Abstract
Glycans are carbohydrate modifications typically found on proteins or lipids, and can act as ligands for glycan-binding proteins called lectins. Glycans and lectins play crucial roles in the function of cells and organs, and in the immune system of animals and humans. Viral pathogens use glycans and lectins that are encoded by their own or the host genome for their replication and spread. Recent advances in glycobiological research indicate that glycans and lectins mediate key interactions at the virus-host interface, controlling viral spread and/or activation of the immune system. This review reflects on glycan–lectin interactions in the context of viral infection and antiviral immunity. A short introduction illustrates the nature of glycans and lectins, and conveys the basic principles of their interactions. Subsequently, examples are discussed highlighting specific glycan–lectin interactions and how they affect the progress of viral infections, either benefiting the host or the virus. Moreover, glycan and lectin variability and their potential biological consequences are discussed. Finally, the review outlines how recent advances in the glycan–lectin field might be transformed into promising new approaches to antiviral therapy.
Keywords: DC-SIGN, collectin, galectin, hemagglutinin, receptor-destroying enzyme, antiviral
Glycans and lectins cover crucial roles in virus biology and their interplay often shapes the virus-host interaction. This review reflects on glycan-lectin interactions in the context of viral infection and anti-viral immunity, and explores potential targets for antiviral strategies.
Introduction
Many emerging and re-emerging viral diseases in animals and humans pose significant global health problems for which novel antiviral measures are in urgent demand. In general, rational design of new prophylactic and curative antiviral strategies requires a detailed knowledge of the viral infection mechanism and the host's innate and adaptive immune defense. Historically, cell biological and microbiological research was mainly focussed on the nucleic acid and protein level. However, over the last few decades it has become clear that also glycans account to a great extent for the structural and functional diversity displayed by animal and human cells and their pathogens. At this moment, glycobiology is one of the most rapidly expanding disciplines in biology. A rapidly evolving array of powerful, novel techniques for the analysis of glycan structure and function (glycomics) has prompted many scientists to apply these tools to the field of virology, and this glycovirological approach has yielded a wealth of information on the various glycobiological aspects of viral infection and antiviral immunity. Particularly fascinating in this context are the many distinct glycan-lectin interactions that may occur during viral infection of a host. Virion-associated glycans often serve as ligands for specific host lectins. Conversely, the glycan portions of host glycoconjugates function as receptors for various viruses that employ viral lectins for host cell entry. This review reflects on glycan–lectin interactions in the context of viral infection and antiviral immunity. Following a general introduction on glycan and lectin biology, specific glycan–lectin interactions are highlighted and discussed within the larger framework of viral infection and immunity. Distinction is made between interactions that benefit the host and interactions that benefit the virus. In addition, different factors that contribute to glycan and lectin variation – and that consequently affect glycan–lectin interactions – are explored and various approaches to modulate specific glycan–lectin interactions in antiviral therapies are briefly discussed.
Glycans vs. lectins
Glycans
Like nucleic acids, proteins and lipids, glycans are essential components of the animal cell and organism. The term ‘glycan’ refers to the carbohydrate portion of glycoproteins and glycolipids typically found at cell surfaces, in extracellular matrices and in cell secretions. Unlike nucleic acid and protein synthesis, the biosynthesis of glycans is not a template-driven process. Instead, glycosylation depends on the concerted action of different glycosyltransferase, glycosidase, and other enzymes. Several important glycan types are exclusively assembled by the enzyme sets present in the endoplasmic reticulum (ER) and the Golgi network: glycan/glycoconjugate synthesis is typically initiated in the ER or early Golgi and gradual processing and diversification – or ‘maturation’ – occurs as these molecules move further through the different enzyme-equipped compartments of the secretory pathway. The variability inherent to glycan synthesis and maturation forms the basis of the considerable diversity and complexity of the glycan repertoires found on animal glycoconjugates (Varki et al., 2009; Taylor & Drickamer, 2011).
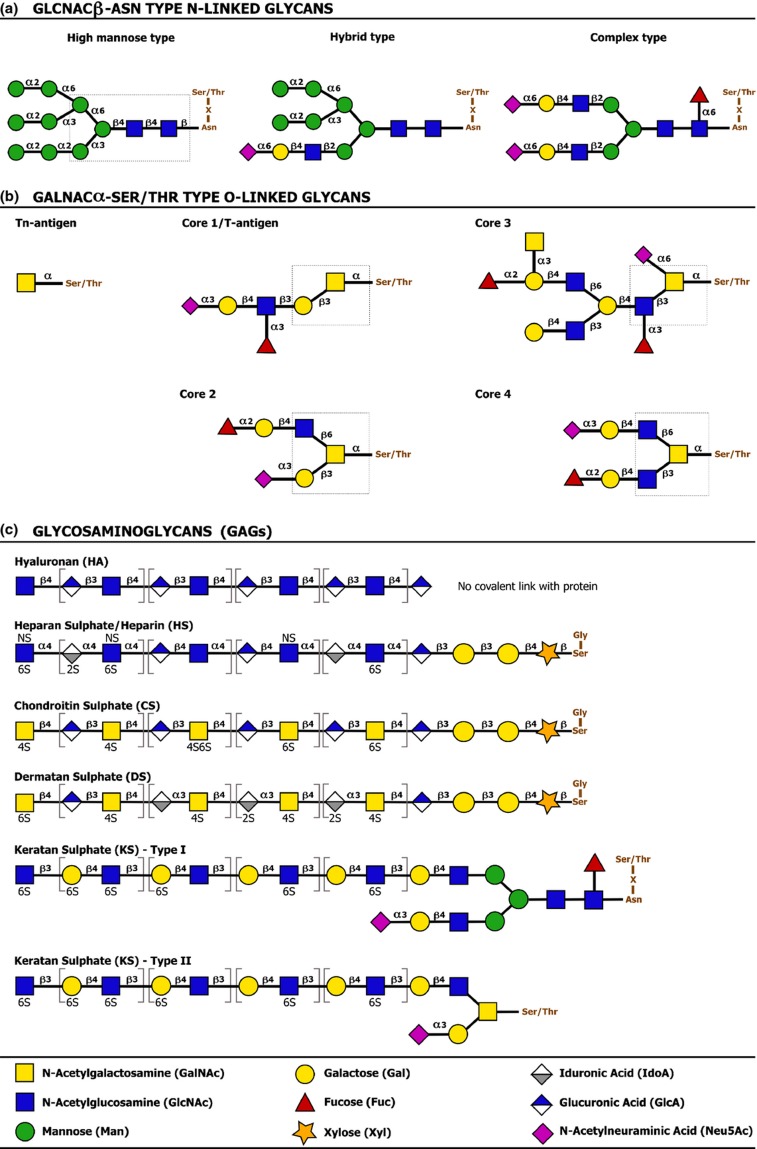
According to the basic glycan ‘core’ structure, the type of molecule the glycans are linked with and the type of the linkage, glycans and glycoconjugates can be categorized in different classes. Two major classes of protein-linked glycosylation are the N- and O-linked glycans. N-linked glycans are covalently linked to the nitrogen atoms of specific amino acid (aa) residues (typically Asparagine) via an N-glycosidic bond. N-acetylglucosamine–to–Asparagine (GlcNAcβ-Asn) type glycans represent the most common form of N-linked protein glycosylation (Weerapana & Imperiali, 2006; Varki et al., 2009; Larkin & Imperiali, 2011; Schwarz & Aebi, 2011; Taylor & Drickamer, 2011). O-linked glycans are covalently attached to the oxygen atoms of specific amino acid residues (typically Serine or Threonine) via an O-glycosidic bond. A common O-glycan type – and potentially the one most studied – is the N-acetylgalactosamine–to–Serine/Threonine (GalNAcα-Ser/Thr) type or mucin-type O-glycan. Other (nonmucin) O-glycan types include α-linked O-mannose, α-linked O-fucose, β-linked O-xylose, β-linked O-GlcNAc (N-acetylglucosamine), α-/β-linked O-galactose, and α-/β-linked O-glucose glycans (Van den Steen et al., 1998; Peter-Katalinic, 2005; Varki et al., 2009; Jensen et al., 2010; Gill et al., 2011; Taylor & Drickamer, 2011). Interestingly, various proteins are also modified with glycosaminoglycans (GAGs), linear polysaccharide chains composed of repeated disaccharide subunits consisting of a uronic acid/galactose residue and an amino sugar. Glycosaminoglycan-carrying glycoproteins are generally referred to as proteoglycans (Prydz & Dalen, 2000; Varki et al., 2009; Taylor & Drickamer, 2011). Figure 1 illustrates the general structure and classification of some common types of protein glycosylation. Similarly as for protein-linked glycosylation, different types of lipid-linked glycosylation can be discerned: the glycosphingolipids (Varki et al., 2009; Taylor & Drickamer, 2011; Yu et al., 2011) and the glycophospholipid anchors or glycosylphosphatidylinositol (GPI) anchors (Paulick & Bertozzi, 2008; Varki et al., 2009; Taylor & Drickamer, 2011) represent important glycolipid classes. Their basic structure is introduced in Fig. 2.
Figure 1.
Classification and basic structure of common types of protein-linked glycosylation. (a) GlcNAcβ-Asn type N-linked glycans are covalently attached to the amide nitrogen atoms of Asn side chains and are almost exclusively found on Asn residues within the sequence Asn-X-Ser/Thr, in which X can be any amino acid except Pro. The nature of the glycan structures that decorate the common glycan core – the glycan part shown in a dashed box – dictates classification of N-linked glycans as high-mannose type, hybrid type or complex type glycans, examples of which are shown in the panel. (b) GalNAcα-Ser/Thr type O-linked glycans have a GalNAc residue α-linked to the oxygen atom of the hydroxyl group of Ser or Thr residues. Unlike for GlcNAcβ-Asn type N-linked protein glycosylation, there are no clear amino acid motifs that mark these O-linked glycosylation sites. A single GalNac residue linked to the Ser/Thr is termed the ‘Tn antigen’. Depending on the basic structure of the glycan core, more complex (extended) O-linked glycans are categorized into different ‘core types’. Cores 1–4 are the most common core structures, but also other core types exist. The Tn antigen and examples of extended core 1, 2, 3, and 4 O-glycans are shown in the panel. The distinct glycan cores are shown in dashed boxes. (c) Glycosaminoglycans (GAGs) are linear polysaccharide chains composed of repeated disaccharide subunits of a uronic acid/galactose residue and an amino sugar. Glycosaminoglycans are classified as hyaluronan (HA), heparan sulfate/heparin (HS), chondroitin sulfate (CS), dermatan sulfate (DS), or keratan sulfate (KS), depending on the structure of their basic disaccharide subunits (shown in square brackets) and further modification (e.g. sulfation at different positions) of the glycan chain. With exception of hyaluronan, all major glycosaminoglycan types are sulfated and occur covalently linked to proteins. HS, CS, and DS are found on Ser-linked xylose residues. Although no unambiguous consensus sequence for xylosylation exists, the Ser attachment site is consistently flanked by a Gly residue at its carboxy-terminal side. As depicted in the figure, heparan sulfate and heparin have the same basic structure. Although they share a common biosynthesis, heparin generally undergoes more extensive sulfation and epimerization of uronic acid to iduronic acid. Moreover, heparin is synthesized only in connective tissue mast cells as part of serglycin proteoglycans, whereas heparan sulfate is synthesized in virtually all mammalian cells. KS is found on Asn-linked N-glycan core structures (KS I) or Ser/Thr-linked O-glycan core 2 structures (KS II). Capping or further modification of the glycosaminoglycan chains – sulfation excepted – is not depicted (adapted from Varki et al., 2009).
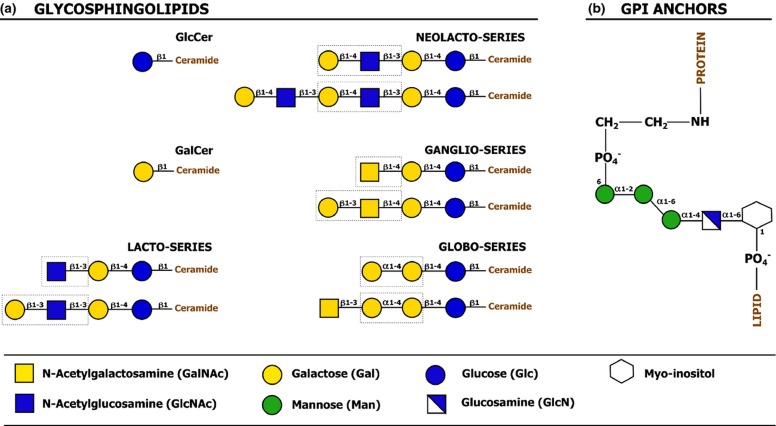
Figure 2.
Classification and basic structure of major types of lipid-linked glycosylation. (a) Glycosphingolipids consist of a hydrophilic glycan moiety linked to a hydrophobic sphingolipid. In higher animals, a ceramide lipid molecule is initially modified with a β-linked glucose or galactose residue, after which further extension and modification of the glycan moiety can occur. Extension to larger glycan chains is common on ceramide-linked glucose residues, whereas further glycan extension on ceramide-linked galactose residues is more rare. Depending on their glycan core structure, glycosphingolipids are classified in ‘series’. The figure depicts a number of glycosphingolipid core structures. The key features that characterize each series are shown in dashed boxes. Core structures can be further modified with sialic acids or sulfate groups, which allows subclassification of glycosphingolipids as neutral (lacking charged carbohydrates or ionic groups), sialylated or sulfated. (b) Glycosylphosphatidylinositol (GPI) anchors are found in association with certain membrane proteins and serve as linkers between the protein and the lipid membrane. Glycosylphosphatidylinositol anchors have a common core structure comprising ethanolamine-PO4-6Manα1-2Manα1-6Manα1-4GlcNα1-6myo-inositol-1-PO4-lipid. Differential derivatization of this common core structure through lipid remodeling and modification of the glycan moiety can cause significant glycosylphosphatidylinositol anchor heterogeneity. The protein is linked to the glycosylphosphatidylinositol anchor via an amide linkage between the C-terminal carboxyl group of the protein and the amino group of phosphatidylethanolamine (adapted from Varki et al., 2009).
Despite their different core structures and linkages to carrier molecules, distinct glycan types can still share conserved structural characteristics as they often follow partially overlapping biosynthetic pathways. Although some glycan features may be exclusively found in one specific glycan class, many (sub)terminal glycan modifications can be found in different glycan classes. Common (sub)terminal modifications include poly-N-acetyllactosamine chains, ABH and Lewis histo-blood group antigens (HBGA), and sialic acids in different linkages (Varki et al., 2009; Taylor & Drickamer, 2011). Consequently, the glycan moieties of glycoproteins and glycolipids often have more in common than one would expect based on their core structure.
Apart from the different glycan types introduced here, several other forms of glycosylation exist. However, an in-depth discussion of these is beyond the scope of this review. For more detailed information on glycan structure and biosynthesis, readers may refer to recent reference works on this topic (Varki et al., 2009; Taylor & Drickamer, 2011).
Not only the animal and human hosts, but also their pathogens can benefit from the fine-tuned cellular biosynthetic pathways that govern glycosylation. This is most obvious in the case of obligatory intracellular pathogens such as viruses. Glycans form an important part of the surface of many viruses. As the glycosylation of viral components is facilitated by the cellular glycosylation machinery, viral glycosylation is similar to that of the host. However, important differences have been noted: Viral glycoproteins are often more heavily glycosylated than their cellular counterparts and the composition of individual glycan chains can diverge greatly between virus and host. The biological basis of this variability is further situated later in this review (see ‘Glycan and lectin variation at the virus level’).
In line with the similarity between viral and host glycosylation, many of the basic functions covered by glycans in normal animal and human physiology and in viral infection biology – which is intrinsically linked to the biology of the host – are essentially the same. Glycans play important structural roles and are for instance implicated in protein folding and solubility, protease resistance, and masking of highly immunogenic protein stretches (‘glycan shielding’) (Varki et al., 2009; Taylor & Drickamer, 2011). Alternatively, glycans can also have nonstructural roles and take part in specific recognition events, in which they usually interact with complementary glycan-binding proteins called lectins (Varki et al., 2009; Taylor & Drickamer, 2011).
Lectins
Lectins may simply be defined as carbohydrate-binding proteins, although some definitions are more restrictive and exclude mono-/oligo-saccharide transport proteins, enzymes, glycan-specific antibodies, and even glycosaminoglycan-binding proteins (Elgavish & Shaanan, 1997; Weis, 1997; Loris, 2002; Varki et al., 2009; Gabius et al., 2011). According to the more strict definitions, glycosaminoglycan-binding proteins and lectins are distinguished based on different factors, including their ligand range, the structural basis of their glycan recognition, and their conservation (Varki et al., 2009). In general, glycosaminoglycan-binding proteins interact with negatively charged glycosaminoglycans via clusters of positively charged aa residues and – with exception of hyaluronan-binding proteins, which seem to share an evolutionarily conserved fold – do not appear to be evolutionarily related to each other (Varki et al., 2009). In contrast, most strict sense lectins belong to protein families with defined ‘carbohydrate recognition domains’ (CRD). The CRDs within a lectin family share structural and functional properties and selectively recognize specific portions of N-glycans, O-glycans, or glycolipids (sometimes also glycosaminoglycans) (Varki et al., 2009). Although some CRDs can efficiently bind monosaccharides, other CRDs show no apparent affinity for monosaccharides and favor oligosaccharide ligands. The latter CRD type often has a preference for ligands with specific linkages between the monosaccharide subunits, as these linkages determine the 3D structure of the glycan ligand and therefore the portions of the glycan that are available for interaction with the CRD.
Interactions between a single CRD and a single mono-/oligo-saccharide (affinity) are often weak, and strong interactions are usually the result of multivalent binding, i.e. the interaction of multiple CRDs with multiple ligands (avidity). Whereas some lectins contain multiple CRDs that can participate in ligand binding, others contain only a single CRD and rely on clustering of individual lectin molecules for high avidity binding. Clustering of CRDs does not only allow stronger interactions with ligands, but also contributes to the specificity/selectivity of interactions at the multivalent level. The relative spacing of the CRDs allows highly avid, multivalent binding to specific saccharide ligands in a certain density and particular presentation. Ultimately, the avidity of lectins for specific glycoconjugates depends on the structure, multivalency, and density of glycans on these molecules (Elgavish & Shaanan, 1997; Weis, 1997; Loris, 2002; Varki et al., 2009; Gabius et al., 2011).
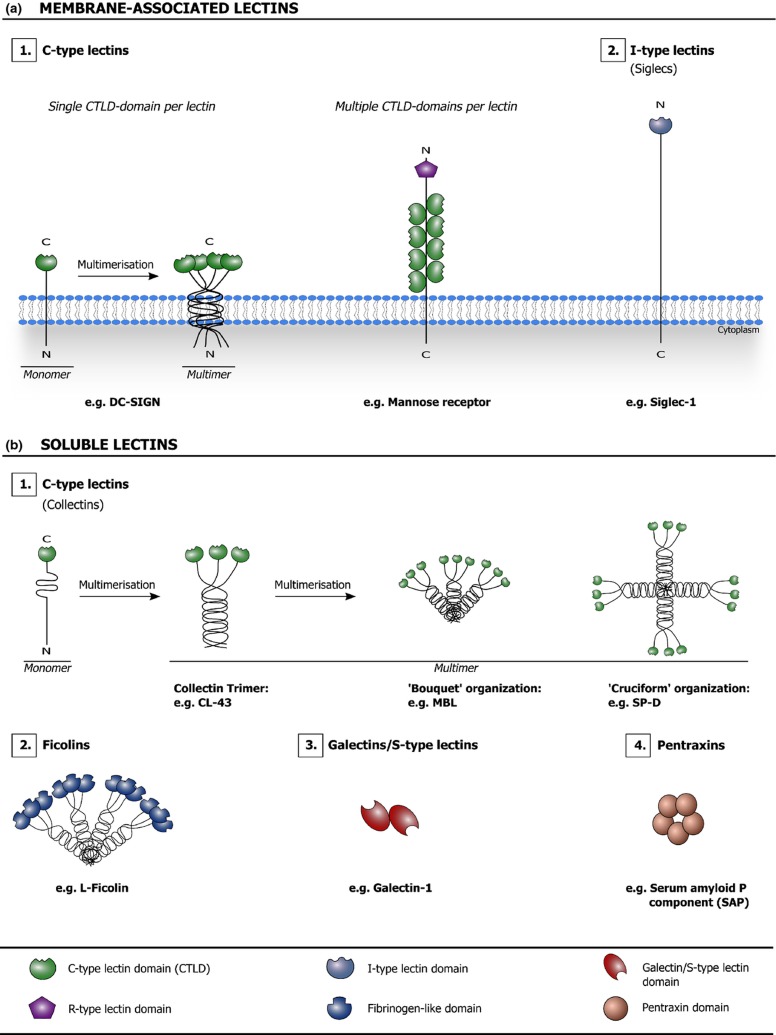
Animal lectins are typically expressed in a cell- and/or tissue-specific manner. They are involved in many different biological processes, including glycoprotein trafficking, cell adhesion and signaling, and their expression is usually tightly regulated (Varki et al., 2009). Particularly striking is the great number of membrane-associated and soluble lectins that are linked with host immunity. Immune system lectins are involved in intercellular communication, positive and/or negative regulation of activation, regulation of inflammation, disposal of damaged and apoptotic cells, etc. Several of these lectins have also been identified as ‘pattern recognition receptors’ (PRRs), which act as molecular sensors for pathogens and endogenous stress signals and often trigger specific immune reactions/mechanisms in response to their detection (Gordon, 2002; Janeway & Medzhitov, 2002; Cambi & Figdor, 2003; McGreal et al., 2004; Cambi et al., 2005; McGreal et al., 2005; Crocker et al., 2007; Crocker & Redelinghuys, 2008; van Kooyk & Rabinovich, 2008; Garcia-Vallejo & van Kooyk, 2009; Geijtenbeek & Gringhuis, 2009; Sato et al., 2009; Bottazzi et al., 2010; Dam & Brewer, 2010; Kumagai & Akira, 2010; Svajger et al., 2010; Davicino et al., 2011; Osorio & Reis e Sousa, 2011; Sancho & Reis e Sousa, 2012). The capacity of many immune system lectins to couple glycan recognition events with specific signaling and/or effector functions gives them a key regulatory position in the immune system. Figure 3 gives a schematic overview of different types of animal lectins that are considered in this review.
Figure 3.
Schematic overview of different types of membrane-associated (a) and soluble (b) animal lectins that are considered in this review. The lectin domains are highlighted and listed in the key. C-type lectin/C-type lectin domain: Lectins are classified as C-type lectins based on their Ca2+-dependency and shared primary structure. In the C-type CRD, a Ca2+ ion is directly involved in carbohydrate binding by making coordination bonds to both the CRD surface and key hydroxyl groups of the carbohydrate. The C-type lectin family contains both membrane-associated (a.1) and soluble (b.1) lectins. The collectins are solubleC-type lectins characterized by the presence of collagen-like domains. R-type lectin domain: This term refers to a CRD that is structurally similar to the CRD in ricin, a toxin found in the plant Ricinus communis. I-type lectin/I-type lectin domain: I-type lectins are glycan-binding proteins that belong to the Ig superfamily, but are not antibodies or T-cell receptors. The ‘sialic acid-binding Ig-like lectin (siglec)’ family of membrane-associated lectins is currently the only well-characterized group of I-type lectins (a.2). Ficolin: Ficolins (b.2) are soluble lectins characterized by the presence of collagen-like domains and fibrinogen-like globular domains with a lectin activity. Galectin/S-type lectin (domain): Galectins (b.3) are soluble lectins that typically bind β-galactose-containing glycoconjugates and show primary structural homology in their CRDs. Galectins were initially referred to as S-type lectins to reflect their sulfhydryl dependency, the presence of cysteine residues and their solubility; however, at present, not all identified galectins fit this initial description anymore. Pentraxin/pentraxin domain: Pentraxins (b.4) are characterized by the presence of pentraxin domains, which contain an eight amino acid long conserved ‘pentraxin signature’ (HxCxS/TWxS, where x is any amino acid) and display an L-type (Legume-type) lectin fold. SAP is a soluble lectin that requires Ca2+ ions for carbohydrate ligand binding (adapted from Fujita, 2002; Varki et al., 2009; Bottazzi et al., 2010).
Lectins play a pivotal role in different aspects of the physiology, including the immune defense against (viral) pathogens. However, it has become apparent that several viruses exploit host lectins to promote their spread. In addition, many viruses encode lectins for the recognition of and infectious entry into target cells. The following section explores how glycan–lectin interactions shape the virus-host interplay, mainly focussing on glycan–lectin interactions directly involving infectious virions.
Glycan–lectin interactions in virus biology
Glycan–lectin interactions that benefit the host
Membrane-associated host lectins can capture viruses for degradation
Lectins cover essential roles in the animal host's immune defense. Importantly, several membrane-associated host (immune system) lectins act as pathogen recognition molecules: they can bind pathogens and activate signaling mechanisms or capture pathogens for subsequent degradation and presentation to cells of the adaptive immune system (e.g. MHCII-restricted presentation of antigens to T cells), resulting in the induction of a pathogen-specific adaptive immune response. Alternatively, pathogens attached to such cell surface lectins may also be directly presented to neighboring immune cells in trans, a process that seems especially significant at sites with a high density of immune cells (e.g. the lymph nodes). Hence, binding of a viral pathogen to membrane-associated (immune system) lectins can lead to its clearance and degradation.
A prominent example is the interaction between the human immunodeficiency virus type 1 (HIV-1) and human langerin, a C-type lectin mainly expressed on Langerhans cells (de Witte et al., 2007; van der Vlist & Geijtenbeek, 2010). de Witte et al. (2007) reported that HIV-1 interacts with langerin via high-mannose glycans on the gp120 envelope protein and is subsequently internalized into Birbeck granules, leading to virus degradation (de Witte et al., 2007). Also DC-SIGN, a mannose-binding C-type lectin mainly expressed on dendritic cells (DCs), can bind and internalize HIV-1 virions for degradation and promotes MHCII-restricted as well as exogenous MHCI-restricted presentation of HIV-1 antigens (Moris et al., 2004, 2006). Similarly, various other membrane-associated host lectins can aid as PRRs in the defense against viral pathogens. However, growing evidence illustrates that many of these lectins, including DC-SIGN, are also abused by viruses to gain access to their target cells and facilitate viral spread, as is discussed further below.
Soluble host lectins can block viral infection and target virions for destruction by the immune system
In contrast to the dual role played by different membrane-associated host lectins, soluble host lectins have mainly been associated with protection against viral infection. Several soluble host lectins have been reported to aid in neutralization and clearance of various viral pathogens. Table 1 gives an overview of membrane-associated and soluble host lectins that are linked with the host's defense against different viruses. Current experimental data strongly implicate these host lectins in the defense against the listed viral pathogens and do not attribute explicit proviral effects to the host lectin – unlike for the lectin-virus pairs listed further in Table 2.
Table 1.
Overview of host lectins that are linked with antiviral defense
| Host lectin | Implicated in defense against … |
| Membrane-associated lectin | |
| Langerin | Human immunodeficiency virusdimt, Measles virus1 |
| Soluble lectin | |
| Collectin-11 (CL-11) | Influenza A virus2,3 |
| Collectin-43 (CL-43) | Bovine rotavirus,4 Influenza A virus4–6 |
| Collectin-46 (CL-46) | Influenza A virus6,7 |
| Conglutinin | Bovine rotavirus,4 Influenza A virus4,6,8–12 |
| Ficolin A | Influenza A virus13 |
| Ficolin-α | Porcine reproductive and respiratory syndrome virus14 |
| H-ficolin | Influenza A virus15 |
| L-ficolin | Hepatitis C virus,16,17 Influenza A virus13 |
| Galectin-1 (Gal-1) | Hendra virus,18 Nipah virusdimt, Parainfluenza virus type 318 |
| Mannose-binding lectin (MBL) | Dengue virus,19,20 Hepatitis C virus,21 Human cytomegalovirus,22 Human immunodeficiency virus,23–29 Influenza A virusdimt, Marburg virus,30 Severe acute respiratory syndrome coronavirus31,32 |
| Serum amyloid P component (SAP) | Influenza A virus,33–36 Influenza B virus,34 Parainfluenza virus type 334 |
| Surfactant protein A (SP-A) | Herpes simplex virus,37–39 Human coronavirus 229E,40 Influenza A virusdimt, Porcine reproductive and respiratory syndrome virus41 |
| Surfactant protein D (SP-D) | Bovine rotavirus,4 Human coronavirus 229E,40 Influenza A virusdimt, Respiratory syncytial virus,42–44 Sendai virus45 |
Current experimental data implicate these host lectins in the defense against the listed viral pathogens and do not attribute explicit proviral effects to the host lectin.
References in Table 1 are listed in Supporting Information, Data S1. dimt: discussed in main text.
Table 2.
Overview of host lectins that have been linked with proviral effects
| Host lectin | Implicated in infection with/spread or persistence of … |
| Membrane-associated lectin | |
| Asialoglycoprotein receptor (ASGPR) | Ebola virus,1–3 Hepatitis A virus,4 Hepatitis B virus,5–10 Marburg virus,2,11 Sendai virus12–14 |
| Blood dendritic cell antigen-2 (BDCA-2) | Hepatitis C virus,15 Human immunodeficiency virus16,17 |
| Dendritic cell immunoreceptor (DCIR) | Hepatitis C virus,15 Human immunodeficiency virus18–20 |
| Dendritic cell-specific intercellular adhesion molecule 3- grabbing nonintegrin (DC-SIGN) | Aura virus,21 Dengue virus,22–34 Ebola virus,1–3,35–41 Feline coronavirus,42,43 Feline immunodeficiency virus,44 Hepatitis C virus,41,45–53 Herpes simplex virus,54 Human coronavirus NL-63,55 Human cytomegalovirus,56–59 Human herpes virus 8/Kaposi's sarcoma-associated herpes virus,60–62 Human immunodeficiency virusdimt, Human T-cell lymphotropic virus,63–65 Influenza A virus,66–68 Junin virus,69 La Crosse virus,70 Lassa virus,71 Lymphocytic choriomeningitis virus,72 Marburg virus,2,41,73 Measles virus,74–79 Porcine reproductive and respiratory syndrome virus,80 Punta Toro virus,81 Respiratory syncytial virus,82 Rift Valley fever virus,70,81 Semliki Forest virus,83 Severe acute respiratory syndrome coronavirus,2,41,55,84–87 Severe fever with thrombocytopenia syndrome virus,70 Simian immunodeficiency virus,1,40,88–99 Sindbis virus,21,100 Toscana virus,81 Uukuniemi virus,81 West Nile virus32,33,101 |
| Liver/lymph node-specific intercellular adhesion molecule 3- grabbing nonintegrin (L-SIGN; DC-SIGN-related protein; DC-SIGNR) | Aura virus,21 Dengue virus,28–30,32,33 Ebola virus,1,2,35,37–41 Hepatitis C virus,45–52,102,103 Human coronavirus NL-63,55 Human coronavirus 229E,104 Human cytomegalovirus,56 Human immunodeficiency virus,1,88,99,105–108 Influenza A virus,66 Junin virus,69 Marburg virus,2,41 Respiratory syncytial virus,82 Semliki Forest virus,83 Severe acute respiratory syndrome coronavirus,2,41,55,86,109,110 Severe fever with thrombocytopenia syndrome virus,70 Simian immunodeficiency virus,88,90,99 Sindbis virus,21,100 West Nile virus32,33 |
| Liver/lymph node sinusoidal endothelial cell C-type lectin (LSECtin) | Ebola virus,3,41,111,112 Lassa virus,71 Lymphocytic choriomeningitis virus,72 Marburg virus,41,112 Severe acute respiratory syndrome coronavirus41 |
| Macrophage Gal/GalNAc-specific C-type lectin (MGL) | Ebola virus,113,114 Influenza A virus,115 Marburg virus73,113 |
| Mannose receptor (MR) | Dengue virus,34 Human immunodeficiency virus,116–123 Influenza A virus,115,124 Visna/Maedi virus125 |
| Paired immunoglobulin-like type 2 receptor alpha (PILR-α) | Herpes simplex virus,126–132 Pseudorabies virus129 |
| Siglec-1 (Sialoadhesin) | Human immunodeficiency virus,133–135 Porcine reproductive and respiratory syndrome virus136–139 |
| Siglec-4 (Myelin-associated glycoprotein; MAG) | Herpes simplex virus,140 Varicella-zoster virus140 |
| Soluble lectin | |
| Galectin-1 (Gal-1) | Human immunodeficiency virusdimt, Human T-cell lymphotropic virus141 |
| Mannose-binding lectin (MBL) | Ebola virus,36,142–144 Hendra virus,142,144 Nipah virus,142,144 West Nile virus144–146 |
| Surfactant protein A (SP-A) | Human immunodeficiency virus,147 Respiratory syncytial virus148–152 |
| Surfactant protein D (SP-D) | Human immunodeficiency virus153,154 |
Although capture of a virus by these lectins may have certain antiviral effects or promote the specific immunity against this pathogen, current experimental data suggest that the listed viruses may also employ these lectins to promote viral infection, spread or persistence.
References in Table 2 are listed in Supporting Information, Data S2. dimt: discussed in main text.
The basic principles of soluble lectin-mediated antiviral protection are most easily conveyed using some specific, well-characterized examples. For instance, various studies point out an important role of surfactant protein A (SP-A), surfactant protein D (SP-D), and mannose-binding lectin (MBL) in the defense against influenza A virus (IAV) infection.
SP-A, SP-D, and MBL are all soluble C-type lectins and share a similar basic structure: lectin monomers – consisting of an N-terminal cysteine-rich domain, a collagen-like domain, a coiled coil neck domain, and a C-terminal CRD – assemble into trimers which, under physiologic conditions, further multimerize via their N-termini to form typical cruciform- (SP-D) or bouquet- (SP-A and MBL) like structures (van de Wetering et al., 2004; Veldhuizen et al., 2011). Despite their similar structure, these lectins show distinct glycan ligand specificities (Veldhuizen et al., 2011) and also their interaction with IAV and their effects on infection and spread appear to differ. Studies using distinct IAV isolates indicate that SP-D and MBL bind mannose-rich glycans on the viral surface glycoproteins hemagglutinin and neuraminidase (a viral lectin and a viral glycosidase, involved in IAV entry and release; see discussion on viral lectins) through their CRDs (Malhotra et al., 1994; Reading et al., 1997; Kase et al., 1999; Hartshorn et al., 2000; Hillaire et al., 2011). Although SP-A may interact with some IAV isolates in a similar manner (Malhotra et al., 1994), binding of this molecule to most of the IAV variants tested to date appears not to involve the lectin activity of SP-A; in contrast, virus binding depends on the interaction of the viral hemagglutinin with a sialylated N-glycan on the SP-A CRD (Hartshorn et al., 1994; Benne et al., 1995, 1997; Hartshorn et al., 1997; van Eijk et al., 2003; Mikerov et al., 2008). In other words: SP-D and MBL binding depend on viral glycosylation, whereas SP-A binding mainly depends on the specificity of the hemagglutinin, and this is mirrored in the spectrum of IAV variants these lectins can effectively bind (Hartshorn et al., 1994; Malhotra et al., 1994; Benne et al., 1995, 1997; Hartshorn et al., 1997; Reading et al., 1997; Kase et al., 1999; Hartshorn et al., 2000; van Eijk et al., 2003; Mikerov et al., 2008; Hillaire et al., 2011). Interestingly, the porcine variant of SP-D appears to combine the above-mentioned IAV-binding functionalities: this molecule may not only bind IAV virions through interaction of its CRD with high mannose glycans on the virion surface (similar to other SP-D molecules), but also through interaction of a sialylated glycan on the lateral surface of its CRD with the IAV hemagglutinin (similar to SP-A) and can therefore bind to a broader array of IAV variants (van Eijk et al., 2002, 2003, 2004; Hillaire et al., 2011).
In vitro assays show that SP-A and SP-D can directly neutralize IAV infectivity (Benne et al., 1995; Reading et al., 1997; Hartshorn et al., 2000; van Eijk et al., 2003; Hawgood et al., 2004; Hillaire et al., 2011). Both SP-A and SP-D inhibit the viral hemagglutinating activity that is required for IAV attachment to target cells (Hartshorn et al., 1994; Malhotra et al., 1994; Benne et al., 1995; Hartshorn et al., 1996, 1997, 2000; van Eijk et al., 2002, 2003, 2004; Mikerov et al., 2008; Hillaire et al., 2011) and SP-D was reported to inhibit the viral neuraminidase (Reading et al., 1997; Hillaire et al., 2011). Interestingly, both lectins also induce viral aggregation (Hartshorn et al., 1994, 1996, 1997; van Eijk et al., 2003) and function as potent opsonins. SP-A for instance was identified as an opsonin for IAV phagocytosis by alveolar macrophages (Benne et al., 1997). Moreover, SP-A and SP-D were shown to enhance IAV binding to neutrophils (Hartshorn et al., 1994, 1996, 1997; van Eijk et al., 2003) and SP-D-IAV complexes were found to internalize upon attachment to neutrophils (Hartshorn et al., 1997; van Eijk et al., 2003). Pre-incubation of IAV with SP-A or SP-D also enhances the virus-induced H2O2 responses in neutrophils (Hartshorn et al., 1994, 1996, 1997; van Eijk et al., 2003) and pre-incubation of the virus with SP-D can protect neutrophils from IAV-induced deactivation (Hartshorn et al., 1994, 1996, 1997). MBL can counteract IAV by roughly the same mechanisms as SP-D (Hartshorn et al., 1993; Anders et al., 1994; Malhotra et al., 1994; Hartshorn et al., 1996, 1997; Reading et al., 1997; Kase et al., 1999), although its ability to activate the complement cascade expands its capabilities (Anders et al., 1994; Reading et al., 1995; Chang et al., 2010). Ligand binding by MBL (or alternatively ficolins) can lead to activation of MBL-associated serine proteases (MASPs) and initiate the complement cascade via the so-called lectin pathway (Blue et al., 2004; Bottazzi et al., 2010). Complement deposition on a virus may interfere directly with crucial steps in the viral infection process (e.g. receptor binding), but can also trigger complement receptor-mediated uptake of the pathogen into immune cells. In addition, for enveloped viruses, complement activation may result in membrane attack complex (MAC) formation on the viral envelope and subsequent virolysis (Blue et al., 2004; Bottazzi et al., 2010).
In line with the available in vitro data, recent work with SP-A-, SP-D-, and MBL-knockout mice also confirmed the antiviral potential of these soluble lectins in vivo (LeVine et al., 2001, 2002; Zhang et al., 2002; Li et al., 2002; Hawgood et al., 2004; LeVine et al., 2004; Kingma et al., 2006; Chang et al., 2010). Noteworthy caveats regarding these in vivo studies are, however, that direct antiviral effects of these lectins can be hard to uncouple from other – e.g. immune-regulatory – effects and that mice do not represent natural hosts for IAV.
Another interesting example of antiviral activity mediated by soluble host lectins was recently reported for Nipah virus (NiV): the physiologic, homodimeric form of the soluble lectin galectin-1 can inhibit NiV envelope protein-mediated membrane fusion (Levroney et al., 2005; Garner et al., 2010). NiV encodes two viral membrane glycoproteins – the attachment protein NiV-G and the fusion protein NiV-F – that mediate viral entry and direct the endothelial cell syncytia formation typically associated with NiV infection (Levroney et al., 2005; Garner et al., 2010; Lee & Ataman, 2011). Binding of NiV-G to cell surface receptors induces a conformational change in NiV-F, thereby activating its fusogenic activity (Levroney et al., 2005; Garner et al., 2010; Lee & Ataman, 2011). Galectin-1 associates with glycans on the NiV envelope proteins and interferes with the membrane fusion process in multiple ways (Levroney et al., 2005; Garner et al., 2010). Not only does galectin-1 binding directly inhibit the crucial conformational change in NiV-F associated with membrane fusion, it also reduces the lateral mobility of NiV-F and NiV-G in the lipid membrane and consequently counteracts the physical separation of NiV-F and NiV-G that is essential for this conformation change (Garner et al., 2010). Moreover, galectin-1 binding impedes endocytosis and maturation of the NiV-F precursor NiV-F0 in infected cells (Garner et al., 2010), further illustrating the capacity of this lectin to block different stages of the viral infection/replication process.
In sum, soluble host lectins can counteract viral infection in various ways: they may directly neutralize virus by destabilizing or aggregating virions, interfere with crucial steps in the viral infection process (e.g. entry), and/or opsonize virus to facilitate uptake and degradation. Upon virus binding, some soluble lectins can trigger complement deposition on the virus, which may inhibit viral infection, enhance viral uptake via complement receptors and subsequent degradation in immune cells, and/or cause virolysis.
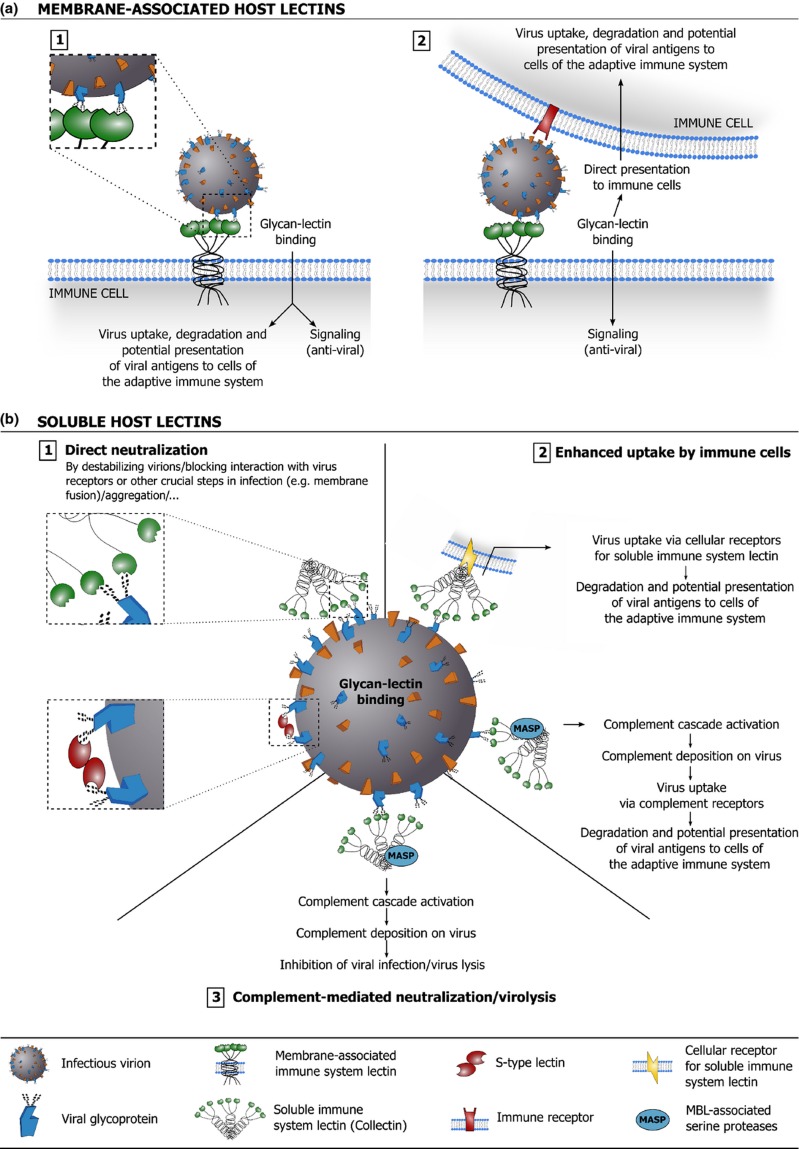
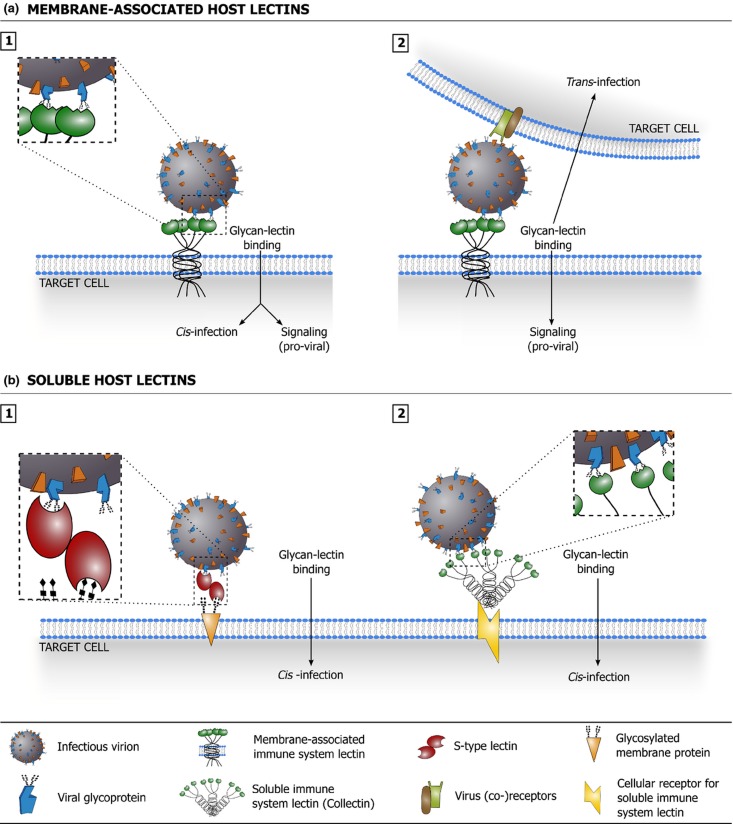
Considering the above, it is clear that lectins contribute significantly to the host's antiviral defense. Host lectins are involved in neutralization and clearance of free virus, immune regulation, and – although not extensively discussed in this overview – the detection and clearance of virus-infected cells. Figure 4 illustrates how membrane-associated and soluble host lectins can aid in antiviral defense.
Figure 4.
Schematic overview of how membrane-associated (a) and soluble (b) host lectins are implicated in antiviral defense. (a.1) Binding of virion-associated glycans with membrane-associated host lectins can lead to virus uptake, degradation, and presentation of viral antigens to cells of the adaptive immune system. Binding may trigger specific signaling that promotes an effective antiviral immunity. (a.2) Binding of virion-associated glycans with membrane-associated host lectins may promote direct presentation of the virus to immune cells in trans. Binding may trigger specific signaling that promotes an effective antiviral immunity. (b.1) Binding of soluble host lectins to virion-associated glycans may interfere directly with viral infection by destabilizing virions, blocking interaction of the virus with its receptors or interfering with other crucial steps in the infection process (e.g. membrane fusion). Soluble host lectins may also aggregate virions, which often negatively impacts viral infectivity (not depicted). (b.2) Soluble host lectins can act as opsonins: lectin binding to virion-associated glycans may facilitate viral uptake in immune cells via lectin receptors, leading to viral degradation and potential presentation of viral antigens to cells of the adaptive immune system. Lectin binding may also trigger complement deposition on the virus (through the lectin pathway) and facilitate viral uptake via complement receptors. (b.3) Detection of virion-associated glycans by soluble host lectins may trigger complement deposition on the virus (through the lectin pathway), which may directly inhibit viral infection and/or elicit lysis of the (enveloped) virus.
Glycan–lectin interactions that benefit the virus
Viruses encode lectins as keys for viral binding and entry into target cells
Like their animal hosts, also viruses can benefit from interactions between glycans and glycan-binding proteins. For instance, many viruses, including HIV-1, herpes simplex virus–1, and Dengue virus, have been shown to interact with glycosaminoglycan molecules present on target cells (Patel et al., 1993; Chen et al., 1997; Krusat & Streckert, 1997; Summerford & Samulski, 1998; Dechecchi et al., 2000, 2001; Vanderheijden et al., 2001; Delputte et al., 2002; Trybala et al., 2002). Viral association with glycosaminoglycans is usually attributed to charge-based attractions between clusters of positively charged aa residues on the virion surface and the negatively charged glycosaminoglycan chains. The interaction with glycosaminoglycans often constitutes the first contact between a virus and its target cell and typically increases infection efficiency. However, it has been documented for several viruses that the ability to interact with glycosaminoglycans can result from adaptation to growth in cell culture (Sa-Carvalho et al., 1997; Klimstra et al., 1998; Hulst et al., 2000; Mandl et al., 2001). Clearly, use of primary virus isolates is of crucial importance when assessing the occurrence and relevance of such interactions in vivo.
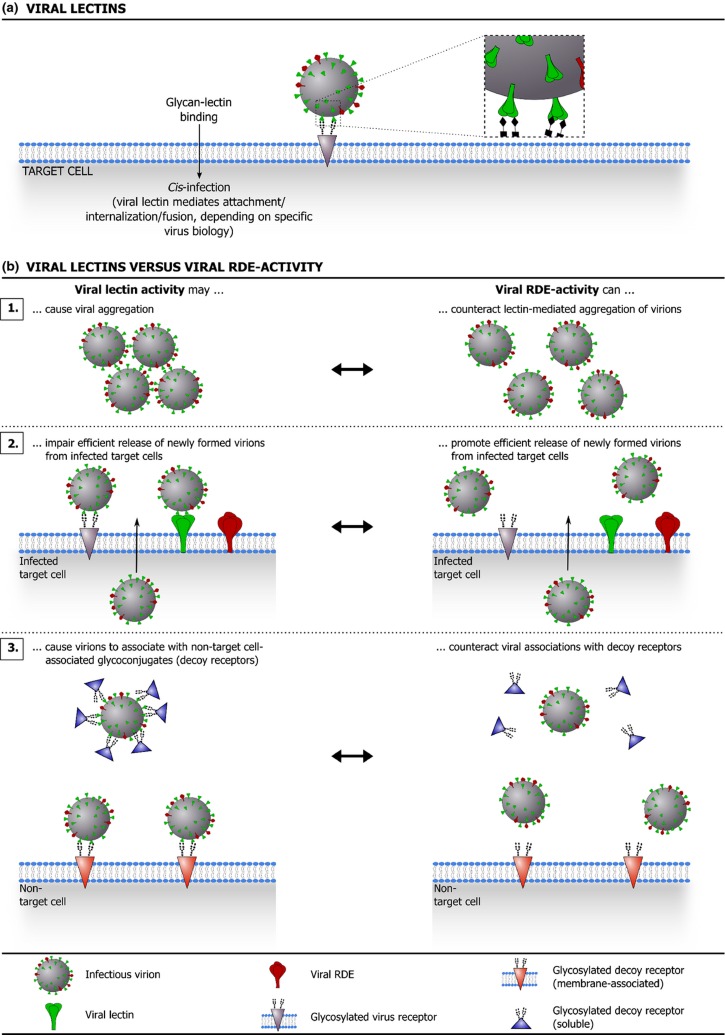
Aside from potential glycosaminoglycan-binding capacity, several viruses are endowed with a true lectin activity: they carry virally encoded lectins on their surface and use these as keys to gain entry into their target cells (Fig. 5a). Similar to animal lectins, such virally encoded lectins often possess characteristic glycan binding regions (‘glycan binding pockets’) and recognize specific portions of protein- and lipid-linked glycans. The hemagglutinin protein of IAV is generally regarded as the prototype of a viral lectin. Hemagglutinin is a membrane glycoprotein that forms noncovalently linked homotrimers in the (viral) membrane and is responsible for both virus attachment and penetration (Skehel & Wiley, 2000; Harrison, 2008; Gamblin & Skehel, 2010). The mature hemagglutinin protein consists of two disulfide-linked subunits, termed HA1 and HA2 (Skehel & Wiley, 2000; Harrison, 2008; Gamblin & Skehel, 2010). The HA1 subunit forms the globular ‘head’ region of hemagglutinin that covers the lectin function of this molecule: sialic acid-binding pockets in the membrane distal part of HA1 allow interaction with sialic acid-containing receptors on target cells (Skehel & Wiley, 2000; Harrison, 2008; Gamblin & Skehel, 2010). Variation in the HA1 subunit determines the affinity and specificity (e.g. α2-3- vs. α2-6-linked sialic acids) of this molecule (Skehel & Wiley, 2000; Gamblin & Skehel, 2010). As seen for animal lectins, also hemagglutinin binds with its glycan counterparts with a relatively low affinity and efficient virus attachment and entry depends on the interaction of multiple hemagglutinin molecules with multiple sialic acid-containing receptors (Skehel & Wiley, 2000; Gamblin & Skehel, 2010). The stalk-like HA2 subunit of hemagglutinin mediates the pH-dependent fusion process upon internalization of the IAV virion in the endosomal compartment of the target cell (Skehel & Wiley, 2000; Harrison, 2008; Gamblin & Skehel, 2010).
Figure 5.
(a) illustrates how viral lectins promote target cell infection. (b) shows how many viruses that employ viral lectins also benefit from a matching receptor-destroying enzyme (RDE) activity, which provides a counterweight against (high avidity) lectin activity. (a) Interaction of viral lectins with glycosylated receptors on a target cell promotes viral entry and infection (attachment/internalization/fusion, depending on specific virus biology). (b) Although they clearly benefit the virus, the use of (high avidity) viral lectins comes with a price. For instance, viral lectin activity can cause virions to aggregate (b.1) and can impair efficient release of newly formed virions from (glycosylated) infected cells (b.2). Moreover, binding of viral lectins to nontarget cell-associated glycoconjugates (decoy receptors) can prevent the virus from efficiently targeting susceptible host cells (b.3). Intriguingly, several lectin-carrying viruses are also equipped with an RDE that matches the specificity of the viral lectin and provides a counterweight against lectin-mediated glycan binding. In fact, for viruses equipped with both viral lectins and RDEs, a functional balance between these molecules appears to be an important determinant of the viral (replicative) fitness.
Similar to IAV, various other enveloped [e.g. influenza B and C viruses (Nakada et al., 1984;Herrler et al., 1985a; Rogers et al., 1986; Vlasak et al., 1987; Herrler et al., 1988; Herrler & Klenk, 1991; Herrler et al., 1991; Rosenthal et al., 1998; Lamb & Krug, 2001; Suzuki & Nei, 2002; Wang et al., 2007b; Wang et al., 2008a), mumps virus (Bowden et al., 2010; Harrison et al., 2010; Chang & Dutch, 2012)] as well as nonenveloped [e.g. murine norovirus (Taube et al., 2009, 2012), feline calicivirus (Stuart & Brown, 2007), and rhesus rotavirus (Dormitzer et al., 2002), among others (Taube et al., 2010)] viruses employ sialic acid-binding viral lectins to infect target cells. Importantly however, viral lectins with a different glycan specificity have also been identified. For instance, many viruses – including human noroviruses (Estes et al., 2006; Le Pendu et al., 2006; Cao et al., 2007; Tan & Jiang, 2007; Bu et al., 2008; Choi et al., 2008; Donaldson et al., 2008; Shirato, 2011), rabbit hemorrhagic disease viruses (Ruvoen-Clouet et al., 2000; Rademacher et al., 2008; Guillon et al., 2009; Nystrom et al., 2011), and the rhesus monkey Tulane virus (Farkas et al., 2010) – were found to interact with specific HBGA types.
Although a broad spectrum of viruses has evolved to use viral lectins to secure efficient target cell infection, the use of viral lectins for cellular attachment comes with a price. The glycan receptors for viral lectins are not necessarily target cell-specific and, whereas low affinity/avidity interactions may be reversible, high affinity/avidity binding of viral lectins to nontarget cell-associated glycoconjugates (‘decoy receptors’) can prevent the virus from efficiently targeting susceptible host cells. In line with this, it was shown for IAV that interaction of the viral lectin hemagglutinin with soluble, sialylated host glycoproteins – e.g. SP-A (cfr. supra) or α2-macroglobulin – can interfere with the viral hemagglutinating activity that is crucial for receptor binding (Rogers et al., 1983; Pritchett & Paulson, 1989; Ryan-Poirier & Kawaoka, 1991; Matrosovich et al., 1992; Ryan-Poirier & Kawaoka, 1993; Hartshorn et al., 1994; Malhotra et al., 1994; Benne et al., 1995; Gimsa et al., 1996; Benne et al., 1997; Hartshorn et al., 1997; Matrosovich et al., 1998; van Eijk et al., 2003; Mikerov et al., 2008; Chen et al., 2010; Cwach et al., 2012).
Although the presence of ‘high avidity’ glycan decoys invariably puts a strain on viral infection efficiency, this burden may be lighter on viruses that are equipped with a receptor destroying enzyme (RDE) that matches the specificity of the viral lectin. Intriguingly, the best-known examples in this context are again influenza viruses. As situated above, both influenza A and B viruses display hemagglutinin proteins on their surface, which bind to sialic acids displayed on the host cell surface and mediate pH-dependent fusion of the viral membrane with the host cell membrane (Skehel & Wiley, 2000; Lamb & Krug, 2001; Wang et al., 2007b; Wang et al., 2008a; Harrison, 2008; Gamblin & Skehel, 2010). An RDE activity was mapped to another viral membrane glycoprotein, designated neuraminidase (Gottschalk, 1957; Colman, 1994; Lamb & Krug, 2001; Gamblin & Skehel, 2010). This enzyme removes sialic acid moieties from glycoproteins and glycolipids by catalyzing the hydrolysis of the α-ketosidic linkage to the subterminal sugar residue and consequently destroys potential receptors for the viral hemagglutinin (Gottschalk, 1957; Colman, 1994; Lamb & Krug, 2001; Gamblin & Skehel, 2010). Viral use of an enzyme that can actually destroy receptors for the virus may seem peculiar at first. Importantly, however, the neuraminidase activity can prevent virions from aggregating via hemagglutinin-sialic acid interactions, promotes efficient release of newly formed virions from (sialic acid-carrying) infected cells, and provides a counterweight to the interaction of hemagglutinin molecules with nontarget cell-associated glycans: neuraminidase-mediated removal of sialic acids from decoy receptors prevents virions from establishing high-avidity interactions with these glycoconjugates and may even provide an escape route for virions after hemagglutinin-mediated binding to nontarget cell-associated glycans (Colman, 1994; Suzuki et al., 1994; Gimsa et al., 1996; Barrere et al., 1997; Gamblin & Skehel, 2010). Conceivably, a functional balance between the hemagglutinin and neuraminidase activities is an important determinant of the (replicative) fitness of IAV variants in vivo. Using IAV as a paradigm, Fig. 5b illustrates how viral RDE activity can balance the high avidity of viral lectins where favorable and consequently improve viral infection efficiency.
In contrast to influenza A and B viruses, other viruses combine both lectin and RDE functions in one protein complex. For example, the viral membranes of mumps virus, Newcastle disease virus, Sendai virus, and human parainfluenza virus 3 and 5 are studded with hemagglutinin-neuraminidase proteins (Bowden et al., 2010; Harrison et al., 2010; Chang & Dutch, 2012). Another example is the influenza C virus, which carries the hemagglutination, RDE and fusion protein functions in one single envelope protein named the hemagglutinin-esterase-fusion protein (Nakada et al., 1984; Herrler et al., 1985a, b; Rogers et al., 1986; Vlasak et al., 1987; Herrler et al., 1988; Schauer et al., 1988; Herrler & Klenk, 1991; Herrler et al., 1991; Rosenthal et al., 1998; Pekosz & Lamb, 1999; Lamb & Krug, 2001; Suzuki & Nei, 2002). Whereas the RDE of influenza A and B viruses is a neuraminidase, which cleaves off entire sialic acid residues, the RDE of influenza C functions as a sialate-O-acetylesterase and cleaves off specific O-acetyl groups (Herrler et al., 1985b; Vlasak et al., 1987; Herrler et al., 1988; Schauer et al., 1988; Pekosz & Lamb, 1999). A similar situation is seen for certain coronaviruses and toroviruses that carry an accessory protein called hemagglutinin-esterase on their surface (de Groot, 2006). As the name implies, functional hemagglutinin-esterase proteins combine a hemagglutinating activity with a sialate-O-acetylesterase activity (de Groot, 2006). Interestingly, for some coronaviruses, the hemagglutinin-esterase protein is not the only envelope protein endowed with a lectin activity. For example, the spike proteins of bovine coronavirus and human coronavirus OC43 have been shown to be potent sialic acid-binding lectins (Schultze et al., 1991; Kunkel & Herrler, 1993). Also the spike protein of the transmissible gastroenteritis coronavirus of pigs has been shown to possess such a lectin activity (Schultze et al., 1996; Krempl et al., 1997, 2000; Schwegmann-Wessels et al., 2011). However, transmissible gastroenteritis coronavirus has no hemagglutinin-esterase protein that serves as RDE to counteract the hemagglutinating activity of the spike protein.
Viruses exploit membrane-associated host lectins to promote infection of target cells and avoid immune recognition
Viruses do not only benefit from virally encoded lectins, but can also use host lectins to their advantage. Paradoxically, many of the host lectins that are exploited by viruses form part of the immune system. Although capture of viral pathogens by these lectins may have certain antiviral effects or promote the specific immunity against this pathogen (cfr. supra), many viruses can also employ such interactions to promote efficient infection and spread or to facilitate persistence (Table 2). Virus binding to membrane-associated lectins can lead to concentration of virions at the cell surface and can facilitate infection of target cells. In many cases, host lectins appear to function as true portals for viral entry: the virus binds to the lectin, which drives subsequent internalization of the virus into specific cellular compartments from which the virus can initiate the next stage of infection. However, it has also been shown that lectins present on non-target cells may facilitate infection of target cells, a process called trans-infection. Many membrane-associated (immune system) lectins also participate in specific signaling pathways and engagement of such lectins by a virus may modulate both viral infection and the immune response in favor of the pathogen.
An immune system lectin that can be used as a paradigm in this context is DC-SIGN. This molecule is mainly expressed on dendritic cells (DCs), but expression on distinct other cell-types – including macrophages, B lymphocytes, platelets, and (immortalized) podocytes – has also been described (Geijtenbeek et al., 2000a, b; Soilleux et al., 2002; Granelli-Piperno et al., 2005; Gurney et al., 2005; Chaipan et al., 2006; Rappocciolo et al., 2006; Mikulak et al., 2010; Svajger et al., 2010). Prototypic DC-SIGN molecules consist of a C-terminal C-type CRD, a neck region made up of 7 and a half 23 aa residue repeats, a transmembrane domain, and a cytoplasmic domain containing motifs involved in receptor internalization and signaling (Svajger et al., 2010; Tsegaye & Pohlmann, 2010). Lectin monomers typically multimerize via their neck regions to form tetramers, which in turn organize in nanoclusters on the cell membrane (Svajger et al., 2010; Tsegaye & Pohlmann, 2010; Manzo et al., 2012). DC-SIGN functions as a receptor for various ligands. The T cell-expressed molecule ICAM-3 is probably its most prominent endogenous ligand: DC-SIGN binds ICAM-3 on the T cell surface and thereby contributes to the transient, nonantigen-specific interaction of DC with T cells that is required for efficient screening of MHCII-peptide complexes and eventual T cell priming (Geijtenbeek et al., 2000a; Svajger et al., 2010). Moreover, DC-SIGN has been implicated in various other processes, including DC differentiation, migration, and antigen capture (Svajger et al., 2010). Over the last decade, it has become apparent that DC-SIGN interacts with a wide variety of viral pathogens.
A textbook example of a virus that recruits DC-SIGN is HIV-1 (Lekkerkerker et al., 2006; Wu & KewalRamani, 2006; Piguet & Steinman, 2007; Tsegaye & Pohlmann, 2010; da Silva et al., 2011; van der Vlist et al., 2011). DC-SIGN can bind the enveloped HIV-1 particle and mainly recognizes mannose-rich glycans on the viral envelope glycoprotein gp120 (Curtis et al., 1992; Geijtenbeek et al., 2000a, b; Feinberg et al., 2001; Hong et al., 2002; Lin et al., 2003; Su et al., 2004; Hong et al., 2007). The efficiency of HIV-1 capture by DC-SIGN has been linked to receptor density. Experiments in 293 T-Rex cells using an inducible DC-SIGN expression system have shown that high surface expression levels of DC-SIGN correlate with optimal binding of HIV-1 particles, and that lowering the DC-SIGN expression levels can significantly reduce the efficiency of HIV-1 binding (Pohlmann et al., 2001). These data suggest that the high DC-SIGN surface expression levels on certain (immature) DC subsets are compatible with optimal capture of HIV-1 virions, whereas lower DC-SIGN expression levels on B lymphocytes and especially platelets may be mirrored in a less efficient HIV-1 capture (Baribaud et al., 2002; Boukour et al., 2006; Chaipan et al., 2006; Rappocciolo et al., 2006). Nevertheless, studies have shown that also B lymphocytes and platelets can effectively bind HIV-1 virions via DC-SIGN (Boukour et al., 2006; Chaipan et al., 2006; Rappocciolo et al., 2006). Evidently, the interaction between HIV-1 and DC-SIGN is also critically dependent on the viral glycome. Recent research has shown that virion-associated gp120 of peripheral blood mononuclear cell (PBMC)-grown virus predominantly carries oligomannose N-glycans (Doores et al., 2010; Bonomelli et al., 2011), which constitute optimal ligands for DC-SIGN (van Liempt et al., 2006). Nevertheless, virus originating from different host cell types can display a different glycosylation profile, and this may modulate the efficiency of DC-SIGN recruitment (Lin et al., 2003).
Binding of HIV-1 to DC-SIGN can entail both negative and positive effects for the virus. In DC-SIGN-expressing antigen-presenting cells, most of the DC-SIGN-captured virions appear to be internalized into the endolysosomal pathway and rapidly degraded (Moris et al., 2004; Turville et al., 2004). In line with this, DC-SIGN was found to promote MHCII-restricted presentation of HIV-1 antigens (Moris et al., 2006). Intriguingly however, in cells that co-express CD4 and CCR5/CXCR4 (HIV-1 receptor and co-receptors, respectively), DC-SIGN expression also facilitates HIV-1 fusion: DC-SIGN efficiently captures and concentrates viral particles at the cell surface, and binding of this lectin to the HIV-1 envelope protein appears to increase exposure of the CD4 binding site (Lee et al., 2001; Nobile et al., 2005; Burleigh et al., 2006; Hijazi et al., 2011). Although DC-SIGN-mediated enhancement of HIV-1 fusion may promote MHCI-restricted presentation of HIV-1 antigen (proteasome and TAP-dependent pathway) and activation of cytotoxic T lymphocytes (Moris et al., 2004), enhanced HIV fusion inevitably leads to more efficient infection. Indeed, several studies confirm that DC-SIGN facilitates productive (cis-) infection in DC-SIGN-expressing cells that also co-express CD4 and CCR5/CXCR4 (Lee et al., 2001; Nobile et al., 2005; Burleigh et al., 2006; Hijazi et al., 2011).
DC-SIGN has also been implicated in HIV-1 trans-infection. An initial study showed that DC-SIGN can efficiently capture HIV-1 particles and transfer them to adjacent target T cells, without the need for productive infection of the DC-SIGN-expressing cell (Geijtenbeek et al., 2000b). Subsequent studies on the subject reported DC-SIGN-mediated internalization of infectious HIV-1 virions into low pH nonlysosomal compartments, and advocated that the virus traffics in intracellular compartments towards the zone of T cell contact, where it is released into the infectious synapse (i.e. the contact zone between the virus-loaded cell and the target T cell) (Kwon et al., 2002; McDonald et al., 2003). DC-captured HIV-1 virions as well as T-cell-expressed CD4 and CCR5/CXCR4 were found to concentrate at the DC-T-cell interface, rendering it an ideal micro-environment for efficient infection of target T cells (McDonald et al., 2003). Moreover, it was postulated that DC-SIGN-mediated capture of HIV-1 virions temporarily protects them from degradation and preserves viral infectivity (Geijtenbeek et al., 2000b; Kwon et al., 2002).
The above data supporting DC-SIGN-mediated capture, uptake, intracellular transport and ultimately transfer of intact HIV-1 particles from DCs to target T cells were united in the ‘Trojan horse model’ of mucosal HIV-1 transmission. This model posits that submucosal DCs capture and internalize HIV-1 virions via DC-SIGN and, by homing to the lymph nodes, provide a means of transport for the virus to a compartment rich in target cells. The virus-loaded DCs then interact with CD4+ T cells and the virions are transferred to the target T cell via the infectious synapse, ultimately resulting in efficient target cell infection (Geijtenbeek et al., 2000b; Baribaud et al., 2001; Sewell & Price, 2001). However, recent research is not always in line with this initial model and has challenged several of its key features. Cavrois et al. (2007) reported that HIV-1 trans-infection does not require intracellular virus trafficking, but primarily depends on cell surface-associated virions that reach the infectious synapses via transport on the cell surface (Cavrois et al., 2007). In line with this, Yu et al. (2008) reported that HIV-1 traffics towards the infectious synapse through a specialized, surface-accessible intracellular compartment (Yu et al., 2008). In addition, reports stating that virus capture by DC-SIGN mainly leads to virus internalization into the endolysosomal pathway and subsequent degradation (Moris et al., 2004; Turville et al., 2004; Moris et al., 2006) and that DC-SIGN-mediated trans-infection can only occur within the first hours after virus attachment (Turville et al., 2004) seem to downplay the importance of DC-SIGN-mediated trans-infection for efficient HIV-1 infection and spread. These and other data counter the theory that HIV-1 capture by DCs preserves viral infectivity, and suggest that the presence – and transfer – of infectious virus at later time points may be ascribed to productive DC infection (Turville et al., 2004; Nobile et al., 2005; Burleigh et al., 2006; Wang et al., 2007a). It is also noteworthy that, whereas initial studies identified DC-SIGN as the main factor involved in HIV-1 capture and transmission by DCs (Geijtenbeek et al., 2000b), recent studies also implicate other lectins in this process (Turville et al., 2002; Izquierdo-Useros et al., 2012) or even conclude that DC-SIGN is not involved in DC-mediated HIV-1 trans-infection (Boggiano et al., 2007). Differences in virus strains, cell types, and experimental setup might in part explain these conflicting data. Intriguingly, other recent work indicates that DC-SIGN-expressing B lymphocytes and platelets may effectively capture and transfer infectious HIV-1 via DC-SIGN (Boukour et al., 2006; Chaipan et al., 2006; Rappocciolo et al., 2006), potentially implicating these cells/cell fragments in HIV-1 dissemination in infected patients, although recent work suggests that platelets might negatively regulate viral spread by secretion of CXCL4 (Auerbach et al., 2012; Tsegaye et al., 2013). Clearly, further research is necessary to allow a better understanding of DC-SIGN-mediated HIV-1 trans-infection and its relevance for viral infection and spread in vivo.
Importantly, the role of DC-SIGN in HIV-1 infection appears not to be restricted to purely physical capture of virions for subsequent degradation or cis- or trans-infection. Recruitment of DC-SIGN by HIV-1 also triggers signal transduction that modulates immune responses and infection of DCs and adjacent target cells more indirectly. For example, Hodges et al. (2007) reported that binding of HIV-1 to DC-SIGN compromises DC maturation and primes these cells for trans-infection: Upregulation of CD86 and MHCII is suppressed, whereas synapse formation between DCs and CD4+ T cells is promoted (Hodges et al., 2007). Moreover, HIV-1 binding to DC-SIGN was shown to activate Cdc42 and promote formation of membrane extensions that facilitate HIV-1 transfer to CD4+ lymphocytes (Nikolic et al., 2011). Other work by Gringhuis et al. (2007, 2010) showed that binding of HIV-1 to DC-SIGN triggers Raf-1 dependent signaling, which modulates toll-like receptor (TLR)-elicited signals to induce synthesis of full-length HIV transcripts as well as production of the immunosuppressive cytokine IL-10 (Gringhuis et al., (2007, 2010). In general, the data discussed above suggest that DC-SIGN recruitment by HIV-1 might affect viral infection and transmission, as well as the host defense against this pathogen in several ways.
Over the last decade, DC-SIGN has become a prototype for lectin-mediated cis- and trans-infection and has been implicated in the infection process of various viruses, including HIV, Dengue virus, Ebola virus, and IAV (see Table 2). Importantly, however, DC-SIGN is not the only host lectin that is (ab)used by viruses to promote target cell infection or avoid immune recognition and clearance. Various other membrane-associated host lectins seem to be exploited by viruses – in ways similar to DC-SIGN – to aid cis-infection, trans-infection and/or viral persistence. Analysis of recent literature suggests that membrane-associated host lectins may constitute weak links in the host's defense against viral pathogens (Table 2).
Soluble host lectins can promote viral infection
Although generally implicated in antiviral defense, soluble host lectins may also support viral infection (Table 2). For example, galectin-1 has been proposed to promote HIV-1 infection (Ouellet et al., 2005; Mercier et al., 2008; St-Pierre et al., 2011; Sato et al., 2012). In vitro experiments pointed out that galectin-1 can enhance HIV-1 infection of different cell types – including human lymphoid cell lines, PBMC, CD4+ T lymphocytes, and monocyte-derived macrophages (Ouellet et al., 2005; Mercier et al., 2008) – and increase HIV-1 infection in an ex vivo lymphoid tissue model (Ouellet et al., 2005). Further experiments showed that galectin-1 accelerates virion binding to the target cell surface, probably by crosslinking viral and cellular glycans (Ouellet et al., 2005; Mercier et al., 2008). A more recent study confirmed these findings and showed that galectin-1 binds to clusters of N-linked glycans on the viral gp120 envelope protein in a β-galactoside-dependent manner (St-Pierre et al., 2011). Data from the same study identify the HIV-1 receptor CD4 as a ligand for galectin-1 and suggest that galectin-1 can cross-link gp120 and CD4 (St-Pierre et al., 2011). In sum, it appears that the dimeric lectin galectin-1 can enhance HIV-1 infection efficiency by cross-linking viral and host cell glycans and thereby promoting firmer adhesion of the virus to the target cell surface and facilitating virus-receptor interactions (Ouellet et al., 2005; Mercier et al., 2008; St-Pierre et al., 2011; Sato et al., 2012).
Some studies have also attributed proviral effects to the collectins MBL, SP-A, and SP-D. For some viruses, it was reported that – under certain conditions – viral recognition by collectins may enhance cis- or trans-infection (Hickling et al., 2000; Sano et al., 2003; Gaiha et al., 2008; Brudner et al., 2013; Madsen et al., 2013). It is conceivable that these collectins can bind the virus and subsequently associate with collectin receptors on the surface of target/transmitting cells, thereby concentrating virions at the cell surface and facilitating infection or viral transfer. Nevertheless, involvement of other mechanisms (e.g. collectin-mediated cross-linking of virus- and host cell-displayed glycans, cfr. the galectin-1-HIV-1 example described above) can currently not be excluded. Further research is necessary to elucidate the biology behind the potential proviral effects of collectins and to estimate the occurrence and relevance of these events in an in vivo context.
Figure 6 gives a schematic overview of how membrane-associated and soluble host lectins can be implicated in interactions that benefit the virus and facilitate viral infection and spread.
Figure 6.
Schematic overview of how membrane-associated (a) and soluble (b) host lectins can be implicated in interactions that benefit the virus and facilitate viral infection and spread. (a.1) Binding of virion-associated glycans to membrane-associated host lectins can promote (cis-) infection of the lectin-expressing cell: host lectins may facilitate viral attachment, internalization, and fusion (depending on specific virus biology). Viral attachment to membrane-associated host lectins may trigger signaling mechanisms that facilitate viral infection, spread, and/or immune evasion. (a.2) Binding of virion-associated glycans to membrane-associated host lectins can promote presentation of the virus to susceptible target cells in trans, thereby facilitating target cell infection. Viral attachment to membrane-associated host lectins may trigger signaling mechanisms that facilitate viral infection, spread, and/or immune evasion. (b.1) Multivalent soluble host lectins may facilitate virus attachment and promote viral infection by crosslinking virus- and host cell-displayed glycans. (b.2) Virus recognition by soluble host lectins and subsequent association with target cell-expressed lectin receptors may promote cis-infection of target cells. In a similar manner, soluble host lectins may capture and concentrate virions on a cell surface for subsequent presentation to target cells in trans (not depicted). Moreover, lectin binding can trigger complement deposition on the virus (through the lectin pathway), which may potentially promote cis- or trans-infection via cell surface-expressed complement receptors (not depicted).
Glycan and lectin: the variable parameters in a biological equation
As glycan–lectin interactions often represent key events in viral infection and/or antiviral immunity, variation in glycan or lectin expression and structure – either at the host or at the virus level – may significantly shift the balance between host and pathogen. A basic insight into the nature and origin of this variability is therefore germane to a proper understanding of glycan–lectin interactions in the context of viral infection biology and immunology.
Glycan and lectin variation at the host level
Glycan formation is a very complex and versatile biosynthetic process. In contrast to the primary amino acid sequences of proteins, glycan structures are not directly encoded in the host genome. Instead, they are synthesized in a step-wise manner via the concerted action of various host-encoded glycosyltransferase, glycosidase, and other enzymes. The availability of these glycoenzymes, the availability of precursor molecules and the accessibility of specific glycosylation sites govern (the efficiency of) glycan addition and modification and hence determine glycan variability. The genetic make-up of the host evidently has a major impact on glycosylation, but also other host-related factors can have pronounced effects. Recent research has shown that different cell types within a host can assemble radically different glycomes (Roth, 1996; Haslam et al., 2008) and that factors such as the activation (Comelli et al., 2006; Bax et al., 2007; Haslam et al., 2008) or infection (Lanteri et al., 2003) status of a cell can significantly influence glycosylation processes. Clearly, various biological factors contribute to the high glycan heterogeneity that is seen for many animal glycoconjugates.
Although host glycan variation may influence virtually all viral infections in several ways, its potential impact is probably most evident for viruses that are equipped with viral lectins. A notable example in this context are the noroviruses, a major cause of nonbacterial gastroenteritis in humans. It is well known that the viral capsids of most human noroviruses display an affinity for HBGAs, structurally related but highly polymorphic carbohydrate structures found on proteins and lipids of epithelial cells in the gastrointestinal and respiratory tract, on the surfaces of red blood cells and as free antigens in body fluids such as saliva, blood, and intestinal contents (Bu et al., 2008; Choi et al., 2008; Shirato, 2011). Different noroviruses display distinct HBGA specificities and can be categorized according to the (range of) HBGA structures they preferentially bind (Huang et al., 2005; Shirato, 2011). Human HBGA synthesis is controlled by various enzymes, including the glycosyltransferase enzymes encoded in the ABO, FUT2, and FUT3 gene loci. The presence of variant (functional or nonfunctional) alleles at these and other relevant gene loci is a key determinant of HBGA phenotype, as it controls which ABH and Lewis antigens an individual can synthesize (Le Pendu et al., 2006; Shirato, 2011). Although it is still unclear whether they function as primary receptors for noroviruses, current data indicate that HBGAs are important determinants of the noroviral tissue specificity. Moreover, several studies have established a link between HBGA geno-/phenotype and individual susceptibility to (clinical) infection with specific norovirus variants: HBGA phenotypes matching the specificity of the viral lectin correlate with a higher risk of (clinical) infection, whereas nonmatching HBGA phenotypes correlate with relative resistance (Le Pendu et al., 2006; Shirato, 2011).
Several studies have revealed significant heterogeneity relating to animal lectins. Lectin expression is governed by various genetic and nongenetic (e.g. hormone balance, immune status) factors. Importantly, gene polymorphisms that affect protein expression and/or functionality have been described for several animal lectins, including MBL and DC-SIGN.
For MBL, mutations in the promoter region of the MBL2 gene were found to affect protein expression levels, probably by influencing binding of transcription factors (Eisen & Minchinton, 2003; Dommett et al., 2006; Heitzeneder et al., 2012). Moreover, specific polymorphisms in MBL2 exon 1, encoding the collagen-like domain of MBL, appear to hinder correct and stable oligomerization of MBL protein chains and impede efficient ligand binding and activation of the lectin complement pathway (Eisen & Minchinton, 2003; Dommett et al., 2006; Heitzeneder et al., 2012). Several studies suggest a correlation between MBL deficiency and susceptibility to HIV infection, but conflicting data have been reported and further research is clearly necessary to corroborate this link (Eisen & Minchinton, 2003; Dommett et al., 2006; Heitzeneder et al., 2012).
Similar findings have been recorded for DC-SIGN. Polymorphisms in the promoter region of the DC-SIGN-encoding CD209 gene can affect protein expression levels and have been linked with altered susceptibility to and/or altered disease progression after infection with several viral pathogens, including HIV-1 and Dengue virus (Martin et al., 2004; Sakuntabhai et al., 2005; Koizumi et al., 2007; Selvaraj et al., 2009; Wang et al., 2011; Boily-Larouche et al., 2012). Moreover, distinct gene polymorphisms in the DC-SIGN-encoding region as well as alternative splicing events give rise to different isoforms of the protein, ranging from variants containing single nucleotide polymorphisms (SNPs) to variants with truncated lectin domains, variable numbers of 23-aa-residue repeats in the neck domain, alternative cytoplasmic domains or a lacking transmembrane region (Mummidi et al., 2001; Liu et al., 2004; Serrano-Gomez et al., 2008; Boily-Larouche et al., 2012). Information on the expression and biological activity of most of these DC-SIGN variants is rather limited. Recently, however, Boily-Larouche et al. (2012) reported that naturally occurring genetic variants of DC-SIGN, carrying specific SNPs in the neck domain-encoding exon 4, have an enhanced capacity to capture and transfer HIV-1 virions to CD4+ T lymphocytes. Moreover, Liu et al. (2004) described different neck domain length variants of DC-SIGN – carrying variable numbers of 23-aa-residue repeats in the neck region – and correlated neck domain length heterozygosity with a reduced risk of HIV-1 infection. Recent experimental data provide evidence that naturally occurring DC-SIGN neck domain variants can differ in multimerization competence in the cell membrane and display altered glycan binding capacity (Serrano-Gomez et al., 2008). Moreover, the presence of such neck domain variants appears to modulate multimerization of the prototypic DC-SIGN molecule (Serrano-Gomez et al., 2008). The fact that neck domain variation may influence the presence, stability, and functionality of DC-SIGN multimers on the cell surface can provide a molecular explanation for the link between DC-SIGN polymorphisms and susceptibility to HIV-1 and other pathogens, although further research is needed to substantiate this (Serrano-Gomez et al., 2008).
Glycan and lectin variation at the virus level
Although viruses rely on the host cell machinery for glycoconjugate synthesis, viral glycosylation profiles can significantly differ from the standard glycosylation profile of their host cell. For instance, it is well known that viral glycoproteins are often more heavily glycosylated than host glycoproteins, and that also the nature of their glycan modifications can significantly differ. A prototypic example in this context is the gp120 glycoprotein of HIV-1. The HIV-1 envelope is studded with trimers of noncovalently associated gp120/gp41 heterodimers (White et al., 2010). Gp120 is one of the most heavily N-glycosylated proteins in nature: it contains more than 20 N-linked glycosylation sites, and N-glycans account for about half of its molecular weight (Zhu et al., 2000; Wei et al., 2003; Pantophlet & Burton, 2006; Scanlan et al., 2007). Intriguingly, whereas mammalian glycoproteins typically carry mainly complex type N-glycans, this is not the case for the viral gp120 glycoprotein. Recent reasearch has shown that virion-associated gp120 of PBMC-grown virus – as opposed to recombinantly expressed monomeric gp120 – predominantly carries oligomannose N-glycans (Doores et al., 2010; Bonomelli et al., 2011). The synthesis of this unusual glycosylation profile appears to be partially directed by the structure of the gp120/gp41 spike itself: the presence of a dense N-glycan cluster in gp120, combined with the steric consequences of gp120/gp41 trimerization, seems to hinder further processing of (normally transient) biosynthetic glycan intermediates by ER and Golgi α-mannosidases, ultimately yielding HIV-1 virions with oligomannose-enriched gp120 glycoproteins (Zhu et al., 2000; Doores et al., 2010; Eggink et al., 2010; Bonomelli et al., 2011). Clearly, although viral glycosylation is critically dependent on the glycosylation machinery of the host cell, the genetic and structural background of a virus can have a decisive influence in this process.
Importantly, viral infection itself may also have strong repercussions on the glycosylation biology of a host cell. Considering the restricted glycosylation enzyme and precursor availabilities, it is conceivable that overexpression of viral glycoproteins in an infected target cell can result in an increased glycan heterogeneity of both viral and cellular glycoconjugates. Moreover, viruses may also actively modify the host and viral glycome by modulating the expression of host cell glycoenzymes (Hiraiwa et al., 1997; Cebulla et al., 2000; Hiraiwa et al., 2003) or via expression of virally encoded glycoenzymes in infected cells (Jackson et al., 1999; Willer et al., 1999; Nash et al., 2000; Sujino et al., 2000; Vanderplasschen et al., 2000; Markine-Goriaynoff et al., 2003, 2004a, b).
An additional source of glycan variation can be discerned for viruses that can infect multiple cell types, or even different host species. For instance, it is well known that HIV can productively infect multiple cell types, and that HIV glycosylation is cell type-dependent (Liedtke et al., 1994; Willey et al., 1996; Liedtke et al., 1997; Lin et al., 2003). Cell type-dependent glycosylation differences for HIV have been shown to impact viral interaction with and trans-infection via DC-SIGN (Lin et al., 2003), as well as viral sensitivity to antibody neutralization (Willey et al., 1996). Another, particularly fascinating example in this context is Dengue virus (DV). DV is a mosquito-borne flavivirus that can replicate in mosquitos as well as in humans (Navarro-Sanchez et al., 2003; Dejnirattisai et al., 2011). In humans, immature skin DCs are considered the primary target cell for the virus after a mosquito bite, and DC-SIGN is believed to be the main DV receptor on these cells (Navarro-Sanchez et al., 2003; Dejnirattisai et al., 2011). In a recent study, it was shown that insect cell-derived DV can efficiently infect DCs, whereas DC-derived DV is not able to reinfect DCs (Dejnirattisai et al., 2011). Similarly, insect cell-derived DV could efficiently bind and infect a DC-SIGN-expessing cell line, whereas this was not the case for DC-derived DV (Dejnirattisai et al., 2011). Finally, it was found that insect cell-derived DV predominantly contains high-/pauci-mannose-type N-glycans, whereas DC-derived virus contains only complex type N-glycans (Dejnirattisai et al., 2011). Projected against the background of DV infection, these data outline a tentative model of the first stages of DV infection in humans: during DV replication in mosquito cells, newly formed DV virions obtain mannose-rich glycans. Upon viral transfer to a human host, these virions efficiently infect immature skin DCs via DC-SIGN. Importantly, DV replication in skin DCs yields virions with complex type N-glycans, thus creating a ‘glycan mismatch’ with DC-SIGN. Due to this mismatch, newly synthesized DC-derived virus will not readily reinfect DCs via DC-SIGN, but preferentially infect other potential host cells via other receptors (Dejnirattisai et al., 2011). Although much more research is needed to verify this tentative model, it elegantly illustrates how cell type-dependent glycan variability may impact a viral infection process.
Another notable factor to be considered in the context of virus-related variability is the rapid evolution of many viral pathogens. This seems especially significant for RNA viruses, as these viruses generally evolve more rapidly than DNA viruses due to factors inherent to their biology and infection strategy (Belshaw et al., 2008; Holmes, 2009; Lauring & Andino, 2010). The higher mutation frequency of many RNA viruses directly implies a higher chance for addition or deletion of putative glycosylation sites. As has been shown for IAV, acquisition or deletion of glycosylation sites may affect crucial steps in the viral infection/replication process (e.g. receptor binding, fusion, release of newly formed virions) (Ohuchi et al., 1997; Wagner et al., 2000; Tsuchiya et al., 2002; Kim & Park, 2012), alter the capacity of the virus to avoid induction of/recognition by virus-specific antibodies (glycan shielding) (Wang et al., 2009; Wei et al., 2010; Wanzeck et al., 2011; Kim & Park, 2012; Job et al., 2013; Sun et al., 2013), and modulate viral interaction with various immune system lectins (Reading et al., 2007; Vigerust et al., 2007; Reading et al., 2009; Tate et al., 2011a, b). Clearly, mutational changes in the viral glycome may affect the virus-host interactome in various ways. Ultimately, the net benefit of a specific glycome change will determine if a glycosylation variant may become dominant in the virus population.
However, not only the viral glycosylation status, but also the affinity and specificity of viral lectins for specific glycoconjugates may change as a result of mutations. For instance, ample data show that amino acid changes at specific sites of the IAV hemagglutinin protein can significantly alter its affinity and/or specificity for particular sialic acid-containing receptors – a factor that is crucial for the virus to infect new host species (Skehel & Wiley, 2000; Wagner et al., 2002; Suzuki, 2005; Gamblin & Skehel, 2010). Finally, functional alterations in the viral RDE due to mutations may also have a strong impact on the interaction of viral lectins with host cell glycans. In the case of IAV, balanced lectin and RDE functions appear to be crucial for efficient viral replication. For IAV variants that are well adapted to a certain host species, the substrate specificity and activity of the neuraminidase generally match the ligand specificity and affinity of the hemagglutinin (Wagner et al., 2002). Disruption of this balance – for instance due to reassortment or transmission to a new host species – often results in a decreased replicative fitness (Wagner et al., 2002). Interestingly, however, the virus may overcome this hurdle and evolve towards replicative competence by selecting for compensatory mutations in hemagglutinin and/or neuraminidase that restore the functional balance between these molecules (Wagner et al., 2002). Considering these data on IAV, it is conceivable that the balance between viral lectin and RDE is also an important determinant of the replicative fitness of several other viruses.
On a related note, glycan–lectin interactions in virus biology are typically studied using a limited number of (prototypic) virus variants. Although the information obtained in these studies can often be extrapolated to include other virus variants, there are important exceptions. For IAV, for instance, it is well documented that different virus variants can carry hemagglutinin lectins with distinct glycan ligand specificities and therefore associate with distinct spectra of (decoy) receptors. Moreover, IAV variants can display different glycan arrays on their surface, which has been shown to modulate viral infection, glycan shielding, and recognition by various immune system lectins (cfr. supra). The fact that the specific genetic make-up of a virus determines specificity/affinity of viral lectins, co-directs viral glycosylation, etc., and that this can be mirrored in a distinct virus-host interactome remains an important issue in glycovirology.
In sum, an intricate web of glycan–lectin interactions can modulate viral infection, and host and virus inherent variability in glycans and lectins adds a further layer of complexity to this matter.
Targeting glycan-lectin interactions in antiviral strategies
Considering the pivotal roles of glycan–lectin interactions in many viral infections, interfering with these interactions seems an attractive strategy in the combat against these pathogens. Conversely, strategies that promote recognition of viruses by specific immune system lectins – involved in viral inhibition and clearance – may also prove useful in antiviral therapies. Several possibilities have been and are currently being explored.
Perhaps the most obvious strategy to modulate glycan–lectin binding is the use of molecules that can physically interfere with these interactions. Glycan decoys (e.g. carbohydrate-containing drugs, sugar analogs/glycomimetics) or carbohydrate-binding agents (CBAs) may be used in antiviral therapies to directly block key glycan–lectin interactions at the side of the lectin and at the side of the glycan, respectively. Binding of glycan decoys with a specific lectin can inhibit binding of other ligands by this lectin. For instance, it has been shown that multivalent mannose-containing molecules or mannose-based glycomimetics can compromise binding of HIV-1 gp120 with DC-SIGN (Wang et al., 2008b; Luallen et al., 2009; Martinez-Avila et al., 2009b; Becer et al., 2010) and inhibit DC-SIGN-mediated trans-infection of CD4+ T lymphocytes (Martinez-Avila et al., 2009a; Sattin et al., 2010; Berzi et al., 2012). Likewise, sialic acid-containing glycan decoys and sialic acid analogs are being evaluated for their capacity to block the sialic acid binding site of IAV hemagglutinin to inhibit interaction of the virus with sialic acid-containing receptor molecules on the surface of target cells (Landers et al., 2002; Matrosovich & Klenk, 2003; Matsubara et al., 2010; Papp et al., 2010, 2011).
Alternatively, binding of CBAs to glycans displayed on the virion surface can inhibit viral cis- or trans-infection via host cell lectins. This is elegantly exemplified in several recent studies, showing that mannose- as well as N-acetylglucosamine-specific CBAs can effectively prevent DC-SIGN-mediated HIV-1 capture and subsequent transmission to T lymphocytes (Balzarini et al., 2010; Balzarini et al., 2007a; Bertaux et al., 2007; Huskens et al., 2010; Hoorelbeke et al., 2011; Alexandre et al., 2012). Although CBA binding to host cell-associated glycans may inhibit viruses that employ viral lectins, the potential of this strategy for antiviral therapy remains virtually unexplored. It is noteworthy that the antiviral activity of CBAs or glycan decoys that bind to virion surfaces is not necessarily limited to direct inhibition of crucial glycan–lectin interactions, as they can mask greater portions of the virus and interfere with other crucial (including non-glycan-lectin) interactions or steps in the infection process. Moreover, recent research on HIV-1 highlights the antiviral potential of CBAs from yet another angle. Although the heavily glycosylated HIV-1 gp120 protein generally provides multiple ligands for mannose- and GlcNAc-specific CBAs, prolonged CBA pressure selects for HIV-1 variants with multiple N-glycosylation site deletions in the gp120 protein that are less sensitive to CBA-mediated neutralization (Balzarini et al., 2004, 2005a, b; Witvrouw et al., 2005; Balzarini et al., 2006; Balzarini, 2007b, c; Balzarini et al., 2007b; Huskens et al., 2007). Interestingly, however, deletion of N-glycosylation sites can also increase the immunogenicity of the virus and weaken the glycan shield that protects the virus from recognition by virus-specific antibodies and other (nonlectin) immune receptors (Botarelli et al., 1991; Back et al., 1994; Reitter et al., 1998; Bolmstedt et al., 2001; Kang et al., 2005), and may decrease the efficiency of HIV-1 trans-infection via immune system lectins like DC-SIGN (Hong et al., 2007; Liao et al., 2011). In addition, it appears that accumulation of mutations under CBA pressure is often paralleled by a significant reduction of the viral fitness, which is obviously advantageous in the context of an antiviral treatment (Balzarini et al., 2005a; Balzarini, 2007b, c). For further information on CBAs and their potential in antiviral therapy, readers may refer to recent expert reviews on this topic (Balzarini, 2007c; Francois & Balzarini, 2012).
Although the use of glycan decoys and CBAs may seem the most intuitive strategy to interfere directly with glycan–lectin interaction, other options exist as well. For instance, similar to glycan decoys and CBAs, lectin- or glycoconjugate-specific immunoglobulins may be used to block specific interactions. Evidently, the increasing availability of such ‘direct’ modulators of glycan–lectin interaction is mirrored in an increasing number of potential antiviral applications.
Apart from direct modulation, also strategies that influence glycan–lectin interactions more indirectly can be employed. In fact, many of the molecules used to examine glycan–lectin interactions in vitro suggest themselves as potential therapeutics.
One approach to indirectly govern glycan–lectin interaction is via the use of drugs that alter the host and/or viral glycome. Glycosidases and other enzymes may be used to alter the glycan portions of fully formed and matured glycoconjugates. Alternatively, various drugs may be employed to directly modify glycan synthesis: glycoconjugates produced in the presence of such molecules will obtain aberrant glycosylation, which may promote or annihilate their interaction with specific lectins. Promising results in glycovirological research have highlighted the antiviral potential of such compounds. For instance, ample data indicate that sialidases may be used to counteract infections where sialic acids play important roles as cellular receptors for viral lectins [e.g. IAV binds to sialic acid receptors on the airway epithelium (Skehel & Wiley, 2000; Malakhov et al., 2006; Belser et al., 2007; Harrison, 2008; Chan et al., 2009; Triana-Baltzer et al., 2009a, b, 2010, 2011)] or as viral ligands for host lectins that serve as portals for viral entry [e.g. sialic acids on the porcine reproductive and respiratory syndrome virus bind the macrophage-specific entry mediator sialoadhesin (Delputte & Nauwynck, 2004; Delputte et al., 2007; Van Breedam et al., 2010a, b)]. Sialidase treatment may also enhance recognition of viral glycoconjugates by mannose-specific immune system lectins that can limit viral infection: in vitro experiments have shown that enzymatic removal of sialic acids from the HIV-1 virion surface can significantly enhance virus binding and neutralization by MBL (Hart et al., 2002, 2003). In line with this, production of HIV-1 in the presence of the Golgi α1-2-mannosidase I inhibitor 1-deoxymannojirimycin – which blocks the biosynthesis of complex-type, sialylated oligosaccharides – increased susceptibility of the virus to MBL-mediated neutralization (Hart et al., 2003). Interestingly, 1-deoxymannojirimycin treatment of HIV-1-infected cells was also shown to potentiate the antiviral effects of mannose-specific plant CBAs towards the newly produced HIV-1 virions (Balzarini, 2007c). These and other examples illustrate the potential of these molecules as antiviral drugs.
Considering the various structural and nonstructural roles of glycans, it is clear that glycome modulation can also have effects beyond the alteration of glycan–lectin binding events. For instance, interfering with host cell glycosylation processes using specific inhibitors may inhibit assembly of infectious virions (Leavitt et al., 1977; Katz et al., 1980; Pizer et al., 1980; Herrler & Compans, 1983; Montefiori et al., 1988; Pal et al., 1989; Mehta et al., 1998; Dwek et al., 2002; Wu et al., 2002; Durantel et al., 2007; Lazar et al., 2007; Scanlan et al., 2007; Durantel, 2009; Merry & Astrautsova, 2010). Moreover, glycome modulation may significantly alter the capacity of the virus to evade recognition by virus-specific antibodies and B- and T-cell receptors via glycan shielding (Botarelli et al., 1991; Back et al., 1994; Willey et al., 1996; Reitter et al., 1998; Bolmstedt et al., 2001; Kang et al., 2005; Aguilar et al., 2006; Wang et al., 2009; Francica et al., 2010; Kobayashi & Suzuki, 2012). Clearly, glycome-modifying drugs can counteract viruses in various ways and constitute versatile tools in the control of viral infection.
Also other strategies that can indirectly influence glycan–lectin interactions are certainly worth exploring. For instance, drugs that alter host or viral lectin expression (e.g. cytokines or RNAi) may prove useful in antiviral strategies (Ochiel et al., 2010; Relloso et al., 2002; Ge et al., 2003; Arrighi et al., 2004; Ge et al., 2004; Nair et al., 2005; Yagi et al., 2010; Raza et al., 2011). Furthermore, patients infected with a virus that employs both viral lectins and RDEs may also benefit from treatment with RDE-specific inhibitors, as these can alter the balance between viral lectin and RDE activity which is often crucial for efficient viral replication and spread. Notable examples in this context are the several neuraminidase inhibitors that have been used successfully for the treatment of IAV infections (Kim et al., 1999; Lew et al., 2000; Roberts, 2001; Garman & Laver, 2004; Alymova et al., 2005; von itzstein, 2007; von itzstein & Thomson, 2009; Gamblin & Skehel, 2010; Ikematsu & Kawai, 2011).
In sum, drugs that modulate glycan–lectin interactions – either directly or indirectly – can be powerful instruments in the combat against viral pathogens. However, the antiviral strategies suggested above can also have drawbacks. Intensive use of antiviral therapeutics may elicit rapid selection of drug-resistant virus variants. Another possible drawback relates to the potential off-target effects of these therapies: therapeutics aimed at influencing specific glycan–lectin interactions that play key roles in viral infection processes may also affect general host glycosylation, lectin expression or glycan–lectin interactions that are crucial for normal functioning of the host and its immune defense. It is also conceivable that such drugs, despite their antiviral effects, may benefit the virus in some ways. For instance, although a glycome-modifying drug may promote viral recognition by specific immune system lectins that aid in viral clearance, it may also promote viral interaction with host lectins that can aid in cis- or trans-infection. Ultimately, the potential of specific agents as antiviral drugs depends on their net (antiviral) effects in vivo. Other potential disadvantages concern the pharmacokinetic properties of specific drugs. For instance, if glycan decoys or CBAs used in antiviral therapy show a broad reactivity and can respectively bind with multiple (nontarget) lectins or glycans in the host, it is possible that much of the antiviral effect is lost. Also, when using peptidic CBAs that are not native to the host, an antibody response might be mounted against these components, leading to neutralization and/or faster clearance of the active compound. In spite of these and other potential pitfalls, it is clear that glycan decoys and CBAs, as well as various indirect approaches to modulate glycan–lectin interaction, show potential for the treatment of diverse viral infections, either or not in combination with other antiviral strategies. A good understanding of relevant glycan–lectin interactions facilitates specific targeting of these binding events and can help to minimize possible off-target effects and to reduce the risk of drug resistance.
Concluding remarks
Glycans and lectins cover crucial roles in virus biology and their interplay often shapes the virus-host interaction. In general, the nature of the glycan, the lectin, and the specific conditions under which their interaction occurs determines the outcome of a specific binding event and directs the virus to a certain fate. Based on current knowledge, it is clear that viral lectins generally facilitate viral infection and spread. On the other hand, although it may seem intuitive that host lectin PRRs and other immune system lectins exclusively act in defense of the host, there is ample evidence that contradicts this. Although many host lectins are involved in the induction of an efficacious immune response against viral pathogens, many viruses can also abuse these lectins to promote infection and spread. Intriguingly, analysis of the many studies regarding the role of host lectins in viral infections suggests that soluble host lectins tend to be associated with antiviral activity, whereas membrane-associated host lectins seem to play a more dubious role and are often implicated in pro- as well as antiviral mechanisms (Tables 1 & 2). It is however noteworthy that the information provided in this manuscript reflects current views on glycan–lectin interactions in virus biology, and that future research may alter our understanding and interpretation of specific interactions. The fact that (aspects of) viral glycobiology may change during virus-host co-evolution even advocates periodic re-evaluation of specific glycan–lectin interactions.
The biology of glycans and lectins is complex and has long been poorly accessible to virologists and other scientists outside this field. This situation is changing with the emergence of international glycomics consortia (e.g. Consortium for Functional Glycomics), which can provide state-of-the-art techniques and expertise to analyze and interpret virologically/immunologically relevant glycan–lectin interactions. Still, there are specific pitfalls associated with glycovirological research. In vitro experiments need to be designed and interpreted considering key issues like glycan and lectin variability, the cell-type dependency of host and virus glycosylation and the influence of the lectin-expressing cell type on the final outcome of a glycan–lectin interaction. In addition, the results of in vitro experiments must ultimately be compared with – and re-interpreted in the context of – data obtained in animal models. In fact, our current understanding of specific glycan–lectin interactions in viral infection is mostly based on in vitro experiments, underlining the need for experimental validation of these results in the context of the infected host.
In the context of viral infections, many different (lectin-dependent or -independent) interactions and processes take place simultaneously, resulting in a complex network of virus–host factor interaction, signaling, and effector mechanisms, the net effect of which may benefit the host or the virus. Many of the interactions taking place are not yet well defined and probably more are still unknown. Key to combating viral disease is to make these black boxes more transparent. Synergisms between different branches of life sciences are essential to sustain and advance our knowledge in this important field of research.
Acknowledgements
The authors thank Leslie Bosseler for critical reading of the manuscript and assistance in creating the figures. They also thank Dr Barbara Bottazzi, Dr Alberto Mantovani, and Dr Antonio Inforzato (Istituto Clinico Humanitas, Italy) for their advice on pentraxin biology. W.V.B. was supported by the Flemish Institute for the Promotion of Innovation by Science and Technology (I.W.T.-Flanders; SB 61491 & 63491) and the Special Research Fund of Ghent University. S.P. was supported by the Leibniz Gemeinschaft. H.W.F. was supported by F.W.O.-Vlaanderen and the Special Research Fund of Ghent University. The authors apologize to all colleagues whose work has not been cited due to space limitations.
References
- Aguilar HC, Matreyek KA, Filone CM. et al (2006) N-glycans on Nipah virus fusion protein protect against neutralization but reduce membrane fusion and viral entry. J Virol 80: 4878–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre KB, Gray ES, Mufhandu H, McMahon JB, Chakauya E, O'Keefe BR, Chikwamba R, Morris L. (2012) The lectins griffithsin, cyanovirin-N and scytovirin inhibit HIV-1 binding to the DC-SIGN receptor and transfer to CD4(+) cells. Virology 423: 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alymova IV, Taylor G, Portner A. (2005) Neuraminidase inhibitors as antiviral agents. Curr Drug Targets 5: 401–409. [DOI] [PubMed] [Google Scholar]
- Anders EM, Hartley CA, Reading PC, Ezekowitz RA. (1994) Complement-dependent neutralization of influenza virus by a serum mannose-binding lectin. J Gen Virol 75: 615–622. [DOI] [PubMed] [Google Scholar]
- Arrighi JF, Pion M, Wiznerowicz M. et al (2004) Lentivirus-mediated RNA interference of DC-SIGN expression inhibits human immunodeficiency virus transmission from dendritic cells to T cells. J Virol 78: 10848–10855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach DJ, Lin Y, Miao HY, Cimbro R, DiFiore MJ, Gianolini ME, Furci L, Biswas P, Fauci AS, Lusso P. (2012) Identification of the platelet-derived chemokine CXCL4/PF-4 as a broad-spectrum HIV-1 inhibitor. P Natl Acad Sci USA 109: 9569–9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back NK, Smit L, De Jong JJ, Keulen W, Schutten M, Goudsmit J, Tersmette M. (1994) An N-glycan within the human immunodeficiency virus type 1 gp120 V3 loop affects virus neutralization. Virology 199: 431–438. [DOI] [PubMed] [Google Scholar]
- Balzarini J. (2007a) The alpha(1,2)-mannosidase I inhibitor 1-deoxymannojirimycin potentiates the antiviral activity of carbohydrate-binding agents against wild-type and mutant HIV-1 strains containing glycan deletions in gp120. FEBS Lett 581: 2060–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarini J. (2007b) Carbohydrate-binding agents: a potential future cornerstone for the chemotherapy of enveloped viruses? Antiviral Chem Chemother 18: 1–11. [DOI] [PubMed] [Google Scholar]
- Balzarini J. (2007c) Targeting the glycans of glycoproteins: a novel paradigm for antiviral therapy. Nat Rev Microbiol 5: 583–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarini J, Van Laethem K, Hatse S, Vermeire K, De Clercq E, Peumans W, Van Damme E, Vandamme AM, Bolmstedt A, Schols D. (2004) Profile of resistance of human immunodeficiency virus to mannose-specific plant lectins. J Virol 78: 10617–10627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarini J, Van Laethem K, Hatse S, Froeyen M, Peumans W, Van Damme E, Schols D. (2005a) Carbohydrate-binding agents cause deletions of highly conserved glycosylation sites in HIV GP120: a new therapeutic concept to hit the achilles heel of HIV. J Biol Chem 280: 41005–41014. [DOI] [PubMed] [Google Scholar]
- Balzarini J, Van Laethem K, Hatse S, Froeyen M, Van Damme E, Bolmstedt A, Peumans W, De Clercq E, Schols D. (2005b) Marked depletion of glycosylation sites in HIV-1 gp120 under selection pressure by the mannose-specific plant lectins of Hippeastrum hybrid and Galanthus nivalis. Mol Pharmacol 67: 1556–1565. [DOI] [PubMed] [Google Scholar]
- Balzarini J, Van Laethem K, Peumans WJ, Van Damme EJ, Bolmstedt A, Gago F, Schols D. (2006) Mutational pathways, resistance profile, and side effects of cyanovirin relative to human immunodeficiency virus type 1 strains with N-glycan deletions in their gp120 envelopes. J Virol 80: 8411–8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarini J, Van Herrewege Y, Vermeire K, Vanham G, Schols D. (2007a) Carbohydrate-binding agents efficiently prevent dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN)-directed HIV-1 transmission to T lymphocytes. Mol Pharmacol 71: 3–11. [DOI] [PubMed] [Google Scholar]
- Balzarini J, Van Laethem K, Daelemans D, Hatse S, Bugatti A, Rusnati M, Igarashi Y, Oki T, Schols D. (2007b) Pradimicin A, a carbohydrate-binding nonpeptidic lead compound for treatment of infections with viruses with highly glycosylated envelopes, such as human immunodeficiency virus. J Virol 81: 362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarini J, Francois KO, Van Laethem K, Hoorelbeke B, Renders M, Auwerx J, Liekens S, Oki T, Igarashi Y, Schols D. (2010) Pradimicin S, a highly soluble nonpeptidic small-size carbohydrate-binding antibiotic, is an anti-HIV drug lead for both microbicidal and systemic use. Antimicrob Agents Chemother 54: 1425–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baribaud F, Pohlmann S, Doms RW. (2001) The role of DC-SIGN and DC-SIGNR in HIV and SIV attachment, infection, and transmission. Virology 286: 1–6. [DOI] [PubMed] [Google Scholar]
- Baribaud F, Pohlmann S, Leslie G, Mortari F, Doms RW. (2002) Quantitative expression and virus transmission analysis of DC-SIGN on monocyte-derived dendritic cells. J Virol 76: 9135–9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrere B, Driguez PA, Maudrin J, Doutheau A, Aymard M, Quash G. (1997) A novel synthetic reversible inhibitor of sialidase efficiently blocks secondary but not primary influenza virus infection of MDCK cells in culture. Arch Virol 142: 1365–1380. [DOI] [PubMed] [Google Scholar]
- Bax M, Garcia-Vallejo JJ, Jang-Lee J. et al (2007) Dendritic cell maturation results in pronounced changes in glycan expression affecting recognition by siglecs and galectins. J Immunol 179: 8216–8224. [DOI] [PubMed] [Google Scholar]
- Becer CR, Gibson MI, Geng J, Ilyas R, Wallis R, Mitchell DA, Haddleton DM. (2010) High-affinity glycopolymer binding to human DC-SIGN and disruption of DC-SIGN interactions with HIV envelope glycoprotein. J Am Chem Soc 132: 15130–15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser JA, Lu X, Szretter KJ. et al (2007) DAS181, a novel sialidase fusion protein, protects mice from lethal avian influenza H5N1 virus infection. J Infect Dis 196: 1493–1499. [DOI] [PubMed] [Google Scholar]
- Belshaw R, Gardner A, Rambaut A, Pybus OG. (2008) Pacing a small cage: mutation and RNA viruses. Trends Ecol Evol 23: 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne CA, Kraaijeveld CA, van Strijp JA, Brouwer E, Harmsen M, Verhoef J, van Golde LM, van Iwaarden JF. (1995) Interactions of surfactant protein A with influenza A viruses: binding and neutralization. J Infect Dis 171: 335–341. [DOI] [PubMed] [Google Scholar]
- Benne CA, Benaissa-Trouw B, van Strijp JA, Kraaijeveld CA, van Iwaarden JF. (1997) Surfactant protein A, but not surfactant protein D, is an opsonin for influenza A virus phagocytosis by rat alveolar macrophages. Eur J Immunol 27: 886–890. [DOI] [PubMed] [Google Scholar]
- Bertaux C, Daelemans D, Meertens L. et al (2007) Entry of hepatitis C virus and human immunodeficiency virus is selectively inhibited by carbohydrate-binding agents but not by polyanions. Virology 366: 40–50. [DOI] [PubMed] [Google Scholar]
- Berzi A, Reina JJ, Ottria R. et al (2012) A glycomimetic compound inhibits DC-SIGN-mediated HIV infection in cellular and cervical explant models. AIDS 26: 127–137. [DOI] [PubMed] [Google Scholar]
- Blue CE, Spiller OB, Blackbourn DJ. (2004) The relevance of complement to virus biology. Virology 319: 176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggiano C, Manel N, Littman DR. (2007) Dendritic cell-mediated trans-enhancement of human immunodeficiency virus type 1 infectivity is independent of DC-SIGN. J Virol 81: 2519–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boily-Larouche G, Milev MP, Zijenah LS. et al (2012) Naturally-occurring genetic variants in human DC-SIGN increase HIV-1 capture, cell-transfer and risk of mother-to-child transmission. PLoS One 7: e40706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolmstedt A, Hinkula J, Rowcliffe E, Biller M, Wahren B, Olofsson S. (2001) Enhanced immunogenicity of a human immunodeficiency virus type 1 env DNA vaccine by manipulating N-glycosylation signals. Effects of elimination of the V3 N306 glycan. Vaccine 20: 397–405. [DOI] [PubMed] [Google Scholar]
- Bonomelli C, Doores KJ, Dunlop DC, Thaney V, Dwek RA, Burton DR, Crispin M, Scanlan CN. (2011) The glycan shield of HIV is predominantly oligomannose independently of production system or viral clade. PLoS One 6: e23521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botarelli P, Houlden BA, Haigwood NL, Servis C, Montagna D, Abrignani S. (1991) N-glycosylation of HIV-gp120 may constrain recognition by T lymphocytes. J Immunol 147: 3128–3132. [PubMed] [Google Scholar]
- Bottazzi B, Doni A, Garlanda C, Mantovani A. (2010) An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu Rev Immunol 28: 157–183. [DOI] [PubMed] [Google Scholar]
- Boukour S, Masse JM, Benit L, Dubart-Kupperschmitt A, Cramer EM. (2006) Lentivirus degradation and DC-SIGN expression by human platelets and megakaryocytes. J Thromb Haemost 4: 426–435. [DOI] [PubMed] [Google Scholar]
- Bowden TA, Crispin M, Jones EY, Stuart DI. (2010) Shared paramyxoviral glycoprotein architecture is adapted for diverse attachment strategies. Biochem Soc Trans 38: 1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudner M, Karpel M, Lear C. et al (2013) Lectin-dependent enhancement of Ebola virus infection via soluble and transmembrane C-type lectin receptors. PLoS One 8: e60838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu W, Mamedova A, Tan M, Xia M, Jiang X, Hegde RS. (2008) Structural basis for the receptor binding specificity of Norwalk virus. J Virol 82: 5340–5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh L, Lozach PY, Schiffer C, Staropoli I, Pezo V, Porrot F, Canque B, Virelizier JL, Arenzana-Seisdedos F, Amara A. (2006) Infection of dendritic cells (DCs), not DC-SIGN-mediated internalization of human immunodeficiency virus, is required for long-term transfer of virus to T cells. J Virol 80: 2949–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambi A, Figdor CG. (2003) Dual function of C-type lectin-like receptors in the immune system. Curr Opin Cell Biol 15: 539–546. [DOI] [PubMed] [Google Scholar]
- Cambi A, Koopman M, Figdor CG. (2005) How C-type lectins detect pathogens. Cell Microbiol 7: 481–488. [DOI] [PubMed] [Google Scholar]
- Cao S, Lou Z, Tan M, Chen Y, Liu Y, Zhang Z, Zhang XC, Jiang X, Li X, Rao Z. (2007) Structural basis for the recognition of blood group trisaccharides by norovirus. J Virol 81: 5949–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavrois M, Neidleman J, Kreisberg JF, Greene WC. (2007) In vitro derived dendritic cells trans-infect CD4 T cells primarily with surface-bound HIV-1 virions. PLoS Pathog 3: e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebulla CM, Miller DM, Knight DA, Briggs BR, McGaughy V, Sedmak DD. (2000) Cytomegalovirus induces sialyl Lewis(x) and Lewis(x) on human endothelial cells. Transplantation 69: 1202–1209. [DOI] [PubMed] [Google Scholar]
- Chaipan C, Soilleux EJ, Simpson P. et al (2006) DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets. J Virol 80: 8951–8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RW, Chan MC, Wong AC. et al (2009) DAS181 inhibits H5N1 influenza virus infection of human lung tissues. Antimicrob Agents Chemother 53: 3935–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Dutch RE. (2012) Paramyxovirus fusion and entry: multiple paths to a common end. Viruses 4: 613–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WC, White MR, Moyo P, McClear S, Thiel S, Hartshorn KL, Takahashi K. (2010) Lack of the pattern recognition molecule mannose-binding lectin increases susceptibility to influenza A virus infection. BMC Immunol 11: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Maguire T, Hileman RE, Fromm JR, Esko JD, Linhardt RJ, Marks RM. (1997) Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med 3: 866–871. [DOI] [PubMed] [Google Scholar]
- Chen CH, Zhang XQ, Lo CW, Liu PF, Liu YT, Gallo RL, Hsieh MF, Schooley RT, Huang CM. (2010) The essentiality of alpha-2-macroglobulin in human salivary innate immunity against new H1N1 swine origin influenza A virus. Proteomics 10: 2396–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JM, Hutson AM, Estes MK, Prasad BV. (2008) Atomic resolution structural characterization of recognition of histo-blood group antigens by Norwalk virus. P Natl Acad Sci USA 105: 9175–9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman PM. (1994) Influenza virus neuraminidase: structure, antibodies, and inhibitors. Protein Sci 3: 1687–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comelli EM, Sutton-Smith M, Yan Q. et al (2006) Activation of murine CD4+ and CD8+ T lymphocytes leads to dramatic remodeling of N-linked glycans. J Immunol 177: 2431–2440. [DOI] [PubMed] [Google Scholar]
- Crocker PR, Redelinghuys P. (2008) Siglecs as positive and negative regulators of the immune system. Biochem Soc Trans 36: 1467–1471. [DOI] [PubMed] [Google Scholar]
- Crocker PR, Paulson JC, Varki A. (2007) Siglecs and their roles in the immune system. Nat Rev 7: 255–266. [DOI] [PubMed] [Google Scholar]
- Curtis BM, Scharnowske S, Watson AJ. (1992) Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. P Natl Acad Sci USA 89: 8356–8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cwach KT, Sandbulte HR, Klonoski JM, Huber VC. (2012) Contribution of murine innate serum inhibitors toward interference within influenza virus immune assays. Influenza Other Respir Viruses 6: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva RC, Segat L, Crovella S. (2011) Role of DC-SIGN and L-SIGN receptors in HIV-1 vertical transmission. Hum Immunol 72: 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam TK, Brewer CF. (2010) Lectins as pattern recognition molecules: the effects of epitope density in innate immunity. Glycobiology 20: 270–279. [DOI] [PubMed] [Google Scholar]
- Davicino RC, Elicabe RJ, Di Genaro MS, Rabinovich GA. (2011) Coupling pathogen recognition to innate immunity through glycan-dependent mechanisms. Int Immunopharmacol 11: 1457–1463. [DOI] [PubMed] [Google Scholar]
- de Groot RJ. (2006) Structure, function and evolution of the hemagglutinin-esterase proteins of corona- and toroviruses. Glycoconj J 23: 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MA, de Gruijl T, Piguet V, van Kooyk Y, Geijtenbeek TB. (2007) Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med 13: 367–371. [DOI] [PubMed] [Google Scholar]
- Dechecchi MC, Tamanini A, Bonizzato A, Cabrini G. (2000) Heparan sulfate glycosaminoglycans are involved in adenovirus type 5 and 2-host cell interactions. Virology 268: 382–390. [DOI] [PubMed] [Google Scholar]
- Dechecchi MC, Melotti P, Bonizzato A, Santacatterina M, Chilosi M, Cabrini G. (2001) Heparan sulfate glycosaminoglycans are receptors sufficient to mediate the initial binding of adenovirus types 2 and 5. J Virol 75: 8772–8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W, Webb AI, Chan V, Jumnainsong A, Davidson A, Mongkolsapaya J, Screaton G. (2011) Lectin switching during dengue virus infection. J Infect Dis 203: 1775–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delputte PL, Nauwynck HJ. (2004) Porcine arterivirus infection of alveolar macrophages is mediated by sialic acid on the virus. J Virol 78: 8094–8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delputte PL, Vanderheijden N, Nauwynck HJ, Pensaert MB. (2002) Involvement of the matrix protein in attachment of porcine reproductive and respiratory syndrome virus to a heparinlike receptor on porcine alveolar macrophages. J Virol 76: 4312–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delputte PL, Van Breedam W, Delrue I, Oetke C, Crocker PR, Nauwynck HJ. (2007) Porcine arterivirus attachment to the macrophage-specific receptor sialoadhesin is dependent on the sialic acid-binding activity of the N-terminal immunoglobulin domain of sialoadhesin. J Virol 81: 9546–9550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dommett RM, Klein N, Turner MW. (2006) Mannose-binding lectin in innate immunity: past, present and future. Tissue Antigens 68: 193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson EF, Lindesmith LC, Lobue AD, Baric RS. (2008) Norovirus pathogenesis: mechanisms of persistence and immune evasion in human populations. Immunol Rev 225: 190–211. [DOI] [PubMed] [Google Scholar]
- Doores KJ, Bonomelli C, Harvey DJ, Vasiljevic S, Dwek RA, Burton DR, Crispin M, Scanlan CN. (2010) Envelope glycans of immunodeficiency virions are almost entirely oligomannose antigens. P Natl Acad Sci USA 107: 13800–13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormitzer PR, Sun ZY, Blixt O, Paulson JC, Wagner G, Harrison SC. (2002) Specificity and affinity of sialic acid binding by the rhesus rotavirus VP8* core. J Virol 76: 10512–10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durantel D. (2009) Celgosivir, an alpha-glucosidase I inhibitor for the potential treatment of HCV infection. Curr Opin Investig Drugs 10: 860–870. [PubMed] [Google Scholar]
- Durantel D, Alotte C, Zoulim F. (2007) Glucosidase inhibitors as antiviral agents for hepatitis B and C. Curr Opin Investig Drugs 8: 125–129. [PubMed] [Google Scholar]
- Dwek RA, Butters TD, Platt FM, Zitzmann N. (2002) Targeting glycosylation as a therapeutic approach. Nat Rev Drug Discov 1: 65–75. [DOI] [PubMed] [Google Scholar]
- Eggink D, Melchers M, Wuhrer M. et al (2010) Lack of complex N-glycans on HIV-1 envelope glycoproteins preserves protein conformation and entry function. Virology 401: 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen DP, Minchinton RM. (2003) Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin Infect Dis 37: 1496–1505. [DOI] [PubMed] [Google Scholar]
- Elgavish S, Shaanan B. (1997) Lectin-carbohydrate interactions: different folds, common recognition principles. Trends Biochem Sci 22: 462–467. [DOI] [PubMed] [Google Scholar]
- Estes MK, Prasad BV, Atmar RL. (2006) Noroviruses everywhere: has something changed? Curr Opin Infect Dis 19: 467–474. [DOI] [PubMed] [Google Scholar]
- Farkas T, Cross RW, Hargitt E, 3rd, Lerche NW, Morrow AL, Sestak K. (2010) Genetic diversity and histo-blood group antigen interactions of rhesus enteric caliciviruses. J Virol 84: 8617–8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg H, Mitchell DA, Drickamer K, Weis WI. (2001) Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294: 2163–2166. [DOI] [PubMed] [Google Scholar]
- Francica JR, Varela-Rohena A, Medvec A, Plesa G, Riley JL, Bates P. (2010) Steric shielding of surface epitopes and impaired immune recognition induced by the ebola virus glycoprotein. PLoS Pathog 6: e1001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois KO, Balzarini J. (2012) Potential of carbohydrate-binding agents as therapeutics against enveloped viruses. Med Res Rev 32: 349–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T. (2002) Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev 2: 346–353. [DOI] [PubMed] [Google Scholar]
- Gabius HJ, Andre S, Jimenez-Barbero J, Romero A, Solis D. (2011) From lectin structure to functional glycomics: principles of the sugar code. Trends Biochem Sci 36: 298–313. [DOI] [PubMed] [Google Scholar]
- Gaiha GD, Dong T, Palaniyar N, Mitchell DA, Reid KB, Clark HW. (2008) Surfactant protein A binds to HIV and inhibits direct infection of CD4+ cells, but enhances dendritic cell-mediated viral transfer. J Immunol 181: 601–609. [DOI] [PubMed] [Google Scholar]
- Gamblin SJ, Skehel JJ. (2010) Influenza hemagglutinin and neuraminidase membrane glycoproteins. J Biol Chem 285: 28403–28409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Vallejo JJ, van Kooyk Y. (2009) Endogenous ligands for C-type lectin receptors: the true regulators of immune homeostasis. Immunol Rev 230: 22–37. [DOI] [PubMed] [Google Scholar]
- Garman E, Laver G. (2004) Controlling influenza by inhibiting the virus's neuraminidase. Curr Drug Targets 5: 119–136. [DOI] [PubMed] [Google Scholar]
- Garner OB, Aguilar HC, Fulcher JA. et al (2010) Endothelial galectin-1 binds to specific glycans on nipah virus fusion protein and inhibits maturation, mobility, and function to block syncytia formation. PLoS Pathog 6: e1000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Q, McManus MT, Nguyen T, Shen CH, Sharp PA, Eisen HN, Chen J. (2003) RNA interference of influenza virus production by directly targeting mRNA for degradation and indirectly inhibiting all viral RNA transcription. P Natl Acad Sci USA 100: 2718–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Q, Eisen HN, Chen J. (2004) Use of siRNAs to prevent and treat influenza virus infection. Virus Res 102: 37–42. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Gringhuis SI. (2009) Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol 9: 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. (2000a) Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100: 575–585. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Kwon DS, Torensma R. et al (2000b) DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100: 587–597. [DOI] [PubMed] [Google Scholar]
- Gill DJ, Clausen H, Bard F. (2011) Location, location, location: new insights into O-GalNAc protein glycosylation. Trends Cell Biol 21: 149–158. [DOI] [PubMed] [Google Scholar]
- Gimsa U, Grotzinger I, Gimsa J. (1996) Two evolutionary strategies of influenza viruses to escape host non-specific inhibitors: alteration of hemagglutinin or neuraminidase specificity. Virus Res 42: 127–135. [DOI] [PubMed] [Google Scholar]
- Gordon S. (2002) Pattern recognition receptors: doubling up for the innate immune response. Cell 111: 927–930. [DOI] [PubMed] [Google Scholar]
- Gottschalk A. (1957) Neuraminidase: the specific enzyme of influenza virus and Vibrio cholerae. Biochim Biophys Acta 23: 645–646. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A, Pritsker A, Pack M, Shimeliovich I, Arrighi JF, Park CG, Trumpfheller C, Piguet V, Moran TM, Steinman RM. (2005) Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J Immunol 175: 4265–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TB. (2007) C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity 26: 605–616. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, van der Vlist M, van den Berg LM, den Dunnen J, Litjens M, Geijtenbeek TB. (2010) HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nat Immunol 11: 419–426. [DOI] [PubMed] [Google Scholar]
- Guillon P, Ruvoen-Clouet N, Le Moullac-Vaidye B, Marchandeau S, Le Pendu J. (2009) Association between expression of the H histo-blood group antigen, alpha1,2fucosyltransferases polymorphism of wild rabbits, and sensitivity to rabbit hemorrhagic disease virus. Glycobiology 19: 21–28. [DOI] [PubMed] [Google Scholar]
- Gurney KB, Elliott J, Nassanian H, Song C, Soilleux E, McGowan I, Anton PA, Lee B. (2005) Binding and transfer of human immunodeficiency virus by DC-SIGN+ cells in human rectal mucosa. J Virol 79: 5762–5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SC. (2008) Viral membrane fusion. Nat Struct Mol Biol 15: 690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MS, Sakaguchi T, Schmitt AP. (2010) Paramyxovirus assembly and budding: building particles that transmit infections. Int J Biochem Cell Biol 42: 1416–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart ML, Saifuddin M, Uemura K, Bremer EG, Hooker B, Kawasaki T, Spear GT. (2002) High mannose glycans and sialic acid on gp120 regulate binding of mannose-binding lectin (MBL) to HIV type 1. AIDS Res Hum Retroviruses 18: 1311–1317. [DOI] [PubMed] [Google Scholar]
- Hart ML, Saifuddin M, Spear GT. (2003) Glycosylation inhibitors and neuraminidase enhance human immunodeficiency virus type 1 binding and neutralization by mannose-binding lectin. J Gen Virol 84: 353–360. [DOI] [PubMed] [Google Scholar]
- Hartshorn KL, Sastry K, White MR, Anders EM, Super M, Ezekowitz RA, Tauber AI. (1993) Human mannose-binding protein functions as an opsonin for influenza A viruses. J Clin Invest 91: 1414–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorn KL, Crouch EC, White MR, Eggleton P, Tauber AI, Chang D, Sastry K. (1994) Evidence for a protective role of pulmonary surfactant protein D (SP-D) against influenza A viruses. J Clin Invest 94: 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorn KL, Reid KB, White MR, Jensenius JC, Morris SM, Tauber AI, Crouch E. (1996) Neutrophil deactivation by influenza A viruses: mechanisms of protection after viral opsonization with collectins and hemagglutination-inhibiting antibodies. Blood 87: 3450–3461. [PubMed] [Google Scholar]
- Hartshorn KL, White MR, Shepherd V, Reid K, Jensenius JC, Crouch EC. (1997) Mechanisms of anti-influenza activity of surfactant proteins A and D: comparison with serum collectins. Am J Physiol 273: L1156–L1166. [DOI] [PubMed] [Google Scholar]
- Hartshorn KL, White MR, Voelker DR, Coburn J, Zaner K, Crouch EC. (2000) Mechanism of binding of surfactant protein D to influenza A viruses: importance of binding to haemagglutinin to antiviral activity. Biochem J 351: 449–458. [PMC free article] [PubMed] [Google Scholar]
- Haslam SM, Julien S, Burchell JM, Monk CR, Ceroni A, Garden OA, Dell A. (2008) Characterizing the glycome of the mammalian immune system. Immunol Cell Biol 86: 564–573. [DOI] [PubMed] [Google Scholar]
- Hawgood S, Brown C, Edmondson J, Stumbaugh A, Allen L, Goerke J, Clark H, Poulain F. (2004) Pulmonary collectins modulate strain-specific influenza a virus infection and host responses. J Virol 78: 8565–8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeneder S, Seidel M, Forster-Waldl E, Heitger A. (2012) Mannan-binding lectin deficiency – Good news, bad news, doesn't matter? Clin Immunol 143: 22–38. [DOI] [PubMed] [Google Scholar]
- Herrler G, Compans RW. (1983) Posttranslational modification and intracellular transport of mumps virus glycoproteins. J Virol 47: 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrler G, Klenk HD. (1991) Structure and function of the HEF glycoprotein of influenza C virus. Adv Virus Res 40: 213–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrler G, Rott R, Klenk HD. (1985a) Neuraminic acid is involved in the binding of influenza C virus to erythrocytes. Virology 141: 144–147. [DOI] [PubMed] [Google Scholar]
- Herrler G, Rott R, Klenk HD, Muller HP, Shukla AK, Schauer R. (1985b) The receptor-destroying enzyme of influenza C virus is neuraminate-O-acetylesterase. EMBO J 4: 1503–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrler G, Durkop I, Becht H, Klenk HD. (1988) The glycoprotein of influenza C virus is the haemagglutinin, esterase and fusion factor. J Gen Virol 69: 839–846. [DOI] [PubMed] [Google Scholar]
- Herrler G, Szepanski S, Schultze B. (1991) 9-O-acetylated sialic acid, a receptor determinant for influenza C virus and coronaviruses. Behring Inst Mitt, 89: 177–184. [PubMed] [Google Scholar]
- Hickling TP, Malhotra R, Bright H, McDowell W, Blair ED, Sim RB. (2000) Lung surfactant protein A provides a route of entry for respiratory syncytial virus into host cells. Viral Immunol 13: 125–135. [DOI] [PubMed] [Google Scholar]
- Hijazi K, Wang Y, Scala C. et al (2011) DC-SIGN increases the affinity of HIV-1 envelope glycoprotein interaction with CD4. PLoS One 6: e28307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillaire ML, van Eijk M, van Trierum SE. et al (2011) Assessment of the antiviral properties of recombinant porcine SP-D against various influenza A viruses in vitro. PLoS One 6: e25005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraiwa N, Hiraiwa M, Kannagi R. (1997) Human T-cell leukemia virus-1 encoded Tax protein transactivates alpha 1–>3 fucosyltransferase Fuc-T VII, which synthesizes sialyl Lewis X, a selectin ligand expressed on adult T-cell leukemia cells. Biochem Biophys Res Commun 231: 183–186. [DOI] [PubMed] [Google Scholar]
- Hiraiwa N, Yabuta T, Yoritomi K, Hiraiwa M, Tanaka Y, Suzuki T, Yoshida M, Kannagi R. (2003) Transactivation of the fucosyltransferase VII gene by human T-cell leukemia virus type 1 Tax through a variant cAMP-responsive element. Blood 101: 3615–3621. [DOI] [PubMed] [Google Scholar]
- Hodges A, Sharrocks K, Edelmann M. et al (2007) Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nat Immunol 8: 569–577. [DOI] [PubMed] [Google Scholar]
- Holmes EC. (2009) The Evolution and Emergence of RNA Viruses. Oxford University Press, Oxford. [Google Scholar]
- Hong PW, Flummerfelt KB, de Parseval A, Gurney K, Elder JH, Lee B. (2002) Human immunodeficiency virus envelope (gp120) binding to DC-SIGN and primary dendritic cells is carbohydrate dependent but does not involve 2G12 or cyanovirin binding sites: implications for structural analyses of gp120-DC-SIGN binding. J Virol 76: 12855–12865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong PW, Nguyen S, Young S, Su SV, Lee B. (2007) Identification of the optimal DC-SIGN binding site on human immunodeficiency virus type 1 gp120. J Virol 81: 8325–8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoorelbeke B, Van Damme EJ, Rouge P, Schols D, Van Laethem K, Fouquaert E, Balzarini J. (2011) Differences in the mannose oligomer specificities of the closely related lectins from Galanthus nivalis and Zea mays strongly determine their eventual anti-HIV activity. Retrovirology 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PW, Farkas T, Zhong WM, Thornton S, Morrow AL, Xi J. (2005) Norovirus and histo-blood group antigens: Demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J Virol 79: 6714–6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulst MM, van Gennip HG, Moormann RJ. (2000) Passage of classical swine fever virus in cultured swine kidney cells selects virus variants that bind to heparan sulfate due to a single amino acid change in envelope protein E(rns). J Virol 74: 9553–9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskens D, Van Laethem K, Vermeire K, Balzarini J, Schols D. (2007) Resistance of HIV-1 to the broadly HIV-1-neutralizing, anti-carbohydrate antibody 2G12. Virology 360: 294–304. [DOI] [PubMed] [Google Scholar]
- Huskens D, Ferir G, Vermeire K, Kehr JC, Balzarini J, Dittmann E, Schols D. (2010) Microvirin, a novel alpha(1,2)-mannose-specific lectin isolated from Microcystis aeruginosa, has anti-HIV-1 activity comparable with that of cyanovirin-N but a much higher safety profile. J Biol Chem 285: 24845–24854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikematsu H, Kawai N. (2011) Laninamivir octanoate: a new long-acting neuraminidase inhibitor for the treatment of influenza. Expert Rev Anti Infect Ther 9: 851–857. [DOI] [PubMed] [Google Scholar]
- Izquierdo-Useros N, Lorizate M, Puertas MC. et al (2012) Siglec-1 Is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS Biol 10: e1001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RJ, Hall DF, Kerr PJ. (1999) Myxoma virus encodes an alpha2,3-sialyltransferase that enhances virulence. J Virol 73: 2376–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA, Jr, Medzhitov R. (2002) Innate immune recognition. Annu Rev Immunol 20: 197–216. [DOI] [PubMed] [Google Scholar]
- Jensen PH, Kolarich D, Packer NH. (2010) Mucin-type O-glycosylation–putting the pieces together. FEBS J 277: 81–94. [DOI] [PubMed] [Google Scholar]
- Job ER, Deng YM, Barfod KK, Tate MD, Caldwell N, Reddiex S, Maurer-Stroh S, Brooks AG, Reading PC. (2013) Addition of glycosylation to influenza A virus hemagglutinin modulates antibody-mediated recognition of H1N1 2009 pandemic viruses. J Immunol 190: 2169–2177. [DOI] [PubMed] [Google Scholar]
- Kang SM, Quan FS, Huang C, Guo L, Ye L, Yang C, Compans RW. (2005) Modified HIV envelope proteins with enhanced binding to neutralizing monoclonal antibodies. Virology 331: 20–32. [DOI] [PubMed] [Google Scholar]
- Kase T, Suzuki Y, Kawai T, Sakamoto T, Ohtani K, Eda S, Maeda A, Okuno Y, Kurimura T, Wakamiya N. (1999) Human mannan-binding lectin inhibits the infection of influenza A virus without complement. Immunology 97: 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E, Margalith E, Duksin D. (1980) Antiviral activity of tunicamycin on herpes simplex virus. Antimicrob Agents Chemother 17: 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Park MS. (2012) N-linked glycosylation in the hemagglutinin of influenza A viruses. Yonsei Med J 53: 886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CU, Chen X, Mendel DB. (1999) Neuraminidase inhibitors as anti-influenza virus agents. Antiviral Chem Chemother 10: 141–154. [DOI] [PubMed] [Google Scholar]
- Kingma PS, Zhang L, Ikegami M, Hartshorn K, McCormack FX, Whitsett JA. (2006) Correction of pulmonary abnormalities in Sftpd−/− mice requires the collagenous domain of surfactant protein D. J Biol Chem 281: 24496–24505. [DOI] [PubMed] [Google Scholar]
- Klimstra WB, Ryman KD, Johnston RE. (1998) Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J Virol 72: 7357–7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Suzuki Y. (2012) Evidence for N-glycan shielding of antigenic sites during evolution of human influenza A virus hemagglutinin. J Virol 86: 3446–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi Y, Kageyama S, Fujiyama Y, Miyashita M, Lwembe R, Ogino K, Shioda T, Ichimura H. (2007) RANTES -28G delays and DC-SIGN – 139C enhances AIDS progression in HIV type 1-infected Japanese hemophiliacs. AIDS Res Hum Retroviruses 23: 713–719. [DOI] [PubMed] [Google Scholar]
- Krempl C, Schultze B, Laude H, Herrler G. (1997) Point mutations in the S protein connect the sialic acid binding activity with the enteropathogenicity of transmissible gastroenteritis coronavirus. J Virol 71: 3285–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krempl C, Ballesteros ML, Zimmer G, Enjuanes L, Klenk HD, Herrler G. (2000) Characterization of the sialic acid binding activity of transmissible gastroenteritis coronavirus by analysis of haemagglutination-deficient mutants. J Gen Virol 81: 489–496. [DOI] [PubMed] [Google Scholar]
- Krusat T, Streckert HJ. (1997) Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells. Arch Virol 142: 1247–1254. [DOI] [PubMed] [Google Scholar]
- Kumagai Y, Akira S. (2010) Identification and functions of pattern-recognition receptors. J Allergy Clin Immunol 125: 985–992. [DOI] [PubMed] [Google Scholar]
- Kunkel F, Herrler G. (1993) Structural and functional analysis of the surface protein of human coronavirus OC43. Virology 195: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon DS, Gregorio G, Bitton N, Hendrickson WA, Littman DR. (2002) DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16: 135–144. [DOI] [PubMed] [Google Scholar]
- Lamb RA, Krug RM. (2001) Orthomyxoviridae: The Viruses and their Replication. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- Landers JJ, Cao Z, Lee I, Piehler LT, Myc PP, Myc A, Hamouda T, Galecki AT, Baker JR., Jr (2002) Prevention of influenza pneumonitis by sialic Acid-conjugated dendritic polymers. J Infect Dis 186: 1222–1230. [DOI] [PubMed] [Google Scholar]
- Lanteri M, Giordanengo V, Hiraoka N, Fuzibet JG, Auberger P, Fukuda M, Baum LG, Lefebvre JC. (2003) Altered T cell surface glycosylation in HIV-1 infection results in increased susceptibility to galectin-1-induced cell death. Glycobiology 13: 909–918. [DOI] [PubMed] [Google Scholar]
- Larkin A, Imperiali B. (2011) The expanding horizons of asparagine-linked glycosylation. Biochemistry 50: 4411–4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauring AS, Andino R. (2010) Quasispecies theory and the behavior of RNA viruses. PLoS Pathog 6: e1001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar C, Durantel D, Macovei A, Zitzmann N, Zoulim F, Dwek RA, Branza-Nichita N. (2007) Treatment of hepatitis B virus-infected cells with alpha-glucosidase inhibitors results in production of virions with altered molecular composition and infectivity. Antiviral Res 76: 30–37. [DOI] [PubMed] [Google Scholar]
- Le Pendu J, Ruvoen-Clouet N, Kindberg E, Svensson L. (2006) Mendelian resistance to human norovirus infections. Semin Immunol 18: 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt R, Schlesinger S, Kornfeld S. (1977) Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol 21: 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Ataman ZA. (2011) Modes of paramyxovirus fusion: a Henipavirus perspective. Trends Microbiol 19: 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Leslie G, Soilleux E. et al (2001) cis Expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J Virol 75: 12028–12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekkerkerker AN, van Kooyk Y, Geijtenbeek TB. (2006) Viral piracy: HIV-1 targets dendritic cells for transmission. Curr HIV Res 4: 169–176. [DOI] [PubMed] [Google Scholar]
- LeVine AM, Whitsett JA, Hartshorn KL, Crouch EC, Korfhagen TR. (2001) Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J Immunol 167: 5868–5873. [DOI] [PubMed] [Google Scholar]
- LeVine AM, Hartshorn K, Elliott J, Whitsett J, Korfhagen T. (2002) Absence of SP-A modulates innate and adaptive defense responses to pulmonary influenza infection. Am J Physiol 282: L563–L572. [DOI] [PubMed] [Google Scholar]
- LeVine AM, Elliott J, Whitsett JA, Srikiatkhachorn A, Crouch E, DeSilva N, Korfhagen T. (2004) Surfactant protein-d enhances phagocytosis and pulmonary clearance of respiratory syncytial virus. Am J Respir Cell Mol Biol 31: 193–199. [DOI] [PubMed] [Google Scholar]
- Levroney EL, Aguilar HC, Fulcher JA, Kohatsu L, Pace KE, Pang M, Gurney KB, Baum LG, Lee B. (2005) Novel innate immune functions for galectin-1: galectin-1 inhibits cell fusion by Nipah virus envelope glycoproteins and augments dendritic cell secretion of proinflammatory cytokines. J Immunol 175: 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew W, Chen X, Kim CU. (2000) Discovery and development of GS 4104 (oseltamivir): an orally active influenza neuraminidase inhibitor. Curr Med Chem 7: 663–672. [DOI] [PubMed] [Google Scholar]
- Li G, Siddiqui J, Hendry M, Akiyama J, Edmondson J, Brown C, Allen L, Levitt S, Poulain F, Hawgood S. (2002) Surfactant protein-A–deficient mice display an exaggerated early inflammatory response to a beta-resistant strain of influenza A virus. Am J Respir Cell Mol Biol 26: 277–282. [DOI] [PubMed] [Google Scholar]
- Liao CF, Wang SF, Lin YT, Ho DD, Chen YM. (2011) Identification of the DC-SIGN-interactive domains on the envelope glycoprotein of HIV-1 CRF07_BC. AIDS Res Hum Retroviruses 27: 831–839. [DOI] [PubMed] [Google Scholar]
- Liedtke S, Adamski M, Geyer R, Pfutzner A, Rubsamen-Waigmann H, Geyer H. (1994) Oligosaccharide profiles of HIV-2 external envelope glycoprotein: dependence on host cells and virus isolates. Glycobiology 4: 477–484. [DOI] [PubMed] [Google Scholar]
- Liedtke S, Geyer R, Geyer H. (1997) Host-cell-specific glycosylation of HIV-2 envelope glycoprotein. Glycoconj J 14: 785–793. [DOI] [PubMed] [Google Scholar]
- Lin G, Simmons G, Pohlmann S. et al (2003) Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC-SIGN and DC-SIGNR. J Virol 77: 1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Hwangbo Y, Holte S. et al (2004) Analysis of genetic polymorphisms in CCR5, CCR2, stromal cell-derived factor-1, RANTES, and dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin in seronegative individuals repeatedly exposed to HIV-1. J Infect Dis 190: 1055–1058. [DOI] [PubMed] [Google Scholar]
- Loris R. (2002) Principles of structures of animal and plant lectins. Biochim Biophys Acta 1572: 198–208. [DOI] [PubMed] [Google Scholar]
- Luallen RJ, Fu H, Agrawal-Gamse C, Mboudjeka I, Huang W, Lee FH, Wang LX, Doms RW, Geng Y. (2009) A yeast glycoprotein shows high-affinity binding to the broadly neutralizing human immunodeficiency virus antibody 2G12 and inhibits gp120 interactions with 2G12 and DC-SIGN. J Virol 83: 4861–4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen J, Gaiha GD, Palaniyar N, Dong T, Mitchell DA, Clark HW. (2013) Surfactant Protein D modulates HIV infection of both T-cells and dendritic cells. PLoS One 8: e59047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malakhov MP, Aschenbrenner LM, Smee DF. et al (2006) Sialidase fusion protein as a novel broad-spectrum inhibitor of influenza virus infection. Antimicrob Agents Chemother 50: 1470–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra R, Haurum JS, Thiel S, Sim RB. (1994) Binding of human collectins (SP-A and MBP) to influenza virus. Biochem J 304(Pt 2): 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl CW, Kroschewski H, Allison SL, Kofler R, Holzmann H, Meixner T, Heinz FX. (2001) Adaptation of tick-borne encephalitis virus to BHK-21 cells results in the formation of multiple heparan sulfate binding sites in the envelope protein and attenuation in vivo. J Virol 75: 5627–5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo C, Torreno-Pina JA, Joosten B, Reinieren-Beeren I, Gualda EJ, Loza-Alvarez P, Figdor CG, Garcia-Parajo MF, Cambi A. (2012) The neck region of the C-type lectin DC-SIGN regulates its surface spatiotemporal organization and virus-binding capacity on antigen-presenting cells. J Biol Chem 287: 38946–38955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markine-Goriaynoff N, Georgin JP, Goltz M, Zimmermann W, Broll H, Wamwayi HM, Pastoret PP, Sharp PM, Vanderplasschen A. (2003) The core 2 beta-1,6-N-acetylglucosaminyltransferase-mucin encoded by bovine herpesvirus 4 was acquired from an ancestor of the African buffalo. J Virol 77: 1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markine-Goriaynoff N, Gillet L, Van Etten JL, Korres H, Verma N, Vanderplasschen A. (2004a) Glycosyltransferases encoded by viruses. J Gen Virol 85: 2741–2754. [DOI] [PubMed] [Google Scholar]
- Markine-Goriaynoff N, Gillet L, Karlsen OA, Haarr L, Minner F, Pastoret PP, Fukuda M, Vanderplasschen A. (2004b) The core 2 beta-1,6-N-acetylglucosaminyltransferase-M encoded by bovine herpesvirus 4 is not essential for virus replication despite contributing to post-translational modifications of structural proteins. J Gen Virol 85: 355–367. [DOI] [PubMed] [Google Scholar]
- Martin MP, Lederman MM, Hutcheson HB. et al (2004) Association of DC-SIGN promoter polymorphism with increased risk for parenteral, but not mucosal, acquisition of human immunodeficiency virus type 1 infection. J Virol 78: 14053–14056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Avila O, Bedoya LM, Marradi M, Clavel C, Alcami J, Penades S. (2009a) Multivalent manno-glyconanoparticles inhibit DC-SIGN-mediated HIV-1 trans-infection of human T cells. ChemBioChem 10: 1806–1809. [DOI] [PubMed] [Google Scholar]
- Martinez-Avila O, Hijazi K, Marradi M, Clavel C, Campion C, Kelly C, Penades S. (2009b) Gold manno-glyconanoparticles: multivalent systems to block HIV-1 gp120 binding to the lectin DC-SIGN. Chemistry 15: 9874–9888. [DOI] [PubMed] [Google Scholar]
- Matrosovich M, Klenk HD. (2003) Natural and synthetic sialic acid-containing inhibitors of influenza virus receptor binding. Rev Med Virol 13: 85–97. [DOI] [PubMed] [Google Scholar]
- Matrosovich MN, Gambaryan AS, Chumakov MP. (1992) Influenza viruses differ in recognition of 4-O-acetyl substitution of sialic acid receptor determinant. Virology 188: 854–858. [DOI] [PubMed] [Google Scholar]
- Matrosovich M, Gao P, Kawaoka Y. (1998) Molecular mechanisms of serum resistance of human influenza H3N2 virus and their involvement in virus adaptation in a new host. J Virol 72: 6373–6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T, Onishi A, Saito T, Shimada A, Inoue H, Taki T, Nagata K, Okahata Y, Sato T. (2010) Sialic acid-mimic peptides as hemagglutinin inhibitors for anti-influenza therapy. J Med Chem 53: 4441–4449. [DOI] [PubMed] [Google Scholar]
- McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. (2003) Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300: 1295–1297. [DOI] [PubMed] [Google Scholar]
- McGreal EP, Martinez-Pomares L, Gordon S. (2004) Divergent roles for C-type lectins expressed by cells of the innate immune system. Mol Immunol 41: 1109–1121. [DOI] [PubMed] [Google Scholar]
- McGreal EP, Miller JL, Gordon S. (2005) Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr Opin Immunol 17: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A, Zitzmann N, Rudd PM, Block TM, Dwek RA. (1998) Alpha-glucosidase inhibitors as potential broad based anti-viral agents. FEBS Lett 430: 17–22. [DOI] [PubMed] [Google Scholar]
- Mercier S, St-Pierre C, Pelletier I, Ouellet M, Tremblay MJ, Sato S. (2008) Galectin-1 promotes HIV-1 infectivity in macrophages through stabilization of viral adsorption. Virology 371: 121–129. [DOI] [PubMed] [Google Scholar]
- Merry T, Astrautsova S. (2010) Alternative approaches to antiviral treatments: focusing on glycosylation as a target for antiviral therapy. Biotechnol Appl Biochem 56: 103–109. [DOI] [PubMed] [Google Scholar]
- Mikerov AN, White M, Hartshorn K, Wang G, Floros J. (2008) Inhibition of hemagglutination activity of influenza A viruses by SP-A1 and SP-A2 variants expressed in CHO cells. Med Microbiol Immunol 197: 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulak J, Teichberg S, Arora S, Kumar D, Yadav A, Salhan D, Pullagura S, Mathieson PW, Saleem MA, Singhal PC. (2010) DC-specific ICAM-3-grabbing nonintegrin mediates internalization of HIV-1 into human podocytes. Am J Physiol 299: F664–F673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori DC, Robinson WE, Jr, Mitchell WM. (1988) Role of protein N-glycosylation in pathogenesis of human immunodeficiency virus type 1. P Natl Acad Sci USA 85: 9248–9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moris A, Nobile C, Buseyne F, Porrot F, Abastado JP, Schwartz O. (2004) DC-SIGN promotes exogenous MHC-I-restricted HIV-1 antigen presentation. Blood 103: 2648–2654. [DOI] [PubMed] [Google Scholar]
- Moris A, Pajot A, Blanchet F, Guivel-Benhassine F, Salcedo M, Schwartz O. (2006) Dendritic cells and HIV-specific CD4+ T cells: HIV antigen presentation, T-cell activation, and viral transfer. Blood 108: 1643–1651. [DOI] [PubMed] [Google Scholar]
- Mummidi S, Catano G, Lam L, Hoefle A, Telles V, Begum K, Jimenez F, Ahuja SS, Ahuja SK. (2001) Extensive repertoire of membrane-bound and soluble dendritic cell-specific ICAM-3-grabbing nonintegrin 1 (DC-SIGN1) and DC-SIGN2 isoforms. Inter-individual variation in expression of DC-SIGN transcripts. J Biol Chem 276: 33196–33212. [DOI] [PubMed] [Google Scholar]
- Nair MP, Reynolds JL, Mahajan SD, Schwartz SA, Aalinkeel R, Bindukumar B, Sykes D. (2005) RNAi-directed inhibition of DC-SIGN by dendritic cells: prospects for HIV-1 therapy. AAPS J 7: E572–E578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada S, Creager RS, Krystal M, Aaronson RP, Palese P. (1984) Influenza C virus hemagglutinin: comparison with influenza A and B virus hemagglutinins. J Virol 50: 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash P, Barry M, Seet BT, Veugelers K, Hota S, Heger J, Hodgkinson C, Graham K, Jackson RJ, McFadden G. (2000) Post-translational modification of the myxoma-virus anti-inflammatory serpin SERP-1 by a virally encoded sialyltransferase. Biochem J 347: 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Sanchez E, Altmeyer R, Amara A, Schwartz O, Fieschi F, Virelizier JL, Arenzana-Seisdedos F, Despres P. (2003) Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep 4: 723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic DS, Lehmann M, Felts R, Garcia E, Blanchet FP, Subramaniam S, Piguet V. (2011) HIV-1 activates Cdc42 and induces membrane extensions in immature dendritic cells to facilitate cell-to-cell virus propagation. Blood 118: 4841–4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile C, Petit C, Moris A, Skrabal K, Abastado JP, Mammano F, Schwartz O. (2005) Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN-expressing cells promotes long-term transmission to lymphocytes. J Virol 79: 5386–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystrom K, Le Gall-Recule G, Grassi P. et al (2011) Histo-blood group antigens act as attachment factors of rabbit hemorrhagic disease virus infection in a virus strain-dependent manner. PLoS Pathog 7: e1002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiel DO, Ochsenbauer C, Kappes JC, Ghosh M, Fahey JV, Wira CR. (2010) Uterine epithelial cell regulation of DC-SIGN expression inhibits transmitted/founder HIV-1 trans infection by immature dendritic cells. PLoS One 5: e14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi M, Ohuchi R, Feldmann A, Klenk HD. (1997) Regulation of receptor binding affinity of influenza virus hemagglutinin by its carbohydrate moiety. J Virol 71: 8377–8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio F, Reis e Sousa C. (2011) Myeloid C-type lectin receptors in pathogen recognition and host defense. Immunity 34: 651–664. [DOI] [PubMed] [Google Scholar]
- Ouellet M, Mercier S, Pelletier I, Bounou S, Roy J, Hirabayashi J, Sato S, Tremblay MJ. (2005) Galectin-1 acts as a soluble host factor that promotes HIV-1 infectivity through stabilization of virus attachment to host cells. J Immunol 174: 4120–4126. [DOI] [PubMed] [Google Scholar]
- Pal R, Hoke GM, Sarngadharan MG. (1989) Role of oligosaccharides in the processing and maturation of envelope glycoproteins of human immunodeficiency virus type 1. P Natl Acad Sci USA 86: 3384–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantophlet R, Burton DR. (2006) GP120: Target for neutralizing HIV-1 antibodies. Annu Rev Immunol 24: 739–769. [DOI] [PubMed] [Google Scholar]
- Papp I, Sieben C, Ludwig K, Roskamp M, Bottcher C, Schlecht S, Herrmann A, Haag R. (2010) Inhibition of influenza virus infection by multivalent sialic-acid-functionalized gold nanoparticles. Small 6: 2900–2906. [DOI] [PubMed] [Google Scholar]
- Papp I, Sieben C, Sisson AL, Kostka J, Bottcher C, Ludwig K, Herrmann A, Haag R. (2011) Inhibition of influenza virus activity by multivalent glycoarchitectures with matched sizes. Chembiochem 12: 887–895. [DOI] [PubMed] [Google Scholar]
- Patel M, Yanagishita M, Roderiquez G, Bou-Habib DC, Oravecz T, Hascall VC, Norcross MA. (1993) Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res Hum Retroviruses 9: 167–174. [DOI] [PubMed] [Google Scholar]
- Paulick MG, Bertozzi CR. (2008) The glycosylphosphatidylinositol anchor: a complex membrane-anchoring structure for proteins. Biochemistry 47: 6991–7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekosz A, Lamb RA. (1999) Cell surface expression of biologically active influenza C virus HEF glycoprotein expressed from cDNA. J Virol 73: 8808–8812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter-Katalinic J. (2005) Methods in enzymology: O-glycosylation of proteins. Methods Enzymol 405: 139–171. [DOI] [PubMed] [Google Scholar]
- Piguet V, Steinman RM. (2007) The interaction of HIV with dendritic cells: outcomes and pathways. Trends Immunol 28: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer LI, Cohen GH, Eisenberg RJ. (1980) Effect of tunicamycin on herpes simplex virus glycoproteins and infectious virus production. J Virol 34: 142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann S, Baribaud F, Lee B, Leslie GJ, Sanchez MD, Hiebenthal-Millow K, Munch J, Kirchhoff F, Doms RW. (2001) DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus. J Virol 75: 4664–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett TJ, Paulson JC. (1989) Basis for the potent inhibition of influenza virus infection by equine and guinea pig alpha 2-macroglobulin. J Biol Chem 264: 9850–9858. [PubMed] [Google Scholar]
- Prydz K, Dalen KT. (2000) Synthesis and sorting of proteoglycans. J Cell Sci 113: 193–205. [DOI] [PubMed] [Google Scholar]
- Rademacher C, Krishna NR, Palcic M, Parra F, Peters T. (2008) NMR experiments reveal the molecular basis of receptor recognition by a calicivirus. J Am Chem Soc 130: 3669–3675. [DOI] [PubMed] [Google Scholar]
- Rappocciolo G, Piazza P, Fuller CL, Reinhart TA, Watkins SC, Rowe DT, Jais M, Gupta P, Rinaldo CR. (2006) DC-SIGN on B lymphocytes is required for transmission of HIV-1 to T lymphocytes. PLoS Pathog 2: e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A, Shareef H, Salim H, Khushal R, Bokhari H. (2011) Selection of predicted siRNA as potential antiviral therapeutic agent against influenza virus. Bioinformation 6: 340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading PC, Hartley CA, Ezekowitz RA, Anders EM. (1995) A serum mannose-binding lectin mediates complement-dependent lysis of influenza virus-infected cells. Biochem Biophys Res Commun 217: 1128–1136. [DOI] [PubMed] [Google Scholar]
- Reading PC, Morey LS, Crouch EC, Anders EM. (1997) Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J Virol 71: 8204–8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading PC, Tate MD, Pickett DL, Brooks AG. (2007) Glycosylation as a target for recognition of influenza viruses by the innate immune system. Adv Exp Med Biol 598: 279–292. [DOI] [PubMed] [Google Scholar]
- Reading PC, Pickett DL, Tate MD, Whitney PG, Job ER, Brooks AG. (2009) Loss of a single N-linked glycan from the hemagglutinin of influenza virus is associated with resistance to collectins and increased virulence in mice. Respir Res 10: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitter JN, Means RE, Desrosiers RC. (1998) A role for carbohydrates in immune evasion in AIDS. Nat Med 4: 679–684. [DOI] [PubMed] [Google Scholar]
- Relloso M, Puig-Kroger A, Pello OM, Rodriguez-Fernandez JL, de la Rosa G, Longo N, Navarro J, Munoz-Fernandez MA, Sanchez-Mateos P, Corbi AL. (2002) DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-beta, and anti-inflammatory agents. J Immunol 168: 2634–2643. [DOI] [PubMed] [Google Scholar]
- Roberts NA. (2001) Anti-influenza drugs and neuraminidase inhibitors. Prog Drug Res. Spec No: 35–77. [DOI] [PubMed] [Google Scholar]
- Rogers GN, Pritchett TJ, Lane JL, Paulson JC. (1983) Differential sensitivity of human, avian, and equine influenza A viruses to a glycoprotein inhibitor of infection: selection of receptor specific variants. Virology 131: 394–408. [DOI] [PubMed] [Google Scholar]
- Rogers GN, Herrler G, Paulson JC, Klenk HD. (1986) Influenza C virus uses 9-O-acetyl-N-acetylneuraminic acid as a high affinity receptor determinant for attachment to cells. J Biol Chem 261: 5947–5951. [PubMed] [Google Scholar]
- Rosenthal PB, Zhang X, Formanowski F, Fitz W, Wong CH, Meier-Ewert H, Skehel JJ, Wiley DC. (1998) Structure of the haemagglutinin-esterase-fusion glycoprotein of influenza C virus. Nature 396: 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. (1996) Protein glycosylation in the endoplasmic reticulum and the Golgi apparatus and cell type-specificity of cell surface glycoconjugate expression: analysis by the protein A-gold and lectin-gold techniques. Histochem Cell Biol 106: 79–92. [DOI] [PubMed] [Google Scholar]
- Ruvoen-Clouet N, Ganiere JP, Andre-Fontaine G, Blanchard D, Le Pendu J. (2000) Binding of rabbit hemorrhagic disease virus to antigens of the ABH histo-blood group family. J Virol 74: 11950–11954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan-Poirier KA, Kawaoka Y. (1991) Distinct glycoprotein inhibitors of influenza A virus in different animal sera. J Virol 65: 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan-Poirier KA, Kawaoka Y. (1993) Alpha 2-macroglobulin is the major neutralizing inhibitor of influenza A virus in pig serum. Virology 193: 974–976. [DOI] [PubMed] [Google Scholar]
- Sa-Carvalho D, Rieder E, Baxt B, Rodarte R, Tanuri A, Mason PW. (1997) Tissue culture adaptation of foot-and-mouth disease virus selects viruses that bind to heparin and are attenuated in cattle. J Virol 71: 5115–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuntabhai A, Turbpaiboon C, Casademont I. et al (2005) A variant in the CD209 promoter is associated with severity of dengue disease. Nat Genet 37: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho D, Reis e Sousa C. (2012) Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol 30: 491–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Nagai K, Tsutsumi H, Kuroki Y. (2003) Lactoferrin and surfactant protein A exhibit distinct binding specificity to F protein and differently modulate respiratory syncytial virus infection. Eur J Immunol 33: 2894–2902. [DOI] [PubMed] [Google Scholar]
- Sato S, St-Pierre C, Bhaumik P, Nieminen J. (2009) Galectins in innate immunity: dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs). Immunol Rev 230: 172–187. [DOI] [PubMed] [Google Scholar]
- Sato S, Ouellet M, St-Pierre C, Tremblay MJ. (2012) Glycans, galectins, and HIV-1 infection. Ann NY Acad Sci 1253: 133–148. [DOI] [PubMed] [Google Scholar]
- Sattin S, Daghetti A, Thepaut M, Berzi A, Sanchez-Navarro M, Tabarani G, Rojo J, Fieschi F, Clerici M, Bernardi A. (2010) Inhibition of DC-SIGN-mediated HIV infection by a linear trimannoside mimic in a tetravalent presentation. ACS Chem Biol 5: 301–312. [DOI] [PubMed] [Google Scholar]
- Scanlan CN, Offer J, Zitzmann N, Dwek RA. (2007) Exploiting the defensive sugars of HIV-1 for drug and vaccine design. Nature 446: 1038–1045. [DOI] [PubMed] [Google Scholar]
- Schauer R, Reuter G, Stoll S, Posadas del Rio F, Herrler G, Klenk HD. (1988) Isolation and characterization of sialate 9(4)-O-acetylesterase from influenza C virus. Bio Chem Hoppe-Seyler 369: 1121–1130. [DOI] [PubMed] [Google Scholar]
- Schultze B, Gross HJ, Brossmer R, Herrler G. (1991) The S protein of bovine coronavirus is a hemagglutinin recognizing 9-O-acetylated sialic acid as a receptor determinant. J Virol 65: 6232–6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze B, Krempl C, Ballesteros ML, Shaw L, Schauer R, Enjuanes L, Herrler G. (1996) Transmissible gastroenteritis coronavirus, but not the related porcine respiratory coronavirus, has a sialic acid (N-glycolylneuraminic acid) binding activity. J Virol 70: 5634–5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz F, Aebi M. (2011) Mechanisms and principles of N-linked protein glycosylation. Curr Opin Struct Biol 21: 576–582. [DOI] [PubMed] [Google Scholar]
- Schwegmann-Wessels C, Bauer S, Winter C, Enjuanes L, Laude H, Herrler G. (2011) The sialic acid binding activity of the S protein facilitates infection by porcine transmissible gastroenteritis coronavirus. Virol J 8: 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj P, Alagarasu K, Swaminathan S, Harishankar M, Narendran G. (2009) CD209 gene polymorphisms in South Indian HIV and HIV-TB patients. Infect Genet Evol 9: 256–262. [DOI] [PubMed] [Google Scholar]
- Serrano-Gomez D, Sierra-Filardi E, Martinez-Nunez RT, Caparros E, Delgado R, Munoz-Fernandez MA, Abad MA, Jimenez-Barbero J, Leal M, Corbi AL. (2008) Structural requirements for multimerization of the pathogen receptor dendritic cell-specific ICAM3-grabbing non-integrin (CD209) on the cell surface. J Biol Chem 283: 3889–3903. [DOI] [PubMed] [Google Scholar]
- Sewell AK, Price DA. (2001) Dendritic cells and transmission of HIV-1. Trends Immunol 22: 173–175. [DOI] [PubMed] [Google Scholar]
- Shirato H. (2011) Norovirus and histo-blood group antigens. Jpn J Infect Dis 64: 95–103. [PubMed] [Google Scholar]
- Skehel JJ, Wiley DC. (2000) Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem 69: 531–569. [DOI] [PubMed] [Google Scholar]
- Soilleux EJ, Morris LS, Leslie G. et al (2002) Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol 71: 445–457. [PubMed] [Google Scholar]
- St-Pierre C, Manya H, Ouellet M, Clark GF, Endo T, Tremblay MJ, Sato S. (2011) Host-soluble galectin-1 promotes HIV-1 replication through a direct interaction with glycans of viral gp120 and host CD4. J Virol 85: 11742–11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart AD, Brown TD. (2007) Alpha2,6-linked sialic acid acts as a receptor for Feline calicivirus. J Gen Virol 88: 177–186. [DOI] [PubMed] [Google Scholar]
- Su SV, Hong P, Baik S, Negrete OA, Gurney KB, Lee B. (2004) DC-SIGN binds to HIV-1 glycoprotein 120 in a distinct but overlapping fashion compared with ICAM-2 and ICAM-3. J Biol Chem 279: 19122–19132. [DOI] [PubMed] [Google Scholar]
- Sujino K, Jackson RJ, Chan NW, Tsuji S, Palcic MM. (2000) A novel viral alpha2,3-sialyltransferase (v-ST3Gal I): transfer of sialic acid to fucosylated acceptors. Glycobiology 10: 313–320. [DOI] [PubMed] [Google Scholar]
- Summerford C, Samulski RJ. (1998) Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol 72: 1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XJ, Jayaraman A, Maniprasad P, Raman R, Houser KV, Pappas C, Zeng H, Sasisekharan R, Katz JM, Tumpey TM. (2013) N-linked glycosylation of the hemagglutinin protein influences virulence and antigenicity of the 1918 pandemic and seasonal H1N1 influenza A viruses. J Virol 87: 8756–8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y. (2005) Sialobiology of influenza: molecular mechanism of host range variation of influenza viruses. Biol Pharm Bull 28: 399–408. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Nei M. (2002) Origin and evolution of influenza virus hemagglutinin genes. Mol Biol Evol 19: 501–509. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Tsukimoto M, Kobayashi M, Yamada A, Kawaoka Y, Webster RG, Suzuki Y. (1994) Sialoglycoproteins that bind influenza A virus and resist viral neuraminidase in different animal sera. J Gen Virol 75(Pt 7): 1769–1774. [DOI] [PubMed] [Google Scholar]
- Svajger U, Anderluh M, Jeras M, Obermajer N. (2010) C-type lectin DC-SIGN: an adhesion, signalling and antigen-uptake molecule that guides dendritic cells in immunity. Cell Signal 22: 1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Jiang X. (2007) Norovirus-host interaction: implications for disease control and prevention. Expert Rev Mol Med 9: 1–22. [DOI] [PubMed] [Google Scholar]
- Tate MD, Brooks AG, Reading PC. (2011a) Specific sites of N-linked glycosylation on the hemagglutinin of H1N1 subtype influenza A virus determine sensitivity to inhibitors of the innate immune system and virulence in mice. J Immunol 187: 1884–1894. [DOI] [PubMed] [Google Scholar]
- Tate MD, Job ER, Brooks AG, Reading PC. (2011b) Glycosylation of the hemagglutinin modulates the sensitivity of H3N2 influenza viruses to innate proteins in airway secretions and virulence in mice. Virology 413: 84–92. [DOI] [PubMed] [Google Scholar]
- Taube S, Perry JW, Yetming K. et al (2009) Ganglioside-linked terminal sialic acid moieties on murine macrophages function as attachment receptors for murine noroviruses. J Virol 83: 4092–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube S, Jiang M, Wobus CE. (2010) Glycosphingolipids as receptors for non-enveloped viruses. Viruses 2: 1011–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube S, Perry JW, McGreevy E, Yetming K, Perkins C, Henderson K, Wobus CE. (2012) Murine noroviruses bind glycolipid and glycoprotein attachment receptors in a strain-dependent manner. J Virol 86: 5584–5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor ME, Drickamer K. (2011) Introduction to Glycobiology. Oxford University Press, Oxford. [Google Scholar]
- Triana-Baltzer GB, Gubareva LV, Klimov AI, Wurtman DF, Moss RB, Hedlund M, Larson JL, Belshe RB, Fang F. (2009a) Inhibition of neuraminidase inhibitor-resistant influenza virus by DAS181, a novel sialidase fusion protein. PLoS One 4: e7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triana-Baltzer GB, Gubareva LV, Nicholls JM. et al (2009b) Novel pandemic influenza A(H1N1) viruses are potently inhibited by DAS181, a sialidase fusion protein. PLoS One 4: e7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triana-Baltzer GB, Babizki M, Chan MC. et al (2010) DAS181, a sialidase fusion protein, protects human airway epithelium against influenza virus infection: an in vitro pharmacodynamic analysis. J Antimicrob Chemother 65: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triana-Baltzer GB, Sanders RL, Hedlund M, Jensen KA, Aschenbrenner LM, Larson JL, Fang F. (2011) Phenotypic and genotypic characterization of influenza virus mutants selected with the sialidase fusion protein DAS181. J Antimicrob Chemother 66: 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trybala E, Roth A, Johansson M, Liljeqvist JA, Rekabdar E, Larm O, Bergstrom T. (2002) Glycosaminoglycan-binding ability is a feature of wild-type strains of herpes simplex virus type 1. Virology 302: 413–419. [DOI] [PubMed] [Google Scholar]
- Tsegaye TS, Pohlmann S. (2010) The multiple facets of HIV attachment to dendritic cell lectins. Cell Microbiol 12: 1553–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsegaye TS, Gnirss K, Rahe-Meyer N, Kiene M, Kramer-Kuhl A, Behrens G, Munch J, Pohlmann S. (2013) Platelet activation suppresses HIV-1 infection of T cells. Retrovirology 10: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya E, Sugawara K, Hongo S, Matsuzaki Y, Muraki Y, Li ZN, Nakamura K. (2002) Effect of addition of new oligosaccharide chains to the globular head of influenza A/H2N2 virus haemagglutinin on the intracellular transport and biological activities of the molecule. J Gen Virol 83: 1137–1146. [DOI] [PubMed] [Google Scholar]
- Turville SG, Cameron PU, Handley A, Lin G, Pohlmann S, Doms RW, Cunningham AL. (2002) Diversity of receptors binding HIV on dendritic cell subsets. Nat Immunol 3: 975–983. [DOI] [PubMed] [Google Scholar]
- Turville SG, Santos JJ, Frank I. et al (2004) Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 103: 2170–2179. [DOI] [PubMed] [Google Scholar]
- Van Breedam W, Van Gorp H, Zhang JQ, Crocker PR, Delputte PL, Nauwynck HJ. (2010a) The M/GP(5) glycoprotein complex of porcine reproductive and respiratory syndrome virus binds the sialoadhesin receptor in a sialic acid-dependent manner. PLoS Pathog 6: e1000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breedam W, Delputte PL, Van Gorp H, Misinzo G, Vanderheijden N, Duan X, Nauwynck HJ. (2010b) Porcine reproductive and respiratory syndrome virus entry into the porcine macrophage. J Gen Virol 91: 1659–1667. [DOI] [PubMed] [Google Scholar]
- van de Wetering JK, van Golde LM, Batenburg JJ. (2004) Collectins: players of the innate immune system. Eur J Biochem 271: 1229–1249. [DOI] [PubMed] [Google Scholar]
- Van den Steen P, Rudd PM, Dwek RA, Opdenakker G. (1998) Concepts and principles of O-linked glycosylation. Crit Rev Biochem Mol Biol 33: 151–208. [DOI] [PubMed] [Google Scholar]
- van der Vlist M, Geijtenbeek TB. (2010) Langerin functions as an antiviral receptor on Langerhans cells. Immunol Cell Biol 88: 410–415. [DOI] [PubMed] [Google Scholar]
- van der Vlist M, van der Aar AM, Gringhuis SI, Geijtenbeek TB. (2011) Innate signaling in HIV-1 infection of dendritic cells. Curr Opin HIV AIDS 6: 348–352. [DOI] [PubMed] [Google Scholar]
- van Eijk M, van de Lest CH, Batenburg JJ, Vaandrager AB, Meschi J, Hartshorn KL, van Golde LM, Haagsman HP. (2002) Porcine surfactant protein D is N-glycosylated in its carbohydrate recognition domain and is assembled into differently charged oligomers. Am J Respir Cell Mol Biol 26: 739–747. [DOI] [PubMed] [Google Scholar]
- van Eijk M, White MR, Crouch EC, Batenburg JJ, Vaandrager AB, Van Golde LM, Haagsman HP, Hartshorn KL. (2003) Porcine pulmonary collectins show distinct interactions with influenza A viruses: role of the N-linked oligosaccharides in the carbohydrate recognition domain. J Immunol 171: 1431–1440. [DOI] [PubMed] [Google Scholar]
- van Eijk M, White MR, Batenburg JJ, Vaandrager AB, van Golde LM, Haagsman HP, Hartshorn KL. (2004) Interactions of influenza A virus with sialic acids present on porcine surfactant protein D. Am J Respir Cell Mol Biol 30: 871–879. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y, Rabinovich GA. (2008) Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol 9: 593–601. [DOI] [PubMed] [Google Scholar]
- van Liempt E, Bank CM, Mehta P, Garcia-Vallejo JJ, Kawar ZS, Geyer R, Alvarez RA, Cummings RD, Kooyk Y, van Die I. (2006) Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett 580: 6123–6131. [DOI] [PubMed] [Google Scholar]
- Vanderheijden N, Delputte P, Nauwynck H, Pensaert M. (2001) Effects of heparin on the entry of porcine reproductive and respiratory syndrome virus into alveolar macrophages. Adv Exp Med Biol 494: 683–689. [DOI] [PubMed] [Google Scholar]
- Vanderplasschen A, Markine-Goriaynoff N, Lomonte P. et al (2000) A multipotential beta-1,6-N-acetylglucosaminyl-transferase is encoded by bovine herpesvirus type 4. P Natl Acad Sci USA 97: 5756–5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. (2009) Essentials of Glycobiology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor. [PubMed] [Google Scholar]
- Veldhuizen EJ, van Eijk M, Haagsman HP. (2011) The carbohydrate recognition domain of collectins. FEBS J 278: 3930–3941. [DOI] [PubMed] [Google Scholar]
- Vigerust DJ, Ulett KB, Boyd KL, Madsen J, Hawgood S, McCullers JA. (2007) N-linked glycosylation attenuates H3N2 influenza viruses. J Virol 81: 8593–8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasak R, Krystal M, Nacht M, Palese P. (1987) The influenza C virus glycoprotein (HE) exhibits receptor-binding (hemagglutinin) and receptor-destroying (esterase) activities. Virology 160: 419–425. [DOI] [PubMed] [Google Scholar]
- von Itzstein M. (2007) The war against influenza: discovery and development of sialidase inhibitors. Nat Rev Drug Discov 6: 967–974. [DOI] [PubMed] [Google Scholar]
- von Itzstein M, Thomson R. (2009) Anti-influenza drugs: the development of sialidase inhibitors. Handb Exp Pharmacol 189: 111–154. [DOI] [PubMed] [Google Scholar]
- Wagner R, Wolff T, Herwig A, Pleschka S, Klenk HD. (2000) Interdependence of hemagglutinin glycosylation and neuraminidase as regulators of influenza virus growth: a study by reverse genetics. J Virol 74: 6316–6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R, Matrosovich M, Klenk HD. (2002) Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev Med Virol 12: 159–166. [DOI] [PubMed] [Google Scholar]
- Wang JH, Janas AM, Olson WJ, Wu L. (2007a) Functionally distinct transmission of human immunodeficiency virus type 1 mediated by immature and mature dendritic cells. J Virol 81: 8933–8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Tian X, Chen X, Ma J. (2007b) Structural basis for receptor specificity of influenza B virus hemagglutinin. P Natl Acad Sci USA 104: 16874–16879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Cheng F, Lu M, Tian X, Ma J. (2008a) Crystal structure of unliganded influenza B virus hemagglutinin. J Virol 82: 3011–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SK, Liang PH, Astronomo RD, Hsu TL, Hsieh SL, Burton DR, Wong CH. (2008b) Targeting the carbohydrates on HIV-1: Interaction of oligomannose dendrons with human monoclonal antibody 2G12 and DC-SIGN. P Natl Acad Sci USA 105: 3690–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Chen JR, Tseng YC. et al (2009) Glycans on influenza hemagglutinin affect receptor binding and immune response. P Natl Acad Sci USA 106: 18137–18142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chen RF, Liu JW, Lee IK, Lee CP, Kuo HC, Huang SK, Yang KD. (2011) DC-SIGN (CD209) Promoter -336 A/G polymorphism is associated with dengue hemorrhagic fever and correlated to DC-SIGN expression and immune augmentation. PLoS Negl Trop Dis 5: e934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanzeck K, Boyd KL, McCullers JA. (2011) Glycan shielding of the influenza virus hemagglutinin contributes to immunopathology in mice. Am J Respir Crit Care Med 183: 767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerapana E, Imperiali B. (2006) Asparagine-linked protein glycosylation: from eukaryotic to prokaryotic systems. Glycobiology 16: 91R–101R. [DOI] [PubMed] [Google Scholar]
- Wei X, Decker JM, Wang S. et al (2003) Antibody neutralization and escape by HIV-1. Nature 422: 307–312. [DOI] [PubMed] [Google Scholar]
- Wei CJ, Boyington JC, Dai KF, Houser KV, Pearce MB, Kong WP, Yang ZY, Tumpey TM, Nabel GJ. (2010) Cross-neutralization of 1918 and 2009 influenza viruses: role of glycans in viral evolution and vaccine design. Sci Transl Med 2: 24ra21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis WI. (1997) Cell-surface carbohydrate recognition by animal and viral lectins. Curr Opin Struct Biol 7: 624–630. [DOI] [PubMed] [Google Scholar]
- White TA, Bartesaghi A, Borgnia MJ. et al (2010) Molecular architectures of trimeric SIV and HIV-1 envelope glycoproteins on intact viruses: strain-dependent variation in quaternary structure. PLoS Pathog 6: e1001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer DO, McFadden G, Evans DH. (1999) The complete genome sequence of shope (rabbit) fibroma virus. Virology 264: 319–343. [DOI] [PubMed] [Google Scholar]
- Willey RL, Shibata R, Freed EO, Cho MW, Martin MA. (1996) Differential glycosylation, virion incorporation, and sensitivity to neutralizing antibodies of human immunodeficiency virus type 1 envelope produced from infected primary T-lymphocyte and macrophage cultures. J Virol 70: 6431–6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witvrouw M, Fikkert V, Hantson A, Pannecouque C, O'Keefe BR, McMahon J, Stamatatos L, de Clercq E, Bolmstedt A. (2005) Resistance of human immunodeficiency virus type 1 to the high-mannose binding agents cyanovirin N and concanavalin A. J Virol 79: 7777–7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, KewalRamani VN. (2006) Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev 6: 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SF, Lee CJ, Liao CL, Dwek RA, Zitzmann N, Lin YL. (2002) Antiviral effects of an iminosugar derivative on flavivirus infections. J Virol 76: 3596–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y, Watanabe E, Watari E, Shinya E, Satomi M, Takeshita T, Takahashi H. (2010) Inhibition of DC-SIGN-mediated transmission of human immunodeficiency virus type 1 by Toll-like receptor 3 signalling in breast milk macrophages. Immunology 130: 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HJ, Reuter MA, McDonald D. (2008) HIV traffics through a specialized, surface-accessible intracellular compartment during trans-infection of T cells by mature dendritic cells. PLoS Pathog 4: e1000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu RK, Tsai YT, Ariga T, Yanagisawa M. (2011) Structures, biosynthesis, and functions of gangliosides-an overview. J Oleo Sci 60: 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hartshorn KL, Crouch EC, Ikegami M, Whitsett JA. (2002) Complementation of pulmonary abnormalities in SP-D(−/−) mice with an SP-D/conglutinin fusion protein. J Biol Chem 277: 22453–22459. [DOI] [PubMed] [Google Scholar]
- Zhu XG, Borchers C, Bienstock RJ, Tomer KB. (2000) Mass spectrometric characterization of the glycosylation pattern of HIV-gp120 expressed in CHO cells. Biochemistry 39: 11194–11204. [DOI] [PubMed] [Google Scholar]