Abstract
Background
While most studies focus on pro-allergic cytokines, the protective role of immunosuppressive cytokines in allergic inflammation is not well elucidated. This study was to explore a novel anti-inflammatory role and cellular/molecular mechanism of IL-27 in allergic inflammation.
Methods
A murine model of experimental allergic conjunctivitis (EAC) was induced in BALB/c, C57BL/6 or IL-27Rα-deficient (WSX-1−/−) mice by short ragweed pollen, with untreated or PBS-treated mice as controls. The serum, eyeballs, conjunctiva, cervical lymph nodes (CLNs) were used for study. Gene expression was determined by RT-qPCR, and protein production and activation were evaluated by immunostaining, ELISA and Western blotting.
Results
Typical allergic manifestations and stimulated thymic stromal lymphopoietin (TSLP) signaling and Th2 responses were observed in ocular surface of EAC models in BALB/c and C57BL/6 mice. The decrease of IL-27 at mRNA (IL-27/EBI3) and protein levels were detected in serum, conjunctiva and CLN, as evaluated by RT-qPCR, immunofluorescent staining, ELISA and Western blotting. EAC induced in WSX-1−/− mice showed aggravated allergic signs with higher TSLP-driven Th2-dominant inflammation, accompanied by stimulated Th17 responses, including IL-17A, IL-17F, and transcription factor RORγt. In contrast, Th1 cytokine IFNγ and Treg marker IL-10, with their respective transcription factors T-bet and foxp3, were largely suppressed. Interestingly, imbalanced activation between reduced phosphor (P)-STAT1 and stimulated P-STAT6 were revealed in EAC, especially WSX-1−/−-EAC mice.
Conclusion
These findings demonstrated a natural protective mechanism by IL-27, of which signaling deficiency develops a Th17-type hyperresponse that further aggravates Th2-dominant allergic inflammation.
Keywords: allergy, anti-inflammatory cytokine, IL-27, Th17, Th2
Graphical Abstract
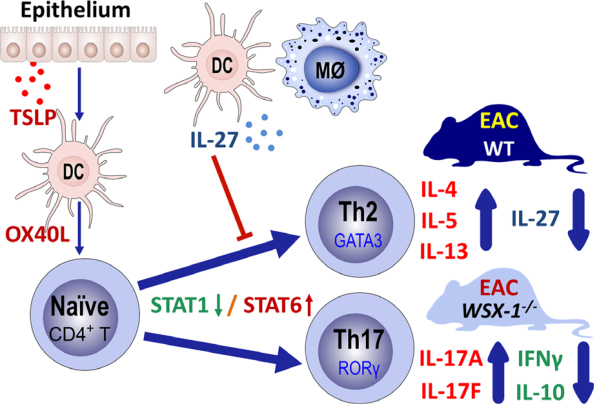
Suppressed expression and production of IL-27 were identified in serum, cervical lymph nodes, and conjunctiva in a murine model of experimental allergic conjunctivitis (EAC) with TSLP-driven Th2-dominant inflammation. The aggravated allergic inflammation was associated with enhanced Th17 hyperresponse and reduced Th1 and Treg cytokines via imbalanced activation of STAT1/STAT6 in WSX-1−/−-EAC mice deficient for IL-27 signaling. The findings uncovered novel anti-inflammatory roles and signaling pathways of IL-27 in allergic inflammation.
1 |. INTRODUCTION
Allergic diseases affect large population worldwide, and the incidence of allergy has steadily increased in the industrialized countries. Recent studies have revealed that the epithelium plays a vital role in innate immunity and serves as a bridge linking innate to adaptive immune responses. Allergic diseases have been recognized as epithelial disorders. The epithelium-derived pro-allergic cytokines, such as thymic stromal lymphopoietin (TSLP) and interleukin (IL)-33, have been recently identified as key initiators that trigger allergic inflammation.
Thymic stromal lymphopoietin has been known to activate dendritic cells (DCs) to produce OX40 ligand (OX40L) that primes naive CD4+ T cells to differentiate into Th2 cells, which produce Th2 allergic cytokines, IL-4, IL-5, and IL-13. Compelling evidence demonstrates that TSLP is a key initiator in allergic inflammation at the interface between epithelial cells and DCs in atopic dermatitis, asthma and allergic conjunctivitis.1–3 Recently, IL-33 was identified as a functional ligand to ST2, a receptor on Th2 cells that mediate allergic inflammatory disease. Epithelium-derived IL-33 has been recognized to trigger allergic diseases including asthma, atopic dermatitis, allergic rhinitis and conjunctivitis.4–6 Our previous studies have revealed two signaling pathways, TSLP/OX40L/OX40 and IL-33/ST2, in allergic inflammation using a murine model of experimental allergic conjunctivitis (EAC) induced by short ragweed (SRW) pollen3,4,7 While it is important to study pro-allergic cytokines and signaling pathways that cause allergic diseases, the protective role of immunosuppressive cytokines in allergic inflammation has been less investigated.
IL-27 is a pleiotropic heterodimeric cytokine, composed of IL-27p28 and Epstein-Barr virus-induced gene 3 (EBI3) subunits. EBI3 functions as a soluble α-receptor, and IL-27 can directly activate its target cells through a high-affinity IL-27 receptor (IL-27R) complex that includes IL-27Rα (known as WSX-1) and glycoprotein 130. Being a heterodimeric cytokine, IL-27 is either grouped into IL-6 or IL-12 family of cytokines.
Recent studies demonstrate that IL-27 displays broad effects on Th1, Th2 and Th17 subsets of T cells, as well as regulatory T cells.8,9 IL-27 may have multifaceted immune-regulatory functions, which may result in either pro-inflammatory or anti-inflammatory effects in relationship to the biological context and experimental models.10–12 IL-27 has been now recognized as an immunosuppressive cytokine that plays protective role in inflammatory and autoimmune diseases.
However, the role of IL-27 in allergic inflammation is not clear. Several reports revealed that IL-27 inhibits Th2 cells and Th2 cytokine production.13,14 In the absence of IL-27-mediated immunosuppression, hyperproduction of various pro-inflammatory cytokines concomitant with severe inflammation in affected organs was observed in IL-27Rα chain (WSX-1−/−)-deficient mice.14,15 IL-27 was found to suppress Th17-cell differentiation16,17 and promote production of IL-10 by CD4+ regulatory T (Treg) cells,18,19 and CD8+ cytotoxic T lymphocytes.20,21
Allergic conjunctivitis is a good model to study allergic diseases, since ocular allergic manifestations are often accompanied by a variety of allergic diseases, and severe cases with corneal irregularity and opacity can cause permanent vision loss. In the murine EAC model, SRW pollen induces typical clinical manifestations simulating to human allergic conjunctivitis, including lid edema, conjunctival redness, chemosis, tearing, and frequent scratching of the eye lids.3,22 The aim of this study was to uncover a protective role and signaling pathway of IL-27 in allergic disease by showing that the IL-27 is suppressed in Th2-dominant EAC model, and IL-27 signaling deficiency further develops enhanced Th17 response that aggravates allergic inflammation.
2 |. METHODS
2.1 |. Animals
The animal protocol for this study was approved by the Institutional Animal Care and Use Committee (IACUC), Center for Comparative Medicine, Baylor College of Medicine. All animals used in this study were maintained in specific pathogen-free conditions in microisolator cages and were treated in accordance with the National Institutes of Health Guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). BALB/c, C57BL/6, and homozygote IL-27Rα-deficient WSX-1−/− female mice aged at 6–8 weeks were purchased from the Jackson Laboratory (Bar Harbor, ME, USA).
2.2 |. A murine EAC model
The mouse EAC model was induced using BALB/c, C57BL/6, and WSX-1−/− mice with previously reported methods.3,22 In brief, mice were immunized with 50 μg of SRW pollen (Greer Lab, Lenoir, NC, USA) by footpad injection on day 0. EAC was induced by given topical applications of 1.5 mg SRW pollen into each eye once at days 10–12. Untreated normal and PBS eyedrop treated SRW-sensitized mice were used as controls. Animals were examined clinically for signs, and a clinical scoring scheme described by Magone et al22 was used to evaluate chemosis, conjunctival redness, lid edema, and tearing. The corneal epithelium, conjunctiva, cervical lymph nodes, and whole eyes were collected for studies. Six mice per group were used in each experiment, and the same experiments were repeated at least 4 times.
2.3 |. Total RNA extraction, reverse transcription (RT), and quantitative real-time PCR (qPCR)
Total RNA was extracted with RNeasy Plus Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions, quantified with a spectrophotometer (NanoDrop ND-2000; Thermo Scientific, Wilmington, DE, USA), and stored at −80°C before use. The first-strand cDNA was synthesized by RT from 1.0 μg of total RNA using Ready-To-Go You-Prime First-Strand Beads as previously described.7 qPCR was performed in StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA), and TaqMan gene expression assays used for this study were listed in Table S1. The results were analyzed by the comparative threshold cycle method and normalized by GAPDH as an internal control.3,23
2.4 |. Enzyme-linked immunosorbent assay (ELISA)
Double-sandwich ELISA kits for mouse IL-27, IL-17, IFNγ, and IL-10 were from BioLegend (San Diego, CA, USA) and performed according to manufacturers’ protocols similar to our previous reports.24,25 Absorbance was read at 450 nm with a reference wavelength of 570 nm by Infinite M200 microplate reader (Tecan US, Inc., Morrisville, NC, USA). The detection ranges of each cytokine by the kits were 19.5–1248, 7.8–1000, 15.6–1000, and 15.6–1000 pg/mL for IL-27, IL-17, IFNγ, and IL-10, respectively.
2.5 |. Immunohistochemical and immunofluorescent staining
Cryosections at 8 μm of mouse eyeballs and CLNs were cut with a cryostat (HM 500; Micron, Waldorf, Germany) and stored at −80°C. Immunohistochemical and immunofluorescent staining were performed as previously described.26,27 Primary antibodies used for this study were listed in Table S2. Secondary antibody alone or isotype IgG were used as negative controls. The results were photographed with an epifluorescence microscope (Eclipse 400; Nikon, Garden City, NY, USA) using a digital camera (DMX 1200; Nikon).
2.6 |. Western blot analysis
Western blot analysis was performed with a previous method.28 Briefly, the cell lysates (50 μg per lane) were separated on a SDS polyacrylamide gel and electronically transferred to PVDF membranes. The membranes were incubated with primary antibodies against IL-27, EBI3 (1:100, 2 μg/mL), or β-actin (1:500, 1 μg/mL) overnight at 4°C and then with horseradish peroxidase-conjugated secondary antibody for 1 hour. The signals were detected with ECL chemiluminescence reagent using an image station 4000R (Eastman Kodak, New Haven, CT, USA).
2.7 |. Statistical analysis
Student’s t test was used to compare differences between two groups. One-way ANOVA test was used to make comparisons among three or more groups, followed by Dunnett’s post hoc test. P values < 0.05 were considered statistically significant.
3 |. RESULTS
3.1 |. Experimental allergic conjunctivitis in a murine model
The typical manifestations of allergic conjunctivitis, including lid edema, conjunctival redness, chemosis, tearing, and frequent scratching of the eye lids, were observed in mice induced by SRW pollen with statistically clinical scores (Figure 1A,B). The mRNA expression and immunoreactivity of pro-allergic cytokine TSLP were significantly stimulated in the corneal and conjunctival epithelia with the increased levels of OX40L, a major downstream molecule of TSLP, in the ocular draining CLNs of EAC mice, when compared with PBS-treated controls (Figure S1).
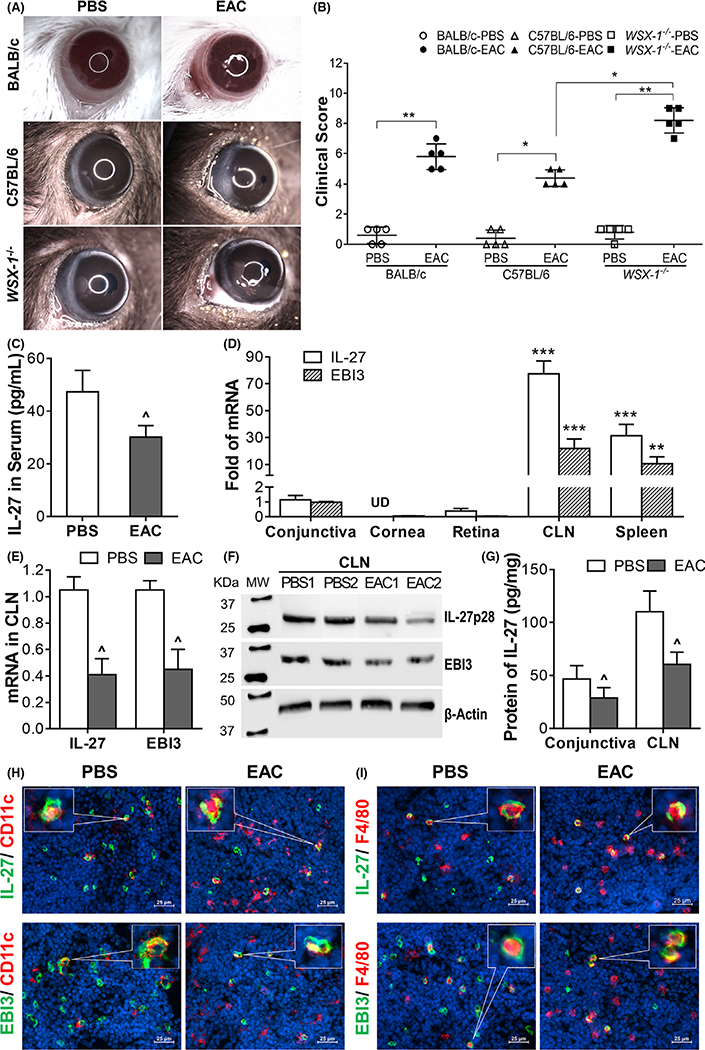
FIGURE 1.
The clinical signs and the suppressed expression of IL-27 in experimental allergic conjunctivitis (EAC) mice. A, The typical manifestations of allergic conjunctivitis, including lid edema, conjunctival redness, chemosis, tearing, and frequent scratching of the eye lids, were observed in mice induced by short ragweed pollen; B, WSX-1−/−-EAC mice had significant higher clinical score, compared with C57BL/6-EAC mice; C, IL-27 in serum by ELISA; D, mRNA levels of IL-27/EBI3 in different tissues of normal mice by RT-qPCR; E, IL-27/EBI3 mRNA levels by CLNs; F-G, IL-27p28/EBI3 protein levels in CLNs by Western blotting with β-actin as control, and by ELISA; H-I, Double staining showing decreased IL-27+ DCs and macrophages in CLNs of EAC. Results shown are the mean ± SD. *P < 0.05, ** P < 0.01, ***P < 0.001; or ^P < 0.05, ^^P < 0.01, n = 5, compared with controls
3.2 |. Suppressed expression and production of IL-27 were identified in serum, CLNs and conjunctiva in EAC mice
Evaluated by ELISA that measures complete IL-27 protein with both subunits, IL-27p28 and EBI3 (Figure 1C), the IL-27 levels in serum were found to decrease significantly to 30.2 ± 4.4 pg/mL in BALB/c-EAC mice from 47.4 ± 8.1 pg/mL in PBS control mice (P < 0.05, n = 10). In order to determine the source of IL-27 in eye, the corneal epithelium, conjunctiva, retina, CLN, and spleen tissues were collected from normal mice. Both transcripts IL-27 and EBI3 of IL-27 gene, coding IL-27p28 and EBI3 chains, respectively, were observed to be expressed highly by CLNs, moderately by spleen, slightly by conjunctiva, and very low levels by retina, but not by corneal epithelium (Figure 1D). CLNs expressed IL-27 and EBI3 transcripts 77.5 ± 9.5- and 22.0 ± 7.1-fold higher than conjunctival expressions.
Serving as draining lymph nodes of ocular surface, CLNs well respond to challenges in ocular disease. Interestingly, IL-27 expression decreased significantly in CLNs of BALB/c-EAC mice when compared with PBS-treated controls. The mRNA levels of IL-27 and EBI3 were reduced by 61% and 57%, respectively (Figure 1E). The two subunits IL-27p28 and EBI3 of IL-27 protein also decreased as shown by Western blot (Figure 1F). The decrease of IL-27 production was confirmed by ELISA that quantifies the total IL-27 protein levels including both chains. IL-27 production by CLNs decreased to 60.5 ± 11.5 pg/mg cellular protein in EAC mice (P < 0.05, n = 6) from 110.3 ± 19.4 pg/mg in PBS controls. Similar results were observed in conjunctiva, where IL-27 levels decreased by 38% in EAC mice (Figure 1G). Immunofluorescent double staining further detected the decrease of IL-27p28 and EBI3 two chains by CD11c+ DCs (Figure 1H) and F4/80+ macrophages (Figure 1I) in CLNs of EAC mice. IL-27p28-positive cells decreased from 63.3% to 25.2% in DCs and from 44.5% to 14.3% in macrophages. EBI-3-positive cells decreased from 55.6% to 15.4% in DCs and from 54.6% to 19.1% in macrophages.
3.3 |. Th2-dominant ocular allergic inflammation was exacerbated in IL-27Rα knockout WSX-1−/− mice with EAC model
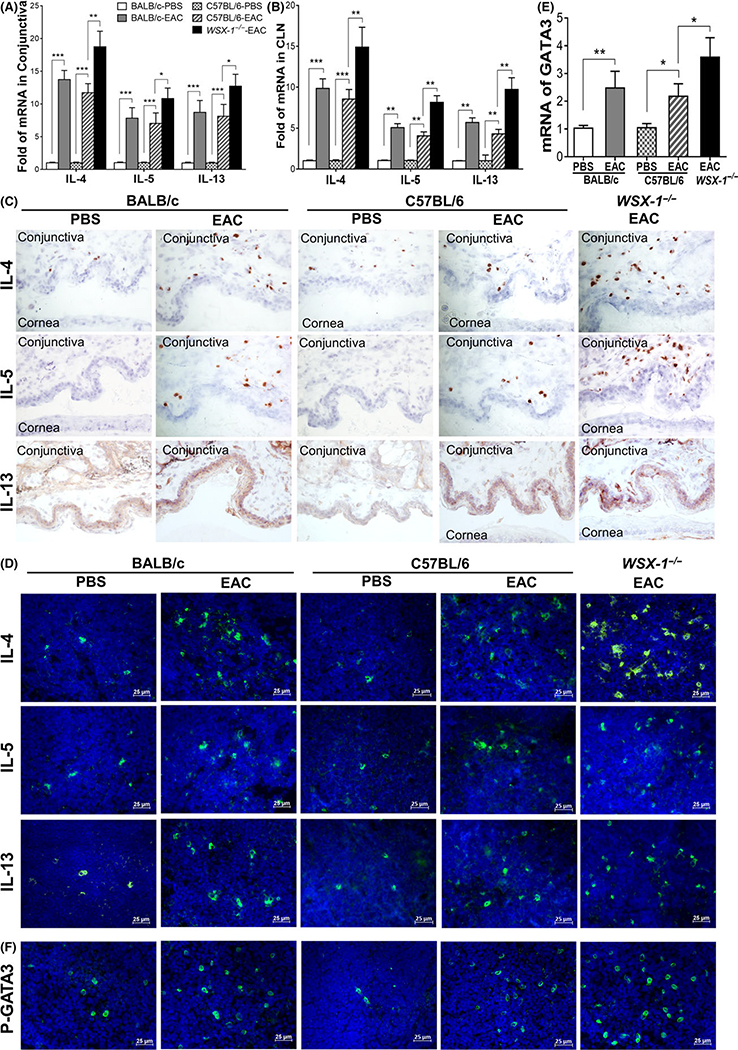
The Th2-dominant inflammation was clearly observed in the EAC models of both BALB/c and C57BL/6 mice, which showed the increased mRNA expression and protein production of Th2 cytokines, IL-4, IL-5, and IL-13, in conjunctival stroma with largely infiltrated lymphocytes, and in the draining CLNs (Figure 2A–D). To confirm the anti-allergic effects of IL-27, IL-27Rα knockout WSX-1−/− mice were induced to be EAC model using same protocol with wildtype C57BL/6 mice as controls. Compared with wild-type C57BL/6-EAC and BALB/c-EAC, WSX-1−/−-EAC showed more severe allergic signs and higher clinical scores with statistical significance (Figure 1A,B), as well as stronger Th2-dominant responses in conjunctiva and CLNs. Th2 cytokine expression was much higher in WSX-1−/−-EAC than wild-type C57BL/6-EAC and BALB/c-EAC mice, showing 18.7 vs 11.7 (C57BL/6)/13.7-fold (BALB/c) of IL-4, 10.8 vs 7.0/7.8-fold of IL-5, and 12.7 vs 8.1/8.7-fold of IL-13, in conjunctiva (Figure 2A), as well as 14.9 vs 8.6/9.8-fold of IL-4, 8.1 vs 4.1/5.0-fold of IL-5, and 9.7 vs 4.3/5.7-fold of IL-13, in CLNs (Figure 2B). Immunohistochemical and immunofluorescent staining confirmed the enhanced increase of Th2 cytokines at protein levels in the conjunctiva (Figure 2C) and CLNs (Figure 2D) of WSX-1−/−-EAC mice.
FIGURE 2.
Exacerbated Th2-dominant inflammation in WSX-1−/−-experimental allergic conjunctivitis (EAC) mice compared with C57BL/6-EAC, BALB/c-EAC, and PBS controls. Production of IL-4, IL-5, and IL-13 by conjunctiva at mRNA (A) and protein levels using immunohistochemical staining (C), as well as by CLNs (B & D); E-F, GATA3 expression and activation by phosphorylated P-GATA3 in CLNs. Results shown are Mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, n = 5, compared with controls
Furthermore, Th2 specific transcription factor GATA-3 was largely activated in EAC mice, especially in WSX-1−/−-EAC mice. As shown in Figure 2E, GATA3 transcripts expressed by CLNs were upregulated to 2.5 ± 0.6-fold in BALB/c-EAC, 2.2 ± 0.4-fold in C57BL/6-EAC, and 3.6 ± 0.7-fold in WSX-1−/−-EAC mice. GATA3 activation was confirmed by immunofluorescent staining in CLNs, which showed significant increase of phosphorylated (P)-GATA3-positive cells in C57BL/6- and BALB/c-EAC, and much more in WSX-1−/−-EAC mice (Figure 2F). These findings indicate the stronger Th2-dominant inflammation in WSX-1−/−-EAC mice deficient to IL-27Rα.
3.4 |. The aggravated allergic inflammation was associated with enhanced Th17 response in WSX-1−/−-EAC mice deficient for IL-27 signaling
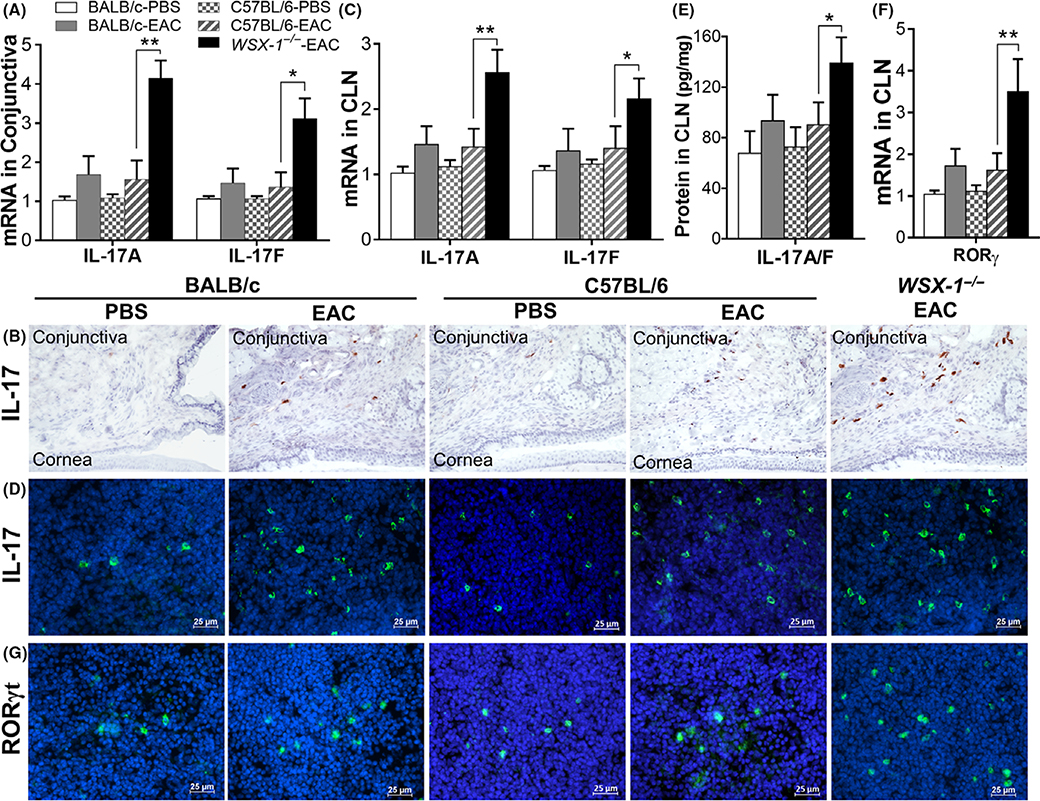
While allergic inflammation is mainly caused by Th2-type response in EAC of wild-type mice, Th17 hyperresponse was observed in WSX-1−/−-EAC mice. IL-17A and IL-17F, two bioactive IL-17 homologues expressed mainly by Th17 cells,29,30 were highly expressed by conjunctival (Figure 3A) and CLN (Figure 3C) cells in WSX-1−/−-EAC mice, compared with wild-type C57BL/6-EAC or BALB/c- EAC and PBS controls. Immunohistochemical staining (Figure 3B) clearly showed markedly increase of IL-17+ cells in conjunctival stroma of WSX-1−/−-EAC mice, representing the infiltrated Th17 cells in the ocular surface. Immunofluorescent staining (Figure 3D) also showed IL-17+ cells increased in CLNs of WSX-1−/−-EAC mice, suggesting that Th17 cells were much more differentiated from naïve T cells when lack of IL-27 signaling. This pattern of IL-17 production was confirmed and quantified using the ELISA kit that measures both IL-17A and IL-17F (Figure 3E). IL-17 protein levels in CLNs significantly increased to 139.2 ± 20.5 pg/mg (P < 0.05, n = 6) in WSX-1−/−-EAC mice from 90.4 ± 17.6 in wild-type C57BL/6-EAC and 93.4 ± 20.6 pg/mg in BALB/c-EAC mice. The IL-17 levels were similar between two wild mice, and increased slightly from their PBS controls, 72.8 ± 15.6 in C57BL/6 and 67.7 ± 17.6 pg/mg in BALB/c mice. Interestingly, Th17 specific transcription factor, retinoic acid-related orphan receptor gamma t (RORγt),31 was found to increase significantly at mRNA (Figure 3F) and protein (IL-17+ immunopositive cells, Figure 3G) levels in CLNs of WSX-1−/−-EAC mice. These findings suggest that the augmented Th17 responses, via increased RORγt, aggravated allergic inflammation in WSX-1−/−-EAC mice lacking IL-27 signaling.
FIGURE 3.
Th17 signaling molecules increased specially in WSX-1−/−-experimental allergic conjunctivitis (EAC) mice compared with C57BL/6-EAC, BALB/c-EAC, and PBS controls. Production of IL-17A and IL-17F at mRNA and protein levels by conjunctiva (A-B) and by CLNs (C-E); F-G, RORγt expression and immunopositive cells in CLNs. Results shown are Mean ± SD. *P < 0.05, **P < 0.01, n = 5, compared with controls
3.5 |. Th1 and Treg cytokines, IFNγ and IL-10, were downregulated in EAC, especially in WSX-1−/− mice
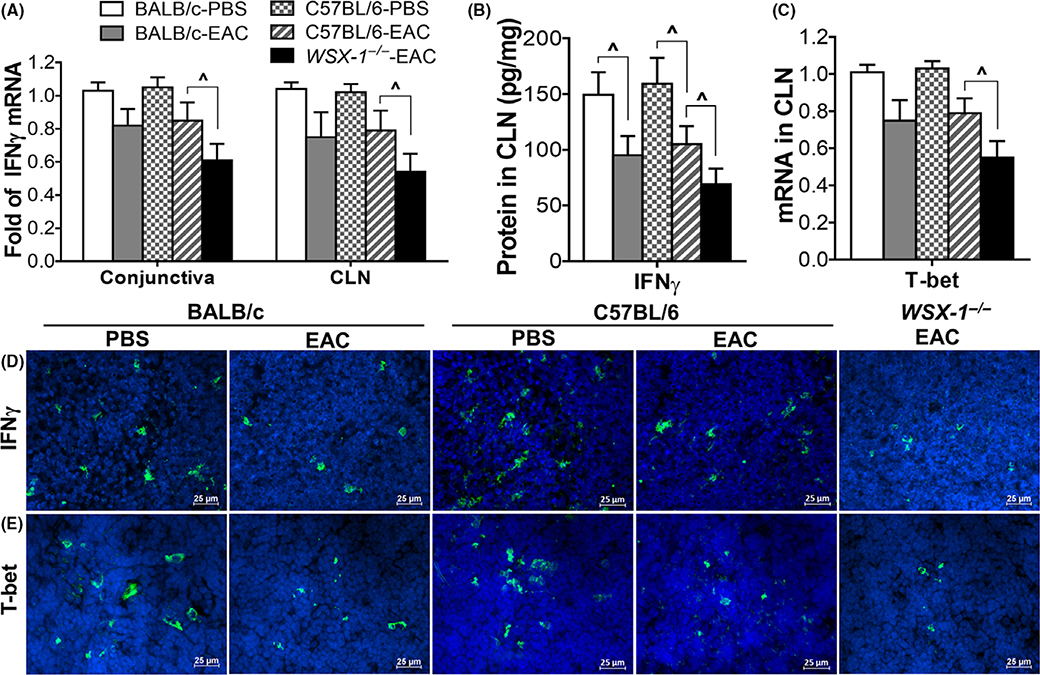
Th1 cytokine IFNγ was significantly downregulated at mRNA and protein levels in WSX-1−/−-EAC mice. Compared with wild-type C57BL/6-EAC, IFNγ mRNA expression was reduced by 39% and 46% (both P < 0.05, n = 6) in conjunctiva and CLN, respectively, of WSX-1−/−-EAC mice (Figure 4A). IFNγ protein production in CLNs from 159.4 ± 23.4 (C57BL/6)/149.4 ± 20.4 (BALB/c) pg/mg in PBS controls decreased significantly to 91.3 ± 16.1 (P < 0.05, n = 6) in C57BL/6-EAC, 88.3 ± 17.1 (P < 0.05, n = 6) in BALB/c-EAC, and 69.1 ± 14.1 pg/mg (P < 0.01, n = 6) in WSX-1−/−-EAC mice (Figure 4B). Th1 specific transcription factor T-bet mRNA was reduced by 41.6% 1 (P < 0.05, n = 6) in CLNs of WSX-1−/−-EAC mice (Figure 4C). Immunofluorescent staining confirmed the decrease of IFNγ+ (Figure 4D) and T-bet+ cells (Figure 4E) in CLNs.
FIGURE 4.
IFNγ was downregulated in pollen-induced experimental allergic conjunctivitis (EAC) model in C57BL/6, BALB/c, and WSX-1−/− mice. IFNγ production at mRNA and protein levels by conjunctiva (A) and CLNs (A,B,D); T-bet expression (C), and immunopositive cells in CLNs (E). Results shown are Mean ± SD. P < 0.05, n = 5, compared with controls
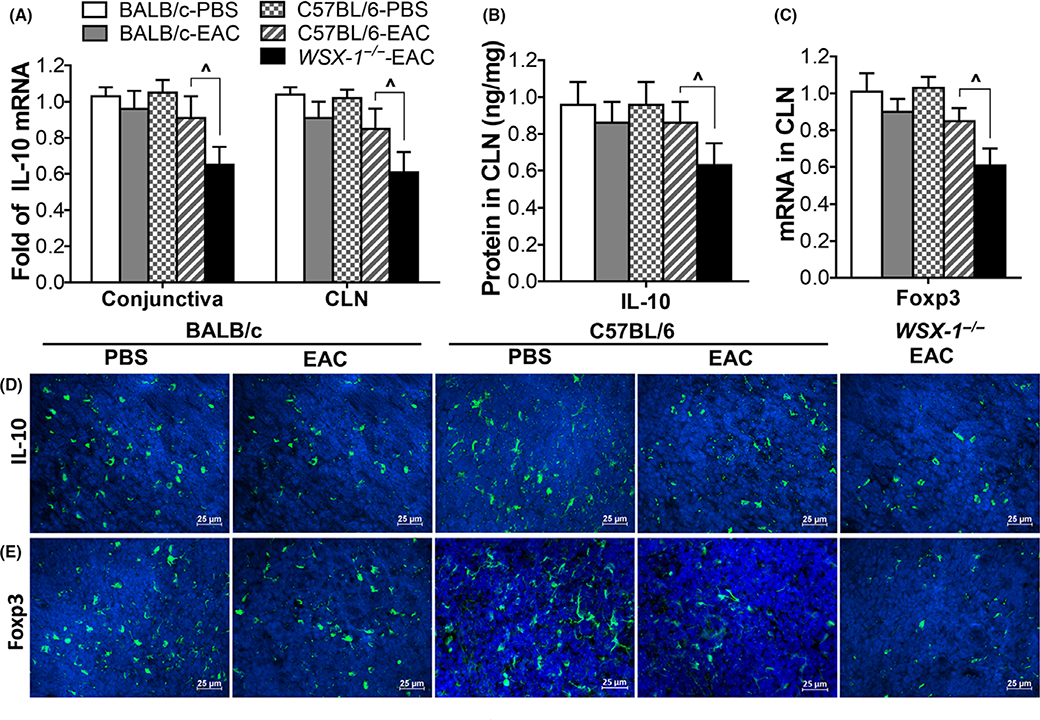
Treg cytokine IL-10 production was found to be suppressed in WSX-1−/−-EAC mice. IL-10 mRNA expression decreased to 0.71-fold (P < 0.05, n = 6) in conjunctiva and 0.63-fold (P < 0.05, n = 6) in CLNs of WSX-1−/−-EAC, significantly lower than PBS controls, C57BL/6-EAC and BALB/c-EAC mice (Figure 5A). ELISA quantified that IL-10 production in CLNs decreased to 0.63 ± 0.12 ng/mg (P < 0.05, n = 6) in WSX-1−/−-EAC mice from 0.96 ± 0.13 ng/mg in controls (Figure 5B). Immunofluorescent staining confirmed the decrease of IL-10+ cell number in CLNs (Figure 5D). Treg cell marker transcription factor foxp3 was also downregulated in WSX-1−/−-EAC mice at mRNA (Figure 5C) and protein levels (Figure 5E). These findings suggest that IL-10 production was decreased in EAC mice deficient to IL-27 signaling.
FIGURE 5.
IL-10 was downregulated in pollen-induced WSX-1−/− experimental allergic conjunctivitis (EAC) mice. IL-10 production at mRNA and protein levels by conjunctiva (A) and CLNs (A,B,D); Foxp3 expression (C) and immunopositive cells in CLNs (E). Results shown are Mean ± SD. P < 0.05, n = 5, compared with controls
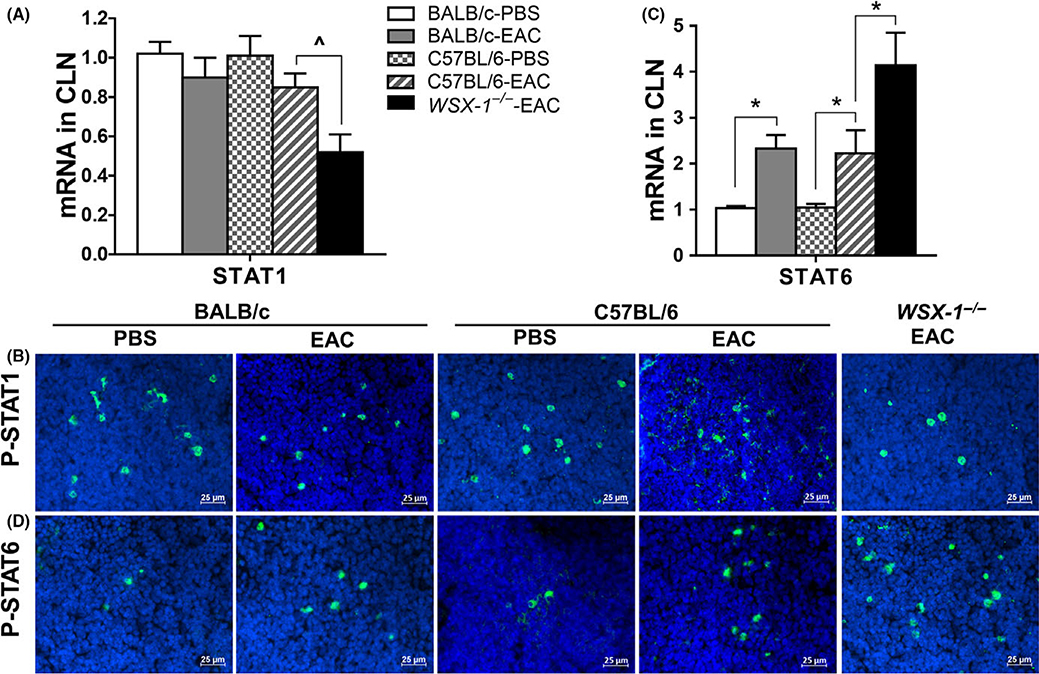
3.6 |. Imbalanced activation between STAT1 and STAT6 was observed in WSX-1−/−-EAC mice
IL-27 has been known to regulate T-cell differentiation via signal transducer and activator of transcription 1 (STAT1).9,32 In murine EAC model, STAT1 expression and activation were found to be downregulated. STAT1 mRNA in CLNs decreased by 48.5% (P < 0.05, n = 6) in WSX-1−/−-EAC mice (Figure 6A), and the cells expressing phosphorylated P-STAT1 were reduced significantly in WSX-1−/−-EAC mice (Figure 6B). In contrast, STAT6 signaling, which is critical to differentiation of Th2 effector cells and hyperresponsiveness in allergic disease,33–35 was upregulated and activated significantly in EAC model. STAT6 transcripts in CLNs increased by 1.9-fold (P < 0.05) in wild-type C57BL/6-EAC and by 4.1-fold (P < 0.01) in WSX-1−/− EAC mice (Figure 6C). The immunopositive cells to phosphorylated P-STAT6 increased significantly, especially in WSX-1−/−-EAC mice (Figure 6D). The findings suggest that the ratio of STAT1/STAT6 activity was largely reduced when IL-27 signaling was suppressed or deficient.
FIGURE 6.
Decreased STAT1 and increased STAT6 in CLNs of WSX-1−/−-experimental allergic conjunctivitis (EAC) mice compared with C57BL/6-EAC, BALB/c-EAC, and PBS controls. Gene expression of STAT1 (A) and STAT6 (C) and phosphorylated P-STAT1 (B) and PSTAT6 (D). Results shown are the Mean ± SD. *P < 0.05, ^P < 0.05, n = 5, compared with controls
4 |. DISCUSSION
IL-27 is a cytokine with strikingly diverse influences on the immune response.9,21,36 Using a well-established murine EAC model,22 we observed the TSLP-driven Th2-dominent allergic inflammation with the significant decrease of IL-27 at mRNA (IL-27/EBI3) and protein levels in serum, conjunctiva and CLN. This allergic inflammation was aggravated by stimulated Th17 response with reduced IFNγ and IL-10, in EAC induced in IL-27R-deficient WSX-1−/− mice, suggesting a protective role of IL-27 in allergic disease.
First, we identified that IL-27 cytokine was significantly suppressed at the mRNA and protein levels in the murine EAC model. IL-27 protein levels in serum decreased significantly when compared with PBS controls; the mRNA levels of IL-27 and EBI3, the two chains of IL-27p28 and EBI3 peptides, and entire IL-27 protein levels were found to be reduced significantly in conjunctiva and draining CLNs (Figure 1). These findings provide strong evidence showing the suppressed IL-27 signaling in Th2-dominant EAC mouse model.
To confirm the inhibitory effects of IL-27 on Th2 development, IL-27Rα knockout WSX-1−/− mice were induced to be EAC model using same protocol. Compared with wild-type C57BL/6-EAC controls and BALB/c-EAC mice, WSX-1−/− EAC mice showed more severe clinical allergic manifestations with highly stimulated expression and production of Th2 cytokines, IL-4, IL-5, and IL-13, as well as the upregulated activation of phosphor GATA3, a Th2 specific transcription factor, in conjunctiva and CLNs (Figure 2). The findings demonstrate that the suppression and depletion of IL-27 signaling exacerbated Th2-domminant inflammation. The notion is supported by previous reports showing that IL-27 has emerged as an important negative regulator of Th2 responses, and the molecular basis for the effects has been revealed by studies showing that IL-27 antagonized expression of GATA3.13,37
Second, we revealed that in addition to Th2-dominant inflammation, Th17 cytokines were upregulated to aggravate allergic inflammation in WSX-1−/−-EAC mice that is completely lack of IL-27 signaling due to IL-27Rα knocked out. Recently, Th17 response has been recognized to be involved in pathogenesis of allergic diseases, such as asthma and dermatitis.38,39 However, the role of Th17 in ocular allergic disease is largely unknown. In our EAC model, Th17 cell number and Th17 cytokine production did not increase significantly in wide type mice, but dramatic stimulation and activation of Th17 cytokines, IL-17A and IL-17F, as well as the specific transcription factor RORγt were noted in WSX-1−/− mice (Figure 3). These findings suggest that Th17 cell development was augmented to aggravate Th2-dominant allergic inflammation when IL-27 signaling is depleted in WSX-1−/−-EAC mice.
Then, we observed the inhibited expression and production of IFNγ and IL-10 in our EAC model, especially in WSX-1−/−EAC mice, suggesting downregulated differentiation of Th1 and Treg cells. The fact that IL-27 promotes naïve CD4+ T cell proliferation and IFNγ production supports the idea that IL-27 facilitates Th1 response.9,32 In our EAC model, Th1 cytokine IFNγ was found to decrease significantly with reduced transcription factor T-bet, especially in WSX-1−/−EAC mice (Figure 4), which indicate that the suppression/depletion of IL-27 signaling leads to impaired response of Th1/Th2 in the bias to Th2-dominant allergic inflammation.
IL-27 has been reported to promote expansion of IL-10, an important anti-inflammatory cytokine,16,40 but reduced IL-10 levels have not been reported by pollen-induced allergic disease, nor in WSX-1−/− mice. In our EAC mice, IL-10 and Treg cell transcription factor foxp3 in draining CLNs were reduced in mRNA and protein levels, especially in WSX-1−/−EAC mice (Figure 5). Inhibition of IL-10 may contribute to stimulated Th2 and Th17 in allergic condition due to suppressed or depleted IL-27 signaling.
Furthermore, imbalanced activation of STAT1 and STAT6 was observed to be a potential transcriptional signaling that mediated the impaired regulation of T-cell differentiation. IL-27 has been known to regulate Th1 and foxp3 via inducing STAT1, and IL-27 was recently reported to attenuate airway inflammation in a mouse asthma model via STAT1.41,42 STAT6 signaling has been recognized to be necessary for the development of Th2 response and allergic inflammation.33,35 However, it is not clear how IL-27 regulates STAT1 and STAT6 both in allergic disease. Using murine EAC model, we revealed that STAT1 mRNA and activated form of phosphorylated STAT1 in CLNs were largely reduced while STATA6 mRNA and activated phosphorylated STAT6 were significantly stimulated, especially in WSX-1−/−EAC mice (Figure 6). The reduced ratio of STAT1 and STAT6 activities suggests a potential mechanism by which the suppression and depletion of IL-27 signaling result in the impaired Tcell regulation and responses. However, our data could not reach the conclusion whether STAT1 and STAT6 were preferentially driving a Th2 or a Th17 cell response. To identify their effects independently, using knockout mice deficient for STAT1 or STAT6 is necessary for future studies.
Our findings in animal model were also supported by recently clinical studies, which investigate part of these topics in human allergic diseases. Significantly decreased levels of serum IL-27 were reported in systemic lupus erythematosus, evaluated in 70 patients and 30 healthy controls by ELISA (mean 899.92 vs 1531.22 pg/mL, P = 0.0005).43 By examining five exons and the boundary intron sequences of IL-27P28, the g.-964A > G polymorphism of IL-27p28 was identified to associate with susceptibility to asthma in a Korean population.44 IL-27p28 polymorphisms rs153109 and rs17855750 were also found to associate with allergic rhinitis susceptibility in Chinese Han population.45
In conclusion, our findings demonstrated a novel anti-inflammatory roles and signaling pathways of IL-27 in allergic inflammation. IL-27 signaling deficiency aggravates Th2-dominant inflammation with enhanced Th17 response, associated with reduced expression of Th1 and Treg cytokines via imbalanced activation of STAT1/ STAT6. Our findings shed light on the understanding of pathogenesis of allergic inflammation and may create new therapeutic targets to treat allergic diseases. However, further studies are necessary to explore how IL-27 is downregulated in DCs and macrophages in allergic diseases, and what are the effects of recombinant IL-27 treatment in EAC model.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by National Institutes of Health, National Eye Institute grants R01 EY023598 (DQL), EY011915 (SCP), and Core Grant for Vision Research EY002520, an unrestricted grant from Research to Prevent Blindness, the Oshman Foundation, and the William Stamps Farish Fund.
Funding information
National Institutes of Health; National Eye Institute, Grant/Award Number: EY002520, EY011915, EY023598; Research to Prevent Blindness; Oshman Foundation; William Stamps Farish Fund
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Liu YJ. TSLP in epithelial cell and dendritic cell cross talk. Adv Immunol. 2009;101:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Z, Hener P, Frossard N, et al. Thymic stromal lymphopoietin overproduced by keratinocytes in mouse skin aggravates experimental asthma. Proc Natl Acad Sci USA. 2009;106:1536–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li D-Q, Zhang L, Pflugfelder SC, et al. Short ragweed pollen triggers allergic inflammation through Toll-like receptor 4-dependent thymic stromal lymphopoietin/OX40 ligand/OX40 signaling pathways. J Allergy Clin Immunol. 2011;128:1318–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Zhang L, Chen X, et al. Pollen/TLR4 innate immunity signaling initiates IL-33/ST2/Th2 pathways in allergic inflammation. Sci Rep. 2016;6:36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo Z, Wu J, Zhao J, et al. IL-33 promotes airway remodeling and is a marker of asthma disease severity. J Asthma. 2014;51:863–869. [DOI] [PubMed] [Google Scholar]
- 6.Cevikbas F, Steinhoff M. IL-33: a novel danger signal system in atopic dermatitis. J Invest Dermatol. 2012;132:1326–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng X, Ma P, de Paiva CS, et al. TSLP and downstream molecules in experimental mouse allergic conjunctivitis. Invest Ophthalmol Vis Sci. 2010;51:3076–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida H, Hunter CA. The immunobiology of interleukin-27. Annu Rev Immunol. 2015;33:417–443. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki Y, Fujio K, Okamura T, Yamamoto K. Interleukin-27 in T cell immunity. Int J Mol Sci. 2015;16:2851–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aparicio-Siegmund S, Garbers C. The biology of interleukin-27 reveals unique pro- and anti-inflammatory functions in immunity. Cytokine Growth Factor Rev. 2015;26:579–586. [DOI] [PubMed] [Google Scholar]
- 11.Hunter CA, Kastelein R. Interleukin-27: balancing protective and pathological immunity. Immunity. 2012;37:960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida H, Nakaya M, Miyazaki Y. Interleukin 27: a double-edged sword for offense and defense. J Leukoc Biol. 2009;86:1295–1303. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimoto T, Yoshimoto T, Yasuda K, Mizuguchi J, Nakanishi K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. J Immunol. 2007;179:4415–4423. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki Y, Inoue H, Matsumura M, et al. Exacerbation of experimental allergic asthma by augmented Th2 responses in WSX-1-deficient mice. J Immunol. 2005;175:2401–2407. [DOI] [PubMed] [Google Scholar]
- 15.Shimanoe Y, Miyazaki Y, Hara H, Inokuchi A, Yoshida H. Amelioration of experimental allergic rhinitis with suppression of topical immune responses by lack of IL-27/WSX-1 signaling. Ann Allergy Asthma Immunol. 2009;102:223–232. [DOI] [PubMed] [Google Scholar]
- 16.Neufert C, Becker C, Wirtz S, et al. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol. 2007;37:1809–1816. [DOI] [PubMed] [Google Scholar]
- 17.Diveu C, McGeachy MJ, Boniface K, et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–5756. [DOI] [PubMed] [Google Scholar]
- 18.Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4 + T cells. J Immunol. 2009;183:2435–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pot C, Apetoh L, Awasthi A, Kuchroo VK. Induction of regulatory Tr1 cells and inhibition of T(H)17 cells by IL-27. Semin Immunol. 2011;23:438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider R, Yaneva T, Beauseigle D, El-Khoury L, Arbour N. IL-27 increases the proliferation and effector functions of human naive CD8 + T lymphocytes and promotes their development into Tc1 cells. Eur J Immunol. 2011;41:47–59. [DOI] [PubMed] [Google Scholar]
- 21.Stumhofer JS, Hunter CA. Advances in understanding the antiinflammatory properties of IL-27. Immunol Lett. 2008;117:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magone MT, Chan CC, Rizzo LV, Kozhich AT, Whitcup SM. A novel murine model of allergic conjunctivitis. Clin Immunol Immunopathol. 1998;87:75–84. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Ruzhi D, Hua X, et al. Blueberry component pterostilbene protects corneal epithelial cells from inflammation via anti-oxidative pathway. Sci Rep. 2016;6:19408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma P, Bian F, Wang Z, et al. Human corneal epithelium-derived thymic stromal lymphopoietin links the innate and adaptive immune responses via TLRs and Th2 cytokines. Invest Ophthalmol Vis Sci. 2009;50:2702–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Lu R, Zhao G, Pflugfelder SC, Li D-Q. TLR-mediated induction of pro-allergic cytokine IL-33 in ocular mucosal epithelium. Int J Biochem Cell Biol. 2011;43:1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HS, Jun SX, de Paiva CS, Chen Z, Pflugfelder SC, Li D-Q. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp Eye Res. 2004;79:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Paiva CS, Chen Z, Corrales RM, Pflugfelder SC, Li D-Q. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005;23:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HS, Luo L, Pflugfelder SC, Li DQ. Doxycycline inhibits TGFbeta1-induced MMP-9 via Smad and MAPK pathways in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:840–848. [DOI] [PubMed] [Google Scholar]
- 29.Liang SC, Long AJ, Bennett F, et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179:7791–7799. [DOI] [PubMed] [Google Scholar]
- 30.Zheng X, Bian F, Ma P, et al. Induction of Th17 differentiation by corneal epithelial-derived cytokines. J Cell Physiol. 2010;222:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007;19:409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeda A, Hamano S, Yamanaka A, et al. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. [DOI] [PubMed] [Google Scholar]
- 33.Stokes K, LaMarche NM, Islam N, Wood A, Huang W, August A. Cutting edge: STAT6 signaling in eosinophils is necessary for development of allergic airway inflammation. J Immunol. 2015;194:2477–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapoval SP, Dasgupta P, Smith EP, et al. STAT6 expression in multiple cell types mediates the cooperative development of allergic airway disease. J Immunol. 2011;186:2571–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chapoval S, Dasgupta P, Dorsey NJ, Keegan AD. Regulation of the Thelper cell type 2 (Th2)/T regulatory cell (Treg) balance by IL-4 and STAT6. J Leukoc Biol. 2010;87:1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Q, Liu J. Regulation and immune function of IL-27. Adv Exp Med Biol. 2016;941:191–211. [DOI] [PubMed] [Google Scholar]
- 37.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci USA. 2003;100:15047–15052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsushita A, Seike M, Hagiwara T, Sato A, Ohtsu H. Close relationship between T helper (Th)17 and Th2 response in murine allergic contact dermatitis. Clin Exp Dermatol. 2014;39:924–931. [DOI] [PubMed] [Google Scholar]
- 39.Li XM, Chen X, Gu W, et al. Impaired TNF/TNFR2 signaling enhances Th2 and Th17 polarization and aggravates allergic airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2017;303:L592–L601. [DOI] [PubMed] [Google Scholar]
- 40.Moon SJ, Park JS, Heo YJ, et al. In vivo action of IL-27: reciprocal regulation of Th17 and Treg cells in collagen-induced arthritis. Exp Mol Med. 2013;45:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su X, Pan J, Bai F, et al. IL-27 attenuates airway inflammation in a mouse asthma model via the STAT1 and GADD45gamma/p38 MAPK pathways. J Transl Med. 2016;14:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ouaked N, Mantel PY, Bassin C, et al. Regulation of the foxp3 gene by the Th1 cytokines: the role of IL-27-induced STAT1. J Immunol. 2009;182:1041–1049. [DOI] [PubMed] [Google Scholar]
- 43.Duarte AL, Dantas AT, de Ataide Mariz H, et al. Decreased serum interleukin 27 in Brazilian systemic lupus erythematosus patients. Mol Biol Rep. 2013;40:4889–4892. [DOI] [PubMed] [Google Scholar]
- 44.Chae SC, Li CS, Kim KM, et al. Identification of polymorphisms in human interleukin-27 and their association with asthma in a Korean population. J Hum Genet. 2007;52:355–361. [DOI] [PubMed] [Google Scholar]
- 45.Shen Y, Yuan XD, Hu D, et al. Association between interleukin-27 gene polymorphisms and susceptibility to allergic rhinitis. Hum Immunol. 2014;75:991–995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.