Abstract
Objective
To assess short term mortality risks and excess mortality associated with exposure to ozone in several cities worldwide.
Design
Two stage time series analysis.
Setting
406 cities in 20 countries, with overlapping periods between 1985 and 2015, collected from the database of Multi-City Multi-Country Collaborative Research Network.
Population
Deaths for all causes or for external causes only registered in each city within the study period.
Main outcome measures
Daily total mortality (all or non-external causes only).
Results
A total of 45 165 171 deaths were analysed in the 406 cities. On average, a 10 µg/m3 increase in ozone during the current and previous day was associated with an overall relative risk of mortality of 1.0018 (95% confidence interval 1.0012 to 1.0024). Some heterogeneity was found across countries, with estimates ranging from greater than 1.0020 in the United Kingdom, South Africa, Estonia, and Canada to less than 1.0008 in Mexico and Spain. Short term excess mortality in association with exposure to ozone higher than maximum background levels (70 µg/m3) was 0.26% (95% confidence interval 0.24% to 0.28%), corresponding to 8203 annual excess deaths (95% confidence interval 3525 to 12 840) across the 406 cities studied. The excess remained at 0.20% (0.18% to 0.22%) when restricting to days above the WHO guideline (100 µg/m3), corresponding to 6262 annual excess deaths (1413 to 11 065). Above more lenient thresholds for air quality standards in Europe, America, and China, excess mortality was 0.14%, 0.09%, and 0.05%, respectively.
Conclusions
Results suggest that ozone related mortality could be potentially reduced under stricter air quality standards. These findings have relevance for the implementation of efficient clean air interventions and mitigation strategies designed within national and international climate policies.
Introduction
Ground level ozone is a highly reactive, oxidative gas commonly found in urban and suburban environments, mostly derived from anthropogenic emissions. Numerous epidemiological studies and several reviews from health and environmental agencies worldwide have reported that exposure to this pollutant is associated with adverse health outcomes, including increased short term mortality and morbidity.1 2 3 4 Evidence on the health impacts related to ozone exposure has important implications in climate change research, as ozone levels are predicted to increase with global warming.5
Short term ozone-mortality associations have been widely assessed in several multi-location time series studies in Europe, the United States, Canada, Latin America, and Asia.2 6 7 8 The general methodological framework consists of pooling location specific estimated risks, accounting for potential heterogeneity in the magnitude of the effect and uncertainty. In addition, the increased statistical power of multi-location analyses allows for the exploration of potentially complex features of the association (ie, non-linearity, delayed effects and harvesting, or differential risks by season).9 10 11 However, previous multi-location studies included a small number of cities and countries, were generally of limited geographical scope, and applied heterogeneous analytical approaches and modelling choices, making it difficult to draw consistent and comprehensive conclusions across different regions of the world.
Although ozone-mortality associations have been widely assessed, results are rarely reported in terms of health impacts, such as excess deaths.12 Available figures are mostly derived from long term exposure metrics and risks estimated in specific subgroups, which are usually extrapolated to the general population.13 14 Quantification of health burdens from air pollution can be extremely useful for the design of efficient public health interventions, including the definition, assessment, and review of air quality standards. Current air quality standards vary greatly between countries, and only a few of them meet the stricter World Health Organization recommendation.15 Comparting the effects on health of ozone levels above different air quality standards can provide valuable insights into potential public health benefits achieved by strengthening current clean air policies. Although a few studies have attempted to tackle this problem, a widespread evaluation across several countries, which would help to identify more affected areas with a greater need for intervention, is still lacking.16 17
We carried out a multi-location time series analysis of mortality associated with short term exposure to ozone using data from 406 cities in 20 countries from multiple geographical regions. Next, we explored potential complexities of the association—namely, non-linearity, mortality displacement, and seasonality. Finally, we quantified the impacts on ozone associated mortality of specific concentration ranges consistent with the current air quality standards levels and then compared these estimates across countries.
Methods
Data collection
We initially extracted data for 434 locations across the 20 countries from the database of the Multi-city Multi-country (MCC) Collaborative Research Network (http://mccstudy.lshtm.ac.uk/) available at the time of the study. These include location specific daily mortality counts and environmental measures (weather and air pollutants) in largely overlapping periods from 1 January 1985 to 31 December 2015. For each location we derived daily time series of ozone (maximum eight hour average), particulate matter with an aerodynamic diameter less than or equal to 10 µm (PM10, per µg/m3, 24 hour average), particulate matter with an aerodynamic diameter less than or equal to 2.5 µm (PM2.5, per µg/m3, 24 hour average), nitrogen dioxide (24 hour average), total mortality, mean temperature (°C), and relative humidity (%). Mortality was represented by all cause deaths in Canada, the Czech Republic, Estonia, France, Germany, Greece, Italy, Japan, Mexico, Portugal, South Africa, South Korea, Sweden, Taiwan, UK, and US, whereas deaths due to non-external causes (eg, excluding self-intentional harm, poisoning) were used in Australia, China, and Spain, and non-external causes other than unintentional injuries in Switzerland (see supplementary eMethods 1 for the specific international classification of diseases codes used in each country). City specific air pollution series were derived from daily measurements of one or more monitors of the national or regional network. When more than one monitor was available, we computed the daily level of each pollutant (24 hour average or eight hour maximum) as the average across monitors of the city, consistent with previous multi-city studies.2 We excluded 28 cities as a result of poor quality data or limited periods (less than three years), with 406 locations included in the final analysis (see supplementary eMethods 1 for a detailed description of the data, exposure assessment, and exclusion criteria).
Statistical analysis
The general statistical framework applied here is an extension of the classic two stage design6 and incorporates complex multivariable associations, hierarchical pooling methods, and the computation of impact measures.18 19 20 Briefly, we first estimated city specific ozone-mortality risks from separate time series regression models and then pooled these through a meta-analysis in the second stage. In a final step, we derived impact estimates, expressed as excess mortality fractions associated with ozone, from the pooled country specific risks and city specific exposure series. Using this general statistical framework, we performed a set of additional and sensitivity analyses to investigate specific features of the association. The analyses were conducted with R software (version 3.5.2) using the dlnm and mixmeta packages.
Main analysis
In the first stage, we performed city specific time series analyses using generalised linear models with quasi-Poisson family. In this type of regression model, to properly scale the standard deviation of the coefficients proportionally to the potential overdispersion, a quasi-likelihood is applied. This phenomenon is common in these types of data, when the variability is larger than that expected under the assumption of a Poisson distribution. We assessed short term ozone-mortality associations using unconstrained distributed lag linear models.11 21 These models account for delayed effects of time varying exposures and quantify net effects over a predefined lag period.20 For the main model, we selected lag 0-1, estimating cumulative associations with the same and previous day’s exposures. The regression model included a natural spline of time with seven degrees of freedom each year, selected based on a quasi-likelihood version of the Akaike information criterion for 4, 6, 7, 8, and 10 degrees of freedom, and indicator variables for the day of the week, to control for long term, seasonal, and weekly variations in risk. Unlike in most previous studies on ozone, we applied a stricter control for temperature by using distributed lag non-linear models, an extension of distributed lag linear models for modelling complex non-linear and lagged associations. Following modelling choices applied in published analyses, we modelled the net temperature-mortality association over lag 0-21 (see supplementary eMethods 2).22
In the second stage we pooled city specific estimates through a multilevel meta-analysis. This novel meta-analytical model defines more complex random effects that can account for variations in risk across two nested grouping levels, represented by cities within countries.19 This approach allowed the derivation of improved estimates of ozone-mortality associations at both city and country level, defined as best linear unbiased predictions. Best linear unbiased predictions borrow information across units within the same hierarchical level and can provide more accurate estimates, especially in locations with small daily mortality counts or short series. We tested the presence of heterogeneity and reported it using multilevel extensions of Cochran Q test and I2 statistic.23 Association estimates, expressed as relative risk of mortality per 10 µg/m3 increase of ozone and 95% confidence interval were derived for each country from the corresponding best linear unbiased predictions.
Risk estimates for ozone related mortality were then translated into impact measures, represented by excess mortality, following a method described elsewhere.18 Briefly, for each city we computed the daily number of deaths attributable to ozone (or daily excess deaths) using the corresponding risk estimate associated with the level of ozone in each day. Regarding the latter, we used country specific best linear unbiased predictions instead of the city specific estimates to avoid imbalances due to selection of cities and periods within each country. City specific estimates were reported as annual average number of excess deaths and 95% confidence intervals, so allowing for a proper comparison between locations with different lengths of study period. Then, country specific impacts were represented by excess mortality fractions (%) computed as the sum of the city specific daily excess deaths divided by the total mortality for each country. We used fractions instead of number of excess deaths, as excess deaths are not comparable across countries given the dependency on the denominator (ie, total mortality), which at the same time depends on the number of locations included. Although no evidence of a “safe” threshold exists, we computed associated deaths only for days with ozone levels above 70 µg/m3, as in previous health impact assessments.4 We considered this counterfactual scenario of 70 µg/m3 because ozone levels below this threshold could be mostly attributed to non-anthropogenic sources. A counterfactual scenario defined at 0 µg/m3 would not be appropriate either as it is not realistic given the ubiquitous presence of low levels of ozone derived from natural sources. We also disaggregated mortality impacts into contributions for exposure ranges above and between current air quality standards: 100 µg/m3 (WHO), 120 µg/m3 (European Union directive), 140 µg/m3 (National Ambient Air Quality Standard (NAAQS) in the US, about 0.070 parts per million), and 160 µg/m3 (Chinese Ambient Air Quality Standard (CAAQS) level 2).15
Additional complexities and sensitivity analyses
We performed a series of additional subanalyses to explore more complex features of the association, such as potential non-linearity, lagged effects, and seasonal differences. Firstly, we modelled exposure-response functions with a non-linear function consisting of a cubic B spline with internal knots at 50 µg/m3 and 60 µg/m3 of ozone. Secondly, we assessed delayed risks and potential mortality displacement by extending the lag dimension of the distributed lag linear model up to 30 days. Lag-response associations were modelled using a natural cubic spline with three internal knots placed at equally spaced lag values in the log scale. Thirdly, we assessed seasonal differences through interaction models between an indicator of season and the distributed lag linear model of ozone, as described elsewhere.24 We derived the ozone-mortality risk for the warm season (June to August in the northern hemisphere, December, January, and February in the southern hemisphere) and cold seasons (the remaining months).
Modelling choices in the main model and extensions previously described were assessed and compared through the quasi-likelihood version of the Akaike information criterion and multivariate extensions of the Wald test. For sensitivity analyses, we first assessed changes in control for time trends and the potential confounding from other air pollutants (PM10, PM2.5, and nitrogen dioxide) and relative humidity by including each of these terms separately in the model. We then assessed the exclusion of a subset of US cities with data for summer only, which were included in the main analysis, and then different modelling approaches to control for temperature. See supplementary eMethods 1 and 2 for a description of the modelling details.
Patient and public involvement
This was a multinational collaboration using aggregated city level mortality and environmental data. Patients and members of the public did not contribute to the steering committee, design, or other areas of the study, which involved complex research methods and analysis.
Results
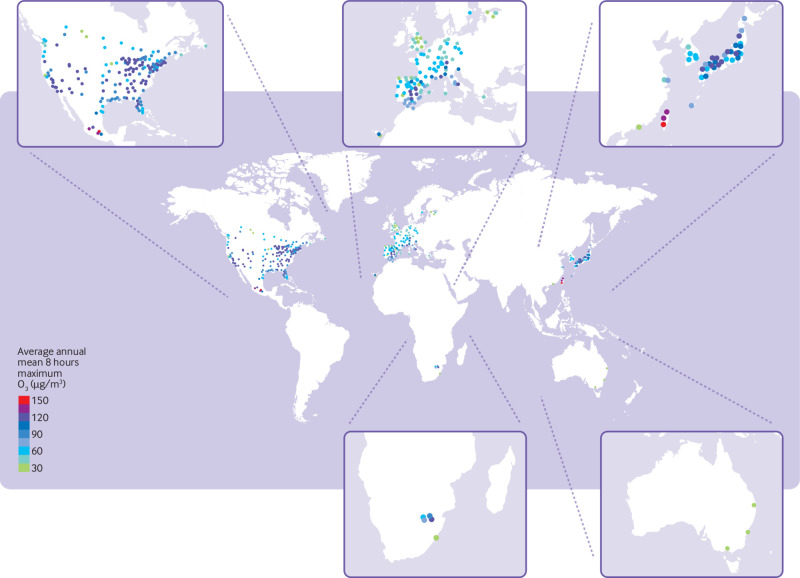
Table 1 provides a summary description of the data included for each country. A total of 45 165 171 deaths were analysed in the 406 cities, with an average time series of 13 years. Average annual mean ozone levels were widely heterogeneous across cities both between and within countries (fig 1). For example, lower levels were registered in Australian and northern European cities, whereas higher annual averages were found in some cities in the central area of the US, in Mexico, and in Taiwan. Supplementary eTable1 provides country specific descriptive summaries of the other air pollutants and humidity and eTable 2 reports the corresponding city specific descriptive results.
Table 1.
Environmental and mortality data
| Countries | No of cities | Period | No of deaths* | Median (interquartile range) No of daily deaths | Median (interquartile range) ozone level (µg/m3)† | Median (interquartile range) mean temperature (°C) |
|---|---|---|---|---|---|---|
| Australia | 3 | 2000-19 | 513 527 | 49.3 (43.7-55.7) | 31.2 (24.2-38.6) | 18.3 (14.8-21.5) |
| Canada | 26 | 1986-2011 | 2 914 630 | 12.8 (10.5-15.3) | 69.2 (53.9-88.4) | 7.3 (−1.0-15.7) |
| China | 3 | 1996-2015 | 780 655 | 87.3 (71.7-140.3) | 49.3 (27.8-77.5) | 20.4 (13.0-25.7) |
| Czech Republic | 1 | 1994-2009 | 214 062 | 36.0 (32.0-41.0) | 69.3 (47.4-95.0) | 9.2 (2.5-15.3) |
| Estonia | 4 | 2002-15 | 80 043 | 5.0 (3.5-6.5) | 48.9 (36.7-61.8) | 6.0 (0.2-13.6) |
| France | 18 | 2000-10 | 1 197 555 | 16.3 (13.7-19.1) | 67.8 (46.8-87.4) | 12.7 (7.6-17.9) |
| Germany | 12 | 1993-2015 | 3 099 176 | 30.4 (26.4-34.8) | 57.1 (35.8-79.2) | 10.5 (4.8-15.9) |
| Greece | 1 | 2001-10 | 287 969 | 78.0 (70.0-87.0) | 75.1 (52.8-97.5) | 17.9 (12.9-24.9) |
| Italy | 9 | 2006-15 | 373 421 | 15.1 (12.6-17.9) | 74.1 (50.5-97.0) | 15.8 (10.2-22.1) |
| Japan | 45 | 2011-15 | 1 856 232 | 22.3 (19.1-25.7) | 78.5 (62.4-98.4) | 16.1 (7.5-22.7) |
| Mexico | 7 | 2000-12 | 2 018 313 | 61.0 (53.7-69.4) | 108.9 (85.1-135) | 18.6 (15.9-20.5) |
| Portugal | 2 | 1997-2012 | 536 958 | 47.0 (41.0-54.0) | 64.2 (50.2-79.2) | 16.1 (12.5-19.6) |
| South Africa | 5 | 2004-13 | 924 478 | 58.4 (48.8-67.0) | 69.5 (52.9-89.5) | 18.3 (14.2-21.2) |
| South Korea | 7 | 1999-2015 | 1 662 199 | 38.3 (34.0-42.7) | 59.5 (42.7-81.9) | 15.1 (5.8-22.1) |
| Spain | 48 | 2004-14 | 1 294 162 | 6.7 (5.1-8.4) | 70.0 (53.9-84.7) | 15.3 (10.3-21.1) |
| Sweden | 1 | 1990-2010 | 201 197 | 26.0 (22.0-30.0) | 61.9 (48.9-76.0) | 6.8 (1.2-13.9) |
| Switzerland | 8 | 1995-2013 | 230 587 | 4.2 (2.9-5.6) | 72.8 (47.0-98.1) | 10.7 (4.4-16.5) |
| Taiwan | 3 | 2008-14 | 443 680 | 57.0 (51.0-63.7) | 109.1 (82.1-138.6) | 24.8 (20-28.2) |
| UK | 15 | 1993-2006 | 2 073 285 | 28.4 (24.5-32.9) | 51.6 (36.7-65.2) | 10.4 (6.5-14.6) |
| USA | 188 | 1985-2006 | 24 463 042 | 16.3 (13.6-19.3) | 80.1 (58.9-104.0) | 14.9 (7.5-21.9) |
Deaths due to non-external causes (Australia, China, Spain, Switzerland (including unintentional injuries)) or to all cause mortality (remaining countries). See supplementary eMethods 1 for a description of the data. Country specific summaries of other air pollutants and relative humidity are provided in supplementary eTable 1 and city specific descriptive summaries are reported in supplementary eTable 2.
Daily maximum eight hour mean.
Fig 1.
Geographical distribution of city specific average annual means of ozone (O3, maximum eight hour average) of 406 cities of the Multi-City Multi-Country Collaborative Research Network included in the study
On average, each 10 µg/m3 increase in ozone was associated with an overall relative risk of mortality of 1.0018 (95% confidence interval 1.0012 to 1.0024) (fig 2). Some heterogeneity was found across country and city specific risks (I2=29.8%, Cochran Q P<0.001). Larger risk estimates were found in the UK (1.0035 (1.0024 to 1.0046)), South Africa (1.0027 (1.0013 to 1.0042)), Estonia (1.0023 (1.0006 to 1.0040)), and Canada (1.0023 (1.0013 to 1.0032)), whereas Australia, China, the Czech Republic, France, Germany, Italy, Japan, South Korea, Sweden, Switzerland, and the US showed similar risks, ranging between 1.0014 and 1.0020. Lower and imprecise associations were estimated for Greece (1.0011 (0.9995 to 1.0028)), Mexico (1.0008 (1.000 to 1.0015)), Portugal (1.0011 (0.9997 to 1.0026)), Spain (1.0006 (0.9992 to 1.0019)), and Taiwan (1.0010 (0.9999 to 1.0021)). Supplementary eFigure 1 provides the corresponding figures with the relative risks for an increase in 10 parts per billion of ozone.
Fig 2.
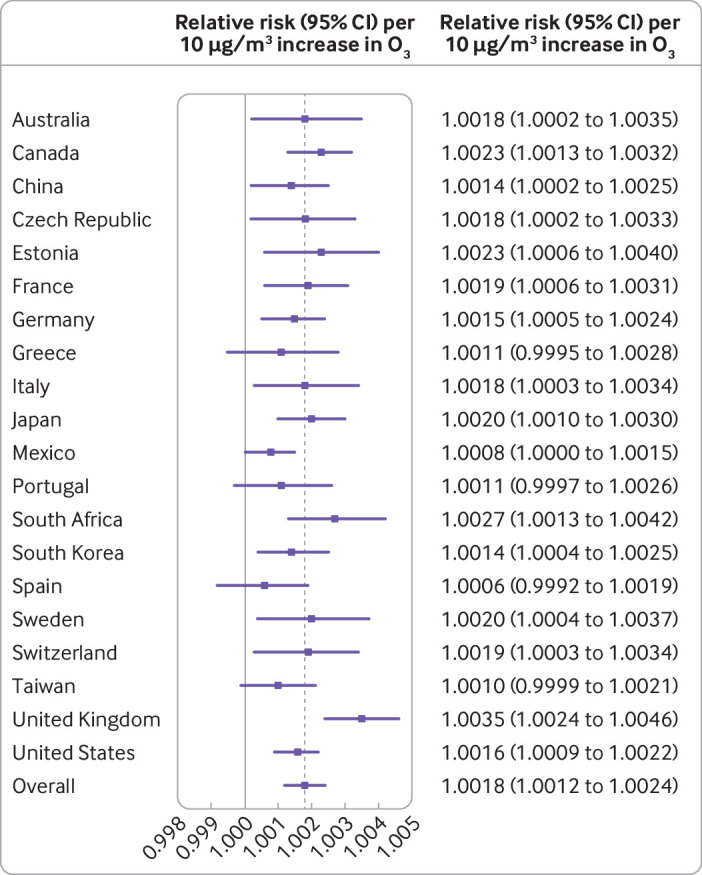
Overall and country specific short term ozone-mortality association, expressed as relative risk per 10 µg/m3 increase in ozone (O3, maximum eight hour average) (lag 01)
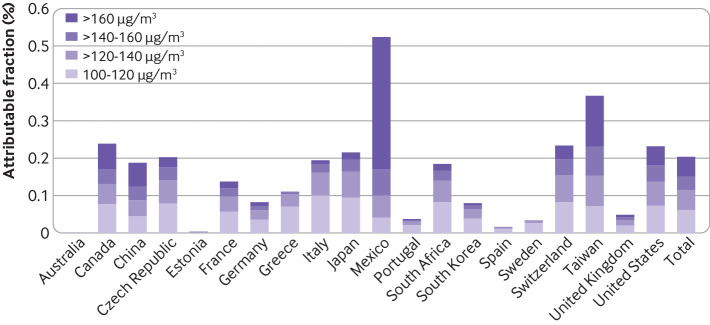
Figure 3 depicts the excess mortality fractions above the WHO guideline and their distribution across intervals between the other air quality standards for each country, whereas supplementary eTable 3 and eTable 4 report the corresponding figures for excess fractions for total ozone (>70 µg/m3) and above and between air quality standards. Table 2 shows fractions and annual number of excess deaths associated with ozone for the total range of exposure and above the WHO guideline for a selection of the main cities in each country and overall across the 406 locations (supplementary eTable5 shows the estimates for all cities). Total mortality associated with ozone greater than 70 µg/m3 accounted for 0.26% of deaths (95% confidence interval 0.24% to 0.28%), which translates into 8203 annual excess deaths (95% confidence interval 3525 to 12 840) across the 406 locations studied (table 2). A substantial residual excess mortality of 0.20% (95% confidence interval 0.18% to 0.22%) corresponding to 6262 (95% confidence interval 1413 to 11 065) annual excess deaths remained when restricting to days with levels above the WHO guideline of 100 µg/m3. This proportion varied greatly by country, with considerably larger fractions in Mexico (0.52% (0.14% to 0.92%)) and Taiwan (0.37% (0.08% to 0.64%)) (fig 3, supplementary eTable 3). A mortality excess around 0.20% was estimated in Canada, China, Italy, Japan, South Africa, Switzerland, and the US, whereas France, Germany, South Korea, and the UK reported smaller percentages, ranging between 0.14% and 0.05% (fig 3, supplementary eTable 3). Imprecise or almost null estimates were found in the Czech Republic, Estonia, Greece, Portugal, Spain, and Sweden (supplementary eTable 3). Overall mortality fractions above more lenient air quality standards (ie, the European Union, NAAQS, and CAAQS) decreased progressively to 0.14%, 0.09%, and 0.05%, respectively (supplementary eTable 3). Only Mexico reported a considerably higher fraction, of 0.35% above the highest air quality standards of 160 µg/m3, although this finding was highly uncertain (black bar in fig 3, supplementary eTable 3). Null excess deaths were found in Australia, as daily exposure levels were all below 70 µg/m3. A similar pattern was found across estimates for the main cities in each country (table 2). A substantial number of annual excess deaths were associated with ozone levels above the WHO guideline—namely, 694 (95% confidence interval 22 to 1317) in the Valley of Mexico, 211 (112 to 307) in Los Angeles, 170 (40 to 304) in Tokyo, 128 (59 to 197) in Toronto, 82 (19 to 148) in Johannesburg, 48 (0 to 96) in Paris, and 37 (15 to 57) in London (table 2). Supplementary eTable 5 shows the corresponding estimates for the 406 cities.
Fig 3.
Overall and country specific excess mortality (%) associated with ozone by specific ranges defined between thresholds consistent with current air quality standards. (No excess mortality associated with ozone was found in Australia, as daily ozone levels were below the maximum background level of 70 µg/m3). 100 µg/m3, World Health Organization guideline; 120 µg/m3, European Union directive; 140 µg/m3 (about 0.070 parts per million); National Ambient Air Quality Standard (NAAQS) in the US; 160 µg/m3 Chinese Ambient Air Quality Standard (CAAQS)
Table 2.
Excess mortality associated with ozone for total (>70 µg/m3) and above World Health Organization guideline of 100 µg/m3 in main cities of each participating country and overall estimates for the 406 cities
| Countries | Cities | Total (>70 µg/m3)* | Above WHO guideline (100 µg/m3) | |||
|---|---|---|---|---|---|---|
| % Excess fraction (95% CI) | No of annual excess deaths (95% CI) | % Excess fraction (95% CI) | No of annual excess deaths (95% CI) | |||
| Australia† | Sydney | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | |
| Canada | Toronto | 0.59 (0.34 to 0.85) | 159 (90 to 228) | 0.48 (0.22 to 0.73) | 128 (59 to 197) | |
| China | Shanghai | 0.32 (0.04 to 0.57) | 117 (15 to 209) | 0.27 (−0.01 to 0.53) | 99 (−4 to 195) | |
| Czech Republic | Prague | 0.27 (0.02 to 0.48) | 38 (3 to 69) | 0.20 (−0.06 to 0.44) | 29 (−9 to 63) | |
| Estonia | Tallinn | 0.01 (0.00 to 0.02) | 1 (0 to 1) | 0.00 (−0.01 to 0.01) | 0 (−1 to 1) | |
| France | Paris | 0.15 (0.05 to 0.26) | 70 (24 to 119) | 0.11 (0.00 to 0.21) | 48 (0 to 96) | |
| Germany | Berlin | 0.12 (0.04 to 0.20) | 46 (14 to 74) | 0.08 (−0.01 to 0.17) | 30 (−3 to 62) | |
| Greece | Athens | 0.16 (−0.07 to 0.41) | 52 (−23 to 132) | 0.11 (−0.13 to 0.37) | 35 (−42 to 117) | |
| Italy | Rome | 0.27 (0.05 to 0.52) | 69 (13 to 132) | 0.19 (−0.05 to 0.44) | 48 (−12 to 111) | |
| Japan | Tokyo | 0.27 (0.14 to 0.40) | 249 (127 to 371) | 0.18 (0.04 to 0.32) | 170 (40 to 304) | |
| Mexico | Valley of Mexico | 0.73 (0.04 to 1.38) | 707 (39 to 1,339) | 0.72 (0.02 to 1.36) | 694 (22 to 1,317) | |
| Portugal | Lisbon | 0.09 (−0.03 to 0.2) | 20 (−6 to 45) | 0.04 (−0.09 to 0.17) | 9 (−20 to 39) | |
| South Africa | City of Johannesburg | 0.32 (0.15 to 0.49) | 121 (59 to 187) | 0.22 (0.05 to 0.39) | 82 (19 to 148) | |
| South Korea | Seoul | 0.10 (0.03 to 0.17) | 41 (13 to 71) | 0.06 (−0.01 to 0.14) | 27 (−3 to 58) | |
| Spain | Madrid | 0.03 (−0.04 to 0.11) | 9 (−12 to 31) | 0.01 (−0.07 to 0.10) | 3 (−21 to 27) | |
| Sweden | Stockholm | 0.10 (0.02 to 0.18) | 10 (2 to 18) | 0.03 (−0.07 to 0.13) | 3 (−7 to 13) | |
| Switzerland | Zurich | 0.31 (0.05 to 0.54) | 13 (2 to 22) | 0.23 (−0.02 to 0.48) | 10 (−1 to 20) | |
| Taiwan | Taipei | 0.34 (−0.05 to 0.72) | 131 (−21 to 276) | 0.28 (−0.11 to 0.67) | 109 (−43 to 258) | |
| UK | London | 0.10 (0.07 to 0.12) | 63 (44 to 81) | 0.06 (0.02 to 0.09) | 37 (15 to 57) | |
| USA | Los Angeles | 0.41 (0.24 to 0.57) | 242 (142 to 335) | 0.36 (0.19 to 0.52) | 211 (112 to 307) | |
| 20 MCC countries‡ | 406 MCC cities | 0.26 (0.24 to 0.28) | 8,203 (3,525 to 12 840) | 0.20 (0.18 to 0.22) | 6,262 (1,413 to 11 065) | |
Total refers to ozone related deaths when levels above 70 µg/m3 (defined as maximum background levels).
No excess mortality associated with ozone were found in Australia, as daily ozone levels were below the maximum background level set up at 70 µg/m3.
Countries contributing to the Multi-City Multi-Country (MCC) Collaborative Research Network included in the present study.
Additional analyses suggested no evidence of non-linearity in the concentration-response association (according to the quasi-likelihood version of the Akaike information criterion) (supplementary eFigure 2). The assessment of the lagged associations confirmed an immediate ozone-mortality association during the first week. However, lag specific estimates below 1 were found after the second week, which resulted in a slightly lower overall cumulative association of 1.0015 (95% confidence interval 0.9991 to 1.0032) when considering the delayed effects over the first 30 days after the exposure. Finally, no evidence of seasonal differences in ozone-mortality association were found (warm season: 1.0012 (95% confidence interval 1.000 to 1.0026); cold season: 1.0015% (1.0006 to 1.0024), Wald test P=0.37).
Results from sensitivity analyses suggest that risk estimates of the main analysis were robust to the different modelling choices related to the control for time trends and adjustment by the three air pollutants and humidity (supplementary eTable 6). However, ozone-mortality risk estimates seemed to be sensitive to the approach to control for temperature (supplementary eFigure 3). We found larger ozone-mortality association estimates using less stringent control, although quasi-likelihood Akaike information criterion values suggested that the model with distributed lag non-linear model of temperature (main model) provided the best fit.
Discussion
On average, this study found an overall short term ozone-mortality association of 1.0018 (95% confidence interval 1.0012 to 1.0024) per 10 µg/m3 increase in ozone. This evidence is supported by previous epidemiological and experimental studies suggesting several pathophysiological mechanisms (e.g. systemic inflammation, haemostatic alterations).25 26 Larger associations were found in previous multi-country studies, including a subset of countries investigated here (eg, relative risk of 1.0022 in APHEA (Air Pollution and Health: A European Approach), 1.0026 in APHENA (Air Pollution and Health: A Combined European and North American Approach), per 10 µg/m3 increase),11 21 or single country studies (eg, relative risk of 1.0025 in the US (originally 1.0052 per 10 parts per billion increase), and China 0.55% per 10 µg/m3 increase, and 1.015 in Italy).27 28 29 Differences in the definition of the exposure variable (eg, moving average, single lag) and modelling approach could explain these discrepancies in the magnitude of the association. For example, compared with previous studies, we applied a stronger control for temperature (ie, distributed lag non-linear models), fully accounting for non-linearity and lagged temperature-mortality associations.22 In fact, results from sensitivity analyses are consistent with previous findings showing that ozone-mortality risk estimates were sensitive to the modelling strategy to control for temperature, reporting larger risks when using simpler approaches (supplementary eFigure 3).27 Moreover, one of the novelties of the applied statistical framework is the use of multilevel meta-analytical models in the second stage, properly accounting for heterogeneity across cities and countries.
Our results showed important differences in the ozone-mortality association across countries. For example, while some areas such as UK, South Africa, Canada, and Estonia reported the largest risk estimates above 1.0020, smaller or imprecise estimates below 1.0011 were found in Greece, Mexico, Spain, and Taiwan. This unclear pattern would suggest that although several community level factors have been proposed as potential modifiers in single country studies (eg, population characteristics), these might not fully characterise differences between countries.30 Future multi-country studies are needed to provide further evidence on the factors defining the level of vulnerability of a population to air pollution.
This study also provides evidence on the potential public health benefits of stricter clean air policies. In particular, we found that 0.20% excess mortality, which translates into more than 6000 deaths each year, related to short term exposure to ozone could have been avoided if ambient levels were below the WHO guideline of 100 µg/m3 in the 406 cities included in the study. Recent reviews found that most of the current air quality standards do not comply with the WHO air quality guideline,15 and that 80% of the world’s population in urban areas are exposed to air pollution levels above this threshold.31 Moreover, an additional 0.06% of excess deaths is associated with ozone levels between 70 µg/m3 and 100 µg/m3. These findings support the WHO initiative of encouraging countries to reconsider current air quality standards and enforce stronger emission restrictions and other public health interventions to meet its recommendations. Additionally, our results have important implications for healthcare practice. Apart from the implementation of clean air policies, individual strategies to reduce personal exposure to air pollutants are also desirable.32 In this regard, clinicians play an important role in counselling patients with potentially a higher susceptibility to adverse health outcomes related to air pollution. For example, professionals can advise sensitive individuals to stay indoors or avoid doing exercise during episodes of high ambient ozone.
Previous studies showed that important health benefits could be achieved if reductions of ozone levels are reached.9 13 16 However, in this multi-country study we compared excess mortality estimates across air quality standards and countries, providing additional insights on specific areas with more urgent need of further interventions. For example, we found that 0.52% of total mortality in Mexico was associated with ozone above the WHO limit, the largest mortality fraction among the studied countries. This was associated with the highest ozone levels registered in the Mexican cities, especially above the 160 µg/m3 limit, which is close to its current air quality standards of 156 µg/m3. This means that attaining the current lenient standards would prevent a substantial proportion of ozone related deaths in this country. In contrast, results for the UK show a lower mortality fraction, despite the strongest ozone-mortality association, owing to the lower ozone levels registered in this country.
Strengths and limitations of this study
This large epidemiological investigation on short term ozone-mortality associations included almost 50 million deaths from 406 cities in 20 countries from different regions across the world. Given its large sample size and wide geographical coverage, we were able to obtain consistent evidence of an association between short term exposure to ozone and total mortality. In addition, we provided ozone related impact estimates, quantified as excess mortality, across different air quality standards, countries and cities, providing evidence with important public health implications.
We were able to explore additional complexities of the association by taking advantage of the large statistical power and advanced statistical techniques. Firstly, our results support the conclusions of previous studies on a generally linear concentration-response functions, with no indication of threshold.9 27 Secondly, we found evidence of a potential mortality displacement in the third and fourth week after the exposure. A similar lag pattern has been previously observed.10 11 However, potential mechanisms explaining this delayed and sustained pattern remain unclear. Finally, we found no evidence of seasonal differences in the ozone-mortality association. Previous multi-site studies have provided conflicting results, with larger risks in cold seasons in Asia27 and in warm seasons in the US and Europe.6 Further analyses are warranted to characterise different patterns across regions.
This study has some limitations. Firstly, our results should not be considered truly global estimates, because several areas of the world such as South America, Africa, and the Middle East are unrepresented or were not assessed. In addition, the reported nationwide results might not be representative of the true impacts for some countries with a limited number of cities included in the study (eg, Sweden, Czech Republic, China). In particular, the estimated number of total excess deaths attributed to ozone should be interpreted as the sum of impacts in the 406 observed locations and not as total estimates across the 20 countries. Although excess fractions could be considered proper representations of the impacts for each country, the total excess number of deaths for each country is highly dependent on the total mortality considered in the study—that is, the number of locations included in each country. Systematic differences could also exist between countries in the characteristics of monitors (type, proximity to study area), study area boundaries, temporal coverage, data processing before data collection, and the collection of mortality data (eg, case ascertainment, codification). However, we ensured that the data fulfilled a minimum set of requirements for quality, a similar definition for the eight hour maximum metric, and location of the monitor (ie, within the study area or close enough to ensure its representativeness). Risks and impact estimates were only reported for total mortality (ie, deaths due to all or non-external causes) and we did not seek to identify the sources of heterogeneity of the results across countries. We acknowledge that the applied approach prevents us from understanding the potential mechanisms or differential susceptibility of the population, together with contextual differences across locations. Further studies are warranted to clarify this complex research question, including, for example, cause specific mortality and morbidity, and more complex two stage analyses. Finally, although the risk estimates were small they apply to the whole population, thus translating into substantial mortality impacts as shown in our estimates of excess mortality. By the same token, owing to the nature of the study design (time series analysis) the obtained excess mortality estimates refer to transient impact measures and not to the mortality burden or person years of life lost attributed to chronic exposure to ozone.33
Conclusions
This large multi-country study provided evidence on the short term association between ozone and mortality. We also show that clean air policies with the enactment of air quality standards can constitute essential public health tools to minimise the health burden. In particular, our results suggest that ozone related health impacts can be largely preventable by attaining effective air quality standards in line with the WHO guideline. Moreover, interventions to further reduce ozone pollution would provide additional health benefits, even in regions that meet current regulatory standards and guidelines. These findings have important implications for the design of future public health actions; particularly, for example, in relation to the implementation of mitigation strategies to reduce the impacts of climate change.
What is already known on this topic
Studies on the short term association between ground level ozone and mortality have been mostly performed in a few locations, in limited geographical areas, and using various designs and modelling approaches
Although most of the studies found positive associations, results are heterogeneous, and a critical comparison across different countries and regions is made difficult by the limited statistical power and differences across studies
Estimates of the association are usually reported as relative risks, a summary measure that does not quantify the actual health impact and makes it difficult to evaluate comparative health benefits of different regulatory limits
What this study adds
This large multi-country study found increased mortality risks associated with exposure to ozone across locations and countries, with an average 0.18% per 10 µg/m3, reinforcing the evidence of a potential causal association
Risk estimates were translated in measures of excess mortality, and it was found that more than 6000 deaths each year, corresponding to 0.20% of the total mortality, would have been avoided in the 406 cities studied if countries had implemented stricter air quality standards compliant with the WHO guideline
Moreover, smaller but still substantial mortality impacts were found below WHO guideline, supporting the WHO initiative of encouraging countries to revisit current air quality guidelines and enforcing stronger emission restrictions to meet these recommendations
Acknowledgments
We thank Meltem Kutlar (Swiss Tropical and Public Health Institute, Basel; University of Basel, Basel, Switzerland), responsible for the LUDOK database (funded by Swiss Federal Office for the Environment) for her support during the literature review.
Web extra.
Extra material supplied by authors
Supplementary information: methods, e-tables 1-6, and e-figures 11-3
Contributors: AG and HK are senior authors and contributed equally to this work. AG, YG, MH, and BA set up the collaborative network. AMV-C, AG, FS, and HK designed the study. AMV-C coordinated the work and took the lead in drafting the manuscript and interpreting the results. AG and FS developed the statistical methods. AMV-C conducted the statistical analysis. BA, AH, FS, AG, KK, ES, MS, AT, CI, VH, AS, JS, NS, RG, and EL provided substantial scientific input in interpreting the results and drafting the manuscript. CL, AM, YG, ST, EL, JK, AU, HO, EI, MP, VH, AS, KK, ES, MS, MS, MH, YH, CFSN, MH, JC, SS, JM, NS, RG, HK, AT, CI, BF, CA, MSR, MR, YLLG, BYC, AZ, JS, MB, and HK provided the data and contributed to the interpretation of the results and to the submitted version of the manuscript. AMV-C, AG, and HK are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work was primarily supported by the UK Medical Research Council (MR/M022625/1 and MR/R013349/1) and by the UK Natural Environment Research Council (NE/R009384/1). HaK was supported by the National Natural Science Foundation of China (91843302 and 91643205) and China Medical Board Collaborating Program (16-250). JM was supported by the Fundação para a Ciência e a Tecnologia (FCT) through the scholarship SFRH/BPD/115112/2016. VH was supported by the Spanish Ministry of Economy, Industry and Competitiveness (MINECO, PCIN-2017-046) and the German Federal Ministry of Education and Research (BMBF, 01LS1201A2). AU and JK were supported by the Czech Science Foundation (18-22125S). HO and EI were supported by the Estonian Ministry of Education and Research (IUT34-17). AT was supported by the Japanese Society for the Promotion of Science invitational fellowships for research in Japan (S18149). YG was supported by the career development fellowship of the Australian National Health and Medical Research Council (APP1107107 and APP1163693). ST was supported by the Science and Technology Commission of Shanghai Municipality (18411951600). HoK was supported by the Global Research Laboratory (#K21004000001-10A0500-0710) through the National Research Foundation of Korea and by the Future Planning and Korea Ministry of Environment as the “Climate Change Correspondence R&D Program” (2013001310002). RMG was supported by a CSIR parliamentary grant. NS is supported by the National Institute of Environmental Health Sciences funded HERCULES Centre (P30ES019776). The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of this manuscript.
This publication was developed under assistance agreement No RD835871 awarded by the US Environmental Protection Agency to Yale University (MLB). It has not been formally reviewed by EPA. The views expressed in this document are solely those of the authors of the publication and do not necessarily reflect those of the Agency. EPA does not endorse any products or commercial services mentioned in this publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from UK Medical Research Council, China Medical Board Collaborating Program, Fundação para a Ciência e a Tecnologia, Spanish Ministry of Economy, Industry and Competitiveness, German Federal Ministry of Education and Research, Czech Science Foundation, Estonian Ministry of Education and Research, Japanese Society for the Promotion of Science, Australian National Health and Medical Research Council, Science and Technology Commission of Shanghai Municipality, Global Research Laboratory, through the National Research Foundation of Korea, Future Planning and Korea Ministry of Environment, CSIR parliamentary grant, and the National Institute of Environmental Health Sciences funded HERCULES Centre; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: Data have been collected within the MCC (Multi-City Multi-Country) Collaborative Research Network (http://mccstudy.lshtm.ac.uk/) under a data sharing agreement and cannot be made publicly available. Researchers can refer to MCC participants listed as coauthors for information on accessing the data for each country. The R code for the analysis is available from the corresponding author.
The lead authors (AG and HK) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Dissemination of the findings will be carried out through press releases by the research institutions of the contributing authors.
Publisher’s note: Published maps are provided without any warranty of any kind, either express or implied. BMJ remains neutral with regard to jurisdictional claims in published maps.
References
- 1. Malig BJ, Pearson DL, Chang YB, et al. A Time-Stratified Case-Crossover Study of Ambient Ozone Exposure and Emergency Department Visits for Specific Respiratory Diagnoses in California (2005-2008). Environ Health Perspect 2016;124:745-53. 10.1289/ehp.1409495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Samet JM, Zeger SL, Dominici F, et al. The National Morbidity, Mortality, and Air Pollution Study. Part II: Morbidity and mortality from air pollution in the United States. Res Rep Health Eff Inst 2000;94:5-70, discussion 71-9. [PubMed] [Google Scholar]
- 3.EPA. Integrated Science Assessment for Ozone and Related Photochemical Oxidants. https://www.epa.gov/isa/integrated-science-assessment-isa-ozone-and-related-photochemical-oxidants (accessed 10 Sep 2019).
- 4.Health risks of air pollution in Europe - HRAPIE. Health risks of air pollution in Europe –HRAPIE project. http://www.euro.who.int/__data/assets/pdf_file/0006/238956/Health_risks_air_pollution_HRAPIE_project.pdf?ua=1,%20GBD (accessed 12 December 2018).
- 5. Orru H, Ebi KL, Forsberg B. The Interplay of Climate Change and Air Pollution on Health. Curr Environ Health Rep 2017;4:504-13. 10.1007/s40572-017-0168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Katsouyanni K, Samet JM, Anderson HR, et al. HEI Health Review Committee Air pollution and health: a European and North American approach (APHENA). Res Rep Health Eff Inst 2009;(142):5-90. [PubMed] [Google Scholar]
- 7. Romieu I, Gouveia N, Cifuentes LA, et al. HEI Health Review Committee Multicity study of air pollution and mortality in Latin America (the ESCALA study). Res Rep Health Eff Inst 2012;171:5-86. [PubMed] [Google Scholar]
- 8. Wong C-M, Vichit-Vadakan N, Kan H, Qian Z. Public Health and Air Pollution in Asia (PAPA): a multicity study of short-term effects of air pollution on mortality. Environ Health Perspect 2008;116:1195-202. 10.1289/ehp.11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bell ML, Peng RD, Dominici F. The exposure-response curve for ozone and risk of mortality and the adequacy of current ozone regulations. Environ Health Perspect 2006;114:532-6. 10.1289/ehp.8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zanobetti A, Schwartz J. Mortality displacement in the association of ozone with mortality: an analysis of 48 cities in the United States. Am J Respir Crit Care Med 2008;177:184-9. 10.1164/rccm.200706-823OC. [DOI] [PubMed] [Google Scholar]
- 11. Samoli E, Zanobetti A, Schwartz J, et al. The temporal pattern of mortality responses to ambient ozone in the APHEA project. J Epidemiol Community Health 2009;63:960-6. 10.1136/jech.2008.084012. [DOI] [PubMed] [Google Scholar]
- 12. Pascal M, Corso M, Chanel O, et al. Aphekom group Assessing the public health impacts of urban air pollution in 25 European cities: results of the Aphekom project. Sci Total Environ 2013;449:390-400. 10.1016/j.scitotenv.2013.01.077. [DOI] [PubMed] [Google Scholar]
- 13. Hubbell BJ, Hallberg A, McCubbin DR, Post E. Health-related benefits of attaining the 8-hr ozone standard. Environ Health Perspect 2005;113:73-82. 10.1289/ehp.7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cohen AJ, Ross Anderson H, Ostro B, et al. The global burden of disease due to outdoor air pollution. J Toxicol Environ Health A 2005;68:1301-7. 10.1080/15287390590936166. [DOI] [PubMed] [Google Scholar]
- 15. Kutlar Joss M, Eeftens M, Gintowt E, Kappeler R, Künzli N. Time to harmonize national ambient air quality standards. Int J Public Health 2017;62:453-62. 10.1007/s00038-017-0952-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berman JD, Fann N, Hollingsworth JW, et al. Health benefits from large-scale ozone reduction in the United States. Environ Health Perspect 2012;120:1404-10. 10.1289/ehp.1104851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lehtomäki H, Korhonen A, Asikainen A, et al. Health Impacts of Ambient Air Pollution in Finland. Int J Environ Res Public Health 2018;15:E736. 10.3390/ijerph15040736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gasparrini A, Leone M. Attributable risk from distributed lag models. BMC Med Res Methodol 2014;14:55. 10.1186/1471-2288-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sera F, Armstrong B, Blangiardo M, Gasparrini A. An extended mixed-effects framework for meta-analysis. Stat Med 2019;38:5429-44. 10.1002/sim.8362 [DOI] [PubMed] [Google Scholar]
- 20. Gasparrini A, Armstrong B. Reducing and meta-analysing estimates from distributed lag non-linear models. BMC Med Res Methodol 2013;13:1. 10.1186/1471-2288-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peng RD, Samoli E, Pham L, et al. Acute effects of ambient ozone on mortality in Europe and North America: results from the APHENA study. Air Qual Atmos Health 2013;6:445-53. 10.1007/s11869-012-0180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gasparrini A, Guo Y, Hashizume M, et al. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet 2015;386:369-75. 10.1016/S0140-6736(14)62114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gasparrini A, Armstrong B, Kenward MG. Multivariate meta-analysis for non-linear and other multi-parameter associations. Stat Med 2012;31:3821-39. 10.1002/sim.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gasparrini A, Guo Y, Hashizume M, et al. Temporal Variation in Heat-Mortality Associations: A Multicountry Study. Environ Health Perspect 2015;123:1200-7. 10.1289/ehp.1409070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Green R, Broadwin R, Malig B, et al. Long- and Short-term Exposure to Air Pollution and Inflammatory/Hemostatic Markers in Midlife Women. Epidemiology 2016;27:211-20. 10.1097/EDE.0000000000000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Day DB, Xiang J, Mo J, et al. Association of Ozone Exposure With Cardiorespiratory Pathophysiologic Mechanisms in Healthy Adults. JAMA Intern Med 2017;177:1344-53. 10.1001/jamainternmed.2017.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen K, Zhou L, Chen X, Bi J, Kinney PL. Acute effect of ozone exposure on daily mortality in seven cities of Jiangsu Province, China: No clear evidence for threshold. Environ Res 2017;155:235-41. 10.1016/j.envres.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bell ML, McDermott A, Zeger SL, Samet JM, Dominici F. Ozone and short-term mortality in 95 US urban communities, 1987-2000. JAMA 2004;292:2372-8. 10.1001/jama.292.19.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stafoggia M, Forastiere F, Faustini A, et al. EpiAir Group Susceptibility factors to ozone-related mortality: a population-based case-crossover analysis. Am J Respir Crit Care Med 2010;182:376-84. 10.1164/rccm.200908-1269OC. [DOI] [PubMed] [Google Scholar]
- 30. Madrigano J, Jack D, Anderson GB, Bell ML, Kinney PL. Temperature, ozone, and mortality in urban and non-urban counties in the northeastern United States. Environ Health 2015;14:3. 10.1186/1476-069X-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization (WHO) Global Urban Ambient Air Pollution Dataset. (Accessed 12 December 2018). https://www.who.int/phe/health_topics/outdoorair/databases/cities/en/.
- 32. Künzli N, Rapp R, Perez L. “Breathe Clean Air”: the role of physicians and healthcare professionals. Breathe (Sheff) 2014;10:214-29 10.1183/20734735.103114. [DOI] [Google Scholar]
- 33. McMichael AJ, Anderson HR, Brunekreef B, Cohen AJ. Inappropriate use of daily mortality analyses to estimate longer-term mortality effects of air pollution. Int J Epidemiol 1998;27:450-3. 10.1093/ije/27.3.450 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: methods, e-tables 1-6, and e-figures 11-3