Abstract
Androgen deprivation therapy is the gold standard for metastatic prostate cancer, which can be achieved either by surgical or medical castration. In this study of 33,585 patients in the National Cancer Database, there was significant decline in the trend of utilization of surgical castration from 8.6% in 2004 to 3.1% in 2014. However, there was no survival difference with surgical castration when compared with medical castration. Increasing the utilization of surgical castration could help reduce health care expenditures. Patients and physicians need to be aware of treatment options and their financial implications.
Background:
Androgen deprivation therapy (ADT) is the gold standard for metastatic prostate cancer, which can be achieved either by surgical or medical castration. In this study, we evaluated the trends of utilization of surgical castration and also assess the survival differences of patients who underwent surgical castration when compared with those who underwent medical castration.
Materials and Methods:
The National Cancer Database was used to identify patients with metastatic prostate cancer from 2004 to 2014. Cochran-Armitage tests were used to assess temporal trends in the proportion of patients receiving surgical castration relative to medical castration. Logistic and Cox regression models were utilized to estimate the odds of utilization of surgical castration and the effect of castration on overall survival (OS).
Results:
A total of 33,585 patients with metastatic prostate cancer were identified; 31,600 (94.1%) had medical castration, and 1985 (5.9%) underwent surgical castration. There was significant decline in the trend of utilization of surgical castration from 8.6% in 2004 to 3.1% in 2014. On multivariable analysis, being of a non-Caucasian race, having lower median income levels, having non-private insurance, and earlier years of diagnosis were found to be associated with increased odds of choosing surgical castration over medical castration. Notably, the odds of surgical castration were lower at academic centers. On univariable analysis, a survival difference between castration modality was evidenced (P < .01); 5-year OS for medical castration and surgical castration were 24.3% and 18.2%, respectively. However, on multivariable analysis, there was no OS difference between surgical castration and medical castration (P = .13).
Conclusions:
In this large contemporary analysis, the utilization of surgical castration has declined over time, with no OS difference when compared with medical castration. Increasing the utilization of surgical castration could help reduce health care expenditures. With rising health care costs, patients and physicians need to be aware of treatment options and their financial implications.
Keywords: Androgen deprivation therapy, Health care costs, Hormonal therapy
Introduction
Prostate cancer is the most common malignancy and second most common cause of cancer-related death in men. In 2018, the American Cancer Society estimated about 164,690 new cases in United States. The incidence has steadily declined since its peak in the early 1990s, coinciding with changing recommendations from the United States Preventive Services Task Force on prostate cancer screening. This was primarily intended to avoid over-diagnosis of early stage and indolent prostate cancer.1,2
Localized prostate cancer is potentially curable with either radiation therapy or radical prostatectomy. Androgen deprivation therapy (ADT) is the gold standard for the treatment of newly diagnosed metastatic prostate cancer or recurrent cancer that cannot be cured with salvage therapy. This can be achieved either by surgical or medical castration. In the United States, surgical castration (bilateral orchiectomy or subcapsular orchiectomy) was the predominant modality of ADT until the late 1980s, and subsequently, this has gradually shifted to medical castration owing to the discovery of long-acting GnRH analogues (goserelin, leuprolide, triptorelin, and histrelin) and GnRH antagonist (degarelix). Both of these modalities are equally effective in achieving castration levels of testosterone (< 50 ng/dL) and have similar efficacy in controlling the cancer.3,4 Medical castration can be achieved with continuous or intermittent dosing of GnRH analogues. Previous studies have shown improvement in quality of life and cost savings with intermittent dosing; however, this also led to an increase in prostate cancer-related deaths.5 Various national guidelines including the National Comprehensive Cancer Network, American Society of Clinical Oncology, and European Association of Urology recommend either medical or surgical modalities as effective therapies. There is no consensus for continuous versus intermittent ADT when using medical castration; however, it is recommended that intermittent dosing should be strongly considered to reduce side effects.6,7 Despite both surgery and medical castration being recommended by the guidelines, the utilization of surgical castration is low.
In this study, we evaluate the trends of utilization of surgical castration and also compare its efficacy with medical castration in 33,585 patients in the National Cancer Database (NCDB). Additionally, we will discuss financial implications of both these modalities in the setting of rising health care costs and its impact on the economy.
Materials and Methods
Data Source
The NCDB is a jointly sponsored cancer database of the American College of Surgeons and the American Cancer Society. The database is one of the largest cancer databases, with more than 34 million records. It collects hospital registry data from more than 1500 Commission on Cancer (COC)-accredited facilities. More than 70 percent of newly diagnosed patients with cancer are represented in this database.8 The data provides several variables including treatment received and overall outcomes, which can be analyzed to identify factors impacting patients’ survival. The data requested from NCDB was provided as the participant user data file, which contains the Health Insurance Portability and Accountability Act-compliant de-identified patient-level data. The most recent data that was available during the time of analysis was from 2004 to 2014.
Cohort Selection
We identified 1,380,357 men based on the International Classification of Disease for Oncology, 3rd edition, code C61.9 (Prostate gland; Prostate, not otherwise specifiec [NOS]) in the database. We included 33,585 adult men with age ≥ 18 years diagnosed with metastatic prostate cancer after excluding patients with localized disease, multiple primary malignant tumors, those who did not receive ADT, and those with incomplete treatment or follow-up information. The histologies included were metastatic adenocarcinoma, ductal carcinoma, acinar cell carcinoma, and adenocarcinoma with mixed subtypes. Transitional, small-cell, pseudosarcomatous, and large-cell carcinoma histologies were excluded from the study. Staging from year 2004 to 2009 was based on the American Joint Committee on Cancer (AJCC) 6th edition, and for men from 2010 to 2014 was based on the AJCC 7th edition. Although there was a change in staging for early stage prostate cancer in AJCC 7th edition when compared with the 6th edition, there was no significant change for metastatic prostate cancer.9 Patients with early stage disease who progressed to have metastatic disease later were excluded from the study as the date of progression was not available. The cohort includes patients who have received chemotherapy and those who received radiation therapy or underwent surgery for the primary tumor. The information regarding the specific type of chemotherapy agent (such as docetaxel, cabazitaxel, and mitoxantrone) and anti-androgen therapy (abiraterone and enzalutamide) is not available in the database. We believe the lack of this specific information is unlikely to impact the study measures. Figure 1 shows the study population who met the inclusion criteria.
Figure 1. Consolidated Standards of Reporting Trials (CONSORT) Diagram for Cohort Selection.
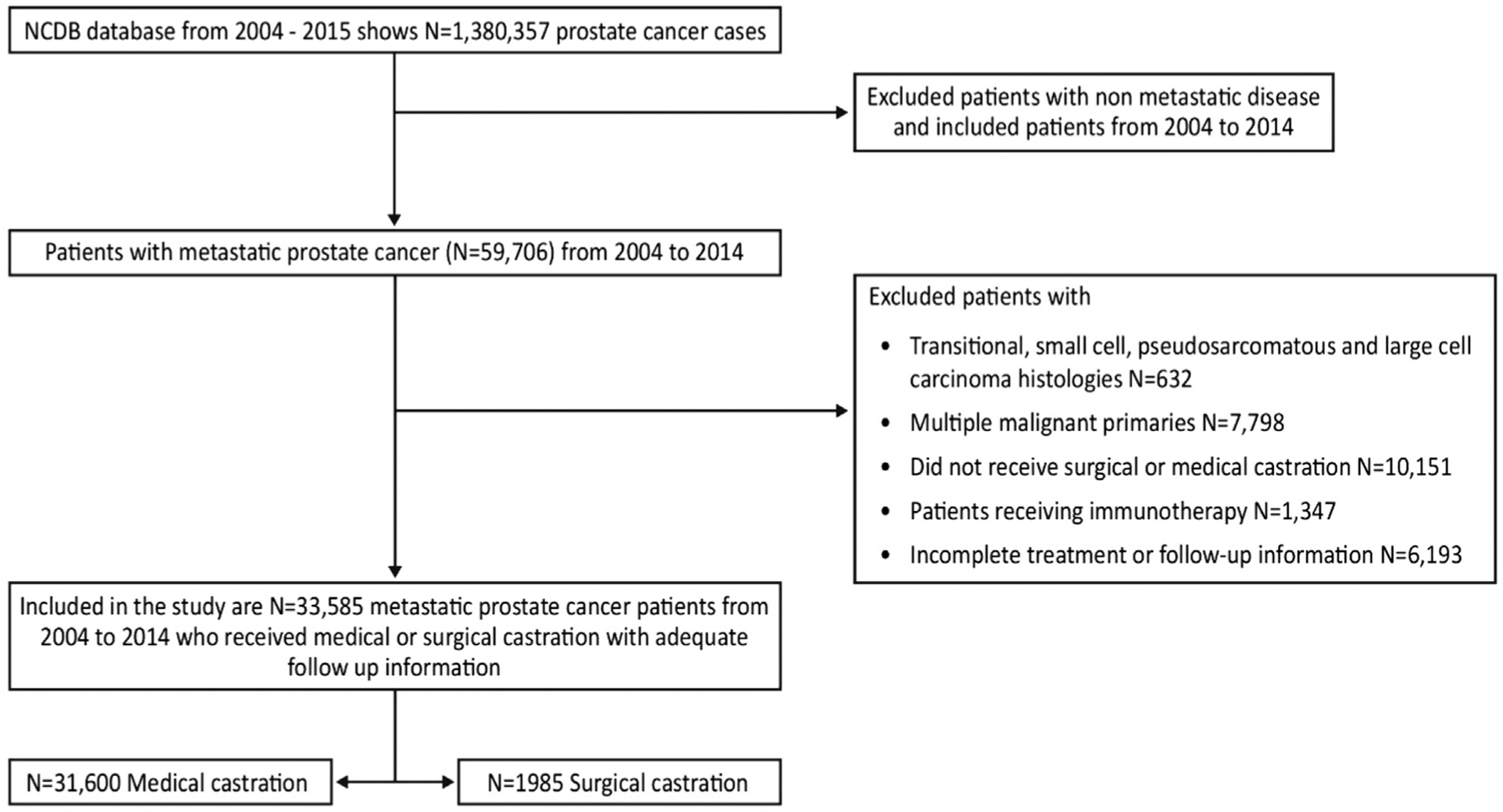
Abbreviation: NCDB = National Cancer Database.
Measures
Cancer registrars documented the modality of castration as surgical castration or medical castration. In the database, surgical castration was defined as endocrine surgery either after bilateral orchiectomy, orchiectomy for a single remaining testicle, or irradiation of both testicles. The remaining patients who did not undergo orchiectomy or who had irradiation of testicles received hormonal therapy for castration. We noted some patients who underwent surgical castration have also received hormonal therapy initially. This, we think, is a standard practice as patients are generally started on hormonal therapy and then, over the course of time, decide to undergo surgical castration.
Covariates
The covariates that were collected are broadly grouped as demographic, socioeconomic, and clinical characteristics. The specific covariates included age, race, facility type, location, primary payer, census median income quartiles, rurality (rural, urban or metro), Charlson-Deyo score, surgery of primary site, receipt of radiation, receipt of chemotherapy, status of patients, and year of diagnosis. The patients were grouped based on age as < 65 and ≥ 65 years. The race is distributed as white, black, and others. The insurance providers were categorized as Medicare, Medicaid, private insurance, and uninsured. Income quartiles are divided into < $38,000, $38,000 to $47,999, $48,000 to $62,999, and ≥ $63,000 median household income, estimated using patients’ ZIP codes. The patients were categorized into rural or urban based on their county of residence as per United States Department of Agriculture ruralurban continuum codes.10 The comorbidities of the patients were assessed based on Charlson/Deyo comorbidity score, which is grouped as 0, 1, 2, or > 2.11 The treating facilities are divided as academic and non-academic based on criteria delineated by the CoC.12 The patients were sorted based on the geographic location into Mountain-Pacific, Central, and New England-Atlantic regions, respectively.
Statistical Analysis
The Cochran-Armitage test was used to assess temporal trends in the proportion of patients receiving surgical castration relative to medical castration. Logistic regression was used to estimate the effects of sociodemographic and clinical variables on receipt of endocrine therapy (surgical vs. medical castration). Cox regression models were utilized to estimate the effect of treatment on overall survival (OS) while adjusting for the potential confounding effects of sociodemographic, clinicopathologic, and other treatment characteristics. Covariates used in the analysis were selected based on previous literature, clinical knowledge, and availability. To account for the possible dependency between patients seen at the same facility, generalized estimating equations with a compound symmetry structure and a robust sandwich variance estimate were used for the logistic and Cox regression models, respectively. Estimated covariate effects are reported as odds ratios or hazard ratios along with 95% confidence intervals. All tests were 2-sided and assessed for significance at the 5% level using SAS v9.4 (SAS Institute, Cary, NC).
Results
Study Population
A total of 33,585 men with metastatic prostate cancer were identified during the 2004 to 2014 period; of these, 31,600 were treated with medical castration, and 1985 underwent surgical castration. Among the men who underwent surgical castration, 846 (42.6%) also received hormonal therapy. Table 1 summarizes their characteristics based on type of castration for different covariates analyzed.
Table 1.
Characteristics of Medical and Surgical Castration Patients
| Type of Castration, n (%) | Total, n (%) | |||
|---|---|---|---|---|
| Medical | Surgical | |||
| Covariate | Level | N = 31,600 | N = 1985 | N = 33,585 |
| Year of diagnosis | 2004–2009 | 13,408 (42.4) | 1132 (57.0) | 14,540 (43.3) |
| 2010–2014 | 18,192 (57.6) | 853 (43.0) | 19,045 (56.7) | |
| Facility type | Not academic/research program | 20,009 (63.4) | 1500 (75.6) | 21,509(64.1) |
| Academic/research program | 11,568 (36.6) | 485 (24.4) | 12,053 (35.9) | |
| Missing | 23 | 0 | 23 | |
| Location | Central | 12,746 (40.4) | 904 (45.5) | 13,650 (40.7) |
| Mountain-Pacific | 5467 (17.3) | 367 (18.5) | 5834 (17.4) | |
| New England-Atlantic | 13,364 (42.3) | 714 (36.0) | 14,078(41.9) | |
| Missing | 23 | 0 | 23 | |
| Age, y | 65+ | 20,847 (66.0) | 1328 (66.9) | 22,175 (66.0) |
| <65 | 10,753 (34.0) | 657 (33.1) | 11,410 (34.0) | |
| Race | Black | 6249 (20.0) | 449 (22.7) | 6698 (20.2) |
| Other | 1064 (3.4) | 81 (4.1) | 1145 (3.4) | |
| White | 23,944 (76.6) | 1444 (73.2) | 25,388 (76.4) | |
| Missing | 343 | 11 | 354 | |
| Primary payer | Not insured | 1840 (5.9) | 236 (12.1) | 2076 (6.3) |
| Private insurance | 8407 (27.1) | 349 (17.9) | 8756 (26.6) | |
| Medicaid-other government | 2631 (8.5) | 207 (10.6) | 2838 (8.6) | |
| Medicare | 18,090 (58.4) | 1153 (59.3) | 19,243 (58.5) | |
| Missing | 632 | 40 | 672 | |
| Census median income quartiles, 2007–2012 | <$38,000 | 6506 (20.9) | 551 (28.2) | 7057 (21.3) |
| $38,000-$47,999 | 7354 (23.6) | 488 (25.0) | 7842 (23.7) | |
| $48,000-$62,999 | 8281 (26.6) | 542 (27.7) | 8823 (26.7) | |
| >$63,000 | 9008 (28.9) | 374 (19.1) | 9382 (28.3) | |
| Missing | 451 | 30 | 481 | |
| Urban/rural, 2013 | Metro | 25,635 (83.8) | 1530 (79.8) | 27,165 (83.6) |
| Urban | 4307 (14.1) | 346 (18.0) | 4653 (14.3) | |
| Rural | 653 (2.1) | 42 (2.2) | 695 (2.1) | |
| Missing | 1005 | 67 | 1072 | |
| Charlson-Deyo score | 0 | 24,500 (77.5) | 1449 (73.0) | 25,949 (77.3) |
| 1 | 4865 (15.4) | 402 (20.3) | 5267 (15.7) | |
| 2 | 1595 (5.0) | 90 (4.5) | 1685 (5.0) | |
| 3+ | 640 (2.0) | 44 (2.2) | 684 (2.0) | |
| Surgery of primary site | No | 28,383 (89.8) | 1636 (82.4) | 30,019 (89.4) |
| Yes | 3217 (10.2) | 349 (17.6) | 3566 (10.6) | |
| Receipt of radiation | No | 22,637 (71.6) | 1528 (77.0) | 24,165 (72.0) |
| Yes | 8963 (28.4) | 457 (23.0) | 9420 (28.0) | |
| Receipt of chemotherapy | No | 29,064 (92.0) | 1895(95.5) | 30,959 (92.2) |
| Yes | 2536 (8.0) | 90 (4.5) | 2626 (7.8) | |
| Status | Alive | 9718 (30.8) | 443 (22.3) | 10,161 (30.3) |
| Dead | 21,882(69.2) | 1542 (77.7) | 23,424 (69.7) | |
The overall trend in the utilization of surgical castration has significantly (P < .01) declined from 2004 to 2014. Specifically, it was 8.6% in 2004 and declined to 3.1% by 2014. The trends along with absolute values are depicted in Figure 2.
Figure 2.
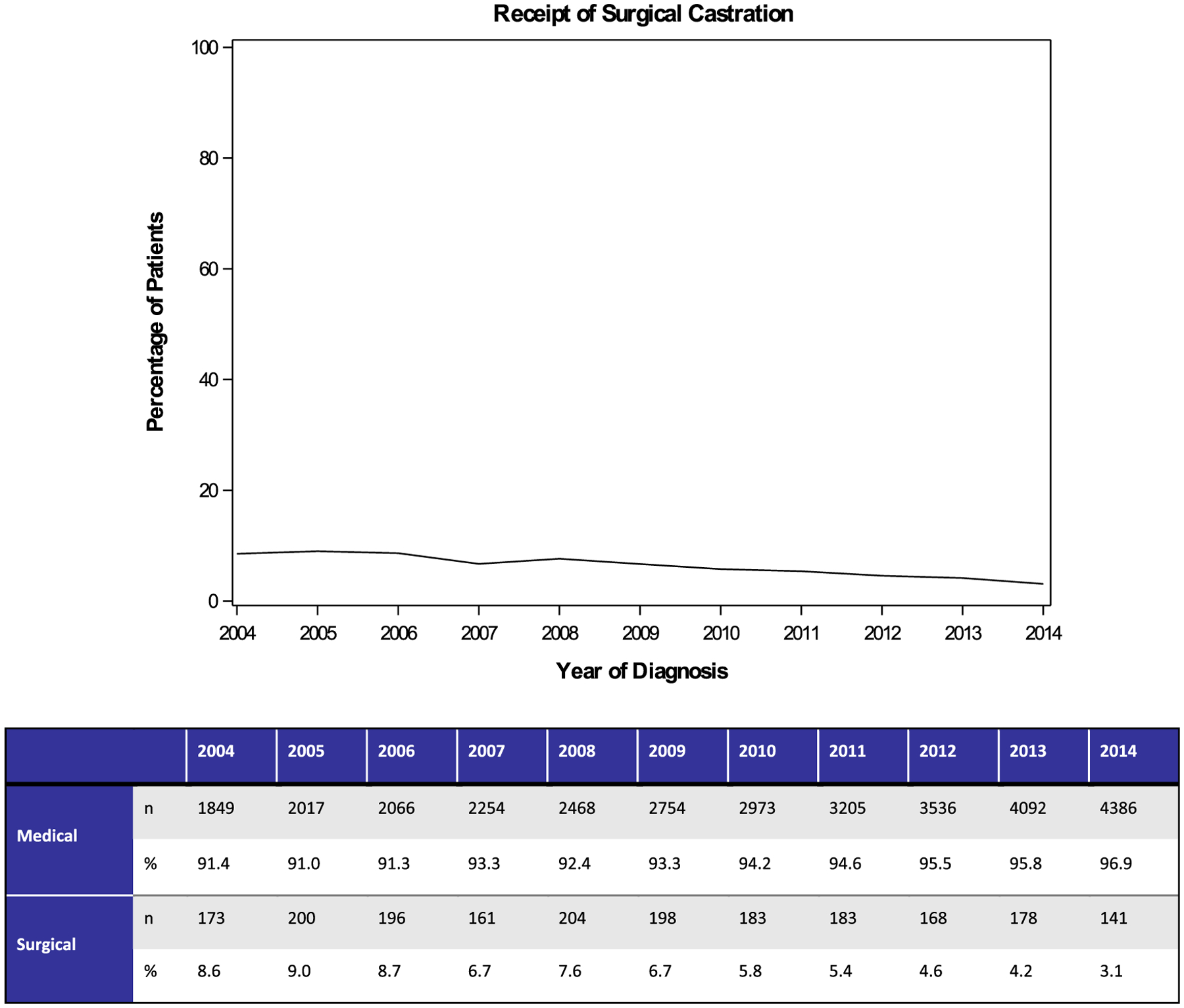
Between 2004 and 2014, a Significant Decline in the Utilization of Surgical Castration Relative to Medical Castration was Evidenced (P < .01)
Of the 1985 men who underwent surgical castration, the majority were white (73.2%), above 65 years (66.9%), had no comorbidities (73%), and were living in an area with an annual income of less than $63,000 (80.9%). Additionally, 79.8% of men from metropolitan areas underwent surgical orchiectomy when compared with men from urban or rural areas. A higher proportion (75.6%) of men treated at non-academic facilities underwent surgical castration when compared with those treated at academic centers.
Odds of Surgical Castration
The univariable and multivariable analyses, as outlined in Table 2 and Figure 3, show that the odds of patients choosing surgical castration over medical castration are higher in non-Caucasians, men living in an area with an annual household income less than $63,000, and those with non-private insurance (Medicare, Medicaid, or uninsured). Interestingly, the odds of undergoing orchiectomy are lower in academic centers. Compared with the New England-Atlantic regions, men in the Central and Mountain-Pacific zones have higher odds of undergoing orchiectomy. The age of the patient (< 65 vs. ≥ 65 years) and their location (ie, metro vs. nonmetro) did not influence the odds of choosing orchiectomy. Patients with a Charlson-Deyo morbidity score of 1 have higher odds of choosing surgical castration. However, beyond the score of 1, the comorbidity index did not show any significant influence on the odds of choosing one procedure over the other relative to patients with no comorbidities. Odds of surgical castration decreased with an increase in year of diagnosis, which is consistent with the declining trend of the surgical castration over the years.
Table 2.
Odds of Surgical Castration
| Univariable | Multivariable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Covariate | Level | Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value | ||
| Facility type | Not academic/research program | 1.57 | 1.28 | 1.93 | <.01 | 1.68 | 1.36 | 2.09 | <.01 |
| Academic/research program | Ref | - | - | - | Ref | - | - | - | |
| Location | Central | 1.28 | 1.06 | 1.54 | .01 | 1.22 | 1.01 | 1.48 | .04 |
| Mountain-Pacific | 1.31 | 1.01 | 1.69 | .04 | 1.34 | 1.04 | 1.73 | .02 | |
| New England-Atlantic | Ref | - | - | - | Ref | - | - | - | |
| Age | 65+ | 0.96 | 0.88 | 1.05 | .41 | 1.09 | 0.95 | 1.25 | .20 |
| <65 | Ref | - | - | - | Ref | - | - | - | |
| Race | Black | 1.30 | 1.15 | 1.46 | <.01 | 1.18 | 1.03 | 1.35 | .02 |
| Other | 1.39 | 1.12 | 1.71 | <.01 | 1.30 | 1.02 | 1.66 | .03 | |
| White | Ref | - | - | - | Ref | - | - | - | |
| Primary payor | Not insured | 2.95 | 2.44 | 3.58 | <.01 | 3.08 | 2.50 | 3.80 | <.01 |
| Medicaid-other government | 1.80 | 1.52 | 2.13 | <.01 | 1.86 | 1.54 | 2.25 | <.01 | |
| Medicare | 1.33 | 1.19 | 1.50 | <.01 | 1.28 | 1.09 | 1.51 | <.01 | |
| Private insurance | Ref | - | - | - | Ref | - | - | - | |
| Census median income quartiles, 2007–2012 | <$38,000 | 1.68 | 1.46 | 1.93 | <.01 | 1.44 | 1.21 | 1.71 | <.01 |
| $38,000-$47,999 | 1.31 | 1.14 | 1.50 | <.01 | 1.18 | 1.01 | 1.38 | .04 | |
| $48,000-$62,999 | 1.33 | 1.19 | 1.50 | <.01 | 1.27 | 1.11 | 1.45 | <.01 | |
| >$68,000 | Ref | - | - | - | Ref | - | - | - | |
| Urban/rural, 2013 | Urban | 1.12 | 0.97 | 1.30 | .11 | 1.05 | 0.89 | 1.23 | .58 |
| Rural | 1.09 | 0.80 | 1.48 | .58 | 1.02 | 0.75 | 1.40 | .89 | |
| Metro | Ref | - | - | - | Ref | - | - | - | |
| Charlson-Deyo score | 1 | 1.32 | 1.18 | 1.48 | <.01 | 1.33 | 1.18 | 1.49 | <.01 |
| 2 | 0.84 | 0.68 | 1.04 | .11 | 0.88 | 0.70 | 1.10 | .26 | |
| 3+ | 1.08 | 0.80 | 1.46 | .61 | 1.12 | 0.82 | 1.53 | .49 | |
| 0 | Ref | - | - | - | Ref | - | - | - | |
| Year of diagnosis | Units = 1 | 0.91 | 0.90 | 0.93 | <.01 | 0.91 | 0.89 | 0.92 | <.01 |
Abbreviations: CI = confidence interval; Ref = reference.
Figure 3. Multivariable Analysis: Odds of Castration.
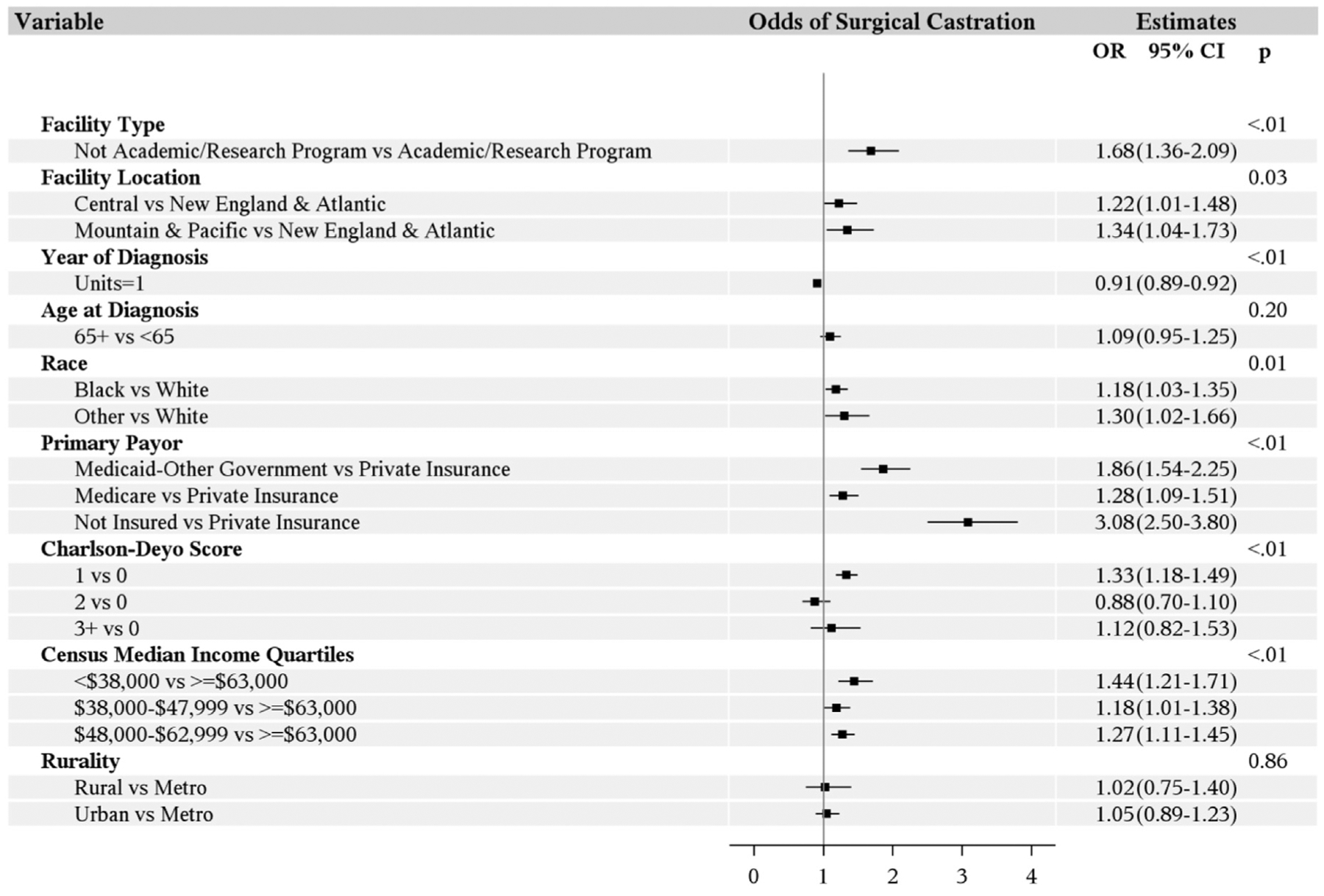
Abbreviations: CI = confidence interval; OR = odds ratio.
Survival Analysis
The 5-year OS in patients undergoing medical and surgical castration was 24.3% and 18.3%, respectively. A significant difference between castration type was found in terms of OS on univariable analysis (P < .01). However, after multivariable analysis, the difference was no longer significant (P = .13) (Figure 4).
Figure 4. Univariable Analysis of Overall Survival.
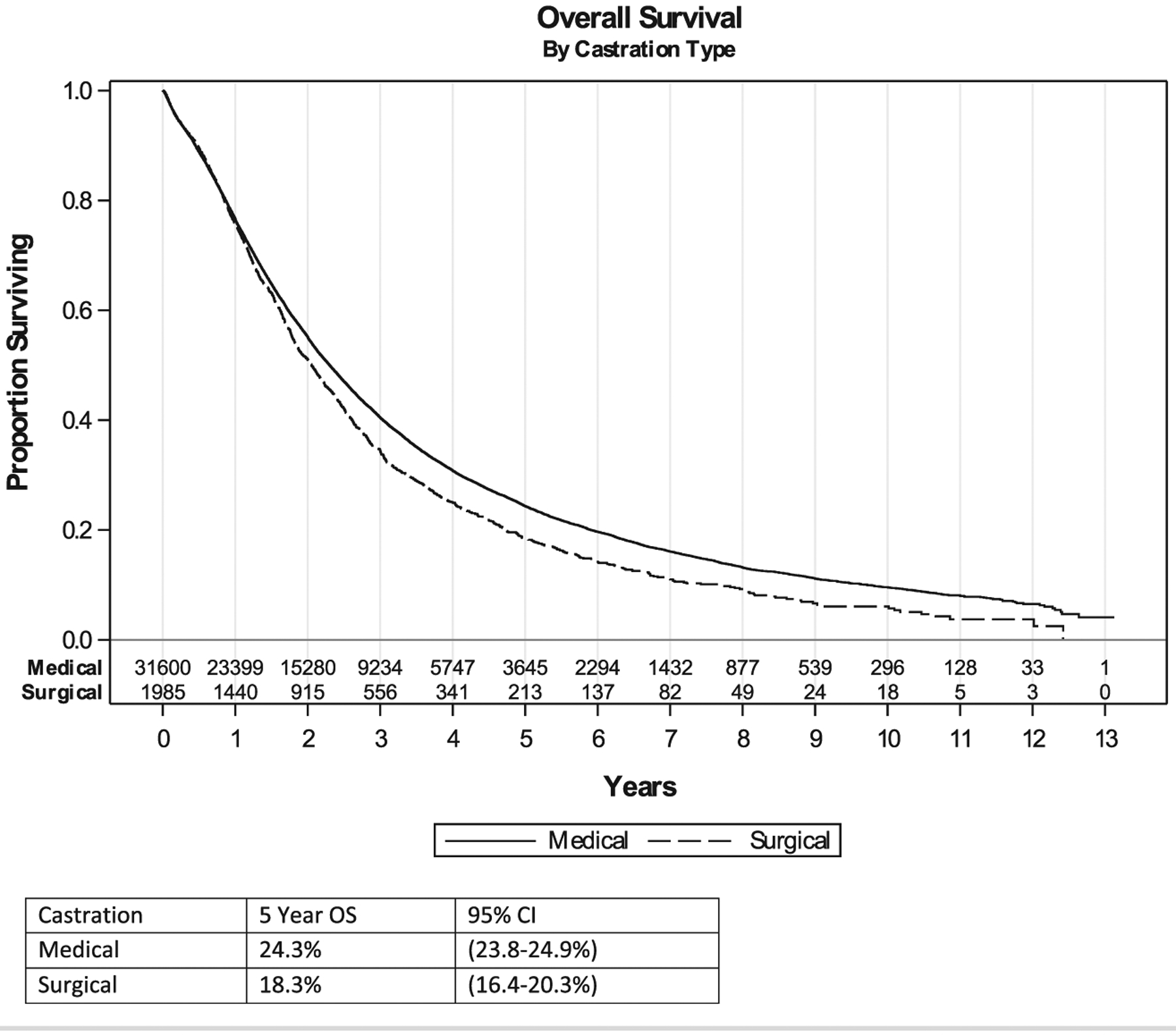
Abbreviations: CI = confidence interval; OS = overall survival.
In addition to castration, we also assessed other variables affecting the survival. We noted significantly lower survival in patients with age ≥ 65 years, in non-academic programs, from areas with relatively low household income (< $48,000), and with non-private payor status (Medicare, Medicaid, or uninsured) (Table 3, Figure 5). Compared with patients with no comorbidities, patients with comorbidities had worse outcomes. Patients who received chemotherapy had lower survival in the multivariable analysis.
Table 3.
Survival Analysis
| Univariable | Multivariable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Covariate | Level | HR | 95% CI | P Value | HR | 95% CI | P Value | ||
| Facility type | Not academic/research program | 1.34 | 1.27 | 1.41 | <.01 | 1.25 | 1.20 | 1.31 | <.01 |
| Academic/research program | Ref | - | - | - | Ref | - | - | - | |
| Location | Mountain-Pacific | 0.93 | 0.87 | 1.00 | .04 | 0.95 | 0.90 | 1.00 | .03 |
| New England-Atlantic | 0.98 | 0.92 | 1.04 | .54 | 0.99 | 0.95 | 1.04 | .73 | |
| Central | Ref | - | - | - | Ref | - | - | - | |
| Age | 65+ | 1.45 | 1.41 | 1.48 | <.01 | 1.21 | 1.16 | 1.26 | <.01 |
| <65 | Ref | - | - | - | Ref | - | - | - | |
| Race | Black | 0.97 | 0.93 | 1.01 | .10 | 0.98 | 0.94 | 1.03 | .43 |
| Other | 0.78 | 0.72 | 0.85 | <.01 | 0.81 | 0.75 | 0.88 | <.01 | |
| White | Ref | - | - | - | Ref | - | - | - | |
| Primary payor | Not insured | 1.18 | 1.09 | 1.27 | <.01 | 1.20 | 1.11 | 1.29 | <.01 |
| Medicaid-other government | 1.22 | 1.15 | 1.31 | <.01 | 1.24 | 1.17 | 1.32 | <.01 | |
| Medicare | 1.54 | 1.49 | 1.59 | <.01 | 1.30 | 1.24 | 1.36 | <.01 | |
| Private insurance | Ref | - | - | - | Ref | - | - | - | |
| Census median income quartiles, 2007–2012 | <$38,000 | 1.11 | 1.06 | 1.17 | <.01 | 1.09 | 1.04 | 1.14 | <.01 |
| $38,000-$47,999 | 1.11 | 1.06 | 1.17 | <.01 | 1.06 | 1.02 | 1.11 | <.01 | |
| $48,000-$62,999 | 1.07 | 1.03 | 1.11 | <.01 | 1.03 | 1.00 | 1.07 | .08 | |
| >$63,000 | Ref | - | - | - | Ref | - | - | - | |
| Urban/rural, 2013 | Urban | 1.02 | 0.98 | 1.07 | .29 | 0.93 | 0.89 | 0.97 | <.01 |
| Rural | 1.03 | 0.94 | 1.14 | .53 | 0.91 | 0.82 | 1.01 | .07 | |
| Metro | Ref | - | - | - | Ref | - | - | - | |
| Charlson-deyo score | 1 | 1.39 | 1.33 | 1.44 | <.01 | 1.33 | 1.28 | 1.38 | <.01 |
| 2 | 1.78 | 1.66 | 1.89 | <.01 | 1.66 | 1.56 | 1.78 | <.01 | |
| 3+ | 2.05 | 1.85 | 2.26 | <.01 | 1.96 | 1.77 | 2.17 | <.01 | |
| 0 | Ref | - | - | - | Ref | - | - | - | |
| Surgery of primary site | Yes | 1.03 | 0.99 | 1.07 | .20 | 0.97 | 0.93 | 1.02 | .24 |
| No | Ref | - | - | - | Ref | - | - | - | |
| Receipt of radiatior | Yes | 0.99 | 0.95 | 1.02 | .50 | 0.99 | 0.96 | 1.03 | .72 |
| No | Ref | - | - | - | Ref | - | - | - | |
| Receipt of chemotherapy | Yes | 0.98 | 0.92 | 1.03 | .39 | 1.14 | 1.08 | 1.20 | <.01 |
| No | Ref | - | - | - | Ref | - | - | - | |
| Type of castration | Surgical | 1.16 | 1.10 | 1.23 | <.01 | 1.05 | 0.99 | 1.11 | .13 |
| Medical | Ref | - | - | - | Ref | - | - | - | |
| Year of diagnosis | Units = 1 | 0.97 | 0.96 | 0.97 | <.01 | 0.97 | 0.96 | 0.97 | <.01 |
Abbreviations: CI = confidence interval; HR = hazard ratio; Ref = reference.
Figure 5. Survival Analysis.
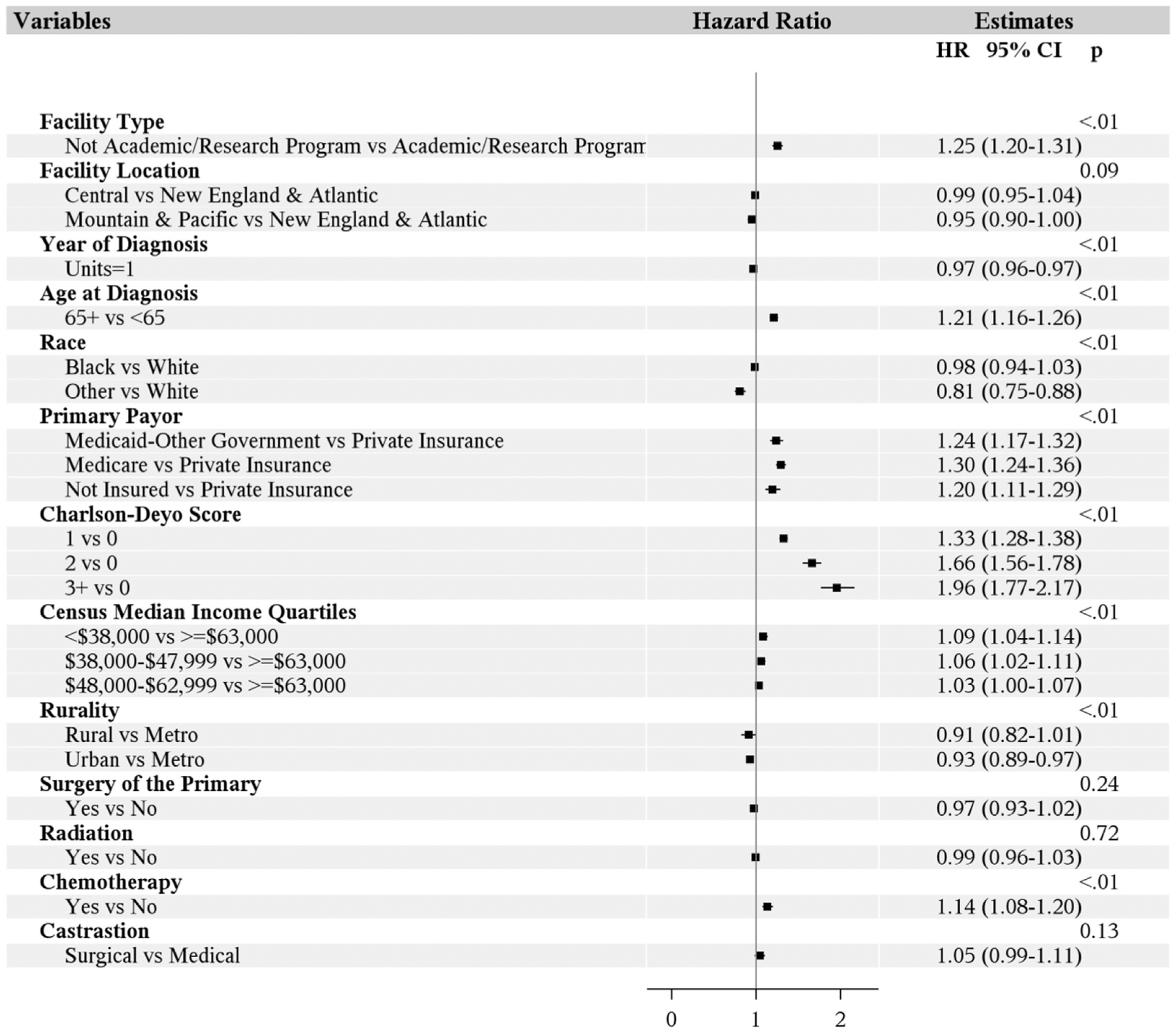
Abbreviations: CI = confidence interval; HR = hazard ratio.
Geographic location of the facility did not significantly influence survival. Blacks have no significant difference in outcomes compared with whites, but other races had significantly better outcomes in comparison with whites. Multivariable analysis shows that urban areas have improved survival in comparison with metropolitan areas, but no significant difference was found in rural areas in comparison with the metropolitan areas. Surgery of the primary site or radiation therapy did not have a significant influence on survival. As the year of diagnosis increases, patients had significantly improved outcomes.
Discussion
Over the past 2 decades, the treatment paradigms have evolved for metastatic prostate cancer with the introduction of potent but rather expensive second-generation anti-androgens such as abiraterone and enzalutamide and with the chemotherapeutic drugs, docetaxel and cabazitaxel. In addition to these agents, the patients also require long-term ADT to lower the testosterone, which could be achieved by a cost-effective surgical castration or expensive hormonal therapy. The effects of continuous ADT with either modality include hot flashes, loss of libido, bone loss, muscle atrophy, and decrease in physical activity. The use of medical ADT allows the option of intermittent dosing. This may reduce costs and side effects but results in a higher prostate cancer-related mortality and is not uniformly recommended for all patients.
Surgical Castration
The efficacy of surgical castration in patients with advanced prostate cancer was first reported by Huggins et al in 1941.13 This discovery was the foundation of prostate cancer management for several decades. Bilateral orchiectomy is a simple, cost-effective outpatient procedure. Also, castration levels of testosterone are reached within 3 to 12 hours as the biological half-life of testosterone is 45 minutes.14 Severe complications are very rare except for minor issues such as wound infection, hematoma, and pain. There is a concern for emotional impact on the mens’ health with the procedure; however, this could be overcome by testicular prosthesis placement or subcapsular orchiectomy.
Medical Versus Surgical Castration
In late 1980s, the advent of GnRH analogues such as goserelin, leuprolide, and triptorelin, and eventually GnRH antagonists such as degarelix as an alternative to surgical castration, led to a surge in medical castration. With GnRH agonists, it is estimated that about 1% to 12.5% of patients do not achieve testosterone levels < 50 ng/mL.15–17
In a systemic review and meta-analysis of more than 6600 patients with advanced prostate cancer, GnRH agonists and anti-androgen therapies were compared with orchiectomy. There was no difference in the 2-year OS between both groups.18 In our analysis as well, there is no significant difference in OS between the 2 groups, which is consistent with the above meta-analysis. Overall, either surgical or medical castration have similar efficacy in advanced prostate cancer.
In 1989, a prospective survey of 159 patients with advanced prostate cancer was conducted regarding their preference of castration with either orchiectomy or a GnRH analog, goserelin. Of the 140 patients who completed the survey, 78% preferred goserelin primarily owing to the avoidance of surgery, as well as the success and convenience of the drug. On the other hand, 22% of patients preferred orchiectomy for castration for the convenience of one-time surgery.19 In concordance to this survey, this trend continues, as is evident in our study where the overall utilization of surgical castration is less than 9%. The odds to prefer surgical castration continues to decline annually. This could be attributed to the perception of orchiectomy as a too-drastic measure with an added emotional impact when compared with medical castration, which has similar efficacy.19,20 Also, it is unknown if patients are provided with these options as equally efficacious with significant cost differences by their physicians.
In our study, we observed that the patients who underwent surgical castration were mostly non-Caucasian, had non-private insurance, lived in lower income (< $63,000) areas, and received care at non-academic centers.
One likely explanation for this observation is that men with low income and government insurance fear the high expenses associated with long-term hormonal therapy and likely prefer orchiectomy. However, this needs to be further validated with prospective surveys of patients and physicians.
Costs of Medical Versus Surgical Castration
In the realm of rising health care expenditure, the financial burden of similarly efficacious treatment options should be considered prior to determining the choice of long-term androgen suppression. Surgical castration expenditures include costs of bilateral orchiectomy, anesthesia, complications of surgery, and follow-up care. Most of these costs will be incurred within the first 6 months postoperatively.21 ADT is one of the costliest treatments for metastatic prostate cancer.22 Whereas costs are incurred mainly within a limited postoperative period in surgical castration, medical castration often requires years of ongoing medication, administration costs, and costs from complications of therapy. The average wholesale price for leuprolide depot is about $1859 per month, and with an annual cost of about $22,300. The average selling price varies from $2500 to $7500 annually.23 A study by Sun et al using the Surveillance, Epidemiology, and End Results (SEER) program showed that total expenditures 1 year after diagnosis of metastatic prostate cancer was $8478.46 with medical castration and $9726.98 after orchiectomy.24 Although the study by Sun et al did not find a financial advantage to orchiectomy, this can be attributed to the limited 12-month follow-up period. Other studies have found that direct treatment costs associated with medical castration range from 6 to 20 times the cost of surgical castration.23 Our study had a 5-year survival rate of 24.3% for those treated with medical castration. Using the previous SEER data, most patient expenditures would be expected to exceed $40,000 within 5 years for this group. Meanwhile, most costs associated with surgical castration would be limited to the $9726.98 incurred within the first year from surgery. Additionally, the study from Sun et al shows a significant increase in fractures, peripheral arterial disease rates, and cardiac-related complications among the medical castration group compared with those undergoing orchiectomy. Overall, surgical castration is an underutilized, safe, and cost-effective approach for metastatic prostate cancer.
Limitations
Although our study has a large number of strengths, it is not entirely devoid of limitations. The primary limitation is its retrospective study design, which is inherently prone to bias. Patient-level data on factors influencing in choosing a castration modality was not available, which is an important limitation of our study. Also, the database does not provide details of individual prostate cancers such as the metastatic burden and does not provide the actual agent or the duration of use for medical castration. The cause of death is not available in the database, which limits cancer-specific survival analysis.
Conclusion
In conclusion, surgical castration is an underutilized, cost-effective treatment for metastatic prostate cancer. In this large contemporary analysis, the utilization of surgical castration has declined over time with no OS difference when compared with medical castration. With rising health care costs, changing the practice by utilizing surgical castration can help reduce health care expenditures. Patients and physicians need to be educated on the financial implications of treatment options. Prospective and patient-level studies should be conducted to identify factors leading to the decline of surgical castration.
Clinical Practice Points.
Prostate cancer is the most common malignancy in men.
Metastatic prostate cancer is treated with ADT, which can be achieved either by surgical castration or medical castration.
In this large contemporary analysis, the utilization of surgical castration has declined over time with no survival difference when compared with medical castration.
Increasing the utilization of surgical castration could help reduce health care expenditures. With rising health care costs, patients and physicians need to be aware of treatment options and their financial implications.
Acknowledgments
The authors from University of Iowa are supported by National Institutes of Health grant P30 CA086862. The research was also supported by University of Iowa Department of Urology Andersen-Hebbeln grant funding for prostate cancer research.
Footnotes
Disclosure
Y. Zakharia reports advisory board for Amgen, Roche Diagnostics, Novartis, Jansen, Eisai, Exelixis, Castle Bioscience, Array, Bayer, Pfizer, Clovis, and EMD Serono; grant/research support (institution clinical trial support) from NewLink Genetics, Pfizer, Exelixis, and Eisai; and DSMC from Jansen. The remaining authors have stated that they have no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.United States Preventive Task Force. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA 2018; 319:1901–13. [DOI] [PubMed] [Google Scholar]
- 3.Vogelzang NJ, Chodak GW, Soloway MS, et al. Goserelin versus orchiectomy in the treatment of advanced prostate cancer: final results of a randomized trial. Urology 1995; 46:220–6. [DOI] [PubMed] [Google Scholar]
- 4.Parmar H, Edwards L, Phillips RH, Allen L, Lightman SL. Orchiectomy versus long-acting D-Trp-6-LHRH in advanced prostatic cancer. Br J Urol 1987; 59:248–54. [DOI] [PubMed] [Google Scholar]
- 5.Niraula S, Le LW, Tannock IF. Treatment of prostate cancer with intermittent versus continuous androgen deprivation: a systematic review of randomized trials. J Clin Oncol 2013; 31:2029–36. [DOI] [PubMed] [Google Scholar]
- 6.Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol 2017; 71:630–42. [DOI] [PubMed] [Google Scholar]
- 7.Loblaw DA, Virgo KS, Nam R, et al. , American Society of Clinical Oncology. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2007 Update of an American Society of Clinical Oncology Practice Guideline. J Clin Oncol 2007; 25:1596–605. [DOI] [PubMed] [Google Scholar]
- 8.Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol 2017; 3:1722–8. [DOI] [PubMed] [Google Scholar]
- 9.American Joint Committee on Cancer. Cancer staging system, Available at: https://cancerstaging.org/CSE/general/Pages/articles.aspx. Accessed: December 3, 2018.
- 10.United States Department of Agriculture Economic Research Service. Rural-urban continuum codes, Available at: https://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx. Accessed: September 7, 2019.
- 11.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000; 53:1258–67. [DOI] [PubMed] [Google Scholar]
- 12.Commission on Cancer. About cancer program categories, Available at: https://www.facs.org/quality-programs/cancer/coc/apply/categories. Accessed: September 7, 2019.
- 13.Huggins C, Stevens RE Jr, Hodges CV. Studies on prostatic cancer: II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg 1941; 43:209–23. [Google Scholar]
- 14.Lin BJ, Chen KK, Chen MT, Chang LS. The time for serum testosterone to reach castrate level after bilateral orchiectomy or oral estrogen in the management of metastatic prostatic cancer. Urology 1994; 43:834–7. [DOI] [PubMed] [Google Scholar]
- 15.Morote J, Esquena S, Abascal JM, et al. Failure to maintain a suppressed level of serum testosterone during long-acting depot luteinizing hormone-releasing hormone agonist therapy in patients with advanced prostate cancer. UrolInt 2006; 77:135–8. [DOI] [PubMed] [Google Scholar]
- 16.Jocham D. Leuprorelin three-month depot in the treatment of advanced and metastatic prostate cancer: long-term follow-up results. UrolInt 1998; 60(Suppl 2): 18–24, discussion: 35. [DOI] [PubMed] [Google Scholar]
- 17.Oefelein MG, Cornum R. Failure to achieve castrate levels of testosterone during luteinizing hormone releasing hormone agonist therapy: the case for monitoring serum testosterone and a treatment decision algorithm. J Urol 2000; 164:726–9. [DOI] [PubMed] [Google Scholar]
- 18.Seidenfeld J, Samson DJ, Hasselblad V, et al. Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Ann Int Med 2000; 132:566–77. [DOI] [PubMed] [Google Scholar]
- 19.Cassileth BR, Soloway MS, Vogelzang NJ, et al. Patients’ choice of treatment in stage D prostate cancer. Urology 1989; 33(5 Suppl):57–62. [DOI] [PubMed] [Google Scholar]
- 20.Loblaw DA, Mendelson DS, Talcott JA, et al. , American Society of Clinical Oncology. American Society of Clinical Oncology recommendations for the initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer. J Clin Oncol 2004; 22:2927–41. [DOI] [PubMed] [Google Scholar]
- 21.Mariani AJ, Glover M, Arita S. Medical versus surgical androgen suppression therapy for prostate cancer: a 10-year longitudinal cost study. J Urol 2001; 165:104–7. [DOI] [PubMed] [Google Scholar]
- 22.Skolarus TA, Zhang Y, Miller DC, Wei JT, Hollenbeck BK. The economic burden of prostate cancer survivorship care. J Urol 2010; 184:532–8. [DOI] [PubMed] [Google Scholar]
- 23.Henry MA, Leung A, Filson CP. Cost considerations for systemic therapy for patients with advanced genitourinary malignancies. Cancer 2018; 124:2897–905. [DOI] [PubMed] [Google Scholar]
- 24.Sun M, Choueiri TK, Hamnvik OP, et al. Comparison of gonadotropin-releasing hormone agonists and orchiectomy: effects of androgen-deprivation therapy. JAMA Oncol 2016; 2:500–7. [DOI] [PubMed] [Google Scholar]


