Abstract
Background:
A number of endocrine disrupting chemicals (EDC) have been associated with gestational diabetes (GDM) risk factors. However, no human study has investigated the association between pregnancy exposure to parabens, a class of EDCs, and pregnancy glucose levels, a risk factor for GDM. Furthermore, little is known about this association in subfertile women—a group at high risk of GDM.
Methods:
A total of 241 women from the Environment and Reproductive Health Study had data available on 1st and/or 2nd trimester urinary methylparaben, propylparaben, and butylparaben concentrations, and blood glucose levels after the glucose loading test (GLT), a non-fasting 50 gram glucose loading test taken at late 2nd trimester. Trimester-specific associations between specific gravity adjusted methylparaben, butylparaben, and propylparaben with adjusted mean of pregnancy glucose levels were evaluated in linear regression models, using quartiles of each paraben’s distribution, and as a paraben mixture, using mutual adjustment and Bayesian kernel machine regression (BKMR), a recently proposed method for investigating chemical mixtures that flexibly models the joint effect of chemicals.
Results:
Investigating parabens one at the time did not provide any significant results. When investigating parabens as a chemical mixture with both multiple regression and BKMR, we observed positive associations of butylparaben (e.g comparing the 4th and 1st quartiles) with glucose levels, for both the 1st trimester (adjusted difference=12.5 mg/dL; 95% CI: 0.9, 24.2) and 2nd trimester (adjusted difference=11.2 mg/dL; 95% CI: 0.2, 22.3), and a negative association between 1st trimester propylparaben and glucose (adjusted difference=−22.3 mg/dL; 95% CI: −43.2, −1.4).
Conclusions:
We found 1st trimester butylparaben and propylparaben urinary concentrations to be associated with glucose levels in a pregnancy cohort of women at high risk of GDM, even after adjusting for potential confounders. Because exposure to parabens is widespread, these findings may suggest further investigating the effects of this chemical class on pregnancy health.
Keywords: gestational diabetes; glucose; endocrine disruptors, environmental epidemiology; pregnancy, parabens
Introduction
Gestational diabetes mellitus (GDM) is one of the most common pregnancy complications, affecting over 9% of all pregnancies in the United States.(1) Some population subgroups, including women with subfertility, are at particularly high-risk of GDM, with an up to 2-fold increased risk of this pregnancy complication.(2) Elevated pregnancy glucose levels, typically assessed in the late second trimester, are the first step in GDM diagnosis, when using a two-step screening method for this pregnancy complication.(3) Studies have suggested that even elevated pregnancy glucose levels that do not cross the clinical threshold for GDM diagnosis may also confer an increased risk of adverse pregnancy and delivery outcomes such as high birth weight,(4) preeclampsia,(5) and perinatal depression.(6) Higher glucose levels in pregnancy are associated with a linear increase in adverse pregnancy and neonatal outcomes including preeclampsia, need for cesarean delivery, large for gestational age infant, neonatal hypoglycemia, and >90th percentile for neonatal skin-fold thickness.(4,7) Furthermore, prenatal depression is more common in women with abnormal glucose levels from glucose loading tests (GLT), even if they don’t develop GDM.(6) Finally, GDM is associated with long-term health risks in mothers and their offspring, including obesity, type 2 diabetes, and atherosclerosis.(8–10)
The rate of GDM has increased over the last decades, suggesting lifestyle and environmental exposures may contribute to the development of this condition.(11,12) In particular, a growing body of literature suggests that environmental chemicals with endocrine disrupting properties may act as metabolic disruptors, with impact on glucose metabolism and risk of diabetes and cardiovascular disease.(13) In the context of pregnancy, these metabolic disruptors may have an impact on risk of GDM.(13) Parabens, chemicals widely used as preservatives in personal care products, pharmaceuticals, and foods, may also operate as endocrine disrupting chemicals (EDCs).(14–16) While phthalates and bisphenol A have been more extensively examined as metabolic disruptors as it relates to pregnancy complications,(17,18) parabens have been relatively understudied. A recent study in a representative sample of the U.S. population in the 2011–2012 National Health and Nutrition Examination Survey (NHANES) detected common parabens such as methylparaben and propylparaben in over 90% of the participants, and butylparaben in over 50%.(19) Parabens are weakly estrogenic, with the ability to bind to both estrogen receptors (ER)α and (ER)β.(17–21) Similar to bisphenol A, parabens may be able to impact glucose metabolism through estrogen-dependent signaling that could alter normal beta cell functioning.(22) As such, exposure to parabens during pregnancy—an increasing insulin resistant state—could impact pregnancy glucose levels and subsequent GDM risk. Yet, no human study has evaluated the association between parabens and pregnancy glucose levels.
Therefore, we evaluated the association between urinary paraben concentrations and blood glucose levels assessed at the standard glucose tolerance test taken at the 2nd trimester of pregnancy utilizing data from a prospective pregnancy cohort of women seeking treatment at a fertility clinic. We assessed whether both 1st and 2nd trimester concentrations of parabens, individually and as a mixture of parabens, were related to this GDM risk factor. To our knowledge, this is the first human study to investigate the association between urinary concentration of parabens and glucose levels during pregnancy among subfertile women, a group of women at high risk of developing GDM.
Methods
Study population
Study participants were women enrolled in the Environment and Reproductive Health (EARTH) Study, an ongoing prospective cohort established in 2004 to evaluate environmental and dietary determinants of fertility. EARTH enrolls women between 18 and 45 years from the Massachusetts General Hospital (MGH) Fertility Center (Boston, MA). For this study, we used data on women enrolled between 2005 and 2015 who had completed at least one in vitro fertilization (IVF) cycle, and had contributed at least one urinary sample during first or/and second trimester. We a priori excluded IVF cycles for which women used an egg donor (n=18) and cryo-thaw cycles (n=34). In total, 241 women with pregnancies that resulted in live births and had data on parabens were included in this study. Only the first pregnancy was evaluated if participating women had more pregnancies during the time study. The study was approved by the Human Studies Institutional Review Boards of the MGH, Harvard T.H. Chan School of Public Health, and the Centers for Disease Control and Prevention (CDC).
Outcome assessment
The main outcome of the study was blood glucose levels assessed at MGH during the 2nd trimester of pregnancy (median: 27 weeks gestation) through a 1-hour non-fasting, 50-gram GLT used as the first step in screening for GDM.(23) We evaluated glucose levels as a continuous outcome, without any transformation because the distribution was not skewed. In sensitivity analyses we also evaluated blood glucose as a binary outcome. For this, we compared women with glucose levels ≥140mg/dL from the GLT to women with glucose levels <140mg/dL; women with results above 140mg/dL are referred for additional GDM screening as a part of the two-step Carpenter-Coustan criteria employed by MGH throughout the entire study.(21) As such, we denote women with glucose levels ≥140mg/dL as having abnormal GLT
Urine sample collection and quantification of paraben concentrations
Urine samples were collected during the 1st and 2nd trimesters of pregnancy (median: 7 and 21 gestation weeks, respectively), with up to two spot urine samples for each time point. Urine was collected in sterile polypropylene cups at the MGH. Specific gravity (SG) was measured within one hour of urine collection, at room temperature, and using a handheld refractometer (National Instrument Company, Inc., Baltimore, MD, USA) calibrated with deionized water before each measurement. Samples were stored at −80 °C and shipped to the CDC for analysis. Urinary concentrations of total (free + conjugated) methylparaben, propylparaben, and butylparaben were quantified as described in detail elsewhere.(24) In brief, 100 μL of urine were treated with β-glucuronidase/sulfatase (Helix pomatia, H1; Sigma Chemical Co, St. Louis, MO, USA) to hydrolyze the paraben-conjugated species. Each paraben was retained and concentrated by online solid phase extraction, separated from each other and other urine matrix components by high-performance liquid chromatography, and detected by isotope dilution negative ion-atmospheric pressure chemical ionization tandem mass spectrometry. The limits of detection (LODs) were 1.0 μg/L for methylparaben, and 0.2 μg/L for propylparaben and butylparaben. All paraben concentrations were adjusted for SG using the formula Pc = P [(1.014 − 1)/(SG – 1)], where Pc is the SG-corrected paraben concentration (μg/L), P is the measured paraben concentration (μg/L), and 1.014 is the mean SG concentration in the study population included in the analysis. When two urine samples were available (about 80% of measurements), we used the geometric mean of the SG-adjusted concentrations as a measure of trimester-specific urinary paraben concentration. For concentrations below LOD, we assigned a value equal to the LOD divided by the square root of 2, prior to SG adjustment.(25)
Covariate assessment
Information on sociodemographic factors, family medical history, and lifestyle factors, were obtained from a brief questionnaire administrated by the study staff at enrollment and from a detailed take-home questionnaire. Pre-pregnancy body mass index (BMI) was calculated as weight (in kilograms) over height (in meters) squared, using anthropometric information measured by the nurse at enrollment. Clinical information was abstracted from the patient’s electronic medical record. Infertility diagnosis by a physician was assigned to each patient based on the Society for Assisted Reproductive Technology. All statistical models were adjusted for the following a priori identified potential confounders based on the literature: maternal age (continuous, years), pre-pregnancy BMI (continuous, kg/m2), total physical activity (continuous, h/week), race (binary, white/non-white), self-reported smoking status (binary, ever/never), education levels (binary, some college degree or lesser vs college graduate or higher), infertility diagnosis (female factor, male factor, or unexplained), number of fetuses (binary, one/more), previous IVF, previous intrauterine insemination (IUI). Since missing data proportion was negligible (<15%), all adjusted models were conducted as complete-case analyses.
Statistical analysis
We present descriptive characteristics in the overall population and by categories of glucose levels from the GLT. For both 1st and 2nd trimesters, we calculated SG-adjusted geometric means, 5th, 25th, 75th and 95th percentiles, as well as the minimum and maximum parabens concentrations.
To relax the assumption of linearity in the dose-response associations, we categorized all paraben biomarkers concentrations into quartiles of their distribution and presented statistical associations by using the lowest quartile as referent category. Information on urinary paraben concentrations at the 1st and 2n medical visits were independently used as two separate exposures. A set of sensitivity analyses was also conducted: i) since the proportion of butylparaben measurements below the LOD was about one third, we replicated the analysis for the single butylparaben by using tertiles of the distribution, so to have all values below the LOD in the referent group; ii) we further excluded all women with a diagnosis of Polycystic Ovary Syndrome (PCOS, n=22); iii) we further excluded women under anti-diabetic medications at baseline (n=7); iv) we evaluated BMI (<25 vs ≥ 25 kg/m2), age (<37, ≥37 years) as potential effect modifier by means of stratified analysis; v) we replicated the main analysis by evaluating glucose as a binary outcome, evaluating the odds of abnormal GLT with multivariable-adjusted logistic regression models; and vi) we further adjusted for calendar year, to evaluate whether eventual changes in paraben levels over the 10 years of recruitment could have influenced the results.
In this first set of analyses, methylparaben, butylparaben, and propylparaben were evaluated as three separated categorical predictors, and linear regression models were used to estimate differences in mean glucose level. Next, we evaluated the simultaneous exposure to the three parabens as a chemical mixture. For this, we first ran a multiple regression mutually-adjusting for all parabens. Next, we used Bayesian kernel machine regression (BKMR), a recently proposed method for investigating chemical mixtures that flexibly models the joint effect of chemicals using a kernel function.(26) BKMR allows the visualization of the exposure-response association for each component of a mixture, while taking into account the correlation between the mixture components. In addition, possible synergistic and non-linear effects can be evaluated. We presented results from this analysis by displaying: i) the paraben-specific dose-response curves; and ii) the difference in pregnancy glucose levels for a change in paraben concentration between the 10th and 90th percentile. For both analyses we set the other components of the mixture at their median values. For all BKMR models, measurements of SG-adjusted parabens concentrations were treated as continuous predictors and log-transformed due to their pronounced right-skewedness.
All analyses were performed in Stata, version 15 (StataCorp, College Station, Texas), with exception of the BKMR models that were conducted using the specific R package bkmr. Statistical tests were two-tailed and all p-values<0.05 were conventionally regarded to as statistically significant.
Results
Descriptive statistics of the study population are shown in Table 1. Women with abnormal GLT had higher BMI, had lower levels of physical activity, were more likely to be Asian, and more likely to have a diagnosis of infertility due to female factor. Other baseline characteristics were similar among women regardless of blood glucose levels. We detected frequently methylparaben and propylparaben (100% and ~98%, respectively), while butylparaben was detected in 64.2% and 64.7% of the urinary samples of the 1st and 2nd trimester, respectively (Table 2). Median differences in the time between exposure and outcome measurements were 20 and 6 weeks (respectively for 1st trimester urinary parabens concentrations and 2nd trimester glucose levels, as well as 2nd trimester urinary parabens concentrations and 2nd trimester glucose levels). Correlations between the two measurements of the same metabolites (between-trimester correlations) were moderate for both butylparaben (r=0.64), propylparaben (r=0.45), and methylparaben (r=0.33).
Table 1.
Baseline characteristics of 241 women included in the analysis, overall, and by impaired glucose tolerance status (glucose from GLT ≥140 mg/dL versus <140 mg/dL)
| Characteristics | Total (n=241) | Glucose <140 (n=199) | Glucose≥140 (n=42) |
|---|---|---|---|
| Maternal age, years (sd) | 35.3 (3,8) | 35.3 (3,8) | 35.7 (3.7) |
| BMI, kg/m2 (sd) | 24.2 (4.8) | 24.0 (4.6) | 25.1 (5.7) |
| Total physical activity, h/week (sd) | 6.7 (6.9) | 6.9 (7.2) | 5.6 (5.0) |
| Smoking, n (%) | |||
| Never | 180 (75) | 151 (76) | 29 (69) |
| Former | 54 (22) | 43 (22) | 11 (26) |
| Current | 7 (3) | 5 (3) | 2 (5) |
| Race, n (%) | |||
| White | 209 (87) | 176 (88) | 33 (79) |
| Black/African American | 5 (2) | 4 (2) | 1 (2) |
| Asian | 17 (7) | 10 (5) | 7 (17) |
| Other | 10 (4) | 9 (5) | 1 (2) |
| Education, n (%) | |||
| High school graduate or less | 23 (10) | 18 (9) | 5 (12) |
| Some college | 6 (2) | 6 (3) | 0 (0) |
| College graduate or higher | 212 (88) | 175 (88) | 37 (88) |
| Infertility diagnosis, n (%) | |||
| Male factor | 77 (32) | 64 (32) | 13 (31) |
| Female factor | 68 (28) | 49 (25) | 19 (45) |
| Unexplained | 96 (40) | 86 (43) | 10 (24) |
| Previous IVF, n(%) | 135 (56) | 111 (56) | 24 (57) |
| Previous IUI, n(%) | 54 (22) | 44 (22) | 10 (24) |
| Currently under medication, n (%) | 7 (3) | 5 (3) | 2 (5) |
| More than one fetus, n (%) | 46 (19) | 36 (18) | 10 (24) |
IVF=in vitro fertilization, IUI=intra uterine insemination, GLT=glucose load test, BMI=body mass index.
Table 2.
Distribution of trimester-specific urinary paraben concentrations (μg/L)
| Paraben type | Geometric mean (μg/L) | Detection frequency | Distribution percentiles (μg/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | 5th | 25th | 50th | 75th | 95th | Max | |||
| 1st trimester (n=204) | |||||||||
| Methylparaben (MP) | 102.9 | 100% | 1.4 | 5.8 | 27 | 113 | 406.2 | 1549.8 | 4770.7 |
| Sg-adjusted MP | 124.1 | 100% | 1.7 | 11.3 | 39.5 | 122.7 | 425.6 | 1443.8 | 3850 |
| Propylparaben (PP) | 19.3 | 98.5% | <LOD* | <LOD | 5 | 20 | 93.1 | 344.1 | 621 |
| SG-adjusted PP | 23.2 | 98.5% | <LOD | <LOD | 7.3 | 25.7 | 110.1 | 331.9 | 855 |
| Butylparaben (BP) | 0.8 | 64.2% | <LOD | <LOD | <LOD | 0.5 | 3.6 | 41.5 | 242 |
| SG-adjusted BP | 0.94 | 64.2% | <LOD | <LOD | <LOD | 0.7 | 3.9 | 34.6 | 239.6 |
| 2nd trimester (n=207) | |||||||||
| Methylparaben (MP) | 89.8 | 100% | 1.1 | 5 | 36.4 | 91 | 290 | 949 | 6098 |
| Sg-adjusted MP | 120.2 | 100% | 0.9 | 9.9 | 51 | 137.1 | 364 | 1092.9 | 4573.6 |
| Propylparaben (PP) | 19 | 98.1% | <LOD | <LOD | 4.4 | 25.7 | 79.4 | 367 | 2291 |
| SG-adjusted PP | 25.3 | 98.1% | <LOD | <LOD | 8.6 | 33.9 | 114.7 | 347 | 1718.4 |
| Butylparaben (BP) | 0.8 | 64.7% | <LOD | <LOD | <LOD | 0.5 | 3.1 | 36.9 | 282 |
| SG-adjusted BP | 1 | 64.7% | <LOD | <LOD | <LOD | 0.8 | 4.9 | 43 | 171.4 |
The limits of detection (LODs) were 1.0 μg/L for methylparaben and 0.20 μg/L for propylparaben and butylparaben. All concentrations below LOD were assigned a value equal to the LOD divided by square root of 2.
Table 3 presents unadjusted and adjusted differences in late 2nd trimester glucose levels across quartiles of 1st and 2nd trimester urinary parabens concentrations, evaluating parabens in separate statistical models. Positive associations were seen between butylparaben and pregnancy glucose levels in both the 1st trimester (comparing the 2nd, 3rd, and 4th quartiles with the 1st quartile of butylparaben, respectively: adj. β=7.5 mg/dL, 95% CI:−3.4,18.9; adj. β=7.7 mg/dL, 95% CI:−3.0, 18.4; adj. β=8.1 mg/dL; 95% CI:−2.6, 18.9) and 2nd trimester (adj. β=13.6 mg/dL, 95% CI:3.5, 23.7; adj. β=11.8 mg/dL, 95% CI:1.7, 22; adj. β=10.1 mg/dL; 95% CI:0.7, 20.2, respectively). No evidence of an association between methylparaben and glucose was observed. On the other hand, 1st trimester urinary propylparaben concentrations had a suggestive inverse association with glucose levels; however, this finding had wide confidence intervals and did not reach statistical significance.
Table 3.
Multivariable-adjusted differences in pregnancy glucose levels in mg/dL from 50-gram GLT across categories of 1st and 2nd trimester urinary paraben concentrations*, evaluated in separate statistical models.
| 1st trimester (n=204) | 2nd trimester (n=207) | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted† | Unadjusted | Adjusted† | |||||
| Glucose levels (mg/dL) (95% CI) | Glucose levels (mg/dL) (95% CI) | |||||||
| Beta (mg/dL) | 95% CI | Beta (mg/dL) | 95% CI | Beta (mg/dL) | 95% CI | Beta (mg/dL) | 95% CI | |
| Methylparaben | ||||||||
| Q1 | 0 | Ref | 0 | Ref | 0 | Ref | 0 | Ref |
| Q2 | −5.1 | (−15.7, 5.4) | −4.4 | (−15.3, 6.5) | −2.8 | (−13.0, 7.4) | −1.6 | (−12.1, 8.8) |
| Q3 | −4.1 | (−14.6, 6.5) | −3.8 | (−14.8, 7.2) | −2.4 | (−12.5, 7.8) | −1.5 | (−11.9, 8.9) |
| Q4 | 0.3 | (−10.2, 10.9) | −1.1 | (−11.8, 9.6) | 2.2 | (−8.1, 12.4) | 0 | (−10.8, 10.8) |
| Propylparaben | ||||||||
| Q1 | 0 | Ref | 0 | Ref | 0 | Ref | 0 | Ref |
| Q2 | −6.9 | (−17.5, 3.6) | −8.2 | (−19.2, 2.7) | −5.9 | (−16.0, 4.2) | −5.7 | (−16.0, 4.6) |
| Q3 | −7.0 | (−17.5, 3.6) | −7.3 | (−18.3, 3.8) | 1.8 | (−8.3, 12.0) | 2.5 | (−7.9, 12.9) |
| Q4 | −4.4 | (−14.9, 6.2) | −6.6 | (−17.3, 4.2) | 2.5 | (−7.7, 12.7) | 1.0 | (−9.8, 11.7) |
| Butylparaben | ||||||||
| Q1 | 0 | Ref | 0 | Ref | 0 | Ref | 0 | Ref |
| Q2 | 8.8 | (−1.7, 19.3) | 7.5 | (−3.4, 18.9) | 12.7 | (2.8, 22.6) | 13.6 | (3.5, 23.7) |
| Q3 | 7.4 | (−3.2, 17.9) | 7.7 | (−3.0, 18.4) | 12.0 | (1.9, 22.1) | 11.8 | (1.7, 22.0) |
| Q4 | 6.7 | (−3.9, 17.2) | 8.1 | (−2.6, 18.9) | 8.7 | (−1.4, 18.8) | 10.1 | (0.7, 20.2) |
| Butylparaben‡ | ||||||||
| T1 | 0 | Ref | 0 | Ref | 0 | Ref | 0 | Ref |
| T2 | 4.7 | (−4.4, 13.8) | 4.1 | (−5.2, 13.4) | 3.8 | (−5.1, 12.6) | 2.2 | (−6.8, 11.2) |
| T3 | 5.8 | (−3.3, 14.9) | 7.1 | (−2.2, 16.4) | 3.0 | (−5.7, 11.7) | 3.5 | (−5.2, 12.2) |
Parabens concentrations log-transformed and SG-adjusted
Models adjusted for physical activity (continuous), maternal age (continuous), BMI (continuous), smoking, race, education, infertility diagnosis, number of fetuses.
Tertiles of Butylparaben
Evaluating butylparabens in tertiles provided smaller estimates but did not change the direction of the associations. Results were similar when excluding women with a diagnosis of PCOS or taking medication at baseline (data not shown). No evidence of effect modification by age, BMI was documented (data not shown). Finally, results were similar when investigating glucose levels as a binary outcome (<140 vs. ≥140mg/dL), and when further adjusting for calendar year (data not shown).
In Table 4, we present the association between pregnancy glucose levels and the parabens mixture evaluated with multiple regression, adjusting for potential confounders and for each paraben in the same model (mutually-adjusted). Methylparaben and propylparaben were highly correlated in both 1st and 2nd trimester samples (r=0.89 and r=0.79, for each trimester respectively), while lower and moderate correlations (r<0.50) were observed between butylparaben and the other two parabens at both time points. We observed positive associations between both 1st and early 2nd trimester butylparaben concentrations and late 2nd trimester pregnancy glucose levels. For example, when comparing the lowest and highest quartiles of 1st and 2nd trimester butylparaben, respectively: adj. β for 1st trimester=12.5 mg/dL, 95% CI:0.9, 24.2; adj. β for 2nd trimester=11.2 mg/dL, 95% CI:0.2,22.3). In addition, a pronounced negative dose-response was observed between 1st trimester propylparaben and glucose levels, with a 22.3 mg/dL lower glucose level when comparing the lowest and highest quartiles (β for 1st trimester=−22.3 mg/dL, 95% CI:−43.2,−1.4).
Table 4.
Multivariable-adjusted differences in glucose levels across categories of 1st and 2nd trimester parabens mixture, evaluated with multiple regression*
| 1st trimester (n=204) | 2nd trimester (n=207) | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted† | Unadjusted | Adjusted† | |||||
| Glucose levels (mg/dL) (95% CI) | ||||||||
| Beta | 95% CI | Beta | 95% CI | Beta | 95% CI | Beta | 95% CI | |
| Methylparaben | ||||||||
| Q1 | 0 | Ref | 0 | Ref | 0 | Ref | 0 | Ref |
| Q2 | −2.3 | (−14.2, 9.6) | −1.6 | (−13.1, 12.4) | −4.5 | (−16.1, 7.0) | −3.7 | (−15.4, 8.0) |
| Q3 | 5.9 | (−10.9, 22.8) | 5.9 | (−10.6, 24.9) | −5.1 | (−17.8, 7.6) | −4.4 | (−17.1, 8.4) |
| Q4 | 14.3 | (−5.8, 34.6) | 13.1 | (−7.9, 34.0) | −2.1 | (−16.9, 12.7) | −4.8 | (−19.8, 10.3) |
| Propylparaben | ||||||||
| Q1 | 0 | Ref | 0 | Ref | 0 | Ref | 0 | Ref |
| Q2 | −9.8 | (−21.4, 1.9) | −11.2 | (−23.4, 1.0) | −5.2 | (−16.4, 6.0) | −5.7 | (−17.1, 6.6) |
| Q3 | −17.0 | (−33.6, −0.4) | −17.7 | (−35.1, −0.3) | 1.8 | (−10.7, 14.4) | 2.2 | (−10.5, 14.8) |
| Q4 | −20.8 | (−40.9, −0.7) | −22.3 | (−43.2, −1.4) | 1.7 | (−12.9, 16.3) | 1.2 | (−13.6, 16.0) |
| Butylparaben | ||||||||
| Q1 | 0 | Ref | 0 | Ref | 0 | Ref | 0 | Ref |
| Q2 | 11.0 | (0.3, 21.6) | 10.0 | (−1.0, 21.0) | 12.3 | (2.2, 22.4) | 13.3 | (3.0, 23.6) |
| Q3 | 9.9 | (−1.0, 20.9) | 10.5 | (−0.6, 21.6) | 12.6 | (2.1, 23.0) | 12.3 | (1.8, 22.9) |
| Q4 | 10.7 | (−0.7, 22.2) | 12.5 | (0.9, 24.2) | 9.4 | (−1.5, 20.3) | 11.2 | (0.2, 22.3) |
Parabens concentrations are log-transformed,SG-adjusted, and mutually adjusted in the same statistical models
Models further adjusted for physical activity (continuous), maternal age (continuous), BMI (continuous), smoking, race, education, infertility diagnosis, number of fetuses.
Results from BKMR were in line with findings from multiple regression, with a positive association between 1st trimester butylparaben and glucose levels, and suggestive inverse association with 1st trimester propylparaben (Figure 1A). Specifically, the difference in glucose between the 10th and 90th percentile of each paraben evaluated at the 1st trimester was −13.9 mg/dL for propylparaben (95% CI: −30.4, 2.6), and 10.2 mg/dL for butylparaben (95% CI:0,20.4) (Figure 2). With BKMR, no clear evidence of associations was seen for 2nd trimester paraben concentrations and later 2nd trimester pregnancy glucose levels (Figure 1B). Also, no evidence of synergistic effects between the three parabens were detected (data not shown).
Figure 1.
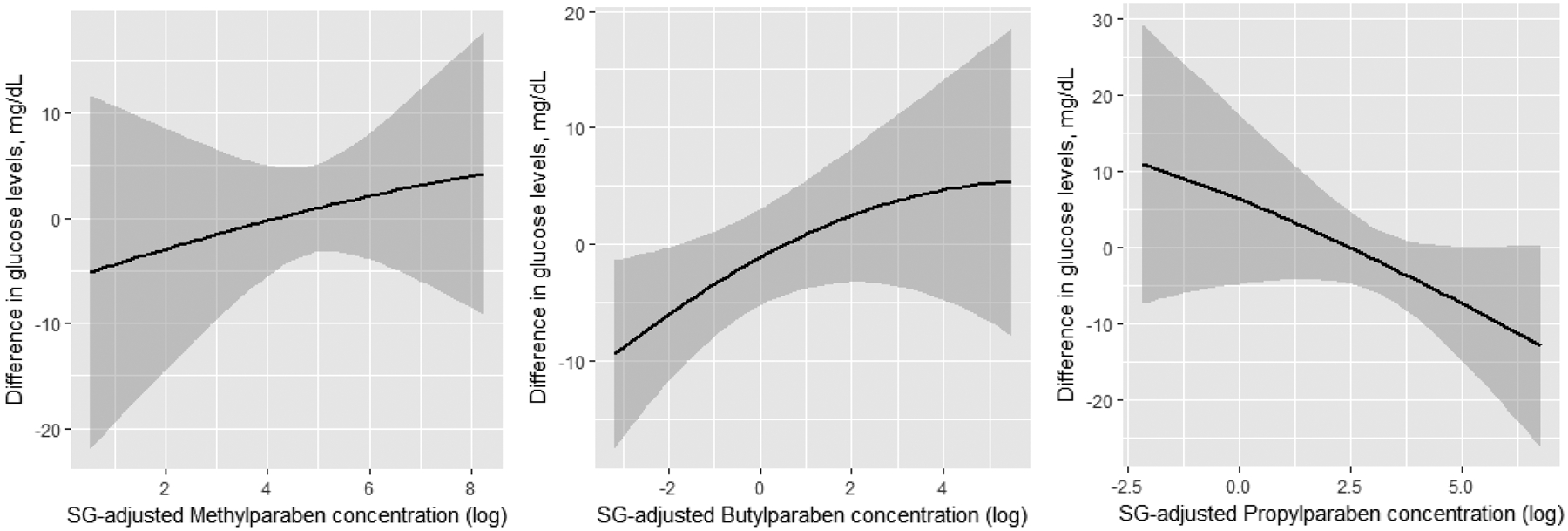
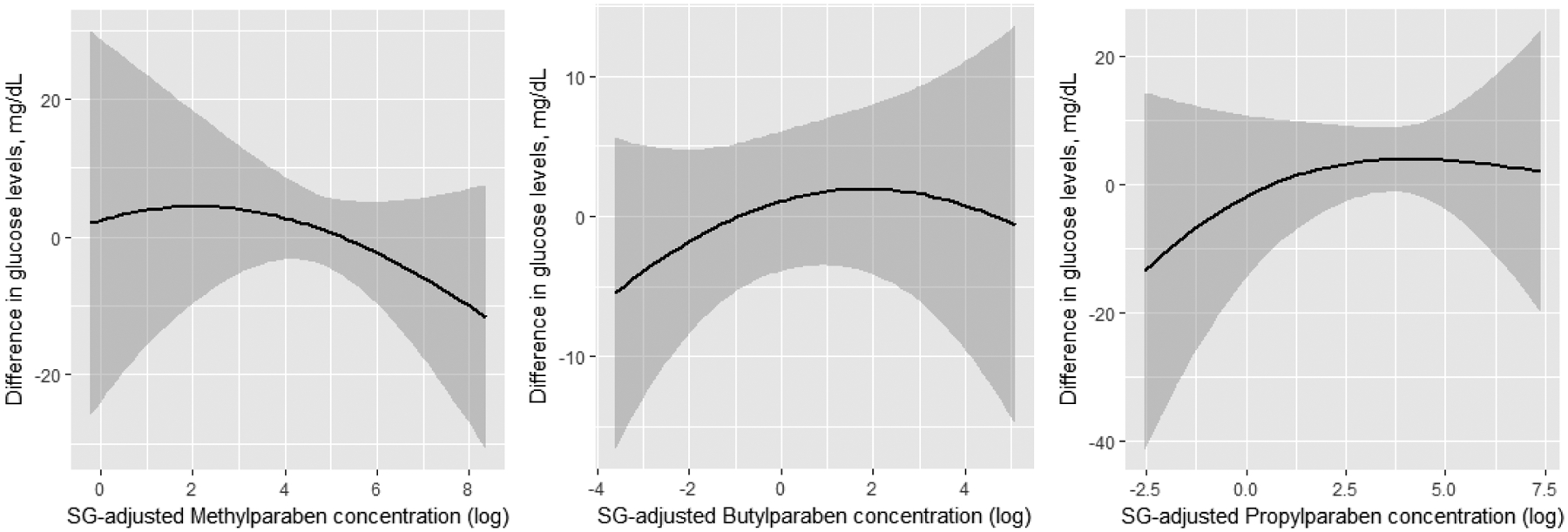
Dose-response associations between urinary parabens concentration (log-transformed and SG adjusted) at 1st (panel A) and 2nd (panel B) trimester, and later 2nd trimester glucose levels. Results obtained by running Bayesian Kernel Machine Regression (BKMR) for the mixture of parabens further adjusting for physical activity (continuous), maternal age (continuous), BMI (continuous), smoking, race, education, infertility diagnosis, number of fetuses.
Figure 2.
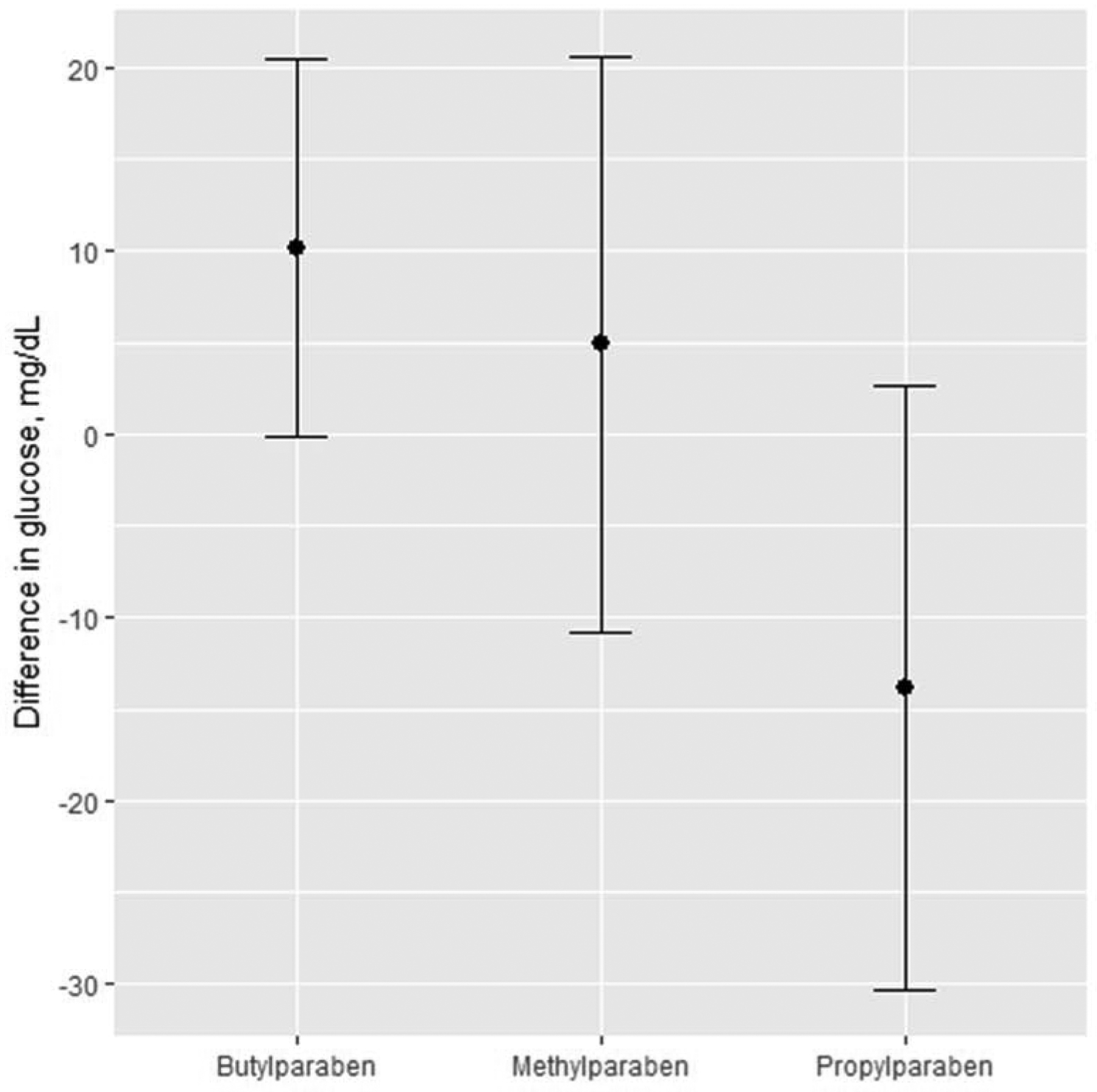
Differences in glucose in mg/dL as a function of 1st trimester paraben concentrations. Point estimates for exposure changes between the 10th and 90th percentile of their distribution.
Discussion
In the present study from a prospective cohort of women from a fertility clinic, we found urinary concentrations of butylparaben to be positively associated with blood glucose levels, when assessed individually and as a mixture with two other parabens. We also found inverse associations between urinary propylparaben concentrations and glucose levels. These associations where more pronounced when assessing parabens as a chemical mixture. Given the ubiquitous nature of parabens exposure, these findings suggest further evaluation of paraben exposures as possibly modifiable risk factors of pregnancy glucose levels in higher-risk women.
Recent studies have suggested that exposure to EDCs, such as phthalates and bisphenol A, may be possible risk factors for GDM.(13,27–29) This is of particular concern in women conceiving with medically assisted reproduction, and women with underlying causes of infertility, who represent a high risk group with an increased risk of not only GDM, but also elevated glucose levels in pregnancy.(30,31) Moreover, such women could be particularly affected by exposure to EDCs that alter glucose metabolism.(32) Parabens are a group of alkyl esters of p-hydroxybenzoic acid commonly used as preservatives in cosmetics, personal care products, pharmaceuticals and food.(33) Methyl, propyl, and butyl parabens have been detected in a large proportion of the U.S. population,(19) thus suggesting widespread exposure to these compounds. Several in vitro and in vivo studies have demonstrated the endocrine disrupting properties of parabens, including their weak estrogenicity; however the estrogenic properties of these chemicals varies by chain length, with butylparaben being the most estrogenic.(34–37)
While a handful of population-based studies have investigated parabens as they relate to pregnancy outcomes,(38–40) limited information exist about the association between paraben concentrations and glucose levels during pregnancy. Such associations may be valid and could operate through a number of pathways. First, parabens can activate glucocorticoid receptors,(41,42) thus interfering with normal glucose regulation. Second, parabens could also act as a thyroid hormone receptor and could alter normal thyroid functioning. Specifically, one study showed inverse associations between parabens and thyroid hormones known to be involved in glucose metabolism.(43) Third, parabens are agonist of peroxisome proliferator–activated receptor, which could alter glucose metabolism through alterations to adipocyte production and insulin resistance.(44) Potentially harmful effects of parabens have also been confirmed by recent human studies.(43,45,46) Interestingly and relevant to the current findings, in vitro studies have suggested that parabens, especially butylparaben, may contribute to obesity,(34,36) a risk factor for GDM.
In this study we observed a positive association between butylparaben and glucose levels. This finding is line with previous studies reporting stronger estrogenic effect and higher glucocorticoid-like activity for butylparaben.(38,41) On the other hand, we also reported a negative association between propylparaben and glucose levels. Of note, parabens may also differ by other properties, with respect to endocrine disruption. For example one study showed that butylparaben was able to increase plasma leptin.(47) Another study showed propylparaben had the ability to perturb mitochondrial function.(48,49) These potential differences could possibly explain differences in the associations between butylparaben and glucose levels and propylparaben and glucose levels. However, reasons for the associations between these parabens and glucose levels, along with the differing results, remain unclear and further work is needed to explain how parabens might be able to perturb glucose metabolism during pregnancy.
This study is subject to several limitations. First, due to the relatively low number of GDM cases we could not focus on clinically-diagnosed GDM and only evaluated continuous glucose levels from the 50-gram GLT that is standardly given to all women as a part of the GDM screening test in late 2nd trimester. Second, our analysis did not include other chemicals such as phthalates and BPA; exposure to these chemicals may correlate with exposure to parabens and be associated with glucose levels. Future studies should evaluate exposure to mixtures of several chemical classes as this relates to GDM. Nevertheless, this is one of the first studies to evaluate paraben mixtures as it relates to pregnancy glucose levels. Third, we evaluated results from the non-fasting GDM screening test that could be influenced by the timing or content of the last meal, information that was unavailable in our dataset. In addition, all our results are based on a population of women seeking care at a fertility clinic, and results may not be generalizable to women who conceived naturally or to subgroups of the population under-represented in our sample (e.g. non-white). Finally, despite having up to two exposure assessments, 1st and 2nd trimester urinary concentrations of parabens were treated separately because previous studies have suggested trimester-specific associations for GDM risk factors. Future studies with larger sample sizes should investigate the trajectory of exposure and take into account changes in the urinary concentrations of parabens occurring during pregnancy. It is also important to mention that parabens are non-persistent chemicals with low to moderate variability, and exposure misclassification may exist. In this study the correlation between paraben concentrations in the first and second trimesters was moderate. That said, we evaluated multiple time periods finding differing associations, which may be suggestive, in agreement with previous studies,(27,29) that second trimester may be a particularly sensitive time window of exposure with respect to the association between parabens and glucose levels in pregnancy. Future studies should focus on known sources of parabens such as personal care products, evaluating whether these change over pregnancies or not.
Our study also has several strengths. First, to our knowledge, this was the first study investigating pregnancy exposure to parabens as it relates to glucose levels in the 2nd trimester of pregnancy, a clinically-relevant indicator of future risk of GDM. Moreover, the prospective design of the study, with two exposure assessments in the 1st and 2n trimesters, allowed us to evaluate a larger and potentially sensitive time window of pregnancy. The prospective nature of the study also strengthens the interpretation of our results by reducing the risk of reverse causation. Third, in addition to evaluating the independent contribution of three parabens, we investigated parabens as a chemical mixture.(50) In particular, by using the recently proposed BKMR, we were able to flexibly evaluate the simultaneous contribution of multiple and highly-correlated exposures, while also taking into account potential non-linear dose-responses and interactions.(26)
In conclusion, we observed a positive association between pregnancy butylparaben urinary concentrations and glucose levels from a late 2nd trimester GDM screening test. We also found a suggestive negative association between propylparaben concentrations during the first trimester and pregnancy glucose levels. These findings suggest that certain parabens may have the ability to affect glucose regulation, with potential downstream impacts on GDM risk and associated sequelae. Subfertile women, given their higher risk of glucose intolerance in pregnancy, may be particularly vulnerable to the effects of these EDCs. Given the widespread use of parabens in consumer products and the increasing prevalence of GDM in U.S. women, future studies would benefit from further investigations of the association between parabens and pregnancy outcomes.
Acknowledgements:
The authors wish to thank Prabha Dwivedi, Xiaoliu Zhou, and Tao Jia for the quantification of chemicals biomarkers at the CDC.
Funding: This work was supported by National Institutes of Health Grants R01ES026166, R01ES022955, R01ES009718, R01ES000002, and P30ES000002 from the National Institute of Environmental Health Sciences.
Abbreviations:
- BKMR
Bayesian kernel machine regression
- EDC
endocrine disrupting chemicals
- GDM
gestational diabetes mellitus
- GLT
glucose loading test
- IVF
in-vitro fertilization
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
Conflict of interest: nothing to declare
References
- 1.DeSisto CL, Kim SY, Sharma AJ. Peer reviewed: Prevalence estimates of gestational diabetes mellitus in the United States, pregnancy risk assessment monitoring system (prams), 2007–2010. Preventing chronic disease. 2014;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luke B, Stern JE, Kotelchuck M, Declercq ER, Cohen B, Diop H. Birth outcomes by infertility diagnosis: analyses of the Massachusetts Outcomes Study of Assisted Reproductive Technologies (MOSART). The Journal of reproductive medicine. 2015. November;60:480. [PMC free article] [PubMed] [Google Scholar]
- 3.Kjos SL, Buchanan TA. Gestational diabetes mellitus. New England journal of medicine. 1999. December 2;341(23):1749–56. [DOI] [PubMed] [Google Scholar]
- 4.HAPO Study Cooperative Research Group, 2008. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med, 2008(358), pp.1991–2002. [DOI] [PubMed] [Google Scholar]
- 5.Bryson CL, Ioannou GN, Rulyak SJ, Critchlow C. Association between gestational diabetes and pregnancy-induced hypertension. American journal of epidemiology. 2003. December 15;158(12):1148–53. [DOI] [PubMed] [Google Scholar]
- 6.Huang T, Rifas‐Shiman SL, Ertel KA, Rich‐Edwards J, Kleinman K, Gillman MW, Oken E, James‐Todd T. Pregnancy hyperglycaemia and risk of prenatal and postpartum depressive symptoms. Paediatric and perinatal epidemiology. 2015. July 1;29(4):281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowe LP, Metzger BE, Dyer AR, Lowe J, McCance DR, Lappin TR, Trimble ER, Coustan DR, Hadden DR, Hod M, Oats JJ. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations of maternal A1C and glucose with pregnancy outcomes. Diabetes care. 2012. March 1;35(3):574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim C, Newton KM and Knopp RH, 2002. Gestational diabetes and the incidence of type 2 diabetes. Diabetes care, 25(10), pp.1862–1868. [DOI] [PubMed] [Google Scholar]
- 9.Gunderson EP, Chiang V, Pletcher MJ, Jacobs DR, Quesenberry CP, Sidney S, Lewis CE. History of gestational diabetes mellitus and future risk of atherosclerosis in mid-life: the Coronary Artery Risk Development in Young Adults study. Journal of the American Heart Association. 2014. April 22;3(2):e000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ACOG Practice Bulletin No. 180: Gestational Diabetes Mellitus. Obstet Gynecol. 2017;130(1):e17–37. [DOI] [PubMed] [Google Scholar]
- 11.Bardenheier BH, Imperatore G, Gilboa SM, Geiss LS, Saydah SH, Devlin HM, Kim SY, Gregg EW. Trends in gestational diabetes among hospital deliveries in 19 US States, 2000–2010. American journal of preventive medicine. 2015. July 1;49(1):12–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavery JA, Friedman AM, Keyes KM, Wright JD, Ananth CV. Gestational diabetes in the United States: temporal changes in prevalence rates between 1979 and 2010. BJOG: An International Journal of Obstetrics & Gynaecology. 2017. April 1;124(5):804–13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehrlich S, Lambers D, Baccarelli A, Khoury J, Macaluso M, Ho SM. Endocrine disruptors: a potential risk factor for gestational diabetes mellitus. American journal of perinatology. 2016. November;33(13):1313–8. [DOI] [PubMed] [Google Scholar]
- 14.Andersen FA. Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. International Journal of Toxicology. 2008;27:1–82. [DOI] [PubMed] [Google Scholar]
- 15.Dodge LE, Kelley KE, Williams PL, Williams MA, Hernández-Díaz S, Missmer SA, et al. Medications as a source of paraben exposure. Reprod Toxicol. 2015;52:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao C, Liu F, Kannan K. Occurrence of and dietary exposure to parabens in foodstuffs from the United States. Environ Sci Technol. 2013;47(8):3918–3925. [DOI] [PubMed] [Google Scholar]
- 17.Cantonwine DE, Meeker JD, Ferguson KK, Mukherjee B, Hauser R, McElrath TF. Urinary concentrations of bisphenol A and phthalate metabolites measured during pregnancy and risk of preeclampsia. Environmental health perspectives. 2016. October;124(10):1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johns LE, Ferguson KK, McElrath TF, Mukherjee B, Meeker JD. Associations between repeated measures of maternal urinary phthalate metabolites and thyroid hormone parameters during pregnancy. Environmental health perspectives. 2016. November;124(11):1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calafat AM, Ye X, Wong LY, Bishop AM, Needham LL. Urinary concentrations of four parabens in the US population: NHANES 2005–2006. Environ Health Perspect. 2010;118(5):679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keller BO, Davidson AGF, Innis SM. Phthalate metabolites in urine of CF patients are associated with use of enteric-coated pancreatic enzymes. Environ Toxicol Pharmacol. 2009;27(3):424–427. [DOI] [PubMed] [Google Scholar]
- 21.Raymond CR, Paul JS, Sian CO. Handbook of pharmaceutical excipients. Am Pharm Assoc Wash DC. 2006;430–433. [Google Scholar]
- 22.Nadal A, Alonso-Magdalena P, Soriano S, Quesada I, Ropero AB. The pancreatic β-cell as a target of estrogens and xenoestrogens: Implications for blood glucose homeostasis and diabetes. Molecular and cellular endocrinology. 2009. May 25;304(1–2):63–8.. [DOI] [PubMed] [Google Scholar]
- 23.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. American Journal of Obstetrics & Gynecology. 1982. December 1;144(7):768–73. [DOI] [PubMed] [Google Scholar]
- 24.Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77(16):5407–5413. [DOI] [PubMed] [Google Scholar]
- 25.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Applied occupational and environmental hygiene. 1990. January 1;5(1):46–51. [Google Scholar]
- 26.Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2014;16(3):493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu YH, Mínguez-Alarcón L, Ford JB, Keller M, Seely EW, Messerlian C, Petrozza J, Williams PL, Ye X, Calafat AM, Hauser R. Trimester-Specific Urinary Bisphenol A Concentrations and Blood Glucose Levels Among Pregnant Women From a Fertility Clinic. The Journal of Clinical Endocrinology & Metabolism. 2017. January 12;102(4):1350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shapiro GD, Dodds L, Arbuckle TE, Ashley-Martin J, Fraser W, Fisher M, Taback S, Keely E, Bouchard MF, Monnier P, Dallaire R. Exposure to phthalates, bisphenol A and metals in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: the MIREC study. Environment international. 2015. October 31;83:63–71. [DOI] [PubMed] [Google Scholar]
- 29.James-Todd TM, Meeker JD, Huang T, Hauser R, Ferguson KK, Rich-Edwards JW, McElrath TF, Seely EW. Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environment international. 2016. November 30;96:118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levran D, Shoham Z, Habib D, Greenwald M, Nebel L, Mashiach S. Glucose tolerance in pregnant women following treatment for sterility. International journal of fertility. 1990;35(3):157–9. [PubMed] [Google Scholar]
- 31.Radon PA, McMahon MJ, Meyer WR. Impaired glucose tolerance in pregnant women with polycystic ovary syndrome. Obstetrics & Gynecology. 1999. August 1;94(2):194–7. [DOI] [PubMed] [Google Scholar]
- 32.Obesity Sam S. and polycystic ovary syndrome. Obesity management. 2007. April 1;3(2):69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, Hauser R. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J Expo Sci Environ Epidemiol. 2014;24(5):459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Ahn KC, Gee NA, Gee SJ, Hammock BD, Lasley BL. Antiandrogenic properties of parabens and other phenolic containing small molecules in personal care products. Toxicol Appl Pharmacol. 2007;221(3):278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terasaka S, Inoue A, Tanji M, Kiyama R. Expression profiling of estrogen-responsive genes in breast cancer cells treated with alkylphenols, chlorinated phenols, parabens, or bis-and benzoylphenols for evaluation of estrogenic activity. Toxicol Lett. 2006;163(2):130–141. [DOI] [PubMed] [Google Scholar]
- 36.Van Meeuwen JA, Van Son O, Piersma AH, De Jong PC, Van Den Berg M. Aromatase inhibiting and combined estrogenic effects of parabens and estrogenic effects of other additives in cosmetics. Toxicol Appl Pharmacol. 2008;230(3):372–382. [DOI] [PubMed] [Google Scholar]
- 37.Hu P, Chen X, Whitener RJ, Boder ET, Jones JO, Porollo A, Chen J, Zhao L. Effects of parabens on adipocyte differentiation. Toxicological sciences. 2012. September 5;131(1):56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aker AM, Watkins DJ, Johns LE, Ferguson KK, Soldin OP, Del Toro LVA, et al. Phenols and parabens in relation to reproductive and thyroid hormones in pregnant women. Environ Res. 2016;151:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Philippat C, Mortamais M, Chevrier C, Petit C, Calafat AM, Ye X, et al. Exposure to Phthalates and Phenols during Pregnancy and Offspring Size at Birth. Environ Health Perspect. 2012. March;120(3):464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mínguez-Alarcón L, Gaskins AJ, Chiu Y-H, Williams PL, Ehrlich S, Chavarro JE, et al. Urinary bisphenol A concentrations and association with in vitro fertilization outcomes among women from a fertility clinic. Hum Reprod. 2015;30(9):2120–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klopčič I, Kolšek K, Dolenc MS. Glucocorticoid-like activity of propylparaben, butylparaben, diethylhexyl phthalate and tetramethrin mixtures studied in the MDA-kb2 cell line. Toxicol Lett. 2015;232(2):376–383. [DOI] [PubMed] [Google Scholar]
- 42.Kolšek K, Gobec M, Raščan IM, Dolenc MS. Screening of bisphenol A, triclosan and paraben analogues as modulators of the glucocorticoid and androgen receptor activities. Toxicol In Vitro. 2015;29(1):8–15. [DOI] [PubMed] [Google Scholar]
- 43.Koeppe ES, Ferguson KK, Colacino JA, Meeker JD. Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007–2008. Sci Total Environ. 2013. February 15;445(Supplement C):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taxvig C, Vinggaard AM, Hass U, Axelstad M, Boberg J, Hansen PR, et al. Do parabens have the ability to interfere with steroidogenesis? Toxicol Sci. 2008;106(1):206–213. [DOI] [PubMed] [Google Scholar]
- 45.Kang H-S, Kyung M-S, Ko A, Park J-H, Hwang M-S, Kwon J-E, et al. Urinary concentrations of parabens and their association with demographic factors: a population-based cross-sectional study. Environ Res. 2016;146:245–251. [DOI] [PubMed] [Google Scholar]
- 46.Meeker JD, Yang T, Ye X, Calafat AM, Hauser R. Urinary Concentrations of Parabens and Serum Hormone Levels, Semen Quality Parameters, and Sperm DNA Damage. Environ Health Perspect. 2011. February;119(2):252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taxvig C, Dreisig K, Boberg J, Nellemann C, Schelde AB, Pedersen D, Boergesen M, Mandrup S, Vinggaard AM. Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARγ activation. Molecular and cellular endocrinology. 2012. September 25;361(1–2):106–15 [DOI] [PubMed] [Google Scholar]
- 48.Golden R, Gandy J, Vollmer G. A review of the endocrine activity of parabens and implications for potential risks to human health. Critical reviews in toxicology. 2005. January 1;35(5):435–58 [DOI] [PubMed] [Google Scholar]
- 49.Prusakiewicz JJ, Harville HM, Zhang Y, Ackermann C, Voorman RL. Parabens inhibit human skin estrogen sulfotransferase activity: possible link to paraben estrogenic effects. Toxicology. 2007. April 11;232(3):248–56. [DOI] [PubMed] [Google Scholar]
- 50.Taylor KW, Joubert BR, Braun JM, Dilworth C, Gennings C, Hauser R, et al. Statistical Approaches for Assessing Health Effects of Environmental Chemical Mixtures in Epidemiology: Lessons from an Innovative Workshop. Environ Health Perspect. 2016. December;124(12):A227–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


