Abstract
Objective
To investigate the association of macronutrient intake with all cause mortality and cardiovascular disease (CVD), and the implications for dietary advice.
Design
Prospective population based study.
Setting
UK Biobank.
Participants
195 658 of the 502 536 participants in UK Biobank completed at least one dietary questionnaire and were included in the analyses. Diet was assessed using Oxford WebQ, a web based 24 hour recall questionnaire, and nutrient intakes were estimated using standard methodology. Cox proportional models with penalised cubic splines were used to study non-linear associations.
Main outcome measures
All cause mortality and incidence of CVD.
Results
4780 (2.4%) participants died over a mean 10.6 (range 9.4-13.9) years of follow-up, and 948 (0.5%) and 9776 (5.0%) experienced fatal and non-fatal CVD events, respectively, over a mean 9.7 (range 8.5-13.0) years of follow-up. Non-linear associations were found for many macronutrients. Carbohydrate intake showed a non-linear association with mortality; no association at 20-50% of total energy intake but a positive association at 50-70% of energy intake (3.14 v 2.75 per 1000 person years, average hazard ratio 1.14, 95% confidence interval 1.03 to 1.28 (60-70% v 50% of energy)). A similar pattern was observed for sugar but not for starch or fibre. A higher intake of monounsaturated fat (2.94 v 3.50 per 1000 person years, average hazard ratio 0.58, 0.51 to 0.66 (20-25% v 5% of energy)) and lower intake of polyunsaturated fat (2.66 v 3.04 per 1000 person years, 0.78, 0.75 to 0.81 (5-7% v 12% of energy)) and saturated fat (2.66 v 3.59 per 1000 person years, 0.67, 0.62 to 0.73 (5-10% v 20% of energy)) were associated with a lower risk of mortality. A dietary risk matrix was developed to illustrate how dietary advice can be given based on current intake.
Conclusion
Many associations between macronutrient intake and health outcomes are non-linear. Thus dietary advice could be tailored to current intake. Dietary guidelines on macronutrients (eg, carbohydrate) should also take account of differential associations of its components (eg, sugar and starch).
Introduction
Achieving important and sustained changes in lifestyle requires clear, consistent public health messages. The general public, however, has been bombarded with confusing and conflicting recommendations on diet. These are due in part to presumptions that the associations between macronutrients and health outcomes are linear across the range of consumption and hold true irrespective of the level of intake of other macronutrients and total energy consumption. Also, dietary recommendations have often focused on single macronutrients in isolation without taking into account the impact of other concurrent macronutrients.
The focus for dietary recommendations has included fat, saturated fat, carbohydrate, and sugar.1 Historical advice to reduce consumption of saturated fat was challenged by recent studies, including meta-analyses of prospective studies,2 3 which led the UK Scientific Advisory Committee on Nutrition (SACN)4 and World Health Organization5 to conclude, based on evidence from both randomised controlled trials and prospective cohort studies, that saturated fat intake is not associated with cardiovascular mortality. At the same time, however, SACN also found inconsistency between randomised controlled trials and prospective cohort studies on the association between saturated fat intake and cardiovascular events (including ischaemic heart disease, cerebrovascular disease, and peripheral vascular disease).4 Whereas randomised controlled trials found that reducing intake of saturated fat could reduce cardiovascular events,6 meta-analyses of prospective cohort studies did not identify any associations.2
More recently, focus has switched to promoting low carbohydrate diets, such as the Atkins diet. Although early evidence suggested such diets were effective in reducing weight,7 8 the more recent Diet Intervention Examining The Factors Interacting with Treatment Success (DIETFITS) trial and a meta-analysis showed no additional benefit compared with low fat diets.9 10 The Prospective Urban Rural Epidemiology (PURE) study reported higher mortality among people consuming high carbohydrate, low fat diets.11 Its models suggested that replacement of carbohydrate with polyunsaturated fat might be associated with a lower risk of mortality and that replacement with saturated fat might be associated with a lower risk of stroke. The Atherosclerosis Risk in Communities (ARIC) study and accompanying meta-analysis also suggested that replacement of carbohydrate with plant, but not animal derived, protein or fat was associated with lower mortality.12 The isocaloric replacement analyses undertaken by PURE and ARIC assumed linearity between macronutrient intake and health outcome, implying that the effect of replacing macronutrients was independent of current levels of intake. Furthermore, in common with most previous studies, PURE and ARIC analysed total carbohydrate rather than its components, sugar, starch, and fibre, which have different associations with mortality and morbidity.13 For example, higher sugar consumption (in sugar sweetened beverages) is associated with greater risk of diabetes, whereas starch is not.14
To deal with these limitations, we used data from the UK Biobank cohort to examine the associations between macronutrients and their components and all cause mortality and cardiovascular disease (CVD); to conduct non-linear isocaloric replacement analyses; and to develop a dietary risk matrix to illustrate how conditional advice can be given.
Methods
Study design
Between 2007 and 2010, UK Biobank recruited 502 536 participants aged 37-73 years from the general population. Participants attended one of 22 assessment centres across England, Scotland, and Wales where they completed a self-administered, touch screen questionnaire and face-to-face interview. Trained staff took a series of measurements, including height, weight, and blood pressure. Ethnicity, smoking status, and alcohol intake were self-reported. Comorbidities and medical history were based on self-report of a doctor diagnosis and verified during the face-to-face interview. Townsend area deprivation index was derived from postcode of residence using aggregated data on unemployment, car and home ownership, and household overcrowding.15 Physical activity level over a typical week was self-reported using the validated international physical activity questionnaire, and the total metabolic equivalent of task (MET) in a week was used in analysis.16 Height was measured to the nearest centimetre using a stadiometer (Seca 202; Hamburg, Germany) and body weight was measured to the nearest 0.1 kg using a body composition analyser (Tanita BC-418; Tokyo, Japan). Body mass index (BMI) was calculated as weight (kg)/(height (m)2 and classified into six categories using WHO criteria: underweight (<18.5), normal weight (18.5 to <25), overweight (25 to <30), obese (30 to <35), obesity class 2 (35 to <40), and obesity class 3 (≥40). Only the assessment at baseline was used in this study.
Dietary information was collected using the Oxford WebQ (www.ceu.ox.ac.uk/research/oxford-webq), a web based 24 hour recall questionnaire developed for use in large population studies. UK Biobank participants were invited to complete the Oxford WebQ on five occasions over five years, and we calculated mean values from the available data. The Oxford WebQ has been validated against an interviewer administered 24 hour dietary recall, producing a mean Spearman correlation coefficient for macronutrients of 0.62 (range 0.54-0.69).17 We estimated nutrient intake using McCance and Widdowson’s Composition of Foods 18 and fibre intake using the Englyst method.19 The intake of monounsaturated fatty acids (MUFA) was calculated by subtracting the intake of saturated fatty acids (SFA) and polyunsaturated fatty acids (PUFA) from total fat intake. The primary exposure variables were the percentages of total energy intake derived from the three macronutrients (carbohydrate, fat, and protein) as well as their components (total sugar, starch, and fibre, and MUFA, PUFA, and SFA). We converted these into quintiles for descriptive statistics. For energy derived from carbohydrate, the cut-off values for quintiles were 40.9%, 45.5%, 49.2%, and 53.4%. The corresponding values for fat were 26.9%, 30.6%, 33.8%, and 37.4%, and for protein were 12.8%, 14.4%, 16.0%, and 18.0%. Participants with implausible values for dietary intake and total energy intake based on the mean of all estimates of dietary intake (ie, up to five separate estimates over five years, n=15 365), defined as <1.1 or >2.5×basal metabolic rate, estimated using the Henry equation,20 were excluded based on previous studies.21
Date of death was obtained from death certificates held within the NHS Information Centre (England and Wales) and the NHS Central Register Scotland (Scotland). Date and cause of hospital admissions were obtained through record linkage to health episode statistics (England and Wales) and Scottish morbidity records (Scotland). Detailed information about the linkage procedures can be found at http://content.digital.nhs.uk/services. At the time of analysis, mortality data were available to 14 February 2018. We therefore censored mortality analysis at this date or at date of death, whichever occurred first. As hospital admission data were available to 31 March 2017, we censored the disease specific outcome analysis at this date or the date of first disease incidence or death, whichever occurred first. We defined incident CVD as incident fatal or non-fatal angina, myocardial infarction, chronic ischaemic heart disease, atrial fibrillation, heart failure, and stroke (ICD-10 (international classification of diseases, 10th revision) codes I20, I21, I25, I48, I50, I60, I61, I63, and I64).
Statistical analyses
Cox proportional hazard models were used with exposure variables fitted on penalised cubic splines. Penalised spline is a variation of basis spline (B spline), with better model fit for most scenarios and comparable with the restricted cubic spline in other scenarios.22 We zeroed the estimated hazard ratio curves at the median of each exposure variable. To examine overall statistical significance as well as non-linearity of the exposures, we used likelihood ratio tests.
In the minimally adjusted model, we adjusted Cox proportional hazard models for total energy intake, age, sex, ethnicity, and area deprivation index (supplementary figs S1 and S2), and in the final model we additionally adjusted for height, BMI categories, systolic blood pressure, alcohol intake, physical activity, smoking status, baseline diabetes, and mental health disorders (eg, depression, anxiety, schizophrenia, and substance and alcohol misuse). In analysis of incident CVD, we excluded participants who reported prevalent CVD at baseline (n=9883). As components of macronutrients were moderately correlated (eg, r=−0.41 for starch and sugar and r=0.49 for MUFA and SFA), we mutually adjusted components of each macronutrient (eg, sugar, starch, and fibre). Because Oxford WebQ, which uses diet recall for a single day, might not be representative of participants’ typical dietary habit, we conducted a sensitivity analysis (n=183 904) to exclude participants (n=11 754) who at any of the five assessments during follow-up reported their previous day’s diet as not being typical. We also explored whether the associations differed by energy intake (1 kcal=4.18 kJ=0.00418 MJ) (men: ≤2500 kcal (n=57 167) v >2500 kcal (n=29 129); women: ≤2000 kcal (n=62 691) v >2000 kcal (n=46 671)), whether the associations of SFA differed by carbohydrate intake (below median (n=97 829) v above median (n=97 829)). Because dietary intake is associated with BMI, which in turn influences health, adjustment for BMI categories might result in over-adjustment bias.23 We therefore compared the associations estimated from the models both with and without BMI categories.
A multivariable nutrient density model was used to estimate the association of isocaloric replacement of macronutrients with outcomes.24 Nutrient density models include both the energy from a macronutrient and the total energy as independent variables. This deals with the potential correlation between diet pattern and total energy intake (eg, dietary fat intake correlates with total energy intake (r=0.20)) while keeping the total energy intake constant (isocaloric). Given the potential non-linear relations, we used penalised cubic splines for all macronutrients. In the model with sugar replaced, we did not include sugar, whereas we included fibre and percentages of energy from starch, SFA, MUFA, PUFA, and proteins as splines, with adjustments of total energy and other covariates. Similar approaches were used for the models with SFA replaced. The hazard ratios produced by these isocaloric substitution analyses are shown conditional on the level of the substituting macronutrient.
To develop a risk matrix for all cause mortality, we ran an additional Cox regression model with dietary variables selected from the previous analysis. To balance simplicity and accuracy, we trichotomised macronutrients and their components using several cut-offs on the basis of percentage of energy intake: sugar (5% to <20%, 20% to <30%, 30% to 45%), starch (10% to <20%, 20% to <30%, 30% to 50%), fibre (5 to <10 g/day, 10 to <15 g/day, 15 to 30 g/day), MUFA (5% to <10%, 10% to <20%, 20% to 25%), PUFA (2% to <5%, 5% to <7%, 7% to 12%), and protein (10% to <14%, 14% to <18%, 18% to 30%). We selected the cut-offs based on change points at the associations with mortality (see fig 1). If no relevant change points were found, we selected cut-offs to balance the sample size between groups. The dietary exposure variables were adjusted for potential sociodemographic and lifestyle confounders (age, sex, diabetes, BMI categories, systolic blood pressure, and smoking) and for each other. We chose these factors because they constitute the office based risk score.25 We collapsed the categories if they had similar hazard ratios. A risk matrix was then constructed, with the cells labelled and coloured on the basis of the estimated hazard ratios. The combination of macronutrients with the lowest hazard ratios was chosen as the reference group, and the percentage increase in hazard of other diets was calculated as the difference in hazard ratios.
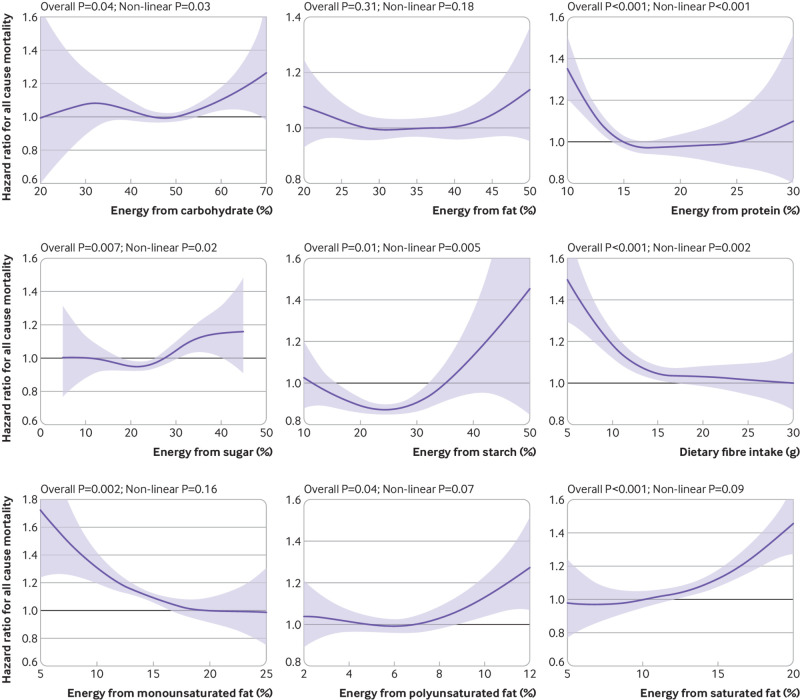
Fig 1.
Association between percentage energy intake from macronutrients and all cause mortality. Analyses adjusted for age, sex, deprivation index, ethnicity, smoking status, height, body mass index categories, systolic blood pressure, baseline diabetes, mental health disorders, total physical activity, daily alcohol intake, and total energy intake. Components of macronutrients (eg, sugar, starch, and fibre) were mutually adjusted. Shaded areas represent 95% confidence intervals
Statistical analyses were performed using R Statistical Software, version 3.5.2, with the package survival.
Patient and public involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing up of results. There are no plans to disseminate the results of the research to study participants or the relevant patient community.
Results
Of the 502 536 UK Biobank participants, 211 023 completed at least one dietary questionnaire. Of these, 15 365 were excluded because of implausible energy intake or dietary intake; leaving 195 658 participants eligible for inclusion in the study. The mean follow-up period for mortality was 10.6 (range 9.4-13.9) years and for CVD incidence was 9.7 (range 8.5-13.0) years. During this period, 4780 (2.4%) participants died and 10 724 (5.5%) had cardiovascular events.
Table 1 shows the characteristics of the cohort by carbohydrate intake. Briefly, participants who derived a higher proportion of energy from carbohydrates were more likely to be women and non-smokers and were more physically active. Carbohydrate intake had a U-shaped relation with deprivation index and an inverse U-shaped relation with total energy intake. Generally, participants with baseline diabetes were less likely to have high carbohydrate intake, and this was the opposite for participants with mental health disorders. Supplementary table S1 shows the median and interquartile range values of the dietary variables, figure S1 the distribution of dietary intake, and tables S2 and S3 the characteristics of the cohort by fat and protein intake.
Table 1.
Characteristics of cohort by quintiles of energy intake from carbohydrates. Values are numbers (percentages) unless stated otherwise
| Characteristics | All (n=195 658) | Quintile groups of energy from carbohydrates (% of total energy intake) | ||||
|---|---|---|---|---|---|---|
| 10.4-40.9 (n=39 132) | >40.9-45.5 (n=39 131) | >45.5-49.2 (n=39 132) | >49.2-53.4 (n=39 131) | >53.4-77.7 (n=39 132) | ||
| Women | 109 362 (55.9) | 19 753 (50.5) | 211 56 (54.1) | 22 249 (56.9) | 22 712 (58.0) | 23 492 (60.0) |
| Mean (SD) age (years) | 56.15 (7.94) | 55.75 (7.85) | 56.17 (7.87) | 56.28 (7.93) | 56.38 (7.96) | 56.17 (8.08) |
| Mean (SD) deprivation index | −1.62 (2.85) | −1.48 (2.91) | −1.68 (2.81) | −1.73 (2.78) | −1.72 (2.81) | −1.48 (2.92) |
| Ethnicity: | ||||||
| White | 187 549 (96.2) | 37 850 (97.0) | 37 896 (97.2) | 37 821 (97.0) | 37 558 (96.3) | 36 424 (93.4) |
| South Asian | 1140 (0.6) | 241 (0.6) | 196 (0.5) | 209 (0.5) | 232 (0.6) | 262 (0.7) |
| Black | 2408 (1.2) | 294 (0.8) | 308 (0.8) | 343 (0.9) | 455 (1.2) | 1008 (2.6) |
| Chinese | 2059 (1.1) | 339 (0.9) | 290 (0.7) | 312 (0.8) | 391 (1.0) | 727 (1.9) |
| Mixed | 523 (0.3) | 90 (0.2) | 109 (0.3) | 94 (0.2) | 105 (0.3) | 125 (0.3) |
| Others | 1296 (0.7) | 187 (0.5) | 187 (0.5) | 217 (0.6) | 271 (0.7) | 434 (1.1) |
| Smoking status: | ||||||
| Never | 110 739 (56.7) | 18 043 (46.2) | 21 085 (54.0) | 22 725 (58.2) | 24 018 (61.5) | 24 868 (63.8) |
| Former | 69 494 (35.6) | 16 413 (42.0) | 14 820 (38.0) | 13 638 (34.9) | 12 721 (32.6) | 11 902 (30.5) |
| Current | 14 937 (7.7) | 4580 (11.7) | 3128 (8.0) | 2678 (6.9) | 2321 (5.9) | 2230 (5.7) |
| Baseline diabetes | 7554 (3.9) | 1780 (4.5) | 1619 (4.1) | 1431 (3.7) | 1312 (3.4) | 1412 (3.6) |
| Baseline mental health disorders | 12 948 (6.6) | 2528 (6.5) | 2439 (6.2) | 2494 (6.4) | 2596 (6.6) | 2891 (7.4) |
| Physical measurements | ||||||
| Mean (SD) height (m) | 1.69 (0.09) | 1.70 (0.09) | 1.70 (0.09) | 1.69 (0.09) | 1.69 (0.09) | 1.68 (0.09) |
| BMI categories: | ||||||
| Underweight (<18.5) | 73 470 (37.6) | 12 546 (32.1) | 14 310 (36.6) | 15 051 (38.5) | 15 747 (40.2) | 15 816 (40.4) |
| Normal weight (18.5 to <25) | 1045 (0.5) | 147 (0.4) | 214 (0.5) | 203 (0.5) | 222 (0.6) | 259 (0.7) |
| Overweight (25 to <30) | 81 688 (41.8) | 17 252 (44.1) | 16 558 (42.3) | 16 175 (41.3) | 15 981 (40.8) | 15 722 (40.2) |
| Obese (≥30)* | 39 455 (20.2) | 9187 (23.5) | 8049 (20.6) | 7703 (19.7) | 7181 (18.4) | 7335 (18.7) |
| Mean (SD) systolic blood pressure (mm hg) | 136.61 (18.29) | 137.57 (18.22) | 136.69 (18.09) | 136.41 (18.23) | 136.42 (18.35) | 135.96 (18.51) |
| Total physical activity (MET-min/week) | 2501.93 (2317.16) | 2406.51 (2287.14) | 2412.20 (2266.11) | 2462.30 (2270.23) | 2547.06 (2313.99) | 2687.08 (2435.30) |
| Mean (SD) dietary intake | ||||||
| Total energy† (kcal/day) | 2006.55 (527.24) | 1927.93 (542.61) | 2026.43 (518.07) | 2044.59 (507.61) | 2043.22 (512.05) | 1990.60 (545.58) |
| Carbohydrate (%) | 49.63 (7.00) | 40.77 (5.31) | 46.44 (3.24) | 49.58 (2.78) | 52.84 (2.53) | 58.54 (3.70) |
| Fat (%) | 33.99 (6.43) | 40.57 (5.58) | 36.73 (4.40) | 34.27 (4.02) | 31.55 (3.79) | 26.84 (4.19) |
| Protein (%) | 16.38 (3.58) | 18.66 (4.09) | 16.83 (3.23) | 16.15 (3.08) | 15.61 (3.03) | 14.62 (3.03) |
| Sugar (%) | 23.62 (6.72) | 18.44 (5.48) | 21.27 (5.13) | 23.30 (5.14) | 25.51 (5.32) | 29.56 (6.62) |
| Starch (%) | 24.10 (5.99) | 21.01 (5.68) | 23.61 (5.18) | 24.51 (5.24) | 25.27 (5.61) | 26.07 (6.81) |
| Fibre (g) | 16.48 (6.17) | 14.02 (5.56) | 15.93 (5.59) | 16.68 (5.74) | 17.51 (6.14) | 18.26 (6.85) |
| Monounsaturated fat (%) | 14.70 (3.19) | 17.93 (2.99) | 15.91 (2.27) | 14.73 (2.06) | 13.52 (1.94) | 11.41 (2.13) |
| Polyunsaturated fat (%) | 6.25 (2.24) | 7.38 (2.39) | 6.78 (2.15) | 6.35 (2.02) | 5.85 (1.93) | 4.90 (1.84) |
| Saturated fat (%) | 13.04 (3.26) | 15.26 (3.28) | 14.04 (2.89) | 13.18 (2.72) | 12.18 (2.62) | 10.52 (2.62) |
| Alcohol (g) | 16.47 (21.14) | 34.57 (28.93) | 20.16 (19.66) | 13.61 (15.53) | 9.25 (12.51) | 4.76 (8.68) |
BMI=body mass index; MET=metabolic equivalent.
Includes World Health Organization obese and obesity classes 2 and 3 categories.
Total energy per day from carbohydrates, fat, and protein.
Figure 1 shows the fully adjusted associations between macronutrient intake and mortality (supplementary fig S2 presents the results for minimally adjusted models). Total fat intake was not significantly associated with all cause mortality. All the remaining macronutrients, except for MUFA, PUFA, and SFA, had non-linear associations with mortality. Risk of death was lowest among participants who consumed 5-20% energy from sugar (2.88 v 3.24 per 1000 person years, average hazard ratio 0.87 (95% confidence interval 0.82 to 0.92), compared with high (35% of energy) intake), 20-30% energy from starch (2.71 v 3.78 per 1000 person years, 0.86 (0.83 to 0.89) compared with low (10% of energy) intake), 15-30 g/day fibre (2.79 v 3.94 per 1000 person years, 0.68 (0.65 to 0.72) compared with low (5 g/day) intake), 20-25% energy from MUFA (2.94 v 3.50 per 1000 person years, 0.58 (0.51 to 0.66) compared with low (5% of energy) intake), 5-7% from PUFA (2.67 v 3.04 per 1000 person years, 0.78 (0.75 to 0.81) compared with high (12% of energy) intake), 5-10% from SFA (2.66 v 3.59 per 1000 person years, 0.67 (0.62 to 0.73) compared with high (20% of energy) intake), and 14-18% from protein (2.73 v 3.41 per 1000 person years, 0.73 (0.70 to 0.75) compared with low (10% of energy) intake). The spline curves can be used to calculate the hazard ratio between any two points. For example, the hazard ratio of 10% energy from starch compared with 25% can be approximated by using the equation: (hazard ratio at 10% of energy)÷(hazard ratio at 25% of energy)≈1.05÷0.9=1.17.
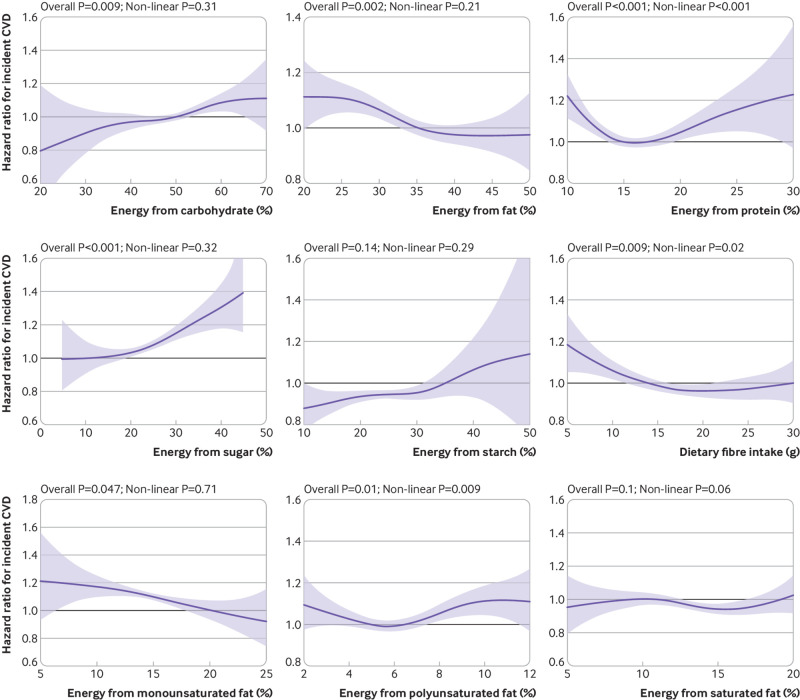
Figure 2 shows the fully adjusted association between macronutrients and incident CVD (supplementary fig S3 presents the results for minimally adjusted models). Intakes of starch and SFA were not associated with CVD even though some part of the confidence intervals did not overlap the null. Total carbohydrate, sugar, and MUFA were linearly associated with CVD, whereas there were non-linear associations for fibre, PUFA, and protein. Risk of CVD was lowest among participants who consumed 5-20% of energy from sugar (7.11 v 7.83 per 1000 person years, average hazard ratio 0.83 (95% confidence interval 0.80 to 0.87) compared with high (35% of energy) intake), 15 to 30 g/day fibre (7.36 v 7.46 per 1000 person years, 0.82 (0.79 to 0.86) compared with low (5 g/day) intake), 20% to 25% from MUFA (6.31 v 7.45 per 1000 person years, 0.80 (0.72 to 0.89) compared with low (5% of energy) intake), 5% to 7% from PUFA (6.94 v 7.61 per 1000 person years, 0.90 (0.87 to 0.92) compared with high (12% of energy) intake), and 14% to 18% from protein (7.12 v 7.35 per 1000 person years, 0.82 (0.80 to 0.84) compared with low (10% of energy) intake).
Fig 2.
Association between percentage energy from macronutrients and incidence of cardiovascular disease (CVD). Analyses were adjusted for age, sex, deprivation index, ethnicity, smoking status, height, body mass index categories, systolic blood pressure, baseline diabetes, mental health disorders, total physical activity, daily alcohol intake, and total energy intake. Components of macronutrients (eg, sugar, starch, and fibre) were mutually adjusted. Shaded areas represent 95% confidence intervals
Similar results were found in sensitivity analysis including only participants who reported their typical diet (supplementary figs S4 and S5). The associations were also similar without adjusting for BMI categories (supplementary figs S6 and S7). Supplementary figures S8 and S9 show that the associations for participants with higher energy intake (men: >2500 kcal; women: >2000 kcal) and with lower energy intake (men: ≤2500 kcal; women: ≤2000 kcal) were largely similar, but the association of sugar with CVD risk seems to be stronger in participants with lower energy intake. Although the association of saturated fat with all cause mortality differed between individuals with high and low carbohydrate intake, no differences were observed for incident CVD (supplementary fig S10).
Because higher sugar intake was associated with a higher risk of all cause mortality and incident CVD, and higher SFA intake was associated with a higher risk of all cause mortality, isocaloric substitution of these two components was investigated. When energy intake remained constant, replacing sugar with starch, MUFA, and protein (fig 3) was associated with lower risk of all cause mortality and incident CVD when replacement was up to 30% of energy from starch, 25% from MUFA, and 20% from protein. The associations with MUFA appeared to be linear, whereas that between protein and CVD appeared to be J-shaped. Replacing sugar with PUFA (fig 3) was associated with an increased risk of both mortality and CVD when PUFA exceeded 6-7% of energy. Replacing sugar with SFA (fig 3) above 10% of energy was associated with a higher risk of mortality and a lower risk of CVD.
Fig 3.
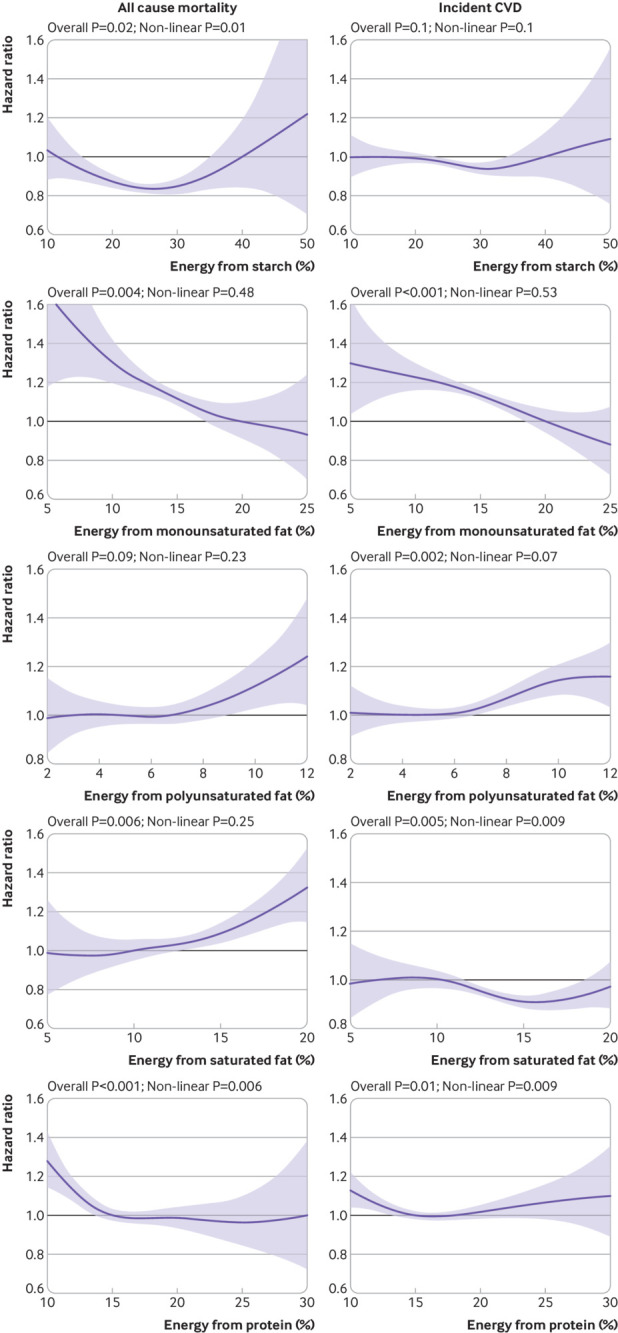
Multivariable isocaloric analysis on replacing sugar intake with starch, monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), saturated fatty acids (SFA), and protein. Outcomes were all cause mortality (left panel) and incident cardiovascular disease (CVD) (right panel). Curves represent hazard ratios conditional on current intake of the replacement macronutrient. For example, for a person having 10% of energy from protein, replacing 5% of energy from fat by protein is associated with lower risk of all cause mortality. Macronutrients shown were mutually adjusted. Additionally, analyses were adjusted for age, sex, deprivation index, ethnicity, smoking status, height, body mass index categories, systolic blood pressure, baseline diabetes, mental health disorders, total physical activity, daily alcohol and fibre intake, and total energy intake. Shaded areas represent 95% confidence intervals
Similarly, replacing SFA with MUFA or with protein (fig 4) was associated with a lower risk of both total mortality and CVD up to 25% energy from MUFA and 15% energy from protein. Replacing SFA with starch (fig 4) was not significantly associated with CVD risk but was associated with a lower risk of mortality up to 30% of energy from starch. Replacing SFA with PUFA (fig 4) was not significantly associated with mortality risk but was associated with a higher risk of CVD. Replacing SFA with sugar (fig 4) was associated with lower all cause mortality up to 25% energy from sugar but was linearly associated with a higher risk of CVD.
Fig 4.
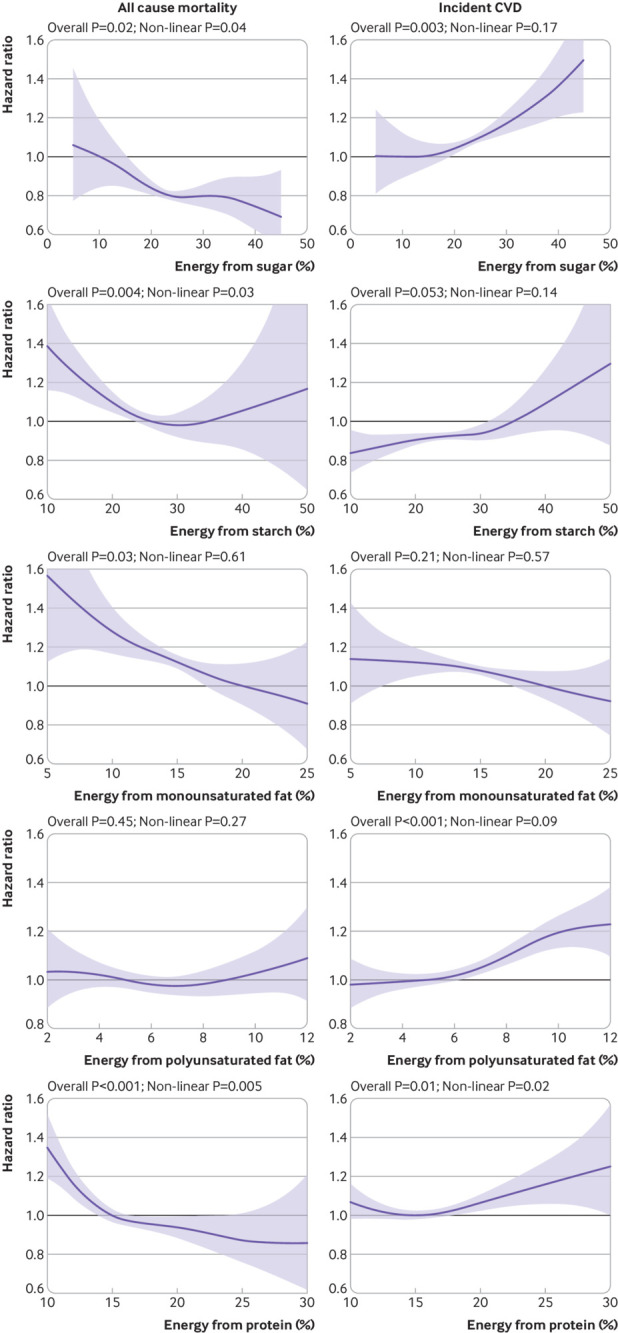
Multivariable isocaloric analysis on replacing intake of saturated fatty acids (SFA) with sugar, starch, monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), and protein. Outcomes were all cause mortality and incident cardiovascular disease (CVD) Curves represent hazard ratios conditional on current intake of the replacement macronutrient. For example, for a person having 10% of energy from protein, replacing 5% of energy from fat by protein is associated with lower risk of all cause mortality. Macronutrients shown were mutually adjusted. Additionally, analyses were adjusted for age, sex, deprivation index, ethnicity, smoking status, height, body mass index categories, systolic blood pressure, baseline diabetes, mental health disorders, total physical activity, daily alcohol and fibre intake, and total energy intake. Shaded areas represent 95% confidence intervals
Sugar, starch, fibre, protein, SFA, MUFA, and PUFA were considered when developing a dietary intake risk matrix. Subsequently, sugar and SFA were dropped from the model because their associations were not significantly independent of the other dietary components (supplementary table S4). The highest two categories of fibre, MUFA, and protein intake were combined because they had comparable hazard ratios. The diet with the lowest hazard ratio of all cause mortality comprised high fibre (10-30 g/day), protein (14-30%), and MUFA (10-25%) and moderate PUFA (5% to <7%) and starch (20% to <30%) intake (supplementary table S4). This was used as the referent dietary pattern in the risk matrix (supplementary fig S11). The cells of the matrix represent alternative, isocaloric combinations of macronutrients and are coloured from green (lowest risk) to red (highest risk) based on the hazard ratios. The numbers inside the cells are the percentage difference in hazard compared with the reference group. Cells with similar colours represent different dietary combinations that are associated with similar levels of risk. For example, people with a diet that was high in protein (≥14%) and starch (≥30%) and low in fibre (<10 g/day), MUFA (<10%), and PUFA (<5%) had a 70% higher risk of mortality compared with the referent diet (supplementary fig S11). A higher fibre intake (≥10 g/day) corresponded to a lower risk; 31% above the referent group. However, a similar lower level of risk, at 29% above the referent group, could be achieved by reducing starch intake to 20% to <30% of total energy, and replacing it with MUFA (up to 10-25% of total energy intake) or PUFA (up to 5% to <7% of total energy intake).
Discussion
In this study, intake of carbohydrate (including sugar, starch, and fibre) and protein were non-linearly associated with all cause mortality, and this finding was similar for intake of fibre, polyunsaturated fatty acids (PUFA), and protein with incident cardiovascular disease (CVD). In contrast, intake of saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and PUFA were linearly associated with all cause mortality, and this finding was similar for intake of total carbohydrate (including sugar) and total fat (including MUFA) with incident CVD. In addition, when participants were compared within the cohort, we found that replacing intake of sugar with starch, MUFA, or protein was associated with a lower risk of all cause mortality and incident CVD when the current intake of starch, MUFA, and protein were low. Similarly, replacing SFA with MUFA or protein was associated with a lower risk of both total mortality and CVD. The results were largely consistent in a series of sensitivity and subgroup analysis.
These findings highlight the complex and diverse associations between macronutrient intake and health outcomes. Previous research has commonly reported linear associations of single macronutrients with health outcomes. In this study we investigated non-linear associations, adjusted the analysis for intake of other macronutrients that could influence the observed associations, and implemented isocaloric replacements based on non-linear and linear associations of macronutrients and health outcomes.
Comparison with other studies
Many of the previous studies11 12 that investigated associations of diet with health did not consider the components of carbohydrates. In our study we identified divergent associations of sugar and starch with all cause mortality, indicating that it could be misleading to provide advice on carbohydrate intake without specifying the components.
We found a J-shaped and positive association between total carbohydrate intake and mortality, similar to that reported in the Prospective Urban Rural Epidemiology (PURE) study.11 In contrast, the Atherosclerosis Risk in Communities (ARIC) study12 reported a U-shaped association after adjusting for sociodemographic characteristics, energy intake, exercise, smoking, and diabetes. We did find a similar U-shaped pattern when we adjusted our analyses for sociodemographic factors (supplementary fig S2). However, the inclusion of additional confounders (such as blood pressure and mental health disorders) resulted in a J-shaped curve, with non-significant changes in risk associated with carbohydrate intake less than 50% of total energy. Although the PURE and ARIC studies11 12 adjusted for several important confounders (including physical activity level and diabetes), they did not adjust for other health conditions such as obesity, hypertension, and depression that might influence dietary choices26 27 and are associated with increased morbidity and mortality.28
In contrast with findings from a recent meta-analysis of observational studies,3 we observed strong and significant associations between SFA consumption and mortality, independent of unsaturated fat consumption. Our finding of a curvilinear relation could explain the lack of association in previous studies, which compared the lowest and highest quintiles3 or assumed linearity.29 30 Also, the inverse association of SFA reported by the PURE study might be an artefact of the truncation used for reporting saturated fat consumption at 18% of total energy,11 thereby masking a U-shaped (albeit non-significant) relation that we found between SFA and CVD.
Although previous studies, such as the pooled analysis of the Nurses’ Health Study and Health Professionals Follow-up Study,31 reported an association between intake of PUFA and lower risk of CVD, we found the opposite. The difference in findings can be related to the use of quintile categories, as well as the proportional intake of subtypes of PUFA in the population. In addition, PUFA comprises different types of fatty acids, and studies have shown that omega-3 PUFA from seafood (eicosapentaenoic and docosahexaenoic acid), but not other PUFA, was associated with incident CVD.32
The association of protein intake with all cause mortality and CVD incidence is relatively less well studied. Consistent with our findings, the pooled analysis of three randomised trials has shown that increased protein intake (by replacing carbohydrate or fat, or both) reduced bodyweight, fat, and total cholesterol among overweight and obese people with increased triacylglycerol levels.33 It should be noted, however, that the source of protein (eg, animal versus plant protein) could play a critical role in the association and this was not investigated in the current study. In prospective cohort studies plant protein has been associated with a lower mortality risk and animal protein with a higher mortality risk.34 35
Implications
In our study, the individual components of macronutrients had different associations with health outcomes even though they were from the same macronutrient family. Given that many of the associations were not linear, any guidance on isocaloric replacement should be based on current intake. For example, although replacing sugar with starch is associated with a lower mortality risk, this is only the case when an individual has a relatively low starch intake. It would be misleading if dietary advice is given without consideration of current intake and the composition of the diet as a whole, even though this can be hard to measure in practice.
In its current format, based on isocaloric replacements and expressed as hazard ratios rather than absolute risk, the risk matrix would be difficult to implement as a real world tool for patients or members of the public. The risk matrix in our study shows that the implications of dietary changes are likely to vary according to the individual’s current diet and therefore, when possible, dietary advice should be personalised.
Strengths and limitations of this study
A strength of this study is that we did not assume linearity between intakes of macronutrients and health outcomes and we adjusted mutually for macronutrient components. We also explored associations with constituent components of macronutrients—for example, starch, sugar, and dietary fibre are components of carbohydrates, each of which has distinctive relations with health outcomes. The possibility of confounding was dealt with through statistical adjustment for a wide range of covariates and through a series of sensitivity analyses. As with any observational study, however, residual confounding is possible, and causation cannot be tested. Also, summary statistics and estimates of absolute risk from this study might not be generalisable even though the personal characteristics of the cohort and estimated effect sizes are similar to those of the general population.36 37 38 As the dietary information used in this study was provided by around half of UK Biobank participants, selection bias is possible. Dietary measurements in our study were derived from 24 hour recall so might not portray participants’ typical intake precisely and could be subject to recall bias.39 Owing to limited statistical power, we did not exclude participants who did not provide multiple dietary records, and some analyses might be underpowered. Further, we were not able to reliably test whether some associations were sex specific. Similarly, associations at the extreme ends of intake (particularly intakes with wide confidence intervals) should be interpreted with caution. Isocaloric replacement analysis is based on comparisons between participants and might not represent real life changes as occurs in randomised controlled trials. We were unable to investigate associations with added sugars, trans fat, types of polyunsaturated fat (omega-3 and omega-6), and animal based versus plant based protein because these data were not available. Also, food source (eg, whole grain versus refined carbohydrate sources) might modify the associations between macronutrient intake and outcomes. The dietary risk matrix was constructed for illustrative purposes rather than as a tool ready for implementation, and the cut-off values have not been validated.
Conclusions
This study found that many of the associations between macronutrient intakes and health (mortality and CVD risk) are non-linear. Thus, dietary advice should be based on current intake. Moreover, components of carbohydrates also displayed differential associations with health outcomes, indicating that dietary guidelines on carbohydrate intake should also take account of the divergent associations of sugar and starch.
What is already known on this topic
Associations between macronutrients and health are often assumed to be linear, especially in isocaloric substitution analysis
Carbohydrate intake was suggested to have a linear or curvilinear linear association with risk of mortality and cardiovascular disease
What this study adds
In this study, many of the associations between macronutrient intakes and health (risk of mortality and cardiovascular disease) were non-linear, and components of carbohydrates (total sugar and starch) had a differential association with health
Dietary advice should be tailored to current intake and consideration given to the components of macronutrients
Acknowledgments
We thank UK Biobank participants. This research has been conducted using the UK Biobank Resource (application No 7155).
Web extra.
Extra material supplied by authors
Supplementary information: tables S1-S4 and figures S1-S11
Contributors: FKH and SRG contributed equally to this work and are joint first authors. JCM, JPP, and CCM contributed equally to this work and are joint senior authors. FKH, SRG, JCM, JPP, and CCM designed the study. FKH, SRG, and CCM conducted the statistical analysis and wrote the first draft. SRG, PW, FPR, HF, HW, JA, DL, NS, JMRG, JCM, and JPP critically revised the manuscript. FKH, JPP and CCM are the guarantors of the manuscript and accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: UK Biobank was established by the Wellcome Trust, Medical Research Council, Department of Health, Scottish government, and Northwest Regional Development Agency. It has also had funding from the Welsh assembly government and the British Heart Foundation.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: UK Biobank was established by the Wellcome Trust medical charity, Medical Research Council, Department of Health, Scottish government, and Northwest Regional Development Agency; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This study was performed under generic ethical approval obtained by UK Biobank from the National Health Service National Research Ethics Service (approval letter ref 11/NW/0382, 17 June 2011).
Data sharing: No additional data available.
The manuscript’s guarantors (JPP and CCM) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Dissemination to participants and related patient and public communities: Results of the study will be linked in the UK Biobank website for research participants and relevant patient and public communities.
References
- 1.FAO. Food-based dietary guidelines: Food and Agriculture Organization of the United Nations; 2018 [updated 2018; cited 2018 27 December]. http://www.fao.org/nutrition/education/food-dietary-guidelines/regions/en.
- 2. Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr 2010;91:535-46. 10.3945/ajcn.2009.27725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Souza RJ, Mente A, Maroleanu A, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ 2015;351:h3978. 10.1136/bmj.h3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.SACN. Saturated fats and health London: Public Health England, 2019.
- 5. WHO Draft WHO Guidelines: Saturated fatty acid and trans-fatty intake for adults and children. WHO, 2018. [PubMed] [Google Scholar]
- 6. Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med 2010;7:e1000252. 10.1371/journal.pmed.1000252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stern L, Iqbal N, Seshadri P, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med 2004;140:778-85. 10.7326/0003-4819-140-10-200405180-00007 [DOI] [PubMed] [Google Scholar]
- 8. Bazzano LA, Hu T, Reynolds K, et al. Effects of low-carbohydrate and low-fat diets: a randomized trial. Ann Intern Med 2014;161:309-18. 10.7326/M14-0180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gardner CD, Trepanowski JF, Del Gobbo LC, et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA 2018;319:667-79. 10.1001/jama.2018.0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA 2014;312:923-33. [DOI] [PubMed] [Google Scholar]
- 11. Dehghan M, Mente A, Zhang X, et al. Prospective Urban Rural Epidemiology (PURE) study investigators Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 2017;390:2050-62. 10.1016/S0140-6736(17)32252-3 [DOI] [PubMed] [Google Scholar]
- 12. Seidelmann SB, Claggett B, Cheng S, et al. Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis. Lancet Public Health 2018;3:e419-28. 10.1016/S2468-2667(18)30135-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reiser S, Hallfrisch J, Michaelis OE, 4th, Lazar FL, Martin RE, Prather ES. Isocaloric exchange of dietary starch and sucrose in humans. I. Effects on levels of fasting blood lipids. Am J Clin Nutr 1979;32:1659-69. 10.1093/ajcn/32.8.1659. [DOI] [PubMed] [Google Scholar]
- 14. SACN Carbohydrates and Health. The Stationery Office, 2015. [Google Scholar]
- 15. Townsend P, Phillimore P, Beattie A. Health and deprivation: inequality and the North. Routledge, 1988. [Google Scholar]
- 16. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381-95. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 17. Liu B, Young H, Crowe FL, et al. Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. Public Health Nutr 2011;14:1998-2005. 10.1017/S1368980011000942 [DOI] [PubMed] [Google Scholar]
- 18. McCance R. McCance and Widdowson’s the composition of foods. Seventh edition Royal Society of Chemistry, 2002. [Google Scholar]
- 19. Englyst HN, Cummings JH. Improved method for measurement of dietary fiber as non-starch polysaccharides in plant foods. J Assoc Off Anal Chem 1988;71:808-14. [PubMed] [Google Scholar]
- 20. Henry CJK. Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr 2005;8(7a):1133-52. 10.1079/PHN2005801 [DOI] [PubMed] [Google Scholar]
- 21. Celis-Morales C, Livingstone KM, Marsaux CF, et al. Design and baseline characteristics of the Food4Me study: a web-based randomised controlled trial of personalised nutrition in seven European countries. Genes Nutr 2015;10:450. 10.1007/s12263-014-0450-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Govindarajulu US, Malloy EJ, Ganguli B, Spiegelman D, Eisen EA. The comparison of alternative smoothing methods for fitting non-linear exposure-response relationships with Cox models in a simulation study. Int J Biostat 2009;5:2. 10.2202/1557-4679.1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009;20:488-95. 10.1097/EDE.0b013e3181a819a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531-40. 10.1093/oxfordjournals.aje.a009849 [DOI] [PubMed] [Google Scholar]
- 25. Ueda P, Woodward M, Lu Y, et al. Laboratory-based and office-based risk scores and charts to predict 10-year risk of cardiovascular disease in 182 countries: a pooled analysis of prospective cohorts and health surveys. Lancet Diabetes Endocrinol 2017;5:196-213. 10.1016/S2213-8587(17)30015-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. NIH Your guide to lowering your blood pressure with DASH. U.S. National Institutes of Health, 2006. [Google Scholar]
- 27.NHS. Treatment of Obesity London: National Health Services, UK; 2016. https://www.nhs.uk/conditions/obesity/treatment/ accessed 12 September 2018.
- 28. Yusuf S, Hawken S, Ôunpuu S, et al. INTERHEART Study Investigators Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937-52. 10.1016/S0140-6736(04)17018-9 [DOI] [PubMed] [Google Scholar]
- 29. de Oliveira Otto MC, Mozaffarian D, Kromhout D, et al. Dietary intake of saturated fat by food source and incident cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr 2012;96:397-404. 10.3945/ajcn.112.037770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jakobsen MU, Overvad K, Dyerberg J, Schroll M, Heitmann BL. Dietary fat and risk of coronary heart disease: possible effect modification by gender and age. Am J Epidemiol 2004;160:141-9. 10.1093/aje/kwh193 [DOI] [PubMed] [Google Scholar]
- 31. Li Y, Hruby A, Bernstein AM, et al. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: a prospective cohort study. J Am Coll Cardiol 2015;66:1538-48. 10.1016/j.jacc.2015.07.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Oliveira Otto MC, Wu JH, Baylin A, et al. Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2013;2:e000506. 10.1161/JAHA.113.000506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clifton PM, Bastiaans K, Keogh JB. High protein diets decrease total and abdominal fat and improve CVD risk profile in overweight and obese men and women with elevated triacylglycerol. Nutr Metab Cardiovasc Dis 2009;19:548-54. 10.1016/j.numecd.2008.10.006 [DOI] [PubMed] [Google Scholar]
- 34. Virtanen HEK, Voutilainen S, Koskinen TT, et al. Dietary proteins and protein sources and risk of death: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr 2019;109:1462-71. 10.1093/ajcn/nqz025 [DOI] [PubMed] [Google Scholar]
- 35. Song M, Fung TT, Hu FB, et al. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern Med 2016;176:1453-63. 10.1001/jamainternmed.2016.4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Collins R. What makes UK Biobank special? Lancet 2012;379:1173-4. 10.1016/S0140-6736(12)60404-8 [DOI] [PubMed] [Google Scholar]
- 37. Bhatnagar P, Wickramasinghe K, Williams J, Rayner M, Townsend N. The epidemiology of cardiovascular disease in the UK 2014. Heart 2015;101:1182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Batty GD, Gale CR, Kivimäki M, et al. Generalisability of Results from UK Biobank: Comparison With a Pooling of 18 Cohort Studies. MedRxiv 3437793 [Preprint]. 2019.
- 39. Poslusna K, Ruprich J, de Vries JH, Jakubikova M, van’t Veer P. Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br J Nutr 2009;101(Suppl 2):S73-85. 10.1017/S0007114509990602 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: tables S1-S4 and figures S1-S11