Abstract
Cannabis legalization and commercialization has resulted in novel alternative cannabis products on the market, including edible and vaporized cannabis, which may appeal to youth with psychiatric problems. Psychiatric comorbidity in adolescent use and poly-use (i.e., use of >2 products) of combustible, edible, and vaporized cannabis products has largely gone uninvestigated. This 2015 cross-sectional survey of Los Angeles, California area adolescents (Mage=16.1, N=3177) characterized associations of various psychiatric problems with use and poly-use of combustible, edible, and vaporized cannabis. Exposure variables included past 30-day non-cannabis substance use (alcohol, e-cigarettes, combustible cigarettes, and nonmedical prescription opioid and stimulant use; yes/no), and psychiatric problems including past-week depressive symptom frequency, past 6-month ADHD symptom and conduct problem frequency, anhedonia, and five dimensions of impulsivity (sensation seeking, perseverance, lack of premeditation, positive urgency, and negative urgency). Outcome variables included past 30-day use (yes/no) of combustible, edible, and vaporized cannabis, independently, and number of cannabis products used (single, dual-use, poly-use). Results showed that all forms of non-cannabis substance use (ORs range: 13.7–36.1) and all psychiatric symptoms and traits (ORs in SD units range: 1.1–2.1) were positively associated with combustible, edible, and vaporized cannabis product use. The magnitude of comorbidity did not significantly differ by cannabis product type used in most cases. Psychiatric comorbidity was typically stronger in poly-product than single-product cannabis use and greater for externalizing-type than internalizing-type psychiatric problems. Practitioners, policy makers, and scientists should be aware that pervasive psychiatric comorbidity may be observed across the spectrum of cannabis product use among adolescents, particularly poly-product users.
Keywords: cannabis, psychiatric comorbidity, adolescents, smoking, vaping, edible
INTRODUCTION
Adolescent cannabis use is highly comorbid with various psychiatric problems, including use of other substances (Gattamorta, Mena, Ainsley, & Santisteban, 2017; Gobbi et al., 2019). Youth with psychiatric or substance use problems who also use cannabis are at a substantially high risk of adverse health, social, occupational, and legal outcomes in adolescence and throughout adulthood (Schulte & Hser, 2014; Volkow, Baler, Compton, & Weiss, 2014). Such adverse outcomes underscore the importance of characterizing psychiatric comorbidity in adolescent cannabis use, particularly in the current milieu in which recent increases in the prevalence of cannabis use in U.S. youth has been observed (Johnston et al., 2019).
Extant analyses of psychiatric comorbidity in adolescent cannabis use predominately study use of combustible traditional plant forms of cannabis only (Gobbi et al., 2019). With cannabis legalization trends, novel non-combustible (i.e., alternative) cannabis products have become increasingly available in the U.S., including cannabinoid-infused food and drinks as well as electronic vaporizers used to heat cannabis plant or liquid extracts into inhalable aerosol with minimal or no combustion (Budney & Borodovsky, 2017). The wide spectrum of different cannabis products currently available also provides the opportunity for poly-cannabis use (i.e., use of >2 different cannabis products). Recent research finds that alternative cannabis product use and poly-cannabis use is not uncommon among adolescents and may be associated with other (non-cannabis) substance use (Kowitt et al., 2019; Meier, Docherty, Leischow, Grimm, & Pardini, 2019; Nguyen et al., 2019). Yet, head-to-head comparisons of the magnitude of association between use of non-cannabis substances and cannabis use across different cannabis products is lacking in the literature, leaving unclear whether co-use is less or more prominent for certain cannabis products. Additionally, there is limited evidence on the association of non-substance-related psychiatric problems that commonly present in adolescence (e.g., depression, conduct problems, Attention Deficit Hyperactivity Disorder [ADHD]) and the underlying vulnerability traits that give rise to behavioral health problems (e.g., impulsivity, anhedonia) with cannabis product use across different cannabis products. These are important gaps; estimates of the extent to which various forms of psychiatric comorbidity (including use of non-cannabis substances, traditional syndrome-based indices of mental health symptoms [e.g., depression and ADHD] and cross-cutting vulnerability traits implicated in multiple psychiatric disorders [e.g., impulsivity, anhedonia; Insel, 2010]) extend to adolescent use and poly-use of across the spectrum of cannabis products are needed to guide pediatric psychiatric and cannabis use health services and policy.
There are reasons to hypothesize that patterns of psychiatric comorbidity may not generalize across combustible, edible, and vaporized cannabis use or across single-product and poly-product forms of cannabis use. Alternative cannabis products may possess unique characteristics that are attractive to a wider segment of the youth population in comparison to combustible cannabis (e.g., combustible may be perceived to have greater respiratory health effects than vaporized and edible products; Budney & Borodovsky, 2017; Popova et al., 2017), edible and vaporized cannabis products are available in palatable preparations not present in traditional combustible cannabis (Budney & Borodovsky, 2017), and vaporized and edible cannabis products lack the smell of smoke and are therefore easier to conceal from authority figures than combustible cannabis (Budney, Sargent, & Lee, 2015). Such unique features of alternative cannabis products may be more salient to youth without (vs. with) certain psychiatric comorbidities (e.g., youth with conduct problems may not necessarily value the ability to conceal cannabis use from authority figures), which could narrow the magnitude of psychiatric-related disparity in cannabis use for alternative products in comparison to combustible cannabis. Additionally, combustible cannabis may result in more efficient cannabinoid delivery than edible cannabis (Newmeyer et al., 2016), which, in turn, could engender greater cannabinoid-induced neuropsychiatric consequences. Poly-product (vs. single-product) cannabis use could lead to increased exposure to a greater diversity of cannabinoid compounds and corresponding neuropsychiatric consequences, which may also appeal to youth with the most extensive psychiatric problems. Consequently, psychiatric comorbidity could be stronger for poly-product than single-product cannabis use.
This cross-sectional survey of 10th grade students in Los Angeles, CA estimated associations of psychiatric comorbidity indicators (use of non-cannabis substances, traditional syndrome-based indices of mental health symptoms, and cross-cutting vulnerability traits implicated in multiple psychiatric disorders) with adolescent use and poly-use of combustible, edible, and vaporized cannabis products. The primary aim was to test whether each psychiatric comorbidity variable predicted odds of cannabis use, focusing on whether the magnitude of this association: 1) differed depending on whether the cannabis product used was a combustible, edible, and vaporized product; and 2) was stronger in the prediction of poly-product (vs. no) cannabis use than the prediction of single-product (vs. no) cannabis use. It was hypothesized that psychiatric comorbidity would be stronger with use of combustible cannabis than alternative cannabis products and with poly-product than single-product cannabis use.
METHODS
Participants and Procedures
Data were drawn from the Happiness and Health Study, a cohort study of students in 10 high schools in the Los Angeles area. Study method details have been previously reported elsewhere (Leventhal et al., 2015). Schools were recruited based on their proximity to the research site and their representation of a diverse cross-section of urban and suburban communities, which collectively form a sociodemographically heterogeneous sample. Paper-and-pencil surveys were administered in students’ classrooms on site. Students not in class during data collections completed abbreviated surveys by telephone, Internet, or mail. Survey items assessing alternative cannabis product use were introduced in the spring 2015 (10th grade) survey. Of 4,100 eligible 9th grade students in 2013, 3,396 (83%) assented and consented to enroll in the cohort. Among 3,251 participants administered the spring 2015 survey, 3,177 provided cannabis use data, constituting this report’s analytic sample. The University of Southern California institutional review board approved the study.
Measures
Cannabis Product Use
Based on the Youth Risk Behavior Surveillance Survey (YRBS) and the Monitoring the Future (MTF) Questionnaires (Eaton, 2010; Johnston et al., 2019), a survey module assessing number of days used in the past 30 days of various substances, with separate items for each substance was administered. Combustible, edible, and vaporized cannabis were assessed in distinct items worded as follows: a) combustible, “smoking marijuana (pot, weed, hash, reefer, or bud),” b) edible, “marijuana or THC food or drinks (pot brownies, edibles, butter, oil),” and c) vaporized, “electronic device to vape liquid THC or hash oil (liquid pot, dabbing, weed pen).” The distribution of past 30-day number of days used responses were highly zero-inflated (86.5%, 92.1%, and 95.1% indicated 0 days of use of combustible, edible, and vaporized cannabis, respectively) and were recoded into binary past 30-day use (yes/no), which served as the primary outcomes in this study; the M(SD) number of days used are reported for descriptive purposes.
Psychiatric Comorbidity Measures
Other substance use
YRBS and MTF derived questions assessing past 30-day use of alcohol, e-cigarettes to vape nicotine, and combustible cigarettes, and nonmedical use of prescription opioids (with item examples “Oxycontin,” “Percocet,” “Vicodin,” and “Codeine”) and stimulants (“Ritalin” “Adderall” “Dexedrine”). Students were instructed to report only use “without a doctor’s orders to get high or have fun.”
Psychiatric symptoms and traits
Several syndrome-based symptom measures appropriate for youth samples were administered. Depressive symptoms was assessed with the Center for Epidemiologic Studies Depression Scale (CESD; Radloff, 1991) which provides past-week symptom frequency self-rating (0= ‘0–1 days’ to 3= ‘5–7 days’) summed across 20 items. Conduct problems was measured using the mean frequency ratings for engaging in 11 conduct problem behaviors (e.g., stealing, lying to parents; 1=Never to 6=Ten or more times) in the past 6 months (Thompson, 2007). The Current Symptoms Self-Report Form (Barkley, 1998) was used to measure Diagnostic and Statistical Manual of Mental Disorders–4th Edition (DSM-IV; American Psychiatric Association, 2004) ADHD symptoms. Respondents rated each symptom’s past 6-month frequency (‘Never or Rare’=0, ‘Sometimes’=1, ‘Often’=2, ‘Very Often’=3). The total score was summed within the 9 inattention (e.g., difficulty organizing and completing tasks) and 9 hyperactivity/impulsivity (e.g., trouble remaining still or with task persistence) symptom domain subscales.
Cross-cutting psychiatric traits were assessed using measures with adequate psychometric properties in prior youth samples (Claes & Muehlenkamp, 2013). Anhedonia was measured using the Snaith-Hamilton Pleasure Scale (SHAPS; Snaith, 1995), which includes 14 self-statements regarding pleasure response to common pleasant experiences (e.g., “I would enjoy seeing others’ smiling faces”; 1=strongly agree, 4=strongly disagree). Subscales from the multidimensional UPPS-P impulsive behavior measure (Whiteside, 2005) was used to assess personality traits indicative of proneness to risky behavior and decision-making stemming cognitive styles and emotional states. We administered the UPPS-P 12-item sensation seeking (e.g., “I generally seek new and exciting experiences and sensations”), 11-item lack of premeditation (e.g., “I usually make up my mind through careful reasoning”), 10-item perseverance (e.g., “I generally like to see things through to the end”), and 14-item positive urgency and 12-item negative urgency scales that assess tendency to act rashly during positive and negative emotional states, respectively. Response to UPPS-P self-statement items are rated from 1=‘Disagree strongly’ to 4=‘Agree strongly.’ The mean response of items within each UPPS-P subscale was calculated.
Demographic Covariates
Demographic measures were included describe the sample and to include as adjustment covariates. At enrollment, participants self-reported their gender, age, race/ethnicity (response options: White, Black, Hispanic, Asian, American Indian/Alaska Native, Native Hawaiian/Pacific Islander, multiethnic or multiracial, or other), parent highest education (six choices: ranging from 8th grade or less to advanced college degree), and eligibility for free/subsidized lunch public program (yes/no; i.e., youth with family income <185% of federal poverty limit). Respondents who selected American Indian/Alaska Native (n=32, 1.0%), Native Hawaiian/Pacific Islander (n=136, 4.4%), or Other (n=48, 1.5%) for the race/ethnicity item, were collapsed into an “Other” category due to smaller sample sizes. To capture education and income in a socioeconomic status (SES) composite variable, we defined ‘high’ SES as youth who reported their parents attended college or higher education level (n=1946, 61.3%) and being ineligible for free/reduced lunch (n=1424, 44.8%) as in prior work (Peters, Bae, Barrington-Trimis, Jarvis, & Leventhal, 2018). Remaining participants were classified ‘low’ SES.
Statistical Analysis
After descriptive analyses, binary logistic regression models were used to estimate the association of a single comorbidity indicator regressor variable with a past 30-day cannabis product use outcome variable (yes/no), with separate models tested for each combination of psychiatric comorbidity variable and cannabis product outcome. We then conducted Generalized Estimating Equation (GEE) models, for which the dataset was structured in ‘long’ format, such that each participant had three rows of data—one for each product. This allowed representation of use of each of the three products via a single cannabis use outcome variable (yes/no) in combination with a new within-participant regressor categorical variable specifying the product (combustible vs. edible vs. vaporized) that the corresponding row’s use observation was representing, with GEEs accounting for within-participant correlation. To determine whether the magnitude—by which each between-subject psychiatric comorbidity regressor variable predicted cannabis use—significantly differed by cannabis product type used, the GEEs tested within-by-between subject regressor interaction terms for each comorbidity indicator (e.g., depressive symptoms × cannabis product type, cigarette use × cannabis product type). Significant interactions indicated that the extent to which the comorbidity regressor predicted cannabis use differed depending on whether the product used was combustible vs. edible vs. vaporized cannabis. Then, standard polytomous logistic regression models were used to examine the association between each comorbidity variable and odds of using one, two, or three (vs. no) cannabis products.
All models included sex, race/ethnicity, and SES as simultaneous regressors and adjusted for age and school fixed effects (ICC for cannabis use within schools = .02, suggesting negligible biasing of error terms due to within-school clustering). Adjusted Odds Ratios (ORs) with 95% Confidence Intervals (CIs) from regression models are reported. Continuous psychiatric symptom and trait variables were rescaled into standard deviation units for regression analyses to facilitate cross-measure interpretation of association estimates. Raw p-values from two-tailed tests were considered statistically significant after correction for multiple testing using the Benjamini-Hochberg method to maintain a .05 study-wide false discovery rate (Jafari, Ansari-Pour, & Jafari, 2019). Analyses were restricted to students with available cannabis product use data and available observations for a respective comorbidity variable, resulting in Ns that varied across models due to varying missingness across study variables (see Table 1). Missing demographic covariate data was handled with multiple imputation (Rubin, 1987). Analyses were conducted using SPSS version 23 (IBM SPSS Inc, Armonk, NY, USA).
Table 1.
Descriptive Statistics of Study Variablesa
| N(%) or M(SD) | Nb Available | Cronbach’s alpha | |
|---|---|---|---|
| Demographics | |||
| Female sex | 1715 (54.0) | 3176 | |
| Age, mean (SD) | 16.09 (.41) | 3154 | |
| High SESc | 1099 (39.9) | 2753 | |
| Race/Ethnicity | |||
| Asian | 537 (17.2) | 3126 | |
| Black | 149 (4.8) | ||
| Hispanic | 1510 (48.3) | ||
| White | 507 (16.2) | ||
| Multiethnic or multiracial | 207 (6.6) | ||
| Otherd | 216 (6.9) | ||
| Past 30-Day Cannabis Product Use | |||
| Combustible | 426 (13.5) | 3164 | |
| Edible | 249 (7.9) | 3169 | |
| Vaporized | 156 (4.9) | 3172 | |
| Total number of cannabis products used | |||
| 1 Product | 208 (6.8) | 3155 | |
| 2 Products | 153 (5.0) | ||
| 3 Products | 99 (3.1) | ||
| Psychiatric Comorbidity Indicatorse | |||
| Past 30-day other substance use | |||
| E-Cigarettes | 277 (8.7) | 3172 | |
| Cigarettes | 154 (4.9) | 3172 | |
| Alcohol | 677 (21.4) | 3162 | |
| Prescription Stimulants | 66 (2.1) | 3171 | |
| Prescription Opioids | 97 (3.1) | 3172 | |
| Psychiatric symptoms and traits, mean (SD) | |||
| Conduct problems | 14.72 (5.87) | 3086 | .84 |
| ADHD inattentive symptoms | 6.82 (5.55) | 2869 | .90 |
| ADHD impulsivity symptoms | 6.76 (5.29) | 2868 | .86 |
| Negative urgency | 24.64 (8.42) | 2845 | .92 |
| Positive urgency | 22.39 (9.30) | 2829 | .96 |
| Lack of premeditation | 24.19 (7.84) | 2816 | .95 |
| Lack of perseverance | 22.71 (5.77) | 2808 | .86 |
| Sensation seeking | 31.92 (9.04) | 2817 | .93 |
| Anhedonia | 24.69 (8.88) | 3084 | .95 |
| Depressive symptoms | 16.19 (12.69) | 3113 | .85 |
Data are expressed as No. (%) unless otherwise indicated.
Number of students with nonmissing data for respective data and denominator for percent calculations.
High SES is defined as parental education equal or higher than some college degree (n = 1946, 61.3%) and family income higher than 185% the US poverty line (i.e., respondents who are not eligible for free or reduced lunch, n = 1424, 44.8%). Low SES is defined as the other respondents.
Other category combines American Indian/Alaska Native (n = 32, 1.0%), Native Hawaiian/Pacific Islander (n = 136, 4.4%), and respondents who did not self-identify with any of the categories provided (n = 48, 1.5%).
See Methods for operational definition of each psychiatric comorbidity variable.
RESULTS
Descriptive Results
Students in the analytic sample (N=3177; M[SD] age = 16.1[0.4] years; 54.0% female) were sociodemographically diverse (Table 1). Past 30-day use prevalence in the overall sample of 3,177 adolescents was 13.5% (n=426), 7.9% (n=249), and 4.9% (n=146) for combustible, edible, and vaporized cannabis products, respectively. Single-product (6.8%; n=208), dual-product (5.0%; n=153), and poly-product (3.1%; n=99) past 30-day cannabis use was of appreciable prevalence in the overall sample. Among past 30-day users of each respective product, number of days used in the past 30 days was moderate for combustible (M[SD]=9.6[9.9]), edible (M[SD]=6.9[8.6]), and vaporized cannabis (M[SD]=8.4[9.3]). Low to moderate levels of psychiatric symptoms and traits were observed on average, but SD estimates indicate substantive inter-individual variability for each indicator. Internal consistency estimates for each multi-item scale indicated adequate internal reliability (Table 1).
Psychiatric Comorbidity with Cannabis Use, by Product Type
Non-cannabis substance use
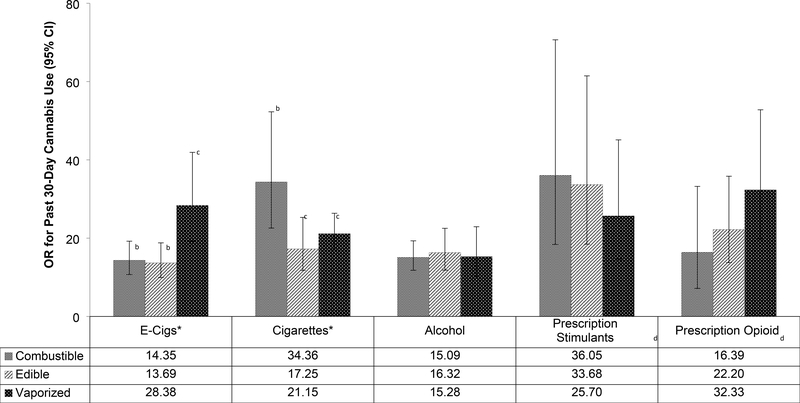
Illustrated in Table 2 and Figure 1, cannabis use prevalence estimates and logistic regression models adjusted for demographic variables found statistically significant large associations between past 30-day use of each non-cannabis substance and past 30-day use of each cannabis product. For example, students who did vs. did not use prescription opioids within the past 30 days had greater odds of reporting past 30-day use of combustible (OR=16.4), edible (OR=22.2) and vaporized cannabis (OR=32.3). Covariate-adjusted comorbidity indicator × cannabis product type interaction tests indicated that past 30-day e-cigarette nicotine use was more strongly associated with past 30-day vaporized (vs. combustible and edible) cannabis use (OR[95%CI]=28.4[19.2–41.9]); prevalence of vaporized cannabis use in e-cigarette users vs. non users, 35.0% vs. 2.0%) than past 30-day use of combustible (OR[95%CI]=14.4[10.7–19.2], 57.8% vs. 9.1%) and edible (OR[95%CI]=13.7[10.0–18.8], 39.0% vs. 4.9%) cannabis, which had association estimates that were not significantly different from one another. Significant interactions were observed demonstrating that cigarette smoking was more strongly associated with use of combustible (OR[95%CI]=34.4[22.6–52.3], 75.3% vs. 10.2%) than edible (OR[95%CI]=17.3[11.8–25.4], 46.8% vs. 5.9%) or vaporized (OR[95%CI]=21.2[17.1–26.4], 38.3% vs. 3.2%) cannabis were observed. Associations of alcohol use and nonmedical use of prescription stimulant and prescription opioids with cannabis use did not significantly differ between cannabis product types.
Table 2.
Past 30-Day Cannabis Product Use and Poly-Use Prevalance,a Stratified by Past 30-Day Non-Cannabis Substance Use
| By cannabis product type |
By number of cannabis products used |
||||||
|---|---|---|---|---|---|---|---|
| Combustible | Edible | Vaporized | No use | Single product | Dual-Product | Poly-Product | |
| E-Cigarettes | |||||||
| User (n=277) | 160 (57.8) | 108 (39.0) | 97 (35.0) | 97 (35.0) | 60 (21.7) | 55 (19.9) | 65 (23.5) |
| Non-user (n=2895) | 264 (9.1) | 141 (4.9) | 59 (2.0) | 2603 (89.9) | 154 (5.3) | 104 (3.6) | 34 (1.2) |
| Combustible cigarettes | |||||||
| User (n=154) | 116(75.3) | 72 (46.8) | 59 (38.3) | 33(21.4) | 37 (24.0) | 42 (27.3) | 42 (27.3) |
| Non-user (n=3018) | 308 (10.2) | 177 (5.9) | 97 (3.2) | 2667 (88.4) | 177 (5.9) | 117 (3.9) | 57 (1.9) |
| Alcohol | |||||||
| User (n=677) | 298 (44.0) | 189 (27.9) | 121 (17.9) | 347 (51.3) | 132 (19.5) | 118 (17.4) | 80 (11.8) |
| Non-user (n=2485) | 121 (4.9) | 57 (2.3) | 33 (1.3) | 2348 (94.5) | 81 (3.3) | 38 (1.5) | 18 (0.7) |
| Prescription Stimulants | |||||||
| User (n=66) | 51 (77.3) | 44 (66.7) | 33 (50.0) | 14 (21.2) | 6 (9.1) | 16 (24.2) | 30 (45.5) |
| Non-user (n=3105) | 372 (12.0) | 204 (6.6) | 123 (4.0) | 2686 (86.5) | 208 (6.7) | 142 (4.6) | 69 (2.2) |
| Prescription Opioids | |||||||
| User (n=97) | 62 (63.9) | 53 (54.6) | 46 (47.4) | 30 (30.9) | 10 (10.3) | 20 (20.6) | 37 (38.1) |
| Non-user (n=3075) | 363 (11.8) | 196 (6.4) | 110 (3.6) | 2669 (86.8) | 205 (6.7) | 139 (4.5) | 62 (2.0) |
Data are expressed as No. (%).
Figure 1.
Association of Past 30-Day Non-Cannabis Substance Use with Past 30-Day Cannabis Use, by Cannabis Product Typea
aEstimates from binary logistic regression generalized estimating equation model regressing past 30 -day use of respective cannabis product on past 30-day use of non-cannabis stubstances adjusting for school and demographic characteristics. Separate models were tested for each combination crossing the specific non-cannabis substance use and each of the three cannabis product use outcomes.
b,c Pairs of products with different letter superscripts demonstrated statistically significant differences in the association of the comorbidity variable with cannabis use in follow-up pairwise contrasts to significant omnibus comorbidity variable × cannabis product type interations.
dNonmedical use of prescription drug to get high and without a doctor’s orders.
*The magnitude of the associations between use of respective substance and cannabis use significantly differed by product type evidence by substance × cannabis product type interaction statistically significant after correction to maintain study-wise false discoverate rate of .05.
Abbreviations: OR = Odds Ratio; CI = Confidence Interval; E-cigs = e-cigarettes
Psychiatric symptoms and traits
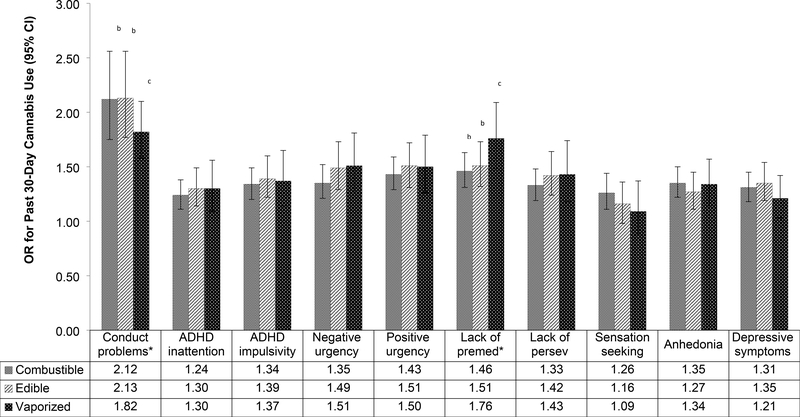
Depicted in Figure 2, there were statistically significant covariate-adjusted associations of each psychiatric symptom and trait score with past 30-day use of each cannabis product. For example, each increase in 1 standard deviation unit in depressive symptoms was associated with greater odds of past 30-day use of combustible (OR=1.31), edible (OR=1.35), and vaporized (OR=1.32) cannabis products. Interaction tests indicated that presence of conduct problems was more strongly associated with combustible (OR[95%CI]=2.1[1.8–2.6]) and edible (OR[95%CI]=2.1[1.8–2.6]) cannabis use than vaporized cannabis (OR[95%CI]=1.8[1.6–2.1]) use; associations with combustible and edible product use did not differ. Lack of premeditation was more strongly associated with past 30-day use of vaporized (OR[95%CI]=1.8[1.5–2.1]) than edible (OR[95%CI]=1.5[1.3–1.7]) or combustible (OR[95%CI]=1.5[1.3–1.6]) cannabis products, which did not significantly differ. Associations of the 8 remaining other psychiatric symptoms and traits with cannabis use did not differ between cannabis product types.
Figure 2.
Association of Psychiatric Symptoms and Traits with Past 30-Day Cannabis Use, by Cannabis Product Typea
aEstimates from binary logistic regression generalized estimating equation model regressing past 30 day use of respective cannabis product on respective psychiatric symptom or trait indicator standardized standard deviation unit score (M=0, SD=1) adjusting for school and demographic characteristics. Separate models were tested for each combination crossing the specific psychiatric symptom/trait and use of each of the three cannabis products.
b,c Pairs of products with different letter superscripts demonstrated statistically significant differences in the association of the comorbidity variable with cannabis use in follow-up pairwise contrasts to significant omnibus comorbidity variable × cannabis product type interations.
*The magnitude of the associations between respective psychiatric symptom/trait and cannabis use significantly differed by product type evidence by substance × cannabis product type interaction statistically significant after correction to maintain study-wise false discoverate rate of .05.
Abbreviations: Premed = premeditation; Persev = perseverance; OR = Odds Ratio; CI = Confidence Interval.
Psychiatric Comorbidity with Cannabis Poly-Product Use
In covariate-adjusted polytomous logistic regression models, past 30-day use of each non-cannabis substance was significantly associated with single-, dual-, and poly-product cannabis use relative to use of no cannabis products (Table 3). For each substance (except e-cigarettes), the 95% CIs of ORs for poly-product, dual-product, and single-product use did not overlap, indicating non-cannabis substance use was associated with successively greater odds of co-use of a greater number of cannabis products. All psychiatric symptoms and traits were significantly associated with single-, dual-, and poly-product cannabis use. For conduct problems, ADHD symptoms, and most of the impulsive behavior symptoms scales, a graded pattern of association was observed with larger ORs with increasing numbers of cannabis products used, with non-overlapping 95% CIs for poly-product and single-product use ORs. Estimates of the association of sensation seeking, anhedonia, and depressive symptoms with cannabis product use did not substantively differ across single-, dual-, and poly-product use.
Table 3.
Association of Psychiatric Comorbidity Indicatorsa with Past 30-Day Use and Poly-Use of Cannabis Products
| Odds Ratio (95%CI) relative to no use of cannabis in any form (Reference)b | |||
|---|---|---|---|
| Single Product Use | Dual-Product Use | Poly-Product Use | |
| Past 30-day non-cannabis substance use (yes vs. no) | |||
| E-cigarettes with nicotine | 10.94 (9.26, 12.91) | 14.97 (12.51, 17.90) | 55.34 (44.46, 68.88) |
| Cigarettes | 18.49 (14.71, 23.23) | 35.36 (28.00, 44.66) | 85.44 (65.99, 110.62) |
| Alcohol | 10.60 (9.25, 12.15) | 19.05 (16.04, 22.62) | 33.05 (26.00, 42.02) |
| Prescription stimulants | 6.89 (4.49, 10.56) | 26.15 (18.47, 37.03) | 117.00 (83.62, 163.69) |
| Prescription opioids | 4.53 (3.25, 6.31) | 14.49 (10.96, 19.16) | 91.72 (69.19, 121.60) |
| Psychiatric symptoms and traits (continuous variables in SD units) | |||
| Conduct problems | 1.91 (1.80, 2.03) | 2.30 (2.16, 2.45) | 2.50 (2.34, 2.67) |
| ADHD inattentive symptoms | 1.19 (1.12, 1.27) | 1.13 (1.05, 1.22) | 1.46 (1.34, 1.58) |
| ADHD impulsivity symptoms | 1.28 (1.20, 1.36) | 1.19 (1.11, 1.28) | 1.55 (1.43, 1.68) |
| Negative urgency | 1.22 (1.14, 1.30) | 1.42 (1.32, 1.53) | 1.59 (1.45, 1.75) |
| Positive urgency | 1.29 (1.21, 1.38) | 1.38 (1.28, 1.48) | 1.63 (1.50, 1.77) |
| Lack of premeditation | 1.39 (1.31, 1.48) | 1.41 (1.31, 1.52) | 1.69 (1.55, 1.84) |
| Lack of perseverance | 1.29 (1.21, 1.38) | 1.31 (1.22, 1.42) | 1.43 (1.30, 1.57) |
| Sensation seeking | 1.19 (1.12, 1.28) | 1.24 (1.15, 1.35) | 1.17 (1.06, 1.29) |
| Anhedonia | 1.28 (1.21, 1.36) | 1.33 (1.24, 1.43) | 1.35 (1.25, 1.47) |
| Depressive symptoms | 1.25 (1.18, 1.33) | 1.36 (1.26, 1.45) | 1.33 (1.21, 1.45) |
See Methods for operational definition of each psychiatric comorbidity variable.
From multinomial logistic regression models regressing number of cannabis products used on psychiatric comorbidity indicators adjusting for gender, race/ethnicity, age, and socioeconomic status; separate models were tested for each respective comorbidity indicator.
DISCUSSION
This study provides new evidence indicating that psychiatric comorbidity with adolescent cannabis use extends to use of novel non-combustible cannabis products that are popular in the current milieu. This study also reports, for the first time, evidence that psychiatric comorbidity is particularly prominent in adolescent poly-product cannabis users who concurrently use two or three forms of cannabis. The current updated picture of psychiatric comorbidity in adolescent cannabis use reinforces long-standing concerns about adolescent psychiatric-cannabis use comorbidity (Hasin, 2017) and raises new concerns about heightened psychiatric comorbidity in poly-cannabis use.
Various psychiatric problems, especially non-cannabis substance use, were associated with incrementally higher probability of use of poly-cannabis use. The substantial comorbidity with poly-cannabis use could indicate that various mechanisms that underlie the transmission of risk from psychopathology to cannabis use (e.g., cannabis use as a maladaptive distress coping strategy, susceptibility to peer influences, lower parental monitoring, genetic vulnerability; Gilman, 2017; Hyman & Sinha, 2009; Santucci, 2012) are elevated in youth with particularly high levels of psychopathology. While this study was not designed to test underlying mechanisms of action is it likely that the accumulation of these mechanisms in youth with more extensive psychopathology could result in greater probability of using a wider variety of cannabis products. It is also possible that poly-use (vs. single-product use) of cannabis could expose the user to a greater diversity of cannabinoids and cannabis-related neurotoxicity, which in turn heightens vulnerability to psychiatric problems and non-cannabis substance use. Future longitudinal research determining the reasons, by testing these hypothesized mechanisms, for substantial psychiatric comorbidity in adolescent poly-cannabis use is warranted.
Two key features of this study enabled a thorough determination of whether psychiatric comorbidity generalizes across adolescent combustible and alternative cannabis product use. First, the comprehensive assessment of other substances, including those contributing to recent public health crises affecting youth (e.g., e-cigarette use, nonmedical prescription opioid use; Scholl, Seth, Kariisa, Wilson, & Baldwin, 2019; U.S. Department of Health and Human Services, 2016), increases relevance of this study. The wide-ranging assessment of common psychiatric symptoms and cross-cutting psychiatric traits implicated in a wide range of behavioral problems provide a detailed characterization of the comorbidity profiles associated with use and poly-use of various cannabis products. Second, the statistical methodology of calculating head-to-head comparisons in the magnitude of associations of comorbidity indicators with use of cannabis in combustible versus edible versus vaporized forms increases ability to make inferences about cross-product generalizability. With these methods, the study showed that for all 16 indicators of psychiatric comorbidity measured here, associations were observed with use of edible and vaporized cannabis and, for the majority of cases, the magnitude of comorbidity did not significantly differ by cannabis product type. Consistent with past results focused on traditional combustible cannabis use (Hasin, 2017), psychiatric comorbidity was generally stronger for externalizing (e.g., non-cannabis substance use, conduct problems, lack of premeditation) than internalizing (e.g., depressive symptoms, anhedonia) problem indicators across each cannabis product type.
The largely concordant pattern of comorbidity across different cannabis products demonstrated here does not parallel the adolescent tobacco product literature, which has shown that psychiatric comorbidity and personality dysfunction may be more prominent with combustible cigarette smoking than vaping e-cigarettes (Leventhal et al., 2015; Wills, Knight, Williams, Pagano, & Sargent, 2015). Smoking combustible cigarettes is believed to be more harmful and socially stigmatized than vaping nicotine in youth (Gorukanti, Delucchi, Ling, Fisher-Travis, & Halpern-Felsher, 2017). It has been hypothesized that the threat of adverse physical or social consequences may be less of a deterrent for teens with (vs. without) psychiatric comorbidity and therefore explains stronger comorbidity with combustible cigarettes than nicotine vaping (Leventhal et al., 2015). Although research in adult cannabis users suggest that the perceptions of respiratory harm is perceived to be lower in vaporized than combustible cannabis (Lee, 2016), whether teens believe vaporized cannabis poses fewer negative consequences than combustible cannabis has not been well-studied. This is of particular importance and high research priority following the E-cigarette or Vaping product use Associated Lung Injury (EVALI) outbreak linked to nearly 3,000 hospitalized cases and 60 deaths, which have primarily been attributed to vitamin E acetate in THC-containing products (Centers for Disease Control and Prevention [CDC], 2020).
While psychiatric comorbidity estimates were often similar across cannabis products, there were two exceptions. First, the comorbidity with conduct problems was less prominent for vaporized than combustible and edible cannabis. It is possible that relative magnitude of comorbidity with vaporized cannabis was lower for these two factors because teens who are less likely to engage in rebellious conduct may be more willing to use a cannabis product (i.e., vaporized cannabis) that may be perceived as less risky than combustible and edible cannabis, which may diminish differences in vaporized cannabis use among adolescents with low versus high conduct problems. Qualitative research suggests that teens may have perceptions of adverse consequences from edible cannabis products, including perceptions of increased risk of excessive intoxication and unpleasant psychoactive effects with edible products (Popova et al., 2017). These perceptions of edible and combustible cannabis being more harmful than vaporized cannabis (Friese, Slater, & Battle, 2017) may be a stronger deterrent for youth conduct problems, resulting in stronger comorbidity estimates between these two factors and use of combustible and edible (vs. vaporized) cannabis products. Second and counter to expectations, the lack of premeditation UPPS scale, which measures impulsive decision making, was more strongly associated with vaporized than combustible and edible cannabis. Hence, despite the connection between impulsivity and conduct problems (Romer, 2010), findings show discordant patterns of relative comorbidity across vaporized and non-vaporized cannabis products. From a broad perspective, all forms of psychiatric comorbidity, including conduct problems and impulsive traits, were associated with all forms of cannabis use.
Cannabis-tobacco product co-use also exhibited non-equivalent comorbidity across different cannabis products. There was substantial clustering of co-use via common administration methods; associations between vaping nicotine e-cigarettes and vaping cannabis and between smoking tobacco and smoking cannabis were particularly robust relative to other forms of cannabis-tobacco product co-use. While associations between adolescent cannabis vaping and e-cigarette use have been previously reported (Morean, 2015), whether the strength of this association was stronger than the corresponding associations of e-cigarette use with combustible tobacco use had not been previously tested. Several companies manufacture cannabinoid-containing vaping solutions that are designed to be compatible for use with nicotine vaping devices and some vaping solutions even contain a mixture of both nicotine and cannabinoids (Giroud et al., 2015; Varlet, 2016). The compatibility and mixing of nicotine and cannabis vaping could increase the probability of poly-substance vaping. Additionally, some youth may simply prefer vaping as a method of administration of both nicotine and cannabis because of the availability of palatable flavors or preference for inhaling aerosol, which is less irritating to the airways than combusted smoke (Budney et al., 2015). Other youth may prefer the sensations of smoking and may therefore be likely to co-use combustible tobacco cigarettes (or perhaps cannabis plant rolled in tobacco cigar leaf casings [i.e., blunts; Cobb, Soule, Rudy, Sutter, & Cohn, 2018]) along with combustible cannabis. Future research testing these hypotheses and other potential explanations of why adolescents who use cannabis and tobacco are more likely to do so with certain product types is warranted.
Study limitations include reliance on self-report measures, raising possibility for reporting biases. Also, the data were collected in a single metropolitan region during 2015. The cannabis landscape has since changed and regional variation in policies and access to commercial cannabis products has increased, leaving unclear the extent of generalizability of the study findings. This study focused on comparing cannabis use across methods of administration and lacked measurement of potentially important diversity in cannabis constituents (THC concentration, presence of other cannabinoids), including assessment of concentrate use that may increase comorbidity (Meier et al., 2019). Because the number of past-30 day users were insufficient to provide power to detect association of psychiatric comorbidity with use frequency among users in this general community sample, cross-product comparisons across different gradations of cannabis use frequency among users were not performed and should be addressed in future work including at risk samples of cannabis users.
Conclusion
In conclusion, this study of Los Angeles area adolescents in 2015 found that use of several non-cannabis substances and various psychiatric symptoms and traits were each positively associated with use of both combustible and non-combustible alternative cannabis products. The magnitude of comorbidity generally did not differ by cannabis product type used, except in a few cases, and was typically stronger in poly-product than single-product cannabis use. Practitioners, policy makers, and scientists should be aware that pervasive psychiatric comorbidity may be observed in adolescents across the spectrum of cannabis product use, especially poly-product use.
Footnotes
Conflict of Interest: The authors have no conflicts of interest relevant to this article to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- American Psychiatric Association (2004). Diagnostic and Statistical Manual of Mental Disorders 4th ed, Text Revision. Washington, DC: American Psychiatric Publishing. [Google Scholar]
- Budney A, & Borodovsky J (2017). The potential impact of cannabis legalization on the development of cannabis use disorders. Preventive Medicine(104), 31–36. 10.1016/j.ypmed.2017.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney A, Sargent J, & Lee D (2015). Vaping cannabis (marijuana): parallel concerns to e-cigs? Addiction, 110(11), 1699–1704. doi: 10.1111/add.13036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes L, & Muehlenkamp J (2013). The Relationship between the UPPS-P Impulsivity Dimensions and Nonsuicidal Self-Injury Characteristics in Male and Female High-School Students. Psychiatry Journal, 5. doi: 10.1155/2013/654847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb CO, Soule EK, Rudy AK, Sutter ME, & Cohn AM (2018). Patterns and Correlates of Tobacco and Cannabis co-use by Tobacco Product Type: Findings from the Virginia Youth Survey. Subst Use Misuse, 53(14), 2310–2319. doi: 10.1080/10826084.2018.1473437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D, Kann L, Kinchen S, Shanklin S, Ross J, Hawkins J, … Wechsler H (2010). Youth risk behavior surveillance - United States, 2009. Morbidity and Mortality Weekly Report. Surveillance Summaries. Retrieved from http://search.proquest.com/docview/733139004/ [PubMed] [Google Scholar]
- Friese B, Slater M, & Battle R (2017). Use of Marijuana Edibles by Adolescents in California. The Journal of Primary Prevention, 38(3), 279–294. doi: 10.1007/s10935-017-0474-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattamorta K, Mena M, Ainsley J, & Santisteban D (2017). The Comorbidity of Psychiatric and Substance Use Disorders Among Hispanic Adolescents. Journal of Dual Diagnosis, 13(5), 254–263. 10.1080/15504263.2017.1343965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman J (2017). Neural Correlates of Social Influence Among Cannabis Users. Current Addiction Reports, 4, 53–61. doi: 10.1007/s40429-017-0141-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroud C, de Cesare M, Berthet A, Varlet V, Concha-Lozano N, Favrat B, & Giroud C (2015). E-Cigarettes: A Review of New Trends in Cannabis Use. International Journal of Environmental Research and Public Health, 12(8), 9988–10008. doi: 10.3390/ijerph120809988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Atkin T, Zytynski T, Wang S, Askari S, Boruff J, & Mayo N (2019). Association of Cannabis Use in Adolescence and Risk of Depression, Anxiety, and Suicidality in Young Adulthood: A Systematic Review and Meta-analysis. JAMA Psychiatry, 76(4), 426–434. doi: 10.1001/jamapsychiatry.2018.4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorukanti A, Delucchi K, Ling P, Fisher-Travis R, & Halpern-Felsher B (2017). Adolescents’ attitudes towards e-cigarette ingredients, safety, addictive properties, social norms, and regulation. Prev Med, 94, 65–71. doi: 10.1016/j.ypmed.2016.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS (2017). U.S. Epidemiology of Cannabis Use and Associated Problems. Neuropsychopharmacology, 43(1), 195–212. doi: 10.1038/npp.2017.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman S, & Sinha R (2009). Stress-related factors in cannabis use and misuse: Implications for prevention and treatment. Journal of Substance Abuse Treatment, 36, 400–413. doi: 10.1016/j.jsat.2008.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, & Wang P (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry, 167(7), 748–751. doi: 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Jafari M, Ansari-Pour N, & Jafari M (2019). Why, When and How to Adjust Your P Values? Cell Journal, 20(4), 604–607. doi: 10.22074/cellj.2019.5992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, & Patrick ME (2019). Monitoring the Future national survey results on drug use, 1975–2018: Overview, key findings on adolescent drug use. Retrieved from: https://files.eric.ed.gov/fulltext/ED594190.pdf. [Google Scholar]
- Kowitt S, Osman A, Meernik C, Zarkin G, Ranney L, Martin J, & Goldstein A (2019). Vaping cannabis among adolescents: prevalence and associations with tobacco use from a cross-sectional study in the USA. BMJ Open, 9(6). doi: 10.1136/bmjopen-2018-028535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Crosier B, Borodovsky J, Sargent J, & Budney A (2016). Online survey characterizing vaporizer use among cannabis users. Drug and Alcohol Dependence, 227–233. doi: 10.1016/j.drugalcdep.2015.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal A, Strong D, Kirkpatrick M, Unger J, Sussman S, Riggs N, … Audrain-McGovern J (2015). Association of Electronic Cigarette Use With Initiation of Combustible Tobacco Product Smoking in Early Adolescence. JAMA, 314(7), 700–707. doi: 10.1001/jama.2015.8950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Strong DR, Sussman S, Kirkpatrick MG, Unger JB, Barrington-Trimis JL, & Audrain-McGovern J (2015). Psychiatric comorbidity in adolescent electronic and conventional cigarette use.. Journal of Psychiatric Research, 73, 71–78. doi: 10.1016/j.jpsychires.2015.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier M, Docherty M, Leischow S, Grimm K, & Pardini D (2019). Cannabis Concentrate Use in Adolescents. Pediatrics, 144(3). 10.1542/peds.2019-0338 [DOI] [PubMed] [Google Scholar]
- Morean M, Kong G, Camenga D, Cavallo D, Krishnan-Sarin S, & Morean M (2015). High School Students’ Use of Electronic Cigarettes to Vaporize Cannabis. Pediatrics, 136(4), 611–616. 10.1542/peds.2015-1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmeyer M, Swortwood M, Barnes A, Abulseoud O, Scheidweiler K, & Huestis M (2016). Free and Glucuronide Whole Blood Cannabinoids’ Pharmacokinetics after Controlled Smoked, Vaporized, and Oral Cannabis Administration in Frequent and Occasional Cannabis Users: Identification of Recent Cannabis Intake. Clinical Chemistry, 62(12), 1579–1592. doi: 10.1373/clinchem.2016.263475 [DOI] [PubMed] [Google Scholar]
- Nguyen N, Barrington-Trimis J, Urman R, Cho J, Mcconnell R, Leventhal A, & Halpern-Felsher B (2019). Past 30-day co-use of tobacco and marijuana products among adolescents and young adults in California. Addictive Behaviors, 98 10.1016/j.addbeh.2019.106053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters E, Bae D, Barrington-Trimis J, Jarvis B, & Leventhal A (2018). Prevalence and Sociodemographic Correlates of Adolescent Use and Polyuse of Combustible, Vaporized, and Edible Cannabis Products. JAMA Network Open, 1(5). 10.1001/jamanetworkopen.2018.2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova L, McDonald EA, Sidhu S, Barry R, Richers Maruyama TA, Sheon NM, & Ling PM (2017). Perceived harms and benefits of tobacco, marijuana, and electronic vaporizers among young adults in Colorado: implications for health education and research. Addiction, 112(10), 1821–1829. doi: 10.1111/add.13854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1991). The use of the Center for Epidemiologic Studies Depression Scale in adolescents and young adults. J Youth Adolesc, 20(2), 149–166. doi: 10.1007/BF01537606 [DOI] [PubMed] [Google Scholar]
- Romer D (2010). Adolescent risk taking, impulsivity, and brain development: implications for prevention. Dev Psychobiol, 52(3), 263–276. doi: 10.1002/dev.20442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB (1987). Multiple Imputation for Nonresponse in Surveys. New Yor: John Wiley & Sons Inc. [Google Scholar]
- Santucci K (2012). Psychiatric disease and drug abuse. Curr Opin Pediatr, 24(2), 233–237. doi: 10.1097/MOP.0b013e3283504fbf [DOI] [PubMed] [Google Scholar]
- Scholl L, Seth P, Kariisa M, Wilson N, & Baldwin G (2019). Drug and Opioid-Involved Overdose Deaths — United States, 2013–2017. Retrieved from MMWR Morb Mortal Wkly Rep: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte M, & Hser Y (2014). Substance Use and Associated Health Conditions throughout the Lifespan. Public Health Reviews. Public Health Reviews, 35(2), 1–27. doi: doi: 10.1007/BF03391702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2016). E-Cigarette Use Among Youth and Young Adults. A Report of the Surgeon General. [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, & Trigwell P (1995). A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale.. The British Journal of Psychiatry: The Journal of Mental Science, 167(1), 99–103. [DOI] [PubMed] [Google Scholar]
- Thompson M, Ho C, & Kingree J (2007). Prospective associations between delinquency and suicidal behaviors in a nationally representative sample. Journal of Adolescent Health, 40(3), 232–237. [DOI] [PubMed] [Google Scholar]
- Varlet V, Concha-Lozano N, Berthet A, Plateel G, Favrat B, De Cesare M, & Giroud C (2016). Drug vaping applied to cannabis: Is “Cannavaping” a therapeutic alternative to marijuana? Sci Rep, 6. doi: 10.1038/srep25599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Compton WM, & Weiss SR (2014). Adverse health effects of marijuana use. N Engl J Med, 370(23), 2219–2227. doi: 10.1056/NEJMra1402309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR, Miller JD, & Reynolds SK (2005). Validation of the UPPS impulsive behaviour scale: a four‐factor model of impulsivity. European Journal of Personality, 19(7), 559–574. [Google Scholar]
- Wills T, Knight R, Williams R, Pagano I, & Sargent J (2015). Risk factors for exclusive e-cigarette use and dual E-cigarette use and tobacco use in adolescents. Pediatrics, 135(1), e43–51. doi: 10.1542/peds.2014-0760 [DOI] [PMC free article] [PubMed] [Google Scholar]