Abstract
Microbiota associated with airborne particulate matter (PM) is an important indicator of indoor pollution as they can be pathogenic and cause serious health threats to the exposed occupants. Present study aimed to investigate the level of culturable microbes associated with PM and their toxicological characterization in urban and rural houses of Pune city. Highest concentration of bacterial aerosols observed to be associated with PM10 size fraction in urban site (2136 ± 285 CFU/m3) whereas maximum fungal concentration has been measured in rural houses (1521 ± 302 CFU/m3). Predominantly found bacterial species were Bacillus sp., S. aureus, and Pseudomonas aeruginosa and fungal species were Aspergillus sp., Cladosporium sp., and Penicillium sp. in both urban and rural residential premises. Concentration of endotoxin measured using the kinetic Limulus Amebocyte Lysate assay exhibited that the level of endotoxin in both urban and rural sites are associated with household characteristics and the activities performed in indoor as well as outdoor. Cell free DTT assay confirmed the ability of these airborne microbes to induce the production of reactive oxygen species (ROS) varying along with the types of microorganisms. On exposure of A549 cells to airborne microbes, a significant decrease in cell viability was observed in terms of both necrosis and apoptosis pathway. Elevated production of nitric oxide (NO) and proinflammatory cytokines in epithelial cells and macrophages clearly suggest the inflammatory nature of these airborne microbes. Results derived from the present study demonstrated that the indoor air of urban and rural houses of Pune is contaminated in terms of microbial load. Therefore, attention should be paid to control the factors favoring the microbial growth in order to safeguard the health of exposed inhabitants.
Keywords: Indoor air quality, PM bound Microbes, Endotoxin, ROS, Cytotoxicity, Inflammation
Graphical abstract
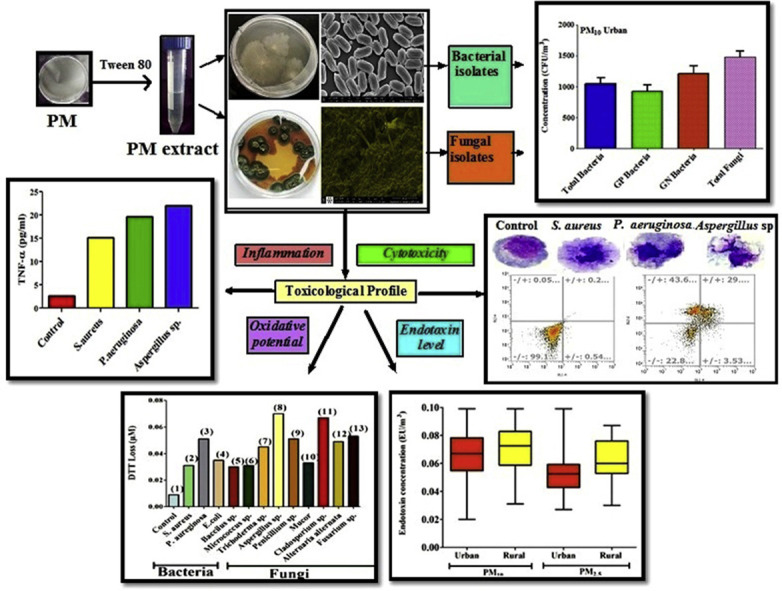
Highlights
-
•
Total culturable microbes associated with PM were detected in indoor environments.
-
•
Predominantly found microbial isolates are S. aureus, P. aeruginosa and Aspergillus sp.
-
•
LAL assay exhibited eminent level of endotoxin in urban and rural houses.
-
•
Annexin study confirmed cell death pathway induced by prevalent airborne microbes.
-
•
Dominantly found microbes exhibited elevated production of proinflammatory mediators.
Present study screened the dynamic behavior of airborne microbes associated with PM collected from rural and urban indoor environments of Pune, India in terms of their measurement, characterization and evaluation of detail toxicological profile (Endotoxin, oxidative potential, pathways of cell death, pro-inflammatory response).
1. Introduction
Poor air quality by particulate pollution is considered as the prevalent environmental health risk globally. Numerous epidemiological studies have revealed the comprehensible relationships of particulate matter (PM) with adverse health outcomes with mortality and morbidity by respiratory and cardiovascular diseases (Hamanaka and Mutlu, 2018; Hime et al., 2018; Shiraiwa et al., 2017). Compared to ambient PM, less attention has been paid to the exposure level of indoor PM and its associated health risk to humans who spend ample amount of their time in various indoor environments. Biological constituents of PM (i.e. bioaerosols) occupying a large portion and are predominantly composed of bacteria, fungal spores, virus, pollens, any fragments from plants, animals or any living organisms with sizes ranging from 50 nm to 10 μm (Gong et al., 2020; Kim et al., 2018). It has been estimated that bioaerosol constitutes 16% mass of the total primary aerosol particles and 4%–11% of the total PM2.5 mass. Chronic and even a momentary exposure to these PM bound microbes can cause numerous diseases viz., asthma, chronic obstructive pulmonary disease (COPD), influenza, allergic rhinitis, eczema, legionellosis, tuberculosis, anthrax, and severe acute respiratory syndrome (Yen et al., 2019; Sedha and Doctor, 2017; Park et al., 2015; Thilsing et al., 2015). No such conclusive evidences are there which can explain the exact mode of action between the bioaerosol exposure and its related toxicity but hypersensitivity, airway inflammation and oxidative stress are considered as three main mechanisms of the diseases caused due to PM bound microbes (Robertson et al., 2019; Liu et al., 2014; Lee, 2011). The endotoxin of bacterial components has been recognized as a predominant factor in causing the hypersensitivity pneumonitis (Peccia et al., 2008). Apart from the allergic reactions and infections, inhalation of bacterial endotoxin can also induce inflammatory responses by activating the toll-like receptor 4 (TLR4) in human cells which further play a crucial role in causing a variety of acute and chronic diseases (Shen et al., 2019; Moretti et al., 2018; Liu et al., 2018). On exposure to PM bound microbes, prime cells such as mononuclear phagocytes and macrophages start responding by secretion of nitric oxide, cytokines tumor necrosis factor - (TNF-α) and interleukin-1- β (IL-1) and prolonged sustain of these inflammatory reactions can damage the surrounding tissues leading to the adverse health effects for occupants residing in indoor environments.
Different Indoor air quality studies have suggested the contribution of bioaersols to about 5–34% of the indoor air pollution (Humbal et al., 2018; Sedha and Doctor, 2017; Mandal and Brandl, 2011). Therefore, it is imperative criterion to take into account, the microbiological air quality in order to ensure the safe indoor environments. Evaluation of inclusive toxicological profile of these bioaerosols is not an easy task due to their complex nature. Precise screening of bioaerosols toxicity cannot be assessed by means of its individual component as toxicity may vary depending on microbial composition and as well as oxidative potential (Humbal et al., 2018). Therefore, detail toxicological evaluation of microbial biomarker is required to assess the effects of this on human health. Recently, research on microbial species in atmosphere has been escalating worldwide but in India, there is a lack of systematic research on the effects of bioaerosol on human health and none originating from the western Indian region. Therefore, methodical research is necessitated in order to understand the role of the bioaerosols for causing the wide range of serious health threats. Looking at the concerns and risks associated with bioaerosols, present study was framed to assess the measurement, seasonal variation, identification and detail immunutoxicological activity (in terms of endotoxin measurement, redox potential, cytotoxicity, apoptosis and inflammatory potential) of airborne microbes associated with PM collected from urban and rural indoor residential environments of Pune which will be effectual to raise the awareness and further address the better indoor air quality for the inhabitants.
2. Materials and methods
2.1. Sample collection and extraction
Pune city has witnessed rapid growth and transformations over the years especially in telecommunication and educational facilities therefore large number of populations have migrated from rural areas resulting a mix of both urban and rural communities in the city. The present study screened the toxicological assessment of microbial components of PM collected from indoor houses of two clusters; urban and rural with diverse local bioaerosol sources based on their separate geographical locations, standard of living and influence of indoor and outdoor activities. PM10 (N = 124) and PM2.5 (N = 128) samples were collected from three houses (U1, U2, U3) in urban area (18°43′30″N, 73°88′87″E) and two houses (R1 and R2) from rural areas (18°38′68″N, 74°03′42″E) of Pune respectively during the study period (March 2016–April 2017).The summary of individual home characteristics along with detailed household and personal daily activities were recorded in 24 h activity log during the period of sampling and presented in Fig. S1. 24 hr indoor PM sampling was carried out using low volume air sampler i.e., Mini Vol TAS (Airmetrics, USA) with a constant flow rate of 5 l/min. Samples were collected on pre-weighed sterile nucleopore filter papers and post sampling; filters were immediately transported to the laboratory and were stored overnight at 4 °C in the refrigerator. On the following day, PM loaded filters were cut into small pieces and transferred to sterile culture tube containing 10 ml of extracting media (0.1% sterile peptone water and 0.01% Tween 80) and vortexed for about 6 h and stored in refrigerator. Meteorological parameters viz. temperature (°C) and relative humidity (RH %) were measured simultaneously during sampling by Aeroqual indoor air quality monitor (IQM 60, flow rate: 1.0 ± 0.05 LPM) (Table S1).
2.2. Preparation and enumeration of microbes associated with PM
Gram negative and Gram positive bacterial fractions associated with PM were cultivated in Eosine Methylene Blue Agar Media (EMB) and Blood Agar media, respectively with 0.5 μgml−1 cyclohexamide added to suppress the fungal growth. Whereas fungal fractions were collected over potato dextrose agar (PDA) media and 0.5 μgml−1 chloramphenicol was added to the media as a bacterial growth inhibitor. Serial dilutions (1:1, 1:10, 1:100) of PM extract were prepared and 100 μl of each dilution were spread plated (in triplicate) on petri plates containing selective culture media. Post spreading, petri plates were kept in incubator for about 2–3 days for bacterial growth and about 7 days for fungal growth. Then the grown microbial colonies were enumerated manually and concentration of culturable microorganism was calculated by CFU count using the total number of counted colonies, total volume of air sampled and sampling duration. CFU count reported in the present study is an average of independent two repeats of same samples collected from rural and urban locations.
2.3. Identification of airborne microbes
Predominant colonies appeared on petri plates were first identified in terms of their morphological characterization (size, shape, colour and appearance) by Field emission scanning electron microscopy (FE-SEM) (FEI Nova Nano SEM 450) and further subjected to 16S rRNA and 18S rRNA Sanger sequencing from a certified laboratory (Xcelris Genomics). The bacterial universal primer 8F/1492R and fungal universal primer 1F/4R was used to amplify the fragments of bacterial 16S rDNA and fungal 18S rDNA subunit respectively. Sequence details of all the primes are listed in Table S2. The amplified fragments were further run on Real-Time PCR System (ABI 3730xl 96, Thermo Fisher Scientific) maintaining the specific PCR condition for bacterial (initial denaturation at 94 °C for 30 s, followed by 25/30 cycles of denaturation at 52 °C for 30 s, annealing at 72 °C for 1 min and extension at 72 C for 1 min) and fungal cultures (initial denaturation at 94 °C for 30 s, followed by 30 cycles of denaturation at 48 °C for 30 s, annealing at 72 °C for 1 min and extension at 72 °C for 7min). Obtained intergenic sequences were deposited in Gen Bank database from National Center of Biotechnology Information BLAST in order to express BLAST percentage identification of the predominantly found microbes in indoor air and that too has been gathered in Table S2.
2.4. Toxicological screening of airborne microbiota
Toxicological screening of bacterial and fungal fractions associated with PM was done in terms of endotoxin measurement, oxidative potential and cytotoxic and inflammatory assessment.
Endotoxin, a cell wall component of gram negative bacteria is a well known pyrogen causing symptomatic effects in the exposed inhabitants. In present study, quantification of endotoxin was assessed as per manufactures instruction in terms of end point chromogenic Limulus Amebocyte Lysate (LAL) assay using endotoxin assay kit (Pyrochrome: Cape Cod Inc.) Briefly, purified extract of Escherichia coli (0113:H10) was used as a control standard endotoxin. PM (100 μl) extract was mixed with LAL reagent (100 μl) followed by incubation at 37 °C for 60 min. Then substrate solution was added to the incubated fraction and vortexed for 10 min to ensure complete mixing. Presence of endotoxins was confirmed by developing yellow colour and mixture was measured spectrophotometrically (PerkinElmer EnSpire 2300) at 405–410 nm. Concentration of endotoxin was calculated from the standard curve prepared using the series of different endotoxin standard concentration i.e., 1.0, 0.5, 0.25, 0.125, 0.0625, 0.03125, 0.015 and 0.007 EU/ml. Quantified values between first and last point of the curve are only accepted and level of endotoxin was expressed as endotoxin units/cubic meter of air (EU/m3).
Oxidative potential (OP) of PM in cell free/abiotic system has been considered as one of the most promising screening method of their biological reactivity (Orevik, 2019). OP describes the inherent ability of particles and their components to induce ROS generation which is an important underlying mechanism for particle toxicity. Therefore, in present study, oxidative potential (OP) of PM10 and PM2.5 samples along with predominantly found microbial species in urban and rural indoor environments were measured by cell free assay i.e., DTT assay and detail methodology for this assay is provided in our earlier publication (Roy et al., 2019).
Evaluation of cytotoxic potential of PM and its associated micro-biota play a vital role in screening of toxicological properties due to their association with genomic instability leading to the cell death. Therefore, in order to detect the cytotoxic effect, cell viability of indoor PM samples and abundantly identified bacterial and fungal species were studied by using MTT assay. About 80% of total collected samples from both urban (three houses; U1, U2 and U3) and rural sites (two houses; R1 and R2) were used for cell culture exposure and data reported for cytotoxic effect is an average values of total samples analyzed at each site. Preparation of A549 cells (lung epithelial cell line) were detailed out in supplementary file. A549 cells were exposed to collected PM samples and a constant dose (10^6 microbes/ml) of microorganisms for 24 h and assayed in three technical and biological replicates. Cells with media acted as a positive control. Briefly, cells were incubated with PM samples and microbial species for 24 h and after that 20 μl of MTT solution was added and further incubated the mixture for 4 h followed by centrifugation. Media was removed after 24 h of exposure and subsequently 100 μl of DMSO was added and finally absorbance was measured at 570 nm by spectrophotometer well plate reader (PerkinElmer EnSpire 2300). Pathways involved in the cell death (in terms of appoptosis and necrosis) on exposure to adequate microbial pathogens can be monitored by widely used phosphatidylserine staining detection method i.e., Annexin PI. Appoptosis is a carefully regulated process of programmed cell death whereas necrosis occurs through differences in plasma membrane integrity and permeability. In present study, cell death on exposure to airborne microbiota was employed by using the Alexa Fluor 488 annexin 5/Dead Cell apoptosis kit with Alexa Fluor 488 annexin v and PI following manufacturer instruction (briefly outlined in supplementary file). The data generated by flow cytometry (Attune NxT Flow cytometer, Thermo Fisher Scientific) are plotted in two-dimensional dot plots in which PI is represented versus Annexin V. These plots can be divided in four regions corresponding to live cells (i.e. annexin V-/PI-), early apoptotic (i.e. annexin V+/PI-), late apoptotic (i.e. annexin V+/PI+) and necrotic (i.e. annexin V-/PI+.)
Apart from oxidative potential and cytotoxic effect, one of the prime toxicity governing factor of airborne microbes is inflammatory potential. Pulmonary cells, such as alveolar epithelial cells and macrophages are first to respond to inhaled airborne microbes, therefore, in present study in order to asses the inflammatory activity, both epithelial cells and macrophages (Mouse RAW264.7 macrophage) were exposed to these airborne microbial species. Inflammatory potential of prevalent gram positive, gram negative bacterial and fungal species associated with PM was measured in terms of analysis of immune response of different cytokines (IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12, TNF-α) in lung epithelial cells by the use of the MACSPTEV Cytokine 12 kit according to the manufacturer instruction. In addition, the expression of cytokines such as IL-6 and TNF- α were also measured on exposure to these microbes to macrophages using cytometric bead array (CBA assay, BD Biosciences) as per the manufacturer’s protocol. Apart from these cytokines, production of highly reactive signalling molecule i.e., nitric oxide (NO) is also considered as an important marker of immunological activation of the cells. Hence, concentration of stable NO2 was estimated by Griess reaction detailed out in supplementary file.
2.5. Quality control
In order to minimise the risk of error, a number of quality control parameters were practiced during the study period from collection of samples, microbial analysis to cell culture conditions and toxicological assays. Flow rate calculations were checked every sampling day to assure that the fluctuations in flow rate of the sampler are within the range. Post sampling, collected filter samples were removed from the samplers immediately and transferred to the sample cassettes cleaned with 70% isopropyl alcohol. All the glassware, reagents and plastic wears used for the microbial and toxicity assays were sterilized and these assays were carried out in laminar hood with aseptic conditions. Field and filter blanks were analyzed once per month for each sampling site; in total, 24 blanks were analyzed. Both field and filter blanks were subjected to similar handling, extraction and treatment (culturing, endotoxin measurement, DTT and MTT assay) like sample filters in order to check for contamination. Mass concentration of field and filter blanks were found to be negligible and no contamination was observed. Proper growth of A549 Cells was daily checked microscopically and cell viability after trypsinization was also checked as an additional quality control parameter.
2.6. Statistical analysis
Data of mass concentration and CFU count of airborne microbes were investigated by descriptive statistics (mean and standard deviation). Statistical analysis of data in terms of student’s t-test and ANOVA was performed using the SPSS software version 20.0. Spearman’s and Pearson’s correlation coefficient was used to determine the correlation between the concentration of microbes and endotoxin with other analyzed parameters. Probability (p) values smaller than 0.01 and 0.05 were considered statistically significant for all analyses.
3. Results and discussion
3.1. Bioaerosol concentration in urban and rural environment
Annual average mass concentration of PM10 and PM2.5 at urban sampling site were 120.9 ± 40.5 μgm−3 and 94.6 μgm−3 respectively while at rural site, concentration of PM10 and PM2.5 was recorded as 142.4 ± 54.3 μgm−3 and 105.8 ± 30.8 μgm−3 respectively. Quantitative analysis revealed that the level of total culturable bacteria (TCB) associated with PM10 at urban houses varied from 323 to 3231 CFU/m3 with an average value of 2136 ± 285 CFU/m3 whereas the concentration of PM2.5 bound bacteria was found to be in the range of 246–2303 with mean value of 1605 ± 239 CFU/m3. Average CFU count of gram negative bacteria (GNB) associated with PM10 and PM2.5 was observed to be 1213 ± 224 and 899 ± 221 CFU/m3 respectively which is higher in comparison with the CFU count of gram positive bacteria (GPB) (PM10 – 923 ± 181 CFU/m3 and PM2.5–706 ± 195 CFU/m3) at urban environment (Table S3a). Average concentration of fungal fraction associated with PM10 and PM2.5 was observed as 1484 ± 301 and 1279 ± 280 CFU/m3, respectively. High bacterial concentration found at urban houses is probably attributed to the human activities such as talking, sneezing, coughing, and their other constant actions. Degradation of wastes carried out in the dump yard located near the urban houses (Fig. S1) provides a good platform for bacterial growth (Balyan et al., 2017; Kumar et al., 2013). In addition, indoor plants and pets present in urban houses are also traced to be the probable fungi and bacteria emitting sources (Prussin and Marr, 2015; Yassin and Almouqatea, 2010).
Level of total culturable bacteria (TCB) associated with PM10 and PM2.5 found in rural environments was exhibited as 1521 ± 206 and 1306 ± 163 CFU/m3 respectively. In rural houses, average concentration of gram positive bacteria associated with both sized PM was found to be higher with an average value of 829 ± 205 CFU/m3 (PM10) and 701 ± 201 CFU/m3 (PM2.5) in comparison with the CFU count of GNB. Concentration of fungal fraction was exhibited the dominance in both PM10 and PM2.5 with mean value of 1794 ± 302 and 1593 ± 300 CFU/m3 respectively (S3 a). Rural houses in present study are comparatively heavily crowded with 5–6 peoples of different age group, health and gender. Therefore, constant human activities of different background people and their activities like burning of oil lamps and incense can cause bacterial emissions (Agarwal et al., 2016). In present study, higher fungal load has been observed in rural houses probably attributed to the outdoor fungal sources such as agricultural activities and cattle sheds (Fig. S1) in close proximity of this site (Kalisa et al., 2019). Natural ventilation in rural houses is driven by prevailing wind flow through open windows and doors leading to higher air exchange rate and therefore more penetration of air from outdoor to indoor may increase in indoor fungal concentration. In addition, air exchange between outdoor and indoor environment i.e., infiltration can also occur through cracks and leaks present in the rural households (Leung, 2015). Previous studies have also reported higher fungal growth in rural environments which is in accordance with present results (Bragoszewska et al., 2016; Canha et al., 2015; Nasir et al., 2013).
The variation in microbial load in urban and rural households of Pune might be due to the different household and personal daily activities and as well as meteorological factors such as temperature and relative humidity (RH). Indoor temperature of all rural and urban housing types was reasonably uniform with an average value of 24.8 °C and 26.7 °C respectively. However, mean value of RH in rural houses (57.7%) was found to be higher than the urban (53.5%) households. In order to check the influence of temperature and relative humidity (RH) on the survival of airborne microbes, correlation between CFU count of bacterial and fungal aerosols with temperature and RH at both sites were calculated in terms of Spearman’s coefficient (Table S6). Statistically significant (p < 0.05) positive correlation has been found between the concentration of microbes in both sites and temperature and RH. Relative humidity is favorable for microbial growth as microbes can absorb this water from their living substrates for their metabolism; therefore increased air humidity helps in growth and release of airborne microbes (Bragoszewska and Pastuszka, 2018). Similarly, low temperature decrease cell membrane fluidity which further inhibits the microbial activity; therefore the increased atmospheric temperature promotes the growth and release of microbes (Bragoszewska et al., 2017).
Due to the absence of epidemiologically established dose-response relationship of most of the microbial agent and diversity between individuals in the response to a particular inhaled microbe, there are no threshold health based reference values concerning the concentration of microorganisms in residential indoor settings. Therefore, data obtained in present study was compared with guidelines provided by some organizations (CEC, WHO, OSHA, ACGIH, OISH, CHMC and CIC) and as well as with other studies performed in indoor environment in India (Chennai, Vishakhapatnam, Delhi) and worldwide (Ethiopia, Egypt, Nigeria and Iran). Microbial concentration in present study was above the threshold values prescribed by these organizations (Table – S4) and according to the sanitary standard of European commission for non-industrial premises (CEC, 1993), these values are considered to be heavily contaminated in terms of bacterial (500–2000 CFU/m3) and fungal (500–2000 CFU/m3) pollution. In addition, bacterial load in present study was found to be higher than Chennai, Vishakhapatnam, Delhi, Ethiopia and Iran, and lower than Nigeria, whereas fungal concentration exhibited higher values than Chennai, Vishakhapatnam, Ethiopia, Nigeria and Iran and lower than the values reported in Delhi and Egypt. Comparative study clearly confirmed the high degree of microbial pollution in urban and rural houses of Pune.
3.2. Seasonal variation in microbial growth
Significant seasonal variation (p < 0.05) in concentration of PM bound bacteria and fungi at urban and rural sites was observed and tabulated in Table S3b. In present study seasons are classified as winter (November–February), summer (March–June) and monsoon (July–October). The clear seasonal pattern for indoor bacteria associated with both sized PM. at urban and rural site was observed increasing from monsoon to winter with maximum concentration found in summer season. During summer season, degree of decomposition of waste in dump yard located near the urban site occurs at a faster rate which eventually causes higher emission of bacteria in urban houses. Predominance of bacterial aerosol in summer season was also reported by Sivasakthivel and Nandini (2017). In case of rural households, during summer season windows and doors are usually kept open which results in higher penetration of outdoor air resulting in higher fungal growth in the environment. In addition, elevated temperature in summer season also provides appropriate conditions for the germination, growth and propagation of airborne fungi (Xie et al., 2018). The concentration of bacteria and fungi in monsoon season remains least at both urban and rural sites due to rain wash effect and similar observation has been reported by other studies (Lal et al., 2017; Blazewicz et al., 2014).
3.3. Microbial identification
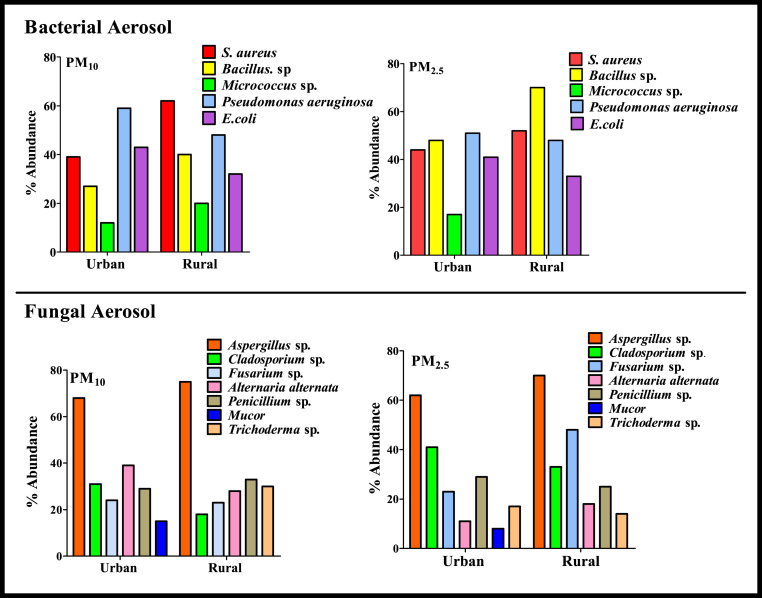
Morphology of bacterial colonies found at the urban and rural sampling sites depicted different shapes viz., like round (cocci) and rod (bacillus) and fungal fractions exhibited the presence of spores and hypehae structure (Fig. S5 a and S5 b). Further, confirmation of these isolates were done in terms of gene sequencing leading to the identification of 5 bacterial and 7 fungal species predominantly found in indoor air. During the study period, common identified bacterias in both rural and urban sites were S. aureus, Bacillus sp., Micrococcus sp., Pseudomonas aeruginosa and E.coli. Among them, two species belong to gram positive Cocci (S. aureus, Micrococcus sp.), Bacillus sp. is spore forming gram positive rod and remaining two Pseudomonas aeruginosa and E.coli. belong to gram negative bacilli. The relative percentage abundance of these microbes associated with PM10 and PM2.5 at urban and rural site is depicted in Fig. 1 . At urban site, Pseudomonas aeruginosa was found to be pre-dominant with 59.2% and 51.3% in PM10 and PM2.5 samples respectively. Whereas, at rural site S. aureus and Bacillus sp. were dominant in PM10 and PM2.5 with percentage abundance of 62.5% and 70% respectively. Profusion of Pseudomonas aeruginosa in urban environment can be a major concern because of its association with respiratory tract infections whereas similarly the dominance of S. aureus in rural houses also may pose health threat to the exposed occupants due to their capability of causing disease through invasion and toxin production such as pneumonia, abscess and diarrhea (Liu et al., 2018; Sheikh et al., 2015). Identified Bacillus species in PM2.5 can survive long in the air and known to cause food poisoning and periodontal disease (Agarwal et al., 2016). Prevalence of similar bacteria aerosols in indoor environments were also reported in previous studies (Agarwal et al., 2016; Sheikh et al., 2015; Gangamma et al., 2011). Among 7 identified species of airborne fungi (Aspergillus sp., Cladosporium sp., Penicillium sp., Alternaria alternata, Fusarium sp., Trichoderma sp. and Mucor), Aspergillus sp. was found to be predominant in both sized PM collected from urban and rural households. Abundance of Aspergillus species may trigger hypersensitivity pneumonitis to the exposed occupants as these species are well-known to play a role in three different clinical settings in humans: opportunistic infections, allergic states and toxicoses by inhalation (Marta Malecka-Adamowicz et al., 2019; Egbuta et al., 2017). In addition, other recognized fungal species such as Penicillium sp, Alternaria alternata and Cladosporium sp have the ability to cause severe episodes of pneumonitis, asthma and rhinitis respectively which can also be a threat for the residents (Agarwal et al., 2016; Priyamvada et al., 2017). Results of the present study exhibited that most of the identified bacterial and fungal species in indoor air of urban and rural houses are pathogenic in nature and therefore more awareness should be paid in order to safeguard the environment.
Fig. 1.
Percentage abundance of bacterial and fungal isolates associated with PM10 and PM2.5 samples collected from urban and rural households.
3.4. Endotoxin measurement
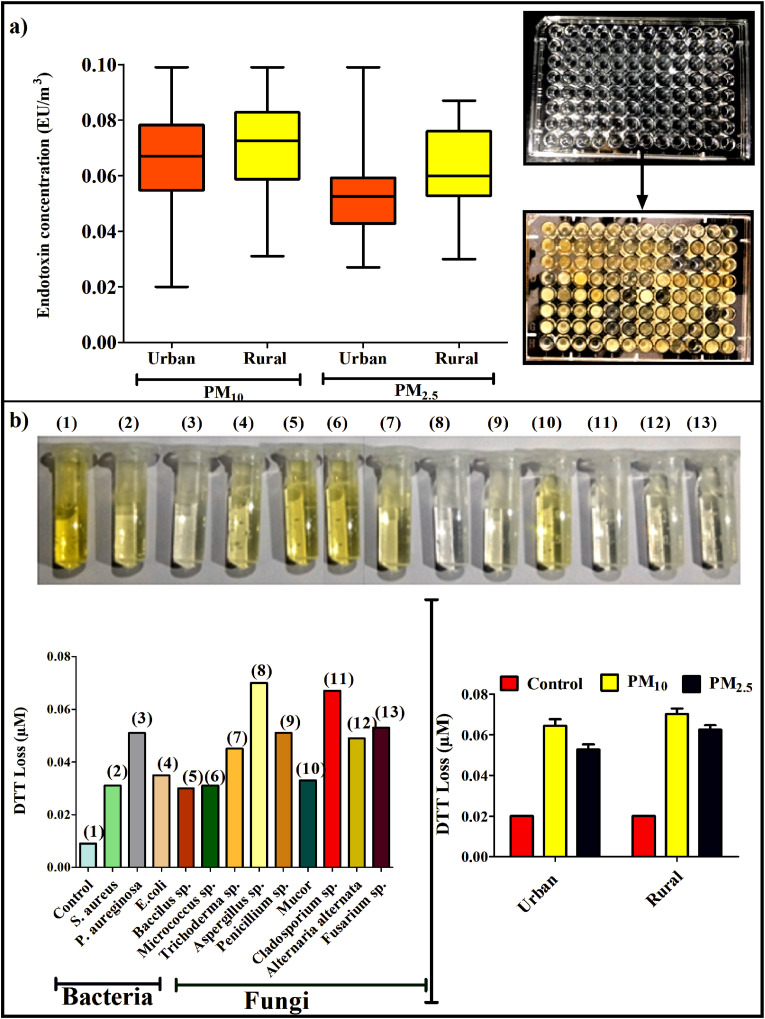
Endotoxin, an important biomarker can be linked to various respiratory outcomes and considered as a potent modifier of acute and chronic toxicity. Mean bacterial endotoxin concentration associated with PM10 and PM2.5 measured at urban site was 0.98 ± 0.34 EU/m3 and 0.77 ± 0.22 EU/m3 respectively while at rural site, the level was recorded as 0.90 ± 0.24 EU/m3 for PM10 samples and 0.69 ± 0.24 EU/m3 for PM2.5 (Fig. 2 a). Overall endotoxin level was little higher in urban houses as compared to the rural one. Endotoxin originates primarily from gram –ve bacteria and in present study concentration of gram –ve bacteria has been found to be maximum in urban houses which supports the comparatively higher level of measured endotoxin at urban site. In present study, urban houses were comprised of pets which have been identified as potential sources of airborne endotoxin (Salonen et al., 2016). In addition to this, carpet flooring in urban houses and habits of the occupants like tobacco smoking (Fig. S1) are also considered as imperative predictors of increased level of endotoxin (Yoda et al., 2016; Salonen et al., 2016). Endotoxin level in rural houses is probably attributed to the agricultural activities, intensive farming operations and biomass burning activities in proximity of the sampling site (Rooij et al., 2017; Yoda et al., 2016). Higher endotoxin concentration was found to be associated with PM10 fraction and similar observation has already been reported in previous studies (Rolph et al., 2018; Lu et al., 2014; Traversi et al., 2011). Endotoxin is made up of lipopolysaccharide which is probably allied with heavy metals and silicates preferably found in larger particles may be resulting into higher concentration of endotoxin in PM10 size fraction (Rolph et al., 2018; Nilsson et al., 2011). Internationally accepted threshold limit of endotoxin exposure indicating the safe level for humans has yet not been established. Although the National Health Council of the Netherlands has prescribed the threshold limit of 90 EU/m3 for endotoxin exposure, but various scholars have suggested that endotoxin level in residential premises ranging from 0.02 to 19.5 EU/m3 could have played significant role in causing sick building symptoms (Roque et al., 2018; Park et al., 2008). In addition, results derived from previous studies demonstrated that low level of endotoxin can also cause adverse health effects (Farokhi et al., 2018; Ryan et al., 2009).
Fig. 2.
a) Endotoxin measurement of PM10 and PM2.5 samples (b) Oxidative potential in terms of DTT loss of PM samples and predominantly found bacterial (2–6) and fungal species (7–13) in indoor environments.
3.5. Oxidative potential of PM bound microbes
Oxidative potential initiated by the formation of reactive oxygen species (ROS) is an important metric associated with various biological responses of PM exposure (Jan et al., 2020; Visentin et al., 2016). These generated ROS are able to cause microbial toxicity in terms of two main mechanisms, oxidative stress and inflammation. Therefore, recognizing the importance of ROS, oxidative reactivity of indoor PM samples and predominantly found 5 bacterial and 7 fungal species in indoor air of urban and rural houses was assessed in terms of DTT assay and depicted in Fig. 2b. Oxidation of DTT can be measured by examining the rate of DTT loss in presence of redox active components (microbial cells) and can be used as a measure of oxidative potential. Intrinsic higher DTT activity (in terms of DTT loss) with respect to control has been observed for both sized PM samples collected from urban and rural households clearly demonstrating their oxidative nature. DTT depletion from blank samples was also measured and no difference was found as compared to control. Generally, the identification of specific components of PM as a contributor to DTT loss is based on the correlation of DTT activity with PM composition. Therefore in present study, in order to identify the role of bacterial and fungal aerosols towards DTT loss, correlation between CFU count of bacterial and fungal composition in both sites and DTT loss in PM samples were calculated in terms of Pearson’s coefficient (Table S6). Significant positive correlation has been observed between DTT loss and microbial concentration in urban (r = 0.63 for bacteria and 0.67 for fungi) and rural (r = 0.6 for bacteria and 0.7 for fungi) houses, clearly supporting the influence of these microbial components of PM on DTT loss. In addition, all of the individual microbes (cultivated from PM extract) predominantly found in indoor air exhibited considerable DTT depletion as compared to the control also suggesting their significant oxidative potential. Fungal isolates have induced higher depletion of DTT in comparison with bacterial cells and amongst all, Aspergillus sp. showed maximum oxidative reactivity followed by Cladosporium sp., Pseudomonas aeruginosa and Fusarium sp. etc. Elevated oxidative potential of Aspergillious species was also previously reported by other studies (Samake et al., 2017; Madsen et al., 2012; Timm et al., 2009) Functional groups present in the cell walls of these microbes can involve in DTT dehydrogenation in terms of redox activity and leading to imbalances in redox cycling and the production of high levels of ROS (Samake et al., 2017). Amongst bacterial isolates, maximum oxidative potential has been exhibited by gram negative bacteria i.e., Pseudomonas aeruginosa which is probably attributed to the oxidative stress induced by Pyocyanin, the most virulence factor produced by this species (Hall et al., 2016). Oxidative potential of these airborne microbes present in urban and rural households reveals the involvement of these microbial cells in induction of ROS generation and their imperative association with serious inflammation related health risk.
3.6. Cellular toxicity: MTT assay and annexing PI
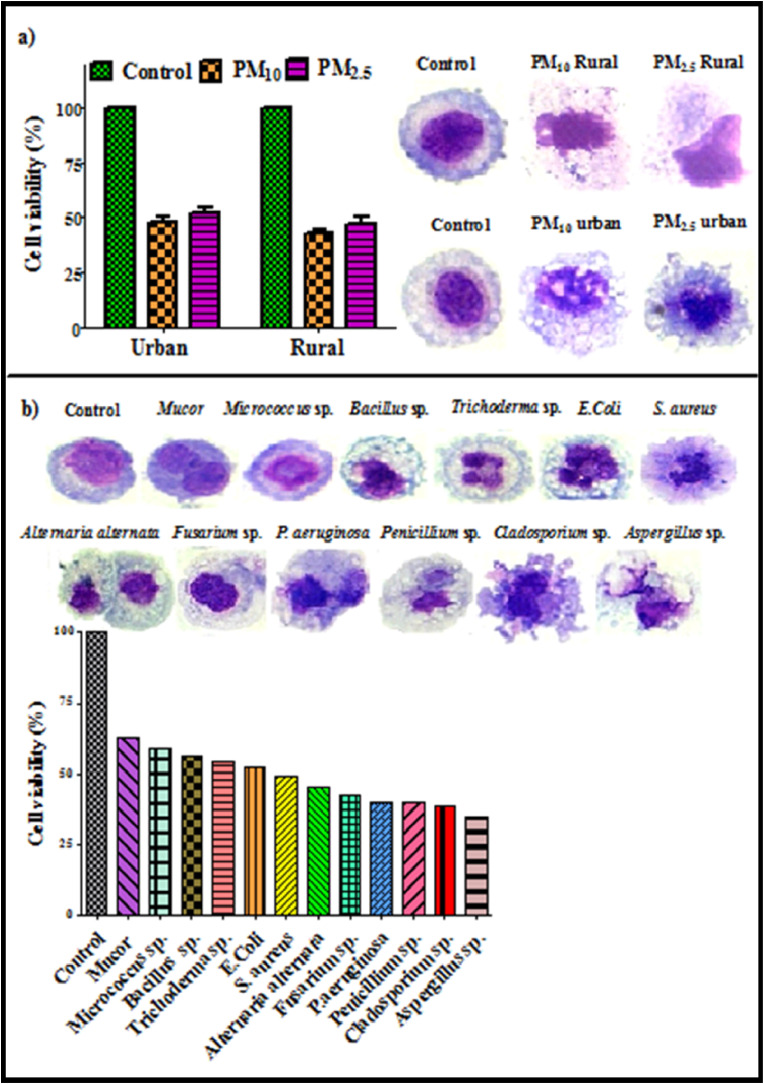
Indoor PM samples and prevalently found microbes associated with PM exhibited considerable cytotoxic response towards A549 cells in both urban and rural households. In both size fraction of PM (75 μg/ml), statistically significant decrease (p < 0.01) in cell viability was observed with maximum cell death in PM10 samples collected from rural houses. Cell viability was decreased to 47.8% and 51.6% for PM10 and PM2.5 samples respectively in urban site whereas in rural site cell viability decreased to 42.4% and 47.1% for PM10 and PM2.5 respectively (Fig. 3 a). The viability from the blank samples from each lot was also calculated and no difference was found as compared to control. Cytotoxic response of PM samples can be characterized by the prominent morphological changes as compared to the untreated cell (control). Microscopic images of A549 cell death on exposure to both PM10 and PM2.5 samples from urban and rural site is depicted in Fig. 3a. Interaction of PM samples with cells causes severe alteration in cell wall, membrane disruption, cell shrinkage and fragmentation of nucleus indicating the prominent feature of cell death. Oxidative stress initiated by the formation of ROS is considered as the most promising mechanism of cytotoxic behavior of indoor PM which probably attributed to the presence of different microbial (bacteria, fungi, pollen) and chemical constituents (metals, PAHs, elemental and organic carbon) in PM. Therefore, in order to address the precise role of microbes associated with PM and bacterial endotoxin for eliciting the cellular toxicity, Pearson’s correlation was calculated in present study (Table S6). Significant negative correlation has been found between A549 cell viability and microbial (r = −0.74 for urban bacteria, r = - 0.69 for rural bacteria; r = - 0.77 for urban fungi, r = - 0.84 for rural fungi) and endotoxin concentration (r = - 0.73 for urban, r = −0.69 for rural) clearly demonstrating the influence of these airborne microbiata and their components in causing the cell death. Further, interaction between individual microbial species abundantly found in indoor air and A549 cells were also evaluated. On exposure of A549 to predominant airborne microbes, significant decrease in cell viability with respect to control was observed. Fungal species induced higher cytotoxic response as compared to the bacterial cells (Fig. 3b) and treated cells appeared to be non-viable with considerable detachment and other morphological changes (Fig. 3b). All together, maximum cell death was observed (65%) by Aspergillus sp. probably due to their ability to produce secondary metabolites which can be responsible for causing cytotoxic effects (Alko and Mehta, 2015) and Mucor was found to be least cytotoxic in nature. Gram negative bacteria (Pseudomonas aeruginosa) exhibited maximum decrease in cell viability among bacterial isolates with alteration of epithelial permeability and finally damage the cytoskeleton.
Fig. 3.
Cytotoxic evaluation (MTT assay) of PM10 and PM2.5 samples (a) and PM associated predominantly found bacterial and fungal species (b) in indoor environments.
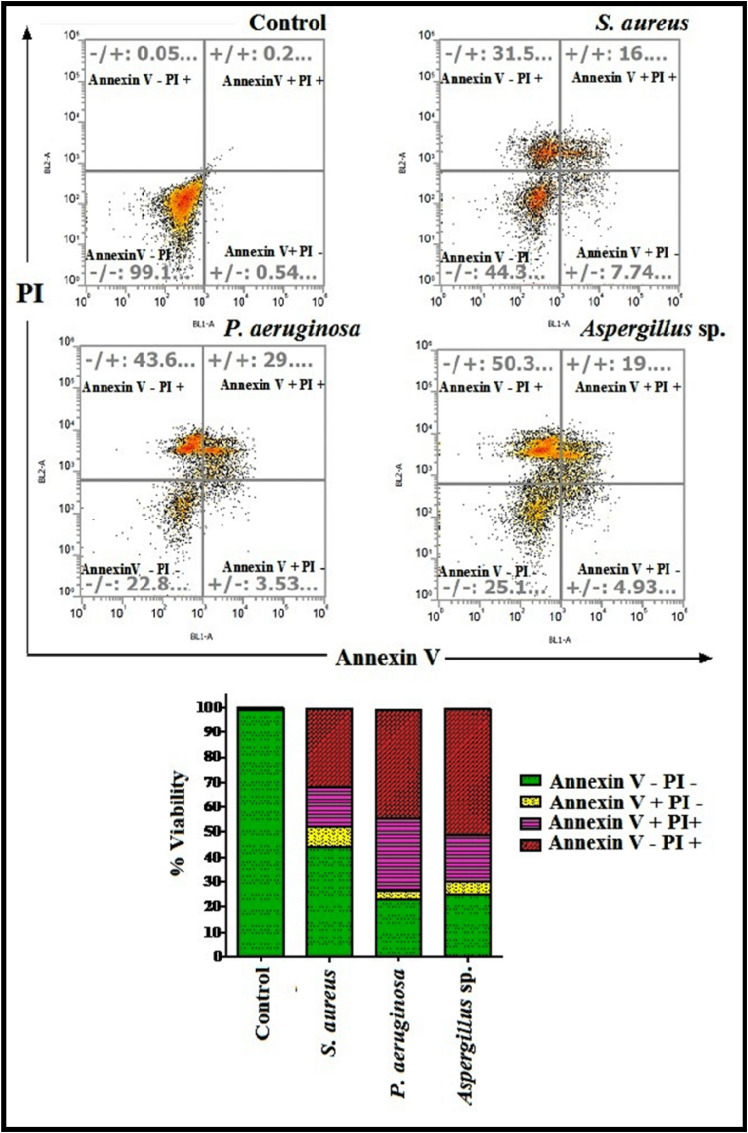
MTT assay revealed the confirmation of cell death induced by microbial load present in indoor air. Commonly cell death involves two primary pathways: apoptosis and necrosis (Turner et al., 2015). In present study, Annexin V assay differentiate between apoptosis and necrosis pathways relating to cell death induced by gram positive (S. aureus), gram negative bacterial (Pseudomonas aeruginosa) and fungal (Aspergillus sp.) species dominantly found in indoor environments and depicted in Fig. 4 . Present findings demonstrated the significant potential of all three chosen airborne microbes to persuade cell death in terms of both necrosis and apoptosis. With respect to the control, percentage cell death has been considerably increased for both bacterial and fungal pathogens. Aspergillus sp. exhibited maximum percentage of necrotic cell death (50.3%) amongst all analyzed microbes. Gram negative bacteria (Pseudomonas aeruginosa) induced 31.5% of necrotic (annexin V-/PI+.)) and 16% of late apoptotic (annexin V+/PI+) cell death where as gram positive bacteria (S. aureus) induced 43.6% necrotic and 29% of late apoptotic cell death. Necrosis is considered as a post apoptotic event in which necrotic cells are associated with both apoptotic (DNA fragmentation) and necrotic features (rupture of cytoplasmic membrane) and pathogenic consequence of persistent apoptotic-necrosis is the leakage of pro-inflammatory mediators leading to acute and chronic inflammation (Oliveira et al., 2015). ROS mediated oxidative stress has comprehensive influence in induction of necrotic cell death (Hea et al., 2017; Choi et al., 2009). Results of DTT assay already demonstrated the highly oxidative nature of Aspergillus sp. and Pseudomonas aeruginosa which can be considered as an imperative factor of causing significant necrotic cell death. Findings from present study established that the cytotoxicity initiated in A549 cells by both bacterial and fungal pathogens can lead to both types of cell death; however, necrosis appeared to be the dominant pathway.
Fig. 4.
Annexin V study of most prevalent PM associated gram positive (S. aureus), gram negative bacterial (P. aeruginosa) and fungal (Aspergillus sp) isolates found in indoor environments.
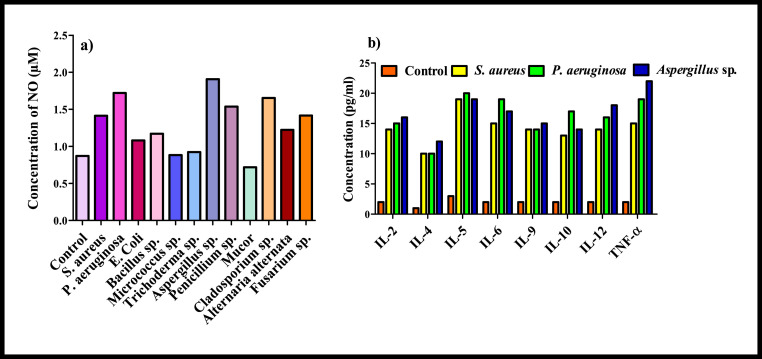
3.7. Inflammation: production of NO and cytokines
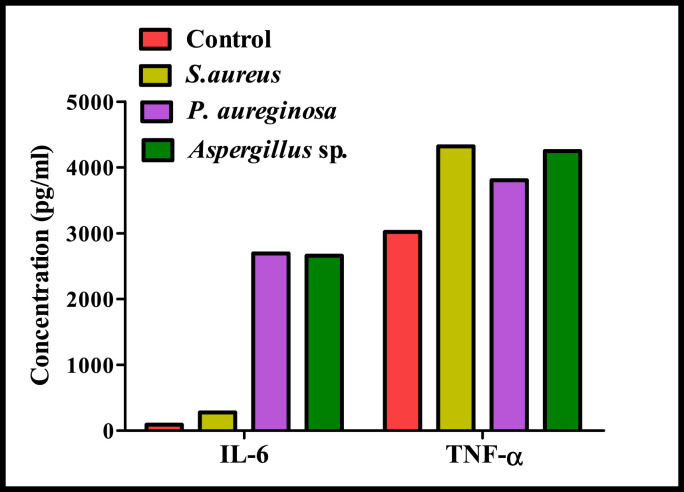
On exposure to microbial pathogens, the immune system responds to irritations through an innate cascade, known as inflammation which is initiated by the release of extracellular mediators. Production of nitric oxide (NO) and proinflammatory cytokines including tumor necrosis factor alpha (TNFα) and various interleukins (IL-6, IL-4, IL-2) are considered as the most potent biomarkers of immunological activation of the cells in the respiratory tract (Huttunen et al., 2012). In present study, mostly all identified microbes in indoor air trigger the production of NO with respect to control suggesting their ability to cause the adverse respiratory health effects (Fig. 5 ). Aspergillus sp. elicited maximum NO production in A549 cells after 24 h exposure with an average value of 1.9 μM. Inflammatory nature of fungal metabolites and their involvement in causing non-allergenic respiratory health effects has been also confirmed previously by Miller et al., 2010. Amongst bacterial isolates, Pseudomonas aeruginosa induced maximum NO production followed by Bacillus sp. and S aureus. In addition to NO, cytokines are considered as the key modulators participating in acute and chronic inflammation (Turner et al., 2014). TNF-α, and IL-6 are believed to be the main mediators of host response towards infectious organisms and higher level of these modulators are reported in response to viral infections, and allergic rhinitis (Skovbjerg et al., 2010; Purokivi et al., 2002). In present study, predominantly found gram positive (S. aureus), gram negative bacterial (Pseudomonas aeruginosa) and fungal (Aspergillus sp.) species associated with PM were subjected to the production of inflammatory cytokines (IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12, TNF-α) in alveolar epithelial cells and the results revealed the significant production of all these cytokines as compared to the corresponding unexposed controls (Fig.3.5). Considerable production of cytokines by these airborne microbes probably is associated with their higher oxidative potential (Viegas et al., 2017). Pseudomonas aeruginosa induced maximum production of IL-5, IL-6 and IL-10 and maximum production of other cytokines (TNF-α, IL-12, IL-2, IL-9) has been triggered by Aspergillus sp. In addition, prominent production of IL-6 and TNF- α as compared to control has been also observed on interaction of chosen microbial with lung macrophages (Fig. 6 ). Significant increase in the expression of representative inflammatory cytokines (IL-6 and TNF- α) clearly indicates acute and short inflammatory response of these airborne microbes of PM. The levels of TNF-α, secreted into the culture medium were highest in GPB treated macrophages whereas, expression of IL-6 was found to be maximum for GNB and Aspergillus sp. treated macrophages. Similar pattern of elevated cytokine (IL-6 and TNF- α) production was also previously reported (Longhin et al., 2016; Skovbjerg et al., 2010). Inflammatory reaction of bacterial isolates is mainly attributed to the binding between different bacterial cell wall components (lipotecichoic acids for GPB and lipopolysaccharide for GNB) and cell surface receptors (Toll like receptor 2 for GPB and Toll like receptor 4 for GNB) of macrophage (Hesslea et al., 2005). Findings in present study clearly indicate the role of inflammation induced by prevalently found indoor bacterial and fungal species as one of the primary response of lung.
Fig. 5.
Production of Inflammatory mediators (NO and cytokines) by prevalent gram positive (S. aureus), gram negative bacterial (P. aeruginosa) and fungal (Aspergillus sp.) fractions of PM in lung epithelial cells.
Fig. 6.
Production of Inflammatory cytokines (IL-6 and TNF- α) by prevalent gram positive (S. aureus), gram negative bacterial (P. aeruginosa) and fungal (Aspergillus sp.) fractions of PM in alveolar macrophages.
4. Conclusion
In summary, results derived from the present study provide an insight into the toxicological characterization of PM and its microbial components collected from urban and rural households of Pune. Results showed that the microbial load in both sampling sites were highly dependent on household characteristics, activities performed by the occupants and as well as the outdoor influences. Mostly identified microbes in indoor air are pathogenic in nature and their elevated levels in urban and rural houses are a real concern for the respiratory health of exposed residents. Significant oxidative potential, inflammatory activity and cytotoxic behavior of prevalently found indoor microbes clearly suggested their hazardous nature and association with various acute and chronic diseases. Findings from present study bridges the existing knowledge gap in the role of airborne microbes for causing wide range of health threats and further could serve as a basis for other bioaerosol studies looking at the role of biogenic components of PM on human health. In addition, detailed understanding of toxicological assessment of airborne microbes in present study will certainly assist the policy makers and environmentalist in the development of appropriate intervention assuring the protection of vulnerable population from adverse health effects of bioaerosols. Bioaerosols are comprised of culturable and non-culturable forms whereas in this study only culturable fractions were monitored therefore the inclusion of non-culturable form is also important to provide an accurate estimation of health risk. Furthermore, in order to reduce the health risk and to establish reliable health based guidelines of bioaerosols, there is a need of more detailed similar studies keeping in view the indoor household conditions.
Acknowledgement
Authors wish to thank Council of Scientific and Industrial Research (CSIR-SRF-09/137/0600/2019 and CSIR- RA-09/137/0601/2019), India and Science and Engineering Research Board (SERB), India (CRG/2019/004189) for financial assistance. Authors are thankful to Department of Chemistry, and Central Instrumentation Facility Savitribai Phule Pune University, Pune for providing necessary facilities (FE-SEM analysis).
Footnotes
This paper has been recommended for acceptance by Eddy Y. Zeng.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envpol.2020.114698.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Agarwal S., Mandal P., Majumdar D., Agarwal S.G., Srivastava A. Characterization of bioaerosols and their relation with OC, EC and carbonyl VOCs at a busy roadside restaurants-cluster in New Delhi. Aerosol Air Qual. Res. 2016;16:3198–3211. [Google Scholar]
- Alko V., Mehta A. Occurrence, detection and detoxification of mycotoxin. J. Biosci. 2015;40(5):943–954. doi: 10.1007/s12038-015-9569-6. [DOI] [PubMed] [Google Scholar]
- Balyan P., Das S., Ghosh C., Banarjee B.D. Spatial variation of biogenic aerosols at different land use configurations in urban Delhi. Int. J. Appl. Environ. Sci. 2017;12(5):731–744. [Google Scholar]
- Blazewicz S.B., Schwartz E., Firestone M.K. Growth and death of bacteria and fungi underlie rainfall-induced carbon dioxide pulses from seasonally dried soil. Ecology. 2014;95(5):1162–1172. doi: 10.1890/13-1031.1. [DOI] [PubMed] [Google Scholar]
- Bragoszewska E., Pastuszka J.S. Influence of meteorological factors on the level and characteristics of culturable bacteria in the air in Gliwice, Upper Silesia (Poland) Aerobiologia. 2018;34:241–255. doi: 10.1007/s10453-018-9510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragoszewska E.B., Manika A., Pastuszka J.S. Bacterial and fungal aerosols in rural nursery schools in southern Poland. Atmosphere. 2016;7:142. doi: 10.3390/atmos7110142. [DOI] [Google Scholar]
- Bragoszewska E.B., Manika A., Pastuszka J.S. Concentration and size distribution of culturable bacteria in ambient air during spring and winter in gliwice: a typical urban area. Atmosphere. 2017;8:239. [Google Scholar]
- Canha N., Almedia S.M., Freitas M.D.C., Woltrbeek T. Assessment of bioaerosols in urban and rural primary schools using passive and active sampling methodologies. Arch. Environ. Protect. 2015;41(4):11–22. [Google Scholar]
- Choi J., Fuentes M., Reich B.J. Spatial–temporal association between fine particulate matter and daily mortality. Comput. Stat. Data Anal. 2009;53:2989–3000. doi: 10.1016/j.csda.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egbuta M.A., Nwanza M., Babalola O.O. Health risks associated with exposure to filamentous fungi. Int. J. Environ. Res. Publ. Health. 2017;14:719. doi: 10.3390/ijerph14070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhi A., Heederik D., Smit L.A.M. Respiratory health effects of exposure to low levels of airborne endotoxin – a systematic review. Environ. Health. 2018;17:14. doi: 10.1186/s12940-018-0360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangamma S., Patil R.S., Mukherji S. Characterization and proinflammatory response of airborne biological particles from wastewater treatment plants. Environ. Sci. Technol. 2011;45:3282–3287. doi: 10.1021/es103652z. [DOI] [PubMed] [Google Scholar]
- Gong J., Qi J., Beibei E., Yin Y., Gao D. Concentration, viability and size distribution of bacteria in atmospheric bioaerosols under different types of pollution. Environ. Pollut. 2020;257:113485. doi: 10.1016/j.envpol.2019.113485. [DOI] [PubMed] [Google Scholar]
- Hall S., McDermott C., Annopkumar-Dukie S., Mcfarland A.J., Forbes A., Perkins A.V. Cellular effects of Pyocyanin, a secreted virulence factor of Pseudomonas aeruginosa. Toxins. 2016;8:236. doi: 10.3390/toxins8080236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka R.B., Mutlu G.M. Particulate matter air pollution: effects on the cardiovascular system. Front. Endocrinol. 2018;16(9):680. doi: 10.3389/fendo.2018.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hea L., Hea T., Farrab S., Jia L., Liua T., Ma Xi. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017;44:532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- Hesslea C.C., Anderssonb B., Wold A.E. Gram-positive and Gram-negative bacteria elicit different patterns of pro-inflammatory cytokines in human monocytes. Cytokine. 2005;30:311–318. doi: 10.1016/j.cyto.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Hime N.J., Marks G.B., Cowie C.T. A comparison of the health effects of ambient particulate matter air pollution from five emission sources. Int. J. Environ. Res. Publ. Health. 2018;15:1206. doi: 10.3390/ijerph15061206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbal C., Gautam S., Trivedi U. A review on recent progress in observations and health effects of bioaerosols. Environ. Int. 2018;118:189–193. doi: 10.1016/j.envint.2018.05.053. [DOI] [PubMed] [Google Scholar]
- Huttunen K., Siponen T., Salonen I., Yli-Tuom T., Aurela M., Dufva H. Low-level exposure to ambient particulate matter is associated with systemic inflammation in ischemic heart disease patients. Environ. Res. 2012;116:44–51. doi: 10.1016/j.envres.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Jan R., Roy R., Bhor R., Pai K., Satsangi P.G. Toxicological screening of airborne particulate matter in atmosphere of Pune: reactive oxygen species and cellular toxicity. Environ. Pollut. 2020 doi: 10.1016/j.envpol.2019.113724. [DOI] [PubMed] [Google Scholar]
- Kalisa E., Archer S., Nagato E., Bizuru E., Lee K., Tang N. Chemical and biological components of urban aerosols in Africa: current status and knowledge gaps. Int. J. Environ. Res. Publ. Health. 2019;16:941. doi: 10.3390/ijerph16060941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.H., Kabir E., Jahan S.A. Airborne bioaerosols and their impact on human health. J. Environ.Sci. 2018;67:23–35. doi: 10.1016/j.jes.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B., Gupta G.P., Singh S., Kulshrestha U.C. Study of abundance and characterization of culturable bioaerosol at Delhi, India. Int. J. Environ. Eng. Manag. 2013;4:219–226. [Google Scholar]
- Lal H., Ghosh B., Srivastava A. Identification and characterization of size segregated bioaerosols at different sites in Delhi. Aerosol Air Qual. Res. 2017;17:1570–1581. [Google Scholar]
- Lee B.U. Life comes from the air: a short review on bioaerosol control. Aerosol Air Qual. Res. 2011;11:921–927. [Google Scholar]
- Leung D.Y.C. Outdoor-indoor air pollution in urban environment: challenges and opportunity. Front. Environ. Sci. 2015;2:1–7. [Google Scholar]
- Liu B., Ichinose T., He M., Kobayashi F., Maki T., Yoshida S. Lung inflammation by fungus, Bjerkandera adusta isolated from Asian sand dust (ASD) aerosol and enhancement of ovalbumin-induced lung eosinophilia by ASD and the fungus in mice. Allergy Asthma Clin. Immunol. 2014;10:1–12. doi: 10.1186/1710-1492-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Zhang X., Zhang H., Yao X., Zhou M., Wang J. Effect of air pollution on the total bacteria and pathogenic bacteria in different sizes of particulate matter. Environ. Pollut. 2018;233:483–493. doi: 10.1016/j.envpol.2017.10.070. [DOI] [PubMed] [Google Scholar]
- Longhin E., Capasso L., Battaglia C., Proverbio M.C., Cosentino C., Cifola I. Integrative transcriptomic and protein analysis of human bronchial BEAS-2B exposed to seasonal urban particulate matter. Environ. Pollut. 2016;209:87–98. doi: 10.1016/j.envpol.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Lu Y., Su Shu, Jin W., Wang B., Li N., Shen H. Characteristics and cellular effects of ambient particulate matter from Beijing. Environ. Pollut. 2014;191:63–69. doi: 10.1016/j.envpol.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Madsen A.M., Tendal K., Schlunssen V., Heltberg I. Organic dust toxic syndrome at a grass seed plant caused by exposure to high concentrations of bioaerosols. Ann. Ocup. Hyg. Health. 2012;56:777–778. doi: 10.1093/annhyg/mes012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecka-Adamowicz M., Kubera L., Jankowiak E., Dembowska E. Microbial diversity of bioaerosol inside sports facilities and antibiotic resistance of isolated Staphylococcus spp. Aerobiologia. 2019;35:731–742. [Google Scholar]
- Mandal J., Brandl H. Bioaerosols in indoor environment - a review with special reference to residential and occupational locations. Open Environ. Biol. Monit. J. 2011;4(1):83–96. [Google Scholar]
- Miller J.D., Sun M., Gilyan A., Roy J., Rand T.G. Inflammation-associated gene transcription and expression in mouse lungs induced by low molecular weight compounds from fungi from the built environment. Chem. Biol. Interact. 2010;183:113–124. doi: 10.1016/j.cbi.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Moretti S., Smets W., Hofman J., Valentine K., Eline M., Dieter O. Human inflammatory response of endotoxin affected by particulate matter-bound transition metals. Environ. Pollut. 2018;244:118–126. doi: 10.1016/j.envpol.2018.09.148. [DOI] [PubMed] [Google Scholar]
- Nasir Z.A., Colbeck I., Ali Z., Ahmed S. Indoor particulate matter in developing countries: a case study in Pakistan and potential intervention strategies. Environ. Res. Lett. 2013;8 [Google Scholar]
- Nilsson S., Merritt A.S., Bellander T. Endotoxins in urban air in Stockholm, Sweden. Atmos. Environ. Times. 2011;45:266–270. [Google Scholar]
- Oliveira P.S.S., Cardoso P.R.G., de Andrade Lima E.V., Pereira M.C., Durate A.L.B.P., Pitta I.R. IL-17A, IL-22, IL-6, and IL-21 serum levels in plaque-type psoriasis in Brazilian patients. Mediat. Inflamm. 2015;1–5 doi: 10.1155/2015/819149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orevik J. Oxidative potential versus biological effects: a review on the relevance of cell-free/abiotic assays as predictors of toxicity from airborne particulate matter. Int. J. Mol. Sci. 2019;20:4772. doi: 10.3390/ijms20194772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.H., Spiegelman D.L., Burge H.A., Gold D.R., Chew G.L., Milton D.K. Longitudinal study of dust and airborne endotoxin in the home. Environ. Health Perspect. 2008;108:1023–1028. doi: 10.1289/ehp.001081023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.W., Park C.W., Lee S.H., Hwang J. Fast monitoring of indoor bioaerosol con-centrations with ATP bioluminescence assay using an electrostatic rod-type sampler. PloS One. 2015;10:1–13. doi: 10.1371/journal.pone.0125251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., Milton D.K., Reponen T., Hill J. A role for environmental engineering and science in preventing bioaerosol-related disease. Environ. Sci. Technol. 2008;42(13) doi: 10.1021/es087179e. 4631-463. [DOI] [PubMed] [Google Scholar]
- Priyamvada H., Akia M., Singh R.K., Ravikrishna R., Verma R.S., Philip L. Terrestrial macrofungal diversity from the tropical dry evergreen biome of southern India and its potential role in Aerobiology. Plus one. 2017;40:1387. doi: 10.1371/journal.pone.0169333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussin A.J., Marr L.C. Sources of airborne microorganisms in the built environment. Microbiome. 2015;3:78. doi: 10.1186/s40168-015-0144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purokivi M.K., Hirvonen M.R., Randell J.T., Roponen M.H., Meklin T.M., Nevalainen A.L. Changes in pro-inflammatory cytokines in association with exposure to moisture-damaged building microbes. Eur. Respir. J. 2002;18:951–958. doi: 10.1183/09031936.01.00201201. [DOI] [PubMed] [Google Scholar]
- Robertson S., Douglasb P., Jarvisc D., Marczylo E. Bioaerosol exposure from composting facilities and health outcomes in workers and in the community: a systematic review update. Int. J. Hyg Environ.Health. 2019;222:364–386. doi: 10.1016/j.ijheh.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Rolph C.A., Gwyther C.L., Tyrrel S.F., Nasir Z.A., Drew G.H., Jackson S.K. Sources of airborne endotoxins in ambient air and exposure of nearby communities—a review. Atmosphere. 2018;9:375. [Google Scholar]
- Rooij M.M.T.D., Heederik D.J.J., Borlee F., Hoek G., Wouters I.M. Spatial and temporal variation in endotoxin and PM10 concentrations in ambient air in a livestock dense area. Environ. Res. 2017;153:161–170. doi: 10.1016/j.envres.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Roque K., Shin K.M., Jo J.H., Lim G.D., Song E.S., Shin S.J. Association between endotoxin levels in dust from indoor swine housing environments and the immune responses of pigs. J. Vet. Sci. 2018;19(3):331–338. doi: 10.4142/jvs.2018.19.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R., Jan J., Gunjal G., Bhor R., Pai K., Satsangi P.G. Particulate matter bound polycyclic Aromatic hydrocarbons: toxicity and health risk assessment of exposed inhabitants. Atmos. Environ. 2019;210:47–57. [Google Scholar]
- Ryan P.H., Bernstein D.I., Lockey J., Reponen T., Levin L., Grinshpun S. Exposure to traffic-related particles and endotoxin during infancy is associated with wheezing at age 3 years. Am. J. Respir. Crit. Care Med. 2009;180:1068–1075. doi: 10.1164/rccm.200808-1307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen H., Duchaine C., Etourneau V., Mazaheri M., Liaitinen S., Cliffor S. Endotoxin levels and contribution factors of endotoxins in resident, school, and office environments: a review. Atmos. Environ. 2016;142:360–369. [Google Scholar]
- Samake A., Uzu G., Calas A., Vince E., Parat S., Jaffrezo J.L. The unexpected role of bioaerosols in the Oxidative Potential of PM. Sci. Rep. 2017;7:10978. doi: 10.1038/s41598-017-11178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedha S., Doctor P. Biological agents - a potential occupational hazard. FPI. 2017;1(3):99–112. [Google Scholar]
- Sheikh H.Q., Aqil A., Kirby A., Hossain F.S. Panton-Valentine leukocidin osteomyelitis in children: a growing threat. Br. J. Hosp. Med. 2015;76:18–24. doi: 10.12968/hmed.2015.76.1.18. [DOI] [PubMed] [Google Scholar]
- Shen F., Zheng Y., Niu M., Zhou F., Wu Y., Wang J. Characteristics of biological particulate matters at urban and rural sites in the North China Plain. Environ. Pollut. 2019;253:567–577. doi: 10.1016/j.envpol.2019.07.033. [DOI] [PubMed] [Google Scholar]
- Shiraiwa M., Ueda K., Pozzer A., Lammel G., Kampf C.J., Fushimi A. Aerosol health effects from molecular to global scales. Environ. Sci. Technol. 2017;51:13545–13567. doi: 10.1021/acs.est.7b04417. [DOI] [PubMed] [Google Scholar]
- Sivasakthivel S., Nandini N. Seasonal variation of airborne viable bacterial pollution in Bengaluru urban, Karnataka, India. Environ. Sci. 2017;13(4):145. [Google Scholar]
- Skovbjerg S., Martner A., Hynsjö Lars B., Hessle C., Olsen I., Dewhirst F.E. Gram-positive and gram-negative bacteria induce different patterns of cytokine production in human mononuclear cells irrespective of taxonomic relatedness. J. Interferon Cytokine Res. 2010;30(1):23–32. doi: 10.1089/jir.2009.0033. [DOI] [PubMed] [Google Scholar]
- Thilsing T., Madsen A.M., Basinas I., Schlünssen V., Tendal K., Bælum J. Dust, endotoxin, fungi, and bacteria exposure as determined by work task, season, and type of plant in a flower greenhouse. Ann. Occup. Hyg. 2015;59(2):142–157. doi: 10.1093/annhyg/meu090. [DOI] [PubMed] [Google Scholar]
- Timm M., Madsen A.M., Hansen J.V., Moesby L., Hansen E.W. Assessment of the total inflammatory potential of bioaerosols by using a granulocyte assay. Appl. Environ. Microbiol. 2009;75:7655–7662. doi: 10.1128/AEM.00928-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversi D., Alessandria L., Gilli S.G. Size-fractionated PM10 monitoring in relation to the contribution of endotoxins in different polluted areas. Atmos. Environ. 2011;45:3515–3521. [Google Scholar]
- Turner M.D., Nedjai B., Hurst T., Pennington D.J. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Turner J., Hernandez M., Snawder J.E., Handorean A., McCabe K.M. A toxicology suite adapted for comparing parallel toxicity responses of model human lung cells to diesel exhaust particles and their extracts. Aerosol Sci. Technol. 2015;49(8):599–610. doi: 10.1080/02786826.2015.1053559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viegas C.S.B., Costa R.M., Santos L., Videria P.A., Silva Z., Macedo A.L. Gla-rich protein function as an anti-inflammatory agent in monocytes/macrophages: implications for calcification-related chronic inflammatory diseases. PloS One. 2017;12(5) doi: 10.1371/journal.pone.0177829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visentin M., Pagnoni A., Sarti E., Pietrogrande M.C. Urban PM2.5 oxidative potential: importance of chemical species and comparison of two spectrophotometric cell-free assays. Environ. Pollut. 2016;219:72–79. doi: 10.1016/j.envpol.2016.09.047. [DOI] [PubMed] [Google Scholar]
- Xie Z., Fan C., Lu R., Wang B., Du S., Jin C. Characteristics of ambient bioaerosols during haze episodes in China: a review. Environ. Pollut. 2018;1–13 doi: 10.1016/j.envpol.2018.09.051. [DOI] [PubMed] [Google Scholar]
- Yassin M.F., Almouqatea S. Assessment of airborne bacteria and fungi in an indoor and outdoor environment. Int. J. Environ. Sci. Technol. 2010;7(3):535–544. [Google Scholar]
- Yen Y.C., Yang C.Y., Mena K.D., Cheng Y.T., Yuan C.S., Chen P.S. Jumping on the bed and associated increases of PM10, PM2.5, PM1, airborne endotoxin, bacteria, and fungi concentrations. Environ. Pollut. 2019;245:799–809. doi: 10.1016/j.envpol.2018.11.053. [DOI] [PubMed] [Google Scholar]
- Yoda Y., Tamura K., Shima M. Airborne endotoxin concentrations in indoor and outdoor particulate matter and their predictors in urban city. Indoor Air. 2016;27(5):955–964. doi: 10.1111/ina.12370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.