Abstract
Obesity confers an increased incidence and poorer clinical prognosis in more than 10 cancer types. Paradoxically, obesity may provide protection from poor outcomes in lung cancer. Mechanisms for the obesity-cancer links are not fully elucidated, with altered glucose metabolism being a promising candidate. Using 18F-fluorodeoxyglucose positron-emission-tomography/computed tomography images from The Cancer Imaging Archive, we explored the relationship between body mass index (BMI) and glucose metabolism in several cancers. In 188 patients (BMI mean [SD] = 27.7 [5.1], range = 17.4–49.3 kg/m2), higher BMI was associated with greater tumor glucose uptake in breast cancer (r = 0.36; P = .02) and with lower tumor glucose uptake in non-small cell lung cancer (r = -0.26; P = .048) using two-sided Pearson correlations. No relationship was observed in soft tissue sarcoma or squamous cell carcinoma. Harnessing the National Cancer Institute’s open-access database, we demonstrate altered tumor glucose metabolism as a potential mechanism for the detrimental and protective effects of obesity on breast and lung cancer, respectively.
Obesity, currently affecting 35–40% of adults in the United States (1), is correlated with an increased incidence and poorer clinical outcomes in more than 10 different types of cancer (2). The mechanisms underlying the obesity-associated cancer risk are currently not well defined, but altered glucose metabolism is characteristic of both increased body mass index (BMI) (3) and cancer (4–7). Distinct metabolic mechanisms exist between tumor types at the cellular level (8,9). Epidemiologic data allude to differences at the population level: obesity is detrimental in many cases (10) but seemingly protective in lung cancer (11).
As a tumor’s avidity for glucose underlies the use of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT), we used PET and CT images to examine tumor glucose metabolism in patients with four cancer types: head and neck squamous cell carcinoma (HNSCC) (12), soft tissue sarcoma (STS) (13), breast cancer (14,15), and non-small cell lung cancer (NSCLC) (16–21). Utilizing the National Cancer Institute-funded open-source database, The Cancer Imaging Archive (TCIA) (22), we performed a retrospective cross-sectional analysis to examine tumor glucose uptake (TGU) in patients with obesity-associated and obesity-independent cancers.
All patients with an 18F-FDG PET/CT scan, sex, height, and weight available from TCIA were studied. All subjects provided informed consent in accordance with each site’s institutional review board. If multiple images were available, the earliest PET and CT scan was used to minimize effects of therapy on the tumor. Power analyses for correlation (β = 0.20 and α = 0.05) necessitated a minimum of 29 patients, thus the four tumor types with no less than 29 unique patient PET and CTs were analyzed. Clinical parameters such as tumor stage and medications were unavailable.
PET and CT images were loaded into ImageJ (NIH, Bethesda, MD, USA) with an open source PET-CT Viewer (23), and tissues were analyzed with a fixed-volume approach (24). Briefly, fixed-volume spheres were drawn to measure maximal glucose uptake in tumor, skeletal muscle (deltoid, infraspinatus, or quadriceps), liver, spleen, and subcutaneous and supraclavicular adipose tissue. Standardized uptake values (SUV) were calculated (25). Because BMI alone is related to SUV in obese patients, the Janmahastian Formula was used to calculate and correct for estimated lean body mass (LBM) (26). The primary endpoint was LBM-corrected SUV (SUL) (in g/mL) of tumors, as related to BMI (in kg/m2). Maximal SUL was chosen to avoid issues from partial volume effects.
Two-sided Pearson correlations were used to measure BMI vs TGU. A two-way ANOVA was used with Tukey multiple comparisons test to examine differences in TGU across cancer types, between tissues, and between sexes. Data were analyzed in GraphPad Prism 7.0 (San Diego, CA, USA), and REMARK reporting guidelines were used where applicable (27). Statistical significance was determined as P values less than .05.
Sex, height, weight, BMI, estimated LBM, and estimated percent body fat (%BF) were reported (Supplementary Table 1, available online). Among 97 women and 91 men, BMI ranged from 17.4% to 49.3 kg/m2 (mean [SD] = 27.7 [5.1]) and %BF ranged from 11.1% to 55.5%. Many STS PET and CT images were taken from waist down, and some breast cancer images were taken from above L3, thus estimated %BF could not be calculated using the L3 vertebral level method (28).
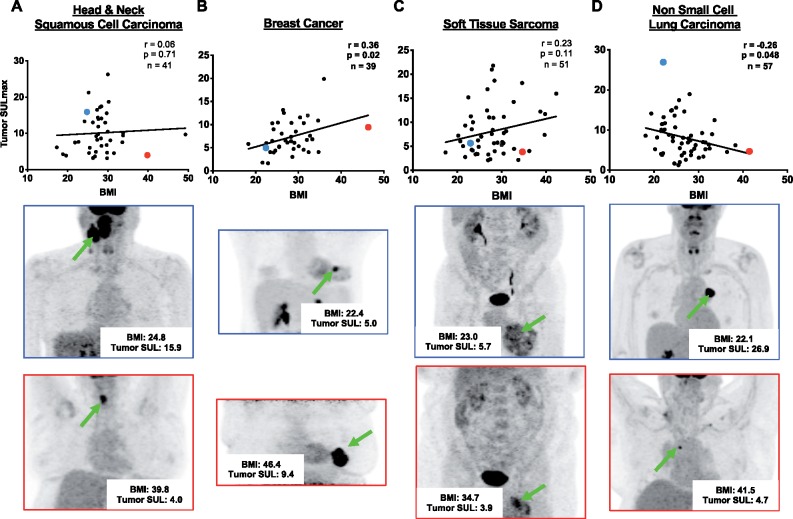
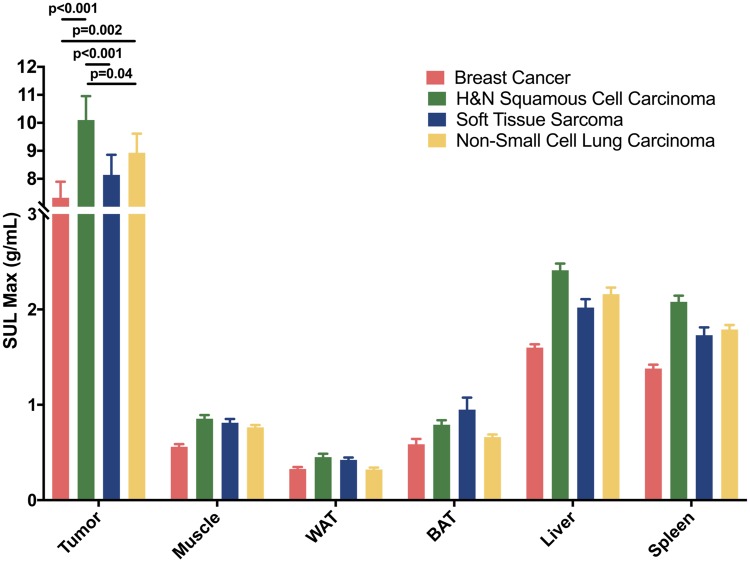
PET and CT data revealed a positive correlation between BMI and TGU in breast cancer (r = 0.36; P = .02), yet a negative correlation in NSCLC (r = −0.26; P = .048) (Figure 1). In these cohorts, we calculated target to background ratio, where tumor SUV was divided by SUV in the descending aorta. Pearson correlations between target to background ratio and BMI for breast cancer (r = 0.25; P= .13) and NSCLC (r = −0.34; P < .001) were calculated. No statistically significant correlations were seen in HNSCC (r = 0.06; P = .71) or STS (r = 0.23; P = .11) (Figure 1). HNSCC TGU was higher than in breast cancer (P < .001), STS (P < .001), and NSCLC (P = .04) (Figure 2). STS TGU was higher than breast cancer TGU (P = .002) (Figure 2). This may be due to a higher proportion of men in the HNSCC group (17.1% female) and STS group (52.9% female) as compared with the 100% female breast cancer cohort, because men had higher glucose uptake in nearly all tissues (Supplementary Table 2, available online). This observation likely reflects men’s higher metabolic rate (29).
Figure 1.
Body mass index vs lean body mass corrected-glucose uptake. Correlations between body mass index (BMI) and lean body mass-corrected maximized standardized uptake value (SULmax) in tumors of (A) head and neck squamous cell carcinoma, (B) breast cancer, (C) soft tissue sarcoma, and (D) non-small cell lung carcinoma. A lean representative subject is highlighted in blue in the scatter plot, the same subject’s maximum intensity projection PET shown beneath with a blue frame, and a green arrow pointing to the tumor. An obese representative subject is highlighted in the red the scatter plot, with his or her maximum intensity projection PET shown beneath with a red frame, and a green arrow pointing to the tumor. A two-sided Pearson correlation was performed, and statistical significance was determined as P < .05. SUL = standardized uptake value.
Figure 2.
Lean body mass-corrected maximal standardized uptake value (SULmax) in tumor; muscle; white adipose tissue (WAT); brown adipose tissue (BAT) from the supraclavicular fat depot; liver; and spleen in all four cancer types. A two-way ANOVA was performed, with Tukey test for multiple comparisons to compare tissue glucose uptake between cancer types. All tests were two-sided, and statistical significance was determined as P < .05. H&N = head and neck.
Tumor SUV intensity has been used as a surrogate for breast cancer time to progression (30). In the PERCIST guidelines (31), lowered SUV in breast cancer is beneficial, whereas treatment nonresponders show no decline in SUV. Further, recent literature has demonstrated the use of the maximum SUV to predict prognosis of NSCLC, with lower pre- and posttreatment SUV showing improved outcomes (32–34). These data suggest tumor glucose metabolism as a link between obesity’s detrimental and protective effects on cancer aggressiveness in breast and NSCLC, respectively.
Our results demonstrate no statistically significant correlation of BMI with TGU in STS (35), but previous reports suggest a weak link between STS severity and obesity (36,37). We detected no correlation between BMI and SUV in HNSCC, supporting a prior study that demonstrates that even in the setting of diabetes, obesity per se is not correlated with HNSCC risk (38). Although our results cannot confirm that TGU is altered by obesity, this study provides a potential explanation for obesity’s role in cancer risk and progression in patients with a wide range of BMIs. Yet, the limited sample size may have reduced the power of our study.
The influence of health and treatment status could not be studied here. Chronic hyperglycemia, higher circulating insulin levels, increased inflammation, elevated glucocorticoids, and more related mechanisms on systemic glucose metabolism in obese patients may impact tumor progression (39). These and other obesity-related factors may reduce TGU in NSCLC and reduce risk (40) and improve survival (41) across all stages and histological subtypes of lung cancer (42). Chemotherapy can increase or decrease tumor and immune cell FDG uptake (43). Antidiabetic therapies [metformin (44) and SGLT2 inhibitors (45)] may also reduce TGU in multiple cancers.
In summary, tumor glucose metabolism was related to BMI in a detrimental and protective manner in breast and lung cancer, respectively. Publicly available PET and CT images were acquired through the National Cancer Institute’s TCIA and analyzed in free open source software, demonstrating the growing utility of shared resources in oncology. Although limited by the availability of clinical information, we highlighted a putative physiological mechanism for obesity’s impact on the aggressiveness of multiple tumor types. Prospective studies should be designed to include clinical parameters to better characterize dysregulated glucose metabolism.
Funding
This study was supported by grants from the US Public Health Service (K99/R00 CA-215315, P50 CA121974-11A1, CTSA UL1TR000142, P30 DK045735), National Institutes of Health Medical Scientist Training Program Training Grant T32GM007205, and by a Yale Cancer Center Innovation Award.
Notes
The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Conflicts of Interest: None.
Author contributions: Conceptualization: BPL and RJP; Data curation: BPL; Formal Analysis: BPL; Funding acquisition: RJP; Investigation: BPL; Methodology: BPL; Visualization: BPL; Writing, original draft: BPL; Writing, review and editing: BPL and RJP.
We would like to thank Ilan Tal for his assistance with image processing and software enhancement.
Supplementary Material
References
- 1. Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL.. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Cancer Institute. Obesity and cancer fact sheet. https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/obesity-fact-sheet. 2017. Accessed August 30, 2019.
- 3. Modan M, Halkin H, Almog S, et al. Hyperinsulinemia. A link between hypertension obesity and glucose intolerance. J Clin Invest. 1985;75(3):809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shaw RJ. Glucose metabolism and cancer. Curr Opin Cell Biol. 2006;18(6):598–608. [DOI] [PubMed] [Google Scholar]
- 5. Bose S, Le A.. Glucose metabolism in cancer In: A Le, ed. The Heterogeneity of Cancer Metabolism. Advances in Experimental Medicine and Biology. New York, NY: Springer International Publishing; 2018:3–12. [DOI] [PubMed] [Google Scholar]
- 6. Wang Y, Nasiri AR, Damsky WE, et al. Uncoupling hepatic oxidative phosphorylation reduces tumor growth in two murine models of colon cancer. Cell Rep. 2018;24(1):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. [DOI] [PubMed] [Google Scholar]
- 8. Cheong H, Lu C, Lindsten T, Thompson CB.. Therapeutic targets in cancer cell metabolism and autophagy. Nat Biotechnol. 2012;30(7):671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rabin-Court A, Rodrigues MR, Zhang X-M, Perry RJ.. Obesity-associated, but not obesity-independent, tumors respond to insulin by increasing mitochondrial glucose oxidation. Plos One. 2019;14(6):e0218126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ.. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. [DOI] [PubMed] [Google Scholar]
- 11. Yang Y, Dong J, Sun K, et al. Obesity and incidence of lung cancer: a meta-analysis. Int J Cancer. 2013;132(5):1162–1169. [DOI] [PubMed] [Google Scholar]
- 12. Grossberg A, Mohamed A, El Halawani H, et al. Data from head and neck cancer CT atlas. 2017. doi:10.7937/k9/tcia.2017.umz8dv6s.
- 13. Vallières M, Freeman CR, Skamene SR, El Naqa I.. A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol. 2015;60(14):5471–5496. [DOI] [PubMed] [Google Scholar]
- 14. Li X, Abramson RG, Arlinghaus LR, Chakravarthy AB, Abramson VG, Sanders M, Yankeelov TE. Data from QIN-Breast. 2016. doi:10.7937/k9/tcia.2016.21juebh0.
- 15. Li X, Abramson RG, Arlinghaus LR, et al. Multiparametric magnetic resonance imaging for predicting pathological response after the first cycle of neoadjuvant chemotherapy in breast cancer. Invest Radiol. 2015;50(4):195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bakr S, Gevaert O, Echegaray S, et al. Data for NSCLC Radiogenomics Collection. Sci Data. 2018;5:180202. [DOI] [PMC free article] [PubMed]
- 17. Gevaert O, Xu J, Hoang CD, Leung AN, et al. Non-small cell lung cancer: identifying prognostic imaging biomarkers by leveraging public gene expression microarray data—methods and preliminary results. Radiology. 2012;264(2):387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Cancer Institute. The Cancer Genome Atlas Program. https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga. Accessed February 21, 2020.
- 19. Madhavi P, Patel S, Tsao AS. Data from anti-PD-1 immunotherapy lung. 2019. doi:10.7937/tcia.2019.zjjwb9ip.
- 20. Albertina B, Watson M, Holback C, et al. Radiology data from The Cancer Genome Atlas Lung Adenocarcinoma [TCGA-LUAD] collection. 2016. doi:10.7937/k9/tcia.2016.jgnihep5.
- 21. Kirk S, Lee Y, Kumar P, et al. Radiology data from The Cancer Genome Atlas Lung Squamous Cell Carcinoma [TCGA-LUSC] collection. 2016. doi:10.7937/k9/tcia.2016.tygkkfmq.
- 22. Clark K, Vendt B, Smith K, et al. The Cancer Imaging Archive (TCIA): maintaining and operating a public information repository. J Digit Imaging. 2013;26(6):1045–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barbaras L, Tal I, Palmer MR, Parker JA, Kolodny GM.. Shareware program for nuclear medicine and PET/CT PACS display and processing. Am J Roentgenol. 2007;188(6):W565–W568. [DOI] [PubMed] [Google Scholar]
- 24. Leitner BP, Huang S, Brychta RJ, et al. Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci. 2017;114(32):8649–8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O JH, Lodge MA, Wahl RL.. Practical PERCIST: a simplified guide to PET response criteria in solid tumors 1.0. Radiology. 2016;280(2):576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tahari AK, Chien D, Azadi JR, Wahl RL.. Optimum lean body formulation for correction of standardized uptake value in PET imaging. J Nucl Med. 2014;55(9):1481–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM.. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst. 2005;97(16):1180–1184. [DOI] [PubMed] [Google Scholar]
- 28. Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE.. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997–1006. [DOI] [PubMed] [Google Scholar]
- 29. Arciero PJ, Goran MI, Poehlman ET.. Resting metabolic rate is lower in women than in men. J Appl Physiol. 1993;75(6):2514–2520. [DOI] [PubMed] [Google Scholar]
- 30. Specht JM, Tam SL, Kurland BF, Gralow JR, et al. Serial 2-[18F] fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET) to monitor treatment of bone-dominant metastatic breast cancer predicts time to progression (TTP). Breast Cancer Res Treat. 2007;105(1):87–94. [DOI] [PubMed] [Google Scholar]
- 31. Wahl RL, Jacene H, Kasamon Y, Lodge MA.. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(suppl 1):122S–150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mu W, Tunali I, Gray JE, Qi J, Schabath MB, Gillies RJ.. Radiomics of 18F-FDG PET/CT images predicts clinical benefit of advanced NSCLC patients to checkpoint blockade immunotherapy. Eur J Nucl Med Mol Imaging. 2019. doi:10.1007/s00259-019-04625-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rossi G, Bauckneht M, Genova C, et al. Comparison between 18F-FDG-PET- and CT-based criteria in non-small cell lung cancer (NSCLC) patients treated with Nivolumab. J Nucl Med. 2019. doi:10.2967/jnumed.119.233056. [DOI] [PubMed] [Google Scholar]
- 34. Brodin NP, Tomé WA, Abraham T, Ohri N.. 18F-fluorodeoxyglucose PET in locally advanced non–small cell lung cancer: from predicting outcomes to guiding therapy. PET Clin. 2020;15(1):55–63. [DOI] [PubMed] [Google Scholar]
- 35. Alamanda VK, Moore DC, Song Y, Schwartz HS, Holt GE.. Obesity does not affect survival outcomes in extremity soft tissue sarcoma. Clin Orthop Relat Res. 2014;472(9):2799–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Montgomery C, Harris J, Siegel E, et al. Obesity is associated with larger soft-tissue sarcomas, more surgical complications, and more complex wound closures (obesity leads to larger soft-tissue sarcomas). J Surg Oncol. 2018;118(1):184–191. [DOI] [PubMed] [Google Scholar]
- 37. Tavani A, Soler M, Vecchia CL, Negri E, Gallus S, Franceschi S.. Body weight and risk of soft-tissue sarcoma. Br J Cancer. 1999;81(5):890–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tseng K-S, Lin C, Lin Y-S, Weng S-F.. Risk of head and neck cancer in patients with diabetes mellitus: a retrospective cohort study in Taiwan. JAMA Otolaryngol Head Neck Surg. 2014;140(8):746–753. [DOI] [PubMed] [Google Scholar]
- 39. Hopkins BD, Goncalves MD, Cantley LC.. Obesity and cancer mechanisms: cancer metabolism. J Clin Oncol. 2016;34(35):4277–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D.. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335(7630):1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lam VK, Bentzen SM, Mohindra P, et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2017;104:52–57. [DOI] [PubMed] [Google Scholar]
- 42. Yang R, Cheung MC, Pedroso FE, Byrne MM, Koniaris LG, Zimmers TA.. Obesity and weight loss at presentation of lung cancer are associated with opposite effects on survival. J Surg Res. 2011;170(1):e75–e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ulaner GA, Lyall A.. Identifying and distinguishing treatment effects and complications from malignancy at FDG PET/CT. RadioGraphics. 2013;33(6):1817–1834. [DOI] [PubMed] [Google Scholar]
- 44. Salani B, Rio AD, Marini C, Sambuceti G, Cordera R, Maggi D.. Metformin, cancer and glucose metabolism. Endocr Relat Cancer. 2014;21(6):R461–R471. [DOI] [PubMed] [Google Scholar]
- 45. Nasiri AR, Rodrigues MR, Li Z, Leitner BP, Perry RJ.. SGLT2 inhibition slows tumor growth in mice by reversing hyperinsulinemia. Cancer Metab. 2019;7(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.