Abstract
Background
Racial disparities in cancer have been attributed to population differences in access to care. Differences in cancer overdiagnosis rates are another, less commonly considered cause of disparities. Here, we examine the contribution of overdiagnosis to observed racial disparities in papillary thyroid cancer and estrogen/progesterone receptor positive (ER/PR+) breast cancer.
Methods
We used Surveillance, Epidemiology, End-Results (SEER) 13 for analysis of white and black non-Hispanic persons with papillary thyroid cancer or ER/PR+ breast cancer (1992–2014). Analyses were performed using SeerStat (v8.3.5, March 2018). All statistical tests were two-sided.
Results
White persons had higher incidence of papillary thyroid cancer than black persons (14.3 vs 7.7 cases per 100 000 age-adjusted population) and ER/PR+ breast cancer (94.8 vs 70.9 cases per 100 000 age-adjusted population) (P < .001). In papillary thyroid cancer, the entire incidence difference was from more frequent diagnosis of 2-cm or less (10.0 vs 4.9 cases per 100 000 population) and localized or regional (13.8 vs 7.4 cases per 100 000 population) cancers in white persons (P < .001), without corresponding excess of metastatic disease, cancers greater than 4 cm, or incidence-based mortality in black persons. In women with ER/PR+ breast cancer, 95% of the incidence difference was from more 2-cm or less (61.2 vs 38.1 cases per 100 000 population) or 2.1- to 5-cm (25.4 vs 23.4 cases per 100 000 population), localized (65.1 vs 43.0 cases per 100 000 population) cancers diagnosed in white women (P < .001), with slightly higher incidence of tumors greater than 5 cm (10.1 vs 9.3 cases per 100 000 population, P < .001) and incidence-based mortality (8.1 vs 7.2 cases per 100 000 population, P < .001) among black women. Overall, 20–30 additional small or localized ER/PR+ breast cancers were diagnosed in white compared with black women for every large or advanced tumor avoided by early detection. Overdiagnosis was estimated 1.3–2.5 times (papillary thyroid cancer) and 1.7–5.7 times (ER/PR+ breast cancer) higher in white compared with black populations.
Conclusions
Differences in low-risk cancer identification among populations lead to overestimation of racial disparities. Estimates of overdiagnosed cases should be considered to improve care and eliminate disparities while minimizing harms of overdiagnosis.
Racial disparities in cancer presentation and survival have been primarily studied in the context of lower levels of access to diagnostic, screening, and treatment modalities. However, racial disparities might also be observed as a result of changes in clinical epidemiology over time. In 1968, Feinstein showed that observed disease incidence rates and survival rates are affected by changing definitions of disease, rates of health-care use, and availability of technology to identify subclinical disease, even if the disease characteristics themselves have not changed (1). For example, wider use of sensitive diagnostic tests can lead to the upstaging of cancers, resulting in stage migration and the spurious appearance of improved survival rates—the so-called “Will Rogers effect.” (2) Later, these observations were extended to include overdiagnosis, the epidemiologic phenomenon in which cancer incidence rates increase because of detection of asymptomatic, early-stage, indolent tumors that were not destined to ever result in symptoms or death (3–6). Increasing overdiagnosis can result from wider use of diagnostic or screening technologies and leads to an increased observed cancer incidence rate despite an unchanged rate of occurrence of the cancer in the population. Overdiagnosis has been described in cancers of the breast, thyroid, prostate, kidney, lung, and skin (melanoma) (3–6). Conceivably, if these epidemiologic factors differ between populations, they could contribute to the appearance of disparities in cancer incidence or survival.
When evaluating cancer disparities, defining the specific contributions of delayed diagnosis and/or overdiagnosis is important, because it informs how much additional screening is likely to help eliminate the disparity. To illustrate, screening might lead to improved detection of potentially lethal disease at an early stage, facilitating curative therapy. Alternatively, if screening leads to increased detection of indolent cancers (overdiagnosis), the group of patients most affected by overdiagnosis will appear to have a higher proportion of early-stage cancers, and survival rates will appear to rise. However, these differences in presentation and survival would be spurious, resulting from increased numbers of overdiagnosed tumors, for which treatment would offer no benefit (7,8). This effect on survival estimates is referred to as “overdiagnosis bias” (9).
In papillary thyroid cancer and estrogen/progesterone receptor positive (ER/PR+) breast cancer, the predominant subtypes of thyroid (86%) and breast (58%) cancers, respectively, there has been recognition of increasing detection of subclinical, indolent disease (overdiagnosis) as well as racial disparities in outcomes (6,10–12). In both cancer types, the incidence is higher in white persons, a higher proportion of black patients present with advanced-stage disease, and black patients experience poorer survival. If these patterns reflect delayed detection in black persons, they could represent the cause of poorer survival with breast and thyroid cancer among black patients in the United States (11,12). On the other hand, if differences in health-care access by race result in higher rates of overdiagnosis in white persons, this could also contribute to the similar appearance of more advanced disease and poorer survival among black persons. Therefore, it is important to examine absolute rates of cancer incidence and mortality (numbers of cases or deaths per unit-population), not solely relative measures such as the proportion of cases presenting at an early stage, or survival rates, which can be influenced by overdiagnosis, lead-time, and length-time biases.
We hypothesized that a portion of the racial disparities observed in papillary thyroid cancer and ER/PR+ breast cancer is attributable to differing rates of overdiagnosis between populations. Here, we estimate the contribution of overdiagnosis to differences in presentation and survival with these cancers among black and white persons in the United States.
Methods
A population-based analysis was performed using the Surveillance, Epidemiology, and End Results (SEER) 13 database (SEERStat v8.3.5; March 2018), which covered a representative 13% of the US population from 1992 to 2014. We analyzed absolute incidence rates (per 100 000, age adjusted to the 2000 US population) of papillary thyroid cancer and ER/PR+ breast cancer and compared white and black non-Hispanic populations with regard to tumor size, American Joint Committee on Cancer seventh edition staging (system in use at the time), and SEER extent of disease (localized, regional, or distant) for 2010–2014. Additionally, we compared the populations in terms of overall survival (relative measure, probability of remaining alive after disease diagnosis), disease-specific survival (relative measure, probability of not dying of cancer after diagnosis), and disease-specific incidence-based mortality (absolute measure, number of patients dying of disease per 100 000 age-adjusted population at risk) in the entire SEER 13 cohort (1992–2014). SEERStat query codes are listed in Supplementary Box 1 (available online). This study used deidentified SEER data via data use agreement with the National Cancer Institute and was deemed exempt from human participant review by the Memorial Sloan Kettering Cancer Center Institutional Review Board.
Statistical Analysis
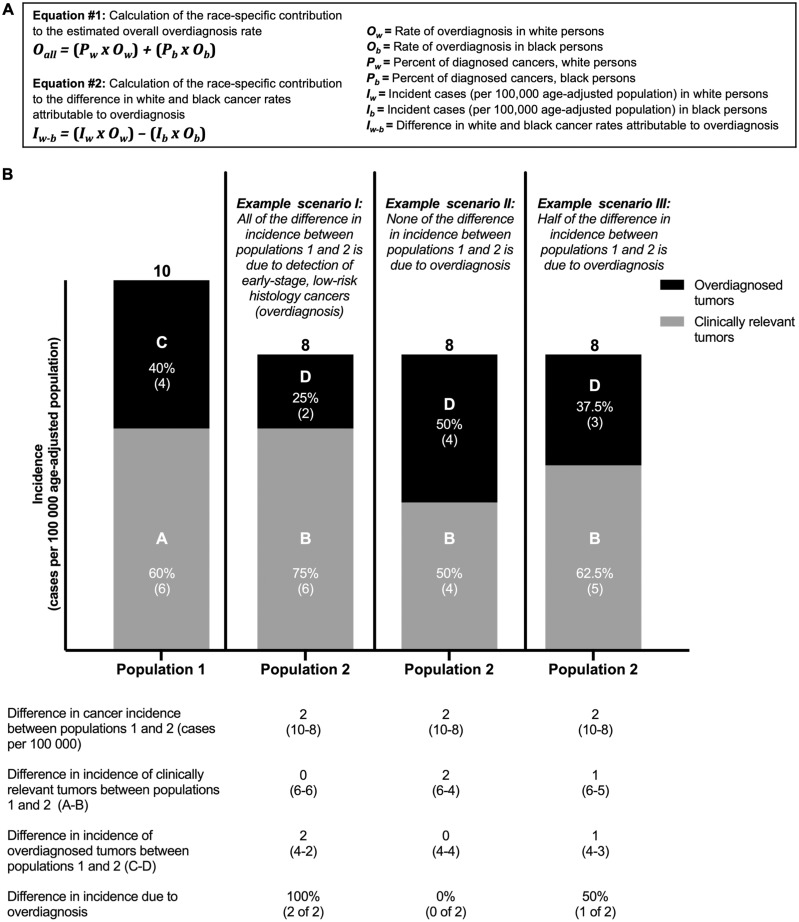
Incidence rates were compared using two-sided χ2 test. Survival rates were compared using log-rank test. Statistical significance was defined as P less than .05. Rates of overdiagnosis were estimated based on the range of reported overall rates of overdiagnosis in the United States (13–15) weighted by population cancer incidence (detailed in Figure 1A). This allowed approximation of the relative contributions of overdiagnosis to the observed differences in cancer incidence (Figure 1B). Survival rates adjusted for overdiagnosis were estimated based on the method of Harding et al. (9):
Figure 1.
Estimating overdiagnosis rates in black and white non-Hispanic patients with papillary thyroid cancer or estrogen/progesterone receptor positive breast cancer. A) Equations used for estimation of overdiagnosis rates. B) Example scenarios of cancer incidence in two populations (population 1 and population 2) with differing underlying rates of overdiagnosis. In scenario I, more overdiagnosis in population 1 creates the appearance of more advanced disease in population 2, when it is in fact unchanged in absolute terms.
Increased observational intensity, such as with screening, may lead to increased overdiagnosis of indolent tumors and/or earlier diagnosis of clinically relevant tumors. To determine the relative contributions of these two processes in populations, we examined the ratio of excess small or localized tumors in one population to excess large or advanced tumors in the other. In a scenario where no overdiagnosis occurs, this ratio would be 1, because each earlier diagnosis would prevent one later diagnosis; if overdiagnosis occurs, this ratio would be greater than 1. In addition, in breast cancer, to examine whether excess detection of small, localized tumors was associated with screening, we also examined patterns of diagnoses in women younger than 40 years, who are not recommended to undergo routine mammographic screening (16).
Results
Papillary Thyroid Cancer
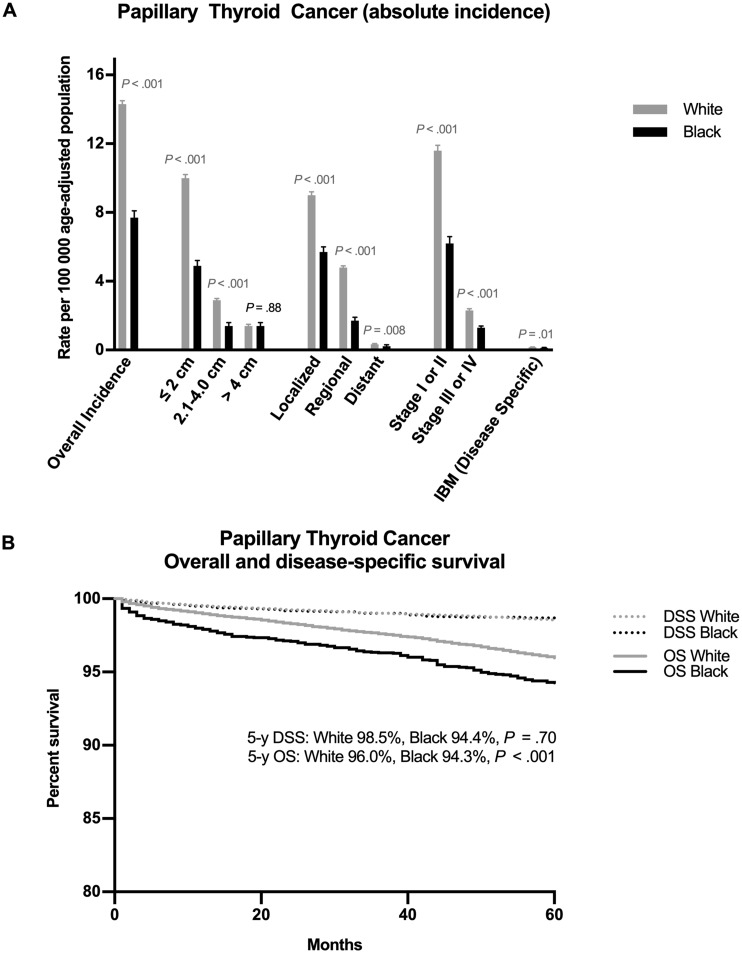
Of 18 875 white and black non-Hispanic patients who presented with papillary thyroid cancer in 2010–2014, 17 177 (91.0%) were white and 1698 (9.0%) black. The overall incidence rate of papillary thyroid cancer in the United States between 2010 and 2014 was markedly higher in white compared with black persons (14.3 vs 7.7 cases per 100 000 age-adjusted population, P < .001) (Figure 2A;Supplementary Table 1, available online). This excess incidence rate of papillary thyroid cancer in white persons was entirely attributable to markedly higher rates of diagnosis of cancers that were 2 cm or less (10.0 vs 4.9 cases per 100 000 population, P < .001), localized or regional (13.8 vs 7.4 cases per 100 000 population, P < .001), and early (I or II) stage (11.6 vs 6.2 cases per 100 000 population, P < .001) in white patients. The incidence rate of large cancers greater than 4 cm was identical in black and white patients (1.4 vs 1.4 cases per 100 000 population, P = .88). In fact, white patients had a slightly higher rate of stage III or IV disease presentation (2.3 vs 1.3 cases per 100 000 population, P < .001) and distant metastasis (.33 vs .22 cases per 100 000 population, P = .008).
Figure 2.
Incidence, incidence-based mortality, and survival with papillary thyroid cancer in white vs black persons. A) Incidence, disease characteristics at presentation, and disease-specific incidence-based mortality of papillary thyroid cancer. B) Overall and disease-specific survival of papillary thyroid cancer. DSS = disease-specific survival; IBM = incidence-based mortality; OS = overall survival. Rates expressed are per 100 000 age-adjusted population, and data presented are from 2010–2014 (incidence) and 1992–2014 (incidence-based mortality and survival).
Overall survival at 5 years was statistically significantly poorer among black patients with papillary thyroid cancer (94.3%, [95% CI = 93.4% to 95.0%] vs 96.0%, [95% CI = 95.8% to 96.2%], P < .001), though there was no difference between black and white patients in terms of 5-year disease-specific survival (98.7%, [95% CI = 98.2% to 99.0%] vs 98.5%, [95% CI = 98.4% to 98.7%], P = .70). Because differences in survival (relative measures) may in part be caused by overdiagnosis, lead-time, or length-time biases, we also examined disease-specific incidence-based mortality rates (absolute measures), which were not higher and, in fact, were slightly lower in the black compared with the white population (.12 vs .16 cases per 100 000 population, P = .01) (Figure 2, A and B; Supplementary Table 1, available online).
These results show a large excess of small localized papillary thyroid cancers being diagnosed in white persons but no corresponding excess of larger or advanced-stage tumors in black persons. Because small papillary thyroid cancers (often indolent with survival nearing 100%) were more frequently diagnosed in white persons, overall survival rates were higher, consistent with overdiagnosis bias (7,17). When examining incidence-based mortality rates, which reflect the number of patients with papillary thyroid cancer dying annually, we observed a slightly lower level of mortality in black persons, indicating that there is not a higher incidence of lethal papillary thyroid cancers in black patients. These data indicate that the entire difference in papillary thyroid cancer incidence and outcomes between white and black persons is attributable to more frequent overdiagnosis in white patients rather than delayed diagnosis in black patients.
Estrogen/Progesterone Receptor Positive Breast Cancer
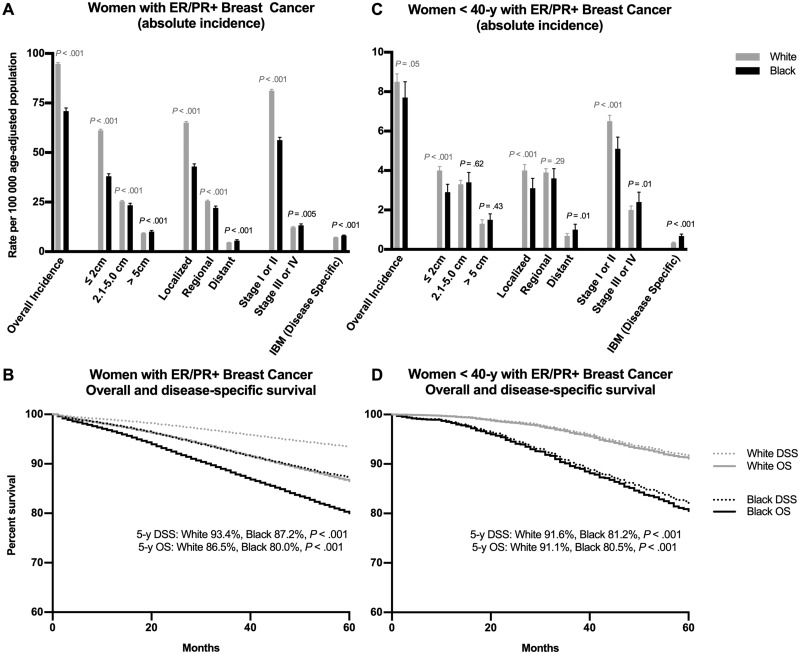
Of 76 388 white and black non-Hispanic women diagnosed with ER/PR+ breast cancer from 2010 to 2014, 68 001 (89.0%) were white and 8387 (11.0%) black. The incidence rate of ER/PR+ breast cancer among women in the United States has historically been higher in white women (18). This was observed in our cohort (94.8 vs 70.9 cases per 100 000 population, P < .001) (Figure 3A;Supplementary Table 2, available online). White women had markedly higher incidence of ER/PR+ breast cancers that were 2 cm or less (61.2 vs 38.1 cases per 100 000 population, P < .001), localized (65.1 vs 43.0 cases per 100 000 population, P < .001), and early (I or II) stage (81.2 vs 56.3 cases per 100 000 population, P < .001). White women had moderately higher incidence of ER/PR+ breast cancers that were 2.1–5 cm (25.4 vs 23.4 cases per 100 000 population, P < .001) as well as moderately higher incidence of presentation with regional disease (25.6 vs 22.1 cases per 100 000 population, P < .001). In contrast, black women had slightly higher incidence of presentation with tumors greater than 5 cm (10.1 vs 9.3 cases per 100 000 population, P < .001) or advanced-stage (III or IV) tumors (13.3 vs 12.3 cases per 100 000 population, P = .005) and distant metastatic disease (5.5 vs 4.6, P < .001).
Figure 3.
Incidence, incidence-based mortality, and survival with estrogen receptor/progesterone receptor positive (ER/PR+) breast cancer in white vs black women. A) Incidence, disease characteristics at presentation, and disease-specific incidence-based mortality with ER/PR+ breast cancer. B) Overall and disease-specific survival with ER/PR+ breast cancer. C) Incidence, incidence-based mortality, and survival of white vs black women younger than 40 years of age with ER/PR+ breast cancer. D) Overall and disease-specific survival of women less than 40 years of age with ER/PR+ breast cancer, white vs black women. DSS = disease-specific survival; IBM = incidence-based mortality; OS = overall survival. Rates expressed are per 100 000 age-adjusted population, and data presented are from 2010–2014 (incidence) and 1992–2014 (incidence-based mortality and survival).
Black women with ER/PR+ breast cancer experienced poorer 5-year overall (80.0%, [95% CI = 79.4% to 80.6%] vs 86.5%, [95% CI = 86.3% to 86.6%], P < .001) and disease-specific survival (87.2%, [95% CI = 86.7% to 87.7%] vs 93.4%, [95% CI = 93.3% to 93.5%], P < .001) compared with white women (Figure 3B, available online). Similarly, disease-specific incidence-based mortality rates among black women with ER/PR+ breast cancer were higher than in white women (8.1 vs 7.2 cases per 100 000 population, P < .001) (Figure 3A, Supplementary Table 2, available online). These differences in tumor size and stage at presentation with ER/PR+ breast cancer are consistent with two co-occurring mechanisms: more widespread overdiagnosis in white women (leading to more small localized early-stage tumors) and more delayed diagnosis in black women (leading to slightly more large or advanced-stage tumors, with slightly higher mortality).
To estimate the relative contributions of these two processes (overdiagnosis and delayed diagnosis), we calculated the ratio of excess small or localized tumors in white women to excess large or advanced tumors in black women (see “Methods”). We found that these ratios were 31.4 (≤5 cm vs >5 cm), 28.4 (localized or regional vs distant), and 24.9 (stage I or II vs III or IV), indicating that approximately 20–30 additional small or localized tumors are diagnosed in white compared with black women for every one large or advanced tumor avoided by early detection. This indicates that the clear majority, but not all (24 of 25, or approximately 95%), of excess small or localized ER/PR+ tumors in white compared with black women are attributable to overdiagnosis, because they are not associated with a corresponding decrease in large or advanced-stage tumors. This suggests that observed racial disparities in ER/PR+ breast cancer incidence are more attributable to differing rates of overdiagnosis than delayed detection of early-stage tumors.
To examine whether excess detection of small or localized tumors was associated with the use of screening, we analyzed trends in women younger than 40 years, who do not undergo routine breast cancer screening (16), and found the white–black difference in overdiagnosis was largely attenuated. There was no statistically significant difference in overall incidence of ER/PR+ breast cancers in white compared with black women (8.5 vs 7.7 cases per 100 000 population, P = .05), and only slightly higher rates in white women of tumors that were 2 cm or less (4.0 vs 2.9, P < .001), localized (4.0 vs 3.1 cases per 100 000 population, P < .001), and stage I or II (6.5 vs 5.1 cases per 100 000 population, P < .001). There was a similar incidence of tumors 2.1–5 cm (3.3 vs 3.4 cases per 100 000 population, P = .62) and greater than 5 cm (1.3 vs 1.5 cases per 100 000 population, P = .43), and a similar incidence of presentation with regional disease (3.9 vs 3.6 cases per 100 000 population, P = .29). There was only a slightly higher incidence of distant metastasis (1.0 vs .69 cases per 100 000 population, P = .01) or stage III or IV disease (2.4 vs 2.0 cases per 100 000 population, P = .03) in black compared with white women (Figure 3, C and D; Supplementary Table 3, available online). In these women younger than 40 years, the ratios of excess small or localized tumors in white women to excess large or advanced tumors in black women were 5.0 (≤5 cm vs >5 cm), 3.9 (localized or regional vs distant), and 3.5 (stage I or II vs III or IV), indicating that only about three to five additional small or localized tumors (compared with 20–30 for women of all ages) are diagnosed for every one large or advanced tumor avoided by early detection. This illustrates that in women younger than 40 years, in whom mammographic screening is not routinely performed, the white–black gap in breast cancer incidence, stage at presentation, and rate of overdiagnosis narrows markedly.
Estimating Overdiagnosis Rates in White Compared With Black Persons With Papillary Thyroid or ER/PR+ Breast Cancer
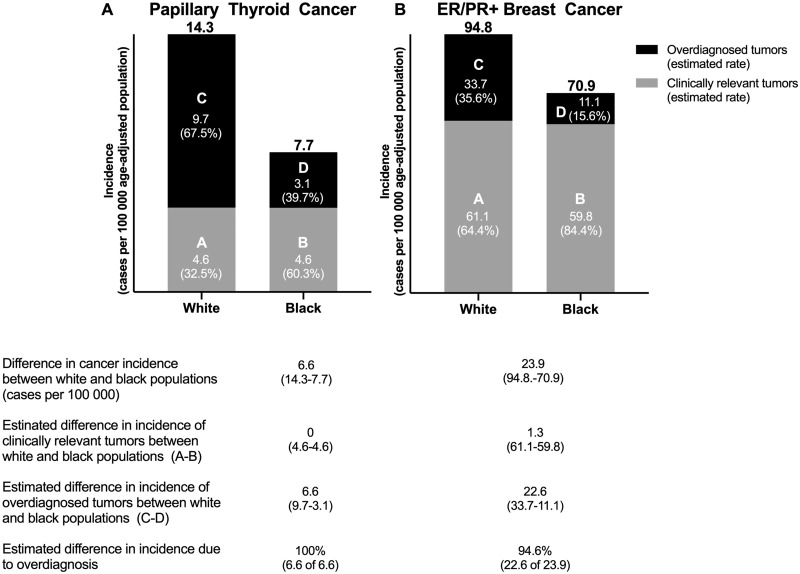
It is estimated that approximately 60–70% of thyroid cancers in the United States are overdiagnosed (15). If the entire excess incidence of papillary thyroid cancer in white compared with black patients (approximately 6.5 per 100 000) is attributable to more frequent overdiagnosis, and if the overall rate of overdiagnosis in papillary thyroid cancer is 65%, the estimated rates of overdiagnosis would be 68% in white patients and 40% in black patients (Figure 4A;Supplementary Figure 1, available online). Depending on the overall rate of overdiagnosis chosen (55–75%), the estimated rate of overdiagnosis of papillary thyroid cancer in white patients would range from 1.3 to 2.5 times that of black patients. Adjusting 20-year survival statistics for these estimated rates of overdiagnosis eliminates the slightly poorer disease-specific survival observed in black patients (Supplementary Figure 1, available online).
Figure 4.
Modeled estimates of the relative contributions of overdiagnosed and clinically significant cases in black and white persons with A) papillary thyroid cancer using a 65% overall estimated overdiagnosis rate and B) ER/PR+ breast cancer (women only) using a 30% overall estimated overdiagnosis rate. ER/PR+ = estrogen receptor/progesterone receptor positive. Rates listed are per 100 000 age-adjusted population.
Recent studies have estimated as many as 20–35% of invasive breast cancers are overdiagnosed (13,14,19,20). Estimates are higher (approximately 50%) for ER/PR+ cancers (21). Based on our previous estimate that 95% of the excess incidence of ER/PR+ breast cancer in white women (approximately 22.7 per 100 000) is due to more widespread overdiagnosis, and conservatively assuming that 30% of ER/PR+ breast cancers are overdiagnosed, we estimate that the overdiagnosis rate is 36% in white women and 16% in black women (Figure 4B;Supplementary Figure 2, available online). Depending on the overall rate of overdiagnosis chosen, the rate of overdiagnosis in white women is estimated at 1.7–5.7 times that of black women. Adjusting 20-year disease-specific survival for this rate of overdiagnosis narrowed the gap in survival rates by almost one-half (43%), with estimated survival rates of 65% in white women and 60% in black women (Supplementary Figure 2, available online).
Discussion
Here, we bring to attention the contribution of overdiagnosis to the appearance of racial disparities in incidence, survival, and mortality rates among US patients with thyroid or breast cancer. Racial disparities in cancer presentation and outcomes are often interpreted as indicative of poorer rates of thyroid and breast cancer detection in black persons. However, we find that the entire black–white disparity in papillary thyroid cancer incidence and survival, and some of this disparity in ER/PR+ breast cancer, is actually due to higher rates of overdiagnosis in white persons. These results, revealing that overdiagnosis is an important force in both breast and thyroid cancer statistics, have implications as interventions to reduce disparities and improve care are developed and prioritized.
Overdiagnosis is common in thyroid and breast cancer and relevant in prostate, renal, and lung cancer as well as melanoma. Overdiagnosis is more common among populations with greater health-care access, because there are more opportunities for the subclinical disease reservoir to be uncovered (6,8,22–24). Because overdiagnosed cancers, by definition, have cancer-specific survival rates approaching 100%, increasing overdiagnosis will create the appearance of higher survival rates and more favorable outcomes. Therefore, overdiagnosis tends to lead to more widespread use of screening techniques, causing a “cycle of positive feedback” that results in increasing overdiagnosis over time (6).
This analysis was necessarily limited in several ways. First, we chose to examine racial disparities in cancer overdiagnosis; however, race is in large part a surrogate for other factors, such as socioeconomic and other health-care access–related variables, which may be responsible for these observations. Additionally, the study was limited to white and black non-Hispanic persons and the predominant subtypes of thyroid and breast cancer, not including other racial or ethnic groups or less common cancer subtypes. Overdiagnosis is unlikely to explain disparities in all cancer types. For cancer types in which overdiagnosis occurs, differential rates of overdiagnosis may create the appearance of disparities; however, in cancer types not prone to overdiagnosis, this would not be expected to be the case. For example, we did not examine aggressive cancer subtypes such as triple-negative breast cancer, which occurs more commonly in black women, does not seem to be as prone to overdiagnosis, and is less frequently detected on screening, given the short lead time of these cancers (21,25,26). Importantly, overdiagnosis cannot be directly measured: it is impossible to ascertain whether an individual tumor was overdiagnosed or clinically relevant, regardless of its size or stage. However, at the population level, we can measure differences in overdiagnosis between populations by comparing rates of mortality and by measuring how many advanced-stage tumors are prevented by the diagnosis of an early-stage tumor. These conclusions are most useful for designing interventions to improve disparities at a population level and are not possible to apply to an individual patient.
Because more frequent overdiagnosis in white persons has the unintended consequence of creating the appearance of improved outcomes in those patients, it is possible that overdiagnosis has “moved the goalposts” of what are believed to be appropriate benchmarks for cancer detection rates. In recent years, screening mammography use in black women has increased and is now nearly equivalent to that of white women (18). However, during this time, the black–white gap in mortality and the absolute incidence of white and black women presenting with late-stage or metastatic disease has remained unchanged. Recent efforts to increase rates of screening appear to have increased rates of overdiagnosis but have not helped narrow the mortality gap, suggesting that improvements in treatment of diagnosed cancers, rather than wider screening, may be more likely to improve outcomes.
Factors previously implicated as causes of the differences in outcomes between black and white women with breast and thyroid cancer include disparities in insurance status, comorbidities, and treatment quality (11,27). Our data do not refute this and indeed emphasize the importance of these factors. We show that efforts to enhance early cancer detection may paradoxically exacerbate the appearance of inequities in cancer diagnosis, and therefore these efforts are unlikely to eliminate disparities in outcomes. Instead, we suggest that efforts to improve access to high-quality treatment for diagnosed cancers, and to address racial disparities in general medical care and the treatment of comorbid conditions, should receive at least as much as, if not more, attention than efforts to increase screening.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health Cancer Center Support Grant (P30 CA008748), the National Institutes of Health (grant numbers T32 CA009685 to ARM, K08 DE024774 and R01 DE027738 to LGTM), and the Frederick Adler Chair Fund (to LGTM).
Notes
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. Authors have declared no conflicts of interest related to this work. LGTM received laboratory research funding from Bristol-Myers Squibb and AstraZeneca unrelated to this work.
Disclaimer: The views expressed do not necessarily represent the views of the Department of Veterans Affairs or the US government.
Supplementary Material
References
- 1. Feinstein AR. Clinical epidemiology: II. The identification rates of disease. Ann Intern Med. 1968;69(5):1037. [Google Scholar]
- 2. Feinstein AR, Sosin DM, Wells CK.. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312(25):1604–1608. [DOI] [PubMed] [Google Scholar]
- 3. Black WC, Welch HG.. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med. 1993;328(17):1237–1243. [DOI] [PubMed] [Google Scholar]
- 4. Carter JL, Coletti RJ, Harris RP.. Quantifying and monitoring overdiagnosis in cancer screening: a systematic review of methods. BMJ. 2015;350:g7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Black WC. Overdiagnosis: an underrecognized cause of confusion and harm in cancer screening. J Natl Cancer Inst. 2000;92(16):1280–1282. [DOI] [PubMed] [Google Scholar]
- 6. Welch HG, Black WC.. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605–613. [DOI] [PubMed] [Google Scholar]
- 7. Ho AS, Davies L, Nixon IJ, et al. Increasing diagnosis of subclinical thyroid cancers leads to spurious improvements in survival rates. Cancer. 2015;121(11):1793–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Welch HG, Brawley OW.. Scrutiny-dependent cancer and self-fulfilling risk factors. Ann Intern Med. 2018;168(2):143–144. [DOI] [PubMed] [Google Scholar]
- 9. Harding CF, Pompei F, Wilson R.. Differences in breast cancer survival by race. JAMA. 2013;310(22):2456–2457. [DOI] [PubMed] [Google Scholar]
- 10. Davies L, Welch HG.. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295(18):2164–2167. [DOI] [PubMed] [Google Scholar]
- 11. Harari A, Li N, Yeh MW.. Racial and socioeconomic disparities in presentation and outcomes of well-differentiated thyroid cancer. J Clin Endocrinol Metab. 2014;99(1):133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Silber JH, Rosenbaum PR, Clark AS, et al. Characteristics associated with differences in survival among black and white women with breast cancer. JAMA. 2013;310(4):389–397. [DOI] [PubMed] [Google Scholar]
- 13. Welch HG, Prorok PC, O’Malley AJ, Kramer BS.. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375(15):1438–1447. [DOI] [PubMed] [Google Scholar]
- 14. Bleyer A, Welch HG.. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367(21):1998–2005. [DOI] [PubMed] [Google Scholar]
- 15. Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L.. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016;375(7):614–617. [DOI] [PubMed] [Google Scholar]
- 16. Siu AL; on behalf of the U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164(4):279–296. [DOI] [PubMed] [Google Scholar]
- 17. Howlader N, Ries LAG, Mariotto AB, Reichman ME, Ruhl J, Cronin KA.. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102(20):1584–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A.. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66(1):31–42. [DOI] [PubMed] [Google Scholar]
- 19. Autier P, Boniol M, Koechlin A, Pizot C, Boniol M.. Effectiveness of and overdiagnosis from mammography screening in the Netherlands: population based study. BMJ. 2017;359:j5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lannin DR. Treatment intensity for mammographically detected tumors: an alternative viewpoint. Ann Surg Oncol. 2018;25(9):2502–2505. [DOI] [PubMed] [Google Scholar]
- 21. Lannin DR, Wang S.. Are small breast cancers good because they are small or small because they are good? N Engl J Med. 2017;376(23):2286–2291. [DOI] [PubMed] [Google Scholar]
- 22. Welch HG, Fisher ES.. Income and cancer overdiagnosis - when too much care is harmful. N Engl J Med. 2017;376(23):2208–2209. [DOI] [PubMed] [Google Scholar]
- 23. Harding C, Pompei F, Burmistrov D, Welch HG, Abebe R, Wilson R.. Breast cancer screening, incidence, and mortality across US counties. JAMA Intern Med. 2015;175(9):1483–1489. [DOI] [PubMed] [Google Scholar]
- 24. Hart JT. The inverse care law. Lancet. 1971;1(7696):405–412. [DOI] [PubMed] [Google Scholar]
- 25. Collett K, Stefansson IM, Eide J, et al. A basal epithelial phenotype is more frequent in interval breast cancers compared with screen detected tumors. Cancer Epidemiol Biomark Prev. 2005;14(5):1108–1112. [DOI] [PubMed] [Google Scholar]
- 26. Farshid G, Walters D.. Molecular subtypes of screen-detected breast cancer. Breast Cancer Res Treat. 2018;172(1):191–199. [DOI] [PubMed] [Google Scholar]
- 27. Jemal A, Robbins AS, Lin CC, et al. Factors that contributed to Black-White disparities in survival among nonelderly women with breast cancer between 2004 and 2013. J Clin Oncol. 2018;36(1):14–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.