Abstract
Hyperferritinemia and pronounced hemophagocytosis help distinguish a subset of patients with a particularly inflammatory and deadly systemic inflammatory response syndrome. Two clinically similar disorders typify these hyperferritinemic syndromes: hemophagocytic lymphohistiocytosis (HLH) and macrophage activation syndrome (MAS). HLH is canonically associated with a complete disturbance of perforin/granzyme-mediated cytotoxicity, whereas MAS occurs in the context of the related rheumatic diseases systemic juvenile idiopathic arthritis and adult-onset Still’s disease, with associated IL-1 family cytokine activation. In practice, however, there are accumulating lines of evidence for innate immune dysregulation in HLH as well as partial impairments of cytotoxicity in MAS, and these mechanisms likely represent only a fraction of the host and environmental factors driving hyperferritinemic inflammation. Herein, we present new findings that highlight the pathogenic differences between HLH and MAS, two conditions that present with life-threatening hyperinflammation, hyperferritinemia and hemophagocytosis.
Keywords: cytotoxicity, hemophagocytic lymphohistiocytosis, IFN-γ, macrophage activation syndrome
Introduction
Systemic inflammation has vexed healers since ancient times, inspiring imagery of sanguine humors run amok in septic patients. Centuries later the hemodynamic changes associated with sepsis were attributed to systemic inflammation in the formulation of the systemic inflammatory response syndrome (SIRS) (1); sepsis can be defined as SIRS in the presence of a defined infection. Intensive clinical study of inflammation in broad sepsis cohorts was initially disappointing, and so the search for the inflammatory underpinnings of SIRS continues. Instructive to this search are advances in our understanding of the hyperferritinemic syndromes, as typified by the overlapping disorders hemophagocytic lymphohistiocytosis (HLH) and macrophage activation syndrome (MAS). The implications of hyperferritinemia and its relationship to inflammation are reviewed elsewhere in this issue (2). Hemophagocytosis is the engulfment of blood cells and their precursors by histiocytes (activated macrophages); lymphohistiocytosis is the histologic co-existence of activated lymphocytes and macrophages. Both HLH and MAS are life-threatening inflammatory states characterized by fever, extreme hyperferritinemia, pancytopenia, coagulopathy, hepatitis, hepatosplenomegaly, increased soluble IL-2 receptor α (IL-2Rα; reflecting lymphocyte activation) and excessive hemophagocytosis (3).
Despite initial disappointments in sepsis, targeted inflammatory cytokine inhibition outside of SIRS has been instructive. TNFα inhibition has been transformative for the treatment of inflammatory arthritis, psoriasis, uveitis and inflammatory bowel disease. Likewise, several monogenic periodic fever syndromes caused by inflammasome hyperactivity respond dramatically to IL-1 inhibition, and this observation helped propel the concept of ‘autoinflammation’ (4). IL-1 family cytokines include IL-1α, IL-1β, IL-18 and IL-33, which bind surface receptors and signal similarly to most toll-like receptors (TLRs) via MyD88 and NF-κB. IL-1β and IL-18 require processing via the inflammasome in order to signal. The inflammasome is a macromolecular structure linking intracellular pathogen or danger sensing with the activation (via caspases) of IL-1β and IL-18. Inflammasome activation also drives a form of inflammatory cell death known as pyroptosis (5).
The disorders at highest risk for MAS, systemic juvenile idiopathic arthritis (SJIA) and adult-onset Still’s disease (AOSD) share some phenotypic similarity with the monogenic ‘inflammasomopathies’ including unexplained fevers, rash and response to IL-1 inhibition (6, 7). However, unlike most autoinflammatory disorders, SJIA and AOSD lack a clear genetic origin and are particularly prone to MAS (8, 9). Recent work increasingly highlights the association of MAS with increased activity of certain cytokines, specifically IL-18 (10, 11) and IFNγ (12).
HLH is a clinically similar hyperferritinemic syndrome that can be familial (FHLH) or seen during infection (often by Epstein–Barr virus [EBV]), malignancy, immunodeficiency or with chimeric antigen receptor T-cell (CAR T-cell) therapy (13, 14). Whereas the mechanisms underlying MAS are just beginning to be defined, the genetic causes for FHLH converge around impairment in the production, transit or function of cytotoxic granules (15). Insights from murine models of HLH have suggested that these granules are both anti-viral (killing infected cells) and anti-inflammatory (terminating immune synapses) (16) and mostly point to the critical importance of antigen-specific CD8 T cells and IFNγ.
In parallel with this work in MAS and HLH, attempts to classify the immune response in sepsis patients have continued. Many sepsis patients develop profound compensatory anti-inflammatory response syndrome and are at risk for opportunistic infection. However, a subset of sepsis patients has a persistent hyperinflammatory response and ultimately poorer outcomes (17–19). Notably, this hyperinflammatory subset has clinical features of MAS, including hyperferritinemia (20) and may respond to IL-1 inhibition (21).
Despite the genetic origins of FHLH and the rheumatic context of MAS, both hyperferritinemic syndromes often have an infectious trigger. An extremely broad variety of viral, bacterial, parasitic and fungal infections have been identified to cause secondary HLH (22), and their pathogenic contributions remain mysterious. The non-viral causes of secondary HLH may be concentrated for intracellular pathogens like Mycobacterium tuberculosis, Ehrlichia, Leptospira, Plasmodia and Toxoplasma. It is tempting to hypothesize that intracellular infection of macrophages drives their aberrant activation and ferritin production, and that these pathogens intentionally drive ferritin synthesis for their own iron needs, but mechanistic data are lacking. Furthermore, the range of HLH-associated pathogens suggests that host factors play the largest role in determining susceptibility to hyperferritinemic inflammation.
In this review, we will examine two competing mechanisms in MAS and HLH: (i) impairment (genetic or acquired) of cytotoxic function and (ii) excessive innate immune activation by IL-1 family cytokines. Ultimately, we find that the evidence indicates a convergence at the level of cytotoxic T lymphocyte (CTL) hyperactivation and cytokine overproduction that may underlie the hyperferritinemic phenotype. Importantly, the mechanisms described are neither detailed nor comprehensive, but represent a model to be confirmed, refined and/or refuted by future work.
Genetic mutations leading to impaired cytotoxic function cause FHLH and may contribute to the pathogenesis of MAS and ‘secondary HLH’
The familial forms of HLH (FHLH) have been recognized since the 1950s as disorders of previously healthy infants and young children who develop persistent and recurrent systemic inflammation with fevers, cytopenias, coagulopathy, liver and neurologic dysfunction (23). The understanding of these rare disorders was transformed by the discovery that FHLH patients had profoundly impaired natural killer (NK) cell cytotoxicity (24), and followed shortly by the identification of perforin (PRF1) deficiency in several patients with FHLH (25). Perforin is encoded by PRF1 and, subsequently, FHLH has been linked to recessively inherited defects in several other genes including UNC13D, STX11 and STXBP2. In addition, other familial disorders with similarity to HLH such as Griscelli syndrome, Hermansky–Pudlak syndrome and Chediak–Higashi syndrome have been linked to biallelic variants in RAB27A, AP3B1 and LYST, respectively (26). Together, these familial hemophagocytic syndromes are all united by genetic defects in specific and essential components of the lymphocyte cytolytic pathway (Table 1), with the degree of cytotoxicity impairment strongly correlated with clinical severity (27).
Table 1.
Lymphocyte cytotoxicity genes linked to HLH and MAS
| Gene | Function | Genetic deficiency | Variants associated with MAS? |
|---|---|---|---|
| PRF1 | Pore formation | FHL-2 | Yes |
| UNC13D | Vesicle maturation and regulation of secretion | FHL-3 | Yes |
| STX11 | Vesicle targeting and fusion | FHL-4 | Yes |
| STXBP2 | Vesicle fusion, interacts with Syntaxin-11 | FHL-5 | Yes |
| RAB27A | Vesicle docking and protein transport | Griscelli syndrome type 2 | Yes |
| AP3B1 | Vesicle trafficking, organelle biogenesis | Hermansky–Pudlak syndrome | No |
| LYST | Vesicle sorting and trafficking in endosomes | Chediak–Higashi syndrome | Yes |
The precise mechanism by which genetic defects in NK cell and CTL function cause hyperferritinemic and hemophagocytic syndromes is not fully understood. Contemporaneous with the description of perforin deficiency causing FHLH, several studies demonstrated that NK cells and CTLs, and specifically perforin, served key roles in restraining T-cell responses during infection (28–31). These essential functions were vividly demonstrated by Jordan et al., who found that perforin-deficient mice infected with lymphocytic choriomeningitic virus (LCMV) developed all cardinal features of FHLH including hyperferritinemia (32). Indeed, this remains the only mouse model that recapitulates all features of HLH (33). Additionally, perforin-deficient mice exhibited excessive early T-cell activation, associated with increased viral antigen presentation and antigenic stimulation, further supporting a key role for NK cells and CTLs in restraining immune activation (16).
Subsequent studies have similarly found that mice deficient in other genes linked to FHLH develop hyperferritinemic, hemophagocytic syndromes upon LCMV infection (27, 34–36). There is also evidence that NK cells and CTLs have distinct pathogenic functions in perforin-deficient mice. CTLs have functions in viral clearance, and CTL depletion greatly diminishes features of FHLH in mice, suggesting that these cells are key drivers of this hyperferritinemic ‘cytokine storm’ (32). In contrast, NK cells limit CTL hyperactivation and down-modulate specific T-cell responses, thus protecting these animals from fatal outcomes (37, 38). This supports a central role for functional NK cell activity in serving as an essential check on the CTL-mediated immunopathology seen in HLH.
There is also evidence supporting a role for NK cells in MAS. The first reports of what we now recognize as MAS described SJIA patients who experienced life-threatening inflammatory episodes with anemia and consumptive coagulopathy, hepatic, neurologic and hematologic dysfunction, and expansion of well-differentiated bone marrow macrophages with hemophagocytic activity (39–42).
The recognition of this and other similarities led Grom and others to propose that MAS represented a secondary or reactive form of HLH (43, 44). In agreement with this, there is substantial evidence of NK cell dysfunction in patients with SJIA and MAS. Several reports have described patients with SJIA and MAS with a mixture of quantitative defects in NK cell numbers and qualitative defects in perforin expression. A subgroup of patients with SJIA also had near-complete absence of CD56bright NK cells, which serve immunoregulatory roles and are often low in HLH (45, 46). A more recent study found that nearly one-third of patients with a history of MAS with suspected but as yet undefined rheumatic disease had persistent functional abnormalities in NK cell granule release (47). Finally, multiple studies have shown that NK cells from SJIA patients have decreased functional responses to IL-18 (48). Together these findings suggest that impaired NK cell function could link MAS to FHLH.
Given this clinical and pathologic overlap, could similar genetic defects in cytolytic pathways be implicated in MAS? Multiple independent cohorts of SJIA and MAS patients have exhibited an enrichment for rare, protein-altering variants in genes linked to FHLH (49–52). In particular, Vastert et al. found a significantly higher prevalence of the perforin gene variant A91V in patients with MAS than in SJIA patients without history of MAS or in healthy controls (50). A recent comprehensive study using whole exome sequencing in a cohort of SJIA and MAS patients found more than one-third carried rare variants in HLH-associated genes. In addition, these patients had a significant enrichment for rare variants in other genes involved in cellular assembly and organization, supporting further as yet undefined genetic links with granule trafficking pathways (51). Finally, while the role of intronic and non-coding genetic variants in MAS is largely unexplored, a recent study intriguingly found that >8% of children with recurrent MAS had deep intronic variants in UNC13D which alter a lymphocyte-specific promoter, highlighting the need for further study of these genomic regions.
Variants in PRF1, UNC13D and RAB27A have also been described in patients with MAS in the setting of other rheumatic diseases (52–56), suggesting that this mechanism may extend beyond SJIA. Most interestingly, cytolytic pathway genetic variants have been recently described in a series of patients with fatal H1N1 influenza infection, most of which would satisfy criteria for HLH or MAS (57). These genetic data further support a central role for cytolytic pathway dysfunction in hyperferritinemic syndromes.
Indeed, there is increasing experimental data to support the idea that even common functional polymorphisms in cytolytic pathway genes can impact NK cell properties. One recent study reported two patients with hyperferritinemic syndromes and a shared heterozygous variant in RAB27A, which was shown to function as a partial dominant negative able to reduce NK cell cytotoxicity and delay granzyme B polarization towards the immunologic synapse (58). This is particularly interesting in light of findings that delayed killing of target cells leads to prolonged NK/target cell ‘immune synapse’ time, resulting in hypersecretion of cytokines, including IFNγ (59). Similarly, heterozygous, dominant-negative mutations have been reported in STXBP2, impairing membrane fusion by arresting the SNARE-complex assembly (60).
Defective killing by NK cells has also been demonstrated for perforin gene variants, including some more common functional polymorphisms. The PRF1 variant A91V (dbSNP rs35947132) is present in between ~1 and 8% of the general population. However, it has also been linked to late-onset FHLH (61), MAS in patients with SJIA (50) and even hematological malignancies (62). Several studies have shown that this variant may impair the structure and function of perforin (57, 62, 63), with recent work also demonstrating mild impairment in function of primary NK cells from healthy A91V carriers (64). These translational findings are further supported by animal models showing that accumulation of heterozygous variants in the cytolytic pathway impairs CTL function (65). Collectively, this work suggests that even heterozygous variants in cytolytic pathway genes may impair NK cell function and could predispose to developing hyperferritinemic syndromes under particular circumstances.
Despite this strong genetic evidence, a majority of patients with MAS do not have identified variants in genes linked to cytolytic dysfunction. Indeed, many SJIA patients rather display transient defects in cytotoxic killing that normalize upon resolution of systemic inflammation (66). Recent work using IL-6 transgenic (IL-6TG) mice may provide a partial explanation for this observation, as well as more widespread impairment in NK cell function in hyperferritinemic syndromes. IL-6TG mice exhibit an enhanced inflammatory response to TLR ligands, in particular LPS, which triggers some clinical features resembling MAS (67). NK cells from IL-6TG mice showed impaired cytotoxicity, with reduced perforin and granzyme expression despite normal degranulation.
Primary human NK cells demonstrated similarly decreased perforin and granzyme expression upon exposure to IL-6, which was restored by treatment with the monoclonal antibody tocilizumab, which blocks IL-6R. IL-6 also reduced perforin expression in primary NK cells from a patient with history of MAS and a partial cytotoxicity defect (58), suggesting that IL-6 could function in synergy with genetic defects to impair cytolytic function. Finally, IL-6 plasma levels during SJIA clinical remission correlated with NK cell function (68). These findings intriguingly suggest that excessive innate immune activation as seen in hyperferritinemic syndromes can itself inhibit key NK cell functions in immune homeostasis and worsen immunopathology.
An emerging role for IL-18 in defining the pathogenesis of MAS
Undoubtedly, cytotoxic impairment is an important host susceptibility factor contributing to the hyperinflammation and hyperferritinemia of HLH, but for many (if not most) patients this is not the whole story. There appears to be a threshold of cytotoxic function, below which patients have increasing risk of developing HLH. As such, complete cytotoxic impairment carries a high penetrance for infantile FHLH, less severe cytotoxic impairments (e.g. Chediak–Higashi syndrome) result in a delayed-onset of HLH, and haploinsufficiency for HLH-associated genes is quite common in the healthy population (64, 69). In fact, most FHLH-associated genes carry a probability of loss-of-function intolerance (pLI) of zero (Table 2) (70). A gene’s pLI refers to the proportion of detected versus expected loss-of-function mutations in a gene within a broad population (http://exac.broadinstitute.org). A pLI >0.9 supports pathogenic haploinsufficiency, whereas a pLI = 0 suggests little evolutionary pressure for two functioning copies of most FHLH genes (Table 2). Thus, the threshold of cytotoxic impairment required to drive HLH may be quite high.
Table 2.
pLIa of familial HLH genes
| Disease | Gene | Protein | pLI |
|---|---|---|---|
| FHLH1 | Unknown | Unknown | – |
| FHLH2 | PRF1 | Perforin | 0.00 |
| FHLH3 | UNC13D | Munc13-4 | 0.00 |
| FHLH4 | STX11 | Syntaxin-11 | 0.02 |
| FHLH5 | STXBP2 | Munc18-2 | 0.00 |
| XLP1b | SH2D1A | SAP | 0.08 |
| XLP2b | XIAP/BIRC4 | XIAP | 0.98 |
| Griscelli | RAB27A | RAB27A | 0.00 |
| Hermansky–Pudlak | AP3B1 | AP-3 | 1.00 |
| Chediak–Higashi | LYST | LYST | 1.00 |
| Phenylketonuria | PAH | Phenylalanine hydroxylase | 0.00 |
| HA20 | TNFAIP3 | A20 | 1.00 |
Assembled from the Exome Aggregation Consortium. PAH and TNFAIP3 are included as controls known to be associated with haplosufficiency and haploinsufficiency, respectively.
aExplanation of pLI available at http://exac.broadinstitute.org/faq and is detailed in Lek et al. (70).
bX-linked disorder.
MAS offers the strongest evidence that host susceptibility pathways distinct from cytotoxic impairment are at work. MAS is not associated with impaired pathogen clearance, and the rheumatic diseases at highest risk for developing MAS (SJIA and AOSD) typically respond to blockade of innate immune cytokines like IL-1β and IL-6. The majority of MAS patients have no genetic cytotoxic defects and no constitutive defects in NK cell function. The argument that the inflammatory milieu of SJIA, specifically excessive IL-6, drives transient cytotoxic dysfunction and MAS ignores the reality that IL-6 levels similar to SJIA occur in many inflammatory disorders that lack hyperferritinemic physiology (10). Most likely, multiple mechanisms (genetic, cytokine, pathogen, etc.) combine to determine both the character and amplitude of the response that, at a certain inflammatory threshold, manifests as MAS or HLH (71). Thus, a fuller understanding of hyperferritinemic physiology requires a mechanistic understanding that includes, but is not limited to, cytotoxic dysfunction.
The same murine models of cytotoxic impairment that have informed our understanding of FHLH also implicate the importance of innate immune mechanisms both for priming hyperinflammatory adaptive responses and for carrying out tissue immunopathology. Specifically, signaling through IL-33 (an alarmin, or danger signal, in the IL-1 family) is necessary for the development of immunopathology in the murine model of perforin deficiency (72). IL-33 signaling (like all IL-1 family cytokines and most TLRs) requires the innate immune signaling adaptor protein MyD88, which is itself essential for the development of murine HLH (73). Finally, models of FHLH all converge on the necessity for IFNγ, which appears to mediate HLH-associated anemia and hemophagocytosis (at least) via effects on macrophages (74, 75).
Models of secondary HLH and MAS variably support the importance of IFNγ, but do not rely on cytotoxicity. In the IL-6TG model, NK cell function was impaired but disease was not shown to depend on that impairment, but rather on IFNγ activity (68, 76). Likewise, in the TLR9-based model of MAS (in which TLR9 is repeatedly stimulated using CpG), NK cell numbers diminish in affected spleens (77), but perforin deficiency does not exacerbate this model (S. W. Canna, unpublished data). Disease in the TLR9-model is unaffected by the absence of adaptive immune cells but requires innate lymphocytes such as NK cells (77). Whether disease in this model requires an intact IL-12–IFNγ axis seems to depend on whether IL-10 is present to inhibit other inflammatory mediators (75). Though Low NK numbers were observed in a murine cytomegalovirus (MCMV)-based model of secondary HLH, eliminating CD8 T cells in this model did not exacerbate disease, suggesting NK cytopenia may be an epiphenomenon. In fact, MCMV induced more severe disease in the absence of IFNγ in this model (78). These models do not undermine the reality that graded cytotoxic impairment contributes to HLH, but rather they suggest that defects other than cytotoxic impairment can proceed (potentially without IFNγ) to the complex immunophenotype observed in MAS and HLH. They also raise the possibility that cytotoxic impairment may in some circumstances be a byproduct, rather than cause, of severe systemic inflammation.
The study of biomarkers in human MAS and HLH has suggested cytokine blockade may be an excellent therapeutic strategy. As described above, IFNγ plays a critical role in models of FHLH and some models of MAS, and these effects appear to be mediated through innate immune cells (74, 77). Similarly, peripheral blood analysis in FHLH and MAS support hyperactivity of IFNγ in the form of highly elevated IFNγ-induced cytokines like CXCL9 (12, 79). Ongoing clinical trials of IFNγ blockade in FHLH appear promising (Clinicaltrials.gov identifier NCT01818492).
Additionally, the calcium-binding proteins S100A8/A9 (calprotectin) and S100A12 (calgranulin) are elevated in active SJIA as opposed to other forms of JIA. S100 elevation may be less specific to SJIA, however, as levels in the autoinflammatory disease familial Mediterranean fever (FMF) are comparable to those in MAS (80, 81). These S100 proteins, when present extracellularly, signal through innate immune receptors like TLR4 and RAGE on myeloid cells to promote inflammatory responses. It is possible that differential up-regulation of S100A8/9 versus S100A12 in specific disease states may provide insight into the preponderance of activated monocytes versus neutrophils, respectively.
Most notably, observations in SJIA–MAS, sepsis and specific monogenic disorders such as NLRC4-MAS and XIAP deficiency (discussed more below) increasingly implicate the inflammasome-activated cytokines IL-1β and IL-18 as important for MAS. Blockade of IL-1β is beneficial to the majority of SJIA patients (7, 82) and, although it did not appreciably prevent MAS in SJIA (83), multiple case reports support the utility of IL-1 blockade in MAS (84). Thus, there is an ongoing clinical trial of IL-1 blockade in MAS (NCT02780583). Importantly, adult sepsis patients with clinical features similar to MAS (hepatobiliary dysfunction and coagulopathy) have enhanced mortality that may be preventable with IL-1 blockade (21). This response is not unique to SJIA, as many monogenic autoinflammatory diseases that are not associated with MAS or HLH respond dramatically to IL-1 inhibition (4).
IL-18 may be the biomarker most specifically linked to patients at risk for MAS (85). Traditionally, IL-18 acts in concert with other cytokines like IL-12 to drive lymphocyte cytotoxicity and cytokine production, most notably IFNγ. However, we are only beginning to appreciate its role in other kinds of inflammatory responses as well as in maintaining mucosal barrier integrity (86). Extremely high levels of IL-18 have been observed in patients with SJIA and AOSD, particularly those with MAS, whereas much more modest elevation of serum IL-18 has been observed in a variety of inflammatory, infectious and malignant diseases (10, 11, 85–87).
IL-18 is inhibited by an endogenous soluble protein called IL-18-binding protein (IL-18BP) such that it is bioactive only when unbound by IL-18BP (87). Detectable ‘free IL-18’ may be specific to SJIA/AOSD and the MAS-prone genetic syndromes (related to NLRC4 or XIAP mutations) where IL-18 is extremely elevated (85, 87, 88). The effects of this chronic, unopposed IL-18 are largely unknown. Elevated free IL-18 was identified in many AOSD patients (87) and a trial of recombinant IL-18BP in AOSD recently completed (NCT02398435). Extremely elevated IL-18 and free IL-18, particularly in relation to IFNγ-induced cytokines like IL-18BP and CXCL9, may be specific to MAS among both hyperferritinemic and autoinflammatory diseases (85). Additionally, two groups have observed failure of SJIA NK cells to respond to IL-18, potentially due to impaired IL-18R signaling (48, 89). Because IL-18 may be important for promoting organ-specific cytotoxicity in some infections (90), chronic IL-18’s effects on the IL-18R could represent another mechanism of acquired NK cell dysfunction in MAS (along with IL-6). Whether such an acquired cytotoxic dysfunction is pathogenic, or simply an epiphenomenon of severe inflammation in some circumstances, remains to be determined.
Two genetic disorders mentioned above, NLRC4-MAS and XIAP deficiency, have recently highlighted the mechanistic importance of the IL-18/MAS association. Patients with NLRC4-MAS have gain-of-function mutations that result in NLRC4 inflammasome hyperactivity and IL-1β and IL-18 overproduction. They can experience life-threatening episodes of MAS accompanied by severe infantile enterocolitis (88, 91–93). Total IL-18 is extremely elevated in these patients and varies very little even between fulminant and quiescent disease (88, 91, 92).
Notably, hyperactivity of inflammasomes other than NLRC4 has been associated with autoinflammatory disease, but not high IL-18 or MAS. Gain-of-function mutations in NLRP3 and MEFV cause the canonical inflammasomopathies cryopyrin-associated periodic syndromes (CAPS) and FMF, respectively. These are diseases of chronic inflammation responsive to IL-1 blockade but rarely associated with MAS (4, 94). Serum IL-18 levels in CAPS and FMF do not approach those of NLRC4-MAS, suggesting that IL-18 may be the necessary link between inflammasome hyperactivity and MAS (85, 92). Mucosal epithelial cells constitutively and highly express pro-IL-18, suggesting a potential source of IL-18 independent of myeloid cell expansion and consistent with such chronic elevation. Elevated serum IL-18 in a murine model of NLRC4 hyperactivity was shown to derive entirely from intestinal epithelia (85), and free IL-18 promoted experimental MAS in two independent animal models (85, 95). Given the chronicity of IL-18 elevation in monogenic forms of MAS, and possibly in SJIA/AOSD, IL-18 may represent a host factor that precedes the development of inflammation (86). IL-1 inhibition has shown benefit in one NLRC4-MAS patient, but a more severely affected patient showed dramatic improvement only when recombinant IL-18BP was added (88). IFNγ blockade may also be beneficial in NLRC4-MAS (96).
HLH in patients with XIAP deficiency [termed X-linked lymphoproliferative disease 2 (XLP2); Table 1] has also been associated with IL-18 levels far beyond those of other causes of HLH (97). XLP2 was originally described as HLH in patients with difficulty clearing primary EBV infection, although it does not appear to induce cytotoxic impairment and reports vary in terms of the rate of association with EBV (98). The mechanisms by which XIAP deficiency might drive IL-18 and/or HLH are less clear than in NLRC4-MAS, but include impaired NOD2 signaling (XIAP is required for NOD2 signaling in vitro), increased apoptosis or failure to efficiently clear EBV (98). Importantly, impaired cytotoxicity is not a feature of either NLRC4-MAS or XIAP deficiency. It is also relevant that not all NLRC4- or XIAP-related inflammatory diseases manifest as MAS/HLH, and IL-18 levels in these milder phenotypes have not been well studied (99–102). The effects of recombinant IL-18BP on NLRC4- and XIAP-mediated inflammation are also under current clinical investigation (NCT03113760).
The dynamics of extreme IL-18 elevation in MAS offer a few insights into its origins and significance. IL-18 appears to be extremely elevated regardless of disease activity or degree of hyperferritinemia in NLRC4-MAS and some patients with XIAP-HLH (88, 91, 92, 97) and circulates in its cleaved form (85). It remains unclear whether this IL-18 remains elevated after interventions such as bone marrow transplantation. By contrast, in MAS associated with SJIA and AOSD, IL-18 levels appear to improve with prolonged disease quiescence (85, 103). Extremely elevated IL-18 can exist without hyperferritinemia, and likewise hyperferritinemia does not rely on extremely elevated IL-18 (as in FHLH). Thus, the association of chronic IL-18 and hyperferritinemia seems unique to patients classified as MAS.
A convergence on T lymphocyte activation and cytokine overproduction
The hyperferritinemic syndromes display an impressive amount of immune complexity and phenotypic variability, even when the genetic origins are known. Indeed, systemic cytokine storm and hyperferritinemia likely represent a final common pathway resulting from many distinct defects including, but not limited to, those we have discussed. We have attempted to summarize the wealth of experimental and observational evidence supporting two competing, non-redundant mechanisms: impairment of granule-mediated cytotoxicity, and pathologic innate immune signaling by the IL-1 family members IL-1, IL-18 and/or IL-33.
On the basis of this evidence, we deduce (at least) two ways in which these mechanisms might converge leading to hyperferritinemia: (i) excessive T lymphocyte activation and (ii) excessive IFNγ activity. Supporting the former, the mechanisms of T-cell activation in FHLH and CAR T-cell HLH are relatively well established (14). In MAS, the evidence for T-cell involvement is more circumstantial, with markedly elevated IL-2Rα levels suggesting shedding by activated T cells (8). Likewise, if IL-18 blockade is acutely beneficial in MAS, as has been observed in NLRC4-MAS (88), then this benefit may be mediated through inhibiting effects on activated T cells, as SJIA NK cells may be chronically IL-18 insensitive (48, 89).
The evidence supporting IFNγ in both MAS and HLH is somewhat stronger. As discussed above, the murine evidence for IFNγ as the quintessential driver of FHLH is ponderous, and IFNγ is an important driver in some models of MAS. Active human MAS and HLH are both associated with elevated CXCL9, ostensibly due to elevated IFNγ activity. Furthermore, IL-18 canonically exerts its inflammatory effects on lymphocytes, promoting cytokine (particularly IFNγ) production and cytotoxicity. A preliminary report also suggested IFNγ blockade was helpful in NLRC4-MAS (96) and further clinical trials in MAS are planned.
Conclusions
Although the mechanisms of hyperferritemic inflammation remain poorly understood, specific but far-reaching insights have been gained via the specific genetic and environmental contexts in which MAS and HLH have been observed. Overall, the state-of-the-art implicates multiple independent pathways, most prominently impaired cytotoxicity in HLH and excessive IL-18 in MAS (Fig. 1), that converge on a pattern of T-cell hyperstimulation, macrophage activation and deadly immunopathology. Several important observations fit less neatly into the paradigm of T-cell activation and IFNγ overproduction, including why certain environmental exposures like EBV or T-cell malignancies trigger hyperferritinemia, the response of some MAS patients to IL-1 inhibition (which may be dose related) and the variable response in animal models to IFNγ blockade. Indeed, a systems-based assessment of the factors driving and restricting MAS and HLH will be required in many circumstances (75, 78). Nonetheless, the rapid pace of discovery in MAS and HLH holds great promise for improving our understanding of the intersecting and complementary roles of cytotoxic dysfunction, IL-1 family signaling, and beyond; the implications of which will have major impacts on the study, diagnosis and treatment of MAS, HLH and systemic inflammation overall.
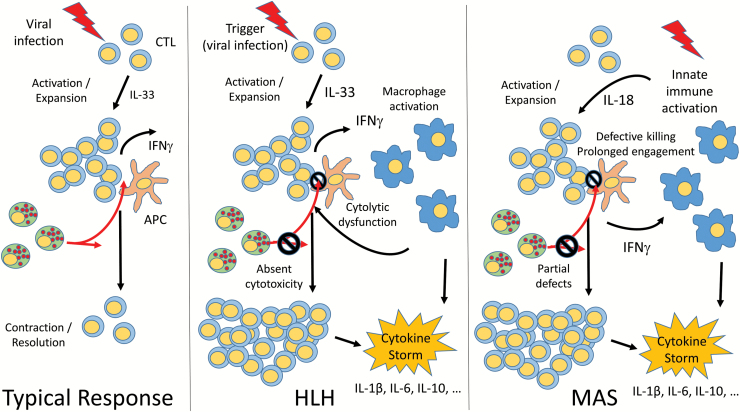
Fig. 1.
Convergent pathways of the hyperferritinemic syndromes HLH and MAS. In a typical immune response, infectious (often viral) triggers induce activation and expansion of specific CD8+ cytotoxic T Lymphocytes (CTLs). Both CD8+ CTLs and NK cells receive signals from antigen-presenting cells (APCs), leading to enhanced IFNγ production and cytolytic killing (red arrows). Contraction and resolution of the immune response also depend upon NK cell killing of activated lymphocytes. In HLH, impaired CTL function leads an inability to kill target cells and excessive IFNγ production. IFNγ has essential roles in activating macrophages, which produce high levels of inflammatory cytokines and further enhance the CTL response. Absent NK cell cytotoxicity further leads to inability to contract the immune response. During MAS, innate immune activation including excessive IL-18 production, drives activation and expansion of CTLs to overproduce IFNγ, which further stimulating macrophages to produce inflammatory cytokines. Partial genetic or acquired CTL and NK cell defects may augment this IFNγ response and similarly impair resolution of inflammation. In both HLH and MAS, this overwhelming immune activation leads to a systemic cytokine storm.
Funding
G.S.S. is supported by a Scientist Development Award from the Rheumatology Research Foundation and a Procter Scholar Award from the Cincinnati Children’s Research Foundation. S.W.C. is supported by the RK Mellon Foundation Institute for Pediatric Research.
Conflicts of interest statement
G.S.S. has received consulting fees from Novartis. S.W.C. has received consulting fees for AB2Bio, Ltd.
References
- 1. Goldstein B., Giroir B., Randolph A., et al. 2005. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 6(1):2 http://www.ncbi.nlm.nih.gov/pubmed/15636651 [DOI] [PubMed] [Google Scholar]
- 2. Kernan K. F. and Carcillo J. A. 2017. Hyperferritinemia and inflammation. Int. Immunol. 29:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ravelli A., Minoia F., Davì S. et al. ; Paediatric Rheumatology International Trials Organisation; Childhood Arthritis and Rheumatology Research Alliance; Pediatric Rheumatology Collaborative Study Group; Histiocyte Society. 2016. 2016 classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a European League against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Arthritis Rheumatol. 68:566. [DOI] [PubMed] [Google Scholar]
- 4. de Jesus A. A., Canna S. W., Liu Y. and Goldbach-Mansky R. 2015. Molecular mechanisms in genetically defined autoinflammatory diseases: disorders of amplified danger signaling. Annu. Rev. Immunol. 33:823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Broz P. and Dixit V. M. 2016. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16:407. [DOI] [PubMed] [Google Scholar]
- 6. Nigrovic P. A., Mannion M., Prince F. H. et al. . 2011. Anakinra as first-line disease-modifying therapy in systemic juvenile idiopathic arthritis: report of forty-six patients from an international multicenter series. Arthritis Rheum. 63:545. [DOI] [PubMed] [Google Scholar]
- 7. Ruperto N., Brunner H. I., Quartier P. et al. ; PRINTO; PRCSG. 2012. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N. Engl. J. Med. 367:2396. [DOI] [PubMed] [Google Scholar]
- 8. Bleesing J., Prada A., Siegel D. M. et al. . 2007. The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor alpha-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum. 56:965. [DOI] [PubMed] [Google Scholar]
- 9. Behrens E. M., Beukelman T., Paessler M. and Cron R. Q. 2007. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J. Rheumatol. 34:1133. [PubMed] [Google Scholar]
- 10. Shimizu M., Nakagishi Y. and Yachie A. 2013. Distinct subsets of patients with systemic juvenile idiopathic arthritis based on their cytokine profiles. Cytokine 61:345. [DOI] [PubMed] [Google Scholar]
- 11. Shimizu M., Nakagishi Y., Inoue N. et al. . 2015. Interleukin-18 for predicting the development of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Clin. Immunol. 160:277. [DOI] [PubMed] [Google Scholar]
- 12. Bracaglia C., de Graaf K., Pires Marafon D. et al. . 2017. Elevated circulating levels of interferon-γ and interferon-γ-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann. Rheum. Dis. 76:166. [DOI] [PubMed] [Google Scholar]
- 13. Henter J. I., Horne A., Aricó M. et al. . 2007. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 48:124. [DOI] [PubMed] [Google Scholar]
- 14. Grupp S. A., Kalos M., Barrett D. et al. . 2013. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 368:1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pachlopnik Schmid J., Schmid J. P., Côte M. et al. . 2010. Inherited defects in lymphocyte cytotoxic activity. Immunol. Rev. 235:10. [DOI] [PubMed] [Google Scholar]
- 16. Lykens J. E., Terrell C. E., Zoller E. E., Risma K. and Jordan M. B. 2011. Perforin is a critical physiologic regulator of T-cell activation. Blood 118:618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castillo L. and Carcillo J. 2009. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/systemic inflammatory response syndrome/multiorgan dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr. Crit. Care Med. 10:387. [DOI] [PubMed] [Google Scholar]
- 18. Carcillo J. A., Halstead E. S., Hall M. W. et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network Investigators. 2017. Three hypothetical inflammation pathobiology phenotypes and pediatric sepsis-induced multiple organ failure outcome. Pediatr. Crit. Care Med. 18:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kyriazopoulou E., Leventogiannis K., Norrby-Teglund A. et al. ; Hellenic Sepsis Study Group. 2017. Macrophage activation-like syndrome: an immunological entity associated with rapid progression to death in sepsis. BMC Med. 15:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garcia P. C., Longhi F., Branco R. G., Piva J. P., Lacks D. and Tasker R. C. 2007. Ferritin levels in children with severe sepsis and septic shock. Acta Paediatr. 96:1829. [DOI] [PubMed] [Google Scholar]
- 21. Shakoory B., Carcillo J. A., Chatham W. W. et al. . 2016. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit. Care Med. 44:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. George M. R. 2014. Hemophagocytic lymphohistiocytosis: review of etiologies and management. J. Blood Med. 5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farquhar J. W. and Claireaux A. E. 1952. Familial haemophagocytic reticulosis. Arch. Dis. Child. 27:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sullivan K. E., Delaat C. A., Douglas S. D. and Filipovich A. H. 1998. Defective natural killer cell function in patients with hemophagocytic lymphohistiocytosis and in first degree relatives. Pediatr. Res. 44:465. [DOI] [PubMed] [Google Scholar]
- 25. Stepp S. E., Dufourcq-Lagelouse R., Le Deist F. et al. . 1999. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science 286:1957. [DOI] [PubMed] [Google Scholar]
- 26. Chandrakasan S. and Filipovich A. H. 2013. Hemophagocytic lymphohistiocytosis: advances in pathophysiology, diagnosis, and treatment. J. Pediatr. 163:1253. [DOI] [PubMed] [Google Scholar]
- 27. Jessen B., Kögl T., Sepulveda F. E., de Saint Basile G., Aichele P. and Ehl S. 2013. Graded defects in cytotoxicity determine severity of hemophagocytic lymphohistiocytosis in humans and mice. Front. Immunol. 4:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gallimore A., Glithero A., Godkin A. et al. . 1998. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 187:1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Binder D., van den Broek M. F., Kägi D. et al. . 1998. Aplastic anemia rescued by exhaustion of cytokine-secreting CD8+ T cells in persistent infection with lymphocytic choriomeningitis virus. J. Exp. Med. 187:1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Badovinac V. P., Tvinnereim A. R. and Harty J. T. 2000. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science 290:1354. [DOI] [PubMed] [Google Scholar]
- 31. Su H. C., Nguyen K. B., Salazar-Mather T. P., Ruzek M. C., Dalod M. Y. and Biron C. A. 2001. NK cell functions restrain T cell responses during viral infections. Eur. J. Immunol. 31:3048. [DOI] [PubMed] [Google Scholar]
- 32. Jordan M. B., Hildeman D., Kappler J. and Marrack P. 2004. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood 104:735. [DOI] [PubMed] [Google Scholar]
- 33. Brisse E., Wouters C. H. and Matthys P. 2015. Hemophagocytic lymphohistiocytosis (HLH): a heterogeneous spectrum of cytokine-driven immune disorders. Cytokine Growth Factor Rev. 26:263. [DOI] [PubMed] [Google Scholar]
- 34. Crozat K., Hoebe K., Ugolini S. et al. . 2007. Jinx, an MCMV susceptibility phenotype caused by disruption of Unc13d: a mouse model of type 3 familial hemophagocytic lymphohistiocytosis. J. Exp. Med. 204:853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pachlopnik Schmid J., Ho C. H., Chrétien F. et al. . 2009. Neutralization of IFNgamma defeats haemophagocytosis in LCMV-infected perforin- and Rab27a-deficient mice. EMBO Mol. Med. 1:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kögl T., Müller J., Jessen B. et al. . 2013. Hemophagocytic lymphohistiocytosis in syntaxin-11-deficient mice: T-cell exhaustion limits fatal disease. Blood 121:604. [DOI] [PubMed] [Google Scholar]
- 37. Chijioke O., Müller A., Feederle R. et al. . 2013. Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein-Barr virus infection. Cell Rep. 5:1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sepulveda F. E., Maschalidi S., Vosshenrich C. A. et al. . 2015. A novel immunoregulatory role for NK-cell cytotoxicity in protection from HLH-like immunopathology in mice. Blood 125:1427. [DOI] [PubMed] [Google Scholar]
- 39. Hadchouel M., Prieur A. M. and Griscelli C. 1985. Acute hemorrhagic, hepatic, and neurologic manifestations in juvenile rheumatoid arthritis: possible relationship to drugs or infection. J. Pediatr. 106:561. [DOI] [PubMed] [Google Scholar]
- 40. Scott J. P., Gerber P., Maryjowski M. C. and Pachman L. M. 1985. Evidence for intravascular coagulation in systemic onset, but not polyarticular, juvenile rheumatoid arthritis. Arthritis Rheum. 28:256. [DOI] [PubMed] [Google Scholar]
- 41. Stéphan J. L., Zeller J., Hubert P., Herbelin C., Dayer J. M. and Prieur A. M. 1993. Macrophage activation syndrome and rheumatic disease in childhood: a report of four new cases. Clin. Exp. Rheumatol. 11:451. [PubMed] [Google Scholar]
- 42. Silverman E. D., Miller J. J. 3rd, Bernstein B. and Shafai T. 1983. Consumption coagulopathy associated with systemic juvenile rheumatoid arthritis. J. Pediatr. 103:872. [DOI] [PubMed] [Google Scholar]
- 43. Grom A. A. and Passo M. 1996. Macrophage activation syndrome in systemic juvenile rheumatoid arthritis. J. Pediatr. 129:630. [DOI] [PubMed] [Google Scholar]
- 44. Ramanan A. V. and Baildam E. M. 2002. Macrophage activation syndrome is hemophagocytic lymphohistiocytosis—need for the right terminology. J. Rheumatol. 29:1105. [PubMed] [Google Scholar]
- 45. Grom A. A., Villanueva J., Lee S., Goldmuntz E. A., Passo M. H. and Filipovich A. 2003. Natural killer cell dysfunction in patients with systemic-onset juvenile rheumatoid arthritis and macrophage activation syndrome. J. Pediatr. 142:292. [DOI] [PubMed] [Google Scholar]
- 46. Villanueva J., Lee S., Giannini E. H. et al. . 2005. Natural killer cell dysfunction is a distinguishing feature of systemic onset juvenile rheumatoid arthritis and macrophage activation syndrome. Arthritis Res. Ther. 7:R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cruikshank M., Anoop P., Nikolajeva O. et al. . 2015. Screening assays for primary haemophagocytic lymphohistiocytosis in children presenting with suspected macrophage activation syndrome. Pediatr. Rheumatol. Online J. 12(Suppl. 1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Put K., Vandenhaute J., Avau A. et al. . 2017. Inflammatory gene expression profile and defective interferon-γ and granzyme K in natural killer cells from systemic juvenile idiopathic arthritis patients. Arthritis Rheumatol. 69:213. [DOI] [PubMed] [Google Scholar]
- 49. Zhang K., Biroschak J., Glass D. N. et al. . 2008. Macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis is associated with MUNC13-4 polymorphisms. Arthritis Rheum. 58:2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vastert S. J., van Wijk R., D’Urbano L. E. et al. . 2010. Mutations in the perforin gene can be linked to macrophage activation syndrome in patients with systemic onset juvenile idiopathic arthritis. Rheumatology (Oxford) 49:441. [DOI] [PubMed] [Google Scholar]
- 51. Kaufman K. M., Linghu B., Szustakowski J. D. et al. . 2014. Whole-exome sequencing reveals overlap between macrophage activation syndrome in systemic juvenile idiopathic arthritis and familial hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. 66:3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang M., Behrens E. M., Atkinson T. P., Shakoory B., Grom A. A. and Cron R. Q. 2014. Genetic defects in cytolysis in macrophage activation syndrome. Curr. Rheumatol. Rep. 16:439. [DOI] [PubMed] [Google Scholar]
- 53. Schulert G. S., Bove K., McMasters R., Campbell K., Leslie N. and Grom A. A. 2015. 11-Month-old infant with periodic fevers, recurrent liver dysfunction, and perforin gene polymorphism. Arthritis Care Res. (Hoboken) 67:1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Filocamo G., Petaccia A., Torcoletti M., Sieni E., Ravelli A. and Corona F. 2016. Recurrent macrophage activation syndrome in spondyloarthritis and monoallelic missense mutations in PRF1: a description of one paediatric case. Clin. Exp. Rheumatol. 34:719. [PubMed] [Google Scholar]
- 55. Tesi B., Sieni E., Neves C. et al. . 2015. Hemophagocytic lymphohistiocytosis in 2 patients with underlying IFN-gamma receptor deficiency. J. Allergy Clin. Immunol. 2015;135:1638-41. doi:10.1016/j.jaci.2014.11.030 [DOI] [PubMed] [Google Scholar]
- 56. Cron R. Q. and Chatham W. W. 2016. Development of spondyloarthropathy following episodes of macrophage activation syndrome in children with heterozygous mutations in haemophagocytic lymphohistiocytosis-associated genes. Clin. Exp. Rheumatol. 34:953. [PubMed] [Google Scholar]
- 57. Schulert G. S., Zhang M., Fall N. et al. . 2016. Whole-exome sequencing reveals mutations in genes linked to hemophagocytic lymphohistiocytosis and macrophage activation syndrome in fatal cases of H1N1 influenza. J. Infect. Dis. 213:1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang M., Bracaglia C., Prencipe G. et al. . 2016. A heterozygous RAB27A mutation associated with delayed cytolytic granule polarization and hemophagocytic lymphohistiocytosis. J. Immunol. 196:2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jenkins M. R., Rudd-Schmidt J. A., Lopez J. A. et al. . 2015. Failed CTL/NK cell killing and cytokine hypersecretion are directly linked through prolonged synapse time. J. Exp. Med. 212:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Spessott W. A., Sanmillan M. L., McCormick M. E. et al. . 2015. Hemophagocytic lymphohistiocytosis caused by dominant-negative mutations in STXBP2 that inhibit SNARE-mediated membrane fusion. Blood 125:1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang K., Jordan M. B., Marsh R. A. et al. . 2011. Hypomorphic mutations in PRF1, MUNC13-4, and STXBP2 are associated with adult-onset familial HLH. Blood 118:5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chia J., Yeo K. P., Whisstock J. C., Dunstone M. A., Trapani J. A. and Voskoboinik I. 2009. Temperature sensitivity of human perforin mutants unmasks subtotal loss of cytotoxicity, delayed FHL, and a predisposition to cancer. Proc. Natl Acad. Sci. USA 106:9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Trambas C., Gallo F., Pende D. et al. . 2005. A single amino acid change, A91V, leads to conformational changes that can impair processing to the active form of perforin. Blood 106:932. [DOI] [PubMed] [Google Scholar]
- 64. House I. G., Thia K., Brennan A. J. et al. . 2015. Heterozygosity for the common perforin mutation, p.A91V, impairs the cytotoxicity of primary natural killer cells from healthy individuals. Immunol. Cell Biol. 93:575. [DOI] [PubMed] [Google Scholar]
- 65. Sepulveda F. E., Garrigue A., Maschalidi S. et al. . 2016. Polygenic mutations in the cytotoxicity pathway increase susceptibility to develop HLH immunopathology in mice. Blood 127:2113. [DOI] [PubMed] [Google Scholar]
- 66. Bryceson Y. T., Pende D., Maul-Pavicic A. et al. . 2012. A prospective evaluation of degranulation assays in the rapid diagnosis of familial hemophagocytic syndromes. Blood 119:2754. [DOI] [PubMed] [Google Scholar]
- 67. Strippoli R., Carvello F., Scianaro R. et al. . 2012. Amplification of the response to Toll-like receptor ligands by prolonged exposure to interleukin-6 in mice: implication for the pathogenesis of macrophage activation syndrome. Arthritis Rheum. 64:1680. [DOI] [PubMed] [Google Scholar]
- 68. Cifaldi L., Prencipe G., Caiello I. et al. . 2015. Inhibition of natural killer cell cytotoxicity by interleukin-6: implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheumatol. 67:3037. [DOI] [PubMed] [Google Scholar]
- 69. Jessen B., Bode S. F., Ammann S. et al. . 2013. The risk of hemophagocytic lymphohistiocytosis in Hermansky-Pudlak syndrome type 2. Blood 121:2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lek M., Karczewski K. J., Minikel E. V. et al. ; Exome Aggregation Consortium. 2016. Analysis of protein-coding genetic variation in 60706 humans. Nature 536:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Strippoli R., Caiello I. and De Benedetti F. 2013. Reaching the threshold: a multilayer pathogenesis of macrophage activation syndrome. J. Rheumatol. 40:761. [DOI] [PubMed] [Google Scholar]
- 72. Rood J. E., Rao S., Paessler M. et al. . 2016. ST2 contributes to T-cell hyperactivation and fatal hemophagocytic lymphohistiocytosis in mice. Blood 127:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Krebs P., Crozat K., Popkin D., Oldstone M. B. and Beutler B. 2011. Disruption of MyD88 signaling suppresses hemophagocytic lymphohistiocytosis in mice. Blood 117:6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zoller E. E., Lykens J. E., Terrell C. E. et al. . 2011. Hemophagocytosis causes a consumptive anemia of inflammation. J. Exp. Med. 208:1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Canna S. W., Wrobel J., Chu N., Kreiger P. A., Paessler M. and Behrens E. M. 2013. Interferon-γ mediates anemia but is dispensable for fulminant Toll-like receptor 9-induced macrophage activation syndrome and hemophagocytosis in mice. Arthritis Rheum. 65:1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Prencipe G., Caiello I., Pascarella A. et al. . 2017. Neutralization of interferon-gamma reverts clinical and laboratory features in a mouse model of macrophage activation syndrome. J. Allergy Clin. Immunol. in press: doi:10.1016/j.jaci.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 77. Behrens E. M., Canna S. W., Slade K. et al. . 2011. Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J. Clin. Invest. 121:2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Brisse E., Imbrechts M., Put K. et al. . 2016. Mouse cytomegalovirus infection in BALB/c mice resembles virus-associated secondary hemophagocytic lymphohistiocytosis and shows a pathogenesis distinct from primary hemophagocytic lymphohistiocytosis. J. Immunol. 196:3124. [DOI] [PubMed] [Google Scholar]
- 79. Takada H., Takahata Y., Nomura A., Ohga S., Mizuno Y. and Hara T. 2003. Increased serum levels of interferon-gamma-inducible protein 10 and monokine induced by gamma interferon in patients with haemophagocytic lymphohistiocytosis. Clin. Exp. Immunol. 133:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Holzinger D., Frosch M., Kastrup A. et al. . 2012. The Toll-like receptor 4 agonist MRP8/14 protein complex is a sensitive indicator for disease activity and predicts relapses in systemic-onset juvenile idiopathic arthritis. Ann. Rheum. Dis. 71:974. [DOI] [PubMed] [Google Scholar]
- 81. Wittkowski H., Frosch M., Wulffraat N. et al. . 2008. S100A12 is a novel molecular marker differentiating systemic-onset juvenile idiopathic arthritis from other causes of fever of unknown origin. Arthritis Rheum. 58:3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Quartier P., Allantaz F., Cimaz R. et al. . 2011. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial). Ann. Rheum. Dis. 70:747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schulert G. S., Minoia F., Bohnsack J. et al. . 2017. Biologic therapy modifies clinical and laboratory features of macrophage activation syndrome associated with systemic juvenile idiopathic arthritis. Arthritis Care Res. 2018;70:409-419. doi:10.1002/acr.23277 [DOI] [PubMed] [Google Scholar]
- 84. Ravelli A., Grom A. A., Behrens E. M. and Cron R. Q. 2012. Macrophage activation syndrome as part of systemic juvenile idiopathic arthritis: diagnosis, genetics, pathophysiology and treatment. Genes Immun. 13:289. [DOI] [PubMed] [Google Scholar]
- 85. Weiss E. S., Girard-Guyonvarc’h C., Holzinger D. et al. . 2018. Interleukin-18 diagnostically distinguishes and pathogenically promotes human and murine macrophage activation syndrome. Blood. doi:10.1182/blood-2017-12-820852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Novick D., Kim S., Kaplanski G. and Dinarello C. A. 2013. Interleukin-18, more than a Th1 cytokine. Semin. Immunol. 25:439. [DOI] [PubMed] [Google Scholar]
- 87. Girard C., Rech J., Brown M. et al. . 2016. Elevated serum levels of free interleukin-18 in adult-onset Still’s disease. Rheumatology (Oxford) 55:2237. [DOI] [PubMed] [Google Scholar]
- 88. Canna S. W., Girard C., Malle L. et al. . 2017. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J. Allergy Clin. Immunol. 139:1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. de Jager W., Vastert S. J., Beekman J. M. et al. . 2009. Defective phosphorylation of interleukin-18 receptor beta causes impaired natural killer cell function in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 60:2782. [DOI] [PubMed] [Google Scholar]
- 90. Maltez V. I., Tubbs A. L., Cook K. D. et al. . 2015. Inflammasomes coordinate pyroptosis and natural killer cell cytotoxicity to clear infection by a ubiquitous environmental bacterium. Immunity 43:987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Romberg N., Al Moussawi K., Nelson-Williams C. et al. . 2014. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat. Genet. 46:1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Canna S. W., de Jesus A. A., Gouni S. et al. . 2014. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat. Genet. 46:1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Liang J., Alfano D. N., Squires J. E. et al. . 2017. Novel NLRC4 mutation causes a syndrome of perinatal autoinflammation with hemophagocytic lymphohistiocytosis, hepatosplenomegaly, fetal thrombotic vasculopathy, and congenital anemia and ascites. Pediatr. Dev. Pathol. 20:498. [DOI] [PubMed] [Google Scholar]
- 94. Rigante D., Emmi G., Fastiggi M., Silvestri E. and Cantarini L. 2015. Macrophage activation syndrome in the course of monogenic autoinflammatory disorders. Clin. Rheumatol. 34:1333. [DOI] [PubMed] [Google Scholar]
- 95. Girard-Guyonvarc’h C., Palomo J., Martin P. et al. . 2018. Unopposed IL-18 signaling leads to severe TLR9-induced macrophage activation syndrome in mice. Blood. doi:10.1182/blood-2017-06-789552. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 96. Bracaglia C., Prencipe G., Gatto A. et al. . 2015. Anti interferon-gamma (IFN gamma) monoclonal antibody treatment in a child with NLRC4-related disease and severe hemophagocytic lymphohistiocytosis (HLH). Pediatr. Blood Cancer 62:S123. [Google Scholar]
- 97. Wada T., Kanegane H., Ohta K. et al. . 2014. Sustained elevation of serum interleukin-18 and its association with hemophagocytic lymphohistiocytosis in XIAP deficiency. Cytokine 65:74. [DOI] [PubMed] [Google Scholar]
- 98. Marsh R. A., Madden L., Kitchen B. J. et al. . 2010. XIAP deficiency: a unique primary immunodeficiency best classified as X-linked familial hemophagocytic lymphohistiocytosis and not as X-linked lymphoproliferative disease. Blood 116:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kitamura A., Sasaki Y., Abe T., Kano H. and Yasutomo K. 2014. An inherited mutation in NLRC4 causes autoinflammation in human and mice. J. Exp. Med. 211:2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Volker-Touw C. M., de Koning H. D., Giltay J. C. et al. . 2017. Erythematous nodes, urticarial rash and arthralgias in a large pedigree with NLRC4-related autoinflammatory disease, expansion of the phenotype. Br. J. Dermatol. 176:244. [DOI] [PubMed] [Google Scholar]
- 101. Kawasaki Y., Oda H., Ito J. et al. . 2017. Identification of a high-frequency somatic NLRC4 mutation as a cause of autoinflammation by pluripotent cell-based phenotype dissection. Arthritis Rheumatol. 69:447. [DOI] [PubMed] [Google Scholar]
- 102. Speckmann C., Lehmberg K., Albert M. H. et al. . 2013. X-linked inhibitor of apoptosis (XIAP) deficiency: the spectrum of presenting manifestations beyond hemophagocytic lymphohistiocytosis. Clin. Immunol. 149:133. [DOI] [PubMed] [Google Scholar]
- 103. Shimizu M., Yokoyama T., Yamada K. et al. . 2010. Distinct cytokine profiles of systemic-onset juvenile idiopathic arthritis-associated macrophage activation syndrome with particular emphasis on the role of interleukin-18 in its pathogenesis. Rheumatology (Oxford) 49:1645. [DOI] [PubMed] [Google Scholar]