 1.06 × 10-3 mm2/s] on the ADC map, which suggests the presence of inflammation; [D] on histopathology, the affected ileum shows mucosal ulcerations [black arrows] and prominent mural thickening in the bowel wall [x 2 H&E]; [E] smooth muscle actin immunostain [SMA] highlights muscle layers in the bowel wall, and there is marked expansion and increase in smooth muscle [coloured in brown] within the muscularis mucosae [*] and muscularis propria [**] layers; [F] Sirius red stain highlights collagen in red, which is predominantly in the submucosa [black arrow] and subserosa [white arrow]. The ratio between normalised SMA and normalized Sirius red was 2.29 [prominent muscle hypertrophy]. H&E, hamatoxylin and eosin.
Figure 3.
Left: 29-yearold female patient with ileal Crohn’s disease and prominent muscle hypertrophy on histopathology. Right: 17-yearold female patient with ileal Crohn’s disease and prominent fibrosis on histopathology. T2-weighted [A, E] and T1-weighted post-contrast [B, F] images demonstrate thicker bowel wall for the patient with prominent muscle hypertrophy [T2w/T1w: 8.2/9.1 mm versus 3.4/2.7 mm]. SMA [smooth muscle actin] stain highlights thickened muscularis mucosae [*] and muscularis propria [**] layers on the left [C], whereas these muscle layers are only minimally thickened on the right [G]. Sirius red stain highlights collagen in red [D, H]. Normalised SMA was higher than normalised Sirius red area in the ileum, showing prominent muscle hypertrophy [left], whereas the normalised area of Sirius red area was greater than nSMA in the ileum showing prominent fibrosis [right].
ADC was significantly lower, and wall thickness on T1W post-contrast imaging and MaRIA score were both significantly higher, in inflammation grades 2/3 compared with grade 1. On multivariate regression analysis, including all the significant parameters in univariate analysis [except MaRIA score which is not independent from the others], only the degree of enhancement was associated with inflammation.
3.1.2. MRI findings according to the degree of oedema
Significantly more ulcerations were found in segments with oedema; and MaRIA/Clermont scores were significantly higher in segments with oedema [Tables 4 and 5].
Table 4.
Imaging parameters stratified by degree of oedema [in 35 patients with CD and 40 ileal segments analysed].
| Parameter | Grade 1 [n*** = 20] | Grade 2/3 [n*** = 20] | p |
|---|---|---|---|
| Length of involved bowel | 8 ± 5.63 | 8.1 ± 5.30 | 0.779 |
| Pattern of enhancement at 25 s* | 0.739 | ||
| • Homogeneous | 7 [37%] | 7 [44%] | |
| • Layered/mucosal only | 12 [63%] | 9 [56%] | |
| Pattern of enhancement at 60 s | 0.527 | ||
| • Homogeneous | 9 [45%] | 12 [60%] | |
| • Layered/mucosal only | 11 [55%] | 8 [40%] | |
| Pattern of enhancement at 180 s** | 0.693 | ||
| • Homogeneous | 14 [74%] | 16 [84%] | |
| • Layered/mucosal only | 5 [26%] | 3 [16%] | |
| Degree of enhancement at 60 s | 0.344 | ||
| • Mild-moderate enhancement | 4 [20%] | 1 [5%] | |
| • Marked enhancement | 2 [10%] | 3 [15%] | |
| Ulceration | 14 [70%] | 16 [80%] | |
| Fistula | 8 [40%] | 16 [80%] | 0.022 |
| Abscess | 8 [40%] | 13 [65%] | 0.205 |
| Comb sign | 4 [20%] | 8 [40%] | 0.301 |
| Upstream dilation | 4 [20%] | 5 [25%] | 1 |
| Enhancement ratio at 25 s* | 191.6 ± 93.1 | 155.6 ± 68.7 | 0.301 |
| Enhancement ratio at 60 s | 202.1 ± 73.6 | 180.6 ± 55.0 | 0.398 |
| Enhancement ratio at 180 s** | 191.4 ± 61.2 | 174.7 ± 53.1 | 0.369 |
| ADC* | 1.12 ± 0.25 | 1.16 ± 0.18 | 0.317 |
| Wall thickness on T1W post-contrast | 7.38 ± 2.29 | 9.29 ± 4.17 | 0.071 |
| Wall thickness on T2WI | 7.21 ± 1.96 | 7.62 ± 2.32 | 0.5 |
| MaRIa score | 24.9 ± 8.1 | 30.4 ± 9.0 | 0.033 |
| Clermont score | 27.7 ± 6.6 | 34.8 ± 7.8 | 0.005 |
Significant p-values are bolded. Quantitative data are presented as mean ± standard deviation. Qualitative data are presented as number of cases [percentage of cases]
*Not available in five patients.
**Not available in two patients.
***Number of bowel segments.
Table 5.
Univariate and multivariate logistic regression between imaging parameters and degree of oedema [in 35 patients with CD and 40 ileal segments analysed].
| Parameter | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR [CI 95%] | p | OR [CI 95%] | p | |
| Length of involved bowel | 1.004 [0.89-1.13] | 0.953 | ||
| Pattern of enhancement at 25 s* | 0.750[0.193-2.92] | 0.678 | ||
| Pattern of enhancement at 60 s | 0.545 [0.16-1.91] | 0.344 | ||
| Pattern of enhancement at 180 s** | 0.525 [0.11-2.60] | 0.430 | ||
| Degree of enhancement at 60 s | 1.715 [0.66-4.44] | 0.267 | ||
| Ulceration | 6.000 [1.46-24.69] | 0.013 | 8.389 [1.55-45.50] | 0.014 |
| Fistula | 2.786 [0.77-10.04] | 0.117 | ||
| Abscess | 2.667 [0.65-10.97] | 0.174 | ||
| Comb sign | 1.333 [0.30-5.93] | 0.705 | ||
| Upstream dilation | 0.815 [0.23-2.86] | 0.749 | ||
| Enhancement ratio at 25 s* | 0.994 [0.99-1.00] | 0.208 | ||
| Enhancement ratio at 60 s | 0.995 [0.99-1.01] | 0.296 | ||
| Enhancement ratio at 180 s | 0.995 [0.98-1.01] | 0.354 | ||
| ADC* | 2.683 [0.11-66.91] | 0.548 | ||
| Wall thickness on T1W post-contrast | 1.260 [0.96-1.66] | 0.100 | ||
| Wall thickness on T2WI | 1.099 [0.816-1.48] | 0.537 | ||
| MaRIa score | 1.088 [1-1.19] | 0.063 | ||
| Clermont score | 1.184 [1.03-1.36] | 0.015 | ||
Significant p-values are bolded.
OR, odds ratio; CI 95%, 95% confidence intervals.
*Not available in five patients
**Not available in two patients
3.1.3. MRI findings according to the degree of muscular hypertrophy and fibrosis
Bowel wall thickness on T2WI was significantly increased in bowel segments with prominent muscle hypertrophy compared with those with prominent fibrosis, whereas there was only a trend to increased bowel wall thickness on T1W post-contrast images. There was no difference in pattern of enhancement, degree of enhancement, and ADC between segments with prominent muscle hypertrophy versus those with prominent fibrosis, whereas there was a trend towards higher MaRIA and Clermont scores in bowel segments with prominent muscle hypertrophy. Bowel wall thickness on T2WI, T1WI, and MaRia and Clermont scores was significantly associated with the ratio between muscle hypertrophy and fibrosis [Tables 6–8, Figs 2 and 3]. The presence of fistulas was also significantly and independently associated with prominent muscle hypertrophy [OR = 14.5; p = 0.017]. ADC was significantly negatively correlated with normalised SMA, and wall thickness on T1WI and MaRIA and Clermont scores was significantly positively correlated with normalised SMA [Table 8]. Normalised SMA was significantly higher in cases of ulceration [p = 0.017] or fistula [p = 0.034], but showed no association with other qualitative parameters. None of the imaging parameters, either quantitative [Table 8] or qualitative [p >0.273], were significantly associated with normalised Sirius red.
Table 6.
Imaging parameters stratified by histopathological grading of prominent muscle hypertrophy or fibrosis [in 35 patients with CD and 40 ileal segments analysed].
| Prominent fibrosis [n*** = 9] | Prominent muscle hypertrophy [n*** = 31] | p | |
|---|---|---|---|
| Length of involved bowel [cm] | 5.22 ± 3.07 | 8.87 ± 5.68 | 0.052 |
| Pattern of enhancement at 25 s* | 0.432 | ||
| • Homogeneous | 5 [56%] | 9 [35%] | |
| • Layered/mucosal only | 4 [44%] | 17 [65%] | |
| Pattern of enhancement at 60 s | 0.457 | ||
| • Homogeneous | 6 [67%] | 15 [48%] | |
| • Layered/mucosal only | 3 [33%] | 16 [52%] | |
| Pattern of enhancement at 180 s** | 0.66 | ||
| • Homogeneous | 7 [87%] | 23 [77%] | |
| • Layered/mucosal only | 1 [12%] | 7 [23%] | |
| Degree of enhancement at 60 s | 0.061 | ||
| • Mild | 3 [33%] | 2 [7%] | |
| • Moderate | 0 [0%] | 5 [16%] | |
| • Marked | 6 [67%] | 24 [77%] | |
| Ulceration | 3 [33%] | 21 [68%] | 0.12 |
| Fistula | 0 [0%] | 9 [29%] | 0.09 |
| Abscess | 0 [0%] | 12 [39%] | 0.037 |
| Comb sign | 1 [11%] | 20 [65%] | 0.007 |
| Upstream dilation | 13 [42%] | 18 [58%] | 1 |
| Enhancement ratio at 25 s* [%] | 196.4 ± 100.7 | 167.8 ± 77.9 | 0.516 |
| Enhancement ratio at 60 s [%] | 196.6 ± 73.5 | 189.9 ± 63.7 | 0.799 |
| Enhancement ratio at 180 s** [%] | 197.9 ± 66.0 | 178.7 ± 54.8 | 0.337 |
| ADC [ × 10-3 mm2/s]* | 1.22 ± 0.34 | 1.12 ± 0.17 | 0.658 |
| Wall thickness on T1WI post-contrast [mm] | 6.62 ± 2.79 | 8.83 ± 3.52 | 0.055 |
| Wall thickness on T2WI [mm] | 6.11 ± 1.85 | 7.79 ± 2.08 | 0.028 |
| MaRIa score | 23.1 ± 7.9 | 29.0 ± 8.8 | 0.059 |
| Clermont score | 26.7 ± 7.8 | 33.0 ± 7.7 | 0.056 |
Significant p-values are bolded. Quantitative data are presented as mean ± standard deviation. Qualitative data are presented as number of cases [percentage of cases].
*Not available in five patients.
**Not available in two patients.
***Number of bowel segments.
Table 8.
Univariate linear regression analysis between imaging parameters and normalised SMA [nSMA] staining, normalised Sirius red [nSirius red], and nSMA/nSirius red ratio [in 35 patients with CD and 40 ileal segments analysed].
| Parameter | nSMA | nSirius red | nSMA/nSirius red ratio | |||
|---|---|---|---|---|---|---|
| R | p | R | p | R | p | |
| Length of involved bowel | 0.221 | 0.171 | 0.004 | 0.983 | 0.170 | 0.205 |
| Enhancement ratio at 25 s* | 0.086 | 0.622 | 0.167 | 0.339 | -0.026 | 0.882 |
| Enhancement ratio at 60 s | 0.132 | 0.419 | 0.112 | 0.491 | 0.057 | 0.725 |
| Enhancement ratio at 180 s | 0.075 | 0.645 | 0.138 | 0.395 | -0.149 | 0.882 |
| ADC* | -0.375 | 0.027 | -0.126 | 0.470 | -0.209 | 0.229 |
| Wall thickness on T1W post-contrast | 0.344 | 0.030 | 0.018 | 0.915 | 0.384 | 0.014 |
| Wall thickness on T2WI | 0.298 | 0.062 | -0.062 | 0.703 | 0.376 | 0.017 |
| MaRIa score | 0.486 | 0.001 | 0.142 | 0.384 | 0.366 | 0.020 |
| Clermont score | 0.557 | 0.001 | 0.173 | 0.319 | 0.344 | 0.043 |
Significant p-values are bolded.
SMA, smooth muscle actin immunostain.
*Not available in five patients.
**Not available in two patients.
Table 7.
Univariate and multivariate logistic regression between imaging parameters and ratio between muscular hypertrophy and fibrosis [in 35 patients with CD and 40 ileal segments analysed].
| Parameter | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR [CI 95%] | p | OR [CI 95%] | p | |
| Length of involved bowel | 1.226 [0.97-1.55] | 0.086 | ||
| Pattern of enhancement at 25 s* | 2.361 [0.50-11.05] | 0.275 | ||
| Pattern of enhancement at 60 s | 2.133 [0.45-10.10] | 0.469 | ||
| Pattern of enhancement at 180 s** | 2.130 [0.22-20.41] | 0.512 | ||
| Degree of enhancement at 60 s | 1.714 [0.34-8.68] | 0.515 | ||
| Ulceration | 4.200 [0.87-20.33] | 0.075 | ||
| Fistula | 14.545 [1.60-131.96] | 0.017 | 9.794 [1.02-93.94] | 0.048 |
| Abscess | Infinity [0-infinity] | 0.999 | ||
| Comb sign | Infinity [0-infinity] | 0.999 | ||
| Upstream dilation | 1.108 [0.248-4.94] | 0.893 | ||
| Enhancement ratio at 25 s* | 0.996 [0.99-1.00] | 0.376 | ||
| Enhancement ratio at 60 s | 0.998 [0.99-1.01] | 0.783 | ||
| Enhancement ratio at 180 s | 0.994 [0.98-1.01] | 0.375 | ||
| ADC* | 0.118 [0.003-4.19] | 0.241 | ||
| Wall thickness on T1W post-contrast | 1.417 [0.98-2.05] | 0.065 | ||
| Wall thickness on T2WI | 1.688 [1.02-2.80] | 0.043 | 1.471 [0.84-2.57] | 0.175 |
| MaRIa score | 1.101 [0.99-1.23] | 0.08 | ||
| Clermont score | 1.150 [1-1.32] | 0.051 | ||
Significant p-values are bolded.
OR, odds ratio; CI 95%, 95% confidence intervals.
*Not available in five patients.
**Not available in two patients.
3.1.4. Area under receiver operating characteristic curve analysis
For detection of inflammation grades 2/3 and grade 1, length of involved bowel, wall thickness on T1w post-contrast imaging, ADC and MaRIA score had significant areas under receiver operating characteristic curves [AUROC]. The association of an ADC lower than 1.11 x 10–3 mm2/s and a MaRIA score higher than 26.1 reached sensitivity of 47% and a specificity of 92% for detecting inflammation grades 2/3; and the association of an ADC lower than 1.11 x 10–3 mm2/s and a wall thickness on T1w post-contrast higher than 5.9 mm reached a sensitivity of 65% and a specificity of 83% for detecting inflammation grades 2/3. For identification of oedema, both MaRIA and Clermont scores had significant AUROC. For differentiation of wall with prominent fibrosis and muscular hypertrophy, only the wall thickness measured on T2WI had a significant AUC [Table 9].
Table 9.
ROC analysis for differentiating grade 1 versus grade 2/3 inflammation, oedema, and prominent muscle hypertrophy versus prominent fibrosis.
| Parameter | Inflammation | Oedema | Prominent muscle hypertrophy versus prominent fibrosis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC | p | Cut-off | Se | Sp | AUC | p | Cut-off | Se | Sp | AUC | p | Cutoff | Se | Sp | |
| Length of involved bowel | 0.7 | 0.036 | 4.5 mm | 88% | 60% | 0.527 | 0.766 | 0.713 | 0.054 | ||||||
| Wall thickness on T2WI | 0.64 | 0.142 | 0.527 | 0.766 | 0.742 | 0.029 | 7.4 mm | 61% | 89% | ||||||
| Wall thickness on T1WI post-contrast | 0.725 | 0.018 | 5.9 mm | 72% | 73% | 0.651 | 0.102 | 0.711 | 0.056 | ||||||
| ADC | 0.728 | 0.029 | 1.11 × 10-3 mm2/s | 70% | 75% | 0.602 | 0.305 | 0.556 | 0.637 | ||||||
| MaRIA score | 0.736 | 0.013 | 26.1 | 96% | 53% | 0.697 | 0.032 | 23.5 | 90% | 50% | 0.71 | 0.058 | |||
| Clermont score | 0.667 | 0.11 | 0.776 | 0.005 | 28.7 | 90% | 63% | 0.727 | 0.054 | ||||||
Significant p-values are bolded.
Se, sensitivity; Sp, specificity; AUC, area under the curve.
4. Discussion
Our results showed that MRI can predict histopathological composition of ileal CD, including inflammation/oedema and disproportionately increased muscular hypertrophy over fibrosis, with reasonable diagnostic performance. On pathology, stenotic lesions in CD are histopathologically characterised by reduced bowel lumen and thickened bowel wall secondary to fibromuscular hypertrophy, with possible concomitant inflammation. Several pathological studies have reported that smooth muscle hypertrophy contributes to stricture formation, particularly within the muscularis mucosae.5–10 Because of the great variation of the relative composition of different components, CD lesions are highly heterogeneous both radiologically and histopathologically. Our results show that MRI can be useful in estimating the relative composition of the three most important components in ileal CD lesions, including inflammation/oedema, smooth muscle hypertrophy, and fibrosis.
Compared with computed tomography, CT, MRI has the advantage of lack of radiation exposure, higher soft-tissue contrast, and additional information relating to diffusion, enhancement/perfusion, and peristalsis.14 Consequently, MRI plays an important role in CD and is becoming the first-line imaging method for CD evaluation.13–15 MRI is used in CD at every step of patient management: initial assessment for activity, extent of disease, diagnosis of complications [fistulas, abscesses], and follow-up after therapy.13,14,31,32 There are a limited number of studies that have compared MRI with/without DWI with surgical pathological findings in CD.12,21–24,26 Even though used in many centres, the role of DWI in CD is not completely established.
Wall thickness, layered pattern of enhancement, T2 signal intensity, and ADC have been described to be sensitive to inflammation.19–21,23,24,27 In our study, a thicker bowel wall was associated with increased inflammation, with a 5.9-mm threshold on T1W post-contrast imaging, similar to what has been reported by Zappa et al. [reported threshold of approximately 6 mm].23 As reported in previous studies, a layered pattern at the early and parenchymal phases was associated with inflammatory activity.12,23,24 The degree of wall enhancement has variable performance for inflammation.21,23,24 Zappa et al. found that the degree of enhancement at the parenchymal phase [90s after contrast injection] was not associated with inflammation, in contradiction to our findings which found that a marked enhancement at 60s is associated with inflammation.23 The discrepancy may be by various acquisition times and evaluation methods of the degree of enhancement. Our study showed that diffusion was restricted [lower ADC] in bowel segments with inflammation in the context of CD, confirming previous studies in CD.17–19,33–35 For example, Oto et al. showed that mean ADC of inflamed bowel was significantly lower than in non-affected bowel segments [1.59 ± 0.45 versus 2.74 ± 0.45 × 10-3 mm2/s].17 Similarly, Schmid-Tannwald et al. showed that mean ADC was lowest in bowel segments with active inflammation compared with chronically inflamed bowel segments and non-affected bowel segments [1.09 ± 0.18 versus 1.55 ± 0.21 versus 2.18 ± 0.37 × 10-3 mm2/s, respectively].33 Finally, as expected, because they were defined in order to identify the inflammation, higher Clermont and MaRIA scores were associated with inflammation.19,27 This is related to the fact that these scores include wall thickness, enhancement ratio, and presence of ulcers and oedema, which are all markers of inflammation. Similarly those scores were higher in cases of oedema on pathology, which is also associated with inflammation.36
There are conflicting data on imaging assessment of bowel wall fibrosis. Zappa et al. showed that wall thickness and hyperintensity on T2-weighted imaging [T2WI], as well as the presence of fistulous disease, are correlated with histopathological fibrosis score.23 In our study, we did not correlate imaging findings to a semiquantitative fibrosis score, but with Sirius red staining, leading to a difficult comparison with other studies. Similarly to Rimola et al., who showed that the pattern of enhancement at 70 s was not associated with the histopathological fibrosis score, we did not find any relationship between the pattern of enhancement at the parenchymal phase and the Sirius red staining.12 However, unlike in Rimola et al.,12 we did not evaluate the pattern of enhancement at 7 min, as a delayed post-contrast acquisition at 7 min is not part of our routine MRI protocol. Finally, contrary to Tielter et al.,24 we did not find any correlation between ADC and fibrosis. However, our pathological reference was different. Indeed, they used a qualitative score, which mixed fibrosis component and muscle hypertrophy component, whereas we correlated with quantitative Sirius red staining, which reflects only collagen deposition.
There are no published reports on the correlation between imaging and muscle hypertrophy versus fibrosis in CD. Our results suggest that wall thickness on T2w images was increased in bowel segments with prominent muscular hypertrophy, compared with those with prominent fibrosis, which is an intuitive finding, as there is considerable narrowing of the bowel lumen in these cases. The presence of fistulas was also significantly and independently associated with prominent muscle hypertrophy. We did not observe an association between homogeneous pattern of enhancement and prominent fibrosis, although the pattern of enhancement is associated with high fibrosis score in Zappa et al. and in Rimola et al..12,23 However, the comparison between their studies and our study is difficult. Indeed, we used the Sirius red staining, assessed as a quantitative variable, and did not use a semiquantitative fibrosis score as in Zappa et al. and Rimola et al..12,23 Moreover, the Sirius red staining only reflected the fibrosis component, whereas the semiquantitative score used by Rimola et al. mixed fibrosis and muscular hypertrophy. Important as the detection of active inflammatory disease is, we hypothesise that the estimation of the relative composition of smooth muscle and fibrosis is also important when selecting the optimal treatment. Both result from the proliferation of mesenchymal cells [including fibroblasts, myofibroblasts, and smooth muscle cells], followed by the differentiation of myofibroblasts linking to hypertrophy of smooth muscle cells and by the accumulation of excess extracellular matrix proteins [mostly collagen I, III, and V].37
The composition of ileal lesions is important for treatment management. First, inflammation should be treated by medical therapy including anti-inflammatory treatment. Second, the presence of fibrosis and muscular hypertrophy can influence the treatment and surgical planning. Hypothetically, a stricture with prominent muscular hypertrophy will not likely respond to new antifibrotic agents any more than in those with excessive collagen deposition, and may be treated by surgery, whereas a stricture with prominent fibrosis may potentially benefit from new antifibrotic agents.38,39
Besides its retrospective design, our study has several limitations. First, the sample size was relatively small, with only 35 patients and 40 analysed bowel segments. However, we used strict inclusion criteria in order to allow a better correlation between MRI and histopathological findings. Second, the quantitative analysis was performed by only one reader. However, the reproducibility of wall thickness measurement and ADC measurement were previously shown to be high.19,40
In conclusion, MRI predicts the histopathological tissue composition of ileal CD, including inflammation and predominant muscular hypertrophy versus predominant fibrosis, with reasonable accuracy. These findings need to be verified in a prospective study.
Funding
This work was partially supported by the Sanford J Grossman Charitable Trust for Integrative Studies in IBD, Société Française de Radiologie [to MW], and U01 DK62429, U01 DK062422, R01 DK092235 [to JC].
Conflict of Interest
MW: consultant Olea Medical. J-FC: consultant AbbVie, Amgen, Boehringer-Ingelheim, Celgene Corporation, Celltrion, Enterome, Ferring, Genentech, Janssen and Janssen, Medimmune, Merck & Co., Pfizer, Protagonist, Second Genome, Seres, Shire, Takeda, Theradiag, speaker for AbbVie, Ferring, speaker’s bureau for Amgen, research grants AbbVie, Takeda, Janssen and Janssen. BT: grant support Guerbet, Bayer.
Author Contributions
MW: acquisition of data, data analysis, interpretation of data, drafting the article, revising critically the article, final approval of the submitted version. MHK: acquisition of data, data analysis, interpretation of data, drafting the article, revising critically the article, final approval of the submitted version. MC: acquisition of data, final approval of the submitted version. CB: acquisition of data, data analysis, interpretation of data, drafting the article, revising critically the article, final approval of the submitted version. JT: acquisition of data, final approval of the submitted version. XZ: acquisition of data, final approval of the submitted version. HP: acquisition of data, final approval of the submitted version. SH: data analysis, interpretation of data, revising critically the article, final approval of the submitted version. JC: concept and design of the study, final approval of the submitted version. JFC: concept and design of the study, revising critically the article, final approval of the submitted version. NH: concept and design of the study, interpretation of data, revising critically the article, final approval of the submitted version. BT: concept and design of the study, interpretation of data, drafting the article, revising critically the article, final approval of the submitted version.
References
- 1. Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol 2010;105:289–97. [DOI] [PubMed] [Google Scholar]
- 2. Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology 2017;152:340–50.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lenze F, Wessling J, Bremer J et al. Detection and differentiation of inflammatory versus fibromatous Crohn’s disease strictures: prospective comparison of 18F-FDG-PET/CT, MR-enteroclysis, and transabdominal ultrasound versus endoscopic/histologic evaluation. Inflamm Bowel Dis 2012;18:2252–60. [DOI] [PubMed] [Google Scholar]
- 4. Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn’s disease complicated by strictures: a systematic review. Gut 2013;62:1072–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Graham MF, Diegelmann RF, Elson CO et al. Collagen content and types in the intestinal strictures of Crohn’s disease. Gastroenterology 1988;94:257–65. [DOI] [PubMed] [Google Scholar]
- 6. Severi C, Sferra R, Scirocco A et al. Contribution of intestinal smooth muscle to Crohn’s disease fibrogenesis. Eur J Histochem 2014;58:2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graham MF. Pathogenesis of intestinal strictures in Crohn’s disease - an update. Inflamm Bowel Dis 1995;1:220–7. [PubMed] [Google Scholar]
- 8. Koukoulis G, Ke Y, Henley JD, Cummings OW. Obliterative muscularization of the small bowel submucosa in Crohn disease: a possible mechanism of small bowel obstruction. Arch Pathol Lab Med 2001;125:1331–4. [DOI] [PubMed] [Google Scholar]
- 9. Lee EY, Stenson WF, DeSchryver-Kecskemeti K. Thickening of muscularis mucosae in Crohn’s disease. Mod Pathol 1991;4:87–90. [PubMed] [Google Scholar]
- 10. Chen W, Lu C, Hirota C, Iacucci M, Ghosh S, Gui X. Smooth muscle hyperplasia/hypertrophy is the most prominent histological change in Crohn’s fibrostenosing bowel strictures: a semiquantitative analysis by using a novel histological grading scheme. J Crohns Colitis 2017;11:92–104. [DOI] [PubMed] [Google Scholar]
- 11. Chiorean MV, Sandrasegaran K, Saxena R, Maglinte DD, Nakeeb A, Johnson CS. Correlation of CT enteroclysis with surgical pathology in Crohn’s disease. Am J Gastroenterol 2007;102:2541–50. [DOI] [PubMed] [Google Scholar]
- 12. Rimola J, Planell N, Rodríguez S et al. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol 2015;110:432–40. [DOI] [PubMed] [Google Scholar]
- 13. Gourtsoyiannis NC, Papanikolaou N, Karantanas A. Magnetic resonance imaging evaluation of small intestinal Crohn’s disease. Best Pract Res Clin Gastroenterol 2006;20:137–56. [DOI] [PubMed] [Google Scholar]
- 14. Grand DJ, Guglielmo FF, Al-Hawary MM. MR enterography in Crohn’s disease: current consensus on optimal imaging technique and future advances from the SAR Crohn’s disease-focused panel. Abdom Imaging 2015;40:953–64. [DOI] [PubMed] [Google Scholar]
- 15. Bouhnik Y, Carbonnel F, Laharie D et al. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort [CREOLE] study. Gut 2018;67:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taouli B, Beer AJ, Chenevert T et al. Diffusion-weighted imaging outside the brain: Consensus statement from an ISMRM-sponsored workshop. J Magn Reson Imaging 2016;44:521–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oto A, Zhu F, Kulkarni K, Karczmar GS, Turner JR, Rubin D. Evaluation of diffusion-weighted MR imaging for detection of bowel inflammation in patients with Crohn’s disease. Acad Radiol 2009;16:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kiryu S, Dodanuki K, Takao H et al. Free-breathing diffusion-weighted imaging for the assessment of inflammatory activity in Crohn’s disease. J Magn Reson Imaging 2009;29:880–6. [DOI] [PubMed] [Google Scholar]
- 19. Hordonneau C, Buisson A, Scanzi J et al. Diffusion-weighted magnetic resonance imaging in ileocolonic Crohn’s disease: validation of quantitative index of activity. Am J Gastroenterol 2014;109:89–98. [DOI] [PubMed] [Google Scholar]
- 20. Oussalah A, Laurent V, Bruot O et al. Diffusion-weighted magnetic resonance without bowel preparation for detecting colonic inflammation in inflammatory bowel disease. Gut 2010;59:1056–65. [DOI] [PubMed] [Google Scholar]
- 21. Punwani S, Rodriguez-Justo M, Bainbridge A et al. Mural inflammation in Crohn disease: location-matched histologic validation of MR imaging features. Radiology 2009;252:712–20. [DOI] [PubMed] [Google Scholar]
- 22. Taylor SA, Punwani S, Rodriguez-Justo M et al. Mural Crohn disease: correlation of dynamic contrast-enhanced MR imaging findings with angiogenesis and inflammation at histologic examination - pilot study. Radiology 2009;251:369–79. [DOI] [PubMed] [Google Scholar]
- 23. Zappa M, Stefanescu C, Cazals-Hatem D et al. Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn’s disease? A retrospective comparison with surgical pathologic analysis. Inflamm Bowel Dis 2011;17:984–93. [DOI] [PubMed] [Google Scholar]
- 24. Tielbeek JA, Ziech ML, Li Z et al. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur Radiol 2014;24:619–29. [DOI] [PubMed] [Google Scholar]
- 25. Catalano OA, Gee MS, Nicolai E et al. Evaluation of quantitative PET/MR enterography biomarkers for discrimination of inflammatory strictures from fibrotic strictures in Crohn disease. Radiology 2016;278:792–800. [DOI] [PubMed] [Google Scholar]
- 26. Steward MJ, Punwani S, Proctor I et al. Non-perforating small bowel Crohn’s disease assessed by MRI enterography: derivation and histopathological validation of an MR-based activity index. Eur J Radiol 2012;81:2080–8. [DOI] [PubMed] [Google Scholar]
- 27. Rimola J, Rodriguez S, García-Bosch O et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut 2009;58:1113–20. [DOI] [PubMed] [Google Scholar]
- 28. Segnani C, Ippolito C, Antonioli L et al. Histochemical detection of collagen fibers by sirius red/fast green is more sensitive than van Gieson or sirius red alone in normal and inflamed rat colon. PLoS One 2015;10:e0144630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol 1986;103:2787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Icardo JM. Collagen and elastin histochemistry of the teleost bulbus arteriosus: false positives. Acta Histochem 2013;115:185–9. [DOI] [PubMed] [Google Scholar]
- 31. Bruining DH, Bhatnagar G, Rimola J, Taylor S, Zimmermann EM, Fletcher JG. CT and MR enterography in Crohn’s disease: current and future applications. Abdom Imaging 2015;40:965–74. [DOI] [PubMed] [Google Scholar]
- 32. Rimola J, Panés J, Ordás I. Magnetic resonance enterography in Crohn’s disease: optimal use in clinical practice and clinical trials. Scand J Gastroenterol 2015;50:66–73. [DOI] [PubMed] [Google Scholar]
- 33. Schmid-Tannwald C, Schmid-Tannwald CM, Morelli JN et al. The role of diffusion-weighted MRI in assessment of inflammatory bowel disease. Abdom Radiol [NY] 2016;41:1484–94. [DOI] [PubMed] [Google Scholar]
- 34. Buisson A, Hordonneau C, Goutte M, Bommelaer G. What is the role of diffusion-weighted imaging in ileocolonic Crohn’s disease?Inflamm Bowel Dis 2015;21:E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dohan A, Taylor S, Hoeffel C et al. Diffusion-weighted MRI in Crohn’s disease: current status and recommendations. J Magn Reson Imaging 2016;44:1381–96. [DOI] [PubMed] [Google Scholar]
- 36. Morson BS. Histopathology of Crohn’s disease. Proc R Soc Med 1968;61:79–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li C, Kuemmerle JF. Mechanisms that mediate the development of fibrosis in patients with Crohn’s disease. Inflamm Bowel Dis 2014;20:1250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bettenworth D, Rieder F. Reversibility of stricturing Crohn’s disease - fact or fiction?Inflamm Bowel Dis 2016;22:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bettenworth D, Rieder F. Medical therapy of stricturing Crohn’s disease: what the gut can learn from other organs - a systematic review. Fibrogenesis Tissue Repair 2014;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tielbeek JA, Makanyanga JC, Bipat S et al. Grading Crohn disease activity with MRI: interobserver variability of MRI features, MRI scoring of severity, and correlation with Crohn disease endoscopic index of severity. AJR Am J Roentgenol 2013;201:1220–8. [DOI] [PubMed] [Google Scholar]