Abstract
Head and neck squamous cell carcinomas (HNSCC) are the seventh most frequent cancers. Among HNSCCs, oral squamous cell carcinomas (OSCCs) include several anatomical locations of the oral cavity, but exclude the oropharynx. The known risk factors for OSCCs are mainly alcohol consumption and tobacco use for at least 75–80% of cases. In addition to these risk factors, Human papillomavirus (HPV) types 16 and 18, classified as high-risk (HR) HPV genotypes, are considered as risk factors for oropharyngeal cancers, but their role in the development of OSCC remains unclear. We tested the hypothesis of viral etiology in a series of 68 well-characterized OSCCs and 14 potentially malignant disorders (PMD) in non-smoking, non-drinking (NSND) patients using broad-range, sensitive molecular methodologies. Deep-sequencing of the transcriptome did not reveal any vertebrate virus sequences other than HPV transcripts, detected in only one case. In contrast, HPV DNA was detected in 41.2% (28/68) and 35.7% (5/14) of OSCC and PMD cases, respectively. Importantly, 90.9% (30/33) of these belonged to the Betapapillomavirus genus, but no viral transcripts were detected. Finally, high-throughput sequencing revealed reads corresponding to transcripts of the Trichomonas vaginalis virus (TVV), which were confirmed by RT-PCR in two OSCCs. Our results strongly suggest that Alphapapillomavirus genotypes classified as HR are not involved in the development of OSCCs in NSND patients and that known oncogenic infectious agents are absent in these specific OSCCs. Any possible direct or indirect role of Betapapillomavirus genus members and TVV in OSCCs remains speculative and requires further investigation.
Introduction
In 2015, head and neck squamous cell carcinomas (HNSCC), i.e. cancers of the mouth, nose, throat, larynx and sinuses, affected more than 5.5 million people worldwide and caused more than 379,000 deaths [1]. HNSCC is the seventh most frequent cancer and the ninth most frequent cause of death from cancer. Among these cancers, oral squamous cell carcinomas (OSCCs) include cancers of the inner mucosa of the lips, the cheeks and vestibule, the mobile part of the tongue, gums, the floor of the mouth, the intermaxillary region as well as the hard and anterior surfaces of the soft palate, but exclude the oropharynx (i.e. tonsils, base of the tongue, the posterior surface of the soft palate and the walls of the oropharynx) [2]. The estimated annual incidence worldwide of OSCC is approximately 300,000 cases with a mortality rate of about 145,000 [3]. However, the overall burden of this cancer varies across continents, with Asian countries contributing more than half of the cases (56.2%), and Africa showing the lowest percentage of cases at 5.7%. The percentages for Europe and America are 20.4 and 22.7%, respectively [3]. A subset of OSCCs appears on pre-existing lesions with a predisposition for malignant transformation, called “potentially malignant disorders” (PMDs) [4]. This group of oral lesions mainly includes leukoplakia, erythroplakia, lichen planus and some other relatively rare disorders. Among them, leukoplakia is the most common lesion, with an estimated prevalence of 0.5% worldwide [4] and an annual transformation rate of approximately 1% [5], albeit higher in Asian countries [6]. Finally, the presence of epithelial dysplasia is considered the most important indicator of malignant potential [5].
The known risk factors for OSCC are mainly alcohol consumption, tobacco use and chewing betel for at least 75–80% of cases [7–9]. Although the first two main factors are independently associated, they also act synergistically [10]. Moreover, some studies confirm that 10–20% patients suffering from OSCC can be considered as non-smokers and non-drinkers (NSND) [11, 12]. In addition to these risk factors, the Human papillomavirus (HPV) types 16 and 18, classified as high-risk (HR) HPV genotypes, are also considered as risk factors for oropharyngeal cancer, but their role in the development of oral cavity cancer remains unclear [13]. The possibility that HPV may play a role in OSCC was first raised in 1983 [14]; since then, several studies have indicated the presence of HR-HPV DNA in a certain proportion of neoplasias, suggesting that some of them were virally induced cancers. However, for OSCCs, these first results were controversial due to highly variable prevalence rates, ranging from 17 to 85% [15]. The first meta-analysis in 2005 on 60 studies showed that HPV prevalence was higher in oropharyngeal SCCs (OPSCCs) (35.6%) than in OSCCs (23.5%) or laryngeal SCCs (LSCC) (24%) [16]. More recently, another meta-analysis, which included more than 50 studies between 2007 and 2017, showed that the prevalence of HPV was 24.4% in OSCCs with a strong disparity across geographical areas, and the highest prevalence in Asian countries (33.77% of cases) [17]. However, in a number of these studies, no specific information on the anatomical sub-localization of SCC in the tongue (mobile part versus base of the tongue) was available, which may lead to confusion between OPSCCs and OSCCs, and thus an overestimation of the true burden of HPV in OSCCs [18]. In OSCCs, HPV type 16 is the most commonly found genotype followed by types 18, 31 and 33 and a few others [16, 19–22]. The tongue and floor of the mouth are the anatomical locations of the tumors most often found infected with these above-mentioned HR-HPV genotypes [19, 20, 22, 23]. Moreover, histological and epidemiological studies have demonstrated significant trends for decreased tumor differentiation of oral cavity and oropharyngeal carcinomas over the last 30 years [24]. Therefore, the increase in undifferentiated cancers suggests that non-identified infectious agents may be involved in malignant disorders and OSCCs in NSND patients [24–27]. To date, a few studies have already specifically investigated an infectious cause in these NSND patients. For instance, Laco et al. (2010) detected HR-HPV DNA via PCR in only 12.5% of OSCC cases in patients who had no history of smoking or alcohol abuse, whereas Li et al. (2015) did not identify any oncogenic virus in a cohort of 20 OSCCs of the tongue in NS patients [28, 29].
The aim of this study was to test the hypothesis of a viral etiology due to a known viral agent such as HPV or another, unknown or unforeseen, agent associated with an oncogenic process in a series of well-characterized PMDs and OSCCs in NSND patients using very broad and sensitive methodologies. Firstly, HPV was investigated using two different methods. The first one consisted of the use of a DNA microarray (PapilloCheck HPV genotyping kit) that detects 24 genotypes belonging to the Alphapapilliomavirus genus and the second one was based on several PCR systems using consensus HPV primers, followed by amplicon sequencing and analysis of the sequences obtained. Secondly, a broad-spectrum transcriptomic approach (i.e. high-throughput sequencing (HTS) of total RNA), was used to explore other etiological causes. This latter methodology was developed to detect and characterize unknown or variant replicating viruses associated with several human diseases, especially cancers.
Methods and materials
PMD and OSCC samples
At the maxillofacial surgery department at the Pitié-Salpêtrière Hospital (Paris, France), NSND patients with PMD or OSCC were selected for our prospective study according to strict criteria between 2012 and 2015. Patients were included in the study if they were over 18 years of age and were considered NSND following commonly accepted criteria. In addition, we will include in this category all patients whose rare, or very rare, occasional use of alcohol or tobacco suggested that the development of their OSCCs were not related to these two risk factors. Consequently, the patients included in our study were (i) strictly NS or with a consumption equal to or less than 5 packs per year or having stopped smoking at least 15 years prior, regardless of the amount smoked and (ii) strictly ND or with a moderate consumption of 10 or 20 g of alcohol per day or less, with the majority of the cases being less than 1–2 drinks per month for women and men, respectively, or having stopped drinking at least 15 years prior, regardless of initial consumption. The only non-inclusion criteria were previous antecedents of oral cancer. The first criteria for inclusion of PMDs was the clinical diagnosis of leukoplakia. All other PMDs, such as erythroplakia, lichen planus, proliferative verrucous leukoplakia (PVL), etc were excluded. All clinical data related to the patient (age, sex and clinical history) or to his/her PMD or OSCC (anatomical location, malignant tumor classification (TNM) and histological criteria) were compiled in a database managed using the Voozanoo platform (http://www2.voozanoo.net/) on the ICAReB platform at the Institut Pasteur. All samples of patients included in the study were collected during their surgical treatment according to current cancer reference standards. Each investigated tissue sample was harvested from the main tumor removed during surgery. To preserve the nucleic acids as much as possible, the tissues were immediately frozen in dry ice in the operating room and then sent to the Institut Pasteur for long-term storage and molecular investigations. All the bio-resources were managed by the ICAReB platform, comprising RNA/DNA extraction, sample transport, registration and coding with regard to ethical compliance. In parallel to the molecular investigations, the entire tumor was analyzed by anatomical pathologists to determine the histological grade, the margins of the tumor and the two main histological criteria of its severity (perineural invasion and vascular embolus).
DNA extraction and genotyping of HPV using PapilloCheck and PCRs
Frozen tumor tissues samples were stored at -80°C until total genomic DNA extraction using the DNeasy Blood and Tissue kit (Qiagen, CA, USA) according to the manufacturer’s instructions. Extracted DNA was quantified using the Qubit® dsDNA BR Assay kit with the Qubit 2.0 fluorimeter (Life Technologies, CA, USA) and stored at -20°C until molecular investigations such as HPV genotyping. Firstly, this extracted DNA was investigated using the PapilloCheck HPV genotyping kit (Greiner BioOne, Frickenhausen, Germany) according to the manufacturer’s recommendations. This assay first included PCR amplification with fluorescent primers (CY5 fluorophore) specific to the HPV E1 gene and the cellular housekeeping gene ADAT1. The amplification products were then hybridized to a DNA microarray containing sequences for 18 HR genotypes (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 70, 73, 82) and six low-risk (LR) genotypes (HPV6, 11, 40, 42, 43 and 44/55). Secondly, three consensus PCR assays were performed using three sets of degenerate primers (GP5+/6+, CP65-70 and CP66-69) [30, 31]. The first reaction using GP5+/6+ primers was performed in 100 μL using 5 μL of template DNA, 1X PCR buffer (Thermo Fisher Scientific), 3.5 mM MgCl2, 0.2 μM of each deoxynucleotide triphosphate (dNTP), 0.5 μM of each consensus primer GP5+/6+ and 1 U of Ampli Taq Gold DNA polymerase (Thermo Fisher Scientific). The second nested PCR assay was also performed using two sets of degenerate primers (CP65-70 and CP66-69). The reaction was performed in 50 μL including 5 μL of template DNA (either template of DNA or amplicon from PCR using the CP67-70 primers), 1X PCR buffer (Thermo Fisher Scientific), 3.5 mM MgCl2, 0.2 μM of each dNTP, 0.5 μM of each consensus primer and 1 U of Ampli Taq Gold DNA polymerase. Thermal cycling for the three PCRs are described in S1 Table. All PCR products were sequenced using the BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems) according to the manufacturer’s protocol.
Viral metatranscriptomics
Total RNA was extracted from biopsies using the Trizol procedure (Thermo Fischer Scientific), purified on RNeasy column including a DNase treatment (Qiagen), and randomly amplified [32] for each patient individually. High-molecular-weight DNA was used for library construction and high-throughput sequencing (HTS) was subcontracted to DNA Vision (Charleroi, Belgium) on a HiSeq 2000 instrument, generating an average of 92 million reads per sample (min = 46 M; max = 162 M). Sequences were trimmed and then screened for viral sequences using the protein version of RVDB database [33]. Additional screening for bacteria and other non-viral microorganism sequences was performed using Centrifuge [34] and Metaphlan2 [35]. The reads and contigs of interest were mapped using the Geneious mapper of Geneious 11.1.5 [36] with the medium sensitivity/fast parameters.
RT-PCR for detection of HR-HPV and the Trichomonas vaginalis virus
Reverse transcription reactions were performed starting from RNA with the SuperScript III First-Strand Synthesis System (Thermo Fischer Scientific). Then, qPCRs were performed in SYBR Green format with 45 cycles of amplification using primers described in S1 Table. Human beta actin was used as a positive internal control with primers ACTB-fw and ACTB-rv (S2 Table). Primers for the Trichomonas vaginalis virus 1, 2, 3 and 4 (TVV1, 2, 3, 4) were retrieved from [37]. Additional PCR systems for TVV1, 2 and 3 were designed from NGS reads (S2 Table). Finally, primers used for the detection of T. vaginalis (TV) were retrieved from [38] and designed according to conserved region of TV cysteine proteinase 4 (S2 Table).
Ethical considerations
This study was approved by both the French Health Products Safety Agency (then called AFSSAPS, then ANSM since May 2012) on 20 February 2012 (B120225-40) and by the Ile de France VI Ethics Committee on 2 March 2012 (CPP no. 13–12 –IDRCB 2012-A00116-37). Written informed consent was obtained from all patients included in this study.
Results
Description of PMD and OSCC samples
This study included 14 PMD and 68 OSCC cases. The sex ratio (M:F) was 0.16 and 0.94 with an average age of 70 years (+/- 15 years) and 69 years (+/- 15 years) for PMDs and OSCCs, respectively (Table 1), with 86.7% of patients being over 50 years of age in this study. Given that the main inclusion criteria for patients was based on their alcohol and tobacco consumption, 42.8% (6/14) and 45.6% (31/68) of PMD and OSCC patients, respectively, had never been drinkers nor smokers. Moreover, for PMDs, 100% of these cases were observed in females and, for OSCCs, 22.6% (7/31) and 77.4% (24/31) were observed in males and females, respectively. The others were either moderate alcohol and/or tobacco users or stopped drinking or smoking more than 15 years ago. In 78.6% of cases (11/14), PMDs were located on the mobile part of the tongue, with a possible extension to other anatomical locations. On the other hand, 75% (51/68) of the OSCCs were found either in the mobile part of the tongue or in the gums (Table 1). Cheeks were the third identified anatomical location of OSCCs with 10.3% (7/68) and the other localizations represented less than 10% of cases. Histological results of PMD samples showed that they could be separated into simple hyperplasias (n = 3), dysplasias of various grades (I to III) (n = 7), and in situ carcinomas (n = 6), which represented 42.8% (6/14) of cases. In OSCC samples, histological criteria classified lesions according to the level of cell differentiation and keratinization. More than 83.9% (47/56) of OSCCs were keratinized, but data on this criterion could not be obtained for 17.6% (12/68) of cases. In parallel, in 35.3% and 55.9% of cases (24/68 and 38/68), the cells were moderately or well differentiated, respectively. Poorly differentiated cells were found in only 7.3% of cases. Finally, a history of PMD was found in 17.6% (12/68) of the OSCC cases in this study (S5 Table). They had the same anatomical location in 75% of cases (9/12). Moreover, depending on the case, these PMDs appeared to have developed over a period ranging from 1 year to about 20 years (S5 Table).
Table 1. Summary of clinical and pathological data on the potentially malignant disorder (PMD) and oral squamous cell carcinoma (OSCC) cases included in this study.
| PMDs | OSCCs | ||||||
|---|---|---|---|---|---|---|---|
| Overall | Female | Male | Overall | Female | Male | ||
| Number of cases | 14 | 12 | 2 | 68 | 35 | 33 | |
| Average age (years) | 70 | 73 | 50 | 69 | 72 | 65 | |
| Age (years) | Range | 34–84 | 51–84 | 34–67 | 29–91 | 35–91 | 29–88 |
| < 30 | 0 | 0 | 0 | 1 | 0 | 1 | |
| 30–50 | 1 | 0 | 1 | 8 | 3 | 5 | |
| 50–70 | 5 | 4 | 1 | 26 | 14 | 12 | |
| 70 + | 8 | 8 | 33 | 18 | 15 | ||
| Number of patients who have never drunk or smoked | 6 | 6 | 0 | 31 | 24 | 7 | |
| Anatomical location | Inner mucosa of lips | 1 | 1 | 0 | 1 | 1 | 0 |
| Mobile part of the tongue | 11 | 9 | 2 | 25 | 12 | 13 | |
| Floor of the mouth | 1 | 0 | 1 | ||||
| Gum | 1 | 1 | 0 | 26 | 16 | 10 | |
| Cheek mucosa | 1 | 1 | 0 | 7 | 2 | 5 | |
| Intermaxillary region | 4 | 1 | 3 | ||||
| Hard palate of the mouth | 3 | 2 | 1 | ||||
| Soft palate of the mouth | 1 | 1 | 0 | ||||
| Hyperplasia | 3 | 3 | 0 | Not applicable | |||
| Dysplasia Grade I | 4 | 3 | 1 | ||||
| Dysplasia Grade II | 2 | 2 | 0 | ||||
| Dysplasia Grade III | 1 | 1 | 0 | ||||
| In situ Carcinoma | 6 | 5 | 1 | ||||
| Cellular differentiation | Well | Not applicable | 38 | 17 | 21 | ||
| Medium | 24 | 14 | 10 | ||||
| Poor | 5 | 3 | 2 | ||||
| Keratinization | Yes | Not applicable | 47 | 23 | 24 | ||
| No | 9 | 8 | 1 | ||||
| Not Determined | 12 | 5 | 7 | ||||
| Perineural invasion | Yes | Not applicable | 26 | 14 | 12 | ||
| No | 38 | 18 | 20 | ||||
| Not applicable | 4 | ||||||
| Vascular embolus | Yes | Not applicable | 13 | 9 | 4 | ||
| No | 55 | 23 | 29 | ||||
| Not applicable | 3 | ||||||
Search of HPV DNA using conventional methods
At least one HPV type was detected in 35.7% (5/14) and 41.2% (28/68) of PMD and OSCC cases, respectively (See S3 & S4 Tables for results by molecular methods for HPV detection). Of these, 91.2% (31/34) belonged to the Betapapillomavirus genus (β-HPVs) (Table 2, S5 & S6 Tables). Only three HPV genotypes classified as high-risk (HPV16 and HPV33) were identified in three OSCC samples (CE03, CE07 & CE41). Two samples with HPV16 DNA were found in women aged 49 and 61 years, and HPV33 DNA was identified in a 53-year-old male. These three OSCC cases with HR-HPV were located in the mobile part of the tongue and histological analyses showed good differentiation and keratinization. These three HR-HPV accounted for either 4.4% of all OSCC cases or 12% of cases in the mobile part of the tongue. However, for only one of these cases (HPV16, in sample CE41), a PMD lesion had previously been reported at the same anatomical location, with development over less than one year (S6 Table). All the other β-HPVs identified in the OSCCs came from 69.2% (18/26) of cases involving either the mobile part of the tongue or the gum (Table 2). Among these OSCCs in which β-HPV was detected, the cells were moderately or well-differentiated only in 88.5% (23/26) of cases and keratinized in only 84.2% (16/19) of cases (S6 Table). Finally, in 92.3% (24/26) of these cases, no previous PMDs were indicated. In two cases, a PMD had been reported in the same anatomical location (S6 Table). These β-HPVs were also identified in 35.7% (5/14) of PMD cases. All β-HPVs were identified only in PMDs located at the tip or lateral part of the tongue (S5 Table). Moreover, they were found in equivalent proportions as simple hyperplasias or grade I or II (3/5) or in situ carcinomas (2/5). Overall, 15 different genotypes of β-HPV were identified in PMDs and OSCCs. However, approximately 86.7% (13/15) of these genotypes were found only once or twice, such as the HPV9 genotype, which was detected only in one PMD case (PM01). Finally, the HPV20 and HPV36 genotypes represented 46.2% (12/26) of the β -HPVs identified in both PMDs and OSCCs.
Table 2. Summary of the number of Human papillomavirus (HPV) genotypes detected according to anatomical location of the potentially malignant disorders (PMD) and oral squamous cell carcinomas (OSCC) included in this study.
| Anatomical localization | ||||||
|---|---|---|---|---|---|---|
| Overall | Mobile part of the tongue | Gum | Cheek | Others | ||
| PMD | All HPVs | 5 | 5 | |||
| Alphapapillomavirus HPV | 0 | 0 | ||||
| Betapapillomavirus HPV | 5 | 5 | ||||
| OSCC | All HPVs | 29 | 13* | 8 | 5 | 3 |
| Alphapapillomavirus HPV | 3 | 3 | 0 | 0 | 0 | |
| Betapapillomavirus HPV | 26 | 10 | 8 | 5 | 3 | |
* one co-infection with α-& β-HPVs
Viral metatranscriptomics
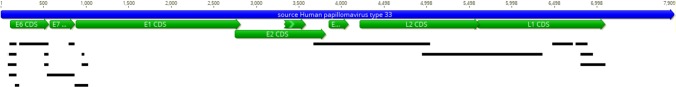
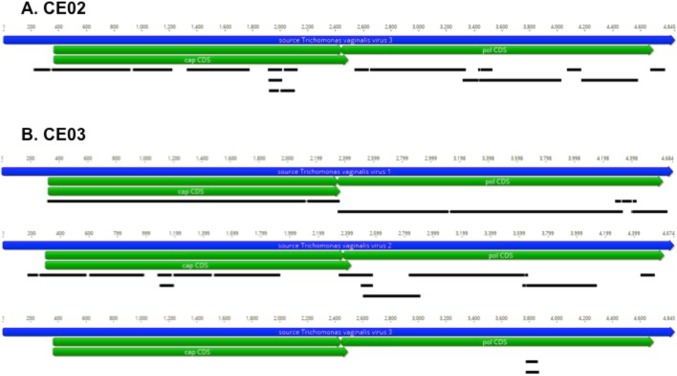
A viral etiological association was investigated using a metatranscriptomics agnostic approach based on the detection of all viral RNAs, in 7 and 19 PMD and OSCC cases, respectively. Most of the PMDs investigated came from the mobile part of the tongue (4/7), but the others came from either the lip, gum or cheek mucosa. Of these, only three β-HPVs had previously been identified using conventional DNA PCR. With the exception of the detection of bacterial transcripts most likely coming from commensal flora (data not shown), these analyses did not reveal any transcripts corresponding to β-HPV or belonging to other viral agents (Table 3). In the OSCCs, 84% of the samples investigated using HTS came mainly from the mobile part of the tongue (9/19) and the gum (8/19). In contrast to the results obtained for PMDs, transcripts corresponding to HPV33 were found in the CE07 sample in which this genotype had previously been detected using PCR (Table 3). These transcripts cover the entire genome with the exception of two viral genes E1 and E2 (Fig 1). However, similarly to PMDs, no other transcripts corresponding either to HPV16 (CE03) or to β-HPVs were detected using HTS, despite a high sequencing depth for the CE03 sample (about 150 million reads; see Table 3). In contrast, in two OSCCs (CE02 and CE03), thousands of unique reads corresponding to the T. vaginalis virus (TVV), a double-stranded RNA virus, belonging to the Totiviridae family, were identified. The analyses showed that these reads corresponded to the TVV1-2-3 and TVV3 viruses in CE03 and CE02, respectively (Fig 2 & Table 3). The presence of these TVV genotypes in these two OSCCs was confirmed either using RT-PCRs from the literature or with specific primers designed from the sequences obtained (Table 4). Moreover, the presence of the parasite itself (TV) was also demonstrated in these two samples by the identification of reads corresponding to specific housekeeping genes, including those of actin and phosphoenolpyruvate carboxykinase (data not shown). However, the presence of TV could not be directly confirmed using a reference RT-PCR [38] (Table 4).
Table 3. Compilation of the results from the analysis of metagenomic data and RT-PCR obtained for the oral squamous cell carcinoma (OSCC) and potentially malignant disorder (PMD) cases analyzed using high-throughput sequencing (HTS).
| Anatomical location | Number of samples | Number of raw HTS reads (10^6) | HTS results (viral hits) (2019) | RT-PCR ACTB | RT-PCR HPV33 | RT-PCR HPV16 E6E7 | |
|---|---|---|---|---|---|---|---|
| PMD | Mobile part of the tongue | PM 01 | 79.9 | Neg | Pos | Neg | Neg |
| PM 04 | 96.0 | Neg | Pos | Neg | Neg | ||
| PM 05 | 97.6 | Neg | Pos | Neg | Neg | ||
| PM 06 | 83.6 | Neg | Pos | Neg | Neg | ||
| Cheek mucosa | PM 02 | 105.4 | Neg | Pos | Neg | Neg | |
| Gum | PM 03 | 53.2 | Neg | Pos | Neg | Neg | |
| Inner mucosa of lips | PM 07 | 82.8 | Neg | Pos | Neg | Neg | |
| OSCC | Gum | CE 01 | 79.8 | Neg | Pos | Neg | Neg |
| CE 02 | 83.4 | TVV3 | Pos | Neg | Neg | ||
| CE 09 | 66.8 | Neg | Pos | Neg | Neg | ||
| CE 13 | 87.7 | Neg | Pos | Neg | Neg | ||
| CE 14 | 97.7 | Neg | Pos | Neg | Neg | ||
| CE 17 | 147.6 | Neg | Pos | Neg | Neg | ||
| CE 26 | 162.0 | Neg | Pos | Neg | Neg | ||
| CE 29 | 57.8 | Neg | Pos | Neg | Neg | ||
| Intermaxillary region | CE 15 | 107.7 | Neg | Pos | Neg | Neg | |
| Mobile part of the tongue | CE 03 | 147.1 | TVV1-2-3 | Pos | Neg | Neg | |
| CE 04 | 83.3 | Neg | Pos | Neg | Neg | ||
| CE 07 | 97.2 | HPV33 | Pos | Pos | Neg | ||
| CE 10 | 112.0 | Neg | Pos | Neg | Neg | ||
| CE 11 | 87.7 | Neg | Pos | Neg | Neg | ||
| CE 20 | 60.4 | Neg | Pos | Neg | Neg | ||
| CE 24 | 114.6 | Neg | Pos | Neg | Neg | ||
| CE 37 | 46.0 | Neg | Pos | Neg | Neg | ||
| Cheek mucosa | CE 06 | 57.0 | Neg | Pos | Neg | Neg | |
| CE12 | 112.2 | Neg | Pos | Neg | Neg |
Fig 1. Mapping of singletons and contigs generated after de novo assembly of reads from sample CE07 on a reference sequence of the Human papillomavirus type 33 (HPV33) genome.
Fig 2.
Mapping of the singletons and contigs generated after de novo assembly of the reads obtained for samples CE02 (A) and CE03 (B) on the reference sequences for Trichomonas vaginalis virus type 1, 2 and 3 genomes.
Table 4. Detection of Trichomonas vaginalis (TV) and the TV virus (TVV) using RT-PCR in oral squamous cell carcinoma (OSCC) cases.
| Trichomonas vaginalis | TVV1 | TVV2 | TVV3 | TVV4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TV-CP4-920*** | TVV1-569** | TVV1-810-199* | TVV1-3036-194* | TVV2-20778-199* | TVV2-20778-202* | TVV2-625** | TVV3-440** | TVV3-4640-193* | TVV3-4640-202* | TVV4-514** | ||
| Gum | CE 02 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Pos | Pos | Neg |
| Mobile part of the tongue | CE 03 | Neg | Neg | Pos | Pos | Pos | Pos | Pos | Pos | Pos | Neg | Neg |
Discussion
In this study, a search of an association with an infectious agent was investigated using various molecular methods in a well-characterized series of PMD and OSCC in NSND patients. The epidemiology of these OSCCs is different from those with known risk factors (alcohol and tobacco). First, these alcohol and tobacco related cancers are generally more common in 60-year-old men and are located mainly in the tongue (25%), the floor of the mouth (17%) and the gums (13%) (unpublished data), whereas our NSND cohort was dominated by women over 70 years of age. Moreover, these cancers in NSND patients were mainly located in the mobile part of the tongue and gums, which represented more than 70% of the cases, with localization in the floor of the mouth being rare. These data confirm that cancers in NSND and SD are epidemiologically different. Therefore, in the absence of the two main known risk factors (i.e. tobacco and alcohol use), we investigated HR-HPV as an infectious etiological cause in our NSND cohort. However, our results differ from previous studies because we focused strictly on lesions localized in the oral cavity in NSND patients. Our investigations revealed HR-HPV DNA in only 4.4% of OSCCs in our cohort. Consequently, our study shows that the potential impact of HR-HPV in NSND patients as an etiological agent is lower compared with the majority of other studies in which the OSCCs studied also included patients with the two main risk factors (alcohol and/or tobacco consumption) [16, 19–22]. Smoking, rather than high-risk sexual behavior or immunodeficiency related to HIV infection, can increase the risk of HR-HPV infection in the oral cavity [23, 39, 40]. However, several studies have shown that the expression of the HPV E6/E7 genes occurs in only 6–7% of cases, confirming that the detection of HPV DNA must be distinguished from its oncogenic activity and that HPV detected may not be biologically active in the majority of OSCCs [20, 41, 42]. In our study, although the DNA of three HR-HPVs was detected, viral transcripts were found only in one case in which the patient was immunodeficient due to HIV infection (HPV33-CE07). No transcript of the HPV16 genotype was detected either using HTS or an RT-PCR specific to this genotype in the other two HR-HPV cases (CE03 and CE41). In conclusion, all our data suggest that the impact of HR-HPV in NSND patients is even lower than previously estimated, because the only OSCC case possibly driven by HR-HPV was identified in a patient co-infected with HIV. These data raise the question of whether OSCCs in which HR-HPV DNA are detected are really HPV-driven, given that the presence of HPV does not mean that it is biologically active.
Nonetheless, this study highlighted the presence of a β-HPV in 35.7% and 41.2% of cases, for PMDs and OSCCs, respectively. Many studies mention that β-HPVs are ubiquitously disseminated throughout the human body, participating in commensal flora (even though β-1 and β-2 HPVs are more prevalent in cutaneous areas whereas β-3 is more abundant in the nasal cavity than on the skin [43, 44]). In addition, these β-HPVs are more often found in keratinized tissues, such as the mobile part of the tongue and gums than in the cheek or the floor of the mouth, which are free of keratin [45]. Some of these cutaneous HPVs are associated with a wide variety of clinical manifestations in the skin [46]. However, the etiological role of these β-HPV in skin cancers is difficult to prove given the high heterogeneity that exists within this group of viruses and that they are found ubiquitously at many anatomical sites [47, 48]. The only evidence of the association between β-1 HPV and skin lesions with carcinogenicity is in epidermodysplasia verruciformis (EV), a rare autosomal recessive hereditary skin disease, in which genotypes HPV5 and HPV8, but also HPV14, HPV20 and some others have been found in these lesions [49]. In addition, viral transcripts and a high number of viral genomes are generally found in EV [50]. However, unlike in EV, for the other β-HPVs detected in various other SCCs of the skin, the viral load is generally less than one copy per cell [51] and no transcript of these papillomaviruses can be identified [52] showing that β-HPVs may not be expressed in these other skin SCCs. Unlike α-HPVs associated with cancers that are transcriptionally active and are absolutely required for cancer maintenance, the role of β-HPVs may be relatively more important for the initiation of cancer than in its maintenance. The E6 protein of β-HPV can target many pathways or proteins such as p300, MAML1 or Notch, which in particular has tumor suppressor gene functions in epithelial cells [53–55]. The effect of E6's interaction with these cellular targets may allow HPV-infected cells to accumulate many mutations that would be necessary and then sufficient for the cell to progress to cancer. However, since these β-HPVs are also found in other compartments, the association with SCC remains questionable. Nevertheless, several studies tentatively associate the circulation of anti-β-HPV antibodies and SCC [56]. For instance, seroconversion seems to increase with age [57, 58] and may be associated with an increased risk of SCC [59, 60]. Although there is no clear evidence that β-HPVs contribute directly to the development of SCCs (with the exception of EV), a recent prospective study showed that the detection of viral DNA, particularly β- and γ-HPV in the oral cavity, is associated with an increased risk of head and neck cancers, particularly with oral cavity cancers [61].
Moreover, this study identified transcripts of TVV in two OSCCs (CE02 and CE03), including one case co-infected with HPV16 (CE03). TV, a flagellated protozoan parasite, is the main non-viral agent responsible for sexually transmitted infections with more than 276 million estimated cases, 90% of which occur in resource-limited countries [62]. In high-grade lesions (HSIL) or cervical cancer, TV co-infects vaginal cells with HPV, but cannot be considered as an HPV cofactor in the genesis of precancerous lesions and their progression to malignancy [63]. However, its role in prostate cancer is more controversial, although it may be associated with a higher risk of high-grade or metastatic prostate cancer [64]. TV can induce the production of a considerable number of different pro-inflammatory cytokines such as IL-6, IL-8, MIP 3-alpha and TvMIF, which is very similar to a human cytokine, HuMIF. All of these cytokines are involved either in carcinogenesis or associated with an unfavorable carcinogenic prognosis [65–71]. Although, to date, TV has never been found associated with OSCCs, these data argue that a chronic infection with TV in the oral cavity may, via chronic inflammation, induce cell proliferation that promotes cancer, particularly in older people.
Finally, this study of the potential infectious cause of this well-characterized series of PMDs and OSCCs, carried out using a wide range of complementary molecular techniques, did not reveal the presence of any other already known oncogenic virus, with the rare exception of HR-HPV. These results are similar to those obtained by Li et al. (2015) on 20 cases of OSCC investigated in NS patients [29]. However, these data contradict those in Laco et al. (2011), which reported a much higher HR-HPV rate (12.5% vs. 4.4%) in OSCCs in NSND patients [28]. Unfortunately, the Laco et al. study did not specify the amount of alcohol the patients had consumed, except that they did not suffer from chronic alcoholism. Although the role of moderate alcohol consumption remains uncertain in the development of OSCC, it has a direct or indirect effect on the epithelium of the oral cavity. Alcohol consumption can act via its main metabolite, acetaldehyde, or alter the metabolism of retinoids or increase its permeability to other carcinogenic agents [72–74]. Moreover, the epithelium of the oral cavity is weakly permissive to HPV infection. The analysis of the microenvironment of the epithelium of the oral cavity confirms the absence of specialized lymphoepithelial tissue, which is present in the oropharynx. This specialized lymphoepithelial tissue fosters the HR-HPV infection of basal keratinocytes of the reticulated crypt epithelium, which promotes the development of carcinoma in these cells [13]. Therefore, the absence of this lymphoepithelial tissue in the oral cavity can explain the very low rate of HR-HPV infection observed in our NSND patients [75]. Hence, alcohol consumption may promote HR-HPV infection of basal keratinocytes in oral mucosa and thus explain the higher rate of HR-HPV infection among drinkers than among NDs in OSCCs.
In conclusion, all the results of this study strongly suggest that HR-HPV is not involved in the development of OSCCs in NSND patients and that known oncogenic infectious agents are absent in these specific OSCCs. Any possible direct or indirect role of β-HPV and TVV in NSND cases of OSCCs remains speculative and requires further investigation.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Valérie Monceaux and Blandine Rimbault of the ICAREb platform for their help in the management of specimens.
Abbreviations
- EV
Epidermodysplasia verruciformis
- HIV
Human immunodeficiency virus
- HNC
head and neck cancer
- HPV
human papillomavirus
- HR
high-risk
- ICAReB
Investigation clinique et accès aux ressources biologiques
- LSCC
laryngeal squamous cell carcinoma
- NSND
non-smoker, non-drinker
- OPSCC
oropharyngeal squamous cell carcinoma
- OSCC
oral squamous cell carcinoma
- PMD
potential malignant disorder
- PVL
proliferative verrucous leukoplakia
- SCC
squamous cell carcinoma
- SD
Smoker, drinker
- TV
Trichomonas vaginalis
- TVV
Trichomonas vaginalis virus
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The French Institut National du Cancer - INCa - had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer. 2010;127(12):2893–917. Epub 2011/02/26. 10.1002/ijc.25516 . [DOI] [PubMed] [Google Scholar]
- 2.Netter FH. Atlas of Human Anatomy. Summit, New Jersey: CIBA-GEIGY; 1989. [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015;136(5):E359–86. Epub 2014/09/16. 10.1002/ijc.29210 . [DOI] [PubMed] [Google Scholar]
- 4.van der Waal I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral oncology. 2009;45(4–5):317–23. Epub 2008/08/05. 10.1016/j.oraloncology.2008.05.016 . [DOI] [PubMed] [Google Scholar]
- 5.Brennan M, Migliorati CA, Lockhart PB, Wray D, Al-Hashimi I, Axell T, et al. Management of oral epithelial dysplasia: a review. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2007;103 Suppl:S19.e1–2. Epub 2007/01/30. 10.1016/j.tripleo.2006.10.015 . [DOI] [PubMed] [Google Scholar]
- 6.Scheifele C, Reichart PA. Is there a natural limit of the transformation rate of oral leukoplakia? Oral oncology. 2003;39(5):470–5. Epub 2003/05/16. 10.1016/s1368-8375(03)00006-x . [DOI] [PubMed] [Google Scholar]
- 7.Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer research. 1988;48(11):3282–7. Epub 1988/06/01. . [PubMed] [Google Scholar]
- 8.Lee KW, Kuo WR, Tsai SM, Wu DC, Wang WM, Fang FM, et al. Different impact from betel quid, alcohol and cigarette: risk factors for pharyngeal and laryngeal cancer. International journal of cancer. 2005;117(5):831–6. Epub 2005/06/16. 10.1002/ijc.21237 . [DOI] [PubMed] [Google Scholar]
- 9.Schutze M, Boeing H, Pischon T, Rehm J, Kehoe T, Gmel G, et al. Alcohol attributable burden of incidence of cancer in eight European countries based on results from prospective cohort study. BMJ (Clinical research ed). 2011;342:d1584 Epub 2011/04/09. 10.1136/bmj.d1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castellsague X, Quintana MJ, Martinez MC, Nieto A, Sanchez MJ, Juan A, et al. The role of type of tobacco and type of alcoholic beverage in oral carcinogenesis. International journal of cancer. 2004;108(5):741–9. Epub 2003/12/30. 10.1002/ijc.11627 . [DOI] [PubMed] [Google Scholar]
- 11.Syrjanen S. Human papillomaviruses in head and neck carcinomas. The New England journal of medicine. 2007;356(19):1993–5. Epub 2007/05/15. 10.1056/NEJMe078004 . [DOI] [PubMed] [Google Scholar]
- 12.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral oncology. 2009;45(4–5):309–16. Epub 2008/09/23. 10.1016/j.oraloncology.2008.06.002 . [DOI] [PubMed] [Google Scholar]
- 13.Marur G, D'Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. The Lancet Oncology. 2010;11(8):781–9. Epub 2010/05/11. 10.1016/S1470-2045(10)70017-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Syrjanen K, Syrjanen S, Lamberg M, Pyrhonen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. International journal of oral surgery. 1983;12(6):418–24. Epub 1983/12/01. 10.1016/s0300-9785(83)80033-7 . [DOI] [PubMed] [Google Scholar]
- 15.Campisi G, Panzarella V, Giuliani M, Lajolo C, Di Fede O, Falaschini S, et al. Human papillomavirus: its identity and controversial role in oral oncogenesis, premalignant and malignant lesions (review). International journal of oncology. 2007;30(4):813–23. Epub 2007/03/03. . [PubMed] [Google Scholar]
- 16.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14(2):467–75. Epub 2005/03/01. 10.1158/1055-9965.epi-04-0551 . [DOI] [PubMed] [Google Scholar]
- 17.Yete S, D'Souza W, Saranath D. High-Risk Human Papillomavirus in Oral Cancer: Clinical Implications. Oncology. 2018;94(3):133–41. Epub 2017/12/15. 10.1159/000485322 . [DOI] [PubMed] [Google Scholar]
- 18.Bertolus C, Goudot P, Gessain A, Berthet N. Clinical relevance of systematic human papillomavirus (HPV) diagnosis in oral squamous cell carcinoma. Infectious agents and cancer. 2012;7(1):13 Epub 2012/06/01. 10.1186/1750-9378-7-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isayeva T, Li Y, Maswahu D, Brandwein-Gensler M. Human papillomavirus in non-oropharyngeal head and neck cancers: a systematic literature review. Head and neck pathology. 2012;6 Suppl 1:S104–20. Epub 2012/07/13. 10.1007/s12105-012-0368-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lingen MW, Xiao W, Schmitt A, Jiang B, Pickard R, Kreinbrink P, et al. Low etiologic fraction for high-risk human papillomavirus in oral cavity squamous cell carcinomas. Oral oncology. 2013;49(1):1–8. Epub 2012/07/31. 10.1016/j.oraloncology.2012.07.002 . [DOI] [PubMed] [Google Scholar]
- 21.Miller CS, Johnstone BM. Human papillomavirus as a risk factor for oral squamous cell carcinoma: a meta-analysis, 1982–1997. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2001;91(6):622–35. Epub 2001/06/13. 10.1067/moe.2001.115392 . [DOI] [PubMed] [Google Scholar]
- 22.Syrjanen S, Lodi G, von Bultzingslowen I, Aliko A, Arduino P, Campisi G, et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: a systematic review. Oral diseases. 2011;17 Suppl 1:58–72. Epub 2011/03/16. 10.1111/j.1601-0825.2011.01792.x . [DOI] [PubMed] [Google Scholar]
- 23.Chung CH, Bagheri A, D'Souza G. Epidemiology of oral human papillomavirus infection. Oral oncology. 2014;50(5):364–9. Epub 2013/10/02. 10.1016/j.oraloncology.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta V, Yu GP, Schantz SP. Population-based analysis of oral and oropharyngeal carcinoma: changing trends of histopathologic differentiation, survival and patient demographics. The Laryngoscope. 2010;120(11):2203–12. Epub 2010/10/13. 10.1002/lary.21129 . [DOI] [PubMed] [Google Scholar]
- 25.Blomberg M, Nielsen A, Munk C, Kjaer SK. Trends in head and neck cancer incidence in Denmark, 1978–2007: focus on human papillomavirus associated sites. International journal of cancer. 2011;129(3):733–41. Epub 2010/09/30. 10.1002/ijc.25699 . [DOI] [PubMed] [Google Scholar]
- 26.Grulich AE, Jin F, Conway EL, Stein AN, Hocking J. Cancers attributable to human papillomavirus infection. Sexual health. 2010;7(3):244–52. Epub 2010/08/20. 10.1071/SH10020 . [DOI] [PubMed] [Google Scholar]
- 27.Ryerson AB, Peters ES, Coughlin SS, Chen VW, Gillison ML, Reichman ME, et al. Burden of potentially human papillomavirus-associated cancers of the oropharynx and oral cavity in the US, 1998–2003. Cancer. 2008;113(10 Suppl):2901–9. Epub 2008/11/05. 10.1002/cncr.23745 . [DOI] [PubMed] [Google Scholar]
- 28.Laco J, Vosmikova H, Novakova V, Celakovsky P, Dolezalova H, Tucek L, et al. The role of high-risk human papillomavirus infection in oral and oropharyngeal squamous cell carcinoma in non-smoking and non-drinking patients: a clinicopathological and molecular study of 46 cases. Virchows Archiv: an international journal of pathology. 2011;458(2):179–87. Epub 2011/01/12. 10.1007/s00428-010-1037-y . [DOI] [PubMed] [Google Scholar]
- 29.Li R, Faden DL, Fakhry C, Langelier C, Jiao Y, Wang Y, et al. Clinical, genomic, and metagenomic characterization of oral tongue squamous cell carcinoma in patients who do not smoke. Head & neck. 2015;37(11):1642–9. Epub 2014/06/24. 10.1002/hed.23807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berkhout RJ, Tieben LM, Smits HL, Bavinck JN, Vermeer BJ, ter Schegget J. Nested PCR approach for detection and typing of epidermodysplasia verruciformis-associated human papillomavirus types in cutaneous cancers from renal transplant recipients. Journal of clinical microbiology. 1995;33(3):690–5. Epub 1995/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snijders PJ, van den Brule AJ, Schrijnemakers HF, Snow G, Meijer CJ, Walboomers JM. The use of general primers in the polymerase chain reaction permits the detection of a broad spectrum of human papillomavirus genotypes. The Journal of general virology. 1990;71 (Pt 1):173–81. Epub 1990/01/01. 10.1099/0022-1317-71-1-173 . [DOI] [PubMed] [Google Scholar]
- 32.Cheval J, Sauvage V, Frangeul L, Dacheux L, Guigon G, Dumey N, et al. Evaluation of high-throughput sequencing for identifying known and unknown viruses in biological samples. Journal of clinical microbiology. 2011;49(9):3268–75. Epub 2011/07/01. 10.1128/JCM.00850-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bigot T, Temmam S, Pérot P, Eloit M. RVDB-prot, a reference viral protein database and its HMM profiles. F1000Research. 2019;8:530 10.12688/f1000research.18776.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim D, Song L, Breitwieser FP, Salzberg SL. Centrifuge: rapid and sensitive classification of metagenomic sequences. Genome research. 2016;26(12):1721–9. Epub 2016/11/18. 10.1101/gr.210641.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Truong DT, Franzosa EA, Tickle TL, Scholz M, Weingart G, Pasolli E, et al. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nature methods. 2015;12(10):902–3. Epub 2015/09/30. 10.1038/nmeth.3589 . [DOI] [PubMed] [Google Scholar]
- 36.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics (Oxford, England). 2012;28(12):1647–9. Epub 2012/05/01. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodman RP, Freret TS, Kula T, Geller AM, Talkington MW, Tang-Fernandez V, et al. Clinical isolates of Trichomonas vaginalis concurrently infected by strains of up to four Trichomonasvirus species (Family Totiviridae). Journal of virology. 2011;85(9):4258–70. Epub 2011/02/25. 10.1128/JVI.00220-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vatanshenassan M, Rezaie S, Mohebali M, Niromand N, Kazemi B, Babaei Z, et al. Trichomonas vaginalis: investigation of a novel diagnostic method in urine samples using cysteine proteinase 4 gene and PCR technique. Experimental parasitology. 2010;126(2):187–90. Epub 2010/05/04. 10.1016/j.exppara.2010.04.021 . [DOI] [PubMed] [Google Scholar]
- 39.Beachler DC, D'Souza G. Oral human papillomavirus infection and head and neck cancers in HIV-infected individuals. Current opinion in oncology. 2013;25(5):503–10. Epub 2013/07/16. 10.1097/CCO.0b013e32836242b4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D'Souza G, Agrawal Y, Halpern J, Bodison S, Gillison ML. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. The Journal of infectious diseases. 2009;199(9):1263–9. Epub 2009/03/27. 10.1086/597755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kouvousi M, Xesfyngi D, Tsimplaki E, Argyri E, Ioannidou G, Ploxorou M, et al. Prevalence of human papillomavirus in 45 greek patients with oral cancer. Journal of oncology. 2013;2013:756510 Epub 2013/03/28. 10.1155/2013/756510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reuschenbach M, Kansy K, Garbe K, Vinokurova S, Flechtenmacher C, Toth C, et al. Lack of evidence of human papillomavirus-induced squamous cell carcinomas of the oral cavity in southern Germany. Oral oncology. 2013;49(9):937–42. Epub 2013/04/24. 10.1016/j.oraloncology.2013.03.451 . [DOI] [PubMed] [Google Scholar]
- 43.Forslund O, Johansson H, Madsen KG, Kofoed K. The nasal mucosa contains a large spectrum of human papillomavirus types from the Betapapillomavirus and Gammapapillomavirus genera. The Journal of infectious diseases. 2013;208(8):1335–41. Epub 2013/07/24. 10.1093/infdis/jit326 . [DOI] [PubMed] [Google Scholar]
- 44.Weissenborn SJ, De Koning MN, Wieland U, Quint WG, Pfister HJ. Intrafamilial transmission and family-specific spectra of cutaneous betapapillomaviruses. Journal of virology. 2009;83(2):811–6. Epub 2008/11/07. 10.1128/JVI.01338-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hampras SS, Rollison DE, Giuliano AR, McKay-Chopin S, Minoni L, Sereday K, et al. Prevalence and Concordance of Cutaneous Beta Human Papillomavirus Infection at Mucosal and Cutaneous Sites. The Journal of infectious diseases. 2017;216(1):92–6. Epub 2017/05/27. 10.1093/infdis/jix245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cardoso JC, Calonje E. Cutaneous manifestations of human papillomaviruses: a review. Acta dermatovenerologica Alpina, Pannonica, et Adriatica. 2011;20(3):145–54. Epub 2011/12/02. . [PubMed] [Google Scholar]
- 47.Nunes EM, Sudenga SL, Gheit T, Tommasino M, Baggio ML, Ferreira S, et al. Diversity of beta-papillomavirus at anogenital and oral anatomic sites of men: The HIM Study. Virology. 2016;495:33–41. Epub 2016/05/11. 10.1016/j.virol.2016.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sichero L, El-Zein M, Nunes EM, Ferreira S, Franco EL, Villa LL. Cervical Infection with Cutaneous Beta and Mucosal Alpha Papillomaviruses. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2017;26(8):1312–20. Epub 2017/04/06. 10.1158/1055-9965.epi-17-0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jablonska S, Dabrowski J, Jakubowicz K. Epidermodysplasia verruciformis as a model in studies on the role of papovaviruses in oncogenesis. Cancer research. 1972;32(3):583–9. Epub 1972/03/01. . [PubMed] [Google Scholar]
- 50.Pfister H. Chapter 8: Human papillomavirus and skin cancer. Journal of the National Cancer Institute Monographs. 2003;(31):52–6. Epub 2003/06/17. 10.1093/oxfordjournals.jncimonographs.a003483 . [DOI] [PubMed] [Google Scholar]
- 51.Weissenborn SJ, Nindl I, Purdie K, Harwood C, Proby C, Breuer J, et al. Human papillomavirus-DNA loads in actinic keratoses exceed those in non-melanoma skin cancers. The Journal of investigative dermatology. 2005;125(1):93–7. Epub 2005/06/29. 10.1111/j.0022-202X.2005.23733.x . [DOI] [PubMed] [Google Scholar]
- 52.Arron ST, Ruby JG, Dybbro E, Ganem D, Derisi JL. Transcriptome sequencing demonstrates that human papillomavirus is not active in cutaneous squamous cell carcinoma. The Journal of investigative dermatology. 2011;131(8):1745–53. Epub 2011/04/15. 10.1038/jid.2011.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McElhinny AS, Li JL, Wu L. Mastermind-like transcriptional co-activators: emerging roles in regulating cross talk among multiple signaling pathways. Oncogene. 2008;27(38):5138–47. Epub 2008/09/02. 10.1038/onc.2008.228 . [DOI] [PubMed] [Google Scholar]
- 54.White EA, Kramer RE, Tan MJ, Hayes SD, Harper JW, Howley PM. Comprehensive analysis of host cellular interactions with human papillomavirus E6 proteins identifies new E6 binding partners and reflects viral diversity. Journal of virology. 2012;86(24):13174–86. Epub 2012/09/28. 10.1128/JVI.02172-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu L, Aster JC, Blacklow SC, Lake R, Artavanis-Tsakonas S, Griffin JD. MAML1, a human homologue of Drosophila mastermind, is a transcriptional co-activator for NOTCH receptors. Nature genetics. 2000;26(4):484–9. Epub 2000/12/02. 10.1038/82644 . [DOI] [PubMed] [Google Scholar]
- 56.Chahoud J, Semaan A, Chen Y, Cao M, Rieber AG, Rady P, et al. Association Between beta-Genus Human Papillomavirus and Cutaneous Squamous Cell Carcinoma in Immunocompetent Individuals-A Meta-analysis. JAMA dermatology. 2016;152(12):1354–64. Epub 2016/01/01. 10.1001/jamadermatol.2015.4530 . [DOI] [PubMed] [Google Scholar]
- 57.Antonsson A, Green AC, Mallitt KA, O'Rourke PK, Pandeya N, Pawlita M, et al. Prevalence and stability of antibodies to 37 human papillomavirus types—a population-based longitudinal study. Virology. 2010;407(1):26–32. Epub 2010/08/21. 10.1016/j.virol.2010.07.046 . [DOI] [PubMed] [Google Scholar]
- 58.Michael KM, Waterboer T, Sehr P, Rother A, Reidel U, Boeing H, et al. Seroprevalence of 34 human papillomavirus types in the German general population. PLoS pathogens. 2008;4(6):e1000091 Epub 2008/06/21. 10.1371/journal.ppat.1000091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karagas MR, Nelson HH, Sehr P, Waterboer T, Stukel TA, Andrew A, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. Journal of the National Cancer Institute. 2006;98(6):389–95. Epub 2006/03/16. 10.1093/jnci/djj092 . [DOI] [PubMed] [Google Scholar]
- 60.Waterboer T, Abeni D, Sampogna F, Rother A, Masini C, Sehr P, et al. Serological association of beta and gamma human papillomaviruses with squamous cell carcinoma of the skin. The British journal of dermatology. 2008;159(2):457–9. Epub 2008/05/28. 10.1111/j.1365-2133.2008.08621.x . [DOI] [PubMed] [Google Scholar]
- 61.Agalliu I, Gapstur S, Chen Z, Wang T, Anderson RL, Teras L, et al. Associations of Oral alpha-, beta-, and gamma-Human Papillomavirus Types With Risk of Incident Head and Neck Cancer. JAMA oncology. 2016. Epub 2016/01/23. 10.1001/jamaoncol.2015.5504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnston VJ, Mabey DC. Global epidemiology and control of Trichomonas vaginalis. Current opinion in infectious diseases. 2008;21(1):56–64. Epub 2008/01/15. 10.1097/QCO.0b013e3282f3d999 . [DOI] [PubMed] [Google Scholar]
- 63.Donders GG, Depuydt CE, Bogers JP, Vereecken AJ. Association of Trichomonas vaginalis and cytological abnormalities of the cervix in low risk women. PloS one. 2013;8(12):e86266 Epub 2014/01/05. 10.1371/journal.pone.0086266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sutcliffe S, Giovannucci E, Alderete JF, Chang TH, Gaydos CA, Zenilman JM, et al. Plasma antibodies against Trichomonas vaginalis and subsequent risk of prostate cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15(5):939–45. Epub 2006/05/17. 10.1158/1055-9965.epi-05-0781 . [DOI] [PubMed] [Google Scholar]
- 65.Azevedo A, Cunha V, Teixeira AL, Medeiros R. IL-6/IL-6R as a potential key signaling pathway in prostate cancer development. World journal of clinical oncology. 2011;2(12):384–96. Epub 2011/12/16. 10.5306/wjco.v2.i12.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fichorova RN, Trifonova RT, Gilbert RO, Costello CE, Hayes GR, Lucas JJ, et al. Trichomonas vaginalis lipophosphoglycan triggers a selective upregulation of cytokines by human female reproductive tract epithelial cells. Infection and immunity. 2006;74(10):5773–9. Epub 2006/09/22. 10.1128/IAI.00631-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garcia AF, Benchimol M, Alderete JF. Trichomonas vaginalis polyamine metabolism is linked to host cell adherence and cytotoxicity. Infection and immunity. 2005;73(5):2602–10. Epub 2005/04/23. 10.1128/IAI.73.5.2602-2610.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kucknoor AS, Mundodi V, Alderete JF. The proteins secreted by Trichomonas vaginalis and vaginal epithelial cell response to secreted and episomally expressed AP65. Cellular microbiology. 2007;9(11):2586–97. Epub 2007/06/26. 10.1111/j.1462-5822.2007.00979.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stark JR, Judson G, Alderete JF, Mundodi V, Kucknoor AS, Giovannucci EL, et al. Prospective study of Trichomonas vaginalis infection and prostate cancer incidence and mortality: Physicians' Health Study. Journal of the National Cancer Institute. 2009;101(20):1406–11. Epub 2009/09/11. 10.1093/jnci/djp306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tesfaye A, Di Cello F, Hillion J, Ronnett BM, Elbahloul O, Ashfaq R, et al. The high-mobility group A1 gene up-regulates cyclooxygenase 2 expression in uterine tumorigenesis. Cancer research. 2007;67(9):3998–4004. Epub 2007/05/08. 10.1158/0008-5472.CAN-05-1684 . [DOI] [PubMed] [Google Scholar]
- 71.Twu O, Dessi D, Vu A, Mercer F, Stevens GC, de Miguel N, et al. Trichomonas vaginalis homolog of macrophage migration inhibitory factor induces prostate cell growth, invasiveness, and inflammatory responses. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(22):8179–84. Epub 2014/05/21. 10.1073/pnas.1321884111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Figuero Ruiz E, Carretero Pelaez MA, Cerero Lapiedra R, Esparza Gomez G, Moreno Lopez LA. Effects of the consumption of alcohol in the oral cavity: relationship with oral cancer. Medicina oral: organo oficial de la Sociedad Espanola de Medicina Oral y de la Academia Iberoamericana de Patologia y Medicina Bucal. 2004;9(1):14–23. Epub 2004/01/06. . [PubMed] [Google Scholar]
- 73.Homann N, Jousimies-Somer H, Jokelainen K, Heine R, Salaspuro M. High acetaldehyde levels in saliva after ethanol consumption: methodological aspects and pathogenetic implications. Carcinogenesis. 1997;18(9):1739–43. Epub 1997/11/05. 10.1093/carcin/18.9.1739 . [DOI] [PubMed] [Google Scholar]
- 74.Seitz HK. Alcohol and retinoid metabolism. Gut. 2000;47(6):748–50. Epub 2000/11/15. 10.1136/gut.47.6.748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lopes V, Murray P, Williams H, Woodman C, Watkinson J, Robinson M. Squamous cell carcinoma of the oral cavity rarely harbours oncogenic human papillomavirus. Oral oncology. 2011;47(8):698–701. Epub 2011/06/22. 10.1016/j.oraloncology.2011.04.022 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.