Abstract
Context
Germline loss-of-function CDKN1B gene variants cause the autosomal dominant syndrome of multiple endocrine neoplasia type 4 (MEN4). Even though pituitary neuroendocrine tumors are a well-known component of the syndrome, only 2 cases of Cushing’s disease (CD) have so far been described in this setting.
Aim
To screen a large cohort of CD patients for CDKN1B gene defects and to determine their functional effects.
Patients
We screened 211 CD patients (94.3% pediatric) by germline whole-exome sequencing (WES) only (n = 157), germline and tumor WES (n = 27), Sanger sequencing (n = 6), and/or germline copy number variant (CNV) analysis (n = 194). Sixty cases were previously unpublished. Variant segregation was investigated in the patients’ families, and putative pathogenic variants were functionally characterized.
Results
Five variants of interest were found in 1 patient each: 1 truncating (p.Q107Rfs*12) and 4 nontruncating variants, including 3 missense changes affecting the CDKN1B protein scatter domain (p.I119T, p.E126Q, and p.D136G) and one 5’ untranslated region (UTR) deletion (c.-29_-26delAGAG). No CNVs were found. All cases presented early (10.5 ± 1.3 years) and apparently sporadically. Aside from colon adenocarcinoma in 1 carrier, no additional neoplasms were detected in the probands or their families. In vitro assays demonstrated protein instability and disruption of the scatter domain of CDKN1B for all variants tested.
Conclusions
Five patients with CD and germline CDKN1B variants of uncertain significance (n = 2) or pathogenic/likely pathogenic (n = 3) were identified, accounting for 2.6% of the patients screened. Our finding that germline CDKN1B loss-of-function may present as apparently sporadic, isolated pediatric CD has important implications for clinical screening and genetic counselling.
Keywords: ACTH, CDKN1B, corticotropinoma, Cushing’s disease, MEN4, pituitary neuroendocrine tumor
The syndrome of multiple endocrine neoplasia type 4 (MEN4, OMIM: 610755) was described in 2006 as a condition caused by germline loss-of-function (LOF) variants in the cyclin-dependent kinase inhibitor 1B gene (CDKN1B, 12p13.1), with clinical manifestations similar to those of multiple endocrine neoplasia type 1 (MEN1) (1). As in MEN1, primary hyperparathyroidism (PHPT) is the most common component of the syndrome, but MEN4 patients display a much more heterogeneous phenotype, including pituitary adenomas (more recently termed “pituitary neuroendocrine tumors” and referred to as PitNETs hereafter), neuroendocrine tumors (NETs), and various benign and malignant neoplasms (1-9). MEN4 is an autosomal dominant disorder with incomplete penetrance, accounting for about 2% of the MEN1 mutation-negative MEN cases (1-3). The relative rarity of this condition and the variability in presentation among the few cases reported in the literature have precluded the establishment of disease-specific guidelines for the treatment, follow-up, and genetic counseling of such patients.
Controlling the function of multiple cyclin-dependent kinases (CDKs)/cyclin complexes, the cyclin-dependent kinase inhibitor CDKN1B (also known as p27Kip1), a member of the Kip/Cip family of CDK inhibitors, acts as a key negative regulator of cell cycle progression from G1 to S phase; its function is often impaired in human neoplasms (10,11). CDKN1B is highly expressed in all the hormone-producing cells of the anterior pituitary, but is significantly reduced in PitNETs, mainly due to post-translational regulatory mechanisms. Among normal pituitary cells, corticotrophs display the lowest CDKN1B levels, and very weak nuclear CDKN1B staining is frequently found in corticotropinomas, inversely correlating with Ki-67 expression (12-14). Interestingly, Cdkn1b knockout mice develop adrenocorticotropin (ACTH)-secreting hyperplasia or adenomas of the pituitary pars intermedia (proopiomelanocortin [POMC] positive) with full penetrance, but no other types of PitNETs (15).
In contrast, germline CDKN1B pathogenic variants have rarely been associated with corticotropinomas in humans (2,16). Most of the cohorts previously screened for CDKN1B variants, however, included a small number of Cushing’s disease (CD) patients (2, 17-19). We aimed to determine the frequency of germline CDKN1B mutations in a large cohort of CD patients and to functionally validate our findings.
Material and Methods
Patients and samples
We studied 211 CD patients who were evaluated at the outpatient clinic and/or admitted for clinical work-up and treatment (n = 208) or whose samples were referred for study (n = 3) at the National Institutes of Health Clinical Research Center between 1997 and 2018. All patients and their parents/guardians provided informed assent or consent and were recruited under the protocol 97-CH-0076 (ClinicalTrials.gov: NCT00001595), approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board. The probands’ parents and siblings were also recruited, when available. Clinical data were obtained from the participants and/or their clinical records. Germline and tumor deoxyribonucleic acid (DNA) samples were extracted as previously described (20). Ribonucleic acid (RNA) was extracted from buffy coats in selected cases, using the RNeasy Plus Mini Kit (QIAGEN 74136).
Some of the patients in our cohort have been screened for other germline defects: AIP, CDKN2C, MEN1, and PRKAR1A (n = 59), CABLES1 (n = 140), somatic and/or germline GPR101 variants (n = 33), and somatic USP8 variants (n = 41) (18,20-22). A case series including 6 MEN1 patients also included here, individual reports of patients with PRKAR1A and USP8 germline defects, and somatic copy number variation (CNV) analyses in 27 patients included in this study have also been published (23-26). Sixty cases were previously unpublished.
Whole-exome sequencing
Whole-exome sequencing (WES) of germline DNA samples from 91 patients and tumor DNA samples from 27 of them was performed at the University of Minnesota Genomics Center (UMGC); WES for 91 additional germline samples was done at Novogene (Beijing, China). In all cases, targeted capture libraries were generated using the Agilent SureSelect Human All Exon v5+UTR (UMGC) or Agilent SureSelect Human All Exon v6 (Novogene) kits. Germline samples were sequenced on an Illumina HiSeq 2000 platform producing 100 bp paired-end reads (UMGC), or on an Illumina HiSeq 2500 platform producing 150 bp paired-end reads (Novogene), while tumor samples were sequenced on a HiSeq 2500 platform producing 125 bp paired-end reads. WES results for all samples were analyzed together. FASTQ files were processed using a Genome Analysis Toolkit v3.7 based pipeline, including BWA-MEM v0.7.17 for alternate contig aware alignment to the hg38 reference genome (GRCh38_full_analysis_set_plus_decoy_hla.fa), Picard Tools v2.6.0 to mark duplicates (http://broadinstitute.github.io/picard/), and Genome Analysis Toolkit for indel realignment, base quality recalibration, genotyping (HaplotypeCaller), variant quality score recalibration, and to split multiallelic sites. The median number of on-target reads generated per sample was 45 million, resulting in median target coverage of 60× (89% of targets covered at >20×).
For CDKN1B, the median percent of bases covered at 20× was 100% for both coding exons (mean for exon 1: 98.9%; mean for exon 2: 99.5%). The third exon had low coverage (median percent of bases covered at 20×: 16.7%, mean: 18%). ANNOVAR was used to determine the effect of coding variants using both the RefSeq and UCSC gene sets, and nonsynonymous variants were annotated using information from dbNSFP (27,28). All variants were annotated for their frequency in public databases and internal WES datasets totaling >10 000 samples. Bioinformatic analyses ruled out rare variants (present in <1% of any variant collection) in other known PitNET-associated genes (eg, AIP, CABLES1, GNAS, GPR101, MEN1, PRKAR1A, USP8, and SDHx). For all samples, a manual check of the WES raw data for CDKN1B was performed using the Integrative Genomics Viewer 2.3.72 platform (Broad Institute) (29). We also included 2 patients for whom clinical WES carried out at GeneDx (Gaithersburg, MD, USA) was reported negative for disease-associated variants; results for CDKN1B were manually checked.
Sanger sequencing
For 6 germline DNA samples, the coding regions and exon–intron junctions of CDKN1B were amplified by end-point polymerase chain reaction (PCR) (GoTaq Green Master Mix, Promega M7123) and subjected to direct bidirectional sequencing (BigDye Terminator 3.1 Cycle Sequencing Kit, Applied Biosystems 4337456) using a 3500xL Genetic Analyzer (Applied Biosystems). Sequences were analyzed using the SeqMan Pro 15.0.1 (DNASTAR) software. Sanger sequencing was likewise used to sequence complementary DNA (cDNA) samples in 5 individuals, confirm WES findings, determine segregation of CDKN1B variants, and investigate loss of heterozygosity (LOH) in corticotropinomas; the USP8 mutational hotspot was also sequenced in the latter. Primers for PCR and Sanger sequencing are listed in Table 1.
Table 1.
Primers used for Sanger sequencing
| Primer name | Gene | Region to amplify | Sequence (5′-3′) | PCR product length |
|---|---|---|---|---|
| CDKN1B_EX1-F1 | CDKN1B | Exon 1 | CAGGTTTGTTGGCAGCAGTA | 406 bp |
| CDKN1B_EX1-R1 | CGGAGCCAAAAGACACAGAC | |||
| CDKN1B_EX1-F2 | CCATTTGATCAGCGGAGACT | 438 bp | ||
| CDKN1B_EX1-R2 | GCCCTCTAGGGGTTTGTGAT | |||
| CDKN1B_EX1-F3 | GAGTTAACCCGGGACTTGGAG | 446 bp | ||
| CDKN1B_EX1-R3 | ATACGCCGAAAAGCAAGCTA | |||
| CDKN1B_EX2-F | Exon 2 | TGACTATGGGGCCAACTTCT | 296 bp | |
| CDKN1B_EX2-R | TTTGCCAGCAACCAGTAAGA | |||
| CDKN1B_EX3-F1 | Exon 3 | CCCCATCAAGTATTTCCAAGC | 410 bp | |
| CDKN1B_EX3-R1 | CCTCCCTTCCCCAAAGTTTA | |||
| CDKN1B_EX3-F2 | TGCCTCTAAAAGCGTTGGAT | 542 bp | ||
| CDKN1B_EX3-R2 | TTTTTGCCCCAAACTACCTG | |||
| CDKN1B_EX3-F3 | GCCCTCCCCAGTCTCTCTTA | 414 bp | ||
| CDKN1B_EX3-R3 | GGTTTTTCCATACACAGGCAAT | |||
| CDKN1B_EX3-F4 | GGAAGGTTCATGTAGAGAAAAGC | 474 bp | ||
| CDKN1B_EX3-R4 | CCTAGGTATTTGCCACTTCACA | |||
| USP8_hs-F | USP8 | Exon 14 hotspot | CTTCCACCCCTCCAACTCAT | 146 bp |
| USP8_hs-R | TGGAGTTACTGTTGGCTTCCT |
In silico analyses
The Alamut Visual 2.9 software (Interactive Biosoftware) and the VarSome platform (30) were used for the annotation and in silico analysis of variants, considering NCBI GenBank NM_004064.4 and UniProt P46527-1 as the reference sequences. Pathogenic associations were searched in the Human Gene Mutation Database (http://www.hgmd.cf.ac.uk/ac/index.php) and ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/), and previous reports in the literature were searched using Genomenon Mastermind (https://www.genomenon.com/mastermind/) and PubMed (https://www.ncbi.nlm.nih.gov/pubmed/). Frequency of the variants in the general population was searched in gnomAD v2.1.1 (31). The Group-Based Prediction System v5.0 platform (http://gps.biocuckoo.cn/index.php) was used to predict protein phosphorylation sites.
Copy number variation analysis and quantitative PCR
Copy number variants (CNVs) were investigated in 194 germline DNA samples by means of droplet digital PCR (ddPCR), using 6-carboxyfluorescein (FAM)-labeled assays binding CDKN1B exon 1, exon 2-intron 2, and exon 3 (TaqMan CNV assays Hs02136152, Hs01660110, and Hs03018768, Applied Biosystems), a 2’-chloro-7’-phenyl-1,4-dichloro-6-carboxyfluorescein (VIC)-labeled RPP30 assay (Applied Biosystems 4403326) as an internal control, HindIII restriction enzyme (New England Biolabs, NEB, R3104), and ddPCR SuperMix for Probes (Bio-Rad 1863024) in a QX200 Droplet Digital PCR System (Bio-Rad). Results were analyzed with the Quanta Soft software 1.7.4.0917 (Bio-Rad). Absolute quantification of CDKN1B expression in cDNA from buffy coats by ddPCR was done using a FAM-labelled CDKN1B assay and a reference VIC-labelled ACTB assay (Hs00153277_m1 and 4326315E, Applied Biosystems).
Immunohistochemistry
Immunostaining for CDKN1B was performed using 1:1000 mouse monoclonal primary antibody (BD Biosciences 610242) and 1:1000 biotinylated secondary antibody/1:500 peroxidase streptavidin (Jackson ImmunoResearch Laboratories 111-065-062 and 016-030-084, respectively); the full protocol has been detailed elsewhere (20). Hematoxylin–eosin and additional immunohistochemistry slides were retrieved from the National Institutes of Health Clinical Research Center pathology archives.
Expression plasmids
The coding DNA sequences (CDSs) of CDKN1B and RHOA (NM_001664.4) were amplified from HEK293 cells (ATCC CRL-1573) cDNA, using Q5 High-Fidelity DNA Polymerase (NEB M0491L), adding a 5′ NcoI restriction site. The blunt-ended amplicons were purified (NucleoSpin Gel and PCR clean-up kit, Macherey-Nagel 740609.50) and NcoI digested (NEB R3193S). The plasmid psF-CMV-NH2-HA-EKT1 (N-terminal HA tag, Oxford Genetics OG324) was digested with NcoI and EcoRV (NEB R3195S) enzymes, dephosphorylated (shrimp alkaline phosphatase, NEB M0371S), purified, and ligated to the CDKN1B CDS (T4 DNA ligase, NEB M0202S). The resulting plasmid was produced into Stellar competent cells (Clontech 636766) and purified (QIAprep Spin Miniprep QIAGEN 27104 and GenElute HP Plasmid Maxiprep Sigma-Aldrich NA0310-1KT kits). Additional plasmids to express CDKN1B variants were created via site-directed mutagenesis (SDM), using the QuikChange II XL kit (Agilent 200521). In addition, the human influenza hemagglutinin (HA) tag in psF-CMV-NH2-HA-EKT1 was deleted and a Flag tag was created using SDM. The plasmid was NcoI/EcoRV digested and dephosphorylated, and finally ligated to the NcoI-digested RHOA insert. All primers used for cloning are described in Table 2.
Table 2.
Primers used for cloning and site-directed mutagenesis
| Primer name | Sequence (5′ to 3′) | Use |
|---|---|---|
| CDKN1B_CDS_NcoI-F | ACCCATGGGAAAGATGTCAAACGTGC | To amplify the CDKN1B CDS, adding a 5′-NcoI restriction enzyme site |
| CDKN1B_CDS-R | TTCATCAAGCAGTGATGTATCTGA | |
| CDKN1B_c.320_320delA-F | CGCAGGAGAGCCGGATGTCAGCGG | To create the c.320delA variant by site-directed mutagenesis |
| CDKN1B_c.320_320delA-R | CCGCTGACATCCGGCTCTCCTGCG | |
| CDKN1B_c.356T>C-F | CCCGGCGGCGCCTTTAACTGGGGCTCC | To create the c.356T>C variant by site-directed mutagenesis. |
| CDKN1B_c.356T>C-R | GGAGCCCCAGTTAAAGGCGCCGCCGGG | |
| CDKN1B_c.376G>C-F | GCGTGTCCTGAGAGTTAGCCGGAGCCC | To create the c.376G>C variant by site-directed mutagenesis |
| CDKN1B_c.376G>C-R | GGGCTCCGGCTAACTCTCAGGACACGC | |
| CDKN1B_c.407A>G-F | GGACCCAAAGACTGGTCCGTCGGACAGCC | To create the c.407A>G variant by site-directed mutagenesis |
| CDKN1B_c.407A>G-R | GGCTGTCCGACGGACCAGTCTTTGGGTCC | |
| RHOA_CDS_NcoI-F | GATTCGTTGCCTGAGCCATGG | To amplify the RHOA CDS, adding a 5′-NcoI restriction enzyme site |
| RHOA_CDS-R | TTAACCGCATAAGGGCTGTG | |
| HA_to_Flag_F | GGAGGTACTCACGATGGATTATAAGGATGACG | To create the Flag by site-directed mutagenesis |
| HA_to_Flag_R | CGTCATCCTTATAATCCATCGTGAGTACCTCC | |
| pSF_delHA-F | GGAGGTACTCACGATGGATTATGCGGATGACG | To delete the HA tag by site-directed mutagenesis |
| pSF_delHA-R | CGTCATCCGCATAATCCATCGTGAGTACCTCC | |
| CMV-F | CGCAAATGGGCGGTAGGCGTG | To verify inserts in plasmids by Sanger sequencing |
| R3 | AGCTGAAGGTACGCTGTATC |
Cell culture and transfection
For POMC and ACTH quantification experiments, 2.5 × 105/well mouse corticotropinoma-derived AtT20/D16vF2 cells (ATCC CRL-1795) were grown for 24 hours in 12-well plates with Dulbecco’s modified Eagle’s medium (ATCC 30-2002) supplemented with 10% fetal bovine serum (Gemini Bio-products 900-208). Cells were then transfected with 1 μg/well plasmid DNA and 2 μL/well TurboFect (Thermo Scientific R0531). After 24 hours, supernatants were collected, cells were lysed in 100 μL/well NP40 lysis buffer (Invitrogen FNN0021) supplemented with protease inhibitors (Roche 11697498001), and proteins were extracted. For cycloheximide (CHX) chase experiments, cells were treated with 25 μM CHX (Sigma-Aldrich C4859-1ML) 24 hours after transfection. Overexpression and CHX chase experiments were done in triplicate and repeated at least 3 times for each condition. For subcellular localization, 5 × 105 cells/well were plated in 6-well plates with coverslips and transfected 24 hours later with 2 μg/well plasmid DNA and 4 μL/well TurboFect. For coimmunoprecipitation, 5 × 106 HEK293 cells (ATCC CRL-1573) were grown for 24 hours in T75 flasks with minimum essential medium (Gibco 11095080) and 10% fetal bovine serum, and then transfected with 10 μg/flask pSF-CMV-NH2-HA-EKT1-Flag-RHOA, 10 μg/flask psF-CMV-NH2-HA-EKT1-CDKN1B (containing the wild-type CDS or each of the variants of interest), and 40 μL/flask TurboFect. Twenty-four hours after transfection cells were lysed in 1 mL of lysis buffer and proteins were extracted.
Immunocytofluorescence
Twenty-four hours after transfection, cells were fixed with 4% formaldehyde, permeabilized with 0.1% triton X-100 in PBS and then blocked with 10% normal goat serum (Vector Laboratories S-1000) in PBS. Cells were then incubated overnight with 1:1000 mouse anti-HA (Sigma-Aldrich H3663) in permeabilization buffer with 10% normal goat serum, and then for 1 hour with 1:500 goat anti-mouse 594-labelled antibody (Abcam ab150120). Cells were counterstained with DAPI (Life Technologies D1306) and mounted on slides (VECTASHIELD HardSet antifade mounting medium, Vector Laboratories S-1000). Immunohistochemistry and immunocytofluorescence images were acquired using a Keyence BZ-X710 microscope and the BZ-X Viewer v01.03.01.01 software (Keyence).
Western blot
For all Western blot (WB) experiments, total proteins were electrophoresed in 10 or 15-well 1 mm NuPAGE 4% to 12% Bis-Tris protein gels (Invitrogen NP0321 and NP0323) and transferred onto nitrocellulose membranes (Thermo Scientific 88018) using a semidry blotter. Membranes from CHX chase experiments were subjected to total protein staining using REVERT (LI-COR 926-11016). All membranes were blocked in Odyssey blocking buffer (LI-COR 927-50000) and incubated overnight with either of the following primary antibodies: 1:5000 rabbit anti-POMC (Abcam ab210605), 1:2000 mouse anti-ACTB (Abcam ab8226), 1:3000 mouse anti-HA (Sigma-Aldrich H3663), 1:3000 rabbit anti-Flag (Sigma-Aldrich F7425), 1:1000 mouse anti-CDK2 (Abcam ab32147), 1:3000 mouse anti-CDKN1B. Near-infrared fluorescent antibodies (LI-COR 926-68170, 926-68171 and 827-08365) were used for detection in an Odyssey Infrared Imaging System (LI-COR). Quantification by means of band densitometry was performed using the Image Studio Lite v5.2.5 software (LI-COR).
ACTH quantification
ACTH was measured in supernatants from transfected AtT20 cells using an enzyme-linked immunosorbent assay (KAMIYA KT-6010).
Coimmunoprecipitation
Total proteins from lysates were precleaned by incubation with 50 μL of protein G sepharose (GE Healthcare 17061801); then, 2 mg of total proteins was incubated with 100 μL protein G sepharose and 5 μg anti-HA antibody overnight. Samples were washed 3 times and then eluted in 30 μL 2× Laemmli buffer for 5 minutes at 95°C. The experiment was done twice for confirmation.
Statistical analyses
All analyses were carried out using the Prism v8.2.1 software (GraphPad Software). Data distribution was analyzed using the Shapiro–Wilk test. Parametric data are presented as mean ± standard deviation and nonparametric data are presented as median and interquartile range. Gene variant frequencies in the study population were compared with frequencies reported in gnomAD using the Fisher exact test or the chi-square test, as appropriate. Clinical parameters were compared among groups using the Mann–Whitney test. For overexpression experiments, comparisons were done using 1-way analysis of variance with Dunnett correction for multiple comparisons. Half-life experiments were analyzed using a 1-phase decay equation, and the degradation speed (K) was compared between each mutant protein and the WT protein using the extra sum-of-squares F test. Results were considered statistically significant when P < .05.
Results
Study cohort
We studied 117 female (55.5%) and 94 (44.5%) male CD cases, including 199 pediatric (≤18 years at disease onset) and 12 adult patients, for a total of 211 individuals. The age at disease onset and age at diagnosis were 10 (8-12.9) and 13 (10.5-15.5) years for pediatric patients and 38.8 ± 9.5 and 42 ± 12.6 for adult patients, respectively. Pituitary microadenomas were found in 179 patients, 25 patients had macroadenomas, and 5 patients had more than 1 tumor (size not available in 2 cases). The median tumor size was 5 (4-8) mm for pediatric and 8 (5-10) mm for adult patients. Seventeen patients had positive family histories: 8 with MEN1 (6 with MEN1 variants), 8 with familial isolated pituitary adenoma (FIPA) of unknown genetic origin, and 1 with pheochromocytoma/paraganglioma and PitNET phenotype of unknown genetic cause. Six patients were simplex cases for germline genetic defects (2 cases with CABLES1 variants, and AIP, MEN1, PRKAR1A, and USP8 variants in 1 patient each), and the rest had apparently sporadic presentation.
Genetic screening results
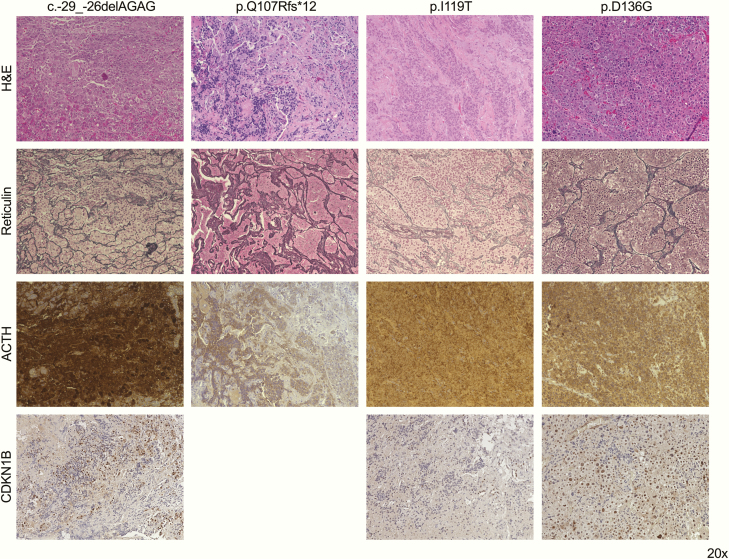
Five heterozygous CDKN1B germline variants of interest were detected by WES in 1 pediatric patient each (2.6% of the 190 patients tested by WES or Sanger sequencing, Table 3), including 1 5′ untranslated region (UTR) variant, as well as 1 frameshift variant and 3 missense variants in exon 1. The 5′ UTR intronic variant c.-29_-26delAGAG has been reported before and its pathogenic potential is well supported by functional analyses and its association with endocrine neoplasms in 3 patients (6,9,32). The frameshift variant c.320delA, p.Q107Rfs*12 has been reported in only 1 individual in gnomAD and has not been associated with MEN4 before. The missense variant c.356T>C, p.I119T, infrequent in the general population (minor allele frequency, MAF: 0.0545%), has been reported in 1 FIPA patient (a second affected family member was not genetically tested) and in patients with nonendocrine neoplasms and other conditions (19,33-37). Functional studies, although not conclusive, point toward a pathogenic potential (19,38). The variant c.376G>C, p.E126Q has not been reported before in MEN4 patients or in gnomAD. Finally, the variant c.407A>G, p.D136G is very infrequent in the general population (MAF: 0.0075%) and has neither been reported in the setting of MEN4 nor functionally characterized. None of the variants found here have been detected in CD before, although c.-29_-26delAGAG and p.I119T have been reported in 1 patient with somatotropinoma each (9,19).
Table 3.
Germline CDKN1B variants of interest in Cushing’s disease patients: evaluation of pathogenicity likelihood
| HGVSa nomenclature: DNA, protein | Location in gene, variant type | dbSNP ID | MAF in gnomAD (%) | Summary of in silico predictions | VarSomeb ACMG classification | Functional data | Clinical associations |
|---|---|---|---|---|---|---|---|
| c.-29_-26delAGAG, p.? | 5′ UTR, intronic. | rs774454456 | Exomes: 0.0376. Genomes: 0.0319. Exomes + genomes: 0.0369. | Mutation taster: disease causing; known disease mutation, protein features might be affected, splice site changes. Alamut: 5′ UTR substitution, the consequence of this change is not predictable, splice changes are unlikely. | Likely benign. | Previously reported c.- 32_-29delGAGA (6) is an equivalent deletion. Predicted altered 5′ UTR secondary structure; reduced mRNA levels and 5′ UTR transcriptional activity in vitro (6,9,32). Disrupts a GRE (9). Cytoplasmic localization by IHC in somatotropinoma (9) and negative staining in parathyroid adenoma (32). LOH in somatotropinoma (9). | Germline variant in one patient with gigantism (9), in one adult patient with gastric carcinoid tumor and PHPT (6), and in one patient with PHPT with a co-existent somatic homozygous frameshift MEN1 variant (32). HGMD (CD119870): disease causing. ClinVar (RCV000354456.1, RCV000210358.1, and RCV000162206.4): conflicting interpretation (likely benign, pathogenic, VUS). |
| c.320delA, p.Q107Rfs*12 | Exon 1, frameshift. | rs755301027 | Exomes: 0.0004. Genomes: n/a. | Mutation taster: disease causing; NMD, aa sequence changed, frameshift, protein features might be affected. Alamut: creates a frame shift starting at Q107; the new reading frame ends in a Stop codon 12 positions downstream. | Likely pathogenic. | n/a | n/a |
| c.356T>C, p.I119T | Exon 1, missense. | rs142833529 | Exomes: 0.0533. Genomes: 0.0637. Exomes + genomes: 0.0545. | Mutation taster: polymorphism; aa sequence changed, known disease mutation. SIFT: tolerated. PolyPhen 2: benign. Provean: neutral. Alamut: weakly conserved nucleotide, moderately conserved aa, moderate physicochemical difference between I and T (Grantham score: 89).c | Likely benign. | Nuclear localization, abnormal migration pattern (19,38), and increased protein stability (19). Loss of interaction with CDK6, but unaltered interaction with CDK1, CDK2, and CDK5 by pulldown assay (38). | Germline variant in the following cases: – One FIPA patient with acromegaly (19). – A 63-year-old woman with unclassified myeloproliferative syndrome (34). – One case of multiple myeloma (37). – One case of premature ovarian failure (36). – A family including 4 siblings with prostate cancer, a sister with lung cancer, and 4 unaffected carriers (33). – One patient from a cohort of secondary and tertiary hyperparathyroidism (35). – Present in a human renal cancer cell line (38). HGMD (CM054649): disease causing. ClinVar (RCV000563714.1, RCV000457158.3, and RCV000344750.1): conflicting interpretation (VUS, likely benign). |
| c.376G>C, p.E126Q | Exon 1, missense. | n/a. | Not found. | Mutation taster: polymorphism; aa sequence changed, protein features might be affected, splice site changes. SIFT: damaging. PolyPhen 2: probably damaging. Provean: neutral. Alamut: moderately conserved nucleotide, highly conserved aa, small physicochemical difference between E and Q (Grantham score: 29).c | VUS | n/a | None reported. Two variants have been described at this position: p.E126D, in a patient with PHPT (48), and p.E126K in a mouth squamous cell carcinoma sample in COSMIC (COSM5970591); somatic status not confirmed (54). |
| c.407A>G, p.D136G | Exon 1, missense. | rs546234840 | Exomes: 0.0069. Genomes: 0.0127. Exomes + genomes: 0.0075. | Mutation taster: disease causing; aa sequence changed, protein features might be affected, splice site changes. SIFT: damaging. PolyPhen 2: benign. Provean: neutral. Alamut: weakly conserved nucleotide, highly conserved aa, moderate physicochemical difference between D and G (Grantham score: 94).c | Likely benign. | n/a | COSMIC (COSM2227662): found in one head/ neck squamous cell carcinoma; somatic status not confirmed (55). ClinVar (RCV000475865.5 and RCV000346316.1): conflicting interpretation (VUS, likely benign). |
aa, amino acid; ACMG, American College of Medical Genetics and Genomics; FIPA, familial isolated pituitary adenoma; GRE, glucocorticoid-response element; MAF, minor allele frequency; MEN, multiple endocrine neoplasia; n/a, not available; NMD, nonsense-mediated mRNA decay; PHPT, primary hyperparathyroidism; UTR, untranslated region; VUS, variant of uncertain significance.
aAll variants were annotated according to the reference sequences NCBI GenBank NM_004064.4 and UniProt P46527-1.
bVarSome employs the Saphetor molecular database, which includes multiple databases for in silico prediction, frequency, disease associations and input from users.
cThe Grantham score calculates the evolutionary distance between two amino acids and ranges from 0 to 215. A larger score represents larger differences between two amino acids in terms of composition, polarity and molecular volume.
Several other germline CDKN1B variants were identified, which we considered as likely benign or benign based on their frequency in the general population and in silico predictions (Table 4). Two common CDKN1B variants (c.-79C>T and c.326T>G, p.V109G) have been associated with cancer risk in multiple studies, but they were not overrepresented in our cohort. Interestingly, 1 patient was heterozygous for c.-79C>T at the germline level and homozygous at the somatic level. Aside from this finding, no other somatic variants were found in the 27 corticotropinoma samples that underwent WES, and no CDKN1B germline CNVs were identified in the samples analyzed.
Table 4.
Additional germline CDKN1B variants and somatic whole-exome sequencing findings
| MAF in gnomAD (%), P value (compared with our cohort) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| HGVS nomenclature: DNA, proteina | Location in gene, variant type | dbSNP ID | MAF in our cohort (%) | Findings in tumor samples (WES) | gnomAD exomes | gnomAD genomes | gnomAD exomes+ genomes | VarSome ACMG classification | Functional data and clinical associations |
| c.-386C>T, p.? | 5′ UTR, intronic. | rs886049078 | 0.2604 | n/a | n/a | 0.0287, P = .1145 | Likely benign. | ClinVar RCV000293046.1: MEN/VUS. | |
| c.-202C>T, p.? | 5′ UTR, intronic. | rs183710253 | 0.7813 | Heterozygous in 1/27 (same as germline). | n/a | 0.7172, P = .7575 | Likely benign. | ClinVar RCV000359300.1: MEN/likely benign. Polymorphism in (56). | |
| c.-126C>T, p.? | 5′ UTR, intronic. | rs549943001 | 0.2551 | n/a | n/a | 0.1179, P = .3761 | Likely benign. | ClinVar RCV000264729.1: MEN/likely benign. | |
| c.-79C>T, p.? | 5′ UTR, intronic. | rs34330 | 28.8265b | Heterozygous in 10/27 and homozygous in 16/27. LOH in one case. | n/a | 30.5275, P = .4673 | Benign. | Reduced transcriptional activity (57, 58). Association with increased risk of hereditary prostate cancer (33), breast cancer (59-61), lung cancer (62), follicular variant of papillary thyroid carcinoma (57), endometrial cancer (63), hepatocellular cancer (64), and neuroblastoma (58). Increased cancer susceptibility, especially in Asians, in a meta-analysis (65). HGMD (CR040861): disease-associated polymorphism with additional supporting functional evidence. | |
| c.24C>T, p.N8= | Exon 1, synonymous. | rs371308246 | 0.2551 | n/a | 0.0040, P = .0171 | 0.0033, P = .0245 | 0.0039, P = 0.0166 | Likely benign. | ClinVar RCV000542803.2: MEN4/ likely benign. |
| c.326T>G, p.V109G | Exon 1, missense. | rs2066827 | 23.2143 | Heterozygous in 10/27 and homozygous in 3/27 (same as germline). | 25.8753, P = .2293 | 36.0140, P < .0001 | 27.0108, P = .0907 | Benign. | Increased colony formation and cell growth rate when overexpressing this variant in AtT20 cells (66). Association with increased risk of squamous cell carcinoma of the head and neck (67), breast cancer progression (68, 69), endometriosis (70), ovarian cancer (71), corticotropinomas (66), papillary thyroid carcinoma (72), prostate cancer (73), and type 2 diabetes mellitus (74). No correlation with overall cancer risk in the general population in a meta-analysis (75). For medullary thyroid carcinoma, both increased risk (76) and association with favorable disease progression (77) have been reported. Reduced risk of prostate cancer (78), ovarian cancer (79), and melanoma (80). Increased risk of aggressive tumors in MEN1 patients (81). HGMD (CM033961): disease-associated polymorphism. ClinVar RCV000244836.2 and RCV000755233.1: not specified/benign, RCV000295040.1: MEN/benign, RCV000568315.1: hereditary cancer-predisposing syndrome/benign. |
| c.475 + 10C>T, p.? | Intron 1, intronic. | rs36101844 | 0.2551 | Heterozygous in 1/27 (same as germline). | 0.0149, P = .0581 | 0.0987, P = .3278 | 0.0247, P = .0937 | Likely benign. | ClinVar: RCV000476950.3: MEN4/benign, RCV000301956.1: MEN/likely benign. |
| c.475 + 49C>T, p.? | Intron 1, intronic. | rs201914302 | 0.2551 | n/a | 0.0099, P = .0397 | 0.0318, P = .1276 | 0.0128, P = .0505 | Likely benign. | COSMIC (COSN10100669): somatic change in one kidney cancer case. |
| c.475 + 105G>C, p.? | Intron 1, intronic. | rs994095606 | 0.2717c | n/a | n/a. | 0.0064, P = .0344 | VUS | n/a | |
| c.476-153T>G, p.? | Intron 1, intronic. | rs142894418 | 0.2717c | n/a | n/a. | 0.1179, P = .358 | Likely benign. | n/a | |
| c.476-77T>C, p.? | Intron 1, intronic. | rs3093731 | 0.8152c | n/a | n/a | 0.2803, P = .0892 | Likely benign. | n/a | |
| c.*9-64A>G, p.? | 3′ UTR, intronic. | rs76967889 | 0.2604 | n/a | n/a | 0.0892, P = .2973 | Likely benign. | n/a | |
| c.*9-38C>T, p.? | 3′ UTR, intronic. | rs4251695 | 0.2604 | n/a | n/a | 0.2706, P>09999 | Likely benign. | n/a | |
| c.*181T>C, p.? | 3′ UTR, intronic. | rs4251696 | 0.2604 | n/a | 0.2769, P>.9999 | 1.0542, P = .1978 | 0.3731, P>.9999 | Likely benign. | ClinVar RCV000333902.1: MEN/benign. |
| c.*452C>T, p.? | 3′ UTR, intronic. | rs762469235 | 0.2577 | n/a | n/a | 0.0127, P = .0596 | Likely benign. | ClinVar RCV000290740.1: MEN/VUS. | |
| c.*956C>A, p.? | 3′ UTR, intronic. | rs7330 | 45.0000d | n/a | n/a | 57.4794, P = .2676 | Benign. | ClinVar RCV000396437.1: MEN/benign. | |
ACMG, American College of Medical Genetics and Genomics; LOH, loss of heterozygosity; MAF, minor allele frequency; MEN, multiple endocrine neoplasia; UTR, untranslated region; VUS, variant of uncertain significance; WES, whole-exome sequencing.
aAll variants were annotated according to the reference sequences NCBI GenBank NM_004064.4 and UniProt P46527-1.
bT is reported as the wild type nucleotide, but it is the minor allele; therefore, frequencies and associations are presented for the T allele.
c Only covered in whole-exome sequencing samples.
dOnly covered in Sanger-sequenced samples.
Patients with CDKN1B putative pathogenic variants
The 5 patients (3 males and 2 females) with CDKN1B putative pathogenic variants had typical pediatric CD clinical presentation and no personal or family history of MEN-associated tumors (Table 5). Their age at disease onset (10.5 ± 1.3 years), age at diagnosis (14.2 ± 1.6 years), and tumor maximum diameter (4.6 ± 2.1 mm) resembled those of the pediatric subset of our cohort (P = .7588, P = .3875 and P = .2074, respectively). At the tumor level, the 3 patients analyzed retained the normal CDKN1B allele. A coexistent somatic, potentially causal USP8 hotspot mutation was found in Case 3. Maternal inheritance was confirmed in Case 2, but variant origin could not be determined for the rest of the cases. When this patient was re-evaluated (due to the unexpected genetic finding), 20 years after the initial diagnosis, he referred to the recent death of his mother due to metastatic colorectal cancer; loss of the normal CDKN1B allele was observed in primary colon and liver metastasis samples.
Table 5.
Clinical summary of patients with CDKN1B variants of interest
| Case no. | CDKN1B variant (origin) | Gender | Race (country of origin) | Age at disease onset/age at diagnosis (years) | Clinical presentation | Biochemistry | Tumor description | Genetic findings in tumor | Treatment and outcomes | Relevant family history | Age at last follow-up (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | c.-29_-26delAGAG, p.? heterozygous (unknown: father n/a, mother wild type). | M | White (USA). | 10/13.6 | Poor linear growth (height -1.7 SDS at first visit), weight gain with central distribution (weight 0.7 SDS, but BMI in the 95th percentile), fatigue, headaches, red striae, dorsocervical fat pad, acanthosis nigricans, hypertension. | Midnight serum cortisol: 20.5 µg/dL; UFC: 156.3 µg/24 h (2.8 × ULN); CRHST: 156.1% increase in cortisol and 258.1% increase in ACTH; BIPSS: 9.6 central/peripheral and 5.5 right/left ACTH ratios. | 3 mm microadenoma (corticotropinoma), no extrasellar extension. | No LOH for CDKN1B variant. USP8 hotspot wild type. | TSS ×1, remission after surgery. | History of hypercholesterolemia in the father. | 14.7 |
| 2 | c.320delA, p.Q107Rfs*12 heterozygous (maternal). | M | White (USA). | 12.5/15.8 | Weight gain with central distribution (weight +2.7 SDS at first visit), poor linear growth (height -1.9 SDS), tinea corporis, proximal muscle weakness, dorsocervical fat pad, thoracic scoliosis, stress fracture of right foot, hypertension. Additional diagnoses: Gilbert’s syndrome, transient ischemic attack, right hydrocele and left epididymal cyst (right testicular biopsy reported normal tissue). | Midnight serum cortisol: 15.6 µg/dL; UFC: 571 µg/24 hours (6.3 × ULN); serum cortisol post-LDDST: 2 µg/dL; CRHST: 127.5% increase in cortisol and 180.4% increase in ACTH; BIPSS: 82.2 central/peripheral and 8.1 right/left ACTH ratios. | 4 mm microadenoma (corticotropinoma), no extrasellar extension. | n/a | TSS ×1, remission after surgery, no evidence of recurrence at age 36 years. | Mother had hypertension, type 2 diabetes mellitus and hypercholesterolemia; deceased due to colon adenocarcinoma, carrier of CDKN1B variant (LOH for CDKN1B variant in primary tumor and liver metastasis tissues). Brother and two children of the patient are apparently healthy carriers of CDKN1B variant. | 36.4 |
| 3 | c.356T>C, p.I119T heterozygous (unknown origin: DNA samples from parents n/a). | F | White (USA). | 10/13.8 | Weight gain with central distribution (weight 4.8 SDS with height –0.5 SDS at first visit), hirsutism, type 2 diabetes mellitus, hypertension.a | Midnight serum cortisol: 7.9 µg/dL; UFC: 148 µg/24 hours (2.6 × ULN); CRHST: 116% increase in cortisol and 533.5% increase in ACTH; BIPSS: 92.4 central/peripheral and 14.2 right/left ACTH ratios.a | 8 mm microadenoma (corticotropinoma) with extension to left cavernous sinus. | No LOH for CDKN1B variant. USP8 p.P720R heterozygous. | TSS ×2, remission after second surgery. | Not significant. | 14.8 |
| 4 | c.376G>C, p.E126Q heterozygous (unknown origin: DNA samples from parents n/a). | F | Unknown (Iraq). | 9/12 | Weight gain with central distribution, hair loss, striae and headaches. | 8 AM serum cortisol: 25 µg/dL; 8 AM ACTH: 274.5 pg/mL; serum cortisol post-LDDST: 20.3 µg/dL. | 5 mm microadenoma (PitNET, no IHC), no extrasellar extension. | n/a | TSS ×1, remission after surgery. | Parents are first cousins (CGH: regions of homozygosity detected, consanguineous parents). Family history of obesity, diabetes and hypertension in multiple members of the family. | 14 |
| 5 | c.407A>G, p.D136G heterozygous (unknown origin: DNA samples from parents n/a). | M | White (Israel). | 11/15.6 | Poor linear growth (height –3.6 SDS at first visit), facial plethora, headaches, dorsocervical fat pad, weight gain (weight –0.8 SDS but BMI in 96th percentile), incidental finding of area of suspected ischemia in the right parietal lobe. | Midnight serum cortisol: 11.2 µg/dL; UFC: 229 µg/24 hours (4.1 × ULN); BIPSS: 27 central/peripheral and 15.6 right/left ACTH ratios. | 3 mm microadenoma (corticotropinoma), no extrasellar extension. | No LOH for CDKN1B variant. USP8 hotspot wild type. | Ketoconazole, TSS ×1, remission after surgery. | Not significant. | 17.3 |
BIPSS, bilateral inferior petrosal sinus sampling; CGH, comparative genomic hybridization; CRHST, CRH stimulation test; IHC, immunohistochemistry; LDDST, low-dose dexamethasone suppression test; LOH, loss of heterozygosity; PitNET, pituitary neuroendocrine tumor; TSS, transsphenoidal surgery; UFC, urinary free cortisol.
aAuxology and biochemistry taken before second surgery (data before first surgery not available).
The histopathological examination confirmed corticotropinomas in 4 patients (Fig. 1); a PitNET was diagnosed in the remaining patient (Case 4), but immunohistochemistry was not available to confirm the clinical diagnosis. CDKN1B immunohistochemistry was performed in 3 patients. In Case 1, moderate to strong nuclear staining was observed in ∼30% of the cells, Case 3 displayed weak to moderate nuclear staining in ∼20% of the cells, and in Case 5 preservation of moderate to strong nuclear staining in ∼55% cells was found; occasional cells with moderate cytoplasmic staining were observed in all cases.
Figure 1.
Immunohistochemistry panel in four corticotropinomas from patients with CDKN1B variants of interest. Hematoxylin–eosin (H&E), reticulin, and ACTH-stained slides were used for the histopathological diagnosis; CDKN1B immunohistochemistry was done for this publication. Magnification for all images: 20×.
Functional characterization of CDKN1B variants
The CDKN1B gene encompasses 3 exons, the first of them including a long 5′ regulatory region (Fig. 2A). A glucocorticoid response element contained in such region is disrupted by the variant found in Case 1 (c.-29_-26delAGAG), leading to reduced CDKN1B transcription. We decided not to perform additional functional experiments for this variant, since its pathogenic potential is already well supported (6,9,32). The frameshift variant was detected at the cDNA level in the 4 variant carriers screened, and CDKN1B expression was similar among variant carriers and 1 wild-type individual from the same family, indicating that this variant does not lead to nonsense-mediated mRNA decay (Fig. 2b).
Figure 2.
Analysis of CDKN1B gene structure, protein domains and conservation, and effects of variants on subcellular localization. (A) The CDKN1B gene (12p13.1) includes 3 exons, 2 of which (exons 1 and 2) are translated into a 198 amino acid protein. Exon 1 includes the 5′ regulatory region that contains a glucocorticoid response element. In addition to multiple phosphorylation sites scattered throughout the protein (yellow rectangles) the following features have been identified in CDKN1B (22.1 kDa): a CDK inhibition domain (residues 25-93) (51), a nuclear export signal (NES, 32-45) (52), a scatter domain (118-158) (44), and a nuclear localization signal (NLS,152-168) (53). The position of the variants found in our patients has been annotated in the figure. (B) Both the CDKN1B frameshift variant p.Q107Rfs*12 and the wild-type sequence were detected in cDNA from variant carriers (proband and three family members); a wild-type chromatogram is presented for comparison. Using qPCR, CDKN1B was expressed at similar levels in variant carriers and in 1 family member not carrying the variant. (C) The human CDKN1B protein was annealed with orthologs from multiple species using the UniProt BLAST function. The 3 missense variants identified in our patients affect moderately (I119) or highly conserved residues (E126 and D136) localized within the scatter domain. (D) Subcellular localization of CDKN1B variants overexpressed in AtT20 cells. An anti-HA antibody was used to detect the HA-tagged CDKN1B proteins and nuclei were counterstained with DAPI. Images were obtained using the Texas Red (560/40 nm) and DAPI (360/40 nm) filters. Magnification for all images: 60×.
Along with multiple phosphorylation sites, a nuclear export signal and a nuclear localization signal (NLS), the CDKN1B protein contains 2 main domains with well-defined functions: an N-terminal cyclin-dependent kinase (CDK) inhibition domain, and a C-terminal scatter domain. The variant identified in case 2, p.Q107Rfs*12, disrupts the reading frame causing an early stop codon; the resulting truncated protein lacks the totality of the scatter domain. The 3 missense variants identified in our patients affect moderately (I119) or highly conserved residues (E126 and D136) localized within the scatter domain (Fig. 2C).
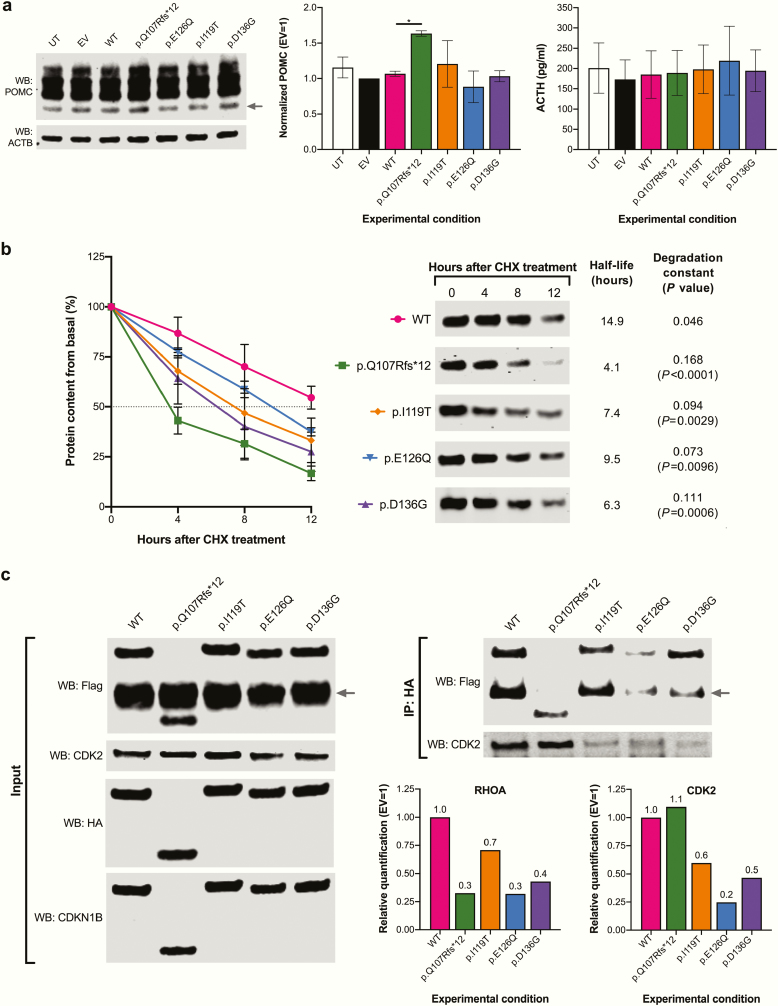
We first sought to determine whether the variants found in our patients could alter the subcellular localization of CDKN1B (Fig. 2D). By performing protein overexpression and immunocytofluorescence in AtT20 cells, we observed exclusively nuclear expression of the wild-type CDKN1B protein and the p.I119T and p.D136G variants, predominance of nuclear staining with moderate cytoplasmic staining for p.E126Q, and purely cytoplasmic expression of p.Q107Rfs*12. To investigate a possible effect of CDKN1B in hormonal production in AtT20 cells, we once more overexpressed the wild-type proteins and the putative pathogenic variants (Fig. 3A). No effect of the wild-type protein and the missense variants on POMC protein expression or ACTH secretion was observed; however, p.Q107Rfs*12 overexpression resulted in significantly increased POMC expression. Since the endogenous Cdkn1b expression was not silenced, this finding could be due to interference of the truncated protein with the function of the endogenous protein.
Figure 3.
Functional validation of CDKN1B variants of interest. (A) Quantification of POMC protein expression and ACTH secretion in cells overexpressing CDKN1B (wild type and variants). The 29.4-kDa WB band corresponding to POMC (arrow) was quantified and divided by the value to the ACTB protein in the same sample; results were normalized to the empty vector control (20 μg/lane total proteins were loaded). POMC was significantly higher in the cells overexpressing p.Q107Rfs*12 (1.62) compared with wild type (1.08, P = .0451) after Dunnett correction for multiple comparisons. ACTH was quantified in cell supernatants using an enzyme-linked immunosorbent assay kit. (B) CHX chase for CDKN1B proteins overexpressed in HEK293 cells, harvested at 0, 4, 8, and 12 hours after treatment. WB bands were normalized to total protein for the corresponding lanes at each time point. Half-life curves, representative WB images, half-life values and degradation constants are presented. P values reflect comparisons versus wild type. (C) CDKN1B-RHOA and CDKN1B-CDK2 coimmunoprecipitation experiment. WB images are presented for input (left) and coimmunoprecipitated samples (right); 40 μg/lane total proteins were loaded for input samples and 25-μL eluates were loaded for coimmunoprecipitation samples. The EKT1 site present in the HA-containing plasmid is very similar to the Flag tag and is therefore recognized by the anti-Flag antibody. The size of the overexpressed proteins was predicted using the Compute pI/Mw tool Expasy tool (https://web.expasy.org/compute_pi/). The Flag-tagged RHOA protein (arrow) is 24.1 kDa, the HA-tagged wild-type CDKN1B and the variants p.I119T, p.E126Q and p.D136G are 25.4 kDa; the variant p.I119T displayed slightly delayed migration. The variant p.Q107Rfs*12 is 17 kDa. The endogenous human CDK2 is 33.9 kDa.
We then performed a CHX chase experiment to assess the effect of our CDKN1B variants on protein stability (Fig. 3B). Not surprisingly, p.Q107Rfs*12 displayed the shortest half-life and the highest degradation speed among the variants tested, but all the missense variants also displayed significantly increased degradation compared with the wild-type protein. Finally, we tested and quantified the direct interaction between RHOA and the CDKN1B variants, which is expected to occur via the scatter domain (Fig. 3C). All the overexpressed CDKN1B proteins were successfully detected by WB; as expected, p.Q107Rfs*12 led to the expression of a truncated protein. As previously shown by other authors, p.I119T displayed slightly delayed migration (19,38). A novel phosphorylation site that could be targeted by multiple protein kinases was in silico predicted for this variant, which could explain the altered migration pattern. We observed significantly reduced interaction between p.Q107Rfs*12 and RHOA (70% reduction compared with wild type). The missense variants p.E126Q and p.D136G also displayed greatly reduced interaction (–70% and –60%, respectively) compared with wild type, while p.I119T displayed only a 30% reduction. Endogenous CDK2 (interacting with the N-terminal domain) was detected in the same membranes, finding slightly increased binding to p.Q107Rfs*12 (+10%) and reduced binding to the rest of the proteins (–40%, –80%, and –50% for p.I119T, p.E126Q and p.D136G, respectively).
Discussion
We have identified 5 CD cases associated with germline CDKN1B variants that are pathogenic/likely pathogenic or of uncertain significance (VUS) among 190 patients screened (2.6%). This was unexpected given that previous studies have reported a very low prevalence of CDKN1B variants among patients with PitNETs (18). This frequency is similar to the estimated prevalence of MEN1 and AIP genetic defects among all patients with PitNETs (39). Our results might be explained by the selection of the study cohort, since this is, to our knowledge, the largest cohort of CD patients ever screened for CDKN1B genetic defects.
PitNETs have been found in 13 other individuals out of 46 patients with MEN4 phenotypes or germline CDKN1B defects reported in the literature: 5 with acromegaly, 4 with nonfunctioning PitNETs, 2 with CD, 1 with gigantism, and 1 with prolactinoma (1,2,4,7,9,16,19). Of note, the patient with gigantism presented sporadically and 2 cases of acromegaly and 1 prolactinoma presented within 2 different FIPA families, although segregation was not proven in 1 family and was negative in the other (19). The remaining PitNETs were diagnosed in patients with a personal or family history of other NETs. Thus, the estimated penetrance of pituitary disease among MEN4 patients is 28.3%, although this might not be an accurate calculation, given the scarcity of reported cases and the variability in the clinical and genetic screening among case reports (16,40).
To our knowledge, only 2 CD cases had been previously reported in patients with germline CDKN1B defects, both of them in adult women with heterozygous frameshift variants: a patient with CD, small cell neuroendocrine cervical carcinoma and PHPT, with the variant p.S27Gfs*104 (with LOH in the corticotropinoma) (2), and a patient with CD and PHPT and the variant p.L41Nfs*83 who was part of a family with 13 affected members (4 with PitNETs) (16). In contrast, our patients presented with apparently sporadic, early-onset, isolated CD, a clinical phenotype that had not been associated before with CDKN1B LOF in the literature. Although CD is so far the only MEN4 feature in our patients, they were still young at last follow-up (Table 5) and are, therefore, at risk of developing additional neoplasms in the future.
Although the origin of the variants could not be determined in most of our patients, there was no evidence of MEN4-associated neoplasms in the patients’ families based on the clinical history. The only additional neoplasm detected in association with one CDKN1B variant was colon adenocarcinoma in the mother of Case 3. CDKN1B is not among the most frequently mutated genes in sporadic colorectal cancer, but low CDKN1B expression is a marker of disease progression (11,41). Interestingly, a recent study linked a germline CDKN1B missense variant with familial colorectal cancer affecting 3 generations of a single kindred (42). Despite the possibility of coincidental occurrence of this common neoplasm in our patient, we believe that the possible role of CDKN1B germline loss of function in colorectal cancer deserves further investigation.
Most variants so far associated with MEN4 do not truncate the CDKN1B protein, and in fact in our cohort we found 4 nontruncating variants (1 short deletion in the 5′ UTR and 2 missense variants in the scatter domain) and only 1 truncating variant (a frameshift variant leading to an early stop codon). To date, there is not enough evidence as to support specific phenotype–genotype correlations, as it has been proposed for MEN2 (43), although the MEN4 kindred with the most affected individuals so far reported carried a truncating variant (16).
Given that the 5′ UTR variant found in our study had been functionally characterized before, we decided to characterize only the frameshift and missense variants detected. As expected, when overexpressed in murine corticotroph cells, p.Q107Rfs*12 localized only in the cytoplasm, due to loss of the NLS. Interestingly, p.E126Q was detected in the nucleus and cytoplasm, while p.I119T and p.D136G had exclusively nuclear localization. In proliferating cells, CDKN1B accumulates in the nucleus bound to cyclin D1/CDK4, and via interaction with the cyclin E/CDK2 complex through its N-terminal domain, CDKN1B promotes G1 arrest. In turn, cyclin E/CDK2 phosphorylate CDKN1B in T187, and the phosphorylated protein is translocated to the cytoplasm, where it undergoes ubiquitination and proteasomal degradation (11). In corticotropinomas, T187-phosphorylated CDKN1B is increased, probably due to upregulation of cyclin E (14). Our experiments showed reduced CDK2 binding for all the missense variants, which could result in defective cell cycle control. The unbound CDKN1B would be expected to be exported to the cytoplasm for degradation, and, indeed, we observed increased protein degradation for all the variants tested. The truncated protein studied binds CDK2 in vitro, also undergoes rapid degradation and does not localize to the nucleus.
The C-terminal half of CDKN1B contains the scatter domain (residues 118-158), which directly interacts with RHOA GTPase inhibiting its activation, leading to increased cell motility (44,45). In addition, phosphorylation by AKT1 (T157), and subsequent binding to 14-3-3 via the scatter domain results in CDKN1B cytoplasmic sequestration and ubiquitination (46). Therefore, the regulation of protein localization and protein levels seems to require an intact scatter domain, independently of the cell cycle inhibitory function. Our coimmunoprecipitation experiments demonstrated reduced RHOA binding for all the variants tested, indicating deletion or disruption of the scatter domain produced by the amino acid substitutions. Although multiple other proteins are known to interact with CDKN1B through this domain, the specific interacting partners and functions relevant for corticotroph function are unknown (15). Two other germline missense variants in the CDKN1B scatter domain have been reported in the literature: p.E126D in a patient with PHPT, and p.P133T in 2 patients with PHPT and 1 patient with PHPT, thyroid cancer, and meningioma (47-49).
No gene copy number gains or losses were found in the 194 patients screened for CNVs. We found LOH for a common variant in 1 of the 27 patients that underwent both germline and tumor WES; this sample did not display chromosomal instability in a recent WES-based CNV study (26). To our knowledge, 12p deletion affecting CDKN1B is not a frequent finding in PitNETs, and CGH analysis will be required in this sample to determine the extent of the genomic defect. Also, LOH was observed in the colon adenocarcinoma tissue but not in corticotropinoma samples. It has been suggested that CDKN1B haploinsufficiency is enough to cause loss of its tumor suppression function (15). Since additional “second hit” mechanisms were not investigated, the absence of LOH neither supports nor contradicts variant pathogenicity.
We applied the American College of Medical Genetics and Genomics criteria for variant classification (50) using our results, the variant frequencies, and previously reported clinical associations (Table 6). This resulted in 1 variant (p.Q107Rfs*12) being classified as pathogenic, 2 (c.-29_-26delAGAG and p.E126Q) as likely pathogenic, and 2 more (p.I119T and p.D136G) as VUS. In addition to p.I119T, Case 3 carried a somatic USP8 hotspot variant, making it impossible to be certain of whether the CDKN1B variant, the USP8 variant, or both, were responsible for the disease. For now, all these patients should be considered as MEN4 cases and followed up accordingly. Genetic and clinical screening of additional family members, however, are currently only justified for individuals at risk of carrying pathogenic or likely pathogenic variants.
Table 6.
Summary of functional studies in CDKN1B variants and variant classification
| CDKN1B variant | LOH | IHC | Migration in denaturing protein gel | CHX chase | ICF | POMC expression | RHOA binding | CDK2 binding | ACMG classification after functional studies |
|---|---|---|---|---|---|---|---|---|---|
| c.-29_-26delAGAG, p.? | No. | Moderate to strong nuclear CDKN1B staining in ∼30% of cells. | Not done (variant previously functionally characterized). | Likely pathogenic. | |||||
| c.320delA, p.Q107Rfs*12 | Yes (in colon cancer). | n/a | Faster than wt (truncated protein). | Shortened half-life/increased degradation speed. | Cytoplasmic localization. | Increased. | –70% | +10% | Pathogenic. |
| c.356T>C, p.I119T | No. | Weak to moderate nuclear staining in ∼20% of cells. | Slower than wt (possible acquired posttranslational modification). | Shortened half-life/increased degradation speed. | Nuclear localization. | Unchanged. | –30% | –40% | VUS. |
| c.376G>C, p.E126Q | n/a. | n/a | Normal. | Shortened half-life/increased degradation speed. | Nuclear and cytoplasmic localization. | Unchanged. | –70% | –80% | Likely pathogenic. |
| c.407A>G, p.D136G | No. | Moderate to strong nuclear staining in ∼55% of cells. | Normal. | Shortened half-life/increased degradation speed. | Nuclear localization. | Unchanged. | –60% | –50% | VUS. |
ACMG, American College of Medical Genetics and Genomics; CHX, cycloheximide; ICF, immunocytofluorescence; IHC, immunohistochemistry; LOH, loss of heterozygosity; n/a, not available; VUS, variant of uncertain significance; wt, wild type.
Some strengths and limitations of our study should be noted. Strengths include the large number of CD cases and the detailed clinical and laboratory data. While we have done our best to functionally characterize the variants found in our patients, the scarcity of MEN4 cases reported in the literature so far reported complicates the evaluation of pathogenicity, and variant classification could change over time in light of new genetic and clinical reports. Also, our center is a tertiary referral hospital, therefore aggressive or atypical cases of CD might be overrepresented in our cohort. While we acknowledge that our study population might not be representative of the general population of patients with pediatric CD, this inherent selection bias might have been crucial in our ability to identify multiple cases of a rare genetic disease.
In conclusion, 5 cases of MEN4-related CD were identified, accounting for 2.6% of the patients screened. Their rarity in the general population and their functional effects point toward a putative pathogenic role for these variants as causative of MEN4. CDKN1B germline LOF variants are a rare cause of CD that should be kept in mind, particularly when evaluating young patients, due to its implications for clinical screening and genetic counseling.
Acknowledgments
We are grateful with Dr. Lyssikatos Charalampos and Ms. María de la Luz Sierra for their help with the collection and preparation of DNA and histopathological samples used in this study and with Dr. Stephen Marx for his kind help with the preparation of this manuscript. We are also thankful to Dr. Maya B. Lodish and Dr. Christina Tatsi for the stewardship of the clinical studies on pediatric patients with Cushing’s disease at the National Institutes of Health. Finally, we thank Dr. Jerôme Bertherat for his mentorship of Dr. Chasseloup along with Dr. Stratakis.
Financial Support: This work was supported by the Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Institutes of Health. F.C. was the recipient of a master’s degree fellowship from the French Endocrine Society and Novartis Pharma laboratories.
Clinical Trial Information: ClinicalTrials.gov NCT00001595 (submitted on 11 March 1999).
Glossary
Abbreviations
- ACTH
adrenocorticotropin
- CD
Cushing’s disease
- CDKN1B
cyclin-dependent kinase inhibitor 1B gene
- CDS
coding DNA sequence
- CHX
cycloheximide
- CNV
copy number variant
- DNA
deoxyribonucleic acid
- FIPA
familial isolated pituitary adenoma
- LOF
loss-of-function
- LOH
loss of heterozygosity
- MAF
minor allele frequency
- MEN
multiple endocrine neoplasia
- NET
neuroendocrine tumor
- NLS
nuclear localization signal
- PCR
polymerase chain reaction
- PHPT
primary hyperparathyroidism
- PitNET
pituitary neuroendocrine tumor
- RNA
ribonucleic acid
- SDM
site-directed mutagenesis
- UMGC
University of Minnesota Genomics Center
- UTR
untranslated region
- VUS
variants of uncertain significance
- WES
whole-exome sequencing
Additional Information
Disclosure Summary: The authors have nothing to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Pellegata NS, Quintanilla-Martinez L, Siggelkow H, et al. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci U S A. 2006;103(42):15558-15563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Georgitsi M, Raitila A, Karhu A, et al. Germline CDKN1B/p27Kip1 mutation in multiple endocrine neoplasia. J Clin Endocrinol Metab. 2007;92(8):3321-3325. [DOI] [PubMed] [Google Scholar]
- 3. Agarwal SK, Mateo CM, Marx SJ. Rare germline mutations in cyclin-dependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. J Clin Endocrinol Metab. 2009;94(5):1826-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Molatore S, Marinoni I, Lee M, et al. A novel germline CDKN1B mutation causing multiple endocrine tumors: clinical, genetic and functional characterization. Hum Mutat. 2010;31(11):E1825-E1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belar O, De La Hoz C, Pérez-Nanclares G, Castaño L, Gaztambide S; Spanish MEN1 Group Novel mutations in MEN1, CDKN1B and AIP genes in patients with multiple endocrine neoplasia type 1 syndrome in Spain. Clin Endocrinol (Oxf). 2012;76(5):719-724. [DOI] [PubMed] [Google Scholar]
- 6. Malanga D, De Gisi S, Riccardi M, et al. Functional characterization of a rare germline mutation in the gene encoding the cyclin-dependent kinase inhibitor p27Kip1 (CDKN1B) in a Spanish patient with multiple endocrine neoplasia-like phenotype. Eur J Endocrinol. 2012;166(3):551-560. [DOI] [PubMed] [Google Scholar]
- 7. Occhi G, Regazzo D, Trivellin G, et al. A novel mutation in the upstream open reading frame of the CDKN1B gene causes a MEN4 phenotype. Plos Genet. 2013;9(3):e1003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pardi E, Mariotti S, Pellegata NS, et al. Functional characterization of a CDKN1B mutation in a Sardinian kindred with multiple endocrine neoplasia type 4 (MEN4). Endocr Connect. 2015;4(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sambugaro S, Di Ruvo M, Ambrosio MR, et al. Early onset acromegaly associated with a novel deletion in CDKN1B 5’UTR region. Endocrine. 2015;49(1):58-64. [DOI] [PubMed] [Google Scholar]
- 10. Polyak K, Kato JY, Solomon MJ, et al. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8(1):9-22. [DOI] [PubMed] [Google Scholar]
- 11. Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8(4):253-267. [DOI] [PubMed] [Google Scholar]
- 12. Dahia PL, Aguiar RC, Honegger J, et al. Mutation and expression analysis of the p27/kip1 gene in corticotrophin-secreting tumours. Oncogene. 1998;16(1):69-76. [DOI] [PubMed] [Google Scholar]
- 13. Lidhar K, Korbonits M, Jordan S, et al. Low expression of the cell cycle inhibitor p27Kip1 in normal corticotroph cells, corticotroph tumors, and malignant pituitary tumors. J Clin Endocrinol Metab. 1999;84(10):3823-3830. [DOI] [PubMed] [Google Scholar]
- 14. Korbonits M, Chahal HS, Kaltsas G, et al. Expression of phosphorylated p27(Kip1) protein and Jun activation domain-binding protein 1 in human pituitary tumors. J Clin Endocrinol Metab. 2002;87(6):2635-2643. [DOI] [PubMed] [Google Scholar]
- 15. Molatore S, Pellegata NS. The MENX syndrome and p27: relationships with multiple endocrine neoplasia. In: Martini L, ed. Prog Brain Res. Vol 182 Oxford, UK: Elsevier; 2010:295-320. [DOI] [PubMed] [Google Scholar]
- 16. Frederiksen A, Rossing M, Hermann P, Ejersted C, Thakker RV, Frost M. Clinical features of multiple endocrine neoplasia type 4: novel pathogenic variant and review of published cases. J Clin Endocrinol Metab. 2019;104(9):3637-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Igreja S, Chahal HS, Akker SA, et al. Assessment of p27 (cyclin-dependent kinase inhibitor 1B) and aryl hydrocarbon receptor-interacting protein (AIP) genes in multiple endocrine neoplasia (MEN1) syndrome patients without any detectable MEN1 gene mutations. Clin Endocrinol (Oxf). 2009;70(2):259-264. [DOI] [PubMed] [Google Scholar]
- 18. Stratakis CA, Tichomirowa MA, Boikos S, et al. The role of germline AIP, MEN1, PRKAR1A, CDKN1B and CDKN2C mutations in causing pituitary adenomas in a large cohort of children, adolescents, and patients with genetic syndromes. Clin Genet. 2010;78(5):457-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tichomirowa MA, Lee M, Barlier A, et al. Cyclin-dependent kinase inhibitor 1B (CDKN1B) gene variants in AIP mutation-negative familial isolated pituitary adenoma kindreds. Endocr Relat Cancer. 2012;19(3):233-241. [DOI] [PubMed] [Google Scholar]
- 20. Hernández-Ramírez LC, Gam R, Valdés N, et al. Loss-of-function mutations in the CABLES1 gene are a novel cause of Cushing’s disease. Endocr Relat Cancer. 2017;24(8):379-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trivellin G, Correa RR, Batsis M, et al. Screening for GPR101 defects in pediatric pituitary corticotropinomas. Endocr Relat Cancer. 2016;23(5):357-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Faucz FR, Tirosh A, Tatsi C, et al. Somatic USP8 gene mutations are a common cause of pediatric Cushing disease. J Clin Endocrinol Metab. 2017;102(8):2836-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hernández-Ramírez LC, Tatsi C, Lodish MB, et al. Corticotropinoma as a component of carney complex. J Endocr Soc. 2017;1(7):918-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Makri A, Bonella MB, Keil MF, et al. Children with MEN1 gene mutations may present first (and at a young age) with Cushing disease. Clin Endocrinol (Oxf). 2018;89(4):437-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cohen M, Persky R, Stegemann R, et al. Germline USP8 mutation associated with pediatric Cushing disease and other clinical features: a new syndrome. J Clin Endocrinol Metab. 2019;104(10):4676-4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tatsi C, Pankratz N, Lane J, et al. Large genomic aberrations in corticotropinomas are associated with greater aggressiveness. J Clin Endocrinol Metab. 2019;104(5):1792-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu X, Jian X, Boerwinkle E. dbNSFP: a lightweight database of human nonsynonymous SNPs and their functional predictions. Hum Mutat. 2011;32(8):894-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robinson JT, Thorvaldsdóttir H, Winckler W, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29(1):24-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kopanos C, Tsiolkas V, Kouris A, et al. VarSome: the human genomic variant search engine. Bioinformatics. 2019;35(11):1978-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karczewski KJ, Francioli LC, Tiao G, et al. Variation across 141 456 human exomes and genomes reveals the spectrum of loss-of-function intolerance across human protein-coding genes. bioRxiv 2019:531210. [Google Scholar]
- 32. Borsari S, Pardi E, Pellegata NS, et al. Loss of p27 expression is associated with MEN1 gene mutations in sporadic parathyroid adenomas. Endocrine. 2017;55(2):386-397. [DOI] [PubMed] [Google Scholar]
- 33. Chang BL, Zheng SL, Isaacs SD, et al. A polymorphism in the CDKN1B gene is associated with increased risk of hereditary prostate cancer. Cancer Res. 2004;64(6):1997-1999. [DOI] [PubMed] [Google Scholar]
- 34. Pappa V, Papageorgiou S, Papageorgiou E, et al. A novel p27 gene mutation in a case of unclassified myeloproliferative disorder. Leuk Res. 2005;29(2):229-231. [DOI] [PubMed] [Google Scholar]
- 35. Lauter KB, Arnold A. Mutational analysis of CDKN1B, a candidate tumor-suppressor gene, in refractory secondary/tertiary hyperparathyroidism. Kidney Int. 2008;73(10):1137-1140. [DOI] [PubMed] [Google Scholar]
- 36. Ojeda D, Lakhal B, Fonseca DJ, et al. Sequence analysis of the CDKN1B gene in patients with premature ovarian failure reveals a novel mutation potentially related to the phenotype. Fertil Steril 2011; 95:2658-2660 e2651. [DOI] [PubMed] [Google Scholar]
- 37. Ruiz-Heredia Y, Sánchez-Vega B, Onecha E, et al. Mutational screening of newly diagnosed multiple myeloma patients by deep targeted sequencing. Haematologica. 2018;103(11):e544-e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gummlich L, Kähne T, Naumann M, Kilic E, Jung K, Dubiel W. New insights into the mechanism of COP9 Signalosome-Cullin-RING ubiquitin-ligase pathway deregulation in urological cancers. Int Rev Cell Mol Biol. 2016;323:181-229. [DOI] [PubMed] [Google Scholar]
- 39. Daly AF, Jaffrain-Rea ML, Ciccarelli A, et al. Clinical characterization of familial isolated pituitary adenomas. J Clin Endocrinol Metab. 2006;91(9):3316-3323. [DOI] [PubMed] [Google Scholar]
- 40. Alrezk R, Hannah-Shmouni F, Stratakis CA. MEN4 and CDKN1B mutations: the latest of the MEN syndromes. Endocr Relat Cancer. 2017;24(10):T195-T208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012; 487(7407):330-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Esteban-Jurado C, Vila-Casadesús M, Garre P, et al. Whole-exome sequencing identifies rare pathogenic variants in new predisposition genes for familial colorectal cancer. Genet Med. 2015;17(2):131-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brandi ML, Gagel RF, Angeli A, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86(12):5658-5671. [DOI] [PubMed] [Google Scholar]
- 44. McAllister SS, Becker-Hapak M, Pintucci G, Pagano M, Dowdy SF. Novel p27(kip1) C-terminal scatter domain mediates Rac-dependent cell migration independent of cell cycle arrest functions. Mol Cell Biol. 2003;23(1):216-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 2004;18(8):862-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fujita N, Sato S, Katayama K, Tsuruo T. Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J Biol Chem. 2002;277(32): 28706-28713. [DOI] [PubMed] [Google Scholar]
- 47. Costa-Guda J, Marinoni I, Molatore S, Pellegata NS, Arnold A. Somatic mutation and germline sequence abnormalities in CDKN1B, encoding p27Kip1, in sporadic parathyroid adenomas. J Clin Endocrinol Metab. 2011;96(4):E701-E706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Elston MS, Meyer-Rochow GY, Dray M, Swarbrick M, Conaglen JV. Early onset primary hyperparathyroidism associated with a novel germline mutation in CDKN1B. Case Rep Endocrinol. 2015;2015:510985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bugalho MJ, Domingues R. Uncommon association of cerebral meningioma, parathyroid adenoma and papillary thyroid carcinoma in a patient harbouring a rare germline variant in the CDKN1B gene. BMJ Case Rep 2016; 2016:bcr2015213934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Richards S, Aziz N, Bale S, et al. ; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Russo AA, Jeffrey PD, Patten AK, Massagué J, Pavletich NP. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature. 1996;382(6589):325-331. [DOI] [PubMed] [Google Scholar]
- 52. Connor MK, Kotchetkov R, Cariou S, et al. CRM1/Ran-mediated nuclear export of p27(Kip1) involves a nuclear export signal and links p27 export and proteolysis. Mol Biol Cell. 2003;14(1):201-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sekimoto T, Fukumoto M, Yoneda Y. 14-3-3 suppresses the nuclear localization of threonine 157-phosphorylated p27(Kip1). EMBO J. 2004;23(9):1934-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Al-Hebshi NN, Li S, Nasher AT, et al. Exome sequencing of oral squamous cell carcinoma in users of Arabian snuff reveals novel candidates for driver genes. Int J Cancer. 2016;139(2): 363-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Martin D, Abba MC, Molinolo AA, et al. The head and neck cancer cell oncogenome: a platform for the development of precision molecular therapies. Oncotarget. 2014;5(19):8906-8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Occhi G, Trivellin G, Ceccato F, et al. Prevalence of AIP mutations in a large series of sporadic Italian acromegalic patients and evaluation of CDKN1B status in acromegalic patients with multiple endocrine neoplasia. Eur J Endocrinol. 2010;163(3):369-376. [DOI] [PubMed] [Google Scholar]
- 57. Landa I, Montero-Conde C, Malanga D, et al. Allelic variant at -79 (C>T) in CDKN1B (p27Kip1) confers an increased risk of thyroid cancer and alters mRNA levels. Endocr Relat Cancer. 2010;17(2):317-328. [DOI] [PubMed] [Google Scholar]
- 58. Capasso M, McDaniel LD, Cimmino F, et al. The functional variant rs34330 of CDKN1B is associated with risk of neuroblastoma. J Cell Mol Med. 2017;21(12):3224-3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ma H, Jin G, Hu Z, et al. Variant genotypes of CDKN1A and CDKN1B are associated with an increased risk of breast cancer in Chinese women. Int J Cancer. 2006;119(9):2173-2178. [DOI] [PubMed] [Google Scholar]
- 60. Driver KE, Song H, Lesueur F, et al. ; Studies in Epidemiology and Risks of Cancer Heredity (SEARCH) Team Association of single-nucleotide polymorphisms in the cell cycle genes with breast cancer in the British population. Carcinogenesis. 2008;29(2):333-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Canbay E, Eraltan IY, Cercel A, et al. CCND1 and CDKN1B polymorphisms and risk of breast cancer. Anticancer Res. 2010;30(7):3093-3098. [PubMed] [Google Scholar]
- 62. Wang W, Spitz MR, Yang H, Lu C, Stewart DJ, Wu X. Genetic variants in cell cycle control pathway confer susceptibility to lung cancer. Clin Cancer Res. 2007;13(19):5974-5981. [DOI] [PubMed] [Google Scholar]
- 63. Cai H, Xiang YB, Qu S, et al. Association of genetic polymorphisms in cell-cycle control genes and susceptibility to endometrial cancer among Chinese women. Am J Epidemiol. 2011;173(11):1263-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu F, Wei YG, Luo LM, et al. Genetic variants of p21 and p27 and hepatocellular cancer risk in a Chinese Han population: a case-control study. Int J Cancer. 2013;132(9):2056-2064. [DOI] [PubMed] [Google Scholar]
- 65. Cheng XK, Wang XJ, Li XD, Ren XQ. Genetic association between the cyclin-dependent kinase inhibitor gene p27/Kip1 polymorphism (rs34330) and cancer susceptibility: a meta-analysis. Sci Rep. 2017;7(1):44871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sekiya T, Bronstein MD, Benfini K, et al. p27 variant and corticotropinoma susceptibility: a genetic and in vitro study. Endocr Relat Cancer. 2014;21(3):395-404. [DOI] [PubMed] [Google Scholar]
- 67. Li G, Sturgis EM, Wang LE, et al. Association between the V109G polymorphism of the p27 gene and the risk and progression of oral squamous cell carcinoma. Clin Cancer Res. 2004;10(12 Pt 1):3996-4002. [DOI] [PubMed] [Google Scholar]
- 68. Schöndorf T, Eisele L, Göhring UJ, et al. The V109G polymorphism of the p27 gene CDKN1B indicates a worse outcome in node-negative breast cancer patients. Tumour Biol. 2004;25(5-6):306-312. [DOI] [PubMed] [Google Scholar]
- 69. Naidu R, Har YC, Taib NA. P27 V109G Polymorphism is associated with lymph node metastases but not with increased risk of breast cancer. J Exp Clin Cancer Res. 2007;26(1):133-140. [PubMed] [Google Scholar]
- 70. Camargo-Kosugi CM, da Silva ID, Sato H, et al. The V109G polymorphism in the p27 gene is associated with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2009;145(2):180-183. [DOI] [PubMed] [Google Scholar]
- 71. Mohamed FZ, Hussien YM, AlBakry MM, Mohamed RH, Said NM. Role of DNA repair and cell cycle control genes in ovarian cancer susceptibility. Mol Biol Rep. 2013;40(5):3757-3768. [DOI] [PubMed] [Google Scholar]
- 72. Halkova T, Dvorakova S, Sykorova V, et al. Polymorphisms in selected DNA repair genes and cell cycle regulating genes involved in the risk of papillary thyroid carcinoma. Cancer Biomark. 2016;17(1):97-106. [DOI] [PubMed] [Google Scholar]
- 73. Schumacher FR, Al Olama AA, Berndt SI, et al. ; Profile Study; Australian Prostate Cancer BioResource (APCB); IMPACT Study; Canary PASS Investigators; Breast and Prostate Cancer Cohort Consortium (BPC3); PRACTICAL (Prostate Cancer Association Group to Investigate Cancer-Associated Alterations in the Genome) Consortium; Cancer of the Prostate in Sweden (CAPS); Prostate Cancer Genome-wide Association Study of Uncommon Susceptibility Loci (PEGASUS); Genetic Associations and Mechanisms in Oncology (GAME-ON)/Elucidating Loci Involved in Prostate Cancer Susceptibility (ELLIPSE) Consortium. Association analyses of more than 140 000 men identify 63 new prostate cancer susceptibility loci. Nat Genet. 2018;50(7):928-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mahajan A, Taliun D, Thurner M, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50(11):1505-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wei F, Xu J, Tang L, et al. p27(Kip1) V109G polymorphism and cancer risk: a systematic review and meta-analysis. Cancer Biother Radiopharm. 2012;27(10):665-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Barbieri RB, Bufalo NE, Secolin R, et al. Polymorphisms of cell cycle control genes influence the development of sporadic medullary thyroid carcinoma. Eur J Endocrinol. 2014;171(6): 761-767. [DOI] [PubMed] [Google Scholar]
- 77. Pasquali D, Circelli L, Faggiano A, et al. CDKN1B V109G polymorphism a new prognostic factor in sporadic medullary thyroid carcinoma. Eur J Endocrinol. 2011;164(3):397-404. [DOI] [PubMed] [Google Scholar]
- 78. Kibel AS, Suarez BK, Belani J, et al. CDKN1A and CDKN1B polymorphisms and risk of advanced prostate carcinoma. Cancer Res. 2003;63(9):2033-2036. [PubMed] [Google Scholar]
- 79. Lu Y, Gao K, Zhang M, et al. Genetic Association Between CDKN1B rs2066827 Polymorphism and Susceptibility to Cancer. Medicine (Baltimore). 2015;94(46):e1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Francisco G, Gonçalves FT, Luiz OC, et al. Polymorphisms in the p27kip-1 and prohibitin genes denote novel genes associated with melanoma risk in Brazil, a high ultraviolet index region. Melanoma Res. 2013;23(3):231-236. [DOI] [PubMed] [Google Scholar]
- 81. Circelli L, Ramundo V, Marotta V, et al. ; Multidisciplinary Group for NeuroEndocrine Tumours of Naples Prognostic role of the CDNK1B V109G polymorphism in multiple endocrine neoplasia type 1. J Cell Mol Med. 2015;19(7): 1735-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]