Abstract
Objective
To investigate the impact of modifications to contemporary cancer protocols, which minimize exposures to cardiotoxic treatments and preserve long term health, on serious cardiac outcomes among adult survivors of childhood cancer.
Design
Retrospective cohort study.
Setting
27 institutions participating in the Childhood Cancer Survivor Study.
Participants
23 462 five year survivors (6193 (26.4%) treated in the 1970s, 9363 (39.9%) treated in the 1980s, and 7906 (33.6%) treated in the 1990s) of leukemia, brain cancer, Hodgkin lymphoma, non-Hodgkin lymphoma, renal tumors, neuroblastoma, soft tissue sarcomas, and bone sarcomas diagnosed prior to age 21 years between 1 January 1970 and 31 December 1999. Median age at diagnosis was 6.1 years (range 0-20.9) and 27.7 years (8.2-58.3) at last follow-up. A comparison group of 5057 siblings of cancer survivors were also included.
Main outcome measures
Cumulative incidence and 95% confidence intervals of reported heart failure, coronary artery disease, valvular heart disease, pericardial disease, and arrhythmias by treatment decade. Events were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events. Multivariable subdistribution hazard models were used to estimate hazard ratios by decade, and mediation analysis examined risks with and without exposure to cardiotoxic treatments.
Results
The 20 year cumulative incidence of heart failure (0.69% for those treated in the 1970s, 0.74% for those treated in the 1980s, 0.54% for those treated in the 1990s) and coronary artery disease (0.38%, 0.24%, 0.19%, respectively), decreased in more recent eras (P<0.01), though not for valvular disease (0.06%, 0.06%, 0.05%), pericardial disease (0.04%, 0.02%, 0.03%), or arrhythmias (0.08%, 0.09%, 0.13%). Compared with survivors with a diagnosis in the 1970s, the risk of heart failure, coronary artery disease, and valvular heart disease decreased in the 1980s and 1990s but only significantly for coronary artery disease (hazard ratio 0.65, 95% confidence interval 0.45 to 0.92 and 0.53, 0.36 to 0.77, respectively). The overall risk of coronary artery disease was attenuated by adjustment for cardiac radiation (0.90, 0.78 to 1.05), particularly among survivors of Hodgkin lymphoma (unadjusted for radiation: 0.77, 0.66 to 0.89; adjusted for radiation: 0.87, 0.69 to 1.10).
Conclusions
Historical reductions in exposure to cardiac radiation have been associated with a reduced risk of coronary artery disease among adult survivors of childhood cancer. Additional follow-up is needed to investigate risk reductions for other cardiac outcomes.
Trial registration
ClinicalTrials.gov NCT01120353.
Introduction
Progress in the treatment of children with cancer has led to an increase in the number of survivors living into adulthood. Although improved survival among children treated in the 1990s compared with the 1980s and 1970s has been shown,1 the impact on the long term health of these individuals remains substantial, with most experiencing chronic health conditions related to previous treatment.2 3 4 5 An array of cardiovascular conditions—a prominent contributor to the overall burden of late health outcomes—has been reported after cancer treatment, including: cardiomyopathy, cardiac arrhythmias, and coronary artery, valvular, and pericardial diseases.6 7 8 Understanding the trajectory of these outcomes has become increasingly important as the risks for survivors of more modern, risk adapted treatments might differ from those of their predecessors.
Contemporary cancer treatment has focused on advancing cure rates while attempting to minimize long term adverse effects. Patterns of exposure to cardiotoxic treatment have changed over time, with fewer children receiving chest directed radiation, with lower doses and smaller volumes for those who do, and an increased use of anthracyclines, albeit with reduced cumulative doses as the risk for late onset heart failure became apparent.9 10 The impact of these treatment modifications on the spectrum of late onset cardiovascular conditions in cancer survivors is understudied. The Childhood Cancer Survivor Study, which includes a diverse population of survivors with a diagnosis across three decades, provides an opportunity to examine temporal trends in cardiac outcomes and the effect of changes in treatment over time. We assessed whether changes in exposures to cancer treatment in childhood are associated with altered risks for cardiac events among adult survivors.
Methods
Population
The Childhood Cancer Survivor Study is a multi-institutional retrospective cohort study with longitudinal follow-up of five year survivors of the most common childhood cancers (leukemia, central nervous system tumors, Hodgkin lymphoma, non-Hodgkin lymphoma, renal tumors, neuroblastoma, soft tissue sarcomas, and bone sarcomas) diagnosed before age 21 years at one of 27 participating institutions in the United States and Canada. Initially inclusive of survivors with a diagnosis through 1986, the cohort was expanded to include survivors of childhood cancer with a diagnosis over three decades, from 1 January 1970 through 31 December 1999. Additionally, a random sample of siblings (n=5057) of participating survivors were recruited as a comparison group. Participants completed a baseline questionnaire and up to four follow-up surveys. Cardiac outcomes are assessed by a series of questions such as ‘Have you ever been told by a doctor or healthcare professional that you have, or have had . . . congestive heart failure, a myocardial infarction/coronary heart disease, stiff/leaky heart valves, pericarditis/pericardial constriction, or an arrhythmia?” Details of the Childhood Cancer Survivor Study design, methods, and characteristics of participants and non-participants are reported elsewhere,11 12 and surveys can be viewed at www.ccss.stjude.org. Participants, or parents of those younger than 18 years, provided informed consent, and the protocol was reviewed and approved by the human subjects committees at each participating institution. Minor participants were re-consented at the age of majority.
Cardiac outcomes
Participants completed surveys that included personal information and medical outcomes. Outcomes were self reported and supplemented by data from the National Death Index. Using a well established algorithm, a multidisciplinary team reviewed and adjudicated all conditions graded and scored according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE v4.03) as previously described.3 For this analysis, we included all reported cardiac conditions of grades 3-5, including heart failure, coronary artery disease (including myocardial infarction or coronary revascularization), heart valve replacement, pericardial disease, and arrhythmias requiring pacemakers or cardioversion, or death from any one of these five conditions (supplemental table 1). Among the 470 reported cardiac conditions, most survivors had a single condition (n=377, 80.2%). Some however, reported two (n=79, 16.8%), three (n=9, 1.4%), or four conditions (n=4, 0.85%). One survivor reported having all five conditions (supplemental figure 1).
Cardiovascular risk factors
We considered participants to have diabetes, dyslipidemia, or hypertension if they reported one of these conditions diagnosed by a physician and were taking drugs for the condition (grade 2 or higher). Smoking status was dichotomized as ever or never smoker and we used age at onset and cessation to determine years of smoking. Body mass index (BMI) was categorized as underweight (<18.5 weight (kg)/(height (m)2), normal (18.5-24.9), overweight (25.0-29.9), or obese (≥30). Sex, race/ethnicity, age, and education status at last follow-up were determined from questionnaire responses.
Treatment exposures
Details for treatment were abstracted from the medical records of those survivors who additionally authorized release of the medical record. We calculated the cumulative anthracycline dose (per square meter) in doxorubicin equivalents,13 and the Department of Radiation Physics at MD Anderson Cancer Center estimated the mean dose of heart radiation by reconstructing individual radiation treatments on age specific computational phantoms.14 Treatment decade (1970-79, 1980-89, 1990-99) was assigned based on diagnosis date.
Statistical analysis
Descriptive statistics compared participating and non-participating survivors across treatment decades as well as participants with the sibling comparison group, using χ2 statistics or two sample t tests. Cumulative incidence and 95% confidence intervals were estimated for each cardiac condition, treating all cause death (except death due to the particular outcome analyzed) as a competing risk event, and stratified by cancer diagnosis and treatment decade and compared at 15 years from initial cancer diagnosis using permutation test.
To account for the variation in treatment regimens, we fitted models to clustered (institutions) data and multivariable subdistribution hazards models (Fine and Gray model15) with repeated measurements of time dependent covariates using years smoked, BMI (continuous), diabetes, dyslipidemia, and hypertension at each time point to estimate relative risks and 95% confidence intervals of each cardiac outcome by treatment era, adjusting for sex, race, exercise intensity, age at diagnosis (continuous), and cardiotoxic treatments (cumulative exposure to anthracycline chemotherapy and mean dose of cardiac radiation). We considered death, except for death from the particular outcome analyzed, as a competing risk. Years from diagnosis were used as the timescale, with censoring at time of grade 3-5 event or 15 years from cohort entry. Multiple imputation methods were used for the missing data on cardiotoxic treatment (11% cardiac radiation and 12% anthracycline chemotherapy), and cumulative logit models to create 20 imputed datasets under the missing at random assumption using cancer diagnosis, age at cancer diagnosis, year of diagnosis, and sex as explanatory variables.
Using mediation analysis we examined the relative risk of treatment era on each cardiac condition in models with and without cardiotoxic treatment to assess the extent of attenuation of the regression coefficient, to determine whether reduction of cardiovascular events across five year treatment eras was attributable to changes in the cardiotoxic treatment exposures over the same period. SAS version 9.4 was used for all statistical analyses and R version 3.5.0 for statistical graphics.
Patient and public involvement
Through the completion of regular surveys, the Childhood Cancer Survivor Study has included self reported patient outcomes since its inception in 1994. No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study.
Results
Among 23 462 participants in the Childhood Cancer Survivor Study, 6193 (26.4%) were treated in the 1970s, 9363 (39.9%) in the 1980s, and 7906 (33.7%) in the 1990s (fig 1, table 1). Of the participating survivors more were women (46.3% v 39.9%, P<0.001) and slightly younger at diagnosis (6.1 years (range 0-20.9) v 6.8 years (0-20.9), P<0.001) than non-participants (supplemental table 2). However, although more non-participants than participants had died (17.4% v 12.4%, P<0.001), cardiac deaths did not differ between the two groups (2.8% v 2.6%, P=0.69). Survivors (median age at follow-up 27.7 (8.2-58.3) years) were younger than siblings (32.8 (0.3-62.6) years). Median follow-up time ranged from 11.0 years (diagnosis in the 1990s) to 29.5 years (diagnosis in the 1970s). The number of survivors exposed to chemotherapy containing anthracycline increased from 27.8% to 50.0% to 64.0% across the respective decades. However, those receiving higher doses (≥250 mg/m2) decreased from 16.5% to 11.9% from the 1970s to the 1990s, while the number receiving <250 mg/m2 increased substantially, from 11.3% to 52.1%. Exposure to cardiac radiation declined from more than three quarters (76.8%) of survivors treated in the 1970s to 40.3% of survivors treated in the 1990s. Nearly a quarter (24.2%) of survivors from the 1970s received a mean heart dose of 15 Gy or more, which by the 1990s had decreased by half (12.4%). Supplemental tables 3 and 4 show changes in exposure to anthracycline and cardiac radiation by diagnosis.
Fig 1.
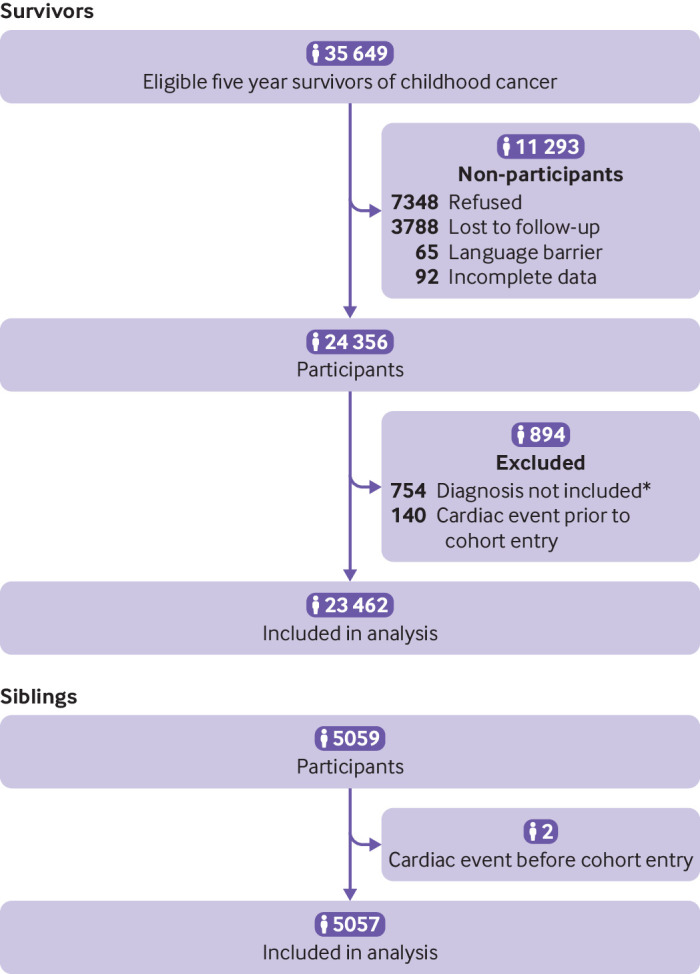
Flow of participants through study. *All soft tissue sarcoma subtypes were included in the Childhood Cancer Survivor Study cohort from 1970-86. However, for the expanded cohort, 1987-99, only rhabdomyosarcoma was included. Thus, to ensure a homogeneous population across decades, non-rhabdomyosarcoma diagnoses were excluded
Table 1.
Demographic and treatment characteristics of survivors of childhood cancer overall and by decade of diagnosis and their siblings. Values are numbers (percentages) unless stated otherwise
| Characteristics | Survivors | Siblings (n=5057) | P value† | |||
|---|---|---|---|---|---|---|
| Overall cohort (n=23 462) | 1970-79 (n=6193)* | 1980-89 (n=9363)* | 1990-99 (n=7906)* | |||
| Sex: | ||||||
| Female | 10 874 (46.3) | 2883 (46.6) | 4292 (45.9) | 3699 (46.4) | 2643 (52.3) | <0.001 |
| Male | 12 588 (53.7) | 3310 (53.4) | 5071 (54.1) | 4207 (53.6) | 2414 (47.7) | |
| Median (range) age at last follow-up (years) | 27.7 (8.2-58.3) | 36.5 (15.5-58.3) | 28.3 (8.2-49.5) | 22.8 (9.4-42.4) | 32.8 (0.3-62.6) | <0.001 |
| Median (range) age at diagnosis (years) | 6.1 (0.0-20.9) | 7.2 (0.0-20.9) | 5.9 (0.0-20.9) | 5.9 (0.0-20.9) | NA | |
| Median (range) time from diagnosis (years) | 20.5 (7.0-39.3) | 29.5 (14.4-39.3) | 22.4 (7.0-29.2) | 11.0 (8.4-24.3) | NA | |
| Race/ethnicity: | ||||||
| Non-Hispanic white | 19 226 (81.2) | 5503 (88.9) | 7744 (82.5) | 5979 (75.4) | 4375 (86.5) | <0.001 |
| Non-Hispanic black | 1495 (6.5) | 241 (3.9) | 577 (6.1) | 677 (8.4) | 151 (3.0) | |
| Hispanic | 1761 (8.1) | 283 (4.6) | 609 (6.7) | 869 (11.4) | 213 (4.2) | |
| Asian/Pacific Islander | 354 (1.7) | 41 (0.7) | 154 (1.7) | 159 (2.1) | 54 (1.1) | |
| Other/unknown | 626 (2.6) | 125 (2.0) | 279 (3.0) | 222 (2.7) | 264 (5.2) | |
| Primary cancer diagnosis: | ||||||
| Leukemia | 7281 (39.5) | 2023 (32.7) | 3314 (40.1) | 1944 (43.0) | NA | |
| Acute lymphoblastic leukemia | 6127 (35.2) | 1818 (29.4) | 2885 (35.9) | 1424 (38.0) | ||
| Acute myelogenous leukemia | 852 (3.2) | 131 (2.1) | 324 (3.2) | 397 (3.8) | ||
| Other | 302 (1.1) | 74 (1.2) | 105 (1.0) | 123 (1.2) | ||
| Brain tumor | 4227 (15.8) | 735 (11.9) | 1498 (14.8) | 1994 (19.1) | ||
| Astrocytoma | 2589 (9.7) | 509 (8.2) | 944 (9.4) | 1136 (10.9) | ||
| Medulloblastoma | 994 (3.7) | 147 (2.4) | 348 (3.4) | 499 (4.8) | ||
| Other | 644 (2.4) | 79 (1.3) | 206 (2.0) | 359 (3.4) | ||
| Lymphoma | 4904 (18.3) | 1545 (25.0) | 1820 (18.0) | 1539 (14.7) | ||
| Hodgkin lymphoma | 2985 (11.2) | 1093 (17.7) | 1053 (10.4) | 839 (8.0) | ||
| Non-Hodgkin lymphoma | 1919 (7.2) | 452 (7.30) | 767 (7.6) | 700 (6.7) | ||
| Kidney tumor | 2130 (8.0) | 531 (8.6) | 870 (8.6) | 729 (7.0) | ||
| Neuroblastoma | 1825 (6.8) | 441 (7.1) | 668 (6.6) | 716 (6.9) | NA | |
| Soft tissue sarcoma | 1153 (4.3) | 364 (5.9) | 443 (4.4) | 346 (3.3) | ||
| Bone cancer | 1942 (7.3) | 554 (9.0) | 750 (7.4) | 638 (6.1) | ||
| Osteosarcoma | 1187 (4.4) | 354 (5.7) | 467 (4.6) | 366 (3.5) | ||
| Ewing sarcoma | 702 (2.6) | 197 (3.2) | 274 (2.7) | 231 (2.2) | ||
| Other | 53 (0.2) | 3 (0.1) | 9 (0.1) | 41 (0.4) | ||
| Therapy type‡: | ||||||
| Radiation only | 81 (0.4) | 24 (0.5) | 33 (0.4) | 24 (0.3) | NA | |
| Surgery only | 1867 (7.7) | 274 (5.2) | 627 (6.9) | 966 (9.7) | ||
| Radiation and surgery | 1885 (7.8) | 782 (14.8) | 770 (8.5) | 333 (3.3) | ||
| Chemotherapy only | 2318 (17.4) | 227 (4.3) | 920 (14.0) | 1171 (27.4) | ||
| Chemotherapy and radiation | 2289 (11.8) | 863 (16.3) | 985 (13.4) | 441 (7.9) | ||
| Chemotherapy and surgery | 4912 (22.0) | 694 (13.1) | 1969 (22.5) | 2249 (26.3) | ||
| Chemotherapy, radiation, and surgery | 7804 (33.0) | 2424 (45.8) | 3011 (34.2) | 2369 (25.1) | ||
| Anthracycline (mg/m2)§: | ||||||
| None | 11 145 (49.0) | 3710 (72.2) | 4248 (50.0) | 3187 (36.0) | NA | |
| <250 | 6190 (36.0) | 581 (11.3) | 2360 (32.2) | 3249 (52.1) | ||
| ≥250 | 3415 (15.0) | 850 (16.5) | 1511 (17.8) | 1054 (11.9) | ||
| Mean heart radiation dose (Gy): | ||||||
| None | 9234 (46.3) | 1219 (23.2) | 3631 (45.2) | 4384 (59.7) | NA | |
| <15 | 8199 (37.8) | 2764 (52.6) | 3343 (39.9) | 2092 (27.9) | ||
| 15 to <35 | 2376 (11.3) | 675 (12.9) | 866 (10.6) | 835 (11.1) | ||
| ≥35 | 1074 (4.6) | 593 (11.3) | 376 (4.3) | 105 (1.3) | ||
| Education: | ||||||
| Some high school | 4263 (20.0) | 674 (11.2) | 1472 (15.7) | 2103 (28.9) | 398 (8.0) | <0.001 |
| High school graduate | 5173 (22.2) | 1490 (24.7) | 2008 (21.5) | 1675 (21.4) | 854 (17.2) | |
| Some college | 5893 (25.8) | 1532 (25.4) | 2309 (25.4) | 2052 (26.4) | 1298 (26.1) | |
| College graduate | 5633 (23.3) | 1553 (25.8) | 2511 (27.4) | 1569 (18.0) | 1623 (32.6) | |
| Postgraduate | 2213 (8.8) | 773 (12.8) | 939 (10.0) | 501 (5.3) | 807 (16.2) | |
| Median (range) body mass index | 24.6 (11.0-63.2) | 25.2 (11.0-63.2) | 24.8 (11.2-61.2) | 24.2 (11.2-62.8) | 23.8 (11.2-60.8) | <0.001 |
| Smoking: | ||||||
| Never | 14 435 (68.4) | 4071 (67.1) | 6243 (68.9) | 4121 (68.8) | 3109 (65.0) | <0.001 |
| Ever | 6654 (31.6) | 1992 (32.9) | 2767 (31.1) | 1895 (31.2) | 1674 (35.0) | |
| Diabetes mellitus: | ||||||
| Yes | 687 (2.8) | 255 (4.1) | 255 (2.7) | 177 (2.1) | 94 (1.9) | <0.001 |
| No | 22 775 (97.2) | 5938 (95.9) | 9108 (97.3) | 7729 (97.9) | 4963 (98.1) | |
| Dyslipidemia: | ||||||
| Yes | 1578 (6.2) | 715 (11.6) | 579 (6.0) | 284 (3.3) | 271 (5.4) | 0.02 |
| No | 21 884 (93.8) | 5478 (88.4) | 8784 (94.0) | 7622 (96.7) | 4786 (94.6) | |
| Hypertension: | ||||||
| Yes | 2232 (9.1) | 914 (14.8) | 853 (9.1) | 465 (5.6) | 437 (8.6) | 0.35 |
| No | 21 230 (90.9) | 5279 (85.2) | 8510 (90.9) | 7441 (94.4) | 4620 (91.4) | |
NA=not applicable.
Sampling weights were applied for all percentages, means, and medians to account for under-sampling of survivors of acute lymphoblastic leukemia (1987-99), using a weight of 1.21 for those aged 0 or 11-20 years at diagnosis and a weight of 3.63 for those aged 1-10 years at diagnosis.
P value comparing overall cohort with siblings
Treatment categories are mutually exclusive. Percentages provided among those with available data on treatment exposure.
Doxorubicin equivalents.
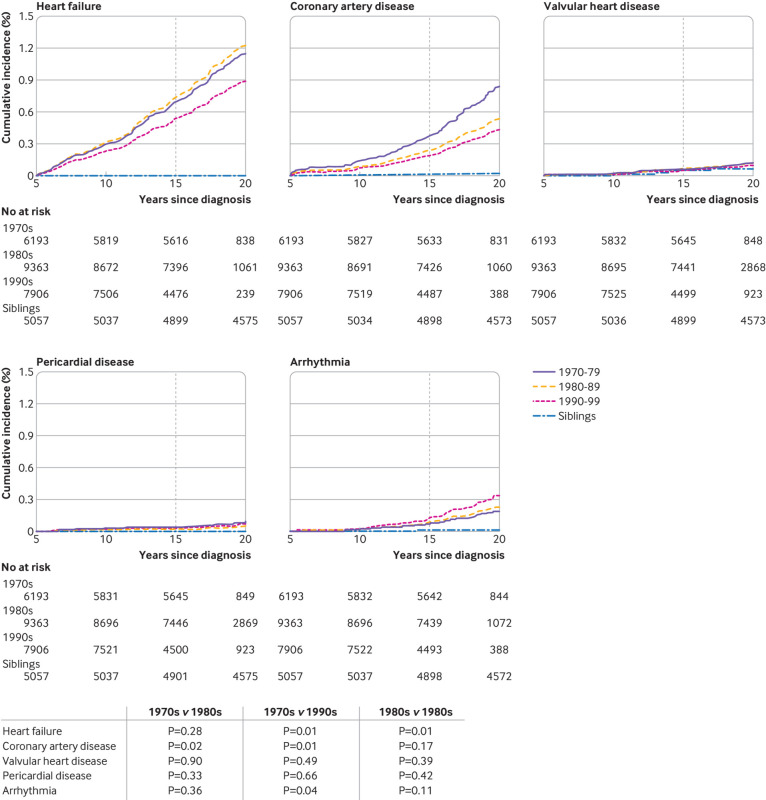
For the overall population, the cumulative incidence of heart failure at 15 years from cancer diagnosis was significantly lower in the 1990s (0.54%) compared with the 1970s (0.69%) (P=0.01) and the 1980s (0.74%) (P=0.01) (fig 2). For coronary artery disease the 20 year incidence decreased successively across all three decades (0.38% in the 1970s, 0.24% in the 1980s, 0.19% in the 1990s). Supplemental table 5 provides the cumulative incidence of each cardiac outcome overall and by primary cancer diagnosis. The prevalence of each cardiac condition was relatively low but substantially raised compared with siblings (fig 2, supplemental table 6). Compared with survivors with a cancer diagnosis in the 1970s, the hazard ratio of adverse cardiac outcomes decreased in the 1990s for heart failure and coronary artery disease (table 2). However, only the decline in coronary artery disease (hazard ratios: 0.53 (95% confidence interval 0.36 to 0.77) in the 1990s and 0.65 (0.45 to 0.92) in the 1980s versus the 1970s) was statistically significant. This was primarily attributable to decreases in the rate of coronary artery disease among survivors of Hodgkin lymphoma (0.77 (0.40 to 1.45) in the 1980s to 0.44 (0.23 to 0.85) in the 1990s). Although the decline for heart failure did not reach statistical significance, the largest decrease was from the 1980s to the 1990s (0.73 (0.52 to 1.02), P=0.07, data not shown). Except for arrhythmias, the risk of each condition consistently decreased across the decades. The only population where an increase was observed was for heart failure among survivors of neuroblastoma (3.22 (0.83 to 12.53) in the 1980s and 5.72 (1.58 to 20.67) in the 1990s versus the 1970s).
Fig 2.
Cumulative incidence of grades 3-5 cardiac outcomes among survivors of cancer in childhood by decade of cancer diagnosis and siblings. Vertical line represents incidence at 15 years from cancer diagnosis (10 years from cohort entry). P values of permutation tests comparing each outcome by decade. Some survivors were censored before reaching 15 years from diagnosis and thus only contributed to the estimation of cumulative incidence curves up to their censoring time
Table 2.
Hazard ratios (95% confidence interval) of cardiac conditions 20 years from diagnosis by treatment era*
| Diagnosis by decade | Hazard ratio (95% CI) | ||||
|---|---|---|---|---|---|
| Heart failure | Coronary artery disease | Valvular heart disease | Pericardial disease | Arrhythmia | |
| All survivors | |||||
| 1970-79 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1980-89 | 1.07 (0.78 to 1.45) | 0.65 (0.45 to 0.92) | 1.08 (0.63 to 1.85) | 0.57 (0.18 to 1.81) | 1.22 (0.69 to 2.17) |
| 1990-99 | 0.77 (0.53 to 1.14) | 0.53 (0.36 to 0.77) | 0.87 (0.33 to 2.33) | 0.80 (0.26 to 2.46) | 1.74 (0.99 to 3.03) |
| Leukemia: | |||||
| 1970-79 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1980-89 | 0.80 (0.39 to 1.66) | 0.69 (0.33 to 1.44) | 0.64 (0.51 to 0.80) | N/E | 0.64 (0.21 to 1.93) |
| 1990-99 | 1.05 (0.36 to 3.12) | 0.83 (0.31 to 2.22) | 3.97 (0.52 to 30.34) | N/E | 0.45 (0.11 to 1.85) |
| CNS tumors: | |||||
| 1970-79 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1980-89 | 0.58 (0.09 to 3.94) | 0.80 (0.19 to 3.43) | N/E | N/E | 1.47 (0.95 to 2.26) |
| 1990-99 | 1.31 (0.21 to 8.39) | 0.60 (0.12 to 2.88) | N/E | N/E | N/E |
| Hodgkin lymphoma: | |||||
| 1970-79 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1980-89 | 1.25 (0.66 to 2.35) | 0.77 (0.40 to 1.45) | 1.59 (0.83 to 3.06) | N/E | 1.24 (0.46 to 3.39) |
| 1990-99 | 0.77 (0.31 to 1.93) | 0.44 (0.23 to 0.85) | 0.41 (0.05 to 3.35) | N/E | 1.50 (0.44 to 5.08) |
| Non-Hodgkin lymphoma: | |||||
| 1970-79 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1980-89 | 0.98 (0.46 to 2.12) | 0.82 (0.25 to 2.75) | N/E | 0.27 (0.02 to 3.52) | 3.30 (0.41 to 26.60) |
| 1990-99 | 0.41 (0.14 to 1.23) | 0.86 (0.23 to 3.22) | N/E | 0.34 (0.06 to 2.0) | 3.08 (0.46 to 20.70) |
| Kidney tumor: | |||||
| 1970-79 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1980-89 | 1.16 (0.42 to 3.23) | N/E | 0.67 (0.09 to 5.03) | N/E | 1.12 (0.83 to 1.51) |
| 1990-99 | 0.35 (0.10 to 1.26) | 1.68 (0.11 to 24.71) | 0.49 (0.04 to 6.56) | N/E | N/E |
| Neuroblastoma: | |||||
| 1970-79 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1980-89 | 3.22 (0.83 to 12.53) | 0.64 (0.17 to 2.43) | N/E | N/E | N/E |
| 1990-99 | 5.72 (1.58 to 20.67) | 0.49 (0.05 to 4.46) | N/E | N/E | N/E |
| Soft tissue sarcoma: | |||||
| 1970-79 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1980-89 | 1.08 (0.30 to 3.85) | 0.47 (0.13 to 1.68) | N/A | 0.84 (0.05 to 15.44) | 0.82 (0.22 to 3.06) |
| 1990-99 | 0.59 (0.24 to 1.45) | N/E | N/A | N/E | N/E |
| Bone cancer: | |||||
| 1970-79 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1980-89 | 1.69 (0.97 to 2.97) | 0.92 (0.38 to 2.24) | N/E | N/E | 2.46 (0.46 to 13.12) |
| 1990-99 | 0.74 (0.26 to 2.09) | 0.53 (0.14 to 2.07) | N/E | N/E | 1.53 (0.26 to 9.15) |
CNS=central nervous system; N/E=not estimable owing to small cell size.
Adjusted for age at diagnosis, race, and sex.
The effects of therapeutic and traditional cardiac risk factors on each cardiac outcome were assessed in adjusted multivariable models (table 3). The hazard ratios for heart failure and coronary artery disease suggested a decline over the treatment eras but were attenuated by the addition of treatment variables (mean cardiac and anthracycline doses) and cardiovascular risk factors. Risk factors associated with heart failure included female sex (1.51 (1.10 to 2.06)), higher exposures to cardiac radiation and anthracycline dose, and reporting a diagnosis of diabetes, dyslipidemia, or hypertension. For coronary artery disease a dose-response was evident with increasing mean exposure to heart radiation (2.26 (1.32 to 3.84) for 15-35 Gy and 5.86 (3.69 to 9.28) for ≥35 Gy). No significant association was found with diabetes mellitus, but dyslipidemia and hypertension (3.49 (2.11 to 5.77) and 4.75 (3.37 to 6.69), respectively) were associated with a significantly increased risk for coronary artery disease. High dose (≥35 Gy) cardiac radiation and hypertension were associated with valvular heart disease (13.97 (6.01 to 32.48) and 3.12 (1.09 to 8.91), respectively). Hypertension was also significantly associated with the development of pericardial disease (6.35 (1.56 to 25.83)). Heart radiation greater than 35 Gy (2.74 (1.01 to 6.81)), high dose (≥250 mg/m2) anthracyclines (3.81 (2.13 to 6.80)), dyslipidemia (3.54 (1.80 to6.95)), and hypertension (2.59 (1.27 to 5.30)) were significantly associated with arrhythmias.
Table 3.
Multivariable analysis of cardiac conditions by treatment era and cardiovascular risk factors 20 years from diagnosis
| Risk factors by treatment era | Hazard ratio (95% CI) | ||||
|---|---|---|---|---|---|
| Heart failure | Coronary artery disease | Valvular heart disease | Pericardial disease | Arrhythmia | |
| Sex: | |||||
| Male | 1.0 | ||||
| Female | 1.51 (1.10 to 2.06) | 0.87 (0.62 to 1.23) | 1.25 (0.74 to 2.12) | 0.68 (0.29 to 1.58) | 1.25 (0.79 to 1.99) |
| Treatment era: | |||||
| 1970-79 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1980-89 | 0.89 (0.67 to 1.17) | 0.66 (0.42 to 1.02) | 1.36 (0.77 to 2.43) | 0.57 (0.16 to 1.97) | 1.16 (0.65 to 2.05) |
| 1990-99 | 0.70 (0.45 to 1.08) | 0.63 (0.36 to 1.08) | 1.33 (0.42 to 4.21) | 0.96 (0.23 to 4.02) | 1.71 (0.96 to 3.07) |
| Mean heart dose (Gy): | |||||
| None | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1-15 | 0.74 (0.54 to 1.03) | 1.31 (0.88 to 1.96) | 1.12 (0.33 to 3.79) | 0.64 (0.19 to 2.20) | 0.97 (0.54 to 1.73) |
| 15.1-34.99 | 1.56 (1.05 to 2.33) | 2.26 (1.32 to 3.84) | 2.03 (0.64 to 6.44) | 0.88 (0.21 to 3.64) | 0.89 (0.37 to 2.18) |
| ≥35 | 3.95 (2.87 to 5.43) | 5.86 (3.69 to 9.28) | 13.97 (6.01 to 32.48) | 2.77 (0.59 to 12.88) | 2.74 (1.10 to 6.81) |
| Anthracycline dose (mg/m2): | |||||
| None | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| <250 | 2.76 (1.93 to 3.97) | 1.42 (0.93 to 2.16) | 1.67 (0.78 to 3.55) | 1.38 (0.43 to 4.49) | 1.38 (0.81 to 2.36) |
| ≥250 | 9.29 (6.01 to 14.37) | 1.77 (1.15 to 2.72) | 2.04 (1.06 to 3.93) | 2.81 (0.85 to 9.34) | 3.81 (2.13 to 6.80) |
| Comorbidities: | |||||
| Diabetes | 2.66 (1.67 to 4.25) | 1.55 (0.67 to 3.58) | 0.79 (0.10 to 6.24) | 2.58 (0.26 to 25.80) | 2.51 (0.82 to 7.71) |
| Dyslipidemia | 2.32 (1.53 to 3.52) | 3.49 (2.11 to 5.77) | 2.43 (0.65 to 9.02) | 1.49 (0.27 to 8.25) | 3.54 (1.80 to 6.96) |
| Hypertension | 4.93 (3.61 to 6.72) | 4.75 (3.37 to 6.69) | 3.12 (1.09 to 8.91) | 6.35 (1.56 to 25.83) | 2.59 (1.27 to 5.30) |
Estimates adjusted for all variables in the table: race, age at diagnosis, body mass index, smoking, and exercise intensity (metabolic hours/week).
Table 4 presents the results of the mediation analyses. After adjustment for age at diagnosis, race, sex, BMI, smoking, exercise intensity, and modifiable risk factors (diabetes, dyslipidemia, and hypertension), the hazard ratios of heart failure and coronary artery disease declined over five year treatment eras. However, only the decline for coronary artery disease (0.80 (0.71 to 0.91) achieved statistical significance. This finding was attenuated by adjustment for exposure to cardiac radiation (0.90 (0.78 to 1.05)), suggesting that decreases in radiation dose or changes in modalities or delivery methods might account for some portion of the treatment era risk for coronary artery disease. Adjustment for exposure to anthracyclines did not attenuate these findings. These results are consistent regardless of whether the model used time since diagnosis or attained age as its timescale adjusted for age at diagnosis. The three variables are linearly dependent and thus only two of the three can be adjusted for in the model. Stratifying the mediation analysis by diagnosis (supplemental table 7) identified that treatment era attenuation in coronary artery disease by exposure to radiation therapy is largely attributable to changes in treatment for Hodgkin lymphoma (0.77 (0.66 to 0.89) without exposure to cardiotoxic treatment and 0.87 (0.69 to 1.10) after adjustment for cardiotoxic treatments).
Table 4.
Hazard ratios of cardiac conditions per five year treatment era
| Five year treatment era (continuous variable) | Hazard ratio (95% CI) | ||||
|---|---|---|---|---|---|
| Heart failure | Coronary artery disease | Valvular heart disease | Pericardial disease | Arrhythmia | |
| Adjusted for demographics and modifiable risk factors | 0.97 (0.88 to 1.06) | 0.80 (0.71 to 0.91) | 0.97 (0.74 to 1.28) | 0.98 (0.68 to 1.40) | 1.17 (0.99 to 1.38) |
| Adjusted for demographics, modifiable risk factors, and cardiac radiation exposure | 0.99 (0.89 to 1.10) | 0.90 (0.78 to 1.05) | 1.18 (0.85 to 1.63) | 1.02 (0.68 to 1.53) | 1.21 (1.03 to 1.43) |
| Adjusted for demographics, modifiable risk factors, and anthracycline exposure | 0.91 (0.82 to 1.02) | 0.79 (0.69 to 0.91) | 0.96 (0.75 to 1.24) | 0.97 (0.67 to 1.37) | 1.15 (0.96 to 1.38) |
| Adjusted for demographics, modifiable risk factors, and cardiotoxic exposures | 0.94 (0.83 to 1.05) | 0.87 (0.74 to 1.03) | 1.13 (0.83 to 1.53) | 0.99 (0.66 to 1.50) | 1.19 (0.99 to 1.43) |
Demographics=age at diagnosis, sex, race, body mass index, smoking, exercise intensity (metabolic hours/week); modifiable risk factors=diabetes, dyslipidemia, and hypertension; cardiotoxic exposures=cardiac radiation and anthracycline.
Discussion
Using the well characterized Childhood Cancer Survivor Study cohort, we assessed long term serious cardiovascular outcomes among survivors of cancer in childhood treated in the 1990s compared with those treated in the 1970s and 1980s, and found evidence suggesting improvements attributable to reductions or changes in exposure to therapeutic radiation. Contemporary therapeutic protocols, while modified to minimize late adverse outcomes, continue to rely heavily on anthracycline based regimens and radiation therapy to maintain successful cure rates. However, analyzing a broad spectrum of cardiovascular outcomes, we found that changes in therapies were associated with a decrease in the incidence of and risks for late cardiotoxicity among long term survivors of childhood cancer. Reductions in the use and doses of radiation have resulted in statistically significant declines in serious coronary artery disease among survivors of Hodgkin lymphoma while declines in other diagnostic groups have been more modest. Notably, for survivors of neuroblastoma, for whom intensified treatments have been associated with more survivors living into adulthood, cardiotoxicity has increased. Traditional cardiovascular risk factors, previously shown to significantly augment treatment induced cardiotoxicity, were also strongly associated with an increased risk for nearly all cardiac outcomes assessed in our young adult cohort; providing a potential therapeutic target to ameliorate outcomes for cancer survivors.
Comparison with other studies
The late morbidity and mortality of therapy related cardiotoxicity, particularly in children with Hodgkin lymphoma, has been well described,6 7 16 17 prompting the introduction of risk adapted or response adapted and immunologic therapies to better refine exposures for these patients. Early recognition of musculoskeletal toxicities associated with high dose radiation,9 followed by increased rates of breast cancer among young women previously irradiated for a childhood cancer18 motivated changes in dose and treatment fields as well as chemotherapy only treatment strategies. Radiation doses were reduced, and are now eliminated, for many low risk patients,19 whereas anthracyclines were added and doses intensified. Reports of radiation induced coronary artery disease, both clinical and subclinical, and particularly among younger patients, further encouraged exposure to radiation to be minimized.20 21 Our study shows that reduced exposure to cardiotoxic treatment modalities have been associated with a reduced risk of myocardial infarction or coronary revascularization among survivors of Hodgkin lymphoma. A reduction in the risk of heart failure was evident overall and among survivors of Hodgkin lymphoma in our cohort but did not meet statistical significance, likely the result of the intensified use of anthracyclines during the same period that radiation exposure was being reduced. Notably, the risk for heart failure was observed to further decrease from the 1980s into the 1990s, suggesting a favorable trend. In contrast, Dutch investigators recently reported that survivors from the 1970s had a lower cumulative incidence of heart failure compared with those treated in the 1980s and 1990s.22 However, the temporal pattern of reduction in exposure to radiotherapy (defined as maximum prescribed heart dose) in Dutch survivors was of a smaller magnitude (with no chest radiotherapy ranging from 65.6% in the 1970s to 82.2% in the 1990s) than in the Childhood Cancer Survivor Study cohort (ranging from 23% to 60%) utilizing detailed heart dosimetry measurements. Additionally, we accounted for exercise intensity, reported to moderate cardiovascular outcomes in survivors of Hodgkin lymphoma,23 as well as traditional cardiovascular risk factors such as smoking, BMI, diabetes, dyslipidemia, and hypertension. Further longitudinal follow-up will be required to determine whether these apparent reductions in risk for heart failure among survivors from the 1990s achieve statistical significance with aging of the population.
While reductions in adverse cardiac outcomes were seen across the decades, the opposite was true for survivors of neuroblastoma, with a statistically significant fivefold increased risk of heart failure for those treated in the 1990s. This corresponds with the intensification of neuroblastoma therapy during this decade with the addition of myeloablative therapy, autologous stem cell rescue, and isotretinoin, which significantly improved outcomes for children with high risk neuroblastoma.24 25 Improved survival rates have increased the number of long term survivors at risk for adverse outcomes. Our study only included survivors with a diagnosis before 1999, thus patients with neuroblastoma treated with immunotherapy (anti-disialoganglioside antibody)26 were not included in this analysis. Current studies focus on modifying the acute toxicities associated with delivery of anti-disialoganglioside antibody, but successful delivery of immunotherapy, for neuroblastoma or other diagnoses, might alter the rate of heart failure for these survivors in the future.
Therapeutic interventions for cardiac toxicity induced by cancer therapy remain elusive and have largely relied on strategies used in other populations, commonly with age related cardiac declines. The pathophysiology in our relatively young population (median age 27.7 (range 8.2-58.3) years), though incompletely understood, is likely different from that of the general population. Importantly, our study supports the evidence of the contribution traditional cardiovascular risk factors have for cancer survivors. Despite adjustment for personal factors, treatment related factors, smoking, BMI, and exercise, dyslipidemia and hypertension remained strongly associated with nearly all cardiac outcomes. When stratification was by exposure to cardiac radiation or anthracycline chemotherapy, similar associations with dyslipidemia and hypertension were previously reported in the Childhood Cancer Survivor Study cohort.27 We found that the risk persists despite adjustment for treatment era. Furthermore, a previous study identified a 2.6-fold increased risk for hypertension among survivors of childhood cancer of all diagnoses, and importantly, a high prevalence (48%) of survivors who were unaware of their hypertension.28 Although studies are underway to ameliorate or prevent therapy induced cardiac toxicity, more aggressive surveillance or treatment of traditional cardiovascular risks, or both, combined with modifications of upfront exposures, might further mitigate the rate of adverse cardiac outcomes.
Strengths and limitations of this study
Although the large population of childhood cancer survivors followed across three decades in the Childhood Cancer Survivor Study cohort permits assessment of trends in a variety of late cardiac outcomes, several limitations need to be considered. Despite the large cohort, 31% of eligible survivors chose not to participate. It is possible that the health status of these individuals differs from those who participated, thus potentially biasing our estimates. Cardiac outcomes are self reported, and validation was not feasible. To minimize misclassification, however, we restricted the analyses to clinically symptomatic cardiac events requiring drug or surgical management (grades 3-5). Our results should be considered in the context of declining all cause mortality in survivors of childhood cancer over the past several decades (1970-99).1 In view of this, we assessed whether the declines in risk over the treatment eras reported here differed from those in rates over the same period, the latter being unaffected by changes in competing risk. Specifically, we compared the era effect estimates from the Fine and Gray models (risk) with those from Cox regression models (rate) with the same adjustment variables. The two sets of estimates were almost identical, suggesting that although the mortality rate declined substantially over time, our findings were not appreciably influenced by it. Additionally, while we show improvement in cardiac outcomes for more recently treated survivors, caution should be exercised in the interpretation of the results, recognizing that this does not account for increased survival from childhood cancer or the enhanced awareness of cardiovascular disease after cancer therapy. Improved survival and health screening of cancer survivors could have identified more cardiac events. Our analysis included death from other causes as a competing risk, and cardiovascular screening is more likely to identify asymptomatic disease at a less severe stage, before becoming grade 3-5 and requiring medical intervention.
Conclusions
We observed that among adult survivors of childhood cancer the risk for coronary artery disease was significantly decreased. This decrease could be associated with historical reductions in exposure to cardiac radiation, particularly among survivors of Hodgkin lymphoma. This is important as childhood cancer survival rates increase and these young adults prematurely acquire cardiovascular disease. While additional longitudinal follow-up is needed to establish whether similar reductions in the cumulative incidence of heart failure can be confirmed in multivariable analysis, these results suggest that efforts to modify cancer therapies in children and promote health surveillance for survivors are beginning to show benefits not only in overall survival but also in late adverse cardiac effects. Continued follow-up is needed to determine if these positive trends persist over time.
What is already known on this topic
Adult survivors of cancer in childhood have substantial morbidity related to past therapy
Cardiovascular conditions such as cardiomyopathy, cardiac arrhythmias, and coronary artery, valvular, and pericardial diseases are prominent contributors to the burden of late health outcomes
Patterns of exposure to cardiotoxic treatment have changed over time as contemporary cancer protocols have focused on advancing cure rates while attempting to minimize long term adverse effects
What this study adds
Efforts to modify cancer therapies in children and promote health surveillance are beginning to show benefits for survivors of childhood cancer
Reductions in cardiotoxic exposures have been associated with declines in serious cardiac outcomes, particularly for the radiation associated risk of coronary artery disease among survivors of Hodgkin lymphoma in childhood
Web extra.
Extra material supplied by authors
Supplemental information: Figure showing number of cardiac conditions reported by participants in Childhood Cancer Survivor Study
Supplemental information: additional tables 1-7
Contributors: DAM, YY, LLR, KCO, MMH, and GTA conceived and designed the study. DAM, GH, KKN, YY, RMH, WML, MMH, and GTA analyzed the data and interpreted the results. DAM, GH, KKN, YY, MMH, and GTA drafted the manuscript and completed critical revisions. DAM, GH, KKN, MJE, YY, DD, RMH, WML, LSC, ET, TMG, LLR, KCO, MMH, and GTA reviewed and revised the manuscript and approved the final manuscript for submission. DAM attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. DAM is the guarantor.
Funding: The Childhood Cancer Survivor Study is supported by the National Cancer Institute grant CA55727 (to GTA, principal investigator), the Cancer Center Support (CORE) grant (CA21765) to St Jude Children’s Research Hospital (to CW Roberts, principal investigator) and the American Lebanese Syrian Associated Charities (ALSAC), Memphis, TN. The funders of the study had no role in the study design; data collection, analysis, or interpretation; or in writing of the report; and in the decision to submit the article for publication. All authors had independence from funders and full access to the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and accuracy of the data analysis.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare support from the National Cancer Institute (CA55727, to GTA, principal investigator) for the Childhood Cancer Survivor Study; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: The Childhood Cancer Survivor Study is approved by the St Jude Children’s Research Hospital Human Research Protection Program as well as the human subjects committees at each participating institution.
Data sharing: Data are available per the Childhood Cancer Survivor Study resource sharing plan. Public access data tables are posted on the Childhood Cancer Survivor Study website (https://ccss.stjude.org/data-and-analysis/public-access-data-tables).
The study guarantor (DAM) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Research results are regularly disseminated through the Childhood Cancer Survivor Study website (www.ccss.stjude.org) and regular participant newsletters.
References
- 1. Armstrong GT, Chen Y, Yasui Y, et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. N Engl J Med 2016;374:833-42. 10.1056/NEJMoa1510795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 2013;309:2371-81. 10.1001/jama.2013.6296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oeffinger KC, Mertens AC, Sklar CA, et al. Childhood Cancer Survivor Study Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006;355:1572-82. 10.1056/NEJMsa060185 [DOI] [PubMed] [Google Scholar]
- 4. Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet 2017;390:2569-82. 10.1016/S0140-6736(17)31610-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gibson TM, Mostoufi-Moab S, Stratton KL, et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970-99: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol 2018;19:1590-601. 10.1016/S1470-2045(18)30537-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 2009;339:b4606. 10.1136/bmj.b4606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mulrooney DA, Armstrong GT, Huang S, et al. Cardiac Outcomes in Adult Survivors of Childhood Cancer Exposed to Cardiotoxic Therapy: A Cross-sectional Study. Ann Intern Med 2016;164:93-101. 10.7326/M15-0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van der Pal HJ, van Dalen EC, van Delden E, et al. High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol 2012;30:1429-37. 10.1200/JCO.2010.33.4730 [DOI] [PubMed] [Google Scholar]
- 9. Hudson MM, Neglia JP, Woods WG, et al. Lessons from the past: opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer 2012;58:334-43. 10.1002/pbc.23385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Green DM, Kun LE, Matthay KK, et al. Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr Blood Cancer 2013;60:1083-94. 10.1002/pbc.24487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol 2002;38:229-39. 10.1002/mpo.1316 [DOI] [PubMed] [Google Scholar]
- 12. Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol 2009;27:2308-18. 10.1200/JCO.2009.22.3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feijen EA, Leisenring WM, Stratton KL, et al. Equivalence Ratio for Daunorubicin to Doxorubicin in Relation to Late Heart Failure in Survivors of Childhood Cancer. J Clin Oncol 2015;33:3774-80. 10.1200/JCO.2015.61.5187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res 2006;166:141-57. 10.1667/RR3525.1 [DOI] [PubMed] [Google Scholar]
- 15. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496-509 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 16. Reulen RC, Winter DL, Frobisher C, et al. British Childhood Cancer Survivor Study Steering Group Long-term cause-specific mortality among survivors of childhood cancer. JAMA 2010;304:172-9. 10.1001/jama.2010.923 [DOI] [PubMed] [Google Scholar]
- 17. Henson KE, Reulen RC, Winter DL, et al. Cardiac Mortality Among 200 000 Five-Year Survivors of Cancer Diagnosed at 15 to 39 Years of Age: The Teenage and Young Adult Cancer Survivor Study. Circulation 2016;134:1519-31. 10.1161/CIRCULATIONAHA.116.022514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhatia S, Robison LL, Oberlin O, et al. Breast cancer and other second neoplasms after childhood Hodgkin’s disease. N Engl J Med 1996;334:745-51. 10.1056/NEJM199603213341201 [DOI] [PubMed] [Google Scholar]
- 19. Metzger ML, Weinstein HJ, Hudson MM, et al. Association between radiotherapy vs no radiotherapy based on early response to VAMP chemotherapy and survival among children with favorable-risk Hodgkin lymphoma. JAMA 2012;307:2609-16. 10.1001/jama.2012.5847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hancock SL, Donaldson SS, Hoppe RT. Cardiac disease following treatment of Hodgkin’s disease in children and adolescents. J Clin Oncol 1993;11:1208-15. 10.1200/JCO.1993.11.7.1208 [DOI] [PubMed] [Google Scholar]
- 21. Mulrooney DA, Nunnery SE, Armstrong GT, et al. Coronary artery disease detected by coronary computed tomography angiography in adult survivors of childhood Hodgkin lymphoma. Cancer 2014;120:3536-44. 10.1002/cncr.28925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Feijen EAML, Font-Gonzalez A, Van der Pal HJH, et al. DCOG‐LATER Study Group Risk and Temporal Changes of Heart Failure Among 5-Year Childhood Cancer Survivors: a DCOG-LATER Study. J Am Heart Assoc 2019;8:e009122. 10.1161/JAHA.118.009122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones LW, Liu Q, Armstrong GT, et al. Exercise and risk of major cardiovascular events in adult survivors of childhood hodgkin lymphoma: a report from the childhood cancer survivor study. J Clin Oncol 2014;32:3643-50. 10.1200/JCO.2014.56.7511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matthay KK, Villablanca JG, Seeger RC, et al. Children’s Cancer Group Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N Engl J Med 1999;341:1165-73. 10.1056/NEJM199910143411601 [DOI] [PubMed] [Google Scholar]
- 25. Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. J Clin Oncol 2009;27:1007-13. 10.1200/JCO.2007.13.8925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu AL, Gilman AL, Ozkaynak MF, et al. Children’s Oncology Group Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010;363:1324-34. 10.1056/NEJMoa0911123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol 2013;31:3673-80. 10.1200/JCO.2013.49.3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gibson TM, Li Z, Green DM, et al. Blood Pressure Status in Adult Survivors of Childhood Cancer: A Report from the St. Jude Lifetime Cohort Study. Cancer Epidemiol Biomarkers Prev 2017;26:1705-13. 10.1158/1055-9965.EPI-17-0510 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental information: Figure showing number of cardiac conditions reported by participants in Childhood Cancer Survivor Study
Supplemental information: additional tables 1-7