Abstract
Background
Proliferative diabetic retinopathy (PDR) is a progressive stage of diabetic retinopathy featured by the formation of neovascular and proliferative membrane. Vascular endothelial growth factor (VEGF) acts as a pivot factor in the development of neovascularization. This study was to investigate the changes of intravitreal VEGF concentrations of severe PDR after intravitreal injection of conbercept (IVC) and its potential advantages to the following vitrectomy.
Methods
This was a prospective, interventional, randomized controlled study. Sixty eyes (60 patients) with severe PDR and 20 eyes from 20 patients with rhegmatogenous retinal detachment complicated with proliferative vitreoretinopathy were enrolled in this study. PDR eyes were randomly assigned to three groups by sortation randomization method with 20 eyes in each based on the interval of preoperative IVC (group A: 7 days, group B: 14 days, group C: non-IVC). Another 20 eyes without diabetes were enrolled as the non-diabetic control group (group D), receiving PPV directly. Vitreous specimens of all 80 patients were collected and evaluated afterwards. The intravitreal VEGF concentration of the four groups, and the total surgical time and the intraoperative bleeding rate of the PDR groups were recorded.
Results
The mean intravitreal VEGF concentrations of groups A–D were 66.6 ± 43.3, 93.1 ± 52.3, 161.4 ± 106.1 and 1.8 ± 1.2 pg/mL, respectively. It increased significantly in PDR patients (groups A, B and C) (P = 0.002, <0.001, and <0.001, respectively). PDR patients with preoperative IVC (groups A and B) presented significantly lower VEGF concentrations (P < 0.001 and 0.001), intraoperative bleeding rates (P = 0.004) and total surgical time (P < 0.001, P = 0.003) compared with group C. No statistical differences were presented between groups A and B on the three parameters.
Conclusion
Seven days and 14 days of preoperative IVC are equally efficient and safe for the vitrectomy of severe PDR patients through decreasing vitreous VEGF concentrations, intraoperative bleeding rate and total surgical times.
Keywords: Anti-vascular endothelial growth factor, Diabetic retinopathy/proliferative diabetic retinopathy, Vitreous humor, Vascular endothelial growth factor
Introduction
Diabetic retinopathy (DR) is a serious, vision-threatening retinal complication of diabetes, remains one of the leading causes of legally blind in the working age population worldwide,[1] accounting for 92.4 million adults, posing a significant public health issue.[2] Proliferative diabetic retinopathy (PDR) is a progressive stage of DR due to retinal ischemia and is characterized by neovascularization (NV), vitreous hemorrhage (VH) and tractional retinal detachment (TRD), which are all main causes of vision loss of patients with diabetes.[3] Pars plana vitrectomy (PPV) has been considered as an effective method for the management of PDR.[4] However, vitreous hemorrhage due to neovascularization makes the operation more difficult to complete by obscuring the surgical field and increasing the chance of iatrogenic retinal tear, thus dramatically prolongs the operative time and improve the incidence of postoperative inflammation and recurrent TRD.
Vascular endothelial growth factor (VEGF) acts as a pivot factor in the development of neovascularization.[5–7] Previous studies reported the introduction of anti-VEGF agents as an adjunctive therapy before the surgery would significantly diminish intraoperative bleeding, shorten surgical time, and consequently improve the surgical outcomes.[8–12] Conbercept is a recombinant fusion protein that consists of the VEGF binding domains of the human VEGF receptor 1 and VEGF receptor 2 combined with the Fc portion of human immunoglobulin G1.[13] It has been widely used for ophthalmic treatment.[14–16]
Pretreatment with intravitreal injection of conbercept (IVC) has already been shown to prevent intraoperative hemorrhage and to facilitate the following PPV due to the regression of neovascularization. But how long will this benefit persist and whether prolonged interval of preoperative IVC will bring extra advantages remains unclear. Previous studies proved that the concentration of conbercept started decreasing from the seventh day after intravitreal injection in mice eyes.[17] However, the corresponding changes of intravitreal VEGF concentration in PDR patients were still not reported. So, we conducted a prospective randomized controlled study to investigate the changes of intravitreal VEGF concentration in patients with severe PDR after IVC and explore its advantages of facilitating the following PPV process.
Methods
Ethical approval
The current study complied with the Declaration of Helsinki and was approved by the Ethics committee of Peking Union Medical Collage Hospital (No. HS-1035). All patients enrolled provided written informed consent.
Patients
Eighty eyes of 80 consecutive of patients (60 PDR patients and 20 non-diabetic patients) were enrolled prospectively from February 2016 to October 2018. The inclusion criteria of patients with PDR included: well controlled type 2 diabetic patients, with fasting blood-glucose (FBG) ≤7.8 mmol/L and 2h-postprandial blood glucose (2h-PBG) ≤11.1 mmol/L; ophthalmic diagnosis should be severe PDR and needed surgical intervention, which referred to PDR complicated with the formation of proliferative fibrotic membrane or TRD confirmed by ophthalmoscopy examination or ultrasound B-scan. Exclusion criteria included: patients with type 1 diabetes; systemic and previous treatment with anti-VEGF therapy; previous ocular surgery; complicated with NVG. Sixty eyes (60 patients) with severe PDR needed surgical interventions were enrolled and assigned to three groups by sortation randomization method by one doctor (Bing Li), with 20 eyes in each based on the period of preoperative IVC (group A: 7 days; group B: 14 days; group C: non-IVC). The administrated dosage of IVC was 0.5 mg/0.05 mL.Twenty eyes (20 patients without diabetes) diagnosed as rhegmatogenous retinal detachment (RD) complicated with proliferative vitreoretinopathy (PVR) were enrolled as the control group (Group D).
Physical and ophthalmic examinations
All 60 patients with severe PDR in groups A–C received complete ophthalmic examinations including best-corrected visual acuity (BCVA), intraocular pressure measurement, ophthalmoscopy, fundus color photography and ultrasound B scan. Systemic evaluations including FBG, 2h-PBG, medical histories of diabetes and ophthalmic conditions were recorded at the time of enrollment. Twenty patients of group D underwent normal preoperative ophthalmic and systemic examination.
Vitreous samples collection and assessment
All surgeries were performed by the same rich experienced vitreoretinal surgeon (Jun-Jie Ye) using 23G vitrectomy system. 0.5 mL vitreous aqueous were collected at the beginning of PPVs and equally divided into two sterile 1 mL ep-tubes for each patient. All samples were cryopreserved into a –80°C refrigerator immediately waiting for assessment. The concentrations of VEGF in vitreous were measured by enzyme-linked immunosorbent assay (ELISA) using kits for human VEGF (human VEGF ELISA Kit; RapidBio Lab, Calabasas, CA, USA) performed by the same technologist (Zi-Jian Guo) in the clinical laboratory of Peking Union Medical College Hospital. The mean value of two samples from each patient was accepted for final statistic evaluation.
Intraoperative bleeding and total surgical time assessments
In this study, we introduced the complexity score to assess the difficulties of the surgeries between the PDR groups.[18] The three indexes were recorded in the PPVs of severe PDR (groups A–C), including complexity scores of the surgery, intraoperative bleeding and total surgical time assessments. Since the main harmfulness of active bleeding during the surgeries came from the disturbance of operation technique, we assessed the condition of macroscopic bleeding in the surgery especially during the process of fibrotic membrane dissection, and the techniques used for hemostasis were also recorded. Hemostatic strategies were performed with a consequence of elevating infusion pressure, endodiathermy, and fluid-air exchange.
Statistical analysis
SPSS 23.0 (IBM SPSS Statistics for Windows, Version 23.0, Armonk, NY: IBM Corp, USA) was used as the statistical analysis software for assessments in this study. The Kolmogorov-Smirnova tests were performed for normality test of the data. The Chi-squared test was performed for comparison of classified data like gender distributions, panretinal photocoagulation (PRP) rate, and intraoperative bleeding rate. The analysis of variance was performed to test continuous variable including duration of diabetes malleus, age, VEGF concentrations and total surgical time. Least Significance Difference (LSD) test was performed for post hoc test between different groups. Data are presented as frequencies or as the mean ± standard deviation. Two-tailed probabilities of less than 0.05 were considered to be statistical significance.
Results
Sixty patients with severe PDR enrolled consisted of 32 males and 28 females aged from 30.0 to 68.0 years, with the mean age of 50.6 ± 15.6 years. Twenty patients with RD complicated by PVR enrolled consisted of 9 males and 11 females aged from 26.0 to 78.0 years, with the mean age of 54.3 ± 15.1 years. General states of patients in four groups were listed in Table 1. General physical conditions including the complexity scores present no statistic differences among the four groups.
Table 1.
Characteristics of 60 PDR patients and 20 non-diabetic patients.
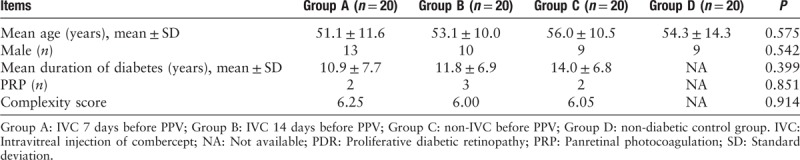
Intravitreal VEGF concentration
The mean intravitreal VEGF concentrations of groups A–D were 66.6 ± 43.3, 93.1 ± 52.3, 161.4 ± 106.1, and 1.8 ± 1.2 pg/mL, respectively. Vitreous VEGF levels increased significantly in patients with PDR (groups A, B, and C) than those in non-diabetic group (group D) (F = 22.0, P values were 0.002, <0.001, and <0.001 respectively). PDR with preoperative IVC (groups A and B) presented significantly lower VEGF concentrations compared with those in non-IVC group (P values were <0.001 and 0.001). However, no statistical difference was presented between groups A and B (P = 0.187). Data distribution of VEGF concentrations is shown in Figure 1.
Figure 1.
Effects of IVC on the concentration of intravitreal VEGF. The concentration of VEGF was detected by ELISA. The plot showed increased expressions of VEGF in patients with PDR (groups A, B, and C) than those in non-diabetic group (group D). Seven (Group A) or 14 (Group B) days of preoperative IVC dramatically decreased the VEGF levels compared with those in non- preoperative IVC patients (group C). However, no statistical differences were presented between group A and B. IVC: Intravitreal injection of combercept; VEGF: Vascular endothelial growth factor; PDR: Proliferative diabetic retinopathy.
Intraoperative bleeding and total surgical time
Intraoperative bleeding occurred in six (6/20), six (6/20) and 15 (15/20) cases in groups A–C respectively. Intraoperative bleeding in the 12 cases of groups A and B were very slight and could stop spontaneously without any applications of hemostatic techniques. However, among the 15 cases with intraoperative bleeding in group C, spontaneous hemostasis occurred only in one patient; elevating infusion pressure, endodiathermy and fluid-air exchange were performed to handle the active bleeding in seven cases (7/15), two cases (2/15) and five cases (5/15), respectively [Figure 2]. PDR patients with preoperative IVC (groups A and B) presented with significantly lower frequencies of intraoperative bleeding than that of non-preoperative IVC (P = 0.004). However, there was no statistical difference between group A and group B (P = 1.000).
Figure 2.
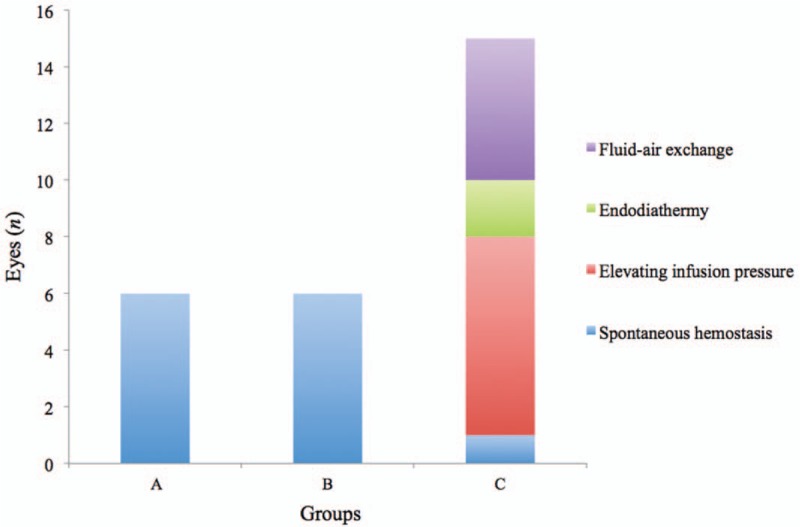
Frequencies of intraoperative bleeding in PDR groups (groups A–C) and techniques used for hemostasis. Ten cases with preoperative IVC in group A and B presented slight intraoperative bleeding and resolved spontaneously. However, different hemostatic techniques had to be adopted in 11 of the 12 cases without preoperative IVC in group C. Group A: IVC 7 days before PPV; Group B: IVC 14 days before PPV. IVC: Intravitreal injection of combercept; PDR: Proliferative diabetic retinopathy.
Mean total surgical time of groups A–C were 54.0 ± 12.6, 59.0 ± 17.8, and 78.9 ± 24.1 min, respectively. Total surgical time of patients from groups A and B were significantly shorter than that in Group C (P < 0.001, P = 0.003). No statistical difference presented between groups A and B (P = 0.403).
Adverse events
No cases were observed to have development or progression of TRD or other adverse events following IVC treatment in groups A and B.
Discussion
The purpose of this study was to investigate the intravitreal VEGF concentrations of patients with severe PDR after IVC for 7 or 14 days, and further evaluate its influence on the following PPV in the aspects of intraoperative bleeding and total surgical time. VEGF is considered to be a major promoter for the process of PDR.[19,20] We also confirmed that the vitreous VEGF level in patients with PDR increased a lot comparing with non-diabetic control group and reached 343.0 pg/mL, with a mean value of 161.4 pg/mL. Previous reports of VEGF concentration in patients with PDR varied a lot, with a mean value of 260.8 to 1880.0 pg/mL ranged from 20.0 to 11,200.0 pg/mL.[21–24] The large diversity of enrolled patient may explain part of this phenomenon. Like VH, TRD, and NVG could all present at the stage of PDR but represent different degrees of ischemic intraocular conditions with different VEGF level consequently. To prevent the bias mentioned above, we chose relatively homogeneous PDR patients with proliferative fibrotic membrane or TRD, without NVG. PDR with non-absorbable VH but not complicated with proliferative fibrotic membrane or TRD were excluded, to assure the statement of PDR and surgical difficulty to be comparable, so that VEGF concentration and the two intraoperative indexes were comparable between the PDR groups. Hence, our data was not so scattered as those previous studies. Furthermore, since the membrane dissection was an unpreventable technique for this group of patients, the pretreatment of IVC would be of great necessary since active bleeding would be of great harmfulness to this elaborate task in order to prevent iatrogenic retinal breaks.
With more binding site to human VEGF receptor, conbercept is considered to have higher binding affinity, which makes it possible for extend treatment interval to three months in other neovascularization disease like neovascular age-related macular degeneration (nAMD).[18] Diabetic animal models reported that IVC decreased the intravitreal VEGF level significantly and lasted for more than 60 days.[25] But its duration of intravitreal effect in diabetic patients remains unclear. In our study, the intravitreal VEGF level of the two groups of PDR with IVC were significantly lower than that of PDR without IVC, which proved its effect within 14 days in PDR patients. Furthermore, although no statistic differences presented between the two groups of PDR with IVC, a tendency of higher VEGF level was presented in the group with IVC 14 days preoperatively. This may attribute to the decrease of intravitreal conbercept concentration, which was verified to start diminishing from the seventh day after intravitreal injection in diabetic animal model[17] and the effect of anti-VEGF diminished consequently.
Anti-VEGF therapy has been accepted as an effective adjunct to the surgical PDR intervention by facilitating the operation.[8–12,26] However, the timing of preoperative anti-VEGF differed a lot among previous studies, most of which preferred to perform the anti-VEGF therapy 7 days prior to the PPV, mainly based on the half-life of anti-VEGF agents and the VEGF levels in the vitreous, which may reflect the activity of NV. A recent study[22] proved the intraoperative concentration of VEGF decreased 2 to 5 days after intravitreal injection of bevacizumab (IVB) in patients with PDR. They also set an experimental group with IVB 14 days prior to PPV, but found no statistical significance compared with PDR without IVB. In our study, the two PDR groups with IVC beforehand (7 days and 14 days) both presented significantly lower VEGF levels compared to the PDR group without IVC. Although conbercept has a comparable half-life to that of bevacizumab,[25,27] it can remain the VEGF level at a low level for a longer time, even till 14 days after administration. We attribute it to a higher binding affinity conbercept possesses than bevacizumab does.[25]
In traditional PPV without adjunctive anti-VEGF therapy, intraoperative conditions of active bleeding were quiet common, which may lead to diminished clarity of operating fields and accuracy, as well as prolong the surgical time. In severe cases, intraoperative hemorrhage could not be resolved even with applications of multiple hemostatic techniques. Although it could be resolved temporarily, residual blood components like platelet would induce recurrent proliferative fibrotic membrane or even TRD promoted by cytokines. In our study, beside the investigation of VEGF level in different groups, we also introduced two indexes to assess the facilitation of decreased VEGF level resulted from preoperative IVC for the following PPV, which will practically benefit the surgeons and patients in clinical practice. In the PDR groups, eyes with preoperative IVC (7 days or 14 days) were presented with less intraoperative bleeding, which could stop spontaneously without the applications of hemostatic techniques. On the contrary, eyes without preoperative IVC got more chance (70%) of intraoperative bleeding, which should be handled by elevating infusion pressure, endodiathermy or even fluid-air exchange measurements with different proportions. With these time consuming hemostatic techniques, the total surgical time were further prolonged nearly half an hour as our results showed, which is comparable to the previous studies.[28] However, the intraoperative bleeding and total surgical time were comparable in patients with PDR received IVC 7 or 14 days preoperatively, which indicated that the two intervals would both be beneficial for facilitating the following surgery. We investigated the intravitreal VEGF levels of PDR patients after IVC. We also introduced a further investigation about the relationship between VEGF level and intraoperative conditions including intraoperative bleeding and total surgical time.
In conclusion, our results showed that the VEGF concentrations in vitreous of PDR were significantly higher than those of non-diabetic PVR patients. Seven days and 14 days of preoperative IVC are equally efficient and safe for the vitrectomy of severe PDR patients through decreasing vitreous VEGF concentrations, intraoperative bleeding rate and total surgical time. Results suggest that the combination of preoperative IVC and PPV might benefit patients with severe PDR.
Acknowledgements
The authors thank Prof. Ying-Chun Xu for providing us the opportunity to work together and finish this work.
Conflicts of interest
The authors have no proprietary or commercial interests in any materials discussed in this article.
Footnotes
How to cite this article: Li B, Li MD, Ye JJ, Chen Z, Guo ZJ, Di Y. Vascular Endothelial Growth Factor Concentration in Vitreous Humor of Patients with Severe Proliferative Diabetic Retinopathy after Intravitreal Injection of Conbercept as an Adjunctive Therapy for Vitrectomy. Chin Med J 2020;133:664–669. doi: 10.1097/CM9.0000000000000687
Bing Li and Meng-Da Li contributed equally to this work.
References
- 1.Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol 2007; 14:179–183. doi: 10.1080/09286580701396720. [DOI] [PubMed] [Google Scholar]
- 2.Yang WY, Lu JM, Weng JP, Jia WP, Ji LN, Xiao JZ, et al. Prevalence of diabetes among men and women in China. New Engl J Med 2010; 362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 3.Liu L, Wu XM, Liu LM, Geng J, Yuan Z, Shan ZY, et al. Prevalence of diabetic retinopathy in Mainland China: a meta-analysis. Plos One 2012; 7:e45264.doi: 10.1371/journal.pone.0045264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blankenship GW, Machemer R. Long-term diabetic vitrectomy results - report of 10 year follow-up. Ophthalmology 1985; 92:503–506. [DOI] [PubMed] [Google Scholar]
- 5.Shi L, Huang YF. Postvitrectomy diabetic vitreous hemorrhage in proliferative diabetic retinopathy. J Res Med Sci 2012; 17:865–871. [PMC free article] [PubMed] [Google Scholar]
- 6.Wakabayashi Y, Usui Y, Okunuki Y, Ueda S, Kimura K, Muramatsu D, et al. Intraocular VEGF level as a risk factor for postoperative complications after vitrectomy for proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 2012; 53:6403–6410. doi: 10.1167/iovs.12-10367. [DOI] [PubMed] [Google Scholar]
- 7.Wang XQ, Wang GB, Wang Y. Intravitreous vascular endothelial growth factor and hypoxia-inducible factor 1a in patients with proliferative diabetic retinopathy. Am J Ophthalmol 2009; 148:883–889. doi: 10.1016/j.ajo.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Su L, Ren X, Wei H, Zhao L, Zhang X, Liu J, et al. Intravitreal Conbercept (Kh902) for surgical treatment of severe proliferative diabetic retinopathy. Retina 2016; 36:938–943. doi: 10.1097/IAE.0000000000000900. [DOI] [PubMed] [Google Scholar]
- 9.Smith JM, Steel DH. Anti-vascular endothelial growth factor for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy. Cochrane Database Syst Rev 2015; 8:CD008214.doi: 10.1002/14651858.CD008214.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pakzad-Vaezi K, Albiani DA, Kirker AW, Merkur AB, Kertes PJ, Eng KT, et al. A randomized study comparing the efficacy of bevacizumab and ranibizumab as pre-treatment for pars plana vitrectomy in proliferative diabetic retinopathy. Ophthalmic Surg Lasers Imaging Retina 2014; 45:521–524. doi: 10.3928/23258160-20∗141118-06. [DOI] [PubMed] [Google Scholar]
- 11.Zhao XY, Xia S, Wang EQ, Chen YX. Efficacy of intravitreal injection of bevacizumab in vitrectomy for patients with proliferative vitreoretinopathy retinal detachment: a meta-analysis of prospective studies. Retina 2018; 38:462–470. doi: 10.1097/IAE.0000000000001584. [DOI] [PubMed] [Google Scholar]
- 12.Yang KB, Zhang H, Li SJ, Cao JJ, Cheng LN, Lin YX, et al. Conbercept and ranibizumab pretreatments in vitrectomy with silicone oil infusion for severe diabetic retinopathy. J Ocul Pharmacol Ther 2019; 35:161–167. doi: 10.1089/jop.2018.0093. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Yu D, Yang C, Xia Q, Li W, Liu B, et al. The pharmacology study of a new recombinant human VEGF receptor-fc fusion protein on experimental choroidal neovascularization. Pharm Res 2009; 26:204–210. doi: 10.1007/s11095-008-9718-9. [DOI] [PubMed] [Google Scholar]
- 14.Liu K, Song Y, Xu G, Ye J, Wu Z, Liu X, et al. Conbercept for treatment of neovascular age-related macular degeneration: results of the randomized phase 3 PHOENIX study. Am J Ophthalmol 2019; 197:156–167. doi: 10.1016/j.ajo.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Zhang C, Hua R. Clinical effectiveness of ranibizumab and conbercept for neovascular age-related macular degeneration: a meta-analysis. Drug Des Devel Ther 2018; 12:3625–3633. doi: 10.2147/DDDT.S176021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang F, Qin X, Lu J, Song P, Li M, Ma X. Optical coherence tomography predictors of short-term visual acuity in eyes with macular edema secondary to retinal vein occlusion treated with intravitreal conbercept. Retina 2019; ahead of print. doi: 10.1097/IAE.0000000000002444. [DOI] [PubMed] [Google Scholar]
- 17.Du L, Peng H, Wu Q, Zhu M, Luo D, Ke X, et al. Observation of total VEGF level in hyperglycemic mouse eyes after intravitreal injection of the novel anti-VEGF drug conbercept. Mol Vis 2015; 21:185–193. [PMC free article] [PubMed] [Google Scholar]
- 18.Grigorian RA, Castellarin A, Fegan R, Seery C, Del Priore LV, Von Hagen S, et al. Epiretinal membrane removal in diabetic eyes: comparison of viscodissection with conventional methods of membrane peeling. Br J Ophthalmol 2003; 87:737–741. doi: 10.1136/bjo.87.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller JW, Adamis AP, Aiello LP. Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes Metab Rev 1997; 13:37–50. doi: 10.1002/(sici)1099-0895(199703)13:1<37::aid-dmr174>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 20.Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol 2001; 280:C1358–1366. doi: 10.1152/ajpcell.2001.280.6.C1358. [DOI] [PubMed] [Google Scholar]
- 21.Wang JX, Chen S, Jiang F, You CY, Mao CJ, Yu JG, et al. Vitreous and plasma VEGF levels as predictive factors in the progression of proliferative diabetic retinopathy after vitrectomy. PLoS One 2014; 9:e110531.doi: 10.1371/journal.pone.0110531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arimura N, Otsuka H, Yamakiri K, Sonoda Y, Nakao S, Noda Y, et al. Vitreous mediators after intravitreal bevacizumab or triamcinolone acetonide in eyes with proliferative diabetic retinopathy. Ophthalmology 2009; 116:921–926. doi: 10.1016/j.ophtha.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 23.Wakabayashi Y, Usui Y, Tsubota K, Ueda S, Umazume K, Muramatsu D, et al. Persistent overproduction of intraocular vascular endothelial growth factor as a cause of late vitreous hemorrhage after vitrectomy for proliferative diabetic retinopathy. Retina 2017; 37:2317–2325. doi: 10.1097/IAE.0000000000001490. [DOI] [PubMed] [Google Scholar]
- 24.Ma Y, Zhang Y, Zhao T, Jiang YR. Vascular endothelial growth factor in plasma and vitreous fluid of patients with proliferative diabetic retinopathy patients after intravitreal injection of bevacizumab. Am J Ophthalmol 2012; 153:307–313. e2. doi: 10.1016/j.ajo.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Lei N, Zhang M, Li Y, Xiao H, Hao X. Pharmacokinetics of a long-lasting anti-VEGF fusion protein in rabbit. Exp Eye Res 2012; 97:154–159. doi: 10.1016/j.exer.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Manabe A, Shimada H, Hattori T, Nakashizuka H, Yuzawa M. Randomized controlled study of intravitreal bevacizumab 0.16 mg injected one day before surgery for proliferative diabetic retinopathy. Retina 2015; 35:1800–1807. doi: 10.1097/IAE.0000000000000577. [DOI] [PubMed] [Google Scholar]
- 27.Sinapis CI, Routsias JG, Sinapis AI, Sinapis DI, Agrogiannis GD, Pantopoulou A, et al. Pharmacokinetics of intravitreal bevacizumab (Avastin(R)) in rabbits. Clin Ophthalmol 2011; 5:697–704. doi: 10.2147/OPTH.S19555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang ZH, Liu HY, Hernandez-Da Mota SE, Romano MR, Falavarjani KG, Ahmadieh H, et al. Vitrectomy with or without preoperative intravitreal bevacizumab for proliferative diabetic retinopathy: a meta-analysis of randomized controlled trials. Am J Ophthalmol 2013; 156:106–115. e2. doi: 10.1016/j.ajo.2013.02.008. [DOI] [PubMed] [Google Scholar]


