Abstract
Background:
Although behavior therapy reduces tic severity, it is unknown whether it improves co-occurring psychiatric symptoms and functional outcomes for adults with Tourette’s Disorder (TD). This information is essential for effective treatment planning. This study examined the effects of behavior therapy on psychiatric symptoms and functional outcomes in older adolescents and adults with TD.
Method:
122 individuals with TD or a chronic tic disorder participated in a clinical trial comparing behavior therapy to psychoeducation and supportive therapy. At baseline, posttreatment, and follow-up visits, participants completed assessments of tic severity, co-occurring symptoms (inattention, impulsiveness, hyperactivity, anger, anxiety, depression, obsessions and compulsions) and psychosocial functioning. We compared changes in tic severity, psychiatric symptoms, and functional outcomes using repeated measure and one-way analysis of variance.
Results:
At posttreatment, participants receiving behavior therapy reported greater reductions in obsessions compared to participants in supportive therapy (=.04, p=.04). Across treatments, a positive treatment response on the Clinical Global Impression of Improvement scale was associated with reduced disruption in family life (=.05, p=.02) and improved functioning in a parental role (=.37, p=.02). Participants who responded positively to 8 sessions of behavior therapy had an improvement in tic severity (=0.75, p<.001), inattention (=0.48, p<.02), and functioning (=.39–.42, p<.03–.04) at the 6-month follow-up.
Conclusion:
Behavior therapy has a therapeutic benefit for co-occurring obsessive symptoms in the short-term, and reduces tic severity and disability in adults with TD over time. Additional treatments may be necessary to address co-occurring symptoms and improve functional outcomes.
Keywords: behavior therapy, comprehensive behavioral intervention for tics, comorbidity, functioning, disability
Introduction
Tourette’s Disorder and persistent (chronic) tic disorders (collectively referred to as TD) are neuropsychiatric conditions affecting 0.5%−0.8% of the population (Knight et al., 2012, Scharf et al., 2015), and characterized by the presence of motor and/or phonic tics (American Psychiatric Association, 2013). In addition to tics, individuals with TD often present with co-occurring psychiatric conditions [e.g., attention deficit hyperactivity disorder (ADHD), obsessive-compulsive disorder (OCD), anxiety disorders, depressive disorders](Hirschtritt et al., 2015). Tics can lead to impairment across multiple domains of functioning at higher rates than the general population (Evans et al., 2016). For instance, an adult with TD may have a repetitive head jerk and arm movements that cause physical pain and impede performance at work. Alternatively, another patient with TD may have vocal tics that cause difficulty in social situations and/or disrupt family life. However, in addition to the impact of tics on functioning, the co-occurrence of psychiatric conditions can exacerbate functional disability experienced by individuals with TD (Cavanna et al., 2013; Eapen et al., 2016). For example, Eapen and colleagues (2001) found that in addition to tic severity, obsessive-compulsive symptoms, anxiety, and depression predicted poor quality of life in older adolescents and adults with TD (Eapen et al., 2001). Similarly, Müller-Vahl and colleagues (2010) identified that tic severity and depression predicted the quality of life among adults with TD (Müller-Vahl et al., 2010). Indeed, Jalenques and colleagues (2012) found depression to be the primary predictor of quality of life across all functional domains in older adolescents and adults with TD (Jalenques et al., 2012). Collectively, tics, co-occurring psychiatric conditions, and functional disability contribute to a poor quality of life for many individuals with TD (Cavanna et al., 2013, Evans et al., 2016). As such, both tics and co-occurring psychiatric conditions require careful assessment and treatment planning for effective global symptom management.
Tic symptoms usually begin in early school-age years (Bloch and Leckman, 2009), and peak in severity during early adolescence (Bloch and Leckman, 2009, Bloch et al., 2006). For 70% to 80% of youth with TD, tic symptoms subside to mild levels by late adolescence (Bloch et al., 2006, Groth et al., 2017). However, for at least 20–30% of individuals with TD, tics persist into adulthood. Reasons for this are unclear, as predictors and underlying neurobiology of persistent tics in adulthood are poorly understood. Although tics are similar in children and adults with TD (McGuire et al., 2013), treatment response may not be comparable across developmental epochs (Piacentini et al., 2010, Sukhodolsky et al., 2017, Wilhelm et al., 2012). Few studies have tested interventions exclusively in adults with TD. Instead, most studies have examined the efficacy of medications in combined samples of children and adults (see Murphy et al., 2013 for a review of clinical trials in TD). Among the few studies that have included adults, evaluations have focused entirely on tics and did not examine the effect of interventions on co-occurring psychiatric symptoms or psychosocial functioning. Thus, it is unclear whether improvement in tic severity also confers improvement for other clinical factors that contribute to functional impairment and quality of life. Indeed, overall impairment is greater in adults with TD and co-occurring ADHD and/or obsessive-compulsive symptoms compared to adults with TD alone regardless of tic severity (Haddad et al., 2009, Thibert et al., 1995). Follow-up studies in older adolescents further support that co-occurring psychiatric symptom profiles conferred greater impairment than tics as well (Groth et al., 2017).
Behavior therapy based on habit reversal training (HRT) is a psychosocial intervention that has empirical support for reducing tic severity in children and adults with TD (Piacentini et al., 2010, Wilhelm et al., 2012). In a multi-site trial of children with TD, 8 sessions of behavior therapy reduced tic severity (d=.68) but did not improve co-occurring psychiatric symptoms or functional outcomes (p=.12–.96) after 10 weeks of treatment, when compared to supportive therapy (Piacentini et al., 2010, Woods et al., 2011). However, the subgroup of youth who had a positive response to behavior therapy after 8 sessions exhibited significant reductions in anxiety, obsessive-compulsive symptom severity, and disruptive behavior (Woods et al., 2011). Up to six months after acute treatment, parents of youth who had a positive treatment response to behavior therapy reported improvements in social functioning with peers. These results suggest that behavior therapy has positive effects on co-occurring psychiatric symptoms and functional outcomes in youth with TD. Although tic-specific behavioral strategies are the focus of behavior therapy for TD, a reduction in co-occurring psychiatric symptoms and improved functioning is not uncommon. For instance, as youth learn to manage tic symptoms using behavioral skills, they may become less anxious around peers and more willing to participate in social activities. Alternatively, relaxation training skills taught in HRT may be used to manage anxiety symptom severity. Furthermore, parents and youth may generalize the behavioral principles provided in function-based strategies to disruptive behaviors and obsessive-compulsive symptoms (e.g., identify antecedent behaviors to disruptive behaviors/obsessive-compulsive symptoms and implement alternative responses).
Despite its importance and clinical relevance, it remains unknown whether behavior therapy produces parallel improvement in co-occurring psychiatric symptoms and functional outcomes in adults with TD. This information is essential for personalized treatment planning, as it helps patients appropriately manage treatment expectations and clinicians adapt treatment plans accordingly (i.e., initiation of other therapeutic strategies if behavior therapy does not successfully improve co-occurring psychiatric symptoms or functional outcomes). In a multi-site trial of older adolescents and adults with TD, 8 sessions of behavior therapy reduced tic severity (d=.57) after 10-weeks of treatment in comparison to supportive therapy, with reductions in tic severity found to be stable for up to six months (Wilhelm et al., 2012). Here, we examined the ancillary benefits of behavior therapy in a sample of 122 older adolescents and adults with TD (Wilhelm et al., 2012). Although behavior therapy is a tic-specific intervention, we were interested to identify whether behavior therapy yields any benefit for common co-occurring psychiatric symptoms and/or produces improvement in functional outcomes. As reductions in tics may have direct benefits on co-occurring psychiatric symptoms (e.g., anxiety, depression) and psychosocial functioning, and principles taught in behavior therapy may generalize to other aspects of patients’ lives, we surmised that behavior therapy would positively influence co-occurring psychiatric symptoms and functional outcomes. For those participants who exhibited a positive treatment response to 8 sessions of behavior therapy, we explored the therapeutic improvement in tic severity, co-occurring psychiatric symptoms and functional outcomes at the 6-month follow-up assessment.
Methods
Design
This 10-week randomized controlled trial (RCT; clinicaltrials.gov identifier: NCT00231985) compared a comprehensive behavioral intervention for tics (CBIT) to psychoeducation and supportive therapy (PST). Participants were randomized using a computer algorithm in a 1:1 ratio, stratified within site and by the presence of a tic-suppressing medication. Although the primary outcome was change in tic severity after acute treatment, participants also completed an assessment battery that measured change in co-occurring psychiatric symptoms and functional outcomes. Participants exhibiting a positive treatment response to either intervention received three monthly booster sessions and were invited to return for follow-up assessments to determine the durability of therapeutic effects up to six months later. Meanwhile, treatment non-responders did not complete follow-up assessments. All study assessors who administered clinician-rated measures were masked to participants’ treatment condition.
Participants
Older adolescents and adults with TD (N=122, 63.9% male) were recruited from three sites from December 2005 to May 2009: Massachusetts General Hospital/Harvard Medical School, Yale University, and The University of Texas Health Science Center at San Antonio. Eligible participants were: 16 years of age or older; met diagnostic criteria for Tourette’s Disorder, chronic motor tic disorder, or chronic vocal tic disorder; had moderate tic severity or greater on the Clinical Global Impression - Severity scale (Guy, 1976); had a Yale Global Tic Severity Scale total score ≥ 14 (≥ 10 for those with motor or vocal tics only) (Leckman et al., 1989); were fluent in English; and had an estimated IQ > 80. Individuals with a history of schizophrenia or pervasive developmental disorders were excluded from participation. Concurrent tic medications were acceptable if the medication regimen was stable for at least six weeks with no planned changes in medications during the study.
Participants were 32 years of age on average (M=31.60, SD=13.7, range: 16–69 years) and 80.3% were Caucasian. Tic disorder diagnoses included Tourette’s Disorder (n=103, 84%), chronic motor tic disorder (n=18, 15%), and chronic vocal tic disorder (n=1, 1%). A quarter of participants were on a stable dose of psychotropic medication for tics at baseline (n=31, 25.4%), and almost all participants remained on the same dose throughout the RCT. However, one participant in the PST condition reported a change in tic medication during acute treatment. Co-occurring lifetime psychiatric conditions included: anxiety disorders (n=19, 15.6%); ADHD (n=34, 28 %); OCD (n=22, 18%); major depressive episode (n=24, 20%); substance use disorder (n=9, 7%); and other psychiatric disorders (n=36, 30%). Treatment groups did not differ on baseline demographic or clinical characteristics (see Wilhelm et al. 2012 for details).
Measures
Yale Global Tic Severity Scale (YGTSS; Leckman et al., 1989). The YGTSS is a clinician rating of tic severity over the past week. The YGTSS total tic score is comprised of 10 items that assess the severity of motor and vocal tics across several domains. The YGTSS total tic score has demonstrated good reliability and validity (Leckman et al., 1989, McGuire et al., 2018, Storch et al., 2005).
Clinical Global Impression of Improvement (CGI-I; Guy, 1976). The CGI-I is a clinician rating of improvement relative to baseline on a 7-point scale, which ranges from “very much improved” (1) to “very much worse” (7). Ratings of “much improved” and “very much improved” correspond with a positive treatment response. The CGI-I is well-validated in treatment studies of TD (Jeon et al., 2013, Storch et al., 2011).
Yale-Brown Obsessive Compulsive Scale (Y-BOCS; Goodman et al., 1989). The Y-BOCS is a clinician-administered interview assessing OCD severity over the past week, with a parallel version for youth (Goodman et al., 1989, Scahill et al., 1997). The Y-BOCS total score consists of 10-items rated on a 5-point scale (range: 0–4), with higher scores corresponding to greater OCD severity. The Y-BOCS and its parallel child version have demonstrated reliability and validity (Goodman et al., 1989, Scahill et al., 1997).
Attention Deficit Hyperactivity Disorder Rating Scale-Fourth Edition (ADHD-RS-IV; DuPaul et al., 1998). The ADHD-RS-IV is an 18-item, DSM-IV referenced parent- or self-report measure of ADHD symptom severity. It yields inattention (9-item) and hyperactivity/impulsivity (9-item) subscales, as well as an ADHD total score (18-item). Items are rated on a 4-point scale (range: 0–3) and summed to produce a total score (range: 0–54). This scale has exhibited good reliability in this sample (α=.86–.93) and shown sensitivity to treatment effects in pediatric and adult samples (Scahill et al., 2001, Wilens et al., 1996).
Beck Depression Inventory-Second Edition (BDI-II; Beck et al., 1996). The BDI-II is a reliable, valid, and widely used 21-item self-report measure of depressive symptom severity. Items are rated on a 0-to-3 scale and summed to produce a total score (range 0–63) (Beck et al., 1996, Steer et al., 1998).
Beck Anxiety Inventory (BAI; Steer and Beck, 1997). The BAI is a 21-item self-report of anxiety severity. Items are rated on a 0-to-3 scale and summed to produce a total score (range 0–63). The BAI total score has demonstrated reliability and validity (Hewitt and Norton, 1993).
State-Trait Anger Expression Inventory-Second Edition (STAXI-2; Spielberger, 1999). The STAXI-2 is a reliable and valid self-report consisting of 57 items rated on a 0-to-3 scale. It yields two subscales: state anger and trait anger, as well as a total score. Higher scores reflect greater anger (Lievaart et al., 2016, Spielberger, 1999).
Sheehan Disability Scale (SDS; Leon et al., 1992). The SDS is a 4-item self-report scale of disability. Three items inquire about social, family, and work related disability on a 10-point scale. The final item serves as an overall rating of disability on a 5-point scale. The SDS is reliable, valid, and has been used in treatment studies (Deckersbach et al., 2006, Leon et al., 1992).
Family Assessment Measure (FAM-III) short form (Skinner et al., 1995). The FAM-III is a 14-item self-report scale that provides a global index of family functioning. Items are rated on a 4-point scale, with higher scores reflecting greater impairment in family functioning. The FAM-III short form has established reliability and validity (Skinner et al., 1995), and has been used in treatment studies of TD (Woods et al., 2011).
Social Adjustment Scale Self-Report (SAS-SR; Weissman et al., 1978). The SAS-SR assesses social adjustment across six domains: primary occupation/role (6 items), social-leisure (9 items), extended family (8 items), primary relationship (9 items), parental role (4 items), and family unit (3 items). Items are rated on a 5-point scale, with higher scores reflecting greater impairment in adjustment. Items within each domain are summed and divided by the number of items answered to produce a per item domain and total adjustment scores.
Procedures
After obtaining informed consent and assent, participants completed a screening assessment to confirm study eligibility. Interested and eligible participants returned at baseline to assess psychiatric symptom severity (Y-BOCS, ADHD-RS-IV, BDI-II, and BAI) and overall functioning (SDS, FAM-III, and SAS-SR). Participants were then randomly assigned to receive 8 sessions of CBIT or PST over a 10-week period. The CBIT condition included training to increase awareness of premonitory urges to tic, the development of competing responses to inhibit tic expression, relaxation training, and function-based strategies to manage situations and events that increase tics (Woods et al., 2008). Meanwhile, the PST condition provided psycho-education on the course, genetics, and underlying neurobiology of TD. Additionally in the PST condition, participants received supportive psychotherapy in which they were allowed to discuss tics and related issues, but were not offered advice or strategies for tic management. Both interventions were guided by a treatment manual with rigorous procedures to ensure protocol fidelity (Wilhelm et al., 2012). After completing the 10-week acute treatment, participants completed assessments of tic severity, measures of psychiatric symptom severity, and measures of overall functioning. Clinician administered scales (YGTSS, CGI-I, and Y-BOCS) were completed by independent evaluators masked to treatment condition. Participants who exhibited a treatment response in tic severity on the CGI-I (i.e., an IE rating of much improved or very much improved from baseline) were invited to return for follow-up assessments up to six months later to evaluate the durability of acute treatment improvement.
Analytic Plan
There were no significant between-group differences in baseline characteristics including tic medication status (see Wilhelm et al., 2012). The intent-to-treat principle was applied for acute treatment analyses, whereby data were analyzed on all participants with at least one post-randomization treatment session (see Figure 1). Treatment effects from baseline to posttreatment were examined using repeated measures analysis of variance (ANOVA). The model included fixed effects for treatment condition and outcome variables. Treatment responder status effects were also tested from baseline to posttreatment using repeated measures ANOVA, with fixed effects for treatment responder status at 10-week and outcome variables.
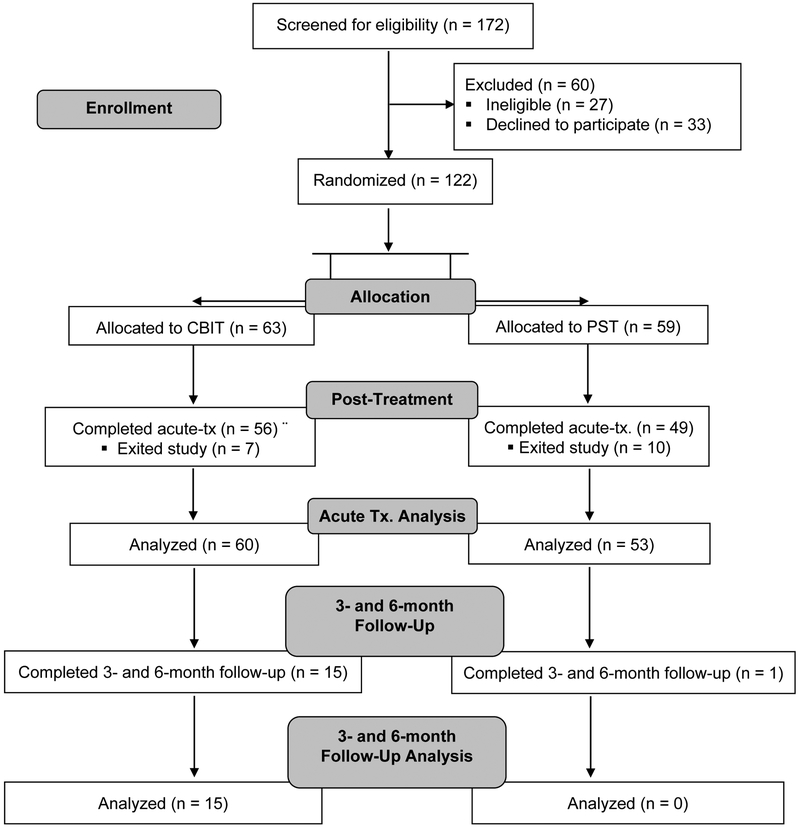
Figure 1.
CONSORT diagram of participant flow through the trial.
Note. CBIT = Comprehensive Behavioral Intervention for Tics; PST = Psychoeducation and Supportive Therapy; Tx. = Treatment
Although 38.1% of individuals in the CBIT group were considered treatment responders on the CGI-I at the posttreatment assessment, only 15 participants completed long-term follow-up assessments (see Figure 1). Meanwhile, only 6.8% of individuals in the PST group were considered treatment responders on the CGI-I at the posttreatment assessment, with only two participants returning for both follow-up assessments. Given our exploratory aim to examine the long-term improvement of co-occurring psychiatric and functional outcomes, completer analyses were conducted for follow-up assessments and focused on the 6-month follow-up assessment. As there were few PST treatment responders, follow-up analyses focused exclusively on participants who exhibited a treatment response to CBIT. For these analyses, scores were compared across baseline, posttreatment, and the 6-month follow-up using a repeated measures ANOVA. Post-hoc comparisons with Bonferroni correction were performed to assess differences in outcomes between baseline and posttreatment, and baseline and the 6-month follow-up. Chi-square tests and repeated measures ANOVAs examined the effects of age and characteristics associated with participants who completed and did not complete the 6-month follow-up assessment.
Results
Posttreatment Improvement on Secondary Psychiatric Symptoms and Functional Outcomes
As reported in Wilhelm et al. (2012), CBIT was associated with a significant decrease in tic severity on YGTSS total tic score (M=24.0, SD=6.47 to M=17.8, SD=7.32) from baseline to posttreatment compared to PST (M=21.8, SD=6.59 to M=19.3, SD=7.40; p<.001, effect size=0.57). When comparing improvement between groups after acute treatment, participants receiving CBIT (n=63) exhibited greater reductions in the severity of obsessions relative to those receiving PST (n=59, see Table 1). In general however, CBIT and PST did not differ in improvement on other co-occurring psychiatric symptoms and functional outcomes after acute treatment (see Table 1).
Table 1.
Adjusted Means and Standard Errors for Baseline to 10-week Change in Psychiatric symptoms and Functioning by Treatment Assignment
| Measure | CBIT (n = 63) |
PST (n = 59) |
F | Sig. |
Partial η2 |
||
|---|---|---|---|---|---|---|---|
| Baseline | Posttreatment | Baseline | Posttreatment | ||||
| Psychiatric Symptoms | |||||||
| Yale-Brown Obsessive Compulsive Scale | |||||||
| Total | 5.88 (1.07) | 3.43 (0.94) | 4.90 (1.14) | 4.06 (1.00) | 2.75 | .10 | .03 |
| Obsessions | 2.20 (0.53) | 0.89 (0.45) | 1.82 (0.57) | 1.74 (0.48) | 4.48 | .04 | .04 |
| Compulsions | 3.68 (0.63) | 2.54 (0.56) | 3.08 (0.68) | 2.33 (0.60) | 0.37 | .55 | .004 |
| Attention Deficit Hyperactivity Disorder Rating Scale – IV | |||||||
| Total | 15.85 (1.45) | 13.57 (1.35) | 13.38 (1.54) | 12.68 (1.43) | 1.21 | .27 | .01 |
| Hyperactivity/ Impulsivity | 8.50 (0.72) | 7.09 (0.70) | 6.60 (0.76) | 6.09 (0.74) | 1.47 | .23 | .02 |
| Inattention | 7.30 (0.87) | 6.40 (0.79) | 6.79 (0.92) | 6.60 (0.83) | 0.77 | .38 | .01 |
| Beck Depression Inventory-II | |||||||
| Total | 6.19 (0.90) | 4.20 (0.96) | 7.21 (0.96) | 6.02 (0.90) | 0.65 | .42 | .01 |
| Beck Anxiety Inventory | |||||||
| Total | 9.55 (1.18) | 6.04 (1.00) | 7.92 (1.27) | 5.98 (1.07) | 1.57 | .21 | .02 |
| State Trait Anger Expression Inventory | |||||||
| Trait | 17.09 (15.00) | 16.52 (0.84) | 16.52 (0.84) | 15.44 (0.72) | 1.41 | .24 | .01 |
| State | 16.55 (0.49) | 16.64 (0.61) | 17.17 (0.53) | 16.61 (0.67) | 0.53 | .47 | .01 |
| Sheehan Disability Scale | |||||||
| Total | 8.36 (0.90) | 5.00 (0.79) | 8.32 (0.97) | 5.79 (0.85) | 0.56 | .46 | .01 |
| Work | 2.78 (0.35) | 1.56 (0.30) | 2.32 (0.37) | 1.76 (0.32) | 2.04 | .16 | .02 |
| Social | 3.07 (0.38) | 2.11 (0.31) | 3.58 (0.40) | 2.05 (0.33) | 1.69 | .20 | .02 |
| Family | 2.51 (0.36) | 1.33 (0.28) | 2.42 (0.38) | 1.98 (0.30) | 2.29 | .13 | .02 |
| Brief Family Assessment Measure – Brief | |||||||
| Total | 25.51 (0.97) | 24.73 (0.97) | 26.83 (1.02) | 25.54 (1.02) | 0.20 | .66 | .002 |
| Social Adjustment Rating Scale Self-report | |||||||
| Overall | 1.83 (0.06) | 1.71 (0.06) | 1.87 (0.06) | 1.83 (0.06) | 1.71 | .20 | .02 |
| Primary Occupation/Role | 1.83 (0.13) | 1.68 (0.12) | 1.75 (0.14) | 1.65 (0.12) | 0.15 | .70 | .002 |
| Social Leisure | 2.01 (0.08) | 1.95 (0.08) | 2.14 (0.09) | 1.96 (0.08) | 1.22 | .27 | .01 |
| Extended Family | 1.63 (0.06) | 1.49 (0.06) | 1.68 (0.06) | 1.67 (0.06) | 2.10 | .15 | .02 |
| Primary Relationship | 1.76 (0.11) | 1.65 (0.11) | 1.92 (0.10) | 1.97 (0.10) | 1.47 | .23 | .04 |
| Parental Role | 1.15 (0.22) | 1.35 (0.26) | 1.78 (0.17) | 1.67 (0.19) | 0.82 | .38 | .06 |
| Family Unit | 1.84 (0.14) | 1.58 (0.13) | 1.78 (0.16) | 1.69 (0.14) | 1.07 | .30 | .01 |
Note. CBIT = Comprehensive Behavioral Intervention for Tics; PST=Psychoeducation and Supportive Therapy
Comparison of Treatment Responders and Non-Responders at Posttreatment
When comparing improvement between participants who displayed a positive treatment response (n=28) and those who did not (n=94) after acute treatment, there were no differences in improvement of co-occurring psychiatric symptoms between groups (see Table 2). On functional outcomes, treatment responders exhibited greater improvement in family disability on the SDS and adjustment in parenting on the SAS-SR relative to non-responders (Table 2).
Table 2.
Adjusted Means and Standard Errors for Baseline to 10-week Change in Psychiatric symptoms and Functioning by CGI-I Responder Status
| Measure | Non-Responder (n = 94) | Responder (n = 28) | F | Sig. | Partial η2 | ||
|---|---|---|---|---|---|---|---|
| Baseline | Posttreatment | Baseline | Posttreatment | ||||
| Psychiatric Symptoms | |||||||
| Yale-Brown Obsessive Compulsive Scale | |||||||
| Total | 5.54 (0.91) | 4.04 (0.80) | 5.07 (1.51) | 2.86 (1.32) | 0.41 | .52 | .004 |
| Obsessions | 2.39 (0.45) | 1.64 (0.38) | 1.00 (0.74) | 0.32 (0.63) | 0.01 | .91 | <.001 |
| Compulsions | 3.16 (0.54) | 2.40 (0.48) | 4.07 (0.90) | 2.54 (0.80) | 1.19 | .28 | .01 |
| Attention Deficit Hyperactivity Disorder Rating Scale – IV | |||||||
| Total | 14.30 (1.24) | 12.92 (1.15) | 16.00 (2.08) | 14.08 (1.93) | 0.11 | .75 | .001 |
| Hyperactivity/ Impulsivity | 7.32 (0.62) | 6.53 (0.60) | 8.50 (1.04) | 6.96 (1.00) | 0.78 | .38 | .01 |
| Inattention | 6.93 (0.74) | 6.33 (0.67) | 7.50 (1.25) | 7.12 (1.12) | 0.05 | .82 | .001 |
| Beck Depression Inventory-II | |||||||
| Total | 6.75 (0.78) | 5.33 (0.74) | 6.29 (1.26) | 4.54 (1.19) | 0.09 | .77 | .001 |
| Beck Anxiety Inventory | |||||||
| Total | 9.43 (1.02) | 6.32 (0.86) | 7.07 (1.66) | 5.21 (1.40) | 0.78 | .38 | .01 |
| State-Trait Anger Expression Inventory | |||||||
| Trait | 17.04 (0.68) | 15.67 (0.58) | 16.36 (1.08) | 13.93 (0.92) | 1.26 | .26 | .01 |
| State | 17.03 (0.43) | 16.97 (0.53) | 16.39 (0.68) | 15.75 (0.85) | 0.34 | .56 | .003 |
| Functional Outcomes | |||||||
| Sheehan Disability Scale | |||||||
| Total | 8.51 (0.78) | 6.21 (0.67) | 7.96 (1.27) | 3.46 (1.09) | 2.88 | .09 | .03 |
| Work | 2.51 (0.30) | 1.86 (0.26) | 2.71 (0.49) | 1.14 (0.42) | 3.22 | .08 | .03 |
| Social | 3.57 (0.32) | 2.32 (0.26) | 2.64 (0.53) | 1.50 (0.43) | 0.05 | .83 | <.001 |
| Family | 2.43 (0.31) | 1.95 (0.24) | 2.61 (0.50) | 0.82 (0.39) | 5.67 | .02 | .05 |
| Brief Family Assessment Measure – III | |||||||
| Total | 26.49 (0.82) | 25.92 (0.81) | 24.92 (1.38) | 22.76 (1.36) | 1.54 | .22 | .02 |
| Social Adjustment Scale – Self-report | |||||||
| Overall | 1.89 (0.05) | 1.81 (0.05) | 1.76 (0.08) | 1.67 (0.08) | 0.06 | .80 | .001 |
| Primary Occupation/Role | 1.88 (0.11) | 1.71 (0.10) | 1.57 (0.18) | 1.55 (0.16) | 0.93 | .34 | .01 |
| Social Leisure | 2.12 (0.07) | 1.97 (0.06) | 1.94 (0.11) | 1.90 (0.10) | 0.65 | .42 | .01 |
| Extended Family | 1.69 (0.05) | 1.62 (0.05) | 1.55 (0.08) | 1.48 (0.08) | 0.01 | .93 | <.001 |
| Primary Relationship | 1.83 (0.09) | 1.84 (0.09) | 1.88 (0.15) | 1.78 (0.16) | 0.52 | .48 | .02 |
| Parental Role | 1.44 (0.15) | 1.58 (0.17) | 2.25 (0.36) | 1.38 (0.42) | 6.94 | .02 | .37 |
| Family Unit | 1.81 (0.12) | 1.67 (0.11) | 1.82 (0.20) | 1.53 (0.18) | 0.63 | .43 | .01 |
Note. CGI-I = Clinical Global Impressions – Improvement Scale.
Long-term Improvement in Psychiatric and Functional Outcomes for Behavior Therapy
When examining sustained improvement in tic severity, psychiatric symptoms, and functional outcomes among participants who had a treatment response to CBIT, several interesting findings emerged. First, CBIT responders exhibited therapeutic improvement from baseline through the 6-month follow-up on the YGTSS total tic score (d=1.71, p<.001, see Table 3). Post-hoc tests revealed significant reductions from baseline to posttreatment (d=1.88, p<.001), but no significant changes were found from posttreatment to the 6-month follow-up visit (p>.99). There were no significant associations between the change in YGTSS total tic score and change in psychiatric and functional outcomes at posttreatment (r=.01–.58, p>.05) and the 6-month follow-up assessment (r=.01–.44, p>.05). Second, a significant decrease in inattention was identified across assessment visits (see Table 3). Although post-hoc tests revealed no significant difference from baseline to posttreatment (p>.99) or baseline to the 6-month follow-up visit (p=.16), there was a significant difference from posttreatment to the 6-month follow-up (d=.47, p=.03). Finally, there was significant improvement in overall disability at posttreatment and the 6-month follow-up visit (Table 3). Post-hoc tests revealed a reduction at posttreatment (d=.60, p=.02) relative to baseline. When examining disability subscales, work-related disability was found to significantly decrease across time for individuals who showed a treatment response to CBIT at posttreatment (Table 3), with reductions identified at posttreatment (d=.54, p=.04) and the 6-month follow-up assessment (d=.61, p=.03) relative to baseline. Although social-related disability decreased over time, this therapeutic improvement only trended towards significance (p=.06).
Table 3.
Adjusted Means and Standard Errors for Change in Psychiatric symptoms and Functioning across Acute-Treatment and Follow-up Assessments for CBIT Treatment Responders
| Measure | Baseline | Posttreatment | 6-month | F | Sig. | Partial η2 |
|---|---|---|---|---|---|---|
| Tic Severity | ||||||
| Yale Global Tic Severity Scale | ||||||
| Total Tic Score | 25.06 (0.96) | 15.88 (1.58)a | 14.38 (2.16)a | 21.22 | <.001 | .75 |
| Psychiatric Symptoms | ||||||
| Yale-Brown Obsessive Compulsive Scale | ||||||
| Total | 4.88 (1.91) | 2.88 (1.23) | 3.56 (1.24) | 0.91 | .43 | .12 |
| Obsessions | 1.31 (0.82) | 0.50 (0.50) | 0.94 (0.53) | 0.65 | .54 | .09 |
| Compulsions | 3.56 (1.32) | 2.38 (0.95) | 2.63 (0.99) | 0.51 | .61 | .07 |
| Attention Deficit Hyperactivity Disorder Rating Scale – IV | ||||||
| Total | 17.79 (3.52) | 15.14 (2.58) | 11.21 (2.30) | 3.04 | .09 | .34 |
| Hyperactivity/Impulsivity | 9.21 (1.65) | 7.50 (1.38) | 6.07 (1.10) | 1.77 | .21 | .23 |
| Inattention | 8.57 (2.11) | 7.64 (1.50) | 5.14 (1.36)b | 5.51 | .02 | .48 |
| Beck Depression Inventory-II | ||||||
| Total | 5.63 (1.86) | 4.44 (1.94) | 2.88 (0.97) | 2.58 | .11 | .27 |
| Beck Anxiety Inventory | ||||||
| Total | 6.19 (1.44) | 5.19 (1.95) | 4.25 (1.20) | 2.00 | .17 | .22 |
| State Trait Anger Expression Inventory | ||||||
| Trait | 14.57 (1.00) | 12.79 (0.83) | 13.79 (0.92) | 3.01 | .09 | .33 |
| State | 16.64 (0.84) | 15.86 (0.78) | 15.71 (0.46) | 0.57 | .58 | .09 |
| Functional Outcomes | ||||||
| Sheehan Disability Scale | ||||||
| Total | 7.60 (1.68) | 4.00 (1.53)a | 4.20 (1.55) | 4.79 | .03 | .42 |
| Work | 2.60 (0.70) | 1.27 (0.61)a | 1.20 (0.52)a | 4.09 | .04 | .39 |
| Social | 2.67 (0.54) | 1.77 (0.53) | 1.73 (0.59) | 3.49 | .06 | .35 |
| Family | 2.33 (0.74) | 1.00 (0.46) | 1.27 (0.54) | 2.67 | .11 | .29 |
| Brief Family Assessment Measure – Brief | ||||||
| Total | 24.08 (1.92) | 22.67 (1.86) | 23.58 (2.09) | 0.51 | .61 | .09 |
| Social Adjustment Scale – Self-Report | ||||||
| Overall | 1.85 (0.15) | 1.80 (0.17) | 1.69 (0.14) | 1.01 | .40 | .17 |
| Primary Occupation/Role | 1.78 (0.32) | 1.82 (0.34) | 1.78 (0.34) | 0.05 | .95 | .01 |
| Social Leisure | 1.99 (0.18) | 2.02 (0.18) | 1.82 (0.13) | 1.79 | .21 | .23 |
| Extended Family | 1.57 (0.13) | 1.46 (0.14) | 1.41 (0.10) | 1.94 | .19 | .25 |
| Primary Relationship | 1.92 (0.15) | 1.83 (0.16) | 2.00 (0.30) | 0.20 | .83 | .17 |
| Parental Rolec | 1.50 | 1.25 | 1.25 | |||
| Family Unit | 1.52 (0.24) | 1.39 (0.17) | 1.48 (0.25) | 0.21 | .82 | .04 |
Note. CBIT = Comprehensive Behavioral Intervention for Tics;
significantly different from baseline;
significantly different from posttreatment
only 1 participant had complete data on parental role
Age effects and differences between participants who completed follow-up assessments.
Given broad age range of participants, we examined whether there were differential response patterns between adolescent and adult participants. There were no significant differences between adolescents and adults for changes in tic severity or functional outcomes at the posttreatment and 6-month follow-up assessments (p=.05–.98). Similarly, there were no main effects of age category or significant interactions between age category and treatment assignment at the posttreatment or 6-month follow-up for most psychiatric symptoms (p=.05–.99).
However, there were two significant findings regarding the influence of age on psychiatric outcomes. First, at the posttreatment assessment, there were significant interactions between age group and time for YBOCS Total, Obsessions subtotal, and Compulsions subtotal (p=.01 – .03). This suggests that adolescents exhibited greater reductions in OCD symptoms compared to adults. There were no significant interactions between age category and time for the remaining psychiatric symptoms at posttreatment (p=.31–.98). Second, at the 6-month follow-up visit, there was a significant interaction for trait anger, such that adolescents showed greater reductions over time relative to adults (p=.02). There were no other significant interactions between age category and time for psychiatric outcomes at 6-month follow-up assessment.
Given the small sample size of participants completing the 6-month follow-up assessment in the CBIT group, we examined whether differences in demographic or clinical characteristics were present among those participants who completed this assessment and those who did not. Chi-square and t-tests found no significant difference in baseline characteristics between CBIT participants with and without 6-month follow-up data (p=.13–.99). Furthermore, we explored whether there were any differences between those participants who responded to CBIT and completed follow-up assessments and those CBIT treatment responders who did not complete follow-up assessments (i.e., lost to follow-up). Chi-square and t-tests found no significant differences in baseline demographic and clinical characteristics between those CBIT treatment responders who completed follow-up assessments and those who did not (p=.15–.99)
Discussion
Given the common co-occurrence of psychiatric symptoms among patients with TD, and similarities between behavior therapy and other psychosocial interventions, this study examined the effect of behavior therapy on tic severity, co-occurring psychiatric symptoms, and psychosocial functioning in comparison to psychoeducation/supportive therapy for 122 older adolescents and adults with TD. Eight sessions of behavior therapy significantly reduced tic severity in comparison to supportive therapy at the posttreatment assessment (d =.57, Wilhelm et al., 2012), and those individuals who responded to CBIT maintained therapeutic improvement for up to 6-months after weekly behavior therapy discontinued. However, there was limited therapeutic benefit for co-occurring psychiatric symptoms or functional outcomes beyond obsessive symptoms after acute treatment. While improvement in obsessions may be attributed to shared phenomenology between TD and OCD (Miguel et al., 1995), the presence of mild OCD symptoms within the sample prohibits extensive extrapolation of these findings. Although behavior therapy consists of multiple components (Woods et al., 2008), the skills provided in this intervention are largely specific to tic management. Thus, it is not surprising to observe little therapeutic benefit on co-occurring psychiatric symptoms and psychosocial functioning compared to supportive therapy. However, as there are some overlapping therapeutic skills between behavior therapy and cognitive behavior therapy (CBT), it is possible that some therapeutic benefit may be observed for specific psychiatric symptoms that are responsive to CBT. Given that some clinicians express concern that behavior therapy may produce adverse consequences for tics or co-occurring symptoms (e.g., symptom substitution, rebound effect, tic worsening), it is important to note that these findings offer additional evidence that behavior therapy does not adversely affect co-occurring psychiatric symptoms or functional outcomes (Peterson et al., 2016). Indeed, these findings parallel results from a comparable clinical trial of behavior therapy in youth (Woods et al., 2011). Therefore, clinicians providing behavior therapy to patients may not observe much improvement or worsening of co-occurring psychiatric symptoms or psychosocial functioning following acute treatment—unless specific symptoms are being addressed by another therapeutic intervention.
Participants who had a positive treatment response to either therapeutic intervention showed improved adjustment in parental functioning and reduced family disability relative to non-responders at posttreatment. These findings suggest a link between clinically significant reductions in tic symptoms and improvements in parenting/family domains. Improved adjustment in parenting and family disability may stem from perceptions of greater confidence, empowerment, and/or efficacy in the parenting role and/or improved interactions with family members—due to greater control over tics. However, as a positive treatment response was not associated with improved family functioning on the FAM-III, findings are likely circumscribed to participants’ perceptions rather than direct reductions in family dysfunction.
For older adolescents and adults with TD showing a positive treatment response to 8 sessions of behavior therapy, there were significant improvements in tic severity, inattention, and disability at the six-month follow-up visit. As a positive treatment response suggests that participants are better able to manage their tics, these participants may have no longer experienced tic-related interference in attention and disability domains to the same degree as they did at the outset of treatment. For instance, a patient who has good tic awareness and can implement appropriate competing responses—two core skills in behavior therapy—may be less distracted in daily activities and better able to function in work and related situations. This offers two key pieces of clinical information. First, clinicians should note that individuals who positively respond to behavior therapy for tics may not exhibit immediate improvement in disability following acute treatment, but may report improvements in disability as time progresses. Second, improvements following behavior therapy primarily pertained to the domain of disability, and did not appear to extend to other aspects of participants’ clinical presentations. This suggests patients positively responding to behavior therapy may have reduced disability, but may still require further treatment to address other psychiatric symptoms or improve psychosocial functioning. As such, patients with TD who experience therapeutic benefit from behavior therapy may still benefit from targeted psychosocial intervention to achieve global wellness. Indeed, this highlights the potential benefit of combined treatment approaches that integrate evidence-based treatment for TD and co-occurring psychiatric conditions (e.g., behavior therapy with CBT and/or pharmacotherapy integrated into treatment).
Despite considerable study strengths (i.e., rigorous methodology, large sample), several limitations exist. First, the study was designed to examine the efficacy of a 10-week trial of behavior therapy among older adolescents and adults with TD. As such, participants were excluded if co-occurring conditions required more immediate treatment, which may have decreased the number of participants with elevated scores on secondary symptom measures. Second, PST was selected as a comparison intervention to provide support for the overall clinical condition of TD and would be expected to have positive effects on behavior, emotional regulation, and family adaptation. It is not surprising that psychiatric symptoms and psychosocial functioning improved in both groups and differences between the treatment groups were minimal at posttreatment. Third, given that our analyses were exploratory, we did not correct for multiple hypothesis testing. Although this may influence the statistical significance of our findings, it would not diminish the clinical significance of this work. Fourth, the limited improvement following acute treatment may be due to the brief trial duration. Specifically, 8 sessions over 10 weeks may be insufficient to detect differences in improvement for co-occurring psychiatric symptoms and psychosocial functioning between these treatment groups. Thus, it is possible that behavior therapy produces differential improvement in secondary psychiatric symptoms and psychosocial functioning compared to supportive therapy at follow-up visits. However, few participants in the PST condition exhibited a treatment response after 10 weeks of treatment, with only two participants attending follow-up visits. In the absence of longer-term outcomes for PST, it is not entirely certain whether the observed benefits within the behavior therapy group can be fully attributed to the treatment itself. Finally, the small sample size of CBIT treatment responders may have limited our ability to detect clinically and statistically significant differences at the 6-month follow-up assessment. However, effect sizes are provided to help interpret the magnitude treatment effects within the context of limited power at the 6-month follow-up visit. Unfortunately, no information is available regarding whether participants who did not exhibit a treatment response at the posttreatment assessment became treatment responders at a later point. Future research should investigate the long-term outcomes for participants who have received CBIT and PST to determine the degree to which individuals achieve lasting therapeutic benefit from either treatment.
In summary, findings suggest that behavior therapy is a tic-specific treatment with only modest positive and minimal adverse effects on co-occurring psychiatric symptoms and psychosocial functioning compared to supportive therapy. For individuals who exhibit a positive treatment response to behavior therapy, improvements in tic severity, inattention, and disability are evident up to 6-months after the end of acute treatment. Taken together, findings suggest behavior therapy is useful as part of an overall treatment program for older adolescents and adults with TD. However, as behavior therapy did not significantly improve secondary psychiatric symptoms and psychosocial functioning, additional interventions may be needed to address these concerns in older adolescents and adults with TD (e.g., CBT, pharmacotherapy, problem solving skills). Indeed, initial work has been conducted to systematically address tics and co-occurring psychosocial challenges in children with TD (McGuire et al., 2014, Storch et al., 2012). These studies developed modular treatments focused on developing therapeutic skills to manage tics, reduce tic-related impairment, foster resilience, and improve quality of life for youth with TD. Similar studies and treatment approaches are needed in adult populations with TD.
Disclosure and acknowledgments
This work was supported in part by grants and/or contracts to Dr. McGuire [Tourette Association of America (TAA), American Academy of Neurology (AAN), and American Brain Foundation (ABF)], Dr. Ricketts (K23MH113884), Dr. Scahill (R01MH069874), Dr. Wilhelm (R01MH069877), and Dr. Peterson (RO1MH069875). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH, NIH, or other grant organization. The authors report no conflicts of interest associated with this work.
Funding: This work was supported in part by grants from the National Institute of Mental Health (NIMH: R01MH069877 (to Dr. Wilhelm), R01MH069874 (to Dr. Scahill), R01MH69875 (to Dr. Peterson), K23MH113884 (to Dr. Ricketts); and a Clinical Research Training Fellowship from the American Academy of Neurology and Tourette Association of America (to Dr. McGuire).
Financial disclosures for all authors
Dr. McGuire reports receiving research support from the TAA, AAN, and ABF. He has served as a consultant to Bracket, Syneos Health, and Luminopia, and received royalties from Elsevier. Dr. Ricketts has received research support from the NIH and TAA. Dr. Scahill has served as a consultant for Roche, Neuren, Bracket, Coronado, and Supernus as well as participates in the Speakers Bureau of the TAA. Dr. Wilhelm has received research support in the form of free medication and matching placebo for NIMH–funded studies from Forest Laboratories, presenters for the Massachusetts General Hospital Psychiatry Academy in educational programs supported through independent medical education grants from pharmaceutical companies, and salary support from Novartis. She receives royalties from Elsevier Publications, Springer Publications, Guilford Publications, New Harbinger Publications, and Oxford University Press, and speaking honoraria from the IOCDF and the TAA. She received payment from the Association for Behavioral and Cognitive Therapies for her role as Associate Editor for Behavior Therapy as well as from John Wiley & Sons, Inc., for her role as Associate Editor for Depression & Anxiety. Dr. Woods has received speaker’s honoraria from the TAA and royalties from Guilford Press and Oxford University Press. Dr. Piacentini has received grant or research support from the NIMH, Pfizer Pharmaceuticals through the Duke University Clinical Research Institute CAPTN Network, Psyadon Pharmaceuticals, and the TAA. He has received financial support from the Petit Family Foundation and the Tourette Syndrome Association Center of Excellence Gift Fund. He has received royalties from Guilford Press and Oxford University Press. He has served on the speakers’ bureau of the TAA, the International Obsessive Compulsive Disorder Foundation (IOCDF), and the Trichotillomania Learning Center (TLC). Dr. Walkup has received research support from the Hartwell Foundation and the TAA. He is an unpaid advisor to the Anxiety and Depression Association of America (ADAA), the TLC, and the American Foundation for Suicide Prevention. He has received royalties for books from Guilford Press and Oxford University Press and educational materials from Wolters Kluwer. He has served as a paid speaker for the Tourette Syndrome–Centers for Disease Control and Prevention outreach educational programs, the American Academy of Child and Adolescent Psychiatry, and the American Psychiatric Association. Dr. Peterson has received research support and speaker’s honoraria from the TAA and receives royalties from Oxford University Press.
References
- American Psychiatric Association (2013). Diagnostic and Statistic Manual of Mental Disorders. American Psychiatric Publishing: Arlington, VA. [Google Scholar]
- Beck AT, Steer RA & Brown GK (1996). Beck depression inventory-II. San Antonio 78, 490–8. [Google Scholar]
- Bloch MH & Leckman JF (2009). Clinical course of Tourette syndrome. Journal of Psychosomatic Research 67, 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, Peterson BS, Scahill L, Otka J, Katsovich L, Zhang H & Leckman JF (2006). Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Archives of Pediatric and Adolescent Medicine 160, 65–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, David K, Bandera V, Termine C, Balottin U, Schrag A & Selai C (2013). Health-related quality of life in Gilles de la Tourette syndrome: a decade of research. Behavioural neurology 27, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckersbach T, Rauch S, Buhlmann U & Wilhelm S (2006). Habit reversal versus supportive psychotherapy in Tourette’s disorder: a randomized controlled trial and predictors of treatment response. Behaviour Research and Therapy 44, 1079–90. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, McGoey KE, Ikeda MJ & Anastopoulos AD (1998). Reliability and validity of parent and teacher ratings of attention-deficit/hyperactivity disorder symptoms. Journal of Psychoeducational Assessment 16, 55–68. [Google Scholar]
- Evans J, Seri S & Cavanna AE (2016). The effects of Gilles de la Tourette syndrome and other chronic tic disorders on quality of life across the lifespan: a systematic review. European child & adolescent psychiatry 25, 939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman W, Price L, Rasmussen S, Mazure C, Fleischmann R, Hill C, Heninger G & Charney D (1989). The Yale-Brown Obsessive Compulsive Scale: Development, use and reliability. Archives of General Psychiatry 46, 1006–1011. [DOI] [PubMed] [Google Scholar]
- Groth C, Debes NM, Rask CU, Lange T & Skov L (2017). Course of Tourette syndrome and comorbidities in a large prospective clinical study. Journal of the American Academy of Child & Adolescent Psychiatry 56, 304–312. [DOI] [PubMed] [Google Scholar]
- Guy W (1976). Clinical Global Impressions In ECDEU Assessment Manual for Psychopharmacology, pp. 218–222. National Institute for Mental Health: Rockville, MD. [Google Scholar]
- Haddad A, Umoh G, Bhatia V & Robertson M (2009). Adults with Tourette’s syndrome with and without attention deficit hyperactivity disorder. Acta Psychiatrica Scandinavica 120, 299–307. [DOI] [PubMed] [Google Scholar]
- Hewitt PL & Norton GR (1993). The Beck Anxiety Inventory: A psychometric analysis. Psychological Assessment 5, 408–412. [Google Scholar]
- Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C, King RA, Sandor P, McMahon WM & Lyon GJ (2015). Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA psychiatry 72, 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S, Walkup JT, Woods DW, Peterson A, Piacentini J, Wilhelm S, Katsovich L, McGuire JF, Dziura J & Scahill L (2013). Detecting a clinically meaningful change in tic severity in Tourette syndrome: A comparison of three methods. Contemporary clinical trials 36, 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight T, Steeves T, Day L, Lowerison M, Jette N & Pringsheim T (2012). Prevalence of tic disorders: a systematic review and meta-analysis. Pediatric neurology 47, 77–90. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT & Ort SI (1989). The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. Journal of the American Academy of Child & Adolescent Psychiatry 28, 566–573. [DOI] [PubMed] [Google Scholar]
- Leon A, Shear MK, Portera L & Klerman G (1992). Assessing impairment in patients with panic disorder: the Sheehan Disability Scale. Social psychiatry and psychiatric epidemiology 27, 78–82. [DOI] [PubMed] [Google Scholar]
- Lievaart M, Franken IH & Hovens JE (2016). Anger assessment in clinical and nonclinical populations: Further validation of the State–Trait Anger Expression Inventory‐2. Journal of clinical psychology 72, 263–278. [DOI] [PubMed] [Google Scholar]
- McGuire JF, Arnold E, Park JM, Nadeau JM, Lewin AB, Murphy TK & Storch EA (2014). Living with tics: Reduced impairment and improved quality of life for youth with chronic tic disorders. Psychiatry Research 225, 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JF, Nyirabahizi E, Kircanski K, Piacentini J, Peterson AL, Woods DW, Wilhelm S, Walkup JT & Scahill L (2013). A cluster analysis of tic symptoms in children and adults with Tourette syndrome: Clinical correlates and treatment outcome. Psychiatry Research 210, 1198–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JF, Piacentini J, Storch EA, Murphy TK, Ricketts EJ, Woods DW, Walkup JW, Peterson AL, Wilhelm S, Lewin AB, McCracken JT, Leckman JF & Scahill L (2018). A multicenter examination and strategic revisions of the Yale Global Tic Severity Scale. Neurology 90, e1711–e1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel EC, Coffey BJ, Baer L, Savage CR, Rauch SL & Jenike MA (1995). Phenomenology of intentional repetitive behaviors in obsessive-compulsive disorder and Tourette’s disorder. The Journal of clinical psychiatry 56, 246–255. [PubMed] [Google Scholar]
- Murphy TK, Lewin AB, Storch EA & Stock S (2013). Practice parameter for the assessment and treatment of children and adolescents with tic disorders. Journal of the American Academy of Child and Adolescent Psychiatry 52, 1341–59. [DOI] [PubMed] [Google Scholar]
- Peterson AL, McGuire JF, Wilhelm S, Piacentini J, Woods DW, Walkup JT, Hatch JP, Villarreal R & Scahill L (2016). An empirical examination of symptom substitution associated with behavior therapy for Tourette’s Disorder. Behavior therapy 47, 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, Ginsburg GS, Deckersbach T, Dziura J, Levi-Pearl S & Walkup JT (2010). Behavior therapy for children with Tourette disorder: A randomized controlled trial. Journal of the American Medical Association 303, 1929–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill L, Chappell P, Kim Y, Schultz R, Katsovich L, Shepherd E, Arnsten A, Cohen D & Leckman J (2001). Guanfacine in the treatment of children with tic disorders and ADHD: A placebo-controlled study. Am J Psychiatry 158, 1067–1074. [DOI] [PubMed] [Google Scholar]
- Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, Cicchetti D & Leckman JF (1997). Children’s Yale-Brown obsessive compulsive scale: reliability and validity. Journal of the American Academy of Child & Adolescent Psychiatry 36, 844–852. [DOI] [PubMed] [Google Scholar]
- Scharf JM, Miller LL, Gauvin CA, Alabiso J, Mathews CA & Ben‐Shlomo Y (2015). Population prevalence of Tourette syndrome: A systematic review and meta‐analysis. Movement disorders 30, 221–228. [DOI] [PubMed] [Google Scholar]
- Skinner H, Steinhauer PD & Santa-Barbara J (1995). Family Assessment Measure III (FAM III). Multi-Health Systems Inc.: New York. [Google Scholar]
- Spielberger CD (1999). STAXI-2: State-trait anger expression inventory-2: Professional manual. Psychological Assessment Resources Odessa, FL. [Google Scholar]
- Steer RA & Beck AT (1997). Beck Anxiety Inventory.
- Steer RA, Kumar G, Ranieri WF & Beck AT (1998). Use of the Beck Depression Inventory-II with adolescent psychiatric outpatients. Journal of Psychopathology and Behavioral Assessment 20, 127–137. [Google Scholar]
- Storch E, Murphy T, Geffken G, Sajid M, Allen P, Roberti J & Goodman W (2005). Reliability and validity of the Yale Global Tic Severity Scale. Psychological Assessment 17, 486–91. [DOI] [PubMed] [Google Scholar]
- Storch EA, De Nadai AS, Lewin AB, McGuire JF, Jones AM, Mutch PJ, Shytle RD & Murphy TK (2011). Defining treatment response in pediatric tic disorders: A signal detection analysis of the Yale Global Tic Severity Scale. Journal of Child and Adolescent Psychopharmacology 21, 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Morgan JE, Caporino NE, Brauer L, Lewin AB, Piacentini J & Murphy TK (2012). Psychosocial treatment to improve resilience and reduce impairment in youth with tics: An intervention case series of eight youth. Journal of Cognitive Psychotherapy 26, 57–70. [Google Scholar]
- Sukhodolsky DG, Woods DW, Piacentini J, Wilhelm S, Peterson AL, Katsovich L, Dziura J, Walkup JT & Scahill L (2017). Moderators and predictors of response to behavior therapy for tics in Tourette syndrome. Neurology 88, 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibert AL, Day HI & Sandor P (1995). Self-concept and self-consciousness in adults with Tourette syndrome. The Canadian Journal of Psychiatry 40, 35–39. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Prusoff BA, Thompson WD, Harding PS & Myers JK (1978). Social adjustment by self-report in a community sample and in psychiatric outpatients. Journal of Nervous and Mental Disease 166, 317–326. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Prince J & Spencer TJ (1996). Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. The American Journal of Psychiatry 153, 1147–1153. [DOI] [PubMed] [Google Scholar]
- Wilhelm S, Peterson AL, Piacentini J, Woods DW, Deckersbach T, Sukhodolsky DG, Chang S, Liu H, Dziura J, Walkup JT & Scahill L (2012). Randomized trial of behavior therapy for adults with tourette syndrome. Archives of General Psychiatry 69, 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DW, Piacentini J, Chang SW, Deckersbach T, Ginsburg GS, Peterson AL, Scahill LD, Walkup JT & Wilhelm S (2008). Managing Tourette Syndrome: A Behavioral Intervention for Children and Adolescents. Oxford University Press: New York. [Google Scholar]
- Woods DW, Piacentini JC, Scahill L, Peterson AL, Wilhelm S, Chang S, Deckersbach T, McGuire J, Specht M, Conelea CA, Rozenman M, Dzuria J, Liu H, Levi-Pearl S & Walkup JT (2011). Behavior therapy for tics in children: Acute and long-term effects on psychiatric and psychosocial functioning. Journal of Child Neurology 26, 858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]