Abstract
Background
Breast cancer survivors rank fatigue (e.g., decreased vitality) as their number one concern affecting quality of life. Excess adiposity is associated with decreased vitality in breast cancer survivors, yet weight loss intervention trials report inconsistent effects on this parameter.
Methods
This is a secondary analysis of the Exercise and Nutrition to Enhance Recovery and Good Health for You trial, in which 692 overweight or obese breast cancer survivors ≤5 years from diagnosis, initiated weight loss interventions, and completed assessments semi-annually for 2 years. Assessments included the Godin Leisure-Time Exercise Questionnaire and the SF-36 MOS vitality subscale as an inverse measure of fatigue. Multilevel structural equation models estimated the direct effects of physical activity on vitality and indirect effects through body mass index (BMI) changes.
Results
Within-person findings show that at assessments with greater physical activity, BMI was significantly lower (B = −0.07, p < 0.001) and vitality was higher (B = 0.22, p < 0.001). However, there was no direct relationship between lower BMI and higher vitality (B = −0.11, p = 0.262) after controlling for the relationship of physical activity with BMI and physical activity with vitality. The between-person indirect effect of physical activity change through BMI change to vitality was significant (B = 0.03, p < 0.001). Participants whose physical activity was above the mean (B = 0.37, p < 0.001) and whose BMI was below the mean (B = −1.05, p < 0.001) were more likely to report greater vitality.
Conclusion
Improvements in vitality are primarily associated with increases in physical activity rather than BMI changes in this trial. Vitality was lower among survivors with higher BMI, although within-individual changes in BMI had no effect on vitality. Physical activity and weight loss share mechanistic links to vitality with physical activity potentially increasing (e.g., in an additive or synergistic manner) the effect of BMI reduction on vitality.
Keywords: Fatigue, Weight loss, Breast cancer survivor, Physical activity
Improvements in vitality are primarily associated with increases in physical activity rather than BMI changes in a weight loss intervention trial among breast cancer survivors.
Introduction
More than 3 million women in the USA are living with a history of breast cancer [1]. Many of these women experience persistent fatigue (e.g., decreased vitality) months to years after their cancer diagnosis, which exacerbates postcancer disability and reduces quality of life [2–5]. Importantly, breast cancer survivors rank decreased vitality as the number one patient-reported outcome of priority related to quality of life [6]. Inflammation and impairments in immune response, hypothalamic-pituitary axis, neuroendocrine, and metabolic function that are initiated and potentially moderated by cancer therapies have been associated with loss of vitality [7–11]. These are contributors in addition to demographic characteristics (e.g., age), cognitive-behavioral symptoms, excess body weight, and reduced physical endurance that are also notable contributors to this complaint [7, 12]. However, optimizing therapeutic strategies for increasing vitality in cancer survivors is limited by an incomplete knowledge of the complex, multifactorial etiologies responsible for vitality loss. Hence, the National Cancer Institute has identified this knowledge gap as a high research priority [11, 13].
Multiple randomized trials have reported beneficial effects of aerobic physical activity on vitality in cancer survivors [7, 14]. Based on the biobehavioral theoretical model of vitality loss (conceptualized as fatigue) proposed by Al-Majid and Gray [12], exercise after a cancer diagnosis prevents, reverses, or attenuates the vitality loss through biological, psychobehavioral, and functional mechanisms. Although several of these mechanisms may also play a role in the association between excess body weight and vitality in breast cancer survivors [7], no prior model related to this symptom has specifically integrated the potential differential effects of physical activity and weight loss. This absence is noteworthy because weight loss intervention trials have reported inconsistent effects on vitality [15, 16]. Moreover, little is known about the independent effects of physical activity and body weight as exemplified by a single cross-sectional study reporting that body mass index (BMI) and physical activity were independently associated with vitality in endometrial cancer survivors [17]. A better understanding of these relationships in longitudinal cohorts in which body weight and physical activity are changing over time will improve our knowledge base and could result in novel therapeutic targets. Furthermore, the etiologic factors proposed in multiple theoretical models related to feelings of vitality (including those not cancer-specific) have applicability to other chronic conditions [18].
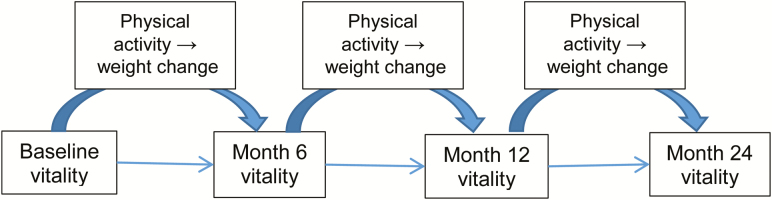
Secondary analyses in large prospective cohorts provide a cost-efficient opportunity to address this limitation. The Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) trial enrolled 692 overweight or obese breast cancer survivors within 5 years of diagnosis, initiated participation in weight loss interventions, and completed assessments semi-annually for 2 years [19]. The primary goal was to achieve and maintain 7% weight loss and 60 minutes of moderate physical activity per day. Participants were randomized to receive a high intensity, 52-contact counseling intervention for weight loss (group, telephone, and email counseling with print materials), or a less-intensive, two-contact session attention control condition that provided similar diet and exercise advice [19]. At 6 months, a significantly higher percentage of body weight was lost in the intensive versus the nonintensive arms (−6.3 ± 0.02% vs. −1.25 ± 0.02%; p < 0.0001); differences remained statistically and clinically significant at 24 months (−4.25 ± 0.03% vs. −1.43 ± 0.03%; p < 0.0001). Moreover, women within the intensive arm reported a baseline to 6-month increase in the SF-36 vitality subscale, going from a mean (standard error of measurement) value of 60.5 (1.36) to 65.1 (1.20), whereas the increase among the nonintensive arm was more modest, that is, from 60.5 (1.37) at baseline to 62.4 (1.23) at 6 months [15]. Importantly, the within-group mean change in vitality, although modest, reached a threshold of clinical significance (p = 0.0508) [19]. The SF-36 vitality subscale has been validated as an inverse measure of fatigue, making it a useful general measure of energy and fatigue [20]. The ENERGY trial’s prospective design, large sample size, and adequate variability in vitality offer a unique opportunity to apply advanced statistical procedures (e.g., multilevel structural equation modeling) to identify the independent effects of BMI reduction versus increased physical activity on the trajectory of vitality in breast cancer survivors. We hypothesized that physical activity and BMI changes could serve as potential mediators of the relationship between the initial status of vitality and the change in vitality by study end as described in the heuristic model provided in Fig. 1 (i.e., physical activity would be directly related to vitality and indirectly related through the mediating effect of BMI).
Fig. 1.
A heuristic conceptual model for testing weight change and physical activity predictors of vitality during a weight loss trial in breast cancer survivors.
Methods
ENERGY was a two-arm, single-blinded randomized controlled trial, the methods and primary outcomes of which have been published previously [19, 21]. The study was conducted at four sites across the USA (the University of California, San Diego, the University of Colorado, Washington University in St Louis, and the University of Alabama at Birmingham) and was approved and monitored by the institutional review boards at each institution, as well as an independent Data Safety and Monitoring Board.
Study participants were adult women, age 21 years or older with a diagnosis of breast cancer within the previous 5 years. Eligible women were those with stage IC-IIIB disease who had completed primary treatment, though some were still receiving endocrine therapy. All participants were either overweight or obese, BMI of 25 to 45 kg/m2. Exclusion criteria were as follows: diagnoses of other malignancies besides breast cancer and nonmelanoma skin cancer, serious psychiatric illness, and any medical condition or circumstances substantially limiting moderate physical activity or ability to adhere or respond to either of the interventions. All eligible women provided written consent and were randomly assigned to either the intensive or nonintensive (control) intervention arms. The study sample for this secondary analysis included 432 women with data available on the primary variables examined in this study through the evaluated time points of baseline, 6 months, 12 month, and 24 months (physical activity, BMI, vitality), as the 18-month ENERGY assessments did not include the vitality (SF-36) measure. The measures examined in this secondary analysis are described below.
Measures
Covariates
Data on cancer stage, date and age at diagnosis, and type of treatment were obtained from cancer registries and/or excerpted from the participant’s medical record. Participants self-reported their menopausal status at baseline. Comorbidities were ascertained using the Lifetime Medical Conditions Questionnaire, which measures the presence of 13 common medical conditions at the time of assessment [22]. Comorbidity was summarized as 0, 1 condition, or 2 or more conditions. Other self-reported covariates included smoking status at baseline categorized as yes/no, beta-blocker/calcium channel blocker use at baseline (yes/no), highest educational attainment, and race and ethnicity. Study arm also was examined as a covariate.
The Center for Epidemiologic Studies Depression Scale was used to assess depressive symptomology and asks participants how often in the past week they have experienced the symptom (responses range from “rarely or none” to “most or all days”) [23, 24]. The scale is scored by tallying all 20 items with a possible range of 0–60 (higher scores indicating more depressive symptoms). The Breast Cancer Prevention Trial Symptom Scales measured concurrent and late effects of cancer and its treatment [25]. Eighteen items measure the degree to which eight domains of symptoms bother a survivor “on average, over the last 4 weeks”: hot flashes (two items), nausea (two items), bladder control (two items), vaginal problems (two items), musculoskeletal pain (three items), cognitive problems (three items), weight problems (two items), and arm problems (two items) [25]. Response options range from “not at all” to “extremely.” Total sum scores of the depression and symptom scales were used as continuous time-varying covariates.
Primary Independent (Exposure) Variable (X)
The primary independent variable (e.g., factor initiating the hypothesized chain of events) for this longitudinal mediation analysis was physical activity as measured by the modified Godin Leisure-Time Exercise Questionnaire (i.e., physical activity as a result of engagement in the intervention effects on vitality independently [direct effect] and through its effects on BMI [indirect effect]). The instrument captures data on both frequency and duration of moderate-to-vigorous physical activity, which were used to calculate weekly minutes of moderate + vigorous physical activity [26]. Physical activity was chosen as the primary independent variable to determine its relationship with vitality independent of and mediated by BMI changes occurring with the intervention.
Mediator (M) Responsible for the Indirect Effect of the Primary Independent Variable on the Outcome Measure
Protocols established for NHANES III were adopted for BMI assessment in this study [27]. Height was measured without shoes at baseline only using a fixed stadiometer upon inhale. Weight was measured in light clothing and without shoes using a calibrated scale. BMI was then calculated for each time point and used as a continuous variable. BMI was hypothesized as a potential mediator of vitality because it was the primary outcome for the original ENERGY trial and has demonstrated associations with vitality and physical activity in prior studies [7, 17, 28].
Outcome Measure (Y)
The primary outcome of energy/fatigue was measured using the four-item vitality subscale of the Medical Outcomes Survey Short Form—36-item questionnaire (SF-36) [29]. The four items were preceded by “How much of the time during the past four weeks…” and include the following questions: “did you feel full of pep?”; “did you have a lot of energy?”; “did you feel worn out?”; and “did you feel tired?”. Item responses range from all of the time to none of the time, and the subscale is scored according to published methods, with higher values indicate greater energy and less fatigue. Because vitality was measured at baseline, 6 months, 12 months, and 24 months, we focus on those measurement points for analysis.
Statistical Analysis
Frequencies and means and standard deviations were evaluated for categorical and continuous covariates measured at baseline, respectively. Univariate analyses were conducted at each time point for the independent variable (originating mediator of the intervention effects; moderate-to-vigorous physical activity), mediator of the independent variable (BMI), and outcome (vitality). Pearson correlations were also conducted for both the within-person and between-person levels.
Multilevel structural equation models were used to estimate the direct effects of physical activity (X) on vitality (Y) and indirect effects through BMI (M). A multilevel approach was used to account for between-person (level 2; j) and within-person (level 1; i) effects (Fig. 1) that are present in repeated measures [30, 31]. The advantages of multilevel structural equation models over regular multilevel regression analyses include more effective estimation of complex models at multiple levels of analysis, allow for examination of the contextual effects across the multiple levels, and allow for testing of direct and indirect effects at both the within- and between-person levels.
We used person-mean centering for the exogenous variables (physical activity and BMI) to establish between-person effects and facilitate interpretation. Overall and time point-specific means of physical activity (X) and BMI (M) were calculated for each person. Level 2 (between-person) is equal to the person-mean centered and Level 1 is the person mean. Covariates included age at survey (continuous), study arm, education, race/ethnicity, stage at diagnosis, treatment received, smoking status, self-reported use of beta-blocker/calcium channel blocker, and comorbidities as dummy variables. Time-varying covariates included continuous measures of depression and symptom scores.
The first step was to fit the between-person and within-person relationship of physical activity (X) to BMI (M). In the second step, we fit the between- and within-person relationship between physical activity (X) and vitality (Y). Next, we fit the between- and within-person relationship between BMI (M) and vitality (Y). Finally, we fit the full between- and within-person physical activity (X) to BMI (M) to vitality (Y) mediation model. Indirect effects were estimated as products of coefficients linking physical activity (X) to BMI (mediator) and to vitality (outcome) [32, 33]. Random intercepts were used, allowing correlation among time points. Monte Carlo bootstrapping was used to construct 95% confidence and test for significance [34]. SAS V9.4 (Cary, NC) was used for data management, and Mplus V7.4 with TYPE=TWOLEVEL was used for the multilevel structural equation model [35].
Alternative models
We tested four alternative models to the one presented in this article. Although we chose BMI because it was the primary outcome for the ENERGY trial, it was possible that body weight change or change in percent body weight may have provided greater variability, thus improving the model. Hence, the first alternative model included weight (in pounds) as the mediator instead of BMI and the second alternative model included percent weight change from baseline. Also, weight loss can contribute to greater physical activity uptake by reducing the work load of weight-bearing physical activity. As such, the third alternative model examined the potential for BMI to serve as the independent variable and physical activity to act as mediator with a similar fourth alternative model including body weight as the independent variable, instead of BMI. We compared Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) of the original model with the alternative models (lower values indicate better fit).
Results
Sample Characteristics
Sample characteristics are provided in Table 1. The mean age at baseline was 57 (SD = 9.1) years. The majority of the participants were non-Hispanic White and had a college education or higher. Approximately one third were former smokers, 31% had one comorbidity, and the majority reported using a beta-blocker or calcium channel blocker at baseline. Half of the participants were diagnosed at Stage II, and most received both chemotherapy and radiation therapy.
Table 1.
Sample characteristics
| Overall (N = 692) | Analytic sample (N = 432) | |
|---|---|---|
| Age at survey | ||
| Mean | 56.2 | 57.3 |
| Standard deviation (SD) | 9.5 | 9.1 |
| Range | 30–82 | 30–82 |
| Months between primary treatment and study entry | ||
| Mean | 25.2 | 26.0 |
| SD (range) | 16 (0–68) | 16.5 (0–68) |
| Race/ethnicity | ||
| Non-Hispanic White | 547 (79.0) | 343 (79.4) |
| African American | 71 (10.3) | 38 (8.8) |
| Hispanic | 46 (6.6) | 30 (6.9) |
| Mixed/other | 28 (4.0) | 19 (4.4) |
| Education | ||
| High school or less | 99 (14.3) | 58 (13.4) |
| More than high school, but not college graduate | 183 (26.5) | 112 (25.9) |
| College graduate | 190 (27.5) | 108 (25.0) |
| Postgraduate degree | 220 (31.8) | 154 (35.7) |
| Smoking status | ||
| Never | 448 (64.7) | 281 (65.1) |
| Former | 220 (31.8) | 139 (32.2) |
| Current | 24 (3.5) | 12 (2.8) |
| Stage at diagnosis | ||
| I | 210 (30.4) | 147 (34.0) |
| II | 358 (51.7) | 214 (49.5) |
| III | 124 (17.9) | 71 (16.4) |
| Treatment received | ||
| Neither radiation nor chemotherapy | 54 (7.8) | 32 (7.4) |
| Chemotherapy only | 136 (19.7) | 85 (19.7) |
| Radiation only | 111 (16.0) | 79 (18.3) |
| Both chemotherapy and radiation | 391 (56.5) | 236 (54.8) |
| Comorbidities | ||
| 0 | 153 (22.1) | 92 (21.3) |
| 1 | 205 (29.6) | 135 (31.3) |
| 2+ | 334 (48.3) | 205 (47.4) |
| Medication | ||
| Beta-blocker/calcium channel blocker use at baseline | 410 (59.3) | 248 (57.0) |
| Breast cancer symptom scale at baseline | ||
| Mean (SD) | 2.1 (0.5) | 2 (0.5) |
| Range | 1–4 | 1–4 |
| Depression scale (CES-D) at baseline | ||
| Mean (SD) | 10.6 (7.1) | 9.8 (6.8) |
| Range | 2–49 | 2–49 |
Means, Standard Deviation, and Pearson Correlations
Table 2 shows physical activity, BMI, and vitality, which demonstrated significant but small-to-moderate correlations at baseline. The strength of correlation between these variables declined over the assessment periods, but correlations between time points within a measure remained significant.
Table 2.
Correlations, means, and standard deviations of within-person and between-person measures
| Within-person | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. PA1a | 2. PA2 | 3. PA3 | 4. PA4 | 5. BM1b | 6. BM2 | 7. BM3 | 8. BM4 | 9. VA1c | 10. VA2 | 11. VA3 | 12. VA4 | |
| 1 | 1 | |||||||||||
| 2 | −0.21*** | 1 | ||||||||||
| 3 | −0.38*** | −0.08 | 1 | |||||||||
| 4 | −0.20*** | −0.47*** | −0.27 | 1 | ||||||||
| 5 | −0.29*** | 0.05 | 0.20*** | −0.06 | 1 | |||||||
| 6 | 0.08 | −0.20*** | −0.06 | 0.10 | −0.04 | 1 | ||||||
| 7 | 0.19*** | −0.08 | −0.31*** | 0.15** | −0.60*** | 0.18*** | 1 | |||||
| 8 | 0.62 | 0.11 | 0.07 | −0.12* | −0.38*** | −0.59*** | −0.22*** | 1 | ||||
| 9 | 0.12* | 0.04 | −0.10 | −0.06 | −0.05 | 0.02 | 0.09 | −0.05 | 1 | |||
| 10 | −0.09 | 0.10 | 0.07 | −0.08 | 0.19*** | −0.06 | −0.15** | −0.02 | 0.59*** | 1 | ||
| 11 | 0.01 | −0.02 | 0.12 | −0.06 | 0.13* | 0.04 | −0.11* | −0.07 | 0.57*** | 0.68*** | 1 | |
| 12 | −0.06 | −0.08 | −0.08 | 0.15*** | 0.03 | 0.11* | 0.01 | −0.12* | 0.50*** | 0.58*** | 0.62*** | 0.90*** |
| Visit 1 | Visit 2 | Visit 3 | Visit 4 | |||||||||
| PA | Mean | −8.3 | 5.9 | 3.17 | −0.07 | |||||||
| SD | 14.1 | 15.0 | 13.3 | 13.5 | ||||||||
| BMI | Mean | 1.0 | −0.29 | −0.4 | −0.08 | |||||||
| SD | 1.6 | 0.94 | 0.93 | 1.1 | ||||||||
| VA | Mean | 61.1 | 67.3 | 64.9 | 64.5 | |||||||
| SD | 20.1 | 18.2 | 19.5 | 19.7 | ||||||||
| Between person | ||||||||||||
| Physical activity | BMI | |||||||||||
| Physical activity | 1 | |||||||||||
| BMI | −0.26*** | 1 | ||||||||||
| Vitality | 0.28*** | −0.12*** | ||||||||||
| Mean | SD | |||||||||||
| Physical activity | 22.8 | 15.5 | ||||||||||
| BMI | 30.8 | 4.8 | ||||||||||
| Vitality | 62.2 | 20.3 | ||||||||||
aPA1–PA4 = moderate-to-vigorous physical activity at time points 1 to 4.
bBM1–BM4 = BMI from time points 1 to 4.
cVA1–VA4: vitality scores from time points 1–4.
*p < 0.05; **p < 0.01; ***p < 0.001.
BMI = body mass index.
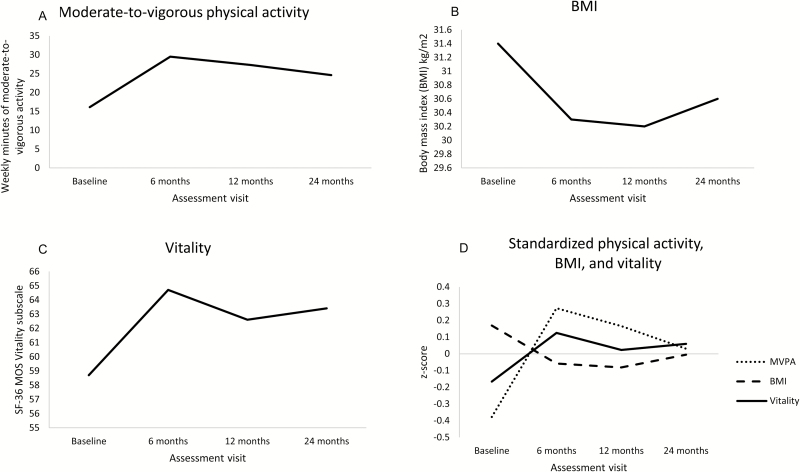
The mean vitality score increased from 61 to 67 from baseline to 6 months and then declined to 64.9, and 64.5 for 12 and 24 months (Table 2 and Fig. 2C). Physical activity also showed a similar pattern of increasing from baseline to 6 months, declining at 12 months, and stabilizing at 24 months (Fig. 2A). The mean BMI declined from baseline to 12 months while showing a slight increase at 24 months (Fig. 2B). Fig. 2D shows the z scores of each measure at each time point, demonstrating that while rates of increase or decline were variable over time, higher vitality scores were reported with greater physical activity and lower BMI.
Fig. 2.
Mean scores of moderate-to-vigorous physical activity (MVPA), body mass index (BMI), vitality. (A) Mean physical activity score across time points; (B) mean BMI score; (C) mean SF-36 vitality score; (D) standardized z scores of physical activity, BMI, vitality.
At baseline, vitality was significantly correlated with depressive symptoms (r = −0.57, p < 0.0001) and breast cancer symptom scores (r = −0.50, p < 0.0001; results not presented in table). Correlations at 6 months, 12, and 24 remained similar for depressive symptom (6 month r = −0.60, 12 month r = −0.61, 24 month r = −0.67, all p < 0.0001) and breast cancer symptom scores (6 month r = −0.53, 12 month r = −0.49, 24 month r = −0.52, all p < 0.0001).
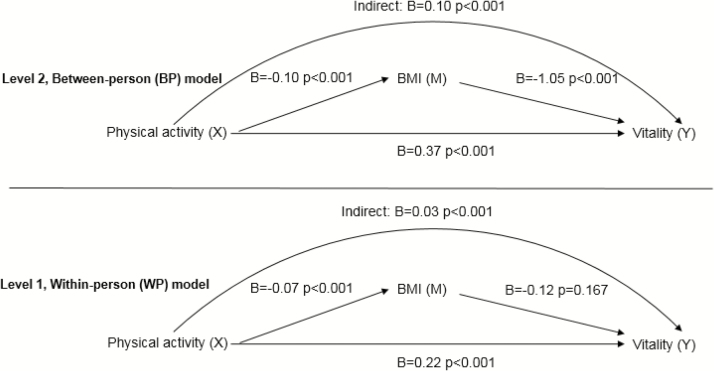
Within-Person Model
The standardized path estimates of within-person model are shown in Table 3 and Fig. 3, Level 1. Findings show that at assessments where participants reported higher physical activity, BMI was significantly lower (B = −0.07, p < 0.001) and vitality was significantly higher (B = 0.22, p < 0.001). However, there was no direct relationship between lower BMI and higher vitality (B = −0.12, p = 0.167) after controlling for the relationship of physical activity with BMI and physical activity with vitality. The within-person indirect effect of physical activity change through BMI change to vitality was significant (B = 0.03, p < 0.001). Table 3 displays the 95% confidence intervals for all effects.
Table 3.
Direct and indirect effects
| Between person | p value | Within person | p value | |
|---|---|---|---|---|
| Beta 95% CI | Beta 95% CI | |||
| Step 1 | ||||
| PA (X) → BMI (M) | ||||
| Direct | −0.09 (−0.11, −0.07) | <0.0001 | −0.06 (−0.08, −0.05) | <0.0001 |
| Step 2 | ||||
| PA (X) → vitality (Y) | ||||
| Direct | 0.41 (0.35, 0.46) | <0.0001 | 0.23 (0.18, 0.28) | <0.0001 |
| Step 3 | ||||
| BMI (M) → Vitality (Y) | ||||
| Direct | −1.74 (−2.21, −1.28) | <0.0001 | −0.26 (−0.44, −0.07) | <0.0001 |
| Step 4: Full | ||||
| PA (X) → BMI (M) → Vitality (Y) | ||||
| Direct: PA (X) → BMI (M) | −0.10 (−0.12, −0.08) | <0.0001 | −0.07 (−0.09, −0.05) | <0.0001 |
| Direct: PA (X)→ Vitality (Y) | 0.37 (0.31, 0.43) | <0.0001 | 0.22 (0.17, 0.27) | <0.0001 |
| Direct: BMI (M) → Vitality (Y) | −1.05 (−1.43, −0.68) | <0.0001 | −0.12 (−0.28, 0.05) | 0.253 |
| Indirect: PA (X) → BMI (M) → Vitality (Y) | 0.10 (0.06,0.14) | <0.0001 | 0.03 (0.02, 0.04) | <0.0001 |
| Contextual indirect | 0.011 (−0.004, 0.019) | 0.262 |
BMI = body mass index; M: Mediator; PA = moderate-to-vigorous physical activity; X = independent variable; Y = outcome variable.
Fig. 3.
Full mediation model (X->M->Y).
Between-Person Model
Table 3 and Fig. 3, Level 2 display the standardized path estimates of the between-person model. Participants with higher than average physical activity (B = 0.37, p < 0.001) and lower than average BMI (−1.05, p < 0.001) were more likely to have greater vitality. Furthermore, the between-person indirect effect of physical activity change through BMI change to vitality was significant (B = 0.10, p < 0.001).
The contextual indirect effect was not significant, indicating that some of the significant mediation is reduced after controlling for repeating physical activity measurements and repeating BMI measurements (B = 0.01, p = 0.262). In the full model of BMI-to-vitality relationship, there was significant between-person effect and no significant within-person effect. Thus, vitality was lower, on average, for persons reporting higher BMI compared with others, but vitality on a given visit was not related to whether BMI was higher than usual on that visit.
Alternative Models
Results for alternative models were consistent with the model presented, though the model fit indices were not as strong. The first and second alternative models with weight and percent body weight change as mediators demonstrated that there were still indirect effects of physical activity through weight or percent weight change. The third and fourth alternative models demonstrated that BMI and weight were directly associated with vitality as the independent variable (X) and that there was an indirect effect of BMI and weight on vitality through physical activity during the intervention. Our original model had improved AIC and BIC values compared with the four alternative models. Values of the original model (AIC: 29,645; BIC: 29,823) were lower than the first (AIC: 40,148; BIC: 40,326), second (AIC: 34,932; BIC: 35,110), third (AIC: 42,028; BIC: 42,206), and fourth models (AIC: 41,912; BIC: 42,090).
Discussion
Findings from our mediation analysis demonstrate that improvements in vitality scores during the ENERGY weight loss intervention are primarily associated with increases in physical activity rather than changes in BMI. This physical activity effect on vitality was partially due to (i.e., mediated by) a lower BMI. Our mediation findings suggest that the magnitude of differences was greater between survivors rather than across an individual survivor’s assessments. This is consistent with the attenuation of cross-sectional associations when tested in prospective study designs in behavioral change research [36]. Overall, results support targeting physical activity as a point of intervention to increase vitality (decrease fatigue) directly during weight loss, with a small indirect effect occurring through moderate weight loss.
Our results are consistent with a cross-sectional study in endometrial cancer survivors reporting that BMI and physical activity were both independently associated with vitality [37]. Our findings also are consistent with meta-analyses of exercise and cancer trials supporting greater improvements in vitality with better exercise adherence [38, 39]. We expanded from these studies by assessing the pathways through which physical activity and BMI changes are associated with vitality. While BMI was not directly associated with vitality at the within-person level in the full mediation model, it should be recognized that there is greater potential to make changes in physical activity of much greater magnitude than for BMI between any two time points, unless drastic weight loss is pursued via bariatric surgery or very low calorie diets, which was not the approach in ENERGY. Given the relationships among physical activity, BMI, and vitality reported herein, the inconsistent effects of weight loss on vitality in cancer survivors may be due, in part, to insufficient levels of exercise adherence achieved in the trials and/or the less prominent role of weight loss in the etiology of vitality. However, further research is needed to better elucidate these relationships for the purpose of optimizing intervention effects on vitality.
Importantly, our data suggest biological, psychobehavioral, and functional mechanisms underlying vitality response that are common to and unique for physical activity and weight loss. Weight loss in obese individuals may increase vitality by decreasing inflammation, work load during weight-bearing activities (e.g., ambulation), and pulmonary restriction caused by excess central adiposity (biological and functional factors). Although physical activity also can reduce inflammation (biological factor), the magnitude of change is greater with weight loss [40]. Physical activity also can further reduce the physiologic strain by improving walking economy (i.e., greater efficiency in oxygen consumption) [41], which can increase vitality through greater exercise self-efficacy [42]. With regard to potential mediators of vitality unique to physical activity, improved rate pressure product (i.e., indicator of myocardial oxygen demand during exercise based on heart rate and blood pressure response) has been reported as mediator of vitality in breast cancer survivors (biological factor) [43]. Importantly, the rate pressure product is a marker for improved hemodynamic responses and autonomic nervous system regulation (including but not limited to the hypothalamic-pituitary axis response), a hypothesized etiologic mechanism in vitality (biological factor influencing psychobehavioral symptoms) [7]. Also, our data are consistent with the proposed mediating role of the hypothalamic-pituitary axis and psychosocial factors because poor vitality, depression, and sleep dysfunction co-occur and often improve with exercise [10, 44]. However, depression worsened in the intensive weight loss group over time in the ENERGY trial [15] suggesting that multiple etiologic mechanisms exist for vitality and possibly explaining the limited effect of weight loss on vitality in our analyses. Furthermore, we have published preliminary data suggesting that the gut microbiota may be a mechanistic link between cardiorespiratory fitness and vitality in breast cancers survivors [45]. Hence, additional research is needed to determine the interrelationships between weight loss, physical activity, vitality, and the gut microbiota.
Several other aspects of weight loss intervention delivery should be considered for breast cancer survivors, as well as for other patients with chronic conditions. First, while we are not able to determine the appropriate “dose” of physical activity (as well as weight loss) needed to improve outcomes, this will be important for future studies to establish benchmarks for clinical recommendations. We might speculate that larger changes in physical activity could result in improved outcomes up to a certain point with the threshold for overtraining (which can cause fatigue) demonstrating individual variability. The ENERGY trial was not designed to establish dose, but results from the trial may be useful to consider when designing future studies examining this issue. Second, there is a question of the ideal timing of intervention delivery (e.g., time from disease diagnosis or completion of treatment). Specifically, at what point in the cancer continuum (or chronic disease trajectory) would the intervention have the largest clinically meaningful effect and be maintained long term? While some studies have indicated that time since diagnosis may influence exercise effects on psychosocial outcomes [26], our study showed that vitality responses to the ENERGY intervention did not differ by time since diagnosis. In a sensitivity analyses, we evaluated the mean physical activity changes, BMI, and vitality changes over time by whether the patient was above or below the median time from treatment to study entry (23 months). We found similar trends in physical activity over time. At study initiation, those who were >23 months from treatment had lower BMI and higher vitality compared with those who were <23 months (Supplementary Figure 1). So, while baseline scores may have differed for BMI and vitality, the magnitude of changes was similar over time. Further, current recommendations suggest that regular physical activity is important across the disease trajectory [46]. Finally, future studies focusing on identifying populations that are most likely to benefit from the intervention are needed to most effectively utilize resources, tailor interventions, and achieve maximum potential gain.
Taken as a whole, the consideration of mediators within the broad categories of biological, psychobehavioral, and functional factors proposed by Al-Majid and Gray remains relevant to physical activity [12]. Because this model was proposed for the purpose of guiding future physical activity research related to vitality in cancer survivors, this report highlights the importance of considering the enhancing or attenuating influence that weight change may have on etiologic factors when applied to overweight or obese breast cancer survivors who are also attempting weight loss. Importantly, our results may have applicability to other chronic conditions. Individuals with chronic health conditions are more likely to report reduced vitality with weight loss often recommended for cardiovascular disease, arthritis, and diabetes [47, 48]. Given that etiologic mechanisms of poor vitality in chronic disease populations overlap with that of cancer survivors [48], the relationships reported here also should be considered when testing the effects of interventions including weight loss on vitality in overweight or obese individuals with other chronic diseases.
We are the first trial to report multilevel, multivariable mediation path analysis to better elucidate the independent effects of physical activity and weight loss on vitality in cancer survivors. The study strengths include a large sample size, prospective data collection strengthening cause and effect conclusions, and state-of-the-art mediation analyses. We acknowledge limitations, which include measurement of physical activity by self-report alone, limited generalizability to other populations, and post-hoc analyses of a trial not originally designed to test the hypotheses examined here. The magnitude of within-person mediation effects may have been larger with alternative measures of weight loss (i.e., body fat percentage lost), more frequent measurement to detect greater variation, or potentially longer follow-up. Additionally, all measures were not assessed at the 18-month time point; therefore, we are unable to evaluate the changes in vitality at this time point.
These limitations notwithstanding, combining physical activity with weight loss can enhance improvements in vitality occurring with weight loss. Physical activity and weight loss share mechanistic links to vitality with physical activity potentially increasing (e.g., in an additive or synergistic manner) the effect of weight loss effects on several of these shared mechanisms. Improvements in cardiorespiratory and muscle fitness that can be achieved with greater physical activity, especially structured exercise training, also exist along with the potential for beneficial effects on autonomic system regulation and the hypothalamic-pituitary axis. Further study aimed at understanding the shared and unique mechanisms will improve the application of theoretical models of decreased vitality and inform future interventions aimed at improving vitality in overweight and obese breast cancer survivors. Moreover, dietary changes are essential for successful weight loss, with physical activity recommended to improve energy balance and maintain lean body mass. These findings suggest that physical activity also may play a critical role in increasing vitality during weight loss and may be another reason to encourage breast cancer survivors to become more physically active.
Supplementary Material
Acknowledgments
The ENERGY trial was supported by National Cancer Institute, Grant No. CA148791. NCI Clinical trials registration information: NCT01112839. Support is acknowledged from the UAB Comprehensive Cancer Core Grant (NCI Cancer Center Support Grant No. CA013148).
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards Dr. Kenzik was funded by the Agency for Health Care Research and Quality, award number K12HS023009. The other authors declare that they have no conflict of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Authors’ Contributions K. M. Kenzik, L. Q. Rogers, and W. Demark-Wahnefried conceptualized and designed this secondary data analysis. W. Demark-Wahnefried, P. A. Ganz, G. Colditz, and C. L. Rock acquired the ENERGY trial data. K. M. Kenzik performed the analysis of the data. All authors contributed to the interpretation of the data, the drafting of the manuscript, and critical revision.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- 1. DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. ca Cancer J Clin. 2016; 66(1): 31–42. [DOI] [PubMed] [Google Scholar]
- 2. Arndt V, Stegmaier C, Ziegler H, Brenner H. A population-based study of the impact of specific symptoms on quality of life in women with breast cancer 1 year after diagnosis. Cancer. 2006; 107(10): 2496–2503. [DOI] [PubMed] [Google Scholar]
- 3. Buffart LM, De Backer IC, Schep G, Vreugdenhil A, Brug J, Chinapaw MJ. Fatigue mediates the relationship between physical fitness and quality of life in cancer survivors. J Sci Med Sport. 2013; 16(2): 99–104. [DOI] [PubMed] [Google Scholar]
- 4. Jones JM, Olson K, Catton P et al. Cancer-related fatigue and associated disability in post-treatment cancer survivors. J Cancer Surviv. 2016; 10(1): 51–61. [DOI] [PubMed] [Google Scholar]
- 5. Bower JE, Ganz PA, Desmond KA et al. Fatigue in long-term breast carcinoma survivors: A longitudinal investigation. Cancer. 2006; 106(4): 751–758. [DOI] [PubMed] [Google Scholar]
- 6. Hollen PJ, Msaouel P, Gralla RJ. Determining issues of importance for the evaluation of quality of life and patient-reported outcomes in breast cancer: Results of a survey of 1072 patients. Breast Cancer Res Treat. 2015; 151(3): 679–686. [DOI] [PubMed] [Google Scholar]
- 7. Bower JE. Cancer-related fatigue—mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014; 11(10): 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism?J Clin Oncol. 2011; 29(26): 3517–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006; 12(9): 2759–2766. [DOI] [PubMed] [Google Scholar]
- 10. Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008; 26(6): 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saligan LN, Kim HS. A systematic review of the association between immunogenomic markers and cancer-related fatigue. Brain Behav Immun. 2012; 26(6): 830–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al-Majid S, Gray DP. A biobehavioral model for the study of exercise interventions in cancer-related fatigue. Biol Res Nurs. 2009; 10(4): 381–391. [DOI] [PubMed] [Google Scholar]
- 13. Barsevick AM, Irwin MR, Hinds P et al. ; National Cancer Institute Clinical Trials Planning Meeting Recommendations for high-priority research on cancer-related fatigue in children and adults. j Natl Cancer Inst. 2013; 105(19): 1432–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012; 11: CD006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demark-Wahnefried W, Colditz GA, Rock CL et al. ; ENERGY Trial Group Quality of life outcomes from the Exercise and Nutrition Enhance Recovery and Good Health for You (ENERGY)-randomized weight loss trial among breast cancer survivors. Breast Cancer Res Treat. 2015; 154(2): 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reeves M, Winkler E, Mccarthy N et al. The living well after Breast Cancer™ pilot trial: A weight loss intervention for women following treatment for breast cancer. Asia Pac J Clin Oncol. 2017; 13(3): 125–136. [DOI] [PubMed] [Google Scholar]
- 17. Courneya KS, Karvinen KH, Campbell KL et al. Associations among exercise, body weight, and quality of life in a population-based sample of endometrial cancer survivors. Gynecol Oncol. 2005; 97(2): 422–430. [DOI] [PubMed] [Google Scholar]
- 18. Mitchell SA. Cancer-related fatigue: State of the science. Pm R. 2010; 2(5): 364–383. [DOI] [PubMed] [Google Scholar]
- 19. Rock CL, Byers TE, Colditz GA et al. ; Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial Group Reducing breast cancer recurrence with weight loss, a vanguard trial: The Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) trial. Contemp Clin Trials. 2013; 34(2): 282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brown LF, Kroenke K, Theobald DE, Wu J. Comparison of SF-36 vitality scale and fatigue symptom inventory in assessing cancer-related fatigue. Support Care Cancer. 2011; 19(8): 1255–1259. [DOI] [PubMed] [Google Scholar]
- 21. Rock CL, Flatt SW, Byers TE et al. Results of the Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) trial: A behavioral weight loss intervention in overweight or obese breast cancer survivors. J Clin Oncol. 2015; 33(28): 3169–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003; 49(2): 156–163. [DOI] [PubMed] [Google Scholar]
- 23. Burnam MA, Wells KB, Leake B, Landsverk J. Development of a brief screening instrument for detecting depressive disorders. Med Care. 1988; 26(8): 775–789. [DOI] [PubMed] [Google Scholar]
- 24. Murphy J. Psychiatric Instrument Development for Primary Care Research: Patient Self-report Questionnaire, Report on Contract 80M01428101D. Bethesda, MD: National Institute of Mental Health; 1982. [Google Scholar]
- 25. Stanton AL, Bernaards CA, Ganz PA. The BCPT symptom scales: A measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005; 97(6): 448–456. [DOI] [PubMed] [Google Scholar]
- 26. Milne HM, Wallman KE, Gordon S, Courneya KS. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: A randomized controlled trial. Breast Cancer Res Treat. 2008; 108(2): 279–288. [DOI] [PubMed] [Google Scholar]
- 27. U.S. Department of Health and Human Services. NHANES III Anthropometric Procedures Video. Washington, DC: U.S. Government Printing Office Stock Number 017 022. [Google Scholar]
- 28. Karvinen KH, Courneya KS, Campbell KL et al. Correlates of exercise motivation and behavior in a population-based sample of endometrial cancer survivors: An application of the theory of planned behavior. Int j Behav Nutr Phys Act. 2007; 4: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992; 30(6): 473–483. [PubMed] [Google Scholar]
- 30. MacKinnon D. Introduction to Statistical Mediation Analysis. New York, NY: Erlbaum; 2008. [Google Scholar]
- 31. MacKinnon DP, Valente MJ. Mediation from multilevel to structural equation modeling. Ann Nutr Metab. 2014; 65(2–3): 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krull JL, MacKinnon DP. Multilevel modeling of individual and group level mediated effects. Multivariate Behav Res. 2001; 36(2): 249–277. [DOI] [PubMed] [Google Scholar]
- 33. Bauer DJ, Preacher KJ, Gil KM. Conceptualizing and testing random indirect effects and moderated mediation in multilevel models: New procedures and recommendations. Psychol Methods. 2006; 11(2): 142–163. [DOI] [PubMed] [Google Scholar]
- 34. Preacher KJ. Multilevel SEM strategies for evaluating mediation in three-level data. Multivariate Behav Res. 2011; 46(4): 691–731. [DOI] [PubMed] [Google Scholar]
- 35. Muthen B, Muthen LK.. Mplus Computer Software. Vol. 7 Los Angeles, CA: Muthen & Muthen; 2012. [Google Scholar]
- 36. Weinstein ND. Misleading tests of health behavior theories. Ann Behav Med. 2007; 33(1): 1–10. [DOI] [PubMed] [Google Scholar]
- 37. Karvinen KH, Courneya KS, North S, Venner P. Associations between exercise and quality of life in bladder cancer survivors: a population-based study. Cancer Epidemiol Biomarkers Prev. 2007; 16(5): 984–990. [DOI] [PubMed] [Google Scholar]
- 38. Puetz TW, Herring MP. Differential effects of exercise on cancer-related fatigue during and following treatment: a meta-analysis. Am j Prev Med. 2012; 43(2): e1–24. [DOI] [PubMed] [Google Scholar]
- 39. Tian L, Lu HJ, Lin L, Hu Y. Effects of aerobic exercise on cancer-related fatigue: A meta-analysis of randomized controlled trials. Support Care Cancer. 2016; 24(2): 969–983. [DOI] [PubMed] [Google Scholar]
- 40. Fisher G, Hyatt TC, Hunter GR, Oster RA, Desmond RA, Gower BA. Effect of diet with and without exercise training on markers of inflammation and fat distribution in overweight women. Obesity (Silver Spring). 2011; 19(6): 1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hunter GR, Fisher G, Neumeier WH, Carter SJ, Plaisance EP. Exercise training and energy expenditure following weight loss. Med Sci Sports Exerc. 2015; 47(9): 1950–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McAuley E, White SM, Rogers LQ, Motl RW, Courneya KS. Physical activity and fatigue in breast cancer and multiple sclerosis: Psychosocial mechanisms. Psychosom Med. 2010; 72(1): 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carter SJ, Hunter GR, McAuley E, Courneya KS, Anton PM, Rogers LQ. Lower rate-pressure product during submaximal walking: A link to fatigue improvement following a physical activity intervention among breast cancer survivors. J Cancer Surviv. 2016; 10(5): 927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mishra SI, Scherer RW, Snyder C et al. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev. 2012; 8: CD008465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Paulsen JA, Ptacek TS, Carter SJ et al. Gut microbiota composition associated with alterations in cardiorespiratory fitness and psychosocial outcomes among breast cancer survivors. Support Care Cancer. 2017; 25(5): 1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schmitz KH, Courneya KS, Matthews C et al. ; American College of Sports Medicine American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010; 42(7): 1409–1426. [DOI] [PubMed] [Google Scholar]
- 47. Bültmann U, Kant I, Kasl SV, Beurskens AJ, van den Brandt PA. Fatigue and psychological distress in the working population: Psychometrics, prevalence, and correlates. J Psychosom Res. 2002; 52(6): 445–452. [DOI] [PubMed] [Google Scholar]
- 48. Fritschi C, Quinn L. Fatigue in patients with diabetes: A review. j Psychosom Res. 2010; 69(1): 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.