Abstract
Uterine transplantation (UTx) associated with IVF restores fertility in women affected by absolute uterine factor infertility (AUFI). Pregnancies achieved both in women undergoing any solid organ transplantation and following IVF are associated with an increased risk of maternal and neonatal complications. This systematic review evaluated this risk in UTx-IVF treated women focusing on the safety and efficacy features of the treatment. Twenty-two studies and three press releases reporting on 52 UTx-IVF treatments were identified. Regarding the safety of treatment, 38/52 (73,1%) of surgical procedures led to the restoration of uterine function in recipients, 12/52 (23,1%) of recipients experienced post-operative complications requiring hysterectomy, and 2/52 (3,8%) of procedures failed before uterine recipients’ surgery due to intra-operative complications. Regarding the efficacy of treatment, results focused on transplanted patients showing full recovery of organ functioning: 16/38 (42,1%) of patients achieved a pregnancy, including two women who gave births twice. UTx-IVF pregnancies led to 16 deliveries and all new-borns were healthy. Six out of 16 (37,5%) UTx pregnancies faced major complications during gestation. Preterm births occurred in 10/16 (62,5%) UTx deliveries. Our data indicates that the risk of gestational and delivery complications deserves important consideration in AUFI women receiving UTx-IVF treatments. However, these observations are preliminary and need to be revised after larger series of data are published.
Introduction
More than 150,000 women of reproductive age in Europe [1], and approximately 1.5 million women worldwide [2] are affected by absolute uterine factor infertility (AUFI) [3,4]. There are many aetiologies of AUFI, which can be categorized into either congenital or acquired causes that preclude the implantation of an embryo or completion of a pregnancy [5]. AUFI may be due to the anatomical absence of the uterus following hysterectomy or to the Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome [1,6]. Alternatively, AUFI may also manifest as uterine dysfunctions caused by radiotherapy, leiomyoma, the Asherman’s syndrome, or congenital uterine malformations occurred as a consequence of disturbances during the formation, of foetal life or in the development or fusion of the Müllerian ducts [1,6]. Adoption and gestational surrogacy have long been the only two options available for women with AUFI who wish to pursue motherhood, and important legal, ethical and social implications define the feasibility of one option or the other [7,8].
Since 2014, the revolutionary procedure of uterus allotransplantation (UTx) in humans [9,10] has allowed women with AUFI to partially overcome such complications, introducing the possibility of giving birth to a genetically related child [11]. To date, UTx represents another quality-of-life-type improvement in transplantation, and a milestone in the gynaecological field. Furthermore, it has endorsed the concept of reproductive surgery that was previously introduced with the strategy of ovarian tissue transplantation [12]. In fact, both transplantation procedures are performed with the intent of restoring fertility in non-life-saving situations, rather than improving the chance of survival in life-saving situations [13,14].
UTx always requires performing IVF from 6 to 18 months before surgery, due to the conditioning regimen for immunosuppressive therapy, and in order to ascertain fertility between AUFI couples, and finally to reduce the risks concerning bleeding and pelvic infections associated with oocyte retrieval. Specifically, the risk of bleeding is related to the presence of abnormal uterine vascular pedicles and anastomosis sites caused by UTx surgery, and the risk of pelvic infections may constitute a complication of surgical retrieval operations [8,9]. It follows that UTx exposes AUFI women to the risk of complications related to solid organ transplantation (SOT) and/or in vitro fertilization (IVF) treatments, despite the fact that the latter are common and well-documented procedures.
The evaluation of IVF complications is crucial, as the primary endpoint of treatment is the delivery of healthy babies from healthy mothers. From this perspective, the clinical use of the UTx-IVF procedure deserves careful consideration, due to the increased risk of obstetric and neonatal complications related to the IVF procedure itself [15–17]. This is of particular relevance to UTx counselling, as the UTx-IVF procedure is applied with an intention-to-treat care, defined by organ function restoration and the delivery of a healthy offspring.
Despite promising initial achievements [18], the UTx-IVF procedure is still at an early clinical experimental stage, and it faces multiple ongoing challenges. The aim of this systematic review was to provide preliminary data on 1) the UTx-IVF safety related to post-operative, maternal and neonatal complications to which the uterus recipients and the intended children may be potentially exposed to, and on 2) the UTx-IVF efficacy, defined as the number of UTx pregnancies and live births actually available for consultation.
Materials and methods
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Individual Participant Data (PRISMA-IPD) guidelines [19]. The review protocol was recorded in PROSPERO [20]; the registry number available for consultation is CRD42016042298 and a copy of the protocol is provided as supporting information (S1 Text). A literature search was performed systematically in order to identify studies involving female patients who underwent UTx to treat AUFI infertility, regardless of whether AUFI was congenital or acquired.
We searched the PubMed, Institute of Biology of the Southern Seas (IBSS), SocINDEX, Institute for Scientific Information, Web of Science, and Google Scholar databases, up to the end of September 2019 by using the following terms alone or in combination: “uterine”, “uterus”, “womb”, “transplant”, “transplantation” and “absolute uterine factor infertility”. A second search was also conducted on common search engines, such as Google, Yahoo, and Mozilla Firefox for the aforementioned terms, in order to identify further cases that were unpublished in scientific reference databases.
All the articles identified by the comprehensive electronic search were examined for the relevance of both title and abstract without any language restriction. Subsequently, articles were screened by full-text availability in English and by content in order to select those eligible for the final analysis. Studies reporting individual data were first considered and used. Additional data on UTx or relevant information from unpublished cases were retrieved by consulting several scientific contributions, such as reviews, original articles and press releases.
In examining the full texts, the following data were extracted to address the primary endpoint of UTx safety: numbers of transplant attempts and the related clinical trial (if registered), country of UTx surgery, donor type, strategy of organ retrieval, graft outcome, graft complications, and the time of graft explantation. To address the secondary outcome of UTx efficacy, the following data were extracted: trial or case number, number of pregnancy attempts by frozen embryo replacement cycles, outcome of the attempt, gestational week at delivery, and obstetric and neonatal complications. The bibliographic search, identification and selection of articles to be included in the final analysis were independently performed by two authors (JD and SPag). Data were independently extrapolated and tabulated by the same authors, and definitively reviewed by a third author (SPal). Any disagreements between operators were assessed, until a consensus among authors was reached.
Initially, the study protocol (PROSPERO ID: CRD42016042298) intended to consider a qualitative and quantitative analysis [19,21]. In addition, a final analysis according to the Oxford Centre for Evidence-based Medicine (OCEM)-Levels of Evidence 2011 guidelines [22] and to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system [23] was initially considered.
Results
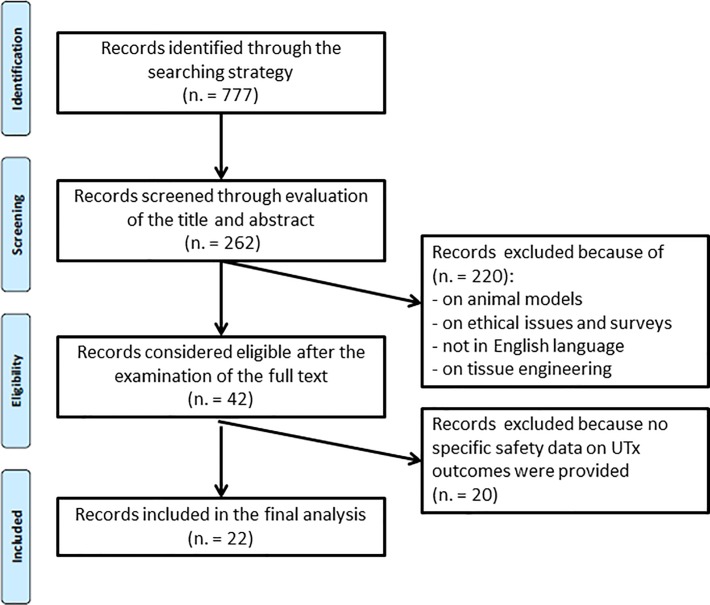
The PRISMA flow diagram (Fig 1) illustrates the total number of studies initially considered, included and excluded in this systematic review. The completed PRISMA-IPD checklist is provided as supporting information (S1 Table).
Fig 1. The PRISMA flow diagram illustrates the total number of studies initially considered, included and excluded in this systematic review.
From a set of 777 studies initially identified through the search strategy, 262 studies were screened throughout the evaluation of title and abstract. After that, 220 studies on animal models, ethical issues, surveys and attitudes, tissue engineering, and in languages other than English were further excluded, including studies providing previously published data without additional and/or new information.
Forty-two studies were then considered eligible for the full-text examination. The final selection included 22 studies: 8 case reports, 6 case series (at least two patients described), 2 articles on the follow-up outcomes of one case report, and 6 reviews/original articles, together with 3 press releases. The final set of studies reporting outcomes on UTx safety and efficacy are cited in Table 1.
Table 1. UTx-IVF safety and efficacy outcomes.
| Reference (n. of registration) | Transplant n. | Country | N. of patients undergoing UTx | Donor type | Donor surgery | Graft outcome | Hysterectomy | Graft complications leading to hysterectomy | Reference of pregnancy | Pregnancy attempts§ | Maternal Complications | Live Birth | Gestational week at birth | Neonatal Complications | Note |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fageeh et al., 2002 [26] | 1 | Saudi Arabia | 1 | LD | LAP | UR | POD99 | Thrombosis of bilateral uterine vessels | / | ||||||
| Okzan et al., 2013 [27] | 2 | Turkey | 1 | DD | LAP | US | / | None | Okzan et al., 2016 [28] | 4 | NA | 0 | / | / | / |
| Brännström et al., 2014 [9] (Trial n. NCT01844362) | 3 | Sweden | 9 | LD | LAP | UR | POD 105 | Persistent intrauterine infection | / | ||||||
| 4 | Sweden | LD | LAP | UR | POD 3 | Thrombosis of the uterine arteries | / | ||||||||
| 5 | Sweden | LD | LAP | US | / | None | Brännström et al.,2015 [10] | 1 | Preeclampsia | 1 | 31+5 | Preterm birth | Maternal URA | ||
| 6 | Sweden | LD | LAP | US | / | None | Brännström et al.,2016 [29] | 1 | Intrahepatic cholestasis | 1 | 34+4 | Preterm birthMild respiratory distress | / | ||
| 7 | Sweden | LD | LAP | US | / | None | Castellόn et al., 2017 [30] | 1 | Preeclampsia Cholestasis PPROM | 1 | 34+5 | Preterm birth | Maternal URA | ||
| 8 | Sweden | LD | LAP | US | / | None | Castellόn et al., 2017 [30] | 2* | Preeclampsia | 2* | 35+3 | Preterm birth | Maternal URA *Delivered a 2nd child | ||
| 9 | Sweden | LD | LAP | US | / | None | Castellόn et al., 2017 [30] | 2* | NR | 2* | 35 | Preterm birth | *Delivered a 2nd child | ||
| 10 | Sweden | LD | LAP | US | / | None | Akouri, 2017 [31] | 1 | NR | 1 | 37 | Preterm birth | / | ||
| 11 | Sweden | LD | LAP | US | / | None | Jones et al., 2019 [32] | 5 | NA | 0 | / | / | / | ||
| Wei et al., 2017 [33] (Trial n. XJZT12Z06) | 12 | China | 2 | LD | RAL | US | / | None | www.scmp.com/news/china/society/article/2183441/chinas-first-womb-transplant-recipient-gives-birth-healthy-baby [34] | 1 | NA | 1 | 34 | Preterm birth | NA |
| 13 | China | LD | RAL | US | / | None | www.scmp.com/news/china/society/article/2183441/chinas-first-womb-transplant-recipient-gives-birth-healthy-baby [34] | 1 | NA | NA | NA | NA | NA | ||
| Flyckt et al., 2017 [35] (Trial n.NCT02573415) | 14 | Cleveland, US | 1 | DD | LAP | UR | POD 12 | Vascular candidal infection | / | ||||||
| Ejzenberg et al., 2019 [36] (National approval n. SNT; 1140/2016) | 15 | Brazil | 1 | DD | LAP | US | / | None | Ejzenberg et al., 2019 [36] | 1 | Pyelonephritis | 1 | 35+3 | Preterm birth | / |
| Testa et al., 2017 [37] (Trial n.NCT02656550) | 16 | Dallas, US | 5 | LD | LAP | UR | POD 6 | Arterial thrombisis | / | ||||||
| 17 | Dallas, US | LD | LAP | UR | POD 12 | Graft ischaemia | / | ||||||||
| 18 | Dallas, US | LD | LAP | UR | POD 14 | Arterial thrombisis | / | ||||||||
| 19 | Dallas, US | LD | LAP | US | / | None | Testa et al.,2018 [38] | 1 | Sub-chorionic haematoma | 1 | 33+1 | Preterm birth | / | ||
| 20 | Dallas, US | LD | LAP | US | / | None | Jones et al., 2019 [32] | 1 | None | 1 | 36+6 | Preterm birth | / | ||
| Personal Communication in Jones et al., 2019 [32] by Johannesson, 2019 | 21 | Dallas, US | 11 | DD | LAP | US | / | None | Personal Communication in Jones et al., 2019 [32] by Johannesson, 2019 | 1 | NR | 0 | NA | / | / |
| 22 | Dallas, US | LD | LAP | US | / | None | 1 | NR | 0 | NA | / | / | |||
| 23 | Dallas, US | DD | LAP | Aborted after donor surgery | Intra-operative | External iliac thrombosis | |||||||||
| 24 | Dallas, US | LD | LAP | US | / | None | 1 | NR | 0 | NA | / | / | |||
| 25 | Dallas, US | LD | LAP | UR | POD 1 | Graft ischemia | |||||||||
| 26 | Dallas, US | LD | LAP | US | / | None | 1 | NR | 0 | NA | / | / | |||
| 27 | Dallas, US | LD | LAP | US | / | None | 1 | NR | 0 | NA | / | / | |||
| 28 | Dallas, US | LD | LAP | US | / | None | 1 | NR | 0 | NA | / | ||||
| 29 | Dallas, US | LD | LAP | UR | POD 3 | Post-operative haemoorhage | |||||||||
| 30 | Dallas, US | LD | LAP | US | / | None | NR | / | 0 | NA | / | / | |||
| 31 | Dallas, US | LD | RAL | US | / | None | NR | / | 0 | NA | / | / | |||
| Chmel et al., 2018 [39] (Trial n.NCT03277430) | 32 | Czech Republic | 9 | LD | LAP | UR | POD 15 | Vascular thrombosis | Chmel et al., 2018 [39] (Trial n.NCT03277430) | ||||||
| 33 | Czech Republic | LD | LAP | US | / | None | 3 | NA | 0 | NA | NA | / | |||
| 34 | Czech Republic | LD | LAP | US | / | None | 3 | NA | 0 | NA | NA | / | |||
| 35 | Czech Republic | LD | LAP | US | / | None | NA | / | NA | / | / | / | |||
| 36 | Czech Republic | LD | LAP | US | / | None | NA | / | NA | / | / | / | |||
| 37 | Czech Republic | DD | LAP | UR | POM 7 | HSV-2 infection | |||||||||
| 38 | Czech Republic | DD | LAP | UR | POD 7 | Vascular thrombosis | |||||||||
| 39 | Czech Republic | DD | LAP | US | / | None | 4 | NA | 0 | NA | NA | / | |||
| 40 | Czech Republic | DD | LAP | US | / | None | 2 | NA | 0 | NA | NA | / | |||
| Brucker et al., 2018 [40] (Trial n. NCT03048396) | 41 | Germany | 3 | LD | LAP | Aborted after donor surgery | Intra-operative | Complications of vessels perfusion | |||||||
| 42 | Germany | LD | LAP | US | / | None | Jones et al., 2019 [32] | 1 | NA | 1 | NA | NA | / | ||
| 43 | Germany | LD | LAP | US | / | None | Jones et al., 2019 [32] | 1 | NA | 0 | NA | NA | / | ||
| Brännström et al., 2018 [24] | 44 | Serbia | 1 | LD | LAP | US | / | None | Brännström et al., 2019 [18] | 1 | NA | 1 | NA | NA | / |
| Puntambekar et al., 2018 [41] Puntambekar et al., 2018 [42] | 45 | India | 4 | LD | RAL | US | / | None | www.dailymail.co.uk/health/article-6393029/Indian-woman-gives-birth-Asias-uterus-transplanted-baby.html [43] | 1 | NR | 1 | NR | NR | / |
| 46 | India | LD | RAL | US | / | None | Jones et al., 2019 [32] | 1 | NR | 0 | NR | NR | / | ||
| 47 | India | LD | RAL | US | / | None | NR | / | 0 | / | / | / | |||
| 48 | India | LD | RAL | US | / | None | NR | / | 0 | / | / | / | |||
| Brännström et al., 2018 [44] Brännström, 2018 [25] (Trial n.02987023) | 49 | Sweden | 4 | LD | RAL | US | / | None | https://nypost.com/2019/04/09/baby-makes-history-afterworlds-first-womb-transplant-performed-by-robot. [45] | 1 | NA | 1 | NA | NA | / |
| 50 | Sweden | LD | RAL | US | / | None | NR | / | 0 | / | / | / | |||
| 51 | Sweden | LD | RAL | US | / | None | NR | / | 0 | / | / | / | |||
| 52 | Sweden | LD | RAL | US | / | None | NR | / | 0 | / | / | / | |||
| TOTAL | 52 | 9 DD 43 LD | 41 LAP 11 RAL | 2 Aborted 12 UR 38 US | 46 | 6 events | 16 births | 10 events |
DD = Deceased donor; LAP = Laparotomy; LD = Living donor; NA = not available; NR = Not reported; POD = post operative day; POM = post operative month; RAL = Robotic Assisted Laparoscopy; UR = uterine removal; URA = unilateral maternal agenesis; US = uterine survival.
§ each attempt is intended as a frozen embryo replacement cycle followed by a single embryo transfer event
Due to the heterogeneity of the available data, mainly limited to case reports, case series or original articles presenting aggregate data, this systematic review reported only a qualitative analysis, as detailed below.
UTx safety outcomes
The technical aspects of UTx surgical approaches in donors and recipients have been described in detail elsewhere [18,24,25]. As shown in Table 1, 41/52 (79%) of UTx procurements were performed by laparotomy retrieval surgery and 11/52 (21%) by partial or complete robotic-assisted laparoscopy. In all reported cases, the uterine placement in recipients was performed in an orthotopic position.
Up to the time of writing, 52 UTx surgical procedures had been carried out in different countries (Table 1). Only 34 UTx procedures were from clinical trials for which a registration number was available for consultation. Forty-three out of 52 uterine procurements were from live donors (LDs) and 9/52 were from deceased donors (DDs).
Positive outcomes, id est graft stability and functionality after surgery, were achieved in 33/43 (77%) of LD procedures and in 5/9 (55,5%) of DD procedures, whereas negative outcomes were reported in 10/43 (23,1%) of LD procedures and 4/9 (44%) of DD procedures. Finally, 12/52 (23%) of UTx recipients underwent hysterectomy post-operatively because of vascular complications such as uterine infarction, thrombosis following complications of graft inflow and outflow, or yeast, bacterial or viral infections. Two out of 52 (4%) of UTx procedures aborted before uterine implantation in the recipient, due to unsuccessful organ and vessel perfusion after donor surgery. The incidence of such complications was higher in the DD UTx recipients compared to the LD UTx recipients [4/9 (44%) vs. 10/43 (23%), respectively].
UTx efficacy outcomes
All of UTx recipients underwent at least one IVF cycle, and had their oocytes retrieved and fertilized prior to uterus transplant (Table 2). Different pools of fertilized oocytes and embryos at cleavage or blastocyst stage were frozen, according to the local cryopreservation practice of each IVF Center. To date, at least 46 UTx pregnancy attempts have been performed involving 29 uterine recipients (Table 1): each attempt is intended as a frozen embryo replacement cycle followed by a single embryo transfer (SET) event. SET events were performed 6–18 months after UTx surgery, once assessed the restoration of uterine function in recipients.
Table 2. Outcomes of IVF cycles.
| Reference (n. of registration) | Number of IVF cycles before UTx surgery | Retrieved oocytes | Number of cryopreserved cells | Cryopreserved cells | Time to first embryo transfer | Note |
|---|---|---|---|---|---|---|
| Fageeh et al., 2002 [26] | NR | NR | NR | NR | / | |
| Okzan et al., 2013 [27] | 3 | At least 15 | 16 | Embryos at cleavage stage | 18 months | |
| Brännström et al., 2014 [9] (Trial n. NCT01844362) | 2–3 | NR | NR | NR | / | |
| 2–3 | NR | NR | NR | / | ||
| 3 | 18 | 11 | Embryos at cleavage stage | 12 months | ||
| 2 | 15 | 6 | Embryos at blastocyst stage | 12 months | ||
| 4 | Embryos at cleavage stage | |||||
| 2–3 | NR | NR | NR | NR | ||
| 2–3 | NR | NR | NR | NR | ||
| 2–3 | NR | NR | NR | NR | ||
| 2–3 | NR | NR | NR | NR | ||
| 2–3 | NR | NR | NR | NR | ||
| Wei et al., 2017 [33] (Trial n. XJZT12Z06) | 2 | 22 | 14 | NR | 12 months | |
| NR | NR | NR | NR | NR | ||
| Flyckt et al., 2017 [35] (Trial n.NCT02573415) | NR | NR | NR | NR | / | |
| Ejzenberg et al., 2019 [36] (National approval n. SNT; 1140/2016) | 1 | 16 | 8 | Embryos at blastocyst stage | 7 months | |
| Testa et al., 2017 [37] (Trial n.NCT02656550) | 1 | 8* | 6^ | Embryos at blastocyst stage | / | *retrieved embryos ^Euploid (PGS tested) |
| 2 | 8* | 4^ | Embryos at blastocyst stage | / | *retrieved embryos ^Euploid (PGS tested) | |
| 2 | 7* | 4^ | Embryos at blastocyst stage | / | *retrieved embryos ^Euploid (PGS tested) | |
| 1 | 8* | 5^ | Embryos at blastocyst stage | 6 months | *retrieved embryos ^Euploid (PGS tested) | |
| 1 | 7* | 5^ | Embryos at blastocyst stage | NR | *retrieved embryos ^Euploid (PGS tested) | |
| Personal Communication in Jones et al., 2019 [32] by Johannesson, 2019 | NR | NR | NR | NR | NR | |
| NR | NR | NR | NR | NR | ||
| NR | NR | NR | NR | / | ||
| NR | NR | NR | NR | NR | ||
| NR | NR | NR | NR | / | ||
| NR | NR | NR | NR | NR | ||
| NR | NR | NR | NR | NR | ||
| NR | NR | NR | NR | NR | ||
| NR | NR | NR | NR | / | ||
| NR | NR | NR | NR | NR | ||
| NR | NR | NR | NR | NR | ||
| Chmel et al., 2018 [39] (Trial n.NCT03277430) | 2 | 23* | 10^ | Embryos at blastocyst stage | / | * fertilized oocytes ^Euploid |
| 1 | 25* | 16^ | Embryos at blastocyst stage | 12–14 months | * fertilized oocytes ^Euploid | |
| 2 | 34* | 12^ | Embryos at blastocyst stage | 12–14 months | * fertilized oocytes ^Euploid | |
| 3 | 24* | 11^ | Embryos at blastocyst stage | NR | * fertilized oocytes ^Euploid | |
| 2 | 22* | 18^ | Embryos at blastocyst stage | NR | * fertilized oocytes ^Euploid | |
| 3 | 22* | 10^ | Embryos at blastocyst stage | / | * fertilized oocytes ^Euploid | |
| 2 | 19* | 11^ | Embryos at blastocyst stage | / | * fertilized oocytes ^Euploid | |
| 1 | 26* | 13^ | Embryos at blastocyst stage | 12–14 months | * fertilized oocytes ^Euploid | |
| 1 | 21* | 12^ | Embryos at blastocyst stage | 12–14 months | * fertilized oocytes ^Euploid | |
| Brucker et al., 2018 [40] (Trial n. NCT03048396) | 2 | 11 | 6 | fertilized oocytes | / | |
| 1 | 18 | 10 | fertilized oocytes | 12 months | ||
| 2 | 9 | 9 | fertilized oocytes | 12 months | ||
| Brännström et al., 2018 [24] | NR | NR | NR | NR | NR | |
| Puntambekar et al., 2018 [41] Puntambekar et al., 2018 [42] | 1 | 7 | 4 | Embryos at cleavage stage | NR | |
| 1 | 11 | 8 | Embryos at cleavage stage | NR | ||
| NR | NR | NR | NR | NR | ||
| NR | NR | NR | NR | NR | ||
| Brännström et al., 2018 [44] Brännström, 2018 [25] (Trial n.02987023) | NR | NR | NR | NR | NR | |
| NR | NR | NR | NR | NR | ||
| NR | NR | NR | NR | NR | ||
| NR | NR | NR | NR | NR |
NA = not applicable; NR = Not reported; PGS = Pre-implantation Genetic Screening; URA = unilateral maternal agenesis.
Based on the total number of patients undergoing UTx surgery, 14/52 (27%) of uterine recipients achieved at least one pregnancy. Based on the total number of patients undergoing UTx surgery followed by the restoration of uterine functioning, the ratio switches from 14/52 to 14/38 (37%) of uterine recipients achieving at least one pregnancy.
Considering the total number of patients undergoing a frozen embryo replacement cycle followed by a SET event for which outcomes were available, the UTx pregnancy rate per embryo transfer was 35,5%, corresponding to 16 clinical pregnancies divided by 45 SET.
All pregnant women delivered healthy babies, including two mothers who gave birth twice and accounting for a total of 16/52 (30,8%) and 16/38 (42,1%) of UTx pregnancies and babies. Maternal complications occurred in 6/16 (37,5%) of UTx-IVF pregnancies. Pre-eclampsia was the most faced gestational complication, as it was reported in 3/16 (19%) of UTx-IVF pregnancies. All deliveries were carried out by elective caesarean sections between gestational weeks 31 and 37. Accordingly, preterm birth was reported in 10/16 (62,5%) deliveries, constituting the most recurrent neonatal complication.
Recovery requiring intensive care services was not reported in either UTx mothers or babies.
Discussion
In the last decade, the UTx-IVF procedure has successfully resulted in several live births, and surgical techniques have been improved for organs of both deceased and living donors. However, the UTx procedure still carries multiple challenges even in the most expert hands. In this systematic review, we collected data on 52 UTx-IVF procedures and 16 live births from an exhaustive bibliographic search conducted by consulting scientific sources and browsing the most common search engines.
The number of studies on UTx is constantly growing, but we identified only a limited set of 22 studies providing data on UTx-IVF cases. By the time of writing, these studies allowed us to objectively make the following estimations: the safety of the UTx-IVF procedure, defined as the number of functioning grafts divided by the number of treated patients, is 73,1% (38/52); the efficacy of the UTx-IVF procedure, defined as the number of live births divided by the number of successfully treated patients, is 42,1% (16/38). Despite the fact that our results were calculated on a small number of cases, they warrant careful consideration prior to expanding the UTx-IVF procedure to new groups of patients in light of the following limitations. Published studies represent only a small percentage of the cases actually performed by the time of writing, and this could influence the accuracy of the estimations of safety and efficacy in both UTx recipients and children, which could be potentially underestimated. As a matter of fact, more than 60 UTx procedures have been performed worldwide, and 18 babies have been delivered, but half of these cases has not yet been scientifically published [32].
The majority of studies does not describe post-operative, maternal and neonatal outcomes simultaneously, probably due to the different timings of occurrence concerning UTx surgery, gestational period and delivery. Data are often published at distance and reported in more than one study, or referred to as personal communications. Furthermore, many studies do not capture the learning curve of the surgical teams at different centers worldwide, and it is to be hoped that further investigations will provide more standardized UTx surgical procedures.
Although the accuracy of UTx-IVF safety and efficacy outcomes cannot be evaluated definitively until UTx centers provide additional, meaningful data from larger series of UTx cases treated homogeneously, we critically evaluate the following aspects.
UTx surgery in recipients is mainly complicated by vascular impairments and uterine infections with a negative impact on graft survival and maintenance [9, 26, 35, 37, 39, 40]. The incidence of post-operative complications leading to hysterectomy in UTx recipients was estimated to be 23%, but this value could be underestimated.
The cohort of UTx patients treated and reported thus far represents only 30% of the entire cohort of UTx patients planned to be enrolled by registered UTx clinical trials (Table 3), together with all individual cases treated following local institutional approvals (Table 4).
Table 3. Registered UTx clinical trials.
| Identifier number* | Institution, State | Enrolled/Estimated enrollment | Donors | Study completion | Outcome measures | Study phase |
|---|---|---|---|---|---|---|
| NCT01844362 | Sahlgrenska University Hospital Gothenburg, Sweden | 10 | LD | April, 2018 | TS, PR, LBR | Active, not recruiting |
| NCT03138226 | Sahlgrenska University Hospital Gothenburg, Sweden | 6 | LD | December 2025 | LBR | Recruiting |
| NCT03277430 | Institute for Clinical and Experimental Medicine Prague, Czech Republic | 20 | LD/DD | December 2025 | TS, LBR LD LBR vs. DD LBR, TC, PC, LD surgery vs. DD surgery | Recruiting |
| NCT02656550 | Baylor University Medical Center, Dallas Texas, US | 10 | LD/DD | January 2026 | LBR | Recruiting |
| NCT03307356 | University of Pennsylvania, Philadelphia Pennsylvania, US | 5 | DD | July 2029 | TS, TC | Recruiting |
| NCT02987023 | Sahlgrenska University Hospital Gothenburg, Sweden | 10 | LD | December 2022 | LBR, newborns follow-up | Recruiting |
| NCT02573415 | Cleveland Clinic, Cleveland Florida, US | 10 | DD | December 2021 | LBR, PC, NC | Recruiting |
| NCT02637674 | Limoges Hospital Limoges, France | 10 | DD | January 2022 | TS, SMC, TC, PR, PC, LBR | Recruiting |
| NCT03252795 | Ghent University Hospital—Women's Clinic Ghent, Belgium | 20 | DD | December 2023 | TS, TC, PR, LBR | Recruiting |
| NCT 03590405 | Sahlgrenska University Hospital Gothenburg, Sweden | 5 | LD | December 2021 | LB | Recruiting |
| NCT03689842 | Hopital Foch | 10 | LD | June 1, 2025 | PR | Recruiting |
| NCT03048396 | University Women's Hospital, Tübingen, Germany | 10 | LD | December 2019 | Number of patients interested in UTx, with a potential donor and having a UTx medical indication | Recruiting by invitation |
| NCT03581019 | Sahlgrenska University Hospital Gothenburg, Sweden | 8 | DD | December 2025 | LB of an healthy child | Recruiting by invitation |
| NCT02741102 | Brigham and Women's Hospital, Boston Massachusetts, US | 10 | LD | January 2023 | LBR, TC, PC, donor QOL, CC, QOL | Not yet recruiting |
| NCT03284073 | Mansoura Urology and Nephrology Center, Mansoura Al-Dakahliya, Egypt | 11 | LD | October 2019 | TS, SMC, PR, LBR | Not yet recruiting |
| NCT02388802 | Imperial College London UK | 10 | DD | January 2020 | TS, PR, LBR | Not yet recruiting |
| NCT02409147 | University of Nebraska Medical Center, Omaha Nebraska, US | 5 | DD | January 2025 | TS | Suspended due to lack of funding |
CC = cost comparison between UTx vs. surrogacy and adoption; DD = deceased donors; LBR = live birth rate; LD = living donors; NC = neonatal complication; PC = pregnancy complications; PR = pregnancy rate; QOL = quality of life; SMC = spontaneous menstruation commencement; TC = transplant complications; TS = transplant success within 12 month post-operatively; UK = United Kingdom; US = United States; UTx = uterus transplantation.
*all trials were recorded on www.ClinicalTrials.gov
Table 4. UTx case studies with Institutional approval.
| Source | Institution, State | N° of treated cases | Donors |
|---|---|---|---|
| Fageeh et al., 2002 [26] | King Fahad Hospital and Research Center, Jeddah, Saudi Arabia | 1 | LD |
| Okzan et al., 2013 [27] | Akdeniz University, Antalya, Turkey | 1 | DD |
| Ejzenberg et al., 2019 [36] | Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo/SP, Brazil | 1 | DD |
| Wei et al., 2017 [41] | Xijing Hospital, The Fourth Military Medical University, Xi'an, People's Republic of China | 1 | LD |
| Brännström, 2018 [25] | Belgrade, Serbia | 1 | LD |
| Puntambekar et al., 2018 [42, 44] | Galaxy CARE Laparoscopy Institute, Pune, India | 4 | LD |
DD = deceased donors; LD = living donors.
Moreover, there are still unpublished data, and data referred to as personal communications to authors that hinder the ability to perform a high-quality assessment of UTx safety in recipients, which will have to be re-assessed in the future after more UTx procedures will be documented. At the same time, insights into post-operative UTx complications are desirable, in light of the positive attitudes towards this promising infertility treatment. UTx is considered more socially and individually acceptable by women of reproductive age than surrogacy and adoption [46–48], and it is at the forefront of the motherhood options considered by AUFI women [49, 50]. Although several ethical [51–55], psychological [56,57] and social [58–62] issues are actively being debated, overall there is great interest among both LDs and potential recipients towards participating in a UTx trial [63,64].
It is well acknowledged that UTx-IVF efficacy depends on favourable surgical UTx outcome, graft function by 1-year post-operation, and delivery of a healthy baby following a successful frozen embryo replacement cycle. Due to this, IVF deserves consideration as it is important to achieve the main reproductive outcome of the entire procedure. To our best knowledge, this is the first study to collect IVF data from UTx patients in a systematic fashion. The recovery of IVF outcomes was complicated, as it carried multiple challenges. IVF data were available for consultation from very few studies, and IVF treatments were applied according to the local clinical practice of single specialty centers. This highlights different cryopreservation policies that may hamper the standardization of the UTx-IVF procedure by the scientific community.
Nonetheless, the current UTx-IVF efficacy is 42,1% (16/38), based on the number of UTx live births per UTx successful procedures. The UTx pregnancy rate per embryo transfer was 35,5% (16/45). This last might be higher than presented as many patients have not yet receive embryo transfer. Furthermore, it is important to underline that not all the embryos transferred were genetically screened for euploidy.
Six out of 16 UTx pregnancies faced major complications during the gestational period [8,9, 32, 29, 36, 38], causing late preterm births in the majority of UTx deliveries [9,32,29,36,38]. Although these outcomes do not seem to impact on the safety of UTx mothers and babies, as no mothers or babies were admitted to intensive care units or experienced major medical problems, further conclusions should not be drawn. This is due to incomplete and/or limited data on UTx deliveries available to date, that negatively influence quality assessments of the outcomes and final estimations of the risk of maternal and neonatal complications in patients undergoing SOT [65,66]. In fact, the risk of complications in SOT pregnancies, such as pre-eclampsia, foetal growth restriction and preterm birth, is increased compared to the risk associated to spontaneous non-transplanted pregnancies [65,66]. Similar data are reported on pregnant women undergoing IVF in comparison to spontaneous conceptions [15–17,67], prompting us to consider UTx-IVF pregnancies at high risk for complications. Given that, careful multidisciplinary counselling is recommended to help patients cope with the reproductive and gestational aspects [68–70].
Specifically, the incidence of pre-eclampsia in healthy women ranges from 3% to 5%, but in SOT patients it accounts for 21.9% liver and 27% renal transplantations respectively [65]. Similarly, the incidence of pre-eclampsia in UTx pregnancies was of 21%. A plausible explanation is that UTx mothers experience the synergistic effect of several risk factors related to this complication, such as the immunosuppression regimen, the presence of a renal disease and the IVF treatment. However, only further analyses from a larger UTx cohort can provide insights to help clarify this issue. In addition, it has been long believed that the development of pre-eclampsia arises from defective placentation following utero-placental hypoperfusion and hypoxia [71,72]. Since no study has so far investigated this relationship in UTx-IVF placentas specifically, major advances regarding the physiology of UTx-placentation could play a pivotal role in the understanding of the occurrence of gestational complications post-UTx surgery, such as pre-eclampsia.
The condition of late preterm birth, that characterizes all UTx babies delivered thus far, is defined by the World Health Organization as the delivery of babies born between the completion of 32 to 37 weeks of gestation. This condition is associated with a lower risk of major medical consequences compared to very preterm births, defined by the World Health Organization as babies born between the completion of 28 to 32 weeks of gestation [73,74]. Accordingly, none of the UTx new-borns were admitted to neonatal intensive care units, and all of them have been reported as healthy babies to date. The same conclusion was reached by Jones et al. (2019). However, a close short- and long-term follow-up is recommended, as late preterm infants are at an increased risk of adverse neonatal outcomes, long-term neurodevelopmental and behavioural sequelae, lower cognitive functioning, and ongoing respiratory morbidities [73].
Conclusions
Taken together, the results of surgical, obstetric and neonatal outcomes highlight that the application of human UTx, as an infertility treatment that aims to solve AUFI, exposes mothers and babies to the risk of complications during the gestational period and at delivery. Our results are in accordance with those recently provided by Jones et al. (2019), and advocate the use of the international registry created within the International Society of Uterus Transplantation. This tool was created with the aim of collecting negative and positive UTx outcomes, so that important advancements in the field of UTx can be made. This approach will contribute to improving research in the field of surgical refinements, uterine bioengineering, donor selection and screening, organ preservation modalities, immunosuppression schedules, patient safety, and short- and long-term follow-ups. An improvement of the reporting system regarding the number of UTx-IVF attempts is desirable to achieve a standardized UTx procedure, increase the transparency of benefits and risks regarding this innovative infertility treatment, and provide better care, encompassing all of the medical, institutional and social parties involved.
Supporting information
(PDF)
(PDF)
Acknowledgments
The authors are grateful to Dr. V. Davoli, Unit of Obstetrics & Gynaecology, Azienda Unità Sanitaria Locale–IRCCS in Reggio Emilia, Italy, for her assistance in collecting the selected studies.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Díaz-García C, Brännström M. Uterus transplantation: potential patients, fertility in animal models and ethics. J Reproduktionsmed Endokrinol. 2013;10: 72–81. [Google Scholar]
- 2.Brännström M. Uterus transplantation. Curr Opin Organ Transplant. 2015;20: 621–628. 10.1097/MOT.0000000000000246 [DOI] [PubMed] [Google Scholar]
- 3.Huet S, Tardieu A, Filloux M, Essig M, Pichon N, Therme JF, et al. Uterus transplantation in France: for which patients?. Eur J Obstet Gynecol Reprod Biol. 2016;205: 7–10. 10.1016/j.ejogrb.2016.08.027 [DOI] [PubMed] [Google Scholar]
- 4.Arian SE, Flyckt RL, Farrell RM, Falcone T, Tzakis AG. Characterizing women with interest in uterine transplant clinical trials in the United States: who seeks information on this experimental treatment?. Am J Obstet Gynecol. 2017;216: 190–191. 10.1016/j.ajog.2016.11.1028 [DOI] [PubMed] [Google Scholar]
- 5.Hur C, Rehmer J, Flyckt R, Falcone T. Uterine factor infertility: a clinical review. Clin Obstet Gynecol. 2019;62: 257–270. 10.1097/GRF.0000000000000448 [DOI] [PubMed] [Google Scholar]
- 6.Dahm-Kähler P, Diaz-Garcia C, Brännström M. Human uterus transplantation in focus. Br Med Bull. 2016;117: 69–78. 10.1093/bmb/ldw002 [DOI] [PubMed] [Google Scholar]
- 7.Grynberg M, Ayoubi JM, Bulletti C, Frydman R, Fanchin R. Uterine transplantation: a promising surrogate to surrogacy?. Ann N Y Acad Sci. 2011;1221: 47–53. 10.1111/j.1749-6632.2011.05952.x [DOI] [PubMed] [Google Scholar]
- 8.Akouri R, Maalouf G, Abboud J, Waked A, Nakad T, Bedran F, et al. Uterus transplantation: an update and the Middle East perspective. Middle East Fertil Soc J. 2017;32: 163–169. [Google Scholar]
- 9.Brännström M, Johannesson L, Dahm-Kähler P, Enskog A, Mölne J, Kvarnström N, First clinical uterus transplantation trial: a six-month report. Fertil Steril. 2014;101: 1228–1236. 10.1016/j.fertnstert.2014.02.024 [DOI] [PubMed] [Google Scholar]
- 10.Brännström M, Johannesson L, Bokström H, Kvarnström N, Mölne J, Dahm-Kähler P, et al. Livebirth after uterus transplantation. Lancet. 2015;385: 607–616. 10.1016/S0140-6736(14)61728-1 [DOI] [PubMed] [Google Scholar]
- 11.Akar ME. Might uterus transplantation be an option for uterine factor infertility?. J Turk Ger Gynecol Assoc. 2015;16: 45–48. 10.5152/jtgga.2015.15107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 2017;34: 325–336. 10.1007/s10815-016-0843-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agich GJ. Extension of organ transplantation: some ethical considerations. Mt Sinai J Med. 2003;70: 141–147. [PubMed] [Google Scholar]
- 14.Silva AF, Carvalho LF. A meta-analysis on uterine transplantation: redefining the limits of reproductive surgery. Rev Assoc Med Bras (1992). 2016;62: 474–477. [DOI] [PubMed] [Google Scholar]
- 15.Palomba S, Homburg R, Santagni S, La Sala GB, Orvieto R. Risk of adverse pregnancy and perinatal outcomes after high technology infertility treatment: a comprehensive systematic review. Reprod Biol Endocrinol. 2016;2016:14:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palomba S, Santagni S, Gibbins K, La Sala GB, Silver RM. Pregnancy complications in spontaneous and assisted conceptions of women with infertility and subfertility factors. A comprehensive review. Reprod Biomed Online. 2016;33: 612–628. 10.1016/j.rbmo.2016.08.007 [DOI] [PubMed] [Google Scholar]
- 17.Palomba S, Santagni S, Daolio J, Gibbins K, Battaglia FA, La Sala GB. Obstetric and perinatal outcomes in subfertile patients who conceived following low technology interventions for fertility enhancement: a comprehensive review. Arch Gynecol Obstet. 2018;297: 33–47. 10.1007/s00404-017-4572-9 [DOI] [PubMed] [Google Scholar]
- 18.Brännström M, Enskog A, Kvarnström N, Ayoubi JM, Dahm-Kähler P. Global results of human uterus transplantation and strategies for pre-transplantation screening of donors. Fertil Steril. 2019;112: 3–10. 10.1016/j.fertnstert.2019.05.030 [DOI] [PubMed] [Google Scholar]
- 19.Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313: 1657–1665. 10.1001/jama.2015.3656 [DOI] [PubMed] [Google Scholar]
- 20.www.crd.york.ac.uk/prospero/
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62: 1006–1012. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 22.Howick J, Glasziou P, Aronson JK. Evidence-based mechanistic reasoning. J R Soc Med. 2010;103: 433–441. 10.1258/jrsm.2010.100146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64: 383–394. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 24.Brännström M, Dahm Kähler P, Greite R, Mölne J, Díaz-García C, Tullius SG. Uterus transplantation: a rapidly expanding field. Transplantation. 2018;102: 569–577. 10.1097/TP.0000000000002035 [DOI] [PubMed] [Google Scholar]
- 25.Brännström M. Current status and future direction of uterus transplantation. Curr Opin Organ Transplant. 2018;23: 592–597. 10.1097/MOT.0000000000000568 [DOI] [PubMed] [Google Scholar]
- 26.Fageeh W, Raffa H, Jabbad H, Marzouki A. Transplantation of the human uterus. Int J Gynaecol Obstet. 2002;76: 245–251. 10.1016/s0020-7292(01)00597-5 [DOI] [PubMed] [Google Scholar]
- 27.Ozkan O, Akar ME, Ozkan O, Erdogan O, Hadimioglu N, Yilmaz M, et al. Preliminary results of the first human uterus transplantation from a multiorgan donor. Fertil Steril. 2013;99: 470–476. 10.1016/j.fertnstert.2012.09.035 [DOI] [PubMed] [Google Scholar]
- 28.Ozkan O, Dogan NU, Ozkan O, Mendilcioglu I, Dogan S, Aydinuraz B, et al. Uterus transplantation: from animal models through the first heart beating pregnancy to the first human live birth. Womens Health (Lond). 2016;12: 442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brännström M, Bokström H, Dahm-Kähler P, Diaz-Garcia C, Ekberg J, Enskog A, et al. One uterus bridging three generations: first live birth after mother-to-daughter uterus transplantation. Fertil Steril. 2016;106: 261–266. 10.1016/j.fertnstert.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 30.Castellón LAR, Amador MIG, González RED, Eduardo MSJ, Díaz-García C, Kvarnström N, et al. The history behind successful uterine transplantation in humans. JBRA Assist Reprod. 2017; 21: 126–134. 10.5935/1518-0557.20170028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akouri R. In British Transplantation Society (BTS) Annual Congress 2017. (Harrogate, 2017). [Google Scholar]
- 32.Jones BP, Saso S, Bracewell-Milnes T, Thum MY, Nicopoullos J, Diaz-Garcia C, et al. Human uterine transplantation: a review of outcomes from the first 45 cases. BJOG. 2019;126: 1310–1319. 10.1111/1471-0528.15863 [DOI] [PubMed] [Google Scholar]
- 33.Wei L, Xue T, Tao K, Zhang G, Zhao GY, Yu SQ, et al. Modified human uterus transplantation using ovarian veins for venous drainage: the first report of surgically successful robotic-assisted uterus procurement and follow-up for 12 months. Fertil Steril. 2017;108: 346–356. 10.1016/j.fertnstert.2017.05.039 [DOI] [PubMed] [Google Scholar]
- 34.www.scmp.com/news/china/society/article/2183441/chinas-first-womb-transplant-recipient-gives-birth-healthy-baby. Accessed on February, 2018.
- 35.Flyckt R, Kotlyar A, Arian S, Eghtesad B, Falcone T, Tzakis A. Deceased donor uterine transplantation. Fertil Steril. 2017;107:e13 10.1016/j.fertnstert.2016.12.009 [DOI] [PubMed] [Google Scholar]
- 36.Ejzenberg D, Andraus W, Baratelli Carelli Mendes LR, Ducatti L, Song A, Tanigawa R, et al. Livebirth after uterus transplantation from a deceased donor in a recipient with uterine infertility. Lancet. 2019;392: 2697–2704. 10.1016/S0140-6736(18)31766-5 [DOI] [PubMed] [Google Scholar]
- 37.Testa G, Koon EC, Johannesson L, McKenna GJ, Anthony T, Klintmalm GB, et al. Living donor uterus transplantation: a single center's observations and lessons learned from early setbacks to technical success. Am J Transplant. 2017;17: 2901–2910. 10.1111/ajt.14326 [DOI] [PubMed] [Google Scholar]
- 38.Testa G, McKenna GJ, Gunby RT, Anthony T, Koon EC, Warren AM, et al. First live birth after uterus transplantation in the United States. Am J Transplant. 2018;18: 1270–1274. 10.1111/ajt.14737 [DOI] [PubMed] [Google Scholar]
- 39.Chmel R, Novackova M, Janousek L, Matecha J, Pastor Z, Maluskova J, et al. Revaluation and lessons learned from the first 9 cases of a Czech uterus transplantation trial: Four deceased donor and 5 living donor uterus transplantations. Am J Transplant. 2018;19: 855–864. 10.1111/ajt.15096 [DOI] [PubMed] [Google Scholar]
- 40.Brucker SY, Brännström M, Taran FA, Nadalin S, Königsrainer A, Rall K, et al. Selecting living donors for uterus transplantation: lessons learned from two transplantations resulting in menstrual functionality and another attempt, aborted after organ retrieval. Arch Gynecol Obstet. 2018;297: 675–684. 10.1007/s00404-017-4626-z [DOI] [PubMed] [Google Scholar]
- 41.Puntambekar S, Puntambekar S, Telang M, Kulkarni P, Date S, Panse M, et al. A novel anastomotic technique for uterine transplant using utero-ovarian veins for venous drainage and internal iliac arteries for perfusion in two laparoscopically harvested uteri. J Minim Invasive Gynecol. 2018;4650: 31431–31436. [DOI] [PubMed] [Google Scholar]
- 42.Puntambekar S, Telang M, Kulkarni P, Puntambekar S, Jadhav S, Panse M, et al. Laparoscopic‐assisted uterus retrieval from live organ donors for uterine transplant: our experience of two patients. J Minim Invasive Gynecol. 2018;25: 622–631. 10.1016/j.jmig.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 43.www.dailymail.co.uk/health/article-6393029/Indian-woman-gives-birth-Asias-uterus-transplanted-baby.html. Accessed on November, 2018.
- 44.Brännström M, Dahm-Kähler P, Kvarnström N. Robotic-assisted surgery in live-donor uterus transplantation. Fertil Steril. 2018;109: 256–257. 10.1016/j.fertnstert.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 45.https://nypost.com/2019/04/09/baby-makes-history-afterworlds-first-womb-transplant-performed-by-robot. Accessed on May, 2019.
- 46.Wennberg AL, Rodriguez-Wallberg KA, Milsom I, Brännström M. Attitudes towards new assisted reproductive technologies in Sweden: a survey in women 30–39 years of age. Acta Obstet Gynecol Scand. 2016;95: 38–44. 10.1111/aogs.12781 [DOI] [PubMed] [Google Scholar]
- 47.Kisu I, Banno K, Soeda E, Kurihara Y, Okushima M, Yamaguchi A, et al. Survey of attitudes toward uterus transplantation among Japanese women of reproductive age: a cross-sectional study. PLoS One. 2016;11:e0156179 10.1371/journal.pone.0156179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hariton E, Bortoletto P, Goldman RH, Farland LV, Ginsburg ES, Gargiulo AR. A Survey of public opinion in the United States regarding uterine transplantation. J Minim Invasive Gynecol. 2018;25: 980–985. 10.1016/j.jmig.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 49.Saso S, Clarke A, Bracewell-Milnes T, Saso A, Al-Memar M, Thum MY, et al. Psychological issues associated with absolute uterine factor infertility and attitudes of patients toward uterine transplantation. Prog Transplant. 2016;26: 28–39. 10.1177/1526924816634840 [DOI] [PubMed] [Google Scholar]
- 50.Chmel R, Novackova M, Pastor Z, Fronek J. The interest of women with Mayer-Rokitansky-Küster-Hauser syndrome and laparoscopic Vecchietti neovagina in uterus transplantation. J Pediatr Adolesc Gynecol. 2018;31: 480–484. 10.1016/j.jpag.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 51.Testa G, Koon EC, Johannesson L. Living donor uterus transplant and surrogacy: ethical analysis according to the principle of equipoise. Am J Transplant. 2017;17: 912–916. 10.1111/ajt.14086 [DOI] [PubMed] [Google Scholar]
- 52.Zaami S, Marinelli E, di Luca NM, Montanari Vergallo G. Ethical and medico-legal remarks on uterus transplantation: may it solve uterine factor infertility?. Eur Rev Med Pharmacol Sci. 2017;21: 5290–5296. 10.26355/eurrev_201711_13854 [DOI] [PubMed] [Google Scholar]
- 53.Testa G, Johannesson L. The ethical challenges of uterus transplantation. Curr Opin Organ Transplant. 2017;22: 593–597. 10.1097/MOT.0000000000000467 [DOI] [PubMed] [Google Scholar]
- 54.Guntram L, Williams NJ. Positioning uterus transplantation as a 'more ethical' alternative to surrogacy: exploring symmetries between uterus transplantation and surrogacy through analysis of a Swedish government white paper. Bioethics. 2018;32: 509–518. 10.1111/bioe.12469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams NJ, Scott R, Wilkinson S. The ethics of uterus transplantation. Bioethics. 2018;32: 478–480. 10.1111/bioe.12530 [DOI] [PubMed] [Google Scholar]
- 56.Järvholm S, Johannesson L, Clarke A, Brännström M. Uterus transplantation trial: psychological evaluation of recipients and partners during the post-transplantation year. Fertil Steril. 2015;104: 1010–1015. 10.1016/j.fertnstert.2015.06.038 [DOI] [PubMed] [Google Scholar]
- 57.Järvholm S, Warren AM, Jalmbrant M, Kvarnström N, Testa G, Johannesson L. Preoperative psychological evaluation of uterus transplant recipients, partners, and living donors: suggested framework. Am J Transplant. 2018;18: 2641–2646. 10.1111/ajt.15039 [DOI] [PubMed] [Google Scholar]
- 58.Allahbadia GN. Human Uterus transplantation: have we opened a Pandora's box?. J Obstet Gynaecol India. 2015;65: 1–4. 10.1007/s13224-015-0674-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blake VK. Financing uterus transplants: the United States context. Bioethics. 2018;32: 527–533. 10.1111/bioe.12506 [DOI] [PubMed] [Google Scholar]
- 60.Tardieu A, Sallée C, Dion L, Piver P, Lavoué V, Gauthier T, et al. Uterus transplantation in transgenders: will it happen one day?. J Gynecol Obstet Hum Reprod. 2019; 48: 5–6. 10.1016/j.jogoh.2018.11.009 [DOI] [PubMed] [Google Scholar]
- 61.Balayla J, Tulandi T, McGill University Uterine Transplantation Exploratory Committee. Provider perceptions, opinions, and insights into uterine transplantation in Canada. J Obstet Gynaecol Can. 2019;41: 428–435. 10.1016/j.jogc.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 62.Guntram L, Zeiler K. The Ethics of the societal entrenchment-approach and the case of live uterus transplantation-IVF. Med Health Care Philos. 2019;22: 557–571. 10.1007/s11019-019-09891-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodrigue JR, Tomich D, Fleishman A, Glazier AK. Vascularized composite allograft donation and transplantation: a survey of public attitudes in the United States. Am J Transplant. 2017;17: 2687–2695. 10.1111/ajt.14302 [DOI] [PubMed] [Google Scholar]
- 64.Johannesson L, Wallis K, Koon EC, McKenna GJ, Anthony T, Leffingwell SG, et al. Living uterus donation and transplantation: experience of interest and screening in a single center in the United States. Am J Obstet Gynecol. 2018;218:331.e1–331.e7. [DOI] [PubMed] [Google Scholar]
- 65.Brosens I, Brosens JJ, Benagiano G. The risk of obstetrical syndromes after solid organ transplantation. Best Pract Res Clin Obstet Gynaecol. 2014;28: 1211–1221. 10.1016/j.bpobgyn.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 66.Durst JK, Rampersad RM. Pregnancy in women with solid organ transplants: a review. Obstet Gynecol Surv. 2015;70: 408–418. 10.1097/OGX.0000000000000194 [DOI] [PubMed] [Google Scholar]
- 67.Paulson R. Pregnancy outcome after assisted reproductive technology Post TW, ed. UpToDate. 2019. Waltham, MA: UpToDate Inc; https://www.uptodate.com (Accessed on January 04, 2019.) [Google Scholar]
- 68.Deshpande NA, Coscia LA, Gomez-Lobo V, Moritz MJ, Armenti VT. Pregnancy after solid organ transplantation: a guide for obstetric management. Rev Obstet Gynecol. 2013;6: 116–225. [PMC free article] [PubMed] [Google Scholar]
- 69.Benagiano G, Brosens I. The multidisciplinary approach. Best Pract Res Clin Obstet Gynaecol. 2014;28: 1114–1122. 10.1016/j.bpobgyn.2014.08.006 [DOI] [PubMed] [Google Scholar]
- 70.Al-Badri M, Kling JM, Vegunta S. Reproductive planning for women after solid-organ transplant. Cleve Clin J Med. 2017;84: 719–728. 10.3949/ccjm.84a.16116 [DOI] [PubMed] [Google Scholar]
- 71.Duan J, Chabot-Lecoanet AC, Perdriolle-Galet E, Christov C, Hossu G, Cherifi A, et al. Utero-placental vascularisation in normal and preeclamptic and intra-uterine growth restriction pregnancies: third trimester quantification using 3D power Doppler with comparison to placental vascular morphology (EVUPA): a prospective controlled study. BMJ Open. 2016;31: 6(3):e009909 10.1136/bmjopen-2015-009909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He C, Shan N, Xu P, Ge H, Yuan Y, Liu Y, et al. Hypoxia-induced Downregulation of SRC-3 Suppresses Trophoblastic Invasion and Migration Through Inhibition of the AKT/mTOR Pathway: Implications for the Pathogenesis of Preeclampsia. Sci Rep. 2019;17: 9(1):10349 10.1038/s41598-019-46699-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Natarajan G, Shankaran S. Short- and long-term outcomes of moderate and late preterm infants. Am J Perinatol. 2016;33: 305–317. 10.1055/s-0035-1571150 [DOI] [PubMed] [Google Scholar]
- 74.Gill JV, Boyle EM. Outcomes of infants born near term. Arch Dis Child. 2017;102: 194–198. 10.1136/archdischild-2015-309584 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.