Abstract
Objective
To delineate the clinical characteristics of patients with coronavirus disease 2019 (covid-19) who died.
Design
Retrospective case series.
Setting
Tongji Hospital in Wuhan, China.
Participants
Among a cohort of 799 patients, 113 who died and 161 who recovered with a diagnosis of covid-19 were analysed. Data were collected until 28 February 2020.
Main outcome measures
Clinical characteristics and laboratory findings were obtained from electronic medical records with data collection forms.
Results
The median age of deceased patients (68 years) was significantly older than recovered patients (51 years). Male sex was more predominant in deceased patients (83; 73%) than in recovered patients (88; 55%). Chronic hypertension and other cardiovascular comorbidities were more frequent among deceased patients (54 (48%) and 16 (14%)) than recovered patients (39 (24%) and 7 (4%)). Dyspnoea, chest tightness, and disorder of consciousness were more common in deceased patients (70 (62%), 55 (49%), and 25 (22%)) than in recovered patients (50 (31%), 48 (30%), and 1 (1%)). The median time from disease onset to death in deceased patients was 16 (interquartile range 12.0-20.0) days. Leukocytosis was present in 56 (50%) patients who died and 6 (4%) who recovered, and lymphopenia was present in 103 (91%) and 76 (47%) respectively. Concentrations of alanine aminotransferase, aspartate aminotransferase, creatinine, creatine kinase, lactate dehydrogenase, cardiac troponin I, N-terminal pro-brain natriuretic peptide, and D-dimer were markedly higher in deceased patients than in recovered patients. Common complications observed more frequently in deceased patients included acute respiratory distress syndrome (113; 100%), type I respiratory failure (18/35; 51%), sepsis (113; 100%), acute cardiac injury (72/94; 77%), heart failure (41/83; 49%), alkalosis (14/35; 40%), hyperkalaemia (42; 37%), acute kidney injury (28; 25%), and hypoxic encephalopathy (23; 20%). Patients with cardiovascular comorbidity were more likely to develop cardiac complications. Regardless of history of cardiovascular disease, acute cardiac injury and heart failure were more common in deceased patients.
Conclusion
Severe acute respiratory syndrome coronavirus 2 infection can cause both pulmonary and systemic inflammation, leading to multi-organ dysfunction in patients at high risk. Acute respiratory distress syndrome and respiratory failure, sepsis, acute cardiac injury, and heart failure were the most common critical complications during exacerbation of covid-19.
Introduction
Coronaviruses are important pathogens of humans and animals that can cause diseases ranging from the common cold to more severe and even fatal respiratory infections. In the past two decades two highly pathogenic human coronaviruses, the coronavirus responsible for severe acute respiratory syndrome (SARS-Cov) and the coronavirus responsible for Middle East respiratory syndrome (MERS-Cov),1 2 have emerged in two separate events. They induced lower respiratory tract infection as well as extrapulmonary manifestations, leading to hundreds or thousands of cases with high mortality rates of up to 50% in certain populations. In December 2019 a new strain of coronavirus, officially named severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2), was first isolated from three patients with coronavirus disease 2019 (covid-19) by the Chinese Center for Disease Control and Prevention,3 4 connected to the cluster of acute respiratory illness cases from Wuhan, China. Recent epidemiological reports have provided evidence for person to person transmission of the SARS-Cov-2 in family and hospital settings.5 6 As of 28 February 2020, the number of patients infected with SARS-Cov-2 has exceeded 83 652 globally, and more than 2858 have now died of covid-19, with the highest mortality rate of 4.47% in Wuhan. On 30 January 2020, the World Health Organization declared that the outbreak of SARS-Cov-2 constituted a public health emergency of international concern.
Evidence indicates that substantial similarities exist between severe acute respiratory syndrome and covid-19. A recent study reported a 79.5% genome sequence identity between SARS-Cov-2 and SARS-Cov, and SARS-Cov-2 was 96% identical in terms of whole genome sequence to a bat coronavirus.7 Clinical and pathological features of patients with covid-19 have recently been reported, showing that the SARS-Cov-2 infection causes clusters of severe and even fatal pneumonia with clinical presentation greatly resembling that of SARS-Cov infection, associated with admission to intensive care units and high mortality.8 The first study of the initial 41 laboratory confirmed cases with covid-19 showed that 28 (68%) of 41 patients had been discharged and six (15%) had died.8 A larger case series involving 138 consecutive patients admitted to hospital with covid-19 showed that 47 (34%) patients were discharged and six died (overall mortality 4.3%).9 Demographic, clinical, laboratory, and radiological differences between patients who were and were not admitted to the intensive care unit have been fully evaluated. Given that the numbers of patients in these studies is relatively small, information about the clinical characteristics of patients who died is scarce. No vaccine or specific antiviral treatment for covid-19 has yet been shown to be effective, so supportive therapy that eases the symptoms and protects multi-organ function may be beneficial. Identifying or more promptly treating patients in high risk groups is crucial to decrease the mortality rate.
In this study, we did a comprehensive evaluation of deceased patients and patients recovered among those with confirmed covid-19 who were previously transferred or admitted to the isolation ward of Wuhan Tongji Hospital, which is one of the designated hospitals assigned by Chinese government for patients severely or critically ill with covid-19. We aimed to compare the demographic, clinical, laboratory, and radiological features of patients with different clinical outcomes.
Methods
Study participants and data collection
From 13 January to 12 February 2020, 799 moderately to severely ill or critically ill patients with confirmed covid-19 were transferred from other hospitals or isolation sites or admitted from fever clinics to Tongji Hospital. Tongji Hospital was urgently reconstructed and has been assigned by Chinese government as a designed hospital for severely or critically ill patients with covid-19. As of 28 February 2020, 113 of the 799 patients had died, with a mortality rate of up to 14.1%, and 161 patients had recovered and been discharged. The remaining 525 patients were still in hospital and receiving medical care. All patients were diagnosed as having covid-19 and classified as being moderately, severely, or critically ill according to the Guidance for Corona Virus Disease 2019 (6th edition) released by the National Health Commission of China.10 All the recovered patients with covid-19 had completely resolved symptoms and signs, had significant improvement in pulmonary and extrapulmonary organ dysfunction, and no longer needed supportive care, with confirmed viral clearance by repeated tests for SARS-Cov-2 before hospital discharge. Written informed consent was waived owing to the rapid emergence of this infectious disease.
We obtained epidemiological, clinical, laboratory, and radiological characteristics, as well as treatment and outcome data, from electronic medical records for deceased patients and recovered patients by using data collection forms. We collected data on demographics, medical history, exposure history, underlying chronic diseases, symptoms and signs, laboratory findings, computed tomographic scans of the chest, and treatment (including antiviral therapy, antibiotics, corticosteroid therapy, and oxygen support) during the hospital admission. The clinical data were monitored up to 28 February 2020. The research team of experienced clinicians from Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology analysed patients’ medical records. A trained team of physicians and researchers independently entered and cross checked data in a computerised database. If the core data were missing, we sent requests for clarification to the coordinators, who subsequently contacted the clinicians responsible for the treatment of the patients. As some patients presented with various forms of disorder of consciousness on admission, we obtained data on their medical histories and pre-admission information through contact with their close relatives and by accessing medical records from previous hospital visits.
The supplementary table shows the criteria and definitions for the diagnosis, clinical classification (mild, moderate, severe, and critically ill),10 and complications (acute respiratory distress syndrome, acute kidney injury, sepsis, shock, acute liver injury, acute heart failure, and cardiac injury)8 11 12 13 for covid-19.
Laboratory measurements
Real time reverse transcription polymerase chain reaction assay for SARS-Cov-2
Throat swab samples were collected for extracting SARS-Cov-2 RNA from patients. The respiratory sample RNA isolation kit (Biogerm, Shanghai, China) was used to extract total RNA within two hours. Briefly, 40 μL of cell lysates were transferred into a collection tube followed by vortex for 10 seconds. After standing at room temperature for 10 minutes, the collection tube was centrifuged at 1000 revolutions per minute for five minutes. The suspension was used for real time reverse transcription polymerase chain reaction (RT-PCR) assay of SARS-Cov-2 RNA. Two target genes—open reading frame 1ab (ORF1ab) and nucleocapsid protein (N)—were simultaneously amplified and tested during the real time RT-PCR assay. Target 1 (ORF1ab) comprised forward primer CCCTGTGGGTTTTACACTTAA, reverse primer ACGATTGTGCATCAGCTGA, and the probe 5′-VIC-CCGTCTGCGGTATGTGGAAAGGTTATGG-BHQ1-3′. Target 2 (N) comprised forward primer GGGGAACTTCTCCTGCTAGAAT, reverse primer CAGACATTTTGCTCTCAAGCTG, and the probe 5′-FAM- TTGCTGCTGCTTGACAGATT-TAMRA-3′. The real time RT-PCR assay was conducted using a SARS-Cov-2 nucleic acid detection kit according to the manufacturer’s protocol (Shanghai Bio-germ Medical Technology company). The reaction mixture contains 12 μL of reaction buffer, 4 μL of enzyme solution, 4 μL of Probe primers solution, 3 μL of diethyl pyrocarbonate treated water, and 2 μL of RNA template. The RT-PCR assay was conducted under the following conditions: incubation at 50°C for 15 minutes and 95°C for five minutes, 40 cycles of denaturation at 94°C for 15 seconds, and extending and collecting fluorescence signal at 55°C for 45 seconds. A cycle threshold value less than 37 was defined as a positive test result, and a cycle threshold value of 40 or more was defined as a negative test. These diagnostic criteria were based on the recommendation by the National Institute for Viral Disease Control and Prevention (China) (http://ivdc.chinacdc.cn/kyjz/202001/t20200121_211337.html). A medium load, defined as a cycle threshold value of 37 to less than 40, required confirmation by retesting.
Clinical laboratory measurements
Initial clinical laboratory investigation included a complete blood count, serum biochemical tests (including liver and kidney function, creatine kinase, lactate dehydrogenase, and electrolytes), a coagulation profile, and cytokine tests. Respiratory specimens, including nasal and pharyngeal swabs, or sputum were tested to exclude evidence of other viral infections, including influenza, respiratory syncytial virus, avian influenza, parainfluenza virus, and adenovirus.
Principles of management of patients
Supportive therapy
Vital signs and oxygen saturation should be monitored (every eight hours; patients with severe disease need continuous monitoring), supportive treatment strengthened, sufficient calories provided, and the stability of the internal environment, such as water, electrolyte, and acid-base balance, maintained. The intake and output volumes should be strictly balanced, especially in critical ill patients.
Oxygen therapy
Supplemental oxygen therapy should be given immediately to patients with hypoxaemia. Oxygen therapy can be started at a flow rate of 5 L/min, and the target oxygen saturation is pulse oxygen saturation ≥90% in non-pregnant adult patients, ≥92-95% in pregnant patients, and ≥94% in patients who are critically ill with severe respiratory distress, shock, or coma.
If standard oxygen therapy fails, mechanical ventilation should be considered; high flow nasal catheter oxygen or non-invasive ventilation (for example, bilevel positive airway pressure mode) can be used. If no improvement is seen within one hour of non-invasive mechanical ventilation, invasive mechanical ventilation should be used. Experienced experts can recommend extracorporeal membrane pulmonary oxygenation according to their evaluation of the patient’s situation.
Empirical antimicrobial therapy
If a history of seasonal or local influenza epidemiology exists, empirical therapy may be considered.
Blood purification therapy
Continuous renal replacement therapy can be used in critically ill patients.
Statistical analysis
We present categorical variables as numbers and percentages and continuous variables as mean and standard deviation if they were normally distributed or median and interquartile range if they were not. We compared means for continuous variables by using independent group t tests when the data were normally distributed; otherwise, we used the Mann-Whitney test. We compared proportions for categorical variables by using the χ2 test. We used Fisher’s exact test in the analysis of contingency tables when the sample sizes were small. For unadjusted comparisons, we considered a two sided P value below 0.05 to be statistically significant. We used SPSS (version 19.0) for all analyses.
Patient and public involvement
This was a retrospective case series study, and no patients were involved in the study design or in setting the research questions or the outcome measures directly. No patients were asked to advise on interpretation or writing up of results.
Results
Demographics and baseline characteristics of deceased patients and recovered patients
From 13 January to 12 February 2020, 799 moderately to severely ill or critically ill patients with confirmed covid-19 were transferred or admitted to Tongji Hospital. As of 28 February 2020, 113 of these patients had died of covid-19 and 161 patients had fully recovered and been discharged. As shown in table 1, the median age of deceased patients was 68 (interquartile range 62.0-77.0) years, which was significantly older than recovered patients (51 (37.0-66.0) years); 94 (83%) deceased patients and 59 (37%) who recovered were aged 60 or older. Male sex was more predominant in deceased patients (83; 73%) than in recovered patients (88; 55%). Overall, 71 (63%) patients who died and 62 (39%) who recovered had at least one chronic medical condition. Hypertension, cardiovascular disease, and cerebrovascular disease were much more frequent among deceased patients (54 (48%), 16 (14%), and 4 (4%)) than among recovered patients (39 (24%), 7 (4%), and 0 (0%)). Few patients had a current or former cigarette smoking history of at least 30 pack years. The proportion of healthcare workers among deceased patients (1; 1%) was significantly lower than among recovered patients (18; 11%). Likewise, the proportion of patients with a history of close contact with previously confirmed patients tended to be lower in deceased patients (44; 12%) than in recovered patients (33; 20%).
Table 1.
Presenting characteristics of patients with coronavirus disease 2019 who died and recovered patients. Values are numbers (percentages) unless stated otherwise
| Total (n=274) | Deaths (n=113) | Recovered patients (n=161) | |
|---|---|---|---|
| Characteristics | |||
| Median (IQR) age, years | 62.0 (44.0-70.0) | 68.0 (62.0-77.0) | 51.0 (37.0-66.0) |
| <40 years | 53 (19) | 0 (0) | 53 (33) |
| 40-60 years | 68 (25) | 19 (17) | 49 (30) |
| ≥60 years | 153 (56) | 94 (83) | 59 (37) |
| Sex: | |||
| Female | 103 (38) | 30 (27) | 73 (45) |
| Male | 171 (62) | 83 (73) | 88 (55) |
| Huanan Seafood Market exposure | 5 (2) | 4 (4) | 1 (1) |
| Close contact with confirmed case | 47 (17) | 14 (12) | 33 (20) |
| Smoking history | 19 (7) | 9 (8) | 10 (6) |
| Current smoker | 12 (4) | 7 (6) | 5 (3) |
| Former smoker | 7 (3) | 2 (2) | 5 (3) |
| Healthcare worker | 19 (7) | 1 (1) | 18 (11) |
| Pregnancy | 4 (1) | 0 (0) | 4 (2) |
| Comorbidities: | 133 (49) | 71 (63) | 62 (39) |
| Hypertension | 93 (34) | 54 (48) | 39 (24) |
| Diabetes | 47 (17) | 24 (21) | 23 (14) |
| Cardiovascular disease | 23 (8) | 16 (14) | 7 (4) |
| Chronic heart failure | 1 (<1) | 1 (1) | 0 (0) |
| Chronic lung diseases | 18 (7) | 11 (10) | 7 (4) |
| Malignancy | 7 (3) | 5 (4) | 2 (1) |
| Hepatitis B virus surface antigen positivity | 11 (4) | 5 (4) | 6 (4) |
| HIV infection | 0 (0) | 0 (0) | 0 (0) |
| Cerebrovascular disease | 4 (1) | 4 (4) | 0 (0) |
| Chronic kidney disease | 4 (1) | 4 (4) | 1 (1) |
| Gastrointestinal diseases | 3 (1) | 1 (1) | 2 (1) |
| Metabolic arthritis | 4 (1) | 1 (1) | 3 (2) |
| Autoimmune disease | 2 (1) | 1 (1) | 1 (1) |
| Signs and symptoms at disease onset | |||
| Fever | 249 (91) | 104 (92) | 145 (90) |
| Cough | 185 (68) | 79 (70) | 106 (66) |
| Fatigue | 137 (50) | 64 (57) | 73 (45) |
| Anorexia | 66 (24) | 31 (27) | 35 (22) |
| Myalgia | 60 (22) | 21 (19) | 39 (24) |
| Dyspnoea | 120 (44) | 70 (62) | 50 (31) |
| Chest tightness | 103 (38) | 55 (49) | 48 (30) |
| Sputum production | 83 (30) | 35 (31) | 48 (30) |
| Haemoptysis | 7 (3) | 4 (4) | 3 (2) |
| Pharyngalgia | 12 (4) | 4 (4) | 8 (5) |
| Diarrhoea | 77 (28) | 27 (24) | 50 (31) |
| Nausea | 24 (9) | 8 (7) | 16 (10) |
| Vomiting | 16 (6) | 6 (5) | 10 (6) |
| Abdominal pain | 19 (7) | 6 (5) | 13 (8) |
| Headache | 31 (11) | 11 (10) | 20 (12) |
| Dizziness | 21 (8) | 10 (9) | 11 (7) |
| Median (IQR) time from onset of symptom to first outpatient visit, days | 4.0 (1.0-7.0) | 4.5 (0.0-7.0) | 4.0 (1.0-6.0) |
| Median (IQR) time from onset of symptom to hospital admission, days | 10.0 (7.0-12.0) | 10.0 (7.0-13.0) | 9.0 (6.0-12.0) |
| Median (IQR) time from onset of symptom to outcome, days | 22.0 (16.8-27.3) | 16.0 (12.0-20.0) | 26.0 (21.8-29.0) |
| Median (IQR) time from hospital admission to outcome, days | 13.0 (6.0-17.0) | 5.0 (3.0-9.3) | 16.0 (14.0-19.0) |
| Vital signs on admission | |||
| Disorders of consciousness | 26 (9) | 25 (22) | 1 (1) |
| Median (IQR) arterial pressure, mm Hg | 129.0 (118.8-143.3) | 137.0 (122.0-147.0) | 125.0 (115.5-136.0) |
| <90 mm Hg | 10 (4) | 8 (7) | 2 (1) |
| 90-140 mm Hg | 181 (66) | 55 (49) | 126 (78) |
| ≥140 mm Hg | 83 (30) | 50 (44) | 33 (20) |
| Median (IQR) heart rate, beat per minute | 94.0 (80.0-108.0) | 101.0 (82.0-111.0) | 91.0 (79.0-104.0) |
| >100 beats per minute | 104 (38) | 56 (50) | 48 (30) |
| Median (IQR) respiratory rate, breaths per minute | 20.0 (20.0-24.0) | 24.0 (20.0-30.0) | 20.0 (20.0-21.0) |
| <24 breaths per minute | 186 (68) | 47 (42) | 139 (86) |
| 24-30 breaths per minute | 53 (19) | 36 (32) | 17 (11) |
| ≥30 breaths per minute | 35 (13) | 30 (27) | 5 (3) |
| Median (IQR) percutaneous oxygen saturation, % | 95.0 (85.0-98.0) | 85.0 (75.0-94.0) | 97.0 (95.3-98.0) |
| ≤93% | 91 (33) | 72 (64) | 19 (12) |
IQR=interquartile range.
Fever and cough were the most prevalent symptoms at disease onset in both deceased patients (104 (92%) and 79 (70%)) and recovered patients (145 (90%) and 106 (66%)), and the proportions of patients reporting these symptoms in the two groups were comparable. Other prevalent symptoms at onset of illness in deceased patients included fatigue, dyspnoea, chest tightness, and sputum production; less common symptoms included anorexia, diarrhoea, and myalgia. Dyspnoea and chest tightness were much more common in deceased patients (70 (62%) and 55 (49%)) than in recovered patients (50 (31%) and 48 (30%)). Twenty five (22%) people who died and only one (1%) who recovered had disorders of consciousness on hospital admission. Nine deceased patients and 16 who recovered had no fever, with fatigue, cough, dyspnoea, myalgia, or diarrhoea as the initial symptoms. Among these, one patient with no symptoms who recovered was diagnosed as having covid-19 during routine physical examination, and another complained only of stinging eyes for two weeks before being admitted to hospital.
Among the deceased patients, the median time from onset of symptoms to hospital admission was 10.0 (interquartile range 7.0-13.0) days, which tended to be longer than for recovered patients (9.0 (6.0-12.0) days). The median time from onset of symptoms to death in deceased patients was 16 (12.0-20.0) days, and the median time from first symptoms to discharge in recovered patients was 26 (21.8-29.0) days. The median time from admission to death was 5 (3.0-9.3) days and the median time from admission to discharge was 16 (14.0-19.0) days.
Measures of vital signs were recorded on the day of hospital admission for all patients. Median systolic blood pressure was significantly higher in deceased patients (137.0 mm Hg) than recovered patients (125.0 mm Hg). More patients who died than who recovered had arterial pressure of 140 mm Hg or higher (50 (44%) v 33 (20%)). Heart rates were much higher in deceased patients (101.0 beats per minute) than in recovered patients (91.0 beats per minute). Respiratory rates were significantly higher in deceased patients (24.0 breaths per minute) than in recovered patients (20.0 breaths per minute). Deceased patients more often developed tachycardia and tachypnoea (respiratory rate ≥24 breaths per minute) (56 (50%) and 66 (58%)) than did recovered patients (48 (30%) and 22 (14%)). Seventy two (64%) deceased patients and only 19 (12%) who recovered had percutaneous oxygen saturation of 93% or below on admission.
Laboratory parameters of deceased patients and recovered patients
We observed substantial differences in laboratory findings between patients who died of covid-19 and those who recovered from it (table 2). Fifty six (50%) deceased patients and only six (4%) who recovered developed leukocytosis (white blood cell count ≥10×109/L). Deceased patients had persistent and more severe lymphopenia than recovered patients; 44 (39%) deceased patients and eight (5%) recovered patients had lymphocyte counts below 0.5×109/L. Median platelet counts were significantly lower in deceased patients.
Table 2.
Laboratory findings on admission of patients with coronavirus disease 2019 who died and recovered patients. Values are numbers (percentages) unless stated otherwise
| Laboratory finding (normal range) | Total (n=274) | Deaths (n=113) | Recovered patients (n=161) |
|---|---|---|---|
| Median (IQR) white blood cell count, ×109/L (3.5-9.5) | 5.9 (4.3-9.2) | 10.2 (6.2-13.6) | 5.0 (3.7-6.3) |
| <4×109/L | 58 (21) | 9 (8) | 49 (30) |
| 4-10×109/L | 154 (56) | 48 (42) | 106 (66) |
| ≥10×109/L | 62 (23) | 56 (50) | 6 (4) |
| Median (IQR) neutrophil count,×109/L (1.8-6.3) | 4.4 (2.8-8.0) | 9.0 (5.4-12.7) | 3.2 (2.4-4.5) |
| >6.3×109/L | 93 (34) | 75 (66) | 17 (11) |
| Median (IQR) lymphocyte count,×109/L (1.1-3.2) | 0.8 (0.6-1.2) | 0.6 (0.4-0.7) | 1.0 (0.7-1.4) |
| ≥1×109/L | 95 (35) | 10 (9) | 85 (53) |
| 0.8-1×109/L | 44 (16) | 16 (14) | 28 (17) |
| 0.5-0.8×109/L | 83 (30) | 43 (38) | 40 (25) |
| <0.5×109/L | 52 (19) | 44 (39) | 8 (5) |
| Median (IQR) monocyte count, ×109/L (0.1-0.6) | 0.4 (0.3-0.5) | 0.4 (0.2-0.6) | 0.4 (0.3-0.5) |
| Median (IQR) haemoglobin, g/L (130-175) | 128.0 (116.0-140.0) | 128.0 (114.0-145.0) | 128.0 (118.0-138.0) |
| Median (IQR) platelet count, ×109/L (125-350) | 179.0 (133.0-235.0) | 156.0 (111.8-219.3) | 198.0 (160.0-256.0) |
| Median (IQR) alanine aminotransferase, U/L (≤41) | 23.0 (15.0-38.0) | 28.0 (18.0-47.0) | 20.0 (14.8-32.0) |
| >41 U/L | 60 (22) | 30 (27) | 30 (19) |
| Aspartate aminotransferase, U/L (≤40) | 30.0 (22.0-46.0) | 45.0 (31.0-67.0) | 25.0 (20.0-33.3) |
| >40 U/L | 84 (31) | 59 (52) | 25 (16) |
| Albumin, g/L (35.0-52.0) | 33.9 (30.3–37.6) | 30.1 (27.9-33.0) | 36.3 (33.7-39.5) |
| <32 g/L | 96 (35) | 74 (65) | 22 (14) |
| Median (IQR) total bilirubin, mmol/L (≤26) | 9.6 (6.7-13.5) | 12.6 (9.4-16.7) | 8.4 (5.8-11.2) |
| Median (IQR) alkaline phosphatase, U/L (40-130) | 68.0 (55.0-87.0) | 76.0 (60.0-118.0) | 64.0 (51.0-77.0) |
| Median (IQR) γ-glutamyl transpeptidase, U/L (10-71) | 33.0 (21.0–51.0) | 42.0 (27.0-70.0) | 28.0 (19.0-45.3) |
| Median (IQR) triglycerides, mmol/L (<1.7) | 1.3 (1.0-2.0) | 1.8 (1.2-2.2) | 1.2 (1.0-1.6) |
| Median (IQR) potassium, mmol/L (3.5-5.1) | 4.1 (3.8-4.6) | 4.3 (3.9-4.9) | 4.1 (3.8-4.4) |
| <3.5 mmol/L | 31 (11) | 14 (12) | 17 (11) |
| 3.5-5.1 mmol/L | 211 (77) | 74 (65) | 137 (85) |
| >5.1 mmol/L | 32 (12) | 25 (22) | 7 (4) |
| Median (IQR) sodium, mmol/L (136-145) | 138.7 (136.3-142.1) | 138.4 (135.8-143.9) | 139.1 (136.6-141.6) |
| >145 mmol/L | 23 (8) | 20 (18) | 3 (2) |
| Median (IQR) blood urea nitrogen, mmol/L (3.1-8.0) | 4.9 (3.5-7.9) | 8.4 (5.7-12.6) | 4.0 (3.0-5.1) |
| Median (IQR) creatinine, μmol/L (59-104) | 76.0 (58.0-94.0) | 88.0 (66.0-114.0) | 66.0 (54.0-84.0) |
| Median (IQR) creatine kinase, U/L (≤190) | 109.0 (53.5-188.0) | 189.0 (94.5-374.5) | 84.0 (50.8-140.3) |
| Median (IQR) lactate dehydrogenase, U/L (135 to 225) | 321.5 (249.8-510.5) | 564.5 (431.0-715.8) | 268.0 (214.3-316.5) |
| >350 U/L | 116 (42) | 93 (82) | 23 (14) |
| Median (IQR) hypersensitive cardiac troponin I, pg/mL (≤15.6) | 8.7 (2.9-33.6) | 40.8 (14.7-157.8) | 3.3 (1.9-7.0) |
| >15.6 pg/mL | 83/203 (41) | 68/94 (72) | 15/109 (14) |
| Median (IQR) N-terminal pro-brain natriuretic peptide, pg/mL (<285) | 267.0 (48.0-821.0) | 800.0 (389.8-1817.5) | 72.0 (20.0-185.0) |
| ≥285 pg/mL | 85/173 (49) | 68/80 (85) | 17/93 (18) |
| Median (IQR) prothrombin time, seconds (11.5-14.5) | 14.3 (13.4-15.4) | 15.5 (14.4-17.3) | 13.9 (13.2-14.4) |
| Median (IQR) international normalised ratio (0.8-1.2) | 1.1 (1.0-1.2) | 1.2 (1.1-1.4) | 1.1 (1.0-1.1) |
| Median (IQR) activated partial thromboplastin time, seconds (29.0- 42.0) | 30.8 (36.6-44.3) | 40.6 (35.6-46.9) | 41.0 (36.9-44.0) |
| Median (IQR) D-dimer, μg/mL (<0.5) | 1.1 (0.5-3.2) | 4.6 (1.3-21.0) | 0.6 (0.3-1.3) |
| >21 μg/mL | 37/247 (15) | 34/97 (35) | 3/150 (2) |
| Median (IQR) procalcitonin, ng/mL (0.02 to 0.05) | 0.09 (0.04-0.23) | 0.33 (0.14-0.65) | 0.05 (0.03-0.08) |
| <0.05 ng/mL | 71/236 (30) | 1/96 (1) | 70/140 (50) |
| 0.05-0.5 ng/mL | 127/236 (54) | 60/96 (63) | 67/140 (48) |
| 0.5-2 ng/mL | 30/236 (13) | 27/96 (28) | 3/140 (2) |
| ≥2 ng/mL | 8/236 (3) | 8/96 (8) | 0/140 (0) |
| Median (IQR) high sensitivity C-reactive protein, mg/L (<1) | 53.4 (18.6-113.0) | 113.0 (69.1-168.4) | 26.2 (8.7-55.8) |
| >100 mg/L | 80/243 (33) | 59/98 (60) | 21/145 (14) |
| Median (IQR) ferritin, μg/L (30-400) | 669.7 (388.8-1494.6) | 1418.3 (915.4-2236.2) | 481.2 (265.1-871.5) |
| Median (IQR) erythrocyte sedimentation rate, mm/h (0-15) | 32.5 (17.3-53.8) | 38.5 (20.5-62.8) | 28.0 (15.8-45.0) |
| Median (IQR) thyroid stimulating hormone, mIU/mL (0.27-4.20) | 1.2 (0.5-2.0) | 0.7 (0.3-1.4) | 1.4 (0.7-2.2) |
| Median (IQR) free triiodothyronine, pmol/L (3.1-6.8) | 3.9 (3.1-4.6) | 2.8 (2.5-3.1) | 4.3 (3.7-4.8) |
| Median (IQR) free thyroxin, pmol/L (12-22) | 17.7 (14.9-19.0) | 15.8 (13.2-18.6) | 18.3 (15.7-19.2) |
| Median (IQR) immunoglobulin A, g/L (0.82-4.53) | 2.2 (1.6-2.8) | 2.4 (1.6-3.3) | 2.1 (1.6-2.8) |
| Median (IQR) immunoglobulin G, g/L (7.51-15.6) | 11.5 (9.3-13.3) | 12.3 (10.1-14.5) | 11.3 (9.3-13.0) |
| Median (IQR) immunoglobulin M, g/L (0.46-3.04) | 1.0 (0.7-1.4) | 1.0 (0.7-1.4) | 1.0 (0.7-1.4) |
| Median (IQR) complement 3, g/L (0.65-1.39) | 0.9 (0.8-1.4) | 0.8 (0.6-0.9) | 0.9 (0.8-1.0) |
| Median (IQR) complement 4, g/L (0.16-0.38) | 0.2 (0.2-0.3) | 0.2 (0.2-0.3) | 0.3 (0.2-0.3) |
| Interleukin 1β ≥5pg/mL | 18/163 (11.0) | 5/53 (9) | 13/110 (12) |
| Median (IQR) interleukin 2 receptor, U/mL (223-710) | 713.0 (502.5-1111.0) | 1189.0 (901.0-1781.0) | 566.5 (448.0-858.3) |
| ≥710 U/L | 84/163 (52) | 43/53 (81) | 41/110 (37) |
| Median (IQR) interleukin 6, pg/mL (<7) | 22.0 (6.1-51.8) | 72.0 (35.6-146.8) | 13.0 (4.0-26.2) |
| ≥7 pg/mL | 119/163 (73) | 53/53 (100) | 66/110 (60) |
| Median (IQR) interleukin 8, pg/mL (<62) | 16.6 (9.2-32.6) | 28.3 (18.7-72.1) | 11.4 (7.8-20.2) |
| ≥62 pg/mL | 24/163 (15) | 15/53 (28) | 9/110 (8) |
| Median (IQR) interleukin 10, pg/mL (<9.1) | 6.7 (5.0-12.2) | 12.8 (8.8-19.6) | 5.0 (5.0-8.4) |
| ≥9.2 pg/mL | 58/163 (36) | 37/53 (70) | 21/110 (19) |
| Median (IQR) tumour necrosis factor α, pg/mL (<8.1) | 8.6 (7.0-12.2) | 11.8 (8.6-17.6) | 7.9 (6.7-9.6) |
| ≥8.1 pg/mL | 93/163 (57) | 41/53 (77) | 52/110 (47) |
| Positive urinary protein | 100/166 (60) | 42/49 (86) | 58/117(50) |
| Positive urinary occult blood | 84/166 (51) | 40/49 (82) | 44/117 (38) |
| Bilateral involvement on chest computed tomography scan | 265 (97) | 113 (100) | 152 (94) |
IQR=interquartile range.
Concentrations of alanine aminotransferase, aspartate aminotransferase, total bilirubin, alkaline phosphatase, and γ-glutamyl transpeptidase were markedly higher in deceased patients than in recovered patients. Fifty nine (52%) deceased patients and 25 (16%) who recovered had abnormal aspartate aminotransferase concentrations (>40 U/L). Albumin concentrations were significantly lower in deceased patients than in recovered patients. Seventy four (65%) deceased patients and 22 (14%) recovered patients developed hypoalbuminaemia (albumin <32 g/L). Concentrations of blood urea nitrogen, creatinine, potassium, triglycerides, creatine kinase, and lactate dehydrogenase were significantly higher in deceased patients than in recovered patients. Concentrations of hypersensitive cardiac troponin I and N-terminal pro-brain natriuretic peptide were markedly higher in deceased patients (40.8 pg/mL and 800.0 pg/mL) than in recovered patients (3.3 pg/mL and 72.0 pg/mL), with eight deceased patients having cardiac troponin I above 1000 pg/mL and two above 10 000 pg/mL. In addition, deceased patients more often had increased cardiac troponin I and N-terminal pro-brain natriuretic peptide concentrations (68/94 (72%) and 68/80 (85%)) than did recovered patients (15/109 (14%) and 17/93 (18%)).
Median prothrombin time was significantly longer in deceased patients than in recovered patients, whereas activated partial thromboplastin time was comparable between the two groups. D-dimer concentrations were markedly greater in deceased patients (4.6 μg/mL) than in recovered patients (0.6 μg/mL). Thirty four (35%) of 97 deceased patients and only three (2%) of 150 recovered patients had D-dimer concentrations above 21μg/mL. Concentrations of procalcitonin, high sensitivity C-reactive protein, and ferritin, as well as erythrocyte sedimentation rate, were significantly higher in deceased patients than in recovered patients. Thirty five (36%) of 96 deceased patients and three (2%) of 140 recovered patients had procalcitonin of 0.5 ng/mL or higher. Thyroid stimulating hormone and free triiodothyronine concentrations were significantly lower in deceased patients (0.7 mIU/mL and 2.8 pmol/L) than in recovered patients (1.4 mIU/mL and 4.3 pmol/L). Serum circulating immunoglobulin A, immunoglobulin G, and immunoglobulin M did not differ significantly between the two groups, whereas complement 3 and complement 4 concentrations were markedly lower in deceased patients than in recovered patients.
Of patients with available data, concentrations of interleukin 2 receptor, interleukin 6, interleukin 8, interleukin 10, and tumour necrosis factor α were significantly higher in deceased patients than in recovered patients. Deceased patients more often had increased concentrations of interleukin 2 receptor, interleukin 6, interleukin 8, interleukin 10, and tumour necrosis factor α than did recovered patients. Most (48/53; 91%) deceased patients had undetectable concentrations of interleukin 1β. Forty two (86%) of 49 deceased patients and 58 (50%) of 117 recovered patients had proteinuria, and 40 (82%) of 49 deceased patients and 44 (38%) of 117 recovered patients showed microscopic haematuria.
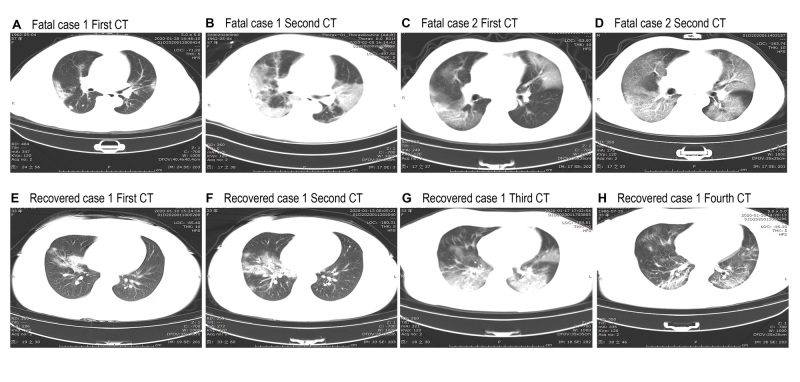
On admission, abnormalities on chest radiographs were seen in all patients (fig 1); 113 (100%) deceased patients and 152 (94%) recovered patients had bilateral involvement on chest radiographs. Typical findings on chest computed tomography images on admission of deceased patients showed bilateral ground glass opacity and subsegmental areas of consolidation (fig 1, A and C), which then progressed rapidly with mass shadows of high density in both lungs (fig 1, B and D). Representative chest computed tomography images of recovered patients showed right middle lobe and lower lobe ground glass opacity and consolidation (fig 1, E and F); then bilateral ground glass opacity and bilateral consolidation of the lungs progressed but right middle lobe consolidation resolved (fig 1, G). Follow-up images showed obviously resolved bilateral ground glass opacity and consolidation (fig 1, H).
Fig 1.
Representative chest computed tomographic images of patients with covid-19 who died and patients who recovered. A-D are chest computed tomograms showing axial view lung window from two deceased patients. Case 1 was a 57 year old women, and case 2 was a 53 year old man. E-H are chest computed tomograms images from a 33 year old woman who recovered. A: image obtained on day 10 after symptom onset shows multiple ground glass opacities and consolidation in bilateral lungs. B: image obtained on day 18 after symptom onset shows progressive multiple ground glass opacities and consolidation in bilateral lungs. C: image obtained on day 9 after symptom onset shows multiple ground glass opacities in bilateral lungs and solid nodule in right lower lobe. D: image obtained on day 13 after symptom onset shows progressive ground glass opacities in bilateral lungs and decreased density of solid nodule in right lower lobe. E: image obtained on day 4 after symptom onset shows right middle lobe and lower lobe consolidation and ground glass opacities. F: image obtained on day 7 after symptom onset shows progressive right middle lobe and lower lobe consolidation and ground glass opacities. G: image obtained on day 11 after symptom onset shows progressive multiple ground glass opacities and consolidation in bilateral lungs and decreased density and range of right middle lobe consolidation. H: after 17 days’ therapy, follow-up computed tomograms show ground glass opacities, and consolidation are obviously resolved in bilateral lungs
Arterial blood gases were measured in 35 deceased patients and 32 recovered patients (table 3). Five (14%) deceased patients and 3 (9%) recovered patients had pH below 7.35, whereas 14 (40%) deceased patients and five (16%) recovered patients had pH 7.45 or above. The median arterial partial pressure of oxygen in deceased patients was 59.2 mm Hg, which was significantly lower than that of recovered patients (121.0 mm Hg). Eighteen of 35 deceased patients and none who recovered had arterial partial pressure of oxygen below 60 mmHg, and all of these had partial pressure of carbon dioxide below 50 mmHg, indicating that they had type I respiratory failure. The median arterial partial pressure of oxygen to fraction of inspired oxygen ratio was significantly lower in deceased patients (105.1) than in recovered patients (350.0). Moreover, all the deceased patients and 14 (44%) recovered patients had an arterial partial pressure of oxygen to fraction of inspired oxygen ratio of 300, indicating that these patients had developed acute respiratory distress syndrome, whereas severe acute respiratory distress syndrome (≤100) developed only in deceased patients (16; 46%). Actual bicarbonate and total carbon dioxide concentration were markedly lower in deceased patients than in recovered patients.
Table 3.
Blood gas analysis of patients with coronavirus disease 2019 who died and recovered patients. Values are numbers (percentages) unless stated otherwise
| Blood gas characteristics (normal range) | Total (n=67) | Deaths (n=35) | Recovered patients (n=32) |
|---|---|---|---|
| Median (IQR) pH (7.35-7.45) | 7.41 (7.39-7.46) | 7.43 (7.40-7.46) | 7.40 (7.39-7.42) |
| <7.35 | 8 (12) | 5 (14) | 3 (9) |
| 7.35-7.45 | 40 (60) | 16 (46) | 24 (75) |
| ≥7.45 | 19 (28) | 14 (40) | 5 (16) |
| Median (IQR) arterial partial pressure of oxygen, mm Hg (80-100) | 82.1 (59.0-128.5) | 59.2 (45.4-78.6) | 121.0 (90.6-163.3) |
| <60 mm Hg | 18/67 (27) | 18/35 (51) | 0/32 (0) |
| Median (IQR) partial pressure of oxygen:fraction of inspired oxygen (400-500) | 193.6 (103.2-341.6) | 105.1 (76.9-169.4) | 350.0 (222.0-417.0) |
| ≤100 | 16/67 (24) | 16/35 (46) | 0/32 (0) |
| 100-300 | 33/67 (49) | 19/35 (54) | 14/32 (44) |
| >300 | 18/67 (27) | 0/35 (0) | 18/32 (56) |
| Median (IQR) partial pressure of carbon dioxide, mm Hg (35-45) | 35.6 (30.2-39.8) | 30.9 (28.9-36.0) | 37.5 (35.0-41.3) |
| <35 mm Hg | 32/67 (48) | 24/35 (69) | 8/32 (25) |
| >50 mm Hg | 3/67 (4) | 2/35 (6) | 1/32 (3) |
| Median (IQR) actual bicarbonate, mmol/L (21.0-28.0) | 22.2 (19.5-24.7) | 20.5 (18.2-24.2) | 22.9 (21.8-25.2) |
| Median (IQR) standard bicarbonate, mmol/L (21.0-25.0) | 23.2 (21.1-25.0) | 22.6 (19.8-24.5) | 23.9 (22.4-25.0) |
| Median (IQR) base excess of blood, mmol/L (−3.0-3.0) | −1.2 (−3.6-0.7) | −2.1 (−4.8-0.8) | −0.6 (−2.4-0.6) |
| Median (IQR) base excess of extracellular fluid, mmol/L (−3.0-3.0) | −1.5 (−4.2-0.9) | −2.8 (−5.7-0.9) | −1.3 (−2.4-0.8) |
| Median (IQR) total carbon dioxide, mmol/L (24.0-32.0) | 20.3 (18.0-22.8) | 18.2 (16.3-21.4) | 21.4 (20.0-23.9) |
IQR=interquartile range.
Complications and primary interventions of deceased patients and recovered patients
Among the deceased patients, respiratory and cardiac complications were numerous (table 4). Common complications observed in deceased patients included acute respiratory distress syndrome (113; 100%), type I respiratory failure (18/35; 51%), sepsis (113; 100%), acute cardiac injury (72/94; 77%), heart failure (41/83; 49%), shock (46; 41%), alkalosis (14/35; 40%), hyperkalaemia (42; 37%), acute kidney injury (28; 25%), and hypoxic encephalopathy (23; 20%). These were significantly more frequent than in recovered patients, showing their potential association with the clinical outcome. Less common complications in deceased patients included acidosis, disseminated intravascular coagulation, and acute liver injury. One patient who died developed gastrointestinal bleeding. Patients with cardiovascular comorbidities were more likely to develop acute cardiac injury and heart failure. In addition, although more deceased patients had chronic hypertension, among patients with available data regardless of history of coexisting cardiovascular disease, cardiac complications were more frequent in deceased patients than in recovered patients.
Table 4.
Complications and treatments of patients with coronavirus disease 2019 who died and recovered patients. Values are numbers (percentages)
| Total (n=274) | Deaths (n=113) | Recovered patients (n=161) | |
|---|---|---|---|
| Complications | |||
| Acute respiratory distress syndrome | 196 (72) | 113 (100) | 83 (52) |
| Type I respiratory failure | 18/67 (27) | 18/35 (51) | 0/32 (0) |
| Acute cardiac injury | 89/203 (44) | 72/94 (77) | 18/109 (17) |
| With history of hypertension or cardiovascular disease | 47/77 (61) | 37/48 (77) | 11/30 (37) |
| Without history of hypertension or cardiovascular disease | 42/126 (33) | 35/46 (76) | 7/80 (9) |
| Heart failure | 43/176 (24) | 41/83 (49) | 3/94 (3) |
| With history of hypertension or cardiovascular disease | 23/67 (34) | 21/42 (50) | 2/25 (8) |
| Without history of hypertension or cardiovascular disease | 21/109 (19) | 20/41 (49) | 1/68 (1) |
| Hypoxic encephalopathy | 24 (9) | 23 (20) | 1 (1) |
| Sepsis | 179 (65) | 113 (100) | 66 (41) |
| Acidosis | 8/67 (12) | 5/35 (14) | 3/32 (9) |
| Alkalosis | 19/67 (28) | 14/35 (40) | 5/32 (16) |
| Acute kidney injury | 29 (11) | 28 (25) | 1 (1) |
| Disseminated intravascular coagulation | 21 (8) | 19 (17) | 2 (1) |
| Hyperkalaemia | 62 (23) | 42 (37) | 22 (14) |
| Shock | 46 (17) | 46 (41) | 0 (0) |
| Acute liver injury | 13 (5) | 10 (9) | 3 (2) |
| Gastrointestinal bleeding | 1 (<1) | 1 (1) | 0 (0) |
| Treatment | |||
| Antiviral therapy | 236 (86) | 89 (79) | 147 (91) |
| Glucocorticoid therapy | 217 (79) | 99 (88) | 118 (73) |
| Antibiotics | 249 (91) | 105 (93) | 144 (89) |
| Intravenous immunoglobulin therapy | 54 (20) | 44 (39) | 59 (37) |
| Interferon inhalation | 89 (32) | 25 (22) | 64 (40) |
| Oxygen treatment | 251 (92) | 113 (100) | 138 (86) |
| High flow nasal cannula | 85 (31) | 77 (68) | 8 (5) |
| Mechanical ventilation | 119 (43) | 93 (82) | 26 (16) |
| Non-invasive | 102 (37) | 76 (67) | 26 (16) |
| Invasive | 17 (6) | 17 (15) | 0 (0) |
| Continuous renal replacement therapy | 3 (1) | 3 (3) | 0 (0) |
| Extracorporeal membrane oxygenation | 1 (<1) | 1 (1) | 0 (0) |
Fewer deceased patients (89; 79%) than recovered patients (147; 91%) received monotherapy or combination therapy with antiviral agents (oseltamivir, arbidol, or lopinavir/ritonavir), whereas more deceased patients (99; 88%) than recovered patients (118; 73%) were given glucocorticoid therapy, considering the severe pneumonia and “cytokine storm” observed in patients who died. One hundred and five (93%) deceased patients and 144 (89%) who recovered received empirical antibacterial therapy (moxifloxacin, cefoperazone, or azithromycin). Forty four (39%) deceased patients and 59 (37%) who recovered received intravenous immunoglobulin therapy. Fewer deceased patients (25; 22%) than recovered patients (64; 40%) received interferon α inhalation treatment. Significantly more deceased patients (93; 82%) than recovered patients (26; 16%) received mechanical ventilation. Invasive mechanical ventilation was needed in 17 (15%) deceased patients, one of whom received extracorporeal membrane pulmonary oxygenation as rescue therapy. Three deceased patients received continuous renal replacement therapy.
Discussion
Our study comprehensively described the major differences in clinical features between the patients who died of covid-19 and those who recovered from it. The median age of deceased patients was significantly older than that of recovered patients. Male sex was more predominant in patients who died than in those who recovered. Chronic hypertension and other cardiovascular comorbidities were more frequent among deceased patients than recovered patients. Symptoms related to hypoxemia were more common in deceased patients than in recovered patients. Deceased patients more often developed systematic inflammation and multi-organ dysfunction than did recovered patients. The indicators of cardiac injury showed more frequent or prominent abnormalities in deceased patients than in recovered patients. The information provided will further enrich knowledge about this critical disease and may consequently help to improve patients’ outcomes and lower the fatality rate.
Comparison with other studies
The clinical spectrum of covid-19 varies widely, ranging from an asymptomatic infection to severe and critical pneumonia with high fatality rates. The Chinese Centers for Disease Control recently reported that most of the confirmed cases were classified as mild or moderate, 13.8% as severe, and only 4.7% as critically ill.14 The overall fatality rate for confirmed covid-19 cases was found to be higher in male than in female patients, with the risk of death rising with age for both sexes. The highest fatality rate was in people aged 80 and above.
The overall mortality rate of covid-19 is much lower than for severe acute respiratory syndrome (10%) and Middle East respiratory syndrome (30%).15 16 However, covid-19 has ultimately proven more deadly as it has spread to many more people globally than did the others, owing to rapid person to person transmission and atypical symptoms at an early stage in certain patients.5 9 Here, we report a relatively high mortality rate for covid-19 of up to 14.1%, which is higher than in recent reports.9 This is partly due to a large proportion of severely or critically ill patients admitted to Tongji Hospital, one of the designated hospitals for severe covid-19, and to the medical resource limitations at the beginning of the covid-19 outbreak. These resources were improved by early February, with prompt supply of medics and medical necessities from across the nation to Wuhan.
In accordance with the recent reports on characteristics of patients with covid-19 who needed management in intensive care units,8 9 17 advanced age (>60), male sex, and comorbidities (particularly hypertension) are believed to be risk factors for severe disease and death from SARS-Cov-2 infection. Thus, early vigilant monitoring along with high quality supportive care are needed in patients at high risk. It is notable that healthcare workers as well as close contacts of previously confirmed patients were likely to have a good outcome, which is consistent with the relatively low fatality rate (0.3%) reported in healthcare workers.18 This could be explained by the fact that in our study the median age of the healthcare workers was much younger than that of the remaining patients (data not shown). It could also be partly due to the lower mortality observed in the second generation of SARS-Cov-2 infection,19 as well as to the early awareness of potential infection in that scenario meaning that people would seek medical care or start treatment promptly. Furthermore, the time from onset of symptoms to hospital admission was longer in deceased patients, as some of them had been in a critical condition before being transferred from other healthcare units to Tongji Hospital. This highlights the need to develop community awareness about prompt seeking of medical care and earlier referral to the intensive care unit for high risk populations.
The incidence of symptoms including fever, cough, fatigue, anorexia, myalgia, and diarrhoea did not differ significantly between deceased patients and recovered patients, whereas dyspnoea, chest tightness, and disorders of consciousness were more common in those who died. Moreover, the vital signs data showed that most deceased patients had tachycardia and/or tachypnoea as well as pulse oxygen saturation of 93% or lower. These signs and symptoms indicated that most deceased patients had been in a severe or critical condition on admission, and the onset of certain symptoms may help physicians to identify the patients at risk of a poor outcome.
The differences in abnormalities of laboratory findings between the deceased patients and the survivors were substantial. Most of the deceased patients and only a few recovered patients developed leukocytosis, and one third of deceased patients and only few who recovered had procalcitonin above 0.5 ng/mL, indicating that a large proportion of deceased patients might have had secondary bacterial infection, which could be strongly associated with death. Deceased patients had persistent and more severe lymphopenia compared with recovered patients, suggesting that a cellular immune deficiency state was associated with poor prognosis. In addition, other common laboratory abnormalities in deceased patients included coagulation disorder (elevation of prothrombin time and D-dimer), impaired liver and kidney function (mild or moderate elevation of alanine aminotransferase, aspartate aminotransferase, total bilirubin, alkaline phosphatase, γ-glutamyl transpeptidase, blood urea nitrogen, and creatinine and frequent hypoalbuminaemia, haematuria, and albuminuria), electrolyte disturbance (hyperkalaemia and hypernatraemia), elevated inflammatory markers (high sensitivity C-reactive protein, ferritin, and erythrocyte sedimentation rate), and cytokine storm. Most notably, markedly higher concentrations of creatine kinase, lactate dehydrogenase, cardiac troponin I, and N-terminal pro-brain natriuretic peptide were seen in deceased patients than in recovered patients. Increase in cardiac troponin I and N-terminal pro-brain natriuretic peptide was much more frequent and significant than that in the recent reports,8 9 likely owing to the relatively small number of deceased patients and more patients at earlier stages of the disease included in those studies.
In the later stages of the disease, patients who die may develop pulmonary and extrapulmonary organ damage, including acute respiratory distress syndrome, type I respiratory failure, sepsis, acute cardiac injury, heart failure, acute kidney injury, hypoxic encephalopathy, shock, acidosis or alkalosis, disseminated intravascular coagulation, and acute liver injury, although the last two complications were less frequent. Development of respiratory, cardiac, and neurological complications is strongly associated with poor outcome in patients with covid-19. Patients with cardiovascular comorbidities were more likely to develop cardiac complications. Cardiac complications were frequent not only in deceased patients with cardiovascular comorbidities but also in those without cardiovascular comorbidities, suggesting that the high risk of cardiac complications in deceased patients could not be entirely ascribed to coexisting cardiovascular disease. Furthermore, in addition to acute respiratory distress syndrome and respiratory failure, acute cardiac injury and heart failure could be major factors contributing to the fatality risk of covid-19 regardless of history of previous cardiovascular disease. However, the pathological report of covid-19 associated with acute respiratory distress syndrome at present shows that pulmonary oedema with hyaline membrane formation in the lungs, but no obvious histological changes in heart tissue, was identified from one single case report.20 This suggests that the underlying mechanism of cardiac injury needs further exploration. The median time from onset of symptoms to death in deceased patients was 16 days, and the median time from first symptoms to discharge in recovered patients was 26 days. In covid-19, the evolution of pulmonary and systemic inflammation in the first two weeks may determine the physiological progression (resolving or progressing) and outcome of disease (death or survival).
To date, no vaccine or specific antiviral treatment for covid-19 has proven to be effective, so supportive therapy that eases the symptoms and protects important organs may be most beneficial. In this study, for patients without second bacterial infection, empirical antimicrobial treatment seemed to be ineffective. Fewer deceased patients than recovered received antiviral monotherapy or combination antiviral therapy, as well as interferon α inhalation treatment. Considering the severe pneumonia and “cytokine storm” observed in deceased patients, more of these patients were given glucocorticoid therapy than recovered patients. Because of hypoxaemia, significantly more deceased patients than recovered received ventilation. We cannot conclude from this study which antivirals given at the right time would be beneficial, or whether steroid use would be beneficial, for patients with covid-19; further investigation is needed.
Substantial similarities exist between covid-19 and severe acute respiratory syndrome, from the virus homology to the potential origin, main transmission route (respiratory droplets), identified receptor (angiotensin converting enzyme 2), clinical manifestation, and disease dynamics.21 Risk factors for severe covid-19 or severe acute respiratory syndrome outcomes are old age and comorbidities. Progression for patients with severe disease follows a similar pattern for both viruses.21 Although both viruses can cause severe and even lethal lower respiratory tract infection and extrapulmonary manifestations, myocardial injury and heart failure are more frequently reported in patients with covid-19, indicating a unique pathophysiology.22 These findings will alert clinicians to pay special attention not only to the development of respiratory dysfunction but also to the signs of cardiac complications.
Limitations of study
Our study has several limitations. Firstly, almost all the deceased patients were classified as being severely or critically ill, whereas a large proportion of recovered patients might be classified as having moderate disease. This patient setting reflects the real world situation where most confirmed cases are mild or moderate. Nevertheless, the high incidence of cardiac complications in deceased patients is of great importance, raising awareness of the need for earlier monitoring and cardiac supportive care. Secondly, nearly a third of deceased patients developed disorders of consciousness on admission, ranging from somnolence to deep coma, which may result in a loss of some information (particularly a detailed history and subjective symptoms). Additionally, some laboratory tests (for example, cardiac troponin I, N-terminal pro-brain natriuretic peptide, and arterial blood gas tests) were not done in all the patients, and missing data or important tests might lead to bias of clinical characteristics. Thirdly, the median length of hospital admission before death was about five days, information on the dynamic changes in laboratory variables in deceased patients was lacking, and the data collected for each patient on admission may have been from different disease stages. Therefore, further study is warranted to gain a better understanding of risk factors for and outcome of covid-19, which ultimately may help to guide efforts aimed at reducing the fatality rate.
Conclusions and policy implications
Certain patients with covid-19, particularly those with advanced age and hypertension, were in a critical condition on admission and progressed rapidly to death within two to three weeks from disease onset. SARS-Cov-2 infection can cause both pulmonary and systemic inflammation, leading to multi-organ dysfunction in high risk populations. In addition to acute respiratory distress syndrome and type I respiratory failure, acute cardiac injury and heart failure may also contribute to the critical illness state associated with high mortality, which highlights the importance of earlier cardiac monitoring and supportive care in such patients.
What is already known on this topic
As of 28 February 2020, more than 2858 people had died of coronavirus disease 2019 (covid-19), with the highest mortality rate of 4.5% in Wuhan, China
Severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) infection causes clusters of severe and even fatal pneumonia
Clinical characteristics of patients with covid-19 who died have not been fully elucidated yet
What this study adds
Certain patients with covid-19, particularly those with advanced age and hypertension, were in a critical condition on admission and progressed rapidly to death within two to three weeks from disease onset
SARS-Cov-2 infection can cause both pulmonary and systemic inflammation, leading to multi-organ dysfunction in high risk populations
In addition to acute respiratory distress syndrome and type I respiratory failure, acute cardiac injury and heart failure may also contribute to the critical illness state associated with high mortality
Acknowledgments
We thank all the patients and their families involved in this study, as well as many doctors, nurses, and civilians working together to fight against SARS-Cov-2.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary table
Contributors: TC, DW, HLC, WMY, DLY, and GC contributed equally to this paper, as did KM, DX, HJY, HWW, and TW. QN designed the study, had full access to all data in the study, and takes responsibility for the integrity and accuracy of the data analysis. TC, DW, HC, WY, DY, and GC contributed to patient recruitment, data collection, data analysis, data interpretation, literature search, and writing of the manuscript. KM, DX, HY, HW, WG, JH, TW, and MH had roles in patient recruitment, data collection, and clinical management. JC, CD, XZ, SL, XL, and JZ had roles in the patient management, data collection, data analysis, and data interpretation. All authors contributed to data acquisition, data analysis, or data interpretation, and all reviewed and approved the final version of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. QN is the guarantor.
Funding: This work was funded by grants from the Tongji Hospital for Pilot Scheme Project and partly supported by the Chinese National Thirteenth Five Years Project in Science and Technology (2017ZX10202201), National Commission of Health, People’s Republic of China. The research was designed, conducted, analysed, and interpreted by the authors entirely independently of the funding sources.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the Tongji Hospital for Pilot Scheme Project and the Chinese National Thirteenth Five Years Project in Science and Technology, National Commission of Health, People’s Republic of China, for the submitted work; no financial relationships with any organisation that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: The case series was approved by the Institutional Review Board of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (TJ-C20200101). Written informed consent was waived owing to the rapid emergence of this infectious disease.
Data sharing: No additional data available.
Transparency declaration: The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: No study participants were involved in the preparation of this article. The results of the article will be summarised in media press releases from the Huazhong University of Science and Technology and presented at relevant conferences.
References
- 1. Drosten C, Günther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003;348:1967-76. . 10.1056/NEJMoa030747 [DOI] [PubMed] [Google Scholar]
- 2. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012;367:1814-20. . 10.1056/NEJMoa1211721 [DOI] [PubMed] [Google Scholar]
- 3. Zhu N, Zhang D, Wang W, et al. China Novel Coronavirus Investigating and Research Team A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med 2020;382:727-33. . 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gralinski LE, Menachery VD. Return of the Coronavirus: 2019-nCoV. Viruses 2020;12:E135. . 10.3390/v12020135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020. . 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020;395:514-23. . 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270-3. . 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. . 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020. . 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.New coronavirus pneumonia prevention and control program (6th ed) (in Chinese). 2020. http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf.
- 11.World Health Organization. Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected.
- 12. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179-84. 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 13. Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J 2006;27:330-7. . 10.1093/eurheartj/ehi631 [DOI] [PubMed] [Google Scholar]
- 14. Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. 2003. https://www.who.int/csr/sars/country/table2004_04_21/en/.
- 16.World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV). 2019. https://www.who.int/emergencies/mers-cov/en/.
- 17. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13. . 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Novel Coronavirus Pneumonia Emergency Response Epidemiology Team [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi 2020;41:145-51. 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 19. Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series [correction in: BMJ 2020;368:m792]. BMJ 2020;368:m606. . 10.1136/bmj.m606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;S2213-2600(20)30076-X. 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilder-Smith A, Chiew CJ, Lee VJ. Can we contain the COVID-19 outbreak with the same measures as for SARS? Lancet Infect Dis 2020;S1473-3099(20)30129-8. 10.1016/S1473-3099(20)30129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu CL, Lu YT, Peng MJ, et al. Clinical and laboratory features of severe acute respiratory syndrome vis-a-vis onset of fever. Chest 2004;126:509-17. . 10.1378/chest.126.2.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary table