Abstract
Antifungal prophylaxis is the standard of care for patients undergoing intensive chemotherapy for haematological malignancy or haematopoietic cell transplantation (HCT). Prophylaxis with azoles reduces invasive fungal infections and may reduce mortality. However, breakthrough infections still occur, and the use of azoles is sometimes complicated by pharmacokinetic variability, drug interactions, adverse events and other issues. Echinocandins are highly active against Candida species, including some organisms resistant to azoles, and have some clinical activity against Aspergillus species as well. Although currently approved echinocandins require daily intravenous administration, the drugs have a favourable safety profile and more predictable pharmacokinetics than mould-active azoles. Clinical data support the efficacy and safety of echinocandins for antifungal prophylaxis in haematology and HCT patients, though data are less robust than for azoles. Notably, sparse evidence exists supporting the use of echinocandins as antifungal prophylaxis for patients with significant graft-versus-host disease (GvHD) after HCT. Two drugs that target (1,3)-β-d-glucan are in development, including an oral glucan synthase inhibitor and an echinocandin with unique pharmacokinetics permitting subcutaneous and weekly administration. Echinocandins are a reasonable alternative to azoles and other agents for antifungal prophylaxis in patients undergoing intensive chemotherapy for haematological malignancy or those receiving HCT, excluding those with significant GvHD.
Introduction
Over the last decade, antifungal prophylaxis has become the standard of care for patients undergoing intensive chemotherapy for haematological malignancies or haematopoietic cell transplantation (HCT).1–8 The impact of antifungal prophylaxis in reducing rates of invasive fungal infections (IFIs) and mortality has been summarized in meta-analyses in adult and paediatric patients.9–11 Several studies have demonstrated the efficacy of azoles as primary antifungal prophylaxis.12 However, IFIs remain a significant cause of morbidity and a leading cause of infection-related mortality.13–16 Pharmacokinetic (PK) variability, drug interactions, adverse events (AEs) and cost may preclude consistent administration of azoles in an increasing number of patients.17–20 Since their introduction 15 years ago, the echinocandins have become increasingly important in our antifungal armamentarium for prophylaxis and treatment (as monotherapy or part of combination therapy). Echinocandins have a relatively broad spectrum of activity and have demonstrated excellent safety and tolerability with few drug interactions.21 A novel echinocandin in development [rezafungin acetate (previously CD101); Cidara Therapeutics, Inc., San Diego, CA, USA] may alleviate the need for daily intravenous (iv) administration and expand the spectrum of coverage for targeted fungal pathogens. We review the currently unmet needs for antifungal prophylaxis in patients with haematological malignancies and HCT and the potential role of echinocandins for this indication.
Rationale for antifungal prophylaxis in haematological malignancies and haematopoietic cell transplantation
Patients with haematological malignancies and HCT are at risk for IFIs caused by opportunistic fungi. These patients have impaired immune defences against fungi owing to their underlying diseases and treatments. For example, patients with acute leukaemia have neutropenia due to marrow infiltration from leukaemia and due to chemotherapy. Lymphopenia, monocytopenia and qualitative defects in phagocytic function are common in patients with lymphoma owing to their underlying disease or treatments (such as corticosteroids and purine analogues). Similarly, cellular immunity is impaired in patients with graft-versus-host disease (GvHD) owing to the pathophysiology of their disease and immunosuppression used for treatment. Furthermore, host factors such as age, comorbidities, iron overload and genetic predisposition as well as exogenous factors such as environmental exposures and presence of iv catheters may increase the risk for IFIs.22 More than one predisposing factor may be present at any given time based on underlying disease and treatment and may affect the types of causative organisms and timing of IFI. Prior to the implementation of empirical antifungal therapy, up to 50% of patients treated for leukaemia were found to have an IFI at autopsy.23 Empirical antifungal therapy reduced the incidence of IFIs during neutropenia.24 The incidence of IFIs was further reduced after the adoption of antifungal prophylaxis.9–11,25,26 However, even in the era of antifungal prophylaxis, IFIs affect quality of life, may delay or preclude potentially curative chemotherapy or HCT, pose a substantial burden for the healthcare system, and remain an important cause of morbidity and mortality.14–16,27,28
Registry studies of IFI in HCT patients provide real-world data showing that: (i) IFIs are more common in allogeneic HCT, particularly recipients of mismatched or unrelated donor allografts; (ii) the majority of IFIs occur late (over 1 month after HCT) with incidence continuing to climb up to 1 year after HCT; (iii) Candida and Aspergillus account for over 85% of IFIs; and (iv) non-Aspergillus moulds (including Mucorales) and Pneumocystis remain rare [1% in a 30 month period after HCT and 1.51% in a 180 day period after the last dose of chemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine and corticosteroids (R-CHOP) in patients with B-cell lymphoma].29–35 The incidence of IFI in multicentre clinical trials may reflect patient selection and controlled monitoring. In the randomized placebo-controlled trials of voriconazole or posaconazole prophylaxis compared with fluconazole, the rate of probable and proven IFIs was 8%–9% in the fluconazole arm and ∼5% in the voriconazole or posaconazole arm, the difference being driven largely by decreases in aspergillosis among those receiving mould-active azoles.36,37
Similar to HCT, the majority of IFIs are caused by Candida and Aspergillus species in patients with haematological malignancies.28,32,38,39 An increase in fluconazole-resistant Candida species, including C. glabrata and C. krusei, has been noted in several studies and has been attributed to azole prophylaxis.40–42 Breakthrough or de novo IFIs by non-Aspergillus moulds also occur but remain relatively infrequent.43,44 Select groups of patients with haematological malignancies and HCT recipients are at risk for pneumonia caused by Pneumocystis jirovecii and require prophylaxis directed against Pneumocystis in addition to traditional antifungal prophylaxis.
Patient-specific considerations in haematological malignancies and haematopoietic cell transplantation
Myeloid malignancies
AML is the most common leukaemia in adults, with an estimated 21 380 new cases (4.2 new cases per 100 000) diagnosed in the USA in 2017.45 Patients with AML are at high risk for IFIs owing to multiple immune defects associated with the underlying malignancy and its treatment. Mucosal integrity is the first line of host defence against invasion by fungal pathogens. Oral and gastrointestinal mucositis due to chemotherapy facilitates translocation of endogenous flora into the bloodstream and is a risk factor for candidaemia and invasive candidiasis (IC). Profound, prolonged neutropenia is commonly caused by both AML and intensive chemotherapy used in its treatment. The duration and severity of neutropenia interact to increase the risk of IFI, particularly mould infections.46 Intrinsic functional defects of neutrophils exist in patients with acute leukaemia, myelodysplastic syndrome (MDS) and pre-leukaemia states, but their clinical significance is poorly defined.47 The risk of IFIs is higher during induction chemotherapy when the typical duration of neutropenia is over 2 weeks compared with consolidation, when the duration of neutropenia is ∼1 week.39,48
MDS is a heterogeneous haematopoietic disease commonly associated with bone marrow failure, peripheral blood cytopenias and progression to AML. The incidence of MDS is similar to that of AML and is estimated at ∼5 per 100 000, representing the most commonly diagnosed myeloid neoplasm in the USA and Europe.49 In comparison with AML patients receiving intensive chemotherapy, patients with MDS have a lower risk of IFIs, possibly because of a stationary neutrophil count and because they have been traditionally managed conservatively with supportive care and growth factors. Low incidence of IFIs has been reported in MDS patients treated with hypomethylating agents, including azacitidine and decitabine, though these populations are less well described and more data are needed.50 A study evaluating over 800 cycles of azacitidine given mostly to patients with MDS without antifungal prophylaxis found that 0.8% were complicated by development of an IFI.51
Acute lymphoblastic leukaemia and other lymphoproliferative malignancies
Approximately 6000 new cases of ALL are diagnosed in the USA annually, of which 60% occur in children and adolescents, with cure rates approaching 90%.52 Adult ALL portends a poorer prognosis.53 T cell function is required for macrophage activation and subsequent fungicidal activity. In the absence of functional T cells, selected fungal pathogens may survive and replicate inside macrophages. Patients with ALL, hairy cell leukaemia and mycosis fungoides have an intrinsic impairment in cellular immunity and are at increased risk for infections by P.jirovecii, Cryptococcus species and endemic fungi. In addition, therapies directed towards lymphoproliferative disorders often include corticosteroids, purine analogues (such as fludarabine and cladribine) and alemtuzumab, resulting in prolonged lymphopenia. Idelalisib, a PI3Kd inhibitor, is associated with a significantly increased risk for P. jirovecii pneumonia (PJP) through unclear mechanisms potentially unrelated to T cell lymphopenia.54 The reported incidence of IFI among ALL patients ranges from 7% to 19% in single-centre studies without routine antifungal prophylaxis.38,55
Patients with lymphoma and other lymphoproliferative disorders characterized by relatively short periods of mild neutropenia develop IFIs infrequently when compared with patients with acute leukaemia.56 IFIs in this patient group are generally due to Candida species; mould infections are uncommon.56 An increased incidence of IFIs is reported in patients with myeloma and chronic lymphocytic leukaemia owing to the cumulative immunosuppressive effects of an ever-expanding number of myeloma-specific therapies.57
Haematopoietic cell transplantation
In 2012, ∼68 000 HCTs, including autologous and allogeneic transplants, were performed worldwide and represent a trend of consistent growth, particularly of allogeneic HCTs, since 2006.58 The increased use of alternative donor sources and reduced-intensity conditioning enables an increasing number of older patients and those with comorbidities to undergo HCT. Furthermore, improved survival of patients with GvHD may further increase the number of individuals at risk for IFIs.59 Periods of risk for IFIs after HCT have been traditionally divided into ‘early’ (pre-engraftment) and ‘late’ (post-engraftment) because of distinct predisposing factors.
Pre-engraftment
The main risk factors for IFIs in the early post-HCT period are neutropenia and mucositis. The duration and severity of neutropenia and mucositis depend on the type of conditioning and the stem cell source. Among HCT patients, IFIs occur less commonly in autologous HCT recipients than in their allogeneic counterparts, since neutropenia typically resolves within 7–10 days among autologous HCT recipients.60,61 Myeloablative conditioning regimens used as part of autologous HCT, including high-dose melphalan and total body irradiation, can cause significant mucositis, which likely predisposes to IC in the absence of prophylaxis.62
Patients undergoing allogeneic HCT have a higher risk for IFIs compared with those undergoing autologous HCT.63–65 Allogeneic HCT recipients develop mucositis not only from conditioning regimens, but also from methotrexate, often used for preventing GvHD.66 Allogeneic HCT patients whose graft is harvested from umbilical cord blood (UCB) and, to a lesser extent, bone marrow, experience delayed engraftment with likely increased short-term risk of IFIs.67,68 Without prophylaxis, IC typically develops before mould infection occurs, likely reflecting the duration of neutropenia and mucositis occurring during the pre-engraftment phase.64
Post-engraftment
The most important risk factors for IFIs after engraftment are receipt of an unrelated donor allograft, development of acute GvHD grades II–IV or extensive chronic GvHD, and treatment with high-dose corticosteroids.31–33 The major effect of corticosteroids on neutrophils appears to be impairment of chemotaxis, which decreases localized inflammatory responses.69,70 However, impairments of phagocytosis, microbicidal activity and antibody-dependent cytotoxicity have also been seen in vitro.70
Acute and chronic GvHD predispose to IC and mould infections, particularly those caused by Aspergillus and agents of mucormycosis.64,65,71 T cell depletion (TCD) of the graft greatly reduces the risk of GvHD, but the resulting prolonged and severe lymphopenia has been associated with late occurrence of mould infections.72 Impaired T cell immunity after HCT is also a risk factor for PJP.73 The period of risk is longer for recipients of TCD allografts or patients who receive prolonged or cumulative high-dose corticosteroids, including patients with GvHD.73–75
Novel therapies for refractory GvHD such as mesenchymal stem cells have been associated with increased risk of IFIs, although it is unclear whether this association reflects cumulative immunosuppression.76
Novel targeted therapies and immunotherapies
Several novel targeted strategies are in development for haematological malignancies. Broadly characterized according to mechanism of action, these strategies employ monoclonal antibodies, bispecific antibodies, molecular targets such as tyrosine kinase inhibitors (TKIs) or checkpoint inhibitors, and adoptive or targeted cellular therapies such as chimeric antigen receptor (CAR)-T cells.77,78 As our understanding of haematological malignancies expands at the genomic and molecular levels, it is likely that these agents will become more broadly applicable in the future. It is still too early to know the net impact of the new agents on IFI and antifungal prophylaxis. Some of the new agents are given in combination with traditional chemotherapies or sequentially, and may be administered long term, making it almost impossible to dissect their relative contribution to IFI risk. The new strategies intersect with IFI prophylaxis both in modulating IFI risk and in introducing new concerns for drug interactions with antifungal prophylaxis and AEs.
For AML, the potential applications of fms-like tyrosine kinase 3 (FLT3) inhibitors are expanding. Novel regimens incorporating FLT3 inhibitors, isocitrate dehydrogenase 1/2 inhibitors, epigenetic therapy and CD33-targeted agents are under intense evaluation.79,80 Strategies for ALL include monoclonal antibodies, antibody–drug conjugates, mechanistic targeting of rapamycin inhibitors, proteasome inhibitors, histone deacetylase inhibitors, Bruton's tyrosine kinase inhibitors, Janus kinase and signal transducer and activator of transcription inhibitors, programmed cell death protein inhibitors and FLT3 inhibitors.81
Many of these agents have significant PK interactions with azoles. A detailed review of interactions is beyond the scope of this review. An illustrative example is the interaction of azoles with TKIs. Exposure to TKIs increases when taken in combination with a strong cytochrome P450 (CYP)3A inhibitor such as voriconazole; however, the appropriate dose adjustment of TKIs for concomitant administration with azoles is less clear.19,82 In contrast, there are no identified interactions between TKI and echinocandins or polyenes.
Prolongation of the QT interval is another potential concern for many TKIs, especially in patients with multiple medications that affect the QT interval. Voriconazole and posaconazole are known to prolong the QT interval.83,84 In contrast, isavuconazole is known to shorten the QT interval.85
Transaminase elevation is common among patients receiving chemotherapy or immunomodulatory therapies and concomitant administration of azoles is often avoided in this setting by the clinicians.78
Current unmet needs in antifungal prophylaxis
Fluconazole, posaconazole, and micafungin are approved by the US FDA for the prevention of IFIs among HCT patients (Table 1).84,86,87 Posaconazole is additionally approved for antifungal prophylaxis in those with haematological malignancies at least 13 years of age with prolonged neutropenia from chemotherapy.84 While the azoles are far more utilized for IFI prophylaxis and are better studied for this indication, these drugs are metabolized by cytochrome P450 isoenzymes, leading to the potential for serious drug interactions with concomitant administration of certain chemotherapeutic agents (including cyclophosphamide, vincristine and TKIs) or other immunosuppressants (including cyclosporine, tacrolimus and sirolimus).19,88–90 QT prolongation may be a limiting factor for patients on multiple medications.
Table 1.
Antifungals approved for IFI prophylaxis
| Parameter | Fluconazole | Posaconazole | Micafungin |
|---|---|---|---|
| FDA-approved indication | Prevention of candidiasis in patients undergoing HCT receiving cytotoxic chemotherapy and/or radiation therapy | Prevention of invasive Aspergillus and Candida infections in patients at high risk due to immunocompromise, such as HCT recipients with GvHD or those with haematological malignancies and prolonged chemotherapy-associated neutropenia | Prevention of Candida infections in patients undergoing HCT |
| Year approved for any indication | 1992 | 2006 (suspension); 2013 (delayed-release tablet); 2014 (iv solution) | 2006 |
| Generic | Yes | No | No |
| Doses and formulations | Oral tablet: 50 mg, 100 mg, 150 mg, 200 mg | Oral suspension: 40 mg/mL (105 mL) | iv solution for reconstitution: 50 mg, 100 mg |
| Oral suspension: 10 mg/mL (35 mL), 40 mg/mL (35 mL) | Delayed-release tablet: 100 mg | ||
| iv solution: 100 mg (50 mL), 200 mg (100 mL), 400 mg (200 mL) | iv solution: 300 mg (16.7 mL) | ||
| Administration | Reliably absorbed orally with or without food | Delayed-release tablets must be given whole; tablets and suspension should be given with food for optimal absorption; iv should be given through central line | Only available iv |
| Dose adjustment for renal insufficiency | Yes | No (theoretical concerns about accumulation of cyclodextrin vehicle when iv formulation given to patients with severe renal insufficiency) | No |
| Dose adjustment for hepatic insufficiency | No | No | No |
| Drug interactions | Mediated by P450 system | Mediated by P450 system | None significant |
Administration of oral posaconazole in particular can be challenging in the presence of mucositis that prevents administration of food or nutritional supplements required for optimal posaconazole absorption. Posaconazole levels <700 ng/mL have been associated with a higher rate of breakthrough IFIs compared with higher levels.91 Therapeutic drug monitoring is sometimes used owing to an association between low drug levels and breakthrough IFIs during prophylaxis, although this monitoring increases provider burden and treatment cost.92 Absorption and adherence have greatly improved with the availability of posaconazole delayed-release tablets; however, administration with food is still recommended for optimal absorption, and discontinuations due to mucositis and colitis limit the utility of posaconazole prophylaxis.93 In a single-centre, prospective study of patients with AML or after HCT taking posaconazole tablets, 80.5% of samples showed a concentration of at least 700 ng/mL at steady state, but 16% of patients stopped posaconazole prematurely owing to colitis, transaminase elevations and mucositis.94 The availability of iv posaconazole alleviates concerns related to absorption; however, cost is a factor limiting its use.
Voriconazole demonstrates wide inter-patient variability in serum concentrations that is due in part to variant CYP2C19 alleles.95 Individuals who are CYP2C19 ultrarapid metabolizers have decreased voriconazole trough concentrations, whereas poor metabolizers have increased trough concentrations and are at increased risk of AEs. Up to one-third of HCT recipients develop biochemical hepatotoxicity while on voriconazole that often leads to discontinuation of voriconazole by the clinicians regardless of causality.17 A long-term safety concern is the association of prolonged exposure to voriconazole with the development of non-melanoma skin cancers in HCT recipients.96 Notably, in randomized controlled trials (RCTs) of prophylaxis with voriconazole or posaconazole compared with fluconazole, the safety profiles of mould-active azoles were similar to those of fluconazole.36,37 However, higher rates of discontinuation of voriconazole have been reported in clinical practice.17
Use of amphotericin B (AmB) and its lipid derivatives for prophylaxis is problematic due to infusional and renal toxicities, as well as insufficient evidence regarding their efficacy.97,98
PJP prophylaxis has historically been considered separately from prevention of IFIs given the unique features of Pneumocystis and the challenges posed by this organism, including the lack of activity of commonly used antifungal agents. PJP prophylaxis is recommended for patients with ALL, HCT recipients and those under treatment with high-dose corticosteroids (generally considered to be at least 20 mg/day of prednisone or an equivalent dose of another corticosteroid for at least 4 weeks) or agents significantly affecting T cell immunity used for treatment of lymphoma and non-haematological malignancies.99 Trimethoprim/sulfamethoxazole is recommended as first-line prophylaxis, but high rates of early withdrawal (31%–56%) have been reported for HCT recipients.99 Mutations conferring resistance to sulfamethoxazole have been recently reported from certain geographic areas.100 Inhaled pentamidine, an alternative to trimethoprim/sulfamethoxazole, frequently causes bronchospasm, needs to be given by a respiratory therapist and requires use of a private room during administration due to its teratogenicity. Dapsone, atovaquone and iv pentamidine are alternatives but may have inferior efficacy compared with trimethoprim/sulfamethoxazole and lack activity against other opportunistic infections (including, in the case of dapsone and pentamidine, Toxoplasma gondii). Atovaquone is associated with poor tolerability and is costly. Thus, there is a need for safer and better-tolerated PJP prophylaxis.
In summary, current prophylactic practices have significantly reduced the rates of IFIs and PJP. However, challenges with pill burden, adherence, safety, tolerability and drug interactions remain. It is thus worthwhile to reflect on the properties of echinocandins that may make them an attractive alternative to azoles or AmB products.
Rationale for echinocandins
Mechanism of action
Echinocandins, semisynthetic cyclic lipopeptides, emerged for clinical use with the approval of caspofungin in 2001, micafungin in 2005 and anidulafungin in 2006.21 These drugs inhibit fungal cell wall synthesis by binding to the (1,3)-β-d-glucan synthase enzyme complex, which is composed of at least two subunits (FKS1p and Rho1p).21,101 This target is not found in mammalian cells and consequently enables this drug class to have a favourable safety profile.21,101 However, echinocandin activity is predicated on the proportion of β-glucan composing the fungal cell wall, resulting in their inactivity against Mucormycetes, Fusarium species or Scedosporium species [owing to reduced (1,3)-β-d-glucan synthase activity] or against Trichosporon species and Cryptococcus species [owing to predominance of (1,6)-β-d-glucan instead].102 Echinocandins are not used to treat endemic fungi because of high MICs for the yeast forms, nor are they currently used to treat P. jirovecii, as previous studies showed limited activity against the trophic form of the biphasic (cyst/trophic) life cycle of Pneumocystis.102–105 Echinocandins have been useful against Candida and Aspergillus species, which are the two primary targets for IFI prophylaxis.
All three echinocandins are fungicidal against Candida species, including those displaying resistance to the azoles, such as C. glabrata, or AmB, such as C. lusitaniae. For Aspergillus species, exposure to the echinocandins leads to lysis of the apical tips of expanding hyphae, alteration of hyphal morphology and modification of cell wall composition and organization.106 Beyond the direct antifungal effect, the echinocandin-induced morphological changes may be able to amplify host immune responses, though this finding is of unclear clinical significance.107 So although fungistatic and with less robust clinical activity in the treatment of invasive aspergillosis, this drug class exhibits excellent in vitro activity against many Aspergillus species, including A. fumigatus, A. flavus, A. niger and A. terreus.108,109
By inhibiting production of glucans incorporated into the cell wall of Pneumocystis cyst forms, echinocandins likely have some activity against this organism.104 While the currently available echinocandins have shown benefit in some pre-clinical studies in treatment of PJP, the utility of echinocandins for PJP prophylaxis has not been formally evaluated in clinical trials. The data on the efficacy of echinocandins as part of combination therapy for PJP in humans is limited and controversial.110
Pharmacokinetics
Owing to their high molecular weights, echinocandins are minimally absorbed after oral administration and are available only in iv formulations.101 These drugs are highly protein bound, display concentration-dependent activity against Candida and Aspergillus species, and distribute well into tissues such as the lung, liver and spleen but have minimal penetration into the CSF, eye and urine.21,101,111 Where the drugs differ from one another is their metabolic pathways, leading to variations in half-lives, drug dosing strategies and drug interaction profiles, as detailed in previously published reviews.21,111 Of the three, caspofungin displays triphasic non-linear PK, whereas both micafungin and anidulafungin exhibit linear PK.21,111 Dose adjustments for any of the three echinocandins are not needed for renal insufficiency, including for patients receiving haemodialysis or continuous renal replacement therapy.86,112,113 While caspofungin requires dose modification for moderate hepatic insufficiency, no data are available for adults with severe hepatic impairment or in paediatric patients with any degree of hepatic impairment.51 No dose adjustments for hepatic insufficiency are needed for micafungin or anidulafungin.86,113 Some PK studies have suggested that echinocandin doses used in clinical practice may be subtherapeutic in some patients.114,115 However, the clinical implications of these data are unclear and current adult dosing recommendations have not been modified based on these data.
In clinical practice, the echinocandins are dosed on a once-daily basis, which can be difficult to maintain in the outpatient setting given the requirement for iv administration. Based on their pharmacodynamic (PD) profile, the AUC/MIC ratio may be the best predictor of clinical outcome when these agents are used.116 Considering their concentration-dependent killing, linear PK, high tissue concentrations and a postulated prolonged post-antifungal effect, higher doses of echinocandins administered several times or once weekly may be a viable, if not better, alternative to daily dosing, though these strategies have not undergone rigorous clinical study and are rarely used as first-line dosing strategies.116–119 Higher intermittent doses of micafungin have demonstrated efficacy against IC in animal models.83,120 Limited clinical studies demonstrate safety and tolerability of higher doses of micafungin in humans, but data supporting the efficacy of alternative administration regimens for antifungal prophylaxis are extremely limited.121 It remains to be seen whether intermittent dosing is a viable strategy for prevention of IFIs. High concentrations of echinocandins can paradoxically lead to a reversal of growth inhibition in vitro, although the clinical relevance of this phenomenon remains unclear.122
Safety profile
Echinocandins are well tolerated such that severe AEs requiring discontinuation occur less frequently when compared with the other antifungal classes.21 Modest elevations of aminotransferases and alkaline phosphatase are the most frequently reported laboratory abnormalities but generally are of little clinical consequence.21,101 While histamine-associated infusion reactions have been reported, they are rare and can be prevented by slowing the infusion rate and providing supportive care as warranted.21,111,123 Injection site pain, uncomplicated gastrointestinal symptoms (such as nausea, vomiting and diarrhoea) and haematological effects (such as anaemia, leukopenia and thrombocytopenia) account for fewer than 10% overall of AEs.21,111
There have been rare case reports of decreased cardiac output, flash pulmonary oedema and haemodynamic instability occurring during echinocandin administration, though causality is difficult to determine.124–126 Histamine release has been postulated as the underlying cause in some cases. Animal studies suggest that there may be a potential for direct mitochondrial injury to cardiac myocytes, particularly for anidulafungin and caspofungin, though only at blood levels rarely if ever seen in humans.124,127 The clinical implications of this research are not understood, and documented cardiac toxicity attributed to echinocandins remains very rare.
A key advantage over the azoles is minimal potential for drug interactions since the echinocandins do not inhibit cytochrome P450 enzyme or P-glycoprotein transport systems.111,123 On the other hand, several drugs (including carbamazepine, dexamethasone, efavirenz, phenytoin and rifampicin) appear to induce the metabolism of caspofungin, so an increased maintenance dose is recommended when any of these drugs is given concurrently with caspofungin.51
Echinocandin resistance
The mechanism of echinocandin resistance involves amino acid alterations in ‘hot spot’ regions of the FKS gene-encoded subunits of glucan synthase and is typically acquired.128 Although the overall prevalence of echinocandin resistance among Candida species is low at 2%–3%, the exception may be C. glabrata, which can also be MDR.128–130 One centre found an increase in echinocandin resistance among the C. glabrata bloodstream isolates from 4.9% in 2001 to 12.3% in 2010.129 This same study found that clinical failure correlated with the presence of FKS mutations and elevated MICs. The increasing use of echinocandins in clinical practice, presence of gastrointestinal reservoirs and poor drug penetration into intra-abdominal infections have been postulated to be clinical factors driving development of echinocandin resistance.128 National surveillance efforts as well as studies to better understand the echinocandin resistance mechanism are ongoing.
Published data on echinocandin prophylaxis
AmB products and azoles have demonstrated significant benefit in reducing rates of IFIs among patients with acute leukaemia and MDS and those undergoing HCT; some studies have also shown reductions in mortality.2,7 Clinical trials and retrospective studies of echinocandin prophylaxis comprise heterogeneous populations and varying echinocandin doses, as well as different comparators and endpoints (Table 2). Studies directly comparing echinocandin prophylaxis with mould-active azoles (such as posaconazole) and data on specific groups, particularly HCT recipients with GvHD, are limited. Nonetheless, the data in aggregate support the safety and efficacy of echinocandins for antifungal prophylaxis in many clinical settings.25
Table 2.
Studies on echinocandin prophylaxis in patients with haematological malignancies or undergoing HCT
| Study citation | Methodology | Setting, population, dates | Arms | Qualitya | Primary endpoint |
|---|---|---|---|---|---|
| Echinocandin versus fluconazole | |||||
| 131 | RCT, blinded | US; multicentre (72 centres); mostly adult; allogeneic or autologous HCT; assessed neutropenic phase; 1999–2000 | Micafungin, (50 mg iv daily; N = 425) vs fluconazole (400 mg iv daily; N = 457) starting ≤48 h after conditioning through engraftment, D + 42, IFI or drug cessation | High | Absence of IFI: micafungin superior, NNT 15 |
| 132 | retrospective, cohort (historical control) | Japan; single-centre; mostly adult; allogeneic HCT; assessed through D + 49; micafungin patients recruited from 2004–07; unknown dates of historical cases; assessed neutropenic phase | Micafungin (100 mg iv daily; N = 41) vs historical control fluconazole (400 mg iv/po daily; N = 29); both started D − 14; both groups changed to fluconazole 200 mg po daily after engraftment and tolerating po intake | Low | Absence of proven, probable, or possible IFI: micafungin superior, NNT 5 |
| 133 | RCT, open label | Japan; multicentre (6 centres); mostly adult; allogeneic or autologous HCT; assessed neutropenic phase; 2004–06 | Micafungin (150 mg iv daily; N = 50) vs fluconazole (400 mg iv daily; N = 50) starting ≤48 h after conditioning through engraftment, D + 42, IFI or drug cessation | Medium | Absence of IFI: no significant difference |
| 134 | RCT, open label | Korea; single-centre; adult; allogeneic or autologous HCT; assessed neutropenic phase; 2010–15 | Micafungin (50 mg iv daily; N = 165) vs fluconazole (400 mg po/iv daily; N = 85) starting ≤24 h after HCT infusion through engraftment, D + 21, IFI or drug cessation | Medium | Incidence of proven or probable IFI: no significant difference |
| Echinocandin versus itraconazole | |||||
| 137 | RCT, open label | US (TX); single-centre; mostly adult; AML or MDS undergoing induction chemotherapy; 2001–03 | Caspofungin (50 mg iv daily; N = 107) vs itraconazole (200 mg iv bid ×2 days then daily; N = 90) starting with induction through resolution of neutropenia, CR, death, change in therapy, IFI, toxicity or through 25 days | Medium | Completion of prophylaxis without IFI: no significant difference |
| 135 | RCT, open label | China; multicentre (10 centres); adults; allogeneic or autologous HCT; assessed neutropenic phase; 2008–09 | Micafungin (50 mg iv daily; N = 136) vs itraconazole (5 mg/kg/day po in 2 divided doses; N = 147) starting within 48 h of beginning of conditioning regimen ending with engraftment, IFI, toxicity, death, withdrawal or other discontinuation | Medium | Absence of IFI: no significant difference |
| Echinocandin versus posaconazole | |||||
| 158 | Retrospective, cohort (historical control) | Austria; single-centre; adults; mixed population (induction and consolidation for acute leukaemia, allo- and auto-HCT, GvHD, some others); 2011–12 (historical control 2008–10); notable differences in baseline characteristics | Micafungin (50 mg iv daily; N = 100) vs posaconazole suspension (200 mg po q8h; N = 202) during neutropenia or other immunosuppression | Low | IFI incidence: no significant difference |
| Echinocandin versus mixed azoles | |||||
| 92 | RCT, open label | Italy; multicentre; adults; mostly AML with some ALL; 2007–09 | Caspofungin (70, 50 mg iv daily; N = 93) vs standard prophylaxis (mostly itraconazole; some posaconazole; unclear others; N = 82) | Medium | Incidence of proven or probable IFIs: no significant difference |
| 138 | Retrospective, cohort | USA (TX); single centre; adults; AML newly diagnosed undergoing induction chemotherapy; 2009–11 | Echinocandin (N = 38) vs voriconazole or posaconazole (N = 42), minimally described | Low | Development of IFI: azole superior |
NNT, number needed to treat; bid, twice daily; po, by mouth; D, day; CR, complete remission.
Double-blinded RCTs were rated as high quality. Other RCTS were rated as medium quality. Non-interventional studies were rated as low quality.
Micafungin is currently FDA approved for the prevention of IFIs among neutropenic HCT patients in the pre-engraftment phase. The most robust data come from studies comparing micafungin and fluconazole. Three RCTs, one blinded and two open-label, along with a retrospective cohort study demonstrate that micafungin is either equivalent or superior to fluconazole in preventing IFIs among neutropenic HCT patients.131–134 An open-label RCT comparing micafungin with itraconazole in the same patient population found similar rates of IFIs between the two arms.135 In a cohort of neutropenic patients undergoing HCT, bridging posaconazole prophylaxis with micafungin improved exposure to antifungal prophylaxis and led to reduced incidence of IFIs compared with posaconazole alone.136
Micafungin has also been studied for prophylaxis of IFIs among patients receiving induction chemotherapy for acute leukaemia and MDS. In two open-label RCTs in patients with acute leukaemia or MDS undergoing induction chemotherapy comparing caspofungin with itraconazole prophylaxis, the incidence of IFIs was similar in both arms.92,137 In a retrospective study, echinocandin-based prophylaxis compared with prophylaxis with voriconazole or posaconazole was associated with a higher risk of IFIs during intensive chemotherapy for AML, although confounding variables could not be excluded.138 Recently, micafungin was compared with posaconazole suspension in an open-label RCT in patients undergoing intensive chemotherapy for acute leukaemia or MDS at Memorial Sloan Kettering Cancer Center.139 The incidence of IFIs was similar between the two arms, but the duration of prophylaxis was longer with micafungin, highlighting the improved safety and tolerability profile of micafungin in this population.
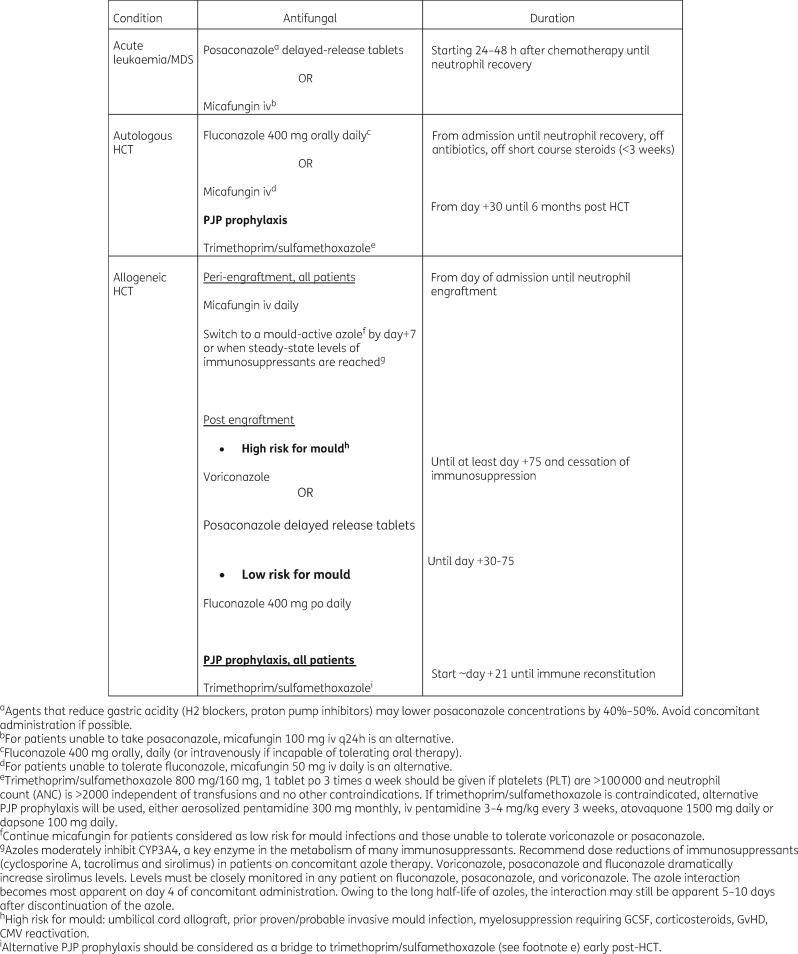
Overall these studies have consistently demonstrated the effectiveness of echinocandins in preventing IFIs among patients with neutropenia due to acute leukaemia and MDS and in the pre-engraftment phase after HCT. Efficacy and safety of micafungin in these patients were confirmed in a recently published meta-analysis of RCTs comparing micafungin with azoles in preventing IFIs among patients receiving chemotherapy, mostly for acute leukaemia, and those undergoing HCT.140 This meta-analysis, in fact, found that micafungin use was associated with lower rates of IFIs and higher rates of treatment success (variably defined) with fewer AEs, and with no difference in mortality.140 However, there is scant evidence for the use of echinocandins in preventing IFIs among patients with significant GvHD after HCT. These data are reflected in society guidelines. National Comprehensive Cancer Network guidelines endorse fluconazole and micafungin as first-line agents for preventing IFIs among patients with ALL and HCT with neutropenia in the pre-engraftment phase.8 Posaconazole is endorsed as first-line prophylaxis for patients with AML and MDS, and for those with significant GvHD, given its robust data in this patient group and its activity against moulds.8 These data are also reflected in the clinical practices of major cancer centres (Figure 1).
Figure 1.
Antifungal prophylaxis strategies at Memorial Sloan Kettering Cancer Center.
High doses of echinocandins
The safety and tolerability of higher doses of micafungin given at different dosing intervals have been explored in several uncontrolled studies with limited sample sizes.141–144 These studies collectively support the safety and tolerability of doses of micafungin between 150 and 300 mg. A recent case series describing 104 patients (84 allogeneic HCT recipients, 20 patients with leukaemia) receiving intermittent administration of high-dose micafungin (at least five doses of 300 mg or more two to three times weekly) mostly for antifungal prophylaxis found few AEs and only a 6% breakthrough IFI rate though there was no comparator group.121
Novel agents in development
The drug development pipeline includes a number of antifungal agents, some of which may become part of future antifungal prophylaxis strategies. Two drugs in development that target (1,3)-β-d-glucan are summarized here, as part of our consideration of echinocandin prophylaxis for patients undergoing HCT and haematological malignancies.
Though not an echinocandin, SCY-078 (Scynexis, Inc., Jersey City, NJ, USA) is an oral glucan synthase inhibitor that, like echinocandins, targets synthesis of glucan.145 The in vitro activity of SCY-078 is also similar to that of current echinocandins in terms of its activity against Candida species (including some isolates with FKS1 hot spot mutations) and Aspergillus species, and poor or absent activity against Mucormycetes and Fusarium species.146–148 SCY-078 has also shown modest activity against Scedosporium prolificans but has not been studied against Pneumocystis species.146In vivo data from a murine model of IC demonstrated SCY-078 efficacy against Candida species, and a Phase 3 trial of this oral glucan synthase inhibitor in patients with refractory or intolerant fungal diseases is under way.145,149
Rezafungin acetate (CD101) (Cidara Therapeutics, Inc., San Diego, CA, USA) is a novel echinocandin in clinical development that is differentiated by its long half-life and a PK/PD profile that demonstrates high plasma drug exposure.150,151 Once-weekly rezafungin achieved exposures well above the targeted AUC/MIC for various Candida species, theoretically predicting a potential to minimize emergence of resistance, though the drug has not yet been tested sufficiently in clinical studies.150,151 Another novel property of rezafungin as an echinocandin is its stability, which enables subcutaneous formulation.151
The potency and spectrum of activity of rezafungin in vitro against common wild-type and antifungal-resistant species of Candida and Aspergillus are comparable to those of other echinocandins.152,153 In neutropenic mouse models of azole-resistant candidiasis and aspergillosis, administration of rezafungin showed comparable efficacy to AmB.154 Once-weekly subcutaneous administration was efficacious as prophylaxis against Candida and Aspergillus in neutropenic mouse models.155 Of special interest are preliminary data in immunosuppressed mice supporting the efficacy of rezafungin in prevention of PJP.156 Preliminary studies demonstrate safety in humans; clinical trials for the treatment of IC are ongoing.150,157
Because of its spectrum of activity, PK and safety, rezafungin could be an attractive single agent for prophylaxis for Candida, Aspergillus and Pneumocystis if clinical trials ultimately support its safety and effectiveness. Well-designed clinical trials are warranted to formally evaluate the potential of rezafungin as prophylaxis in patients with haematological malignancies.
Conclusions
Diverse groups of patients with haematological and oncological disorders are at risk for IFIs, including PJP. Azole-based prophylaxis has reduced the rates of IFIs and mortality, but issues with tolerability, safety and drug interactions may limit its use. IFIs remain leading causes of infection-related mortality in these patients. The expanding clinical applications of molecular and immunomodulatory therapies pose new challenges with regard to drug interactions with azoles. Similarly, there is clearly an unmet need for better prophylaxis for PJP.
Compared with azoles, echinocandins have a more favourable safety profile, increased tolerability, minimal drug interactions and more predictable PK. Robust data support the use of echinocandins for prevention of IFIs among patients with prolonged neutropenia due to leukaemia or the neutropenic phase of HCT, although less data exist to warrant the routine use of echinocandins for prevention of IFIs in patients with GvHD after HCT. Novel antifungal drugs in early stages of development show potential as prophylaxis against Candida and Aspergillus, as well as possibly Pneumocystis in the case of rezafungin. If confirmed in clinical trials, newer antifungal agents might also help reduce pill burden and toxicities and improve adherence, allowing clinicians to provide effective and consistent antifungal prophylaxis to patients undergoing HCT and other treatments for haematological malignancies.
Acknowledgments
Funding
This article is part of a Supplement sponsored by Cidara Therapeutics, Inc. Editorial support was provided by T. Chung (Scribant Medical) with funding from Cidara.
Transparency declarations
G. A. P. has served as a consultant for Astellas and has received research funding from Astellas and Merck. All other authors have none to declare. This article was co-developed and published based on all authors’ approval. The authors received no compensation for their contribution to this Supplement.
References
- 1. Tomblyn M, Chiller T, Einsele H. et al. Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: a global perspective. Preface. Bone Marrow Transplant 2009; 44: 453–5. [DOI] [PubMed] [Google Scholar]
- 2. Ziakas PD, Kourbeti IS, Voulgarelis M. et al. Effectiveness of systemic antifungal prophylaxis in patients with neutropenia after chemotherapy: a meta-analysis of randomized controlled trials. Clin Ther 2010; 32: 2316–36. 10.1016/j.clinthera.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 3. Freifeld AG, Bow EJ, Sepkowitz KA. et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis 2011; 52: 427–31. 10.1093/cid/ciq147 [DOI] [PubMed] [Google Scholar]
- 4. Maertens J, Marchetti O, Herbrecht R. et al. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3–2009 update. Bone Marrow Transplant 2011; 46: 709–18. 10.1038/bmt.2010.175 [DOI] [PubMed] [Google Scholar]
- 5. Fleming S, Yannakou CK, Haeusler GM. et al. Consensus guidelines for antifungal prophylaxis in haematological malignancy and haemopoietic stem cell transplantation, 2014. Intern Med J 2014; 44: 1283–97. 10.1111/imj.12595 [DOI] [PubMed] [Google Scholar]
- 6. Tacke D, Buchheidt D, Karthaus M. et al. Primary prophylaxis of invasive fungal infections in patients with haematologic malignancies. 2014 update of the recommendations of the Infectious Diseases Working Party of the German Society for Haematology and Oncology. Ann Hematol 2014; 93: 1449–56. [DOI] [PubMed] [Google Scholar]
- 7. Ziakas PD, Kourbeti IS, Mylonakis E.. Systemic antifungal prophylaxis after hematopoietic stem cell transplantation: a meta-analysis. Clin Ther 2014; 36: 292–306. 10.1016/j.clinthera.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 8. National Comprehensive Cancer Network. Prevention and Treatment of Cancer-Related Infections Versions 2 .2017. National Comprehensive Cancer Network; https://www.nccn.org/professionals/physician_gls/pdf/infections.pdf. [DOI] [PubMed] [Google Scholar]
- 9. Bow EJ, Laverdiere M, Lussier N. et al. Antifungal prophylaxis for severely neutropenic chemotherapy recipients: a meta analysis of randomized-controlled clinical trials. Cancer 2002; 94: 3230–46. 10.1002/cncr.10610 [DOI] [PubMed] [Google Scholar]
- 10. Fisher BT, Kavcic M, Li Y. et al. Antifungal prophylaxis associated with decreased induction mortality rates and resources utilized in children with new-onset acute myeloid leukemia. Clin Infect Dis 2014; 58: 502–8. 10.1093/cid/cit781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robenshtok E, Gafter-Gvili A, Goldberg E. et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol 2007; 25: 5471–89. 10.1200/JCO.2007.12.3851 [DOI] [PubMed] [Google Scholar]
- 12. Pagano L, Caira M.. The role of primary antifungal prophylaxis in patients with haematological malignancies. Clin Microbiol Infect 2014; 20 Suppl 6: 19–26. [DOI] [PubMed] [Google Scholar]
- 13. Bhatti Z, Shaukat A, Almyroudis NG. et al. Review of epidemiology, diagnosis, and treatment of invasive mould infections in allogeneic hematopoietic stem cell transplant recipients. Mycopathologia 2006; 162: 1–15. 10.1007/s11046-006-0025-x [DOI] [PubMed] [Google Scholar]
- 14. Kontoyiannis DP. Antifungal prophylaxis in hematopoietic stem cell transplant recipients: the unfinished tale of imperfect success. Bone Marrow Transplant 2011; 46: 165–73. 10.1038/bmt.2010.256 [DOI] [PubMed] [Google Scholar]
- 15. Kontoyiannis DP, Lewis RE, Lortholary O. et al. Future directions in mucormycosis research. Clin Infect Dis 2012; 54 Suppl 1: S79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lewis G, Hall P, Eisa N. et al. Acute myelogenous leukemia patients are at low risk for invasive fungal infections after high-dose cytarabine consolidations and thus do not require prophylaxis. Acta Haematol 2010; 124: 206–13. 10.1159/000321504 [DOI] [PubMed] [Google Scholar]
- 17. Amigues I, Cohen N, Chung D. et al. Hepatic safety of voriconazole after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2010; 16: 46–52. 10.1016/j.bbmt.2009.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andes D, Pascual A, Marchetti O.. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother 2009; 53: 24–34. 10.1128/AAC.00705-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haouala A, Widmer N, Duchosal MA. et al. Drug interactions with the tyrosine kinase inhibitors imatinib, dasatinib, and nilotinib. Blood 2011; 117: e75–87. [DOI] [PubMed] [Google Scholar]
- 20. Lewis RE. Importance of pharmacokinetic considerations for selecting therapy in the treatment of invasive fungal infections. Am J Ther 2012; 19: 51–63. 10.1097/MJT.0b013e3181ff7e10 [DOI] [PubMed] [Google Scholar]
- 21. Aguilar-Zapata D, Petraitiene R, Petraitis V.. Echinocandins: the expanding antifungal armamentarium. Clin Infect Dis 2015; 61 Suppl 6: S604–11. [DOI] [PubMed] [Google Scholar]
- 22. Pagano L, Caira M.. Risks for infection in patients with myelodysplasia and acute leukemia. Curr Opin Infect Dis 2012; 25: 612–8. 10.1097/QCO.0b013e328358b000 [DOI] [PubMed] [Google Scholar]
- 23. Pizzo PA, Robichaud KJ, Gill FA. et al. Empiric antibiotic and antifungal therapy for cancer patients with prolonged fever and granulocytopenia. Am J Med 1982; 72: 101–11. 10.1016/0002-9343(82)90594-0 [DOI] [PubMed] [Google Scholar]
- 24. Hughes WT, Armstrong D, Bodey GP. et al. 1997 Guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever. Infectious Diseases Society of America. Clin Infect Dis 1997; 25: 551–73. [DOI] [PubMed] [Google Scholar]
- 25. Wang JF, Xue Y, Zhu XB. et al. Efficacy and safety of echinocandins versus triazoles for the prophylaxis and treatment of fungal infections: a meta-analysis of RCTs. Eur J Clin Microbiol Infect Dis 2015; 34: 651–9. 10.1007/s10096-014-2287-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu SX, Shen JL, Tang XF. et al. Newer antifungal agents for fungal infection prevention during hematopoietic cell transplantation: a meta-analysis. Transplant Proc 2013; 45: 407–14. 10.1016/j.transproceed.2012.07.149 [DOI] [PubMed] [Google Scholar]
- 27. Even C, Bastuji-Garin S, Hicheri Y. et al. Impact of invasive fungal disease on the chemotherapy schedule and event-free survival in acute leukemia patients who survived fungal disease: a case-control study. Haematologica 2011; 96: 337–41. 10.3324/haematol.2010.030825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tang JL, Kung HC, Lei WC. et al. High incidences of invasive fungal infections in acute myeloid leukemia patients receiving induction chemotherapy without systemic antifungal prophylaxis: a prospective observational study in Taiwan. PLoS One 2015; 10: e0128410.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Castro N, Neuville S, Sarfati C. et al. Occurrence of Pneumocystis jiroveci pneumonia after allogeneic stem cell transplantation: a 6-year retrospective study. Bone Marrow Transplant 2005; 36: 879–83. 10.1038/sj.bmt.1705149 [DOI] [PubMed] [Google Scholar]
- 30. Tomonari A, Takahashi S, Ooi J. et al. No occurrence of Pneumocystis jiroveci (carinii) pneumonia in 120 adults undergoing myeloablative unrelated cord blood transplantation. Transpl Infect Dis 2008; 10: 303–7. 10.1111/j.1399-3062.2008.00321.x [DOI] [PubMed] [Google Scholar]
- 31. Girmenia C, Barosi G, Piciocchi A. et al. Primary prophylaxis of invasive fungal diseases in allogeneic stem cell transplantation: revised recommendations from a consensus process by Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol Blood Marrow Transplant 2014; 20: 1080–8. 10.1016/j.bbmt.2014.02.018 [DOI] [PubMed] [Google Scholar]
- 32. Kontoyiannis DP, Marr KA, Park BJ. et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 2010; 50: 1091–100. 10.1086/651263 [DOI] [PubMed] [Google Scholar]
- 33. Neofytos D, Horn D, Anaissie E. et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis 2009; 48: 265–73. 10.1086/595846 [DOI] [PubMed] [Google Scholar]
- 34. Schuster MG, Cleveland AA, Dubberke ER. et al. Infections in hematopoietic cell transplant recipients: results from the organ transplant infection project, a multicenter, prospective, cohort study. Open Forum Infect Dis 2017; 4: ofx050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barreto JN, Ice LL, Thompson CA. et al. Low incidence of Pneumocystis pneumonia utilizing PCR-based diagnosis in patients with B-cell lymphoma receiving rituximab-containing combination chemotherapy. Am J Hematol 2016; 91: 1113–7. 10.1002/ajh.24499 [DOI] [PubMed] [Google Scholar]
- 36. Ullmann AJ, Lipton JH, Vesole DH. et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 2007; 356: 335–47. 10.1056/NEJMoa061098 [DOI] [PubMed] [Google Scholar]
- 37. Wingard JR, Carter SL, Walsh TJ. et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood 2010; 116: 5111–8. 10.1182/blood-2010-02-268151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hammond SP, Marty FM, Bryar JM. et al. Invasive fungal disease in patients treated for newly diagnosed acute leukemia. Am J Hematol 2010; 85: 695–9. 10.1002/ajh.21776 [DOI] [PubMed] [Google Scholar]
- 39. Neofytos D, Lu K, Hatfield-Seung A. et al. Epidemiology, outcomes, and risk factors of invasive fungal infections in adult patients with acute myelogenous leukemia after induction chemotherapy. Diagn Microbiol Infect Dis 2013; 75: 144–9. 10.1016/j.diagmicrobio.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang E, Farmakiotis D, Yang D. et al. The ever-evolving landscape of candidaemia in patients with acute leukaemia: non-susceptibility to caspofungin and multidrug resistance are associated with increased mortality. J Antimicrob Chemother 2015; 70: 2362–8. 10.1093/jac/dkv087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sipsas NV, Lewis RE, Tarrand J. et al. Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001-2007): stable incidence but changing epidemiology of a still frequently lethal infection. Cancer 2009; 115: 4745–52. 10.1002/cncr.24507 [DOI] [PubMed] [Google Scholar]
- 42. Hachem R, Hanna H, Kontoyiannis D. et al. The changing epidemiology of invasive candidiasis: Candida glabrata and Candida krusei as the leading causes of candidemia in hematologic malignancy. Cancer 2008; 112: 2493–9. 10.1002/cncr.23466 [DOI] [PubMed] [Google Scholar]
- 43. Hachem RY, Kontoyiannis DP, Boktour MR. et al. Aspergillus terreus: an emerging amphotericin B-resistant opportunistic mold in patients with hematologic malignancies. Cancer 2004; 101: 1594–600. 10.1002/cncr.20554 [DOI] [PubMed] [Google Scholar]
- 44. Kontoyiannis DP, Lionakis MS, Lewis RE. et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J Infect Dis 2005; 191: 1350–60. 10.1086/428780 [DOI] [PubMed] [Google Scholar]
- 45. Cancer Stat Facts: Acute Myeloid Leukemia (AML) National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program. https://seer.cancer.gov/statfacts/html/amyl.html.
- 46. Portugal RD, Garnica M, Nucci M.. Index to predict invasive mold infection in high-risk neutropenic patients based on the area over the neutrophil curve. J Clin Oncol 2009; 27: 3849–54. 10.1200/JCO.2008.21.0856 [DOI] [PubMed] [Google Scholar]
- 47. Bogomolski-Yahalom V, Matzner Y.. Disorders of neutrophil function. Blood Rev 1995; 9: 183–90. 10.1016/0268-960X(95)90024-1 [DOI] [PubMed] [Google Scholar]
- 48. Vogler WR, Velez-Garcia E, Weiner RS. et al. A phase III trial comparing idarubicin and daunorubicin in combination with cytarabine in acute myelogenous leukemia: a Southeastern Cancer Study Group Study. J Clin Oncol 1992; 10: 1103–11. 10.1200/JCO.1992.10.7.1103 [DOI] [PubMed] [Google Scholar]
- 49. Cogle CR. Fishing for myelodysplastic syndromes finds uncaptured cases by state cancer registries: need for more resources. Cancer 2014; 120: 1614–6. 10.1002/cncr.28638 [DOI] [PubMed] [Google Scholar]
- 50. Caira M, Latagliata R, Girmenia C.. The risk of infections in patients with myelodysplastic syndromes in 2016. Expert Rev Hematol 2016; 9: 607–14. 10.1080/17474086.2016.1181540 [DOI] [PubMed] [Google Scholar]
- 51. Trubiano JA, Dickinson M, Thursky KA. et al. Incidence, etiology and timing of infections following azacitidine therapy for myelodysplastic syndromes. Leuk Lymphoma 2017; 58: 2379–86. 10.1080/10428194.2017.1295141 [DOI] [PubMed] [Google Scholar]
- 52. Hunger SP, Lu X, Devidas M. et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children's oncology group. J Clin Oncol 2012; 30: 1663–9. 10.1200/JCO.2011.37.8018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gokbuget N, Stanze D, Beck J. et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood 2012; 120: 2032–41. 10.1182/blood-2011-12-399287 [DOI] [PubMed] [Google Scholar]
- 54. Sehn LH, Hallek M, Jurczak W. et al. A retrospective analysis of Pneumocystis jirovecii pneumonia infection in patients receiving idelalisib in clinical trials. In: American Society of Hematology 58th Annual Meeting and Exposition, San Diego, CA, USA, 2016. Abstract 3705.
- 55. Mariette C, Tavernier E, Hocquet D. et al. Epidemiology of invasive fungal infections during induction therapy in adults with acute lymphoblastic leukemia: a GRAALL-2005 study. Leuk Lymphoma 2017; 58: 586–93. 10.1080/10428194.2016.1204652 [DOI] [PubMed] [Google Scholar]
- 56. Pagano L, Caira M, Candoni A. et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica 2006; 91: 1068–75. [PubMed] [Google Scholar]
- 57. Nucci M, Anaissie E.. Infections in patients with multiple myeloma. Semin Hematol 2009; 46: 277–88. 10.1053/j.seminhematol.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 58. Niederwieser D, Baldomero H, Szer J. et al. Hematopoietic stem cell transplantation activity worldwide in 2012 and a SWOT analysis of the Worldwide Network for Blood and Marrow Transplantation Group including the global survey. Bone Marrow Transplant 2016; 51: 778–85. 10.1038/bmt.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Khoury HJ, Wang T, Hemmer MT. et al. Improved survival after acute graft-versus-host disease diagnosis in the modern era. Haematologica 2017; 102: 958–66. 10.3324/haematol.2016.156356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jantunen E, Salonen J, Juvonen E. et al. Invasive fungal infections in autologous stem cell transplant recipients: a nation-wide study of 1188 transplanted patients. Eur J Haematol 2004; 73: 174–8. 10.1111/j.1600-0609.2004.00273.x [DOI] [PubMed] [Google Scholar]
- 61. Auner HW, Sill H, Mulabecirovic A. et al. Infectious complications after autologous hematopoietic stem cell transplantation: comparison of patients with acute myeloid leukemia, malignant lymphoma, and multiple myeloma. Ann Hematol 2002; 81: 374–7. 10.1007/s00277-002-0484-1 [DOI] [PubMed] [Google Scholar]
- 62. Wardley AM, Jayson GC, Swindell R. et al. Prospective evaluation of oral mucositis in patients receiving myeloablative conditioning regimens and haemopoietic progenitor rescue. Br J Haematol 2000; 110: 292–9. 10.1046/j.1365-2141.2000.02202.x [DOI] [PubMed] [Google Scholar]
- 63. Orasch C, Weisser M, Mertz D. et al. Comparison of infectious complications during induction/consolidation chemotherapy versus allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2010; 45: 521–6. 10.1038/bmt.2009.187 [DOI] [PubMed] [Google Scholar]
- 64. Harrison N, Mitterbauer M, Tobudic S. et al. Incidence and characteristics of invasive fungal diseases in allogeneic hematopoietic stem cell transplant recipients: a retrospective cohort study. BMC Infect Dis 2015; 15: 584.. 10.1186/s12879-015-1329-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Girmenia C, Raiola AM, Piciocchi A. et al. Incidence and outcome of invasive fungal diseases after allogeneic stem cell transplantation: a prospective study of the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol Blood Marrow Transplant 2014; 20: 872–80. 10.1016/j.bbmt.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 66. Chaudhry HM, Bruce AJ, Wolf RC. et al. The incidence and severity of oral mucositis among allogeneic hematopoietic stem cell transplantation patients: a systematic review. Biol Blood Marrow Transplant 2016; 22: 605–16. 10.1016/j.bbmt.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 67. Takahashi S, Ooi J, Tomonari A. et al. Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood 2007; 109: 1322–30. [DOI] [PubMed] [Google Scholar]
- 68. Chang YJ, Weng CL, Sun LX. et al. Allogeneic bone marrow transplantation compared to peripheral blood stem cell transplantation for the treatment of hematologic malignancies: a meta-analysis based on time-to-event data from randomized controlled trials. Ann Hematol 2012; 91: 427–37. 10.1007/s00277-011-1299-8 [DOI] [PubMed] [Google Scholar]
- 69. Fauci AS, Dale DC, Balow JE.. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med 1976; 84: 304–15. 10.7326/0003-4819-84-3-304 [DOI] [PubMed] [Google Scholar]
- 70. Lionakis MS, Kontoyiannis DP.. Glucocorticoids and invasive fungal infections. Lancet 2003; 362: 1828–38. 10.1016/S0140-6736(03)14904-5 [DOI] [PubMed] [Google Scholar]
- 71. Mikulska M, Raiola AM, Bruno B. et al. Risk factors for invasive aspergillosis and related mortality in recipients of allogeneic SCT from alternative donors: an analysis of 306 patients. Bone Marrow Transplant 2009; 44: 361–70. 10.1038/bmt.2009.39 [DOI] [PubMed] [Google Scholar]
- 72. van Burik JA, Carter SL, Freifeld AG. et al. Higher risk of cytomegalovirus and Aspergillus infections in recipients of T cell-depleted unrelated bone marrow: analysis of infectious complications in patients treated with T cell depletion versus immunosuppressive therapy to prevent graft-versus-host disease. Biol Blood Marrow Transplant 2007; 13: 1487–98. 10.1016/j.bbmt.2007.08.049 [DOI] [PubMed] [Google Scholar]
- 73. Williams KM, Ahn KW, Chen M. et al. The incidence, mortality and timing of Pneumocystis jiroveci pneumonia after hematopoietic cell transplantation: a CIBMTR analysis. Bone Marrow Transplant 2016; 51: 573–80. 10.1038/bmt.2015.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hardak E, Oren I, Dann EJ. et al. The increased risk for Pneumocystis pneumonia in patients receiving rituximab-CHOP-14 can be prevented by the administration of trimethoprim/sulfamethoxazole: a single-center experience. Acta Haematol 2012; 127: 110–4. 10.1159/000334113 [DOI] [PubMed] [Google Scholar]
- 75. Hughes WT, Feldman S, Aur RJ. et al. Intensity of immunosuppressive therapy and the incidence of Pneumocystis carinii pneumonitis. Cancer 1975; 36: 2004–9. 10.1002/cncr.2820360912 [DOI] [PubMed] [Google Scholar]
- 76. Blennow O, Remberger M, Torlen J. et al. Risk factors for invasive mold infections and implications for choice of prophylaxis after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2016; 22: 1684–9. 10.1016/j.bbmt.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 77. Davila ML, Sadelain M.. Biology and clinical application of CAR T cells for B cell malignancies. Int J Hematol 2016; 104: 6–17. 10.1007/s12185-016-2039-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kroschinsky F, Stolzel F, von Bonin S. et al. New drugs, new toxicities: severe side effects of modern targeted and immunotherapy of cancer and their management. Crit Care 2017; 21: 89.. 10.1186/s13054-017-1678-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Leick MB, Levis MJ.. The future of targeting FLT3 activation in AML. Curr Hematol Malig Rep 2017; 12: 153–67. 10.1007/s11899-017-0381-2 [DOI] [PubMed] [Google Scholar]
- 80. Kantarjian H. Acute myeloid leukemia—major progress over four decades and glimpses into the future. Am J Hematol 2016; 91: 131–45. [DOI] [PubMed] [Google Scholar]
- 81. Man LM, Morris AL, Keng M.. New therapeutic strategies in acute lymphocytic leukemia. Curr Hematol Malig Rep 2017; 12: 197–206. 10.1007/s11899-017-0380-3 [DOI] [PubMed] [Google Scholar]
- 82. van Leeuwen RW, van Gelder T, Mathijssen RH. et al. Drug interactions between tyrosine-kinase inhibitors and acid suppressive agents: more than meets the eye—Authors' reply. Lancet Oncol 2014; 15: e470–1. [DOI] [PubMed] [Google Scholar]
- 83. Petraitiene R, Petraitis V, Hope WW. et al. Intermittent dosing of micafungin is effective for treatment of experimental disseminated candidiasis in persistently neutropenic rabbits. Clin Infect Dis 2015; 61 Suppl 6: S643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Noxafil (posaconazole) [package insert]. Whitehouse Station, NJ: Merck & Co., Inc., 2014.
- 85.Cresemba (isavuconazonium sulfate) [package insert]. Northbrook, IL: Astellas Pharma, Inc., 2015.
- 86.Mycamine (micafungin sodium) [package insert]. Northbrook, IL: Astellas Pharma Inc., 2016.
- 87.Diflucan (fluconazole) [package insert]. New York, NY: Pfizer, Inc., 2014.
- 88. Marr KA, Leisenring W, Crippa F. et al. Cyclophosphamide metabolism is affected by azole antifungals. Blood 2004; 103: 1557–9. [DOI] [PubMed] [Google Scholar]
- 89. Bruggemann RJ, Alffenaar JW, Blijlevens NM. et al. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis 2009; 48: 1441–58. [DOI] [PubMed] [Google Scholar]
- 90. Moriyama B, Henning SA, Leung J. et al. Adverse interactions between antifungal azoles and vincristine: review and analysis of cases. Mycoses 2012; 55: 290–7. 10.1111/j.1439-0507.2011.02158.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sengar M, Dsouza S, Deshpande R. et al. Therapeutic drug monitoring of posaconazole in adult acute myeloid leukemia patients receiving posaconazole prophylaxis during induction: experience from a center with high invasive fungal infection burden. Abstract 4005. In: 58th Annual Meeting of the American Society of Hematology, San Diego, CA, USA, 2016.
- 92. Cattaneo C, Monte S, Algarotti A. et al. A randomized comparison of caspofungin versus antifungal prophylaxis according to investigator policy in acute leukaemia patients undergoing induction chemotherapy (PROFIL-C study). J Antimicrob Chemother 2011; 66: 2140–5. 10.1093/jac/dkr271 [DOI] [PubMed] [Google Scholar]
- 93. Kung HC, Johnson MD, Drew RH. et al. Clinical effectiveness of posaconazole versus fluconazole as antifungal prophylaxis in hematology-oncology patients: a retrospective cohort study. Cancer Med 2014; 3: 667–73. 10.1002/cam4.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Deslandes G, Chauvin C, Paré M. et al. Drug monitoring and treatment adherence of posaconazole tablet for prophylaxis of invasive fungal infection in haematological patients, a prospective monocentric observational study of 50 cases (POSANANTES). Abstract P1728 In: 27th European Congress of Clinical Microbiology and Infectious Disease, Vienna, Austria, 2017.
- 95. Moriyama B, Obeng AO, Barbarino J. et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP2C19 and voriconazole therapy. Clin Pharmacol Ther 2017; 102: 45–51. 10.1002/cpt.583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Clancy CJ, Nguyen MH.. Long-term voriconazole and skin cancer: is there cause for concern? Curr Infect Dis Rep 2011; 13: 536–43. [DOI] [PubMed] [Google Scholar]
- 97. Cohen N, Seo SK.. Role of antimicrobial prophylaxis during treatment of adults with acute leukemia. Int J Hematol Oncol 2015; 4: 77–86. 10.2217/ijh.15.7 [DOI] [Google Scholar]
- 98. Cornely OA, Leguay T, Maertens J. et al. Randomized comparison of liposomal amphotericin B versus placebo to prevent invasive mycoses in acute lymphoblastic leukaemia. J Antimicrob Chemother 2017; 72: 2359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Maertens J, Cesaro S, Maschmeyer G. et al. ECIL guidelines for preventing Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother 2016; 71: 2397–404. 10.1093/jac/dkw157 [DOI] [PubMed] [Google Scholar]
- 100. Ponce CA, Chabe M, George C. et al. High prevalence of Pneumocystis jirovecii dihydropteroate synthase gene mutations in patients with a first episode of Pneumocystis pneumonia in Santiago, Chile, and clinical response to trimethoprim-sulfamethoxazole therapy. Antimicrob Agents Chemother 2017; 61: pii=e01290-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chang CC, Slavin MA, Chen SC.. New developments and directions in the clinical application of the echinocandins. Arch Toxicol 2017; 91: 1613–21. 10.1007/s00204-016-1916-3 [DOI] [PubMed] [Google Scholar]
- 102. Hoffman JA, Walsh TJ.. Echinocandins in children. Pediatr Infect Dis J 2011; 30: 508–9. 10.1097/INF.0b013e31821b95e2 [DOI] [PubMed] [Google Scholar]
- 103. Denning DW. Echinocandin antifungal drugs. Lancet 2003; 362: 1142–51. 10.1016/S0140-6736(03)14472-8 [DOI] [PubMed] [Google Scholar]
- 104. Cushion MT, Linke MJ, Ashbaugh A. et al. Echinocandin treatment of pneumocystis pneumonia in rodent models depletes cysts leaving trophic burdens that cannot transmit the infection. PLoS One 2010; 5: e8524.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Linke MJ, Ashbaugh A, Collins MS. et al. Characterization of a distinct host response profile to Pneumocystis murina asci during clearance of Pneumocystis pneumonia. Infect Immun 2013; 81: 984–95. 10.1128/IAI.01181-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kurtz MB, Heath IB, Marrinan J. et al. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-β-d-glucan synthase. Antimicrob Agents Chemother 1994; 38: 1480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hohl TM, Feldmesser M, Perlin DS. et al. Caspofungin modulates inflammatory responses to Aspergillus fumigatus through stage-specific effects on fungal β-glucan exposure. J Infect Dis 2008; 198: 176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Antachopoulos C, Meletiadis J, Sein T. et al. Comparative in vitro pharmacodynamics of caspofungin, micafungin, and anidulafungin against germinated and nongerminated Aspergillus conidia. Antimicrob Agents Chemother 2008; 52: 321–8. 10.1128/AAC.00699-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pfaller MA, Boyken L, Hollis RJ. et al. In vitro susceptibility of clinical isolates of Aspergillus spp. to anidulafungin, caspofungin, and micafungin: a head-to-head comparison using the CLSI M38-A2 broth microdilution method. J Clin Microbiol 2009; 47: 3323–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lobo ML, Esteves F, de Sousa B. et al. Therapeutic potential of caspofungin combined with trimethoprim-sulfamethoxazole for Pneumocystis pneumonia: a pilot study in mice. PLoS One 2013; 8: e70619.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chen SC, Slavin MA, Sorrell TC.. Echinocandin antifungal drugs in fungal infections: a comparison. Drugs 2011; 71: 11–41. 10.2165/11585270-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 112.Cancidas (caspofungin acetate) [package insert]. Whitehouse Station, NJ: Merck & Co., Inc., 2017.
- 113.Eraxis (anidulafungin) [package insert]. New York, NY: Pfizer, Inc., 2006.
- 114. Gumbo T, Hiemenz J, Ma L. et al. Population pharmacokinetics of micafungin in adult patients. Diagn Microbiol Infect Dis 2008; 60: 329–31. 10.1016/j.diagmicrobio.2007.09.018 [DOI] [PubMed] [Google Scholar]
- 115. Yang Q, Wang T, Xie J. et al. Pharmacokinetic/pharmacodynamic adequacy of echinocandins against Candida spp. in intensive care unit patients and general patient populations. Int J Antimicrob Agents 2016; 47: 397–402. [DOI] [PubMed] [Google Scholar]
- 116. Andes D, Diekema DJ, Pfaller MA. et al. In vivo pharmacodynamic characterization of anidulafungin in a neutropenic murine candidiasis model. Antimicrob Agents Chemother 2008; 52: 539–50. 10.1128/AAC.01061-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ernst EJ, Roling EE, Petzold CR. et al. In vitro activity of micafungin (FK-463) against Candida spp.: microdilution, time-kill, and postantifungal-effect studies. Antimicrob Agents Chemother 2002; 46: 3846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Fleischhacker M, Radecke C, Schulz B. et al. Paradoxical growth effects of the echinocandins caspofungin and micafungin, but not of anidulafungin, on clinical isolates of Candida albicans and C. dubliniensis. Eur J Clin Microbiol Infect Dis 2008; 27: 127–31. [DOI] [PubMed] [Google Scholar]
- 119. Groll AH, Gullick BM, Petraitiene R. et al. Compartmental pharmacokinetics of the antifungal echinocandin caspofungin (MK-0991) in rabbits. Antimicrob Agents Chemother 2001; 45: 596–600. 10.1128/AAC.45.2.596-600.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gumbo T, Drusano GL, Liu W. et al. Once-weekly micafungin therapy is as effective as daily therapy for disseminated candidiasis in mice with persistent neutropenia. Antimicrob Agents Chemother 2007; 51: 968–74. 10.1128/AAC.01337-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Neofytos D, Huang YT, Cheng K. et al. Safety and efficacy of intermittent intravenous administration of high-dose micafungin. Clin Infect Dis 2015; 61 Suppl 6: S652–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Steinbach WJ, Lamoth F, Juvvadi PR.. Potential microbiological effects of higher dosing of echinocandins. Clin Infect Dis 2015; 61 Suppl 6: S669–77. [DOI] [PubMed] [Google Scholar]
- 123. Girmenia C, Iori AP.. An update on the safety and interactions of antifungal drugs in stem cell transplant recipients. Expert Opin Drug Saf 2017; 16: 329–39. 10.1080/14740338.2017.1273900 [DOI] [PubMed] [Google Scholar]
- 124. Hindahl CB, Wilson JW.. Flash pulmonary oedema during anidulafungin administration. J Clin Pharm Ther 2012; 37: 491–3. 10.1111/j.1365-2710.2011.01309.x [DOI] [PubMed] [Google Scholar]
- 125. Lichtenstern C, Wolff M, Arens C. et al. Cardiac effects of echinocandin preparations - three case reports. J Clin Pharm Ther 2013; 38: 429–31. 10.1111/jcpt.12078 [DOI] [PubMed] [Google Scholar]
- 126. Fink M, Zerlauth U, Kaulfersch C. et al. A severe case of haemodynamic instability during anidulafungin administration. J Clin Pharm Ther 2013; 38: 241–2. 10.1111/jcpt.12046 [DOI] [PubMed] [Google Scholar]
- 127. Cleary JD, Stover KR.. Antifungal-associated drug-induced cardiac disease. Clin Infect Dis 2015; 61 Suppl 6: S662–8. [DOI] [PubMed] [Google Scholar]
- 128. Perlin DS. Mechanisms of echinocandin antifungal drug resistance. Ann N Y Acad Sci 2015; 1354: 1–11. 10.1111/nyas.12831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Alexander BD, Johnson MD, Pfeiffer CD. et al. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 2013; 56: 1724–32. 10.1093/cid/cit136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Farmakiotis D, Tarrand JJ, Kontoyiannis DP.. Drug-resistant Candida glabrata infection in cancer patients. Emerg Infect Dis 2014; 20: 1833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. van Burik JA, Ratanatharathorn V, Stepan DE. et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis 2004; 39: 1407–16. 10.1086/422312 [DOI] [PubMed] [Google Scholar]
- 132. Hashino S, Morita L, Takahata M. et al. Administration of micafungin as prophylactic antifungal therapy in patients undergoing allogeneic stem cell transplantation. Int J Hematol 2008; 87: 91–7. 10.1007/s12185-007-0011-1 [DOI] [PubMed] [Google Scholar]
- 133. Hiramatsu Y, Maeda Y, Fujii N. et al. Use of micafungin versus fluconazole for antifungal prophylaxis in neutropenic patients receiving hematopoietic stem cell transplantation. Int J Hematol 2008; 88: 588–95. 10.1007/s12185-008-0196-y [DOI] [PubMed] [Google Scholar]
- 134. Park S, Kim K, Jang JH. et al. Randomized trial of micafungin versus fluconazole as prophylaxis against invasive fungal infections in hematopoietic stem cell transplant recipients. J Infect 2016; 73: 496–505. 10.1016/j.jinf.2016.06.011 [DOI] [PubMed] [Google Scholar]
- 135. Huang X, Chen H, Han M. et al. Multicenter, randomized, open-label study comparing the efficacy and safety of micafungin versus itraconazole for prophylaxis of invasive fungal infections in patients undergoing hematopoietic stem cell transplant. Biol Blood Marrow Transplant 2012; 18: 1509–16. 10.1016/j.bbmt.2012.03.014 [DOI] [PubMed] [Google Scholar]
- 136. Vehreschild MJ, von Bergwelt-Baildon M, Tran L. et al. Feasibility and effectiveness of posaconazole prophylaxis in combination with micafungin bridging for patients undergoing allogeneic stem cell transplantation: a 6-yr analysis from the Cologne Cohort for Neutropenic Patients. Eur J Haematol 2014; 93: 400–6. 10.1111/ejh.12368 [DOI] [PubMed] [Google Scholar]
- 137. Mattiuzzi GN, Alvarado G, Giles FJ. et al. Open-label, randomized comparison of itraconazole versus caspofungin for prophylaxis in patients with hematologic malignancies. Antimicrob Agents Chemother 2006; 50: 143–7. 10.1128/AAC.50.1.143-147.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Gomes MZ, Jiang Y, Mulanovich VE. et al. Effectiveness of primary anti-Aspergillus prophylaxis during remission induction chemotherapy of acute myeloid leukemia. Antimicrob Agents Chemother 2014; 58: 2775–80. 10.1128/AAC.01527-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Huang Y, Aufiero K, Halton E. et al. Micafungin vs posaconazole prophylaxis in adult patients with acute leukemia undergoing induction chemotherapy. Abstract T-2033. In: 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, USA, 2014.
- 140. Lee CH, Lin JC, Ho CL. et al. Efficacy and safety of micafungin versus extensive azoles in the prevention and treatment of invasive fungal infections for neutropenia patients with hematological malignancies: a meta-analysis of randomized controlled trials. PLoS One 2017; 12: e0180050.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Andes DR, Reynolds DK, Van Wart SA. et al. Clinical pharmacodynamic index identification for micafungin in esophageal candidiasis: dosing strategy optimization. Antimicrob Agents Chemother 2013; 57: 5714–6. 10.1128/AAC.01057-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Mehta PA, Vinks AA, Filipovich A. et al. Alternate-day micafungin antifungal prophylaxis in pediatric patients undergoing hematopoietic stem cell transplantation: a pharmacokinetic study. Biol Blood Marrow Transplant 2010; 16: 1458–62. 10.1016/j.bbmt.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 143. Ota Y, Tatsuno K, Okugawa S. et al. Relationship between the initial dose of micafungin and its efficacy in patients with candidemia. J Infect Chemother 2007; 13: 208–12. 10.1007/s10156-007-0522-Y [DOI] [PubMed] [Google Scholar]
- 144. Sirohi B, Powles RL, Chopra R. et al. A study to determine the safety profile and maximum tolerated dose of micafungin (FK463) in patients undergoing haematopoietic stem cell transplantation. Bone Marrow Transplant 2006; 38: 47–51. 10.1038/sj.bmt.1705398 [DOI] [PubMed] [Google Scholar]
- 145. Lepak AJ, Marchillo K, Andes DR.. Pharmacodynamic target evaluation of a novel oral glucan synthase inhibitor, SCY-078 (MK-3118), using an in vivo murine invasive candidiasis model. Antimicrob Agents Chemother 2015; 59: 1265–72. 10.1128/AAC.04445-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lamoth F, Alexander BD.. Antifungal activities of SCY-078 (MK-3118) and standard antifungal agents against clinical non-Aspergillus mold isolates. Antimicrob Agents Chemother 2015; 59: 4308–11. 10.1128/AAC.00234-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Pfaller MA, Messer SA, Motyl MR. et al. In vitro activity of a new oral glucan synthase inhibitor (MK-3118) tested against Aspergillus spp. by CLSI and EUCAST broth microdilution methods. Antimicrob Agents Chemother 2013; 57: 1065–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Pfaller MA, Messer SA, Motyl MR. et al. Activity of MK-3118, a new oral glucan synthase inhibitor, tested against Candida spp. by two international methods (CLSI and EUCAST). J Antimicrob Chemother 2013; 68: 858–63. [DOI] [PubMed] [Google Scholar]
- 149. ClinicalTrials.gov. Open-Label Study to Evaluate Efficacy and Safety of SCY-078 in Patients With Refractory or Intolerant Fungal Diseases (FURI) https://clinicaltrials.gov/ct2/show/NCT03059992.
- 150. Sandison T, Ong V, Lee J. et al. Safety and pharmacokinetics of CD101 IV, a novel echinocandin, in healthy adults. Antimicrob Agents Chemother 2017; 61: e01627–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhao Y, Perez WB, Jimenez-Ortigosa C. et al. CD101: a novel long-acting echinocandin. Cell Microbiol 2016; 18: 1308–16. 10.1111/cmi.12640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Pfaller MA, Messer SA, Rhomberg PR. et al. Activity of a long-acting echinocandin (CD101) and seven comparator antifungal agents tested against a global collection of contemporary invasive fungal isolates in the SENTRY 2014 Antifungal Surveillance Program. Antimicrob Agents Chemother 2017; 61: e02045–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Pfaller MA, Messer SA, Rhomberg PR. et al. Activity of a long-acting echinocandin, CD101, determined using CLSI and EUCAST reference methods, against Candida and Aspergillus spp., including echinocandin- and azole-resistant isolates. J Antimicrob Chemother 2016; 71: 2868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ong V, Bartizal K, Miesel L. Efficacy of CD101, a novel echinocandin, in mouse models of aspergillosis and azole-resistant disseminated candidiasis. Abstract 3400. In: 58th Annual Meeting of the American Society of Hematology, San Diego, CA, USA.
- 155. Ong V, Bartizal K, Lopez S. et al. Prophylactic, single-dose, subcutaneous (SC) administration of CD101 shows robust efficacy in neutropenic mouse models of candidiasis and aspergillosis. Abstract EP0703. In: 27th European Congress of Clinical Microbiology and Infectious Disease, Vienna, Austria, 2017.
- 156. Cushion M, Ashbaugh A, Lynch K. et al. Efficacy of CD101, a novel echinocandin, in Prevention of Pneumocystis Pneumonia (PCP): thwarting the biphasic life cycle of pneumocystis. Abstract 3396. In: American Society of Hematology 58th Annual Meeting and Exposition, San Diego, CA, USA, 2016.
- 157. STRIVE : CD101 Compared to Caspofungin Followed by Oral Step Down Treatment in Subjects with Candidemia (ClinicalTrials.gov Identifier: NCT02734862). https://clinicaltrials.gov/ct2/show/NCT02734862.
- 158. Nachbaur D, Angelova O, Orth-Holler D. et al. Primary antifungal prophylaxis with micafungin in patients with haematological malignancies: real-life data from a retrospective single-centre observational study. Eur J Haematol 2015; 94: 258–64. 10.1111/ejh.12426 [DOI] [PubMed] [Google Scholar]