Abstract
Background:
The potentiator ivacaftor (VX-770) has been approved for therapy of 38 cystic fibrosis (CF) mutations (~10% of the patient population) associated with a gating defect of the CF transmembrane conductance regulator (CFTR). Despite the success of VX-770 treatment of patients carrying at least one allele of the most common gating mutation G551D-CFTR, some lung function decline and P. aeruginosa colonization persist. This study aims at identifying potentiator combinations that can considerably enhance the limited channel activity of a panel of CFTR gating mutants over monotherapy.
Methods:
The functional response of 13 CFTR mutants to single potentiators or systematic potentiator combinations was determined in the human bronchial epithelial cell line CFBE41o- and a subset of them was confirmed in primary human nasal epithelia (HNE).
Results:
In six out of thirteen CFTR missense mutants the fractional plasma membrane (PM) activity, a surrogate measure of CFTR channel gating, reached only ~10–50% of WT channel activity upon VX-770 treatment, indicating incomplete gating correction. Combinatorial potentiator profiling and cluster analysis of mutant responses to 24 diverse investigational potentiators identified several compound pairs that improved the gating activity of R352Q-, S549R-, S549N-, G551D-, and G1244E-CFTR to ~70–120% of the WT. Similarly, the potentiator combinations were able to confer WT-like function to G551D-CFTR in patient-derived human nasal epithelia.
Conclusion:
This study suggests that half of CF patients with missense mutations approved for VX-770 administration, could benefit from the development of dual potentiator therapy.
Introduction
More than 2000 mutations have been identified in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which may lead to a loss-of-function of this anion channel at the apical plasma membrane of secretory epithelia [1]. Cystic fibrosis (CF), one of the most common lethal autosomal-recessive diseases, is characterized by a multi-organ pathology affecting the upper- and lower-airways, endocrine system, as well as the intestinal and reproductive tracts. In the lung, lack of functional CFTR expression leads to impaired anion secretion, reduced airway liquid volume and pH, and defective mucociliary clearance resulting in reoccurring infections and uncontrolled inflammation, culminating in lung damage, the main cause for morbidity and mortality in CF [1].
CFTR mutants can be categorized into six classes according to their cellular phenotypes: class I–VI mutants exhibit expression, folding, gating, conductance, quantity and peripheral stability defects, respectively [2]. However, the complexity of CFTR mutant phenotypes at the cellular/molecular levels warranted the introduction of combinatorial categories to guide the development of mutant-specific combination therapy with CFTR modulators [2].
In recent years, efforts have focused on the development of modulators that can alleviate the folding (correctors) or gating (potentiators) defects via direct interaction with CFTR mutants. Presently, a single potentiator drug, ivacaftor (VX-770), has been approved for mono-therapy of 38 mutants with defective channel gating [3, 4]. However, based on electrophysiological studies, the function of the prototypical gating mutant G551D-CFTR is only partially restored upon VX-770 treatment [5, 6]. Combinations of potentiators were recognized to increase the gating efficacy of G551D-, S1251N-, W1282X- and N1303K-CFTR [7–12].
Here we carried out a systematic approach to select efficacious dual potentiator combinations for gating mutants with suboptimal susceptibility to VX-770. The results present a framework for the identification of CFTR mutants that can benefit from dual potentiator treatment and underscore the therapeutic potential of potentiator combinations in CF personalized medicine as soon as multiple gating potentiators enter the clinic.
Methods
Full details are available in the Supplementary Methods.
Abbreviated methods
The isolation of human nasal epithelia (HNE) from healthy and CF human subjects was performed under the protocol and consent form approved by the McGill MUHC Research Ethics Board (MP-37-2018-4227).
HNE were isolated, conditionally reprogrammed, expanded and differentiated on filter supports as described previously [13]. The generation of CFBE41o-(CFBE) cell lines harboring the inducible expression of full-length human WT-CFTR with a 3HA-tag in the fourth extracellular loop has been described [14]. Nucleotide substitutions to generate other CFTR variants were introduced by overlapping PCR mutagenesis. PM density determination [14], short-circuit current (Isc) measurement [15], halide-sensitive YFP quenching assay [15], qPCR [14], CFTR reconstitution in planar phospholipid bilayer [15] and EThcD-MS/MS [16] were performed as described previously.
Results
VX-770 incompletely corrects the functional defect of a subset of gating mutants
To determine the mutation-dependent CFTR gating rescue by VX-770, we determined the biochemical and functional expression of 12 CFTR mutants, which have been approved for VX-770 treatment. To this end, we transduced the human bronchial epithelial cell line CFBE41o-(CFBE) with lentiviral particles that conferred the inducible expression of the 3HA-tagged P67L [15], R117H, G178R, R334W, R347H [17], R352Q, S549N, S549R, G551D [14], G551S, G1244E, S1251N and G1349D CFTR variants (Supplementary Fig. S1a). The M/V status of the polymorphism at position 470, which can affect the mutant biogenesis [18] and modulator response [19], was chosen to reflect the majority distribution in the CF population (Supplementary Table 1) (G. Cutting on behalf of the CFTR2 team, personal communication). In addition, the R334W-CFTR, a conductance mutant [20], was also included.
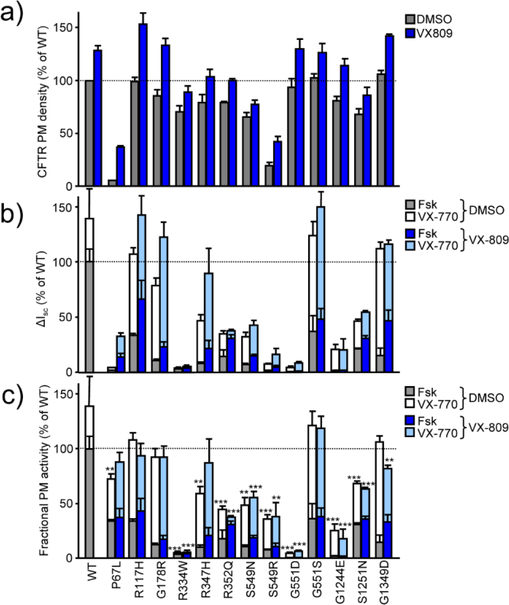
The PM density of most mutants was similar to that of WT-CFTR after normalization for CFTR mRNA content (Supplementary Fig. 1b), suggesting that the mutations do not interfere with folding or stability of the channel (Fig. 1a). The exceptions were the P67L and S549R mutations, which were associated with a VX-809 correctable folding defect [21] (Fig. 1a).
Fig. 1.
Incomplete correction of mutant CFTR gating defect by VX-770. (a) PM density of CFTR mutants alone and after VX-809 (3 μM, 24 hours) treatment, expressed as the percentage of WT-CFTR in CFBE cells (n = 3−4). (b) Mutant CFTR short-circuit current (Isc) after sequential acute addition of 20 μM forskolin (Fsk) and 14.444 μM VX-770 in CFBE with or without VX-809 treatment (n = 3) expressed as percentage of the WT-CFTR function. Measurements were performed in the presence of a basolateral-to-apical chloride gradient after basolateral permeabilization with amphotericin B (100 μM) and inhibition of the epithelial sodium channel ENaC with amiloride (100 μM). (c) The fractional PM activity, calculated as the ratio of Isc (from panel b) and PM density (panel a) normalized to WT, of CFTR mutants as a factor of potentiation with VX-770 and correction with VX-809. Data are means ± SEM of the indicated number (n) of independent experiments. * P < 0.05, ** P < 0.01, *** P < 0.001 by unpaired, two-tailed Student’s t-test.
Next, we determined the cAMP-dependent protein kinase (PKA)-stimulated activation of the mutants. As expected, the short-circuit current (Isc) was increased upon acute VX-770 addition (Fig. 1b, Supplementary Fig. 1c). To compare the mutant and WT activation at the single-channel level, we calculated the fractional PM activity, defined as CFTR mediated Isc divided by the channel PM density in CFBE monolayer, an estimate of the mean channel open probability relative to that of the WT and previously validated on F508del-CFTR [22]. The fractional PM activity determination showed that the R334W, R352Q, S549N, S549R, G551D, G1244E and S1251N mutants activation in the presence of VX-770 reached 5.0 ± 1.1, 44.2 ± 3.3, 48.5 ± 6.7, 35.6 ± 4.2, 4.4 ± 1.1, 25.1 ± 6.0 and 68.1 ± 2.1% of the PKA-activated WT CFTR transport activity, respectively, indicating only partial correction of their gating defects (Fig. 1c).
Systematic approach to identify additive potentiator combinations
To assess whether combinations of potentiators may further improve channel function in comparison to single-potentiator treatment, we selected R334W, S549N, S549R, G551D and G1244E mutants with the most severe fractional PM activity defects. The R347H with a WT-like fractional PM activity served as control. The identification of additive potentiator pairs was performed in a three-stage process.
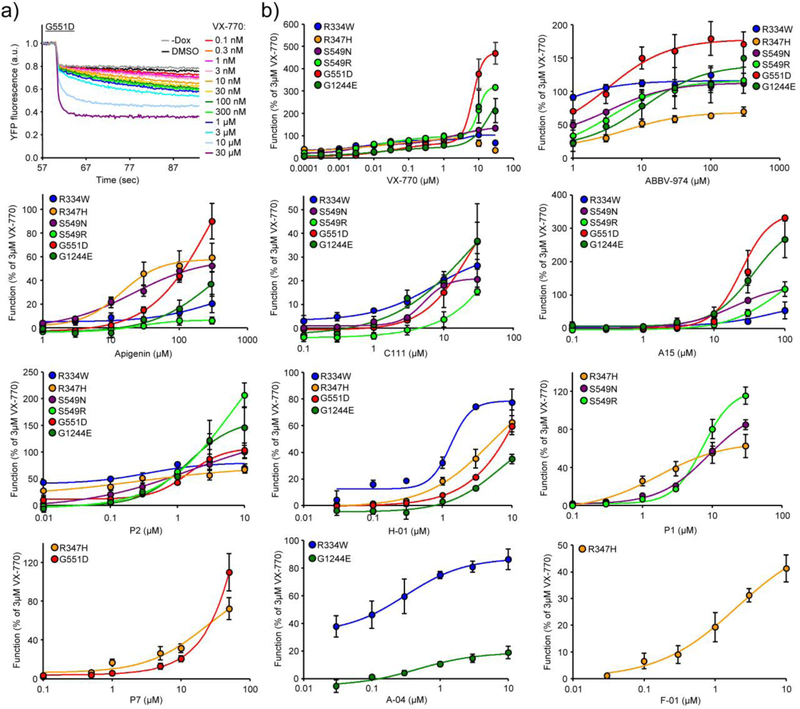
First, the response of PKA-activated mutants to a panel of 24 potentiators was determined using the halide-sensitive yellow fluorescent protein (YFP) quenching assay (Supplementary Table 2). These potentiators included VX-770, ABBV-974 (formerly GLPG1837), a potentiator under development by AbbVie, the P1–P10 investigational potentiator panel made available by the Cystic Fibrosis Foundation, potentiators that do not exhibit a downregulating effect on F508del-CFTR (P12, C-01, D-01, E-01, F-01, H-01 and A-04) [23], A15 that was initially identified as W1282X potentiator [8], apigenin an analog of P6 (genistein) with increased potency for G551D potentiation (Supplementary Fig. 2a and b), and three curcumin derivatives; bisdemethoxycurcumin (bDMC), demethoxycurcumin (C110), as well as the newly synthesized C111 analogues that exhibit higher potency and efficacy for G551D potentiation than curcumin (Supplementary Fig. 2b and c).
Second, we determined the potency of the most efficacious eight potentiators for six gating mutants, using the halide-sensitive YFP quenching assay (Fig. 2a and b, Supplementary Table 3), since both efficacy and potency of VX-770 are mutant-specific (Supplementary Fig. 1c and d). For VX-770 we observed a biphasic channel activation, measured by YFP quenching and Isc assay. The two EC50 values for VX-770 were in the nanomolar and low micromolar range (Supplementary Table 3). This may be the result of unspecific, saturable sequestration of VX-770, considering the compound’s hydrophobic nature and avidity to hydrophobic surfaces, serum proteins and cellular accumulation [24, 25]. The EC50 of ABBV-974 and curcumin analogs were in the low micromolar range and of A15 and apigenin in the mid micromolar range, despite mutant-specific variability (Supplementary Table 3).
Fig. 2.
Potency and efficacy of potentiators for mutant CFTRs. (a) Representative traces of G551D-CFTR function assayed by halide-sensitive YFP quenching in CFBE cells treated with increasing concentrations of VX-770. The YFP quenching kinetics was determined in response to extracellular iodide addition in the presence of forskolin (10 μM), IBMX (250 μM), cpt-cAMP (250 μM), and indicated potentiator concentrations. (b) Dose-response of indicated potentiators for the R334W, R347H, S549N, S549R, G551D, G1244E potentiation measured by halide-sensitive YFP quenching assay in CFBE (n = 3−4). Values are expressed as percentage of the response to 3 μM VX-770 of the respective mutants. Data are means ± SEM of the indicated number of independent experiments.
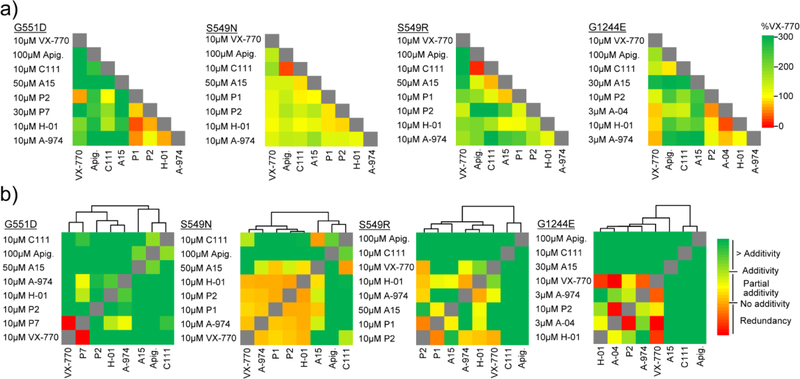
Lastly, we tested the gating effect of potentiator pairs at or near saturating concentrations to minimize possible additivity from their interaction with the same binding site. Increased activation of G551D, S549R, S549N and G1244E was achieved by several potentiator combinations as compared to that of VX-770 treatment alone (Fig. 3a and Supplementary Fig. 3a–e). Two mutants, R334W and R347H, did not respond to combinations, likely due to the R334W conductance defect [20] and the WT-like fractional PM activity of the R347H-CFTR (Supplementary Fig. 3f–i).
Fig. 3.
Combinatorial screen, combinatorial profiling and clustering of mechanistic classes of potentiators for G551D-, S549N-, S549R-, and G1244E-CFTR. (a) Heat map of the effect of potentiator combinations (combinatorial screen) on the PKA-activated channel function of the indicated mutants expressed in CFBE (n = 3−4). (b) Heat map of the combinatorial profiling established by calculating the dual potentiator effect in relation to the theoretical additivity of the compounds. Combinatorial profiles were subsequently used to cluster compounds. Clustering was performed by average linkage analysis and the distance determined by Spearman`s rank correlation. The underlying data are depicted as bar plots in Supplementary Fig. 3b–e. A-974 - ABBV-974, Apig. - apigenin.
To gain insights whether the increase in function of the responsive mutants was due to higher efficacy of single potentiators or simultaneous binding of two potentiators that exert additive effects, the results were analyzed by combinatorial profiling (Fig. 3b). Here we compared the functional gain elicited by potentiator pairs to that of the calculated additive effect of individual compounds. Based on this analysis, we categorized the potentiator combinations as redundant, partially additive, additive or higher than additive (Fig. 3b and Supplementary Fig. 3b–e). Subsequent clustering of the combination profiles revealed potentiators that show comparable interactions with other compounds, and thus likely work by a similar mechanism or compete for an overlapping binding site. Compounds that clustered together were VX-770 and ABBV-974 (Fig. 3b), confirming published results [26–28], as well as P1, P2, P7, H-01 and A-04. Conversely, apigenin, the curcumin analog C111, and for some mutants A15 clustered far from VX-770, ABBV-974 and other investigational potentiators, suggesting a complementary mechanism of action (Fig. 3b).
Potentiator combinations increase the open probability without influencing the phosphorylation of mutant CFTR
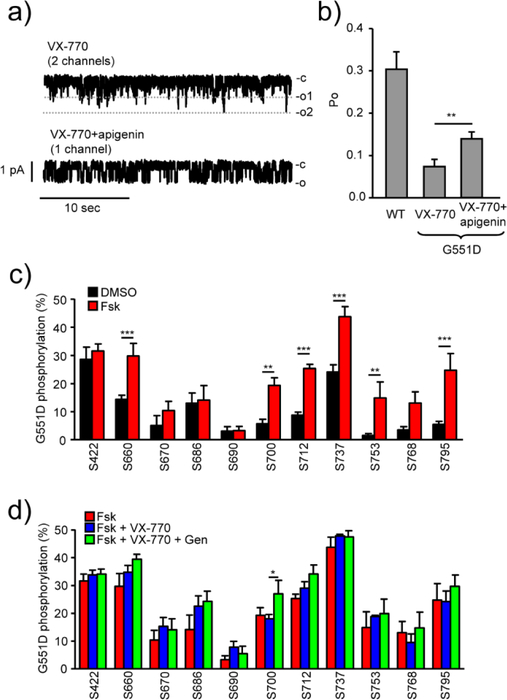
To gain more insights into the effect of potentiator combinations, we determined the single-channel activity of G551D-CFTR in reconstituted planar phospholipid bilayer. The open probability (Po) of phosphorylated G551D-CFTR was ~0.08 in the presence of VX-770, considerably lower than that of the WT (Fig. 4a and b). Upon addition of apigenin the Po increased to 0.14 (Fig. 4a and b). These results suggest that simultaneous and direct binding of both, VX-770 and apigenin, can nearly double the Po of G551D-CFTR.
Fig. 4.
Dual potentiator treatment increases the single-channel function of G551D-CFTR without substantially altering the phospho-occupancy. (a, b) The effect of 3μM VX-770 (n = 19) or VX-770 + 50μM apigenin (n = 18) on the function of protein kinase A–activated G551D-CFTR reconstituted into an artificial phospholipid bilayer. The Po of protein kinase A–activated WT-(n = 13) and G551D-CFTR were analyzed at 28–30°C. Significance between the VX-770 and VX-770+apigenin treated channel Po was calculated by two-sided Mann-Whitney U test (**P < 0.01, b). Representative traces (c - closed, o - open state) are shown in a. (c, d) In vivo phospho-occupancy of the PKA consensus sites in G551D-CFTR expressed in CFBE cells was determined by EThcD-MS/MS. Relative phosphorylation (%) of PKA consensus sites in G551D-CFTR upon DMSO or forskolin (20μM, 5 min) treatment (c), forskolin alone and VX-770 (3μM, 5 min) or VX-770 + genistein (Gen, 50μM, 5 min) in the presence of forskolin (d). Data are means ± SEM of at least three independent experiments. * P < 0.05, ** P < 0.01, *** P < 0.001 by unpaired, two-tailed Student’s t-test.
The potentiation efficacy of VX-770 depends on the extent of phosphorylation in wild-type and several mutant CFTR channels [29, 30], with higher PKA activity exposure showing enhanced VX-770-mediated potentiation in G551D-CFTR. It is unknown whether the gating defect of G551D- and S549N-CFTR is a consequence of impaired phosphorylation and whether dual potentiator could further enhance the channel phosphorylation propensity. To address a possible relationship between the phosphorylation and potentiation efficacy of the channel, we quantified the phospho-occupancy of eleven and ten PKA consensus phosphosites in the affinity purified G551D- and S549N-CFTR, respectively by our recently implemented electron-transfer/higher-energy collision dissociation (EThcD) fragmentation mass spectrometry (EThcD-MS/MS) workflow [16] (Fig. 4c, d and Supplementary Fig. 4a, b). The confidence of these sites was manually verified by their MS/MS spectra (Supplementary Fig. 4c). While under resting conditions, some of the sites showed ~5–30% phospho-occupancy in both mutants, PKA-activation increased the relative phosphorylation levels at six and five sites in G551D- and S549N-CFTR, respectively (Fig. 4c and Supplementary Fig. 4a). The addition of VX-770 alone or in combination with genistein, however, only marginally affected the phospho-occupancy (Fig. 4d and Supplementary Fig. 4b), suggesting that dual-potentiator exposure fails to alter the phosphorylation propensity of the channel and acts downstream.
Functional rescue of CFTR gating mutants by potentiator combinations
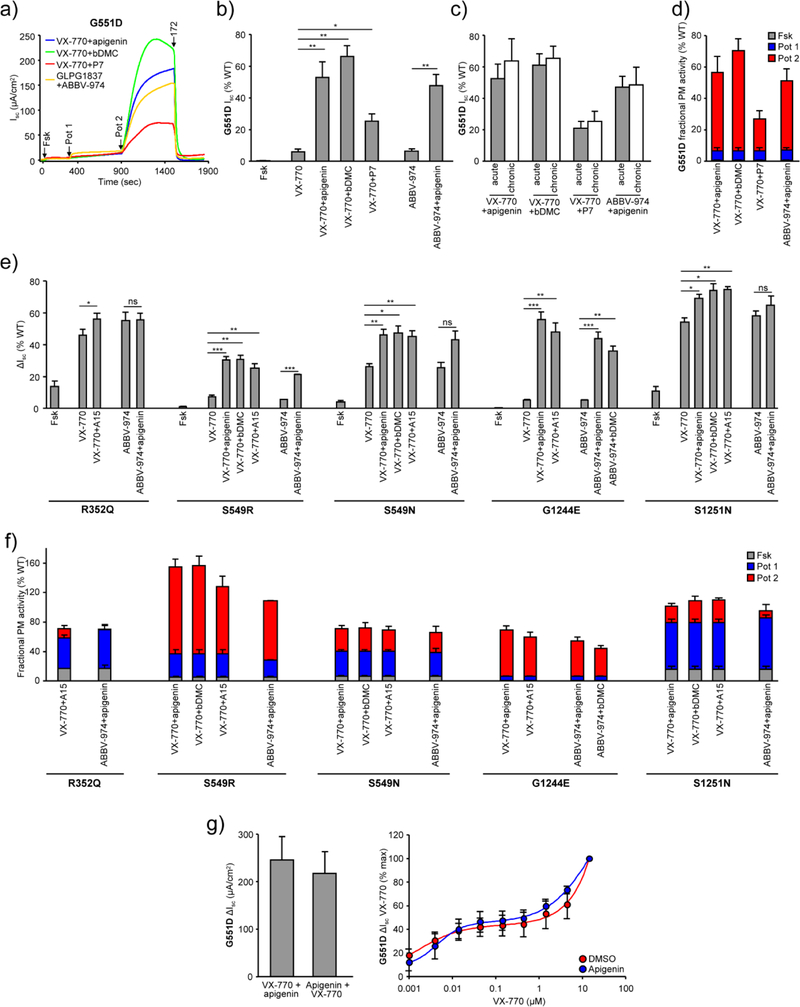
The extent of the mutant functional rescue by dual-potentiator treatment was determined by Isc measurements in CFBE cells. Here we focused on the combination of potentiators which are approved drugs or are in clinical development, i.e. VX-770 or ABBV-974, with potentiators that exhibit a complementary mechanism of action as indicated by the combinatorial profiling, i.e. apigenin, A15 or curcumin analogs. CFTR was phosphorylated by forskolin exposure followed by sequential potentiator addition and the individual compound contribution to the maximal Isc was determined as percentage of WT-controls (Fig. 5a–b, e and Supplementary Fig. 5a). For G551D-CFTR, forskolin-activation resulted in marginal CFTR function, which was increased by acute VX-770 addition to ~6% of WT-function (Fig. 5a and b). Addition of a second potentiator, apigenin, bDMC or P7, increased the G551D-CFTR current to 53%, 66% or 25% of the WT, respectively (Fig. 5a and b). Similar values were obtained for ABBV-974 and apigenin (Fig. 5a and b).
Fig. 5.
Potentiator combinations rescue the function of CFTR gating mutants in CFBE cells. (a) The effect of the indicated single potentiators or potentiator combinations on the Isc of G551D-CFTR. Isc was measured in presence of a basolateral-to-apical chloride gradient after basolateral permeabilization with amphotericin B (100 μM) and inhibition of the epithelial sodium channel ENaC with 100 μM amiloride. (b) Effect of acute addition of forskolin (20 μM) followed by VX-770 (10 μM) or ABBV-974 (10 μM) and apigenin (50 μM), bDMC (10 μM) or P7 (50 μM) as percent of forskolin-activated WT-CFTR (n = 3). (c) Comparison between the acute and chronic (24 hours, VX-770 – 3 μM, ABBV-974 – 3 μM, bDMC - 10 μM, apigenin and P7 – 50 μM) potentiator effect on the acute forskolin-stimulated G551D Isc (n = 3). (d) The fractional PM activity of G551D after forskolin-stimulation alone or in combination with single or dual potentiator treatment expressed as percentage of WT (n = 3). (e) Effect of acute addition of forskolin (20 μM) followed by VX-770 (10 μM) or ABBV-974 (10 μM) and subsequent apigenin (50 μM), bDMC (10 μM) or A15 (50 μM) on the Isc of R352Q, S549R, S549N, G1244E and S1251N expressed as percentage of WT-CFTR function (n = 3). (f) Fractional PM activity of R352Q, S549R, S549N, G1244E and S1251N-CFTR upon forskolin alone or in combination with single or dual potentiator treatment, expressed as percentage of WT (n = 3). (g) Effect of order-of-addition on the efficacy of G551D potentiation (10 μM VX-770, 50 μM apigenin) (left panel, n = 3) and potency of VX-770 in the presence or absence of apigenin (50 μM) (right panel, n = 3). Data in b–g are means ± SEM of the indicated number of independent experiments. * P < 0.05, ** P < 0.01, *** P < 0.001 by unpaired, two-tailed Student’s t-test.
Chronic exposure to potentiators can attenuate the biogenesis and stability of some CFTR mutants, which results in decreased channel-activity [17, 25] and this effect is both mutation and potentiator specific [13]. In case of G551D-CFTR, however, chronic exposure to potentiator combinations did not decrease the maximal channel current (Fig. 5c). This allowed the determination of the fractional PM activity of G551D, which was increased from 6% or 7% of the WT with VX-770 or ABBV-974 mono-therapy to 56%, 70%, 26% and 51% upon dual potentiator treatment with VX-770+apigenin, VX-770+bDMC, VX-770+P7, and ABBV-974+apigenin, respectively.
Similar to G551D and consistent with the results from combinatorial profiling, combining VX-770 or ABBV-974 with apigenin, A15 or bDMC significantly increased the Isc of phosphorylated R352Q, S549R, S549N, G1244E and S1251N-CFTR (Fig. 5e and Supplementary Fig. 5a). Chronic exposure to these potentiator combinations, with the exception of VX-770+A15 in S549N, S549R and S1251N, did not attenuate the maximal channel current (Supplementary Fig. 5b). The maximal fractional PM activity was 71% of the WT current for R352Q, 72% for S549N, >100% for S549R and 69% for G1244E (Fig. 5f). Interestingly, S1251N which responded synergistically to potentiator combinations in rectal organoids [11], exhibited a fractional PM activity of ~80% of WT after single potentiator therapy with VX-770 or ABBV-974 and second potentiator addition led to only a small but significant increase to the WT-level (Fig. 5f).
Interdependence of the potentiator binding/activity was tested in G551D- and S549N-CFTR. Changing the order of VX-770 and apigenin addition neither influenced their combined efficacy, nor the VX-770 potency was altered by the presence of the second potentiator (Fig. 5g and Supplementary Fig. 5c). These results suggest that the two potentiators act on independent, rather than allosterically coupled, binding sites.
Functional correction of CFTR gating defects in human nasal epithelia by potentiator combinations
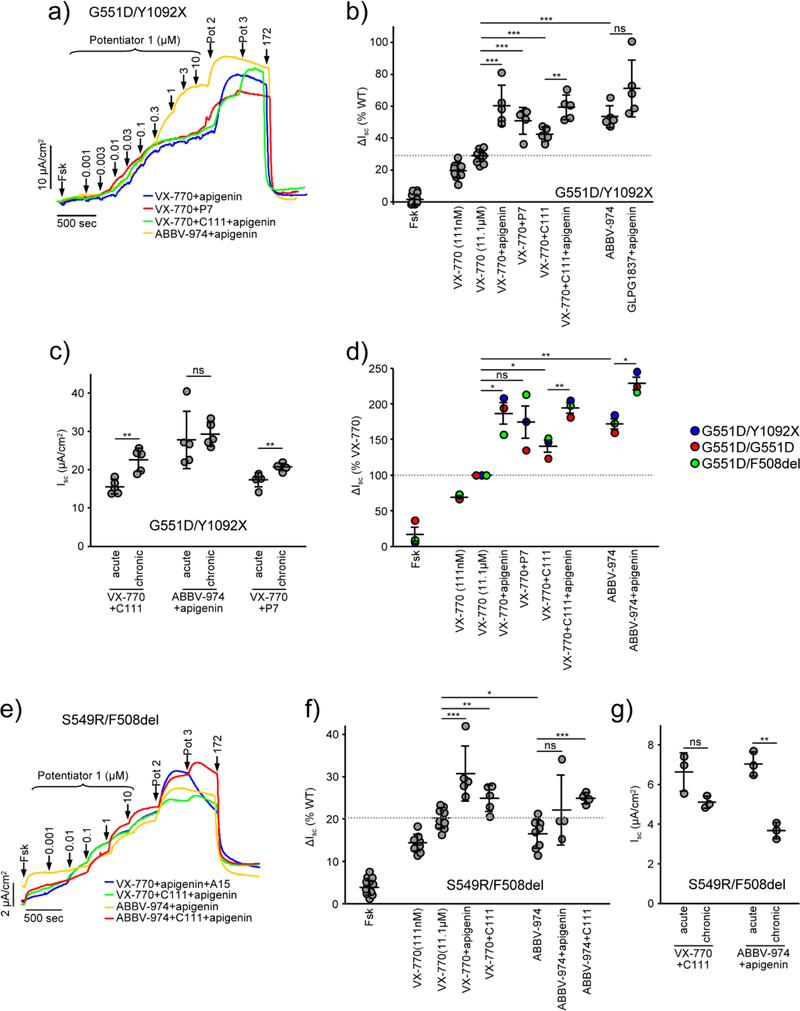
To confirm the efficacy of potentiator combinations in patient-derived cells, CFTR function was monitored in human nasal epithelia (HNE), which were amplified by the conditional reprogramming (CR) technique [31] and characterized by key markers of differentiated epithelia [13] (Supplementary Fig. 6a). HNE were isolated from three CF patients with the G551D mutation on at least one allele, one CF patient with S549R/F508del genotype and five healthy non-CF donors. The CFTR mRNA levels of patient HNE were similar to the WT, with the exception of the compound heterozygote with G551D/Y1092X genotype (Supplementary Fig. 6b), probably due to the reduction of Y1092X mRNA by nonsense-mediated decay [32]. VX-770 increased the forskolin-activated Isc to ~25% relative to that of WT-CFTR in cells with one copy of G551D and ~50% in cells with G551D on both alleles (Fig. 6a, b and Supplementary Fig. 6c). As reported previously [33], ABBV-974 exhibited significantly increased efficacy for G551D-CFTR potentiation (Fig. 6b and Supplementary Fig. 6c), albeit with 7-fold lower potency (EC50 ~300 nM) in comparison to VX-770 (EC50 ~40 nM, Supplementary Fig. 6e and f). Dual potentiator treatment by VX-770+C111, VX-770+apigenin or ABBV-974+apigenin increased the G511D-CFTR Isc to 140%, 186% and 228%, respectively, of VX-770 mono-therapy (Fig. 6d), without reducing the maximal CFTR current upon chronic exposure (Fig. 6c and Supplementary Fig. 6d). Prolonged exposure to some potentiator combinations increased their efficacy (Fig. 6c and Supplementary Fig. 6d), possibly because of their slow intracellular compound accumulation [25].
Fig. 6.
Dual potentiator-mediated rescue of G551D- and S549R-CFTR function in human nasal epithelia (HNE). (a) Effect of indicated potentiator combinations on the Isc of HNE with CFTRG551D/Y1092X genotype. (b) Quantification of the forskolin-(Fsk) and potentiator-stimulated currents (ΔIsc) in HNE with CFTRG551D/Y1092X expressed as percentage of WT-CFTR currents in HNE from five donors. Isc was measured in presence of a basolateral-to-apical chloride gradient. After ENaC inhibition, HNE were exposed to Fsk (20 μM), increasing concentrations of VX-770 or ABBV-974, followed by apigenin (50 μM), C111 (10 μM) or P7 (50 μM). (c) Comparison between the acute and chronic potentiator effect (24 hours, VX-770 and ABBV-974 – 1 μM, bDMC - 10 μM, apigenin and P7 – 50 μM) on the Isc of forskolin-stimulated CFTRG551D/Y1092X HNE. (d) Effect of potentiator combinations on the forskolin-stimulated Isc in HNE from three patients, homo- or heterozygous for the G551D mutation, expressed as percentage of VX-770 induced currents. (e–g) Representative traces (e), effect of acute potentiator combinations (f), and chronic potentiator exposure (g) of the forskolin-stimulated Isc of HNE with CFTRS549R/F508del genotype. Data in b–c and f–g are values from individual filters; solid lines indicate means ± SEM. Data in d are mean values from HNE of three CF patients; solid lines indicate means ± SEM. ns, not significant, * P < 0.05, ** P < 0.01, *** P < 0.001 by unpaired (b–c and f–g) or paired (d), two-tailed Student’s t-test.
Similar results were obtained for S549R/F508del HNE, which were additively potentiated by VX-770+apigenin, VX-770+C111 and ABBV974+C111 combinations, but due to the partial folding defect of S549R, reached only ~30% of the WT current (Fig. 6e and f). Chronic exposure to the potentiator combinations in these HNE resulted in an attenuated maximal CFTR current (Fig. 6g), possibly due to the partial downregulation of F508del-CFTR [17, 25]. In contrast, the phosphorylated channels in homozygous F508del-CFTR human bronchial epithelia did not respond to potentiator combinations (Supplementary Fig. 7), in agreement with the WT-like fractional PM activity of VX-809 corrected and genistein potentiated F508del-CFTR [22].
Discussion
Despite the success of potentiator therapy for G551D-CFTR [3] and other gating mutants, the G551D channel function is only partially normalized by VX-770 [5, 6] and adult G551D patients still experience progressive loss of their lung function [34, 35] and P. aeruginosa infection albeit at reduced frequency [36]. As a strategy to increase the anion secretion, combination of potentiators has been used for some gating mutants [7–12], without a rational for mutant selection. Our results suggest that gating mutants, with a reduced fractional PM activity after mono-potentiator therapy, are more responsive to dual-potentiator treatment. Six (R352Q, S549N, S549R, G551D, G1244E, S1251N) out of twelve gating mutants, for which VX-770 has been approved, exhibited lower fractional PM activity than the WT and were susceptible to potentiator combinations. In contrast, mutants with WT-like fractional PM activity (R347H and F508del) did not respond to second potentiator treatment. A similar phenomenon was observed for R334W, a mutation that likely impairs ion conductance [20] manifesting in severe fractional PM activity defect.
Curcumin and genistein have been proposed to additively potentiate G551D and S1251N-CFTR [11, 12], but results from a recent clinical study showed low efficacy of this potentiator combination in S1251N patients [37] probably due to the low potency and bioavailability of the compounds [38, 39]. As a first step to improve potentiator combination therapy, we identified analogs of both curcumin and genistein which exhibit in the presence of VX-770 a ~10-fold and 2-fold higher potency, respectively. These compounds were included in the established CFTR potentiator panel that was used for the initial screen. Combinatorial profiling of the most efficacious potentiators at near saturating concentrations for channel activation and clustering analysis identified at least two mechanistically distinct groups of potentiator compounds, conceivably having distinct binding sites. The different mechanisms of potentiators in both clusters could be related to three-step process that leads to CFTR activation.
First, the PKA-dependent phosphorylation of the regulatory domain (RD) of CFTR is required to suspend the pore blockage [40, 41]. However, the marginally altered phospho-occupancies of forskolin-treated S549N- and G551D-CFTR show that this step is not influenced by mono- or dual potentiator exposure. Second, RD removal permits the ATP-dependent heterodimerization of the nucleotide-binding domains 1 and 2 (NBD1-NBD2) [40, 41]. Genistein and its analog apigenin, which is present in the second cluster of potentiators, is presumed to bind to the NBD1-NBD2 interface and likely promote dimerization [42]. Third, NBD1-NBD2 interface formation initiates the mechanochemical coupling of CFTR pore opening [41]. VX-770 and ABBV-974 are both in the first cluster of potentiators and act competitively [26, 28]. They bind to the same binding site in the transmembrane domains of CFTR [5, 27, 28] and facilitate gating in an ATP-independent manner [5, 29] by directly influencing pore opening. Curcumin potentiates CFTR in the absence of ATP and the NBD2 domain [43], and is able to cross-link CFTR channels, however, cyclic derivatives of curcumin that have no cross-linking activity are still acting as potentiators [44].
The distinct mechanism of potentiators in both clusters is further supported by their additive effect on mutant Isc, which was absent when using combinations of compounds from the same cluster. Combination of VX-770 or ABBV-974 with apigenin, bDMC or A15 was necessary and sufficient to increase the R352Q, S549N, S549R, G551D, G1244E and S1251N fractional PM activity to ≥70% of the WT. Measurement of single G551D-CFTR channel in phospholipid bilayer suggests that potentiator combinations act by directly binding to and promoting the open probability of CFTR. We failed to detect a beneficial effect of a third potentiator on CFTR activity in HNE from patients with one or two G551D alleles. Dual potentiator treatment increased the CFTR function in HNE from compound heterozygous G551D patients to ≥60% and in homozygous G551D patients to ~100% of the WT channel function, values that are deemed sufficient to alleviate clinical CF symptoms.
In summary, we present a framework for the identification of CFTR mutants that benefit from dual potentiator treatment and a precision medicine approach for the identification of optimized potentiator combinations to improve the efficacy of VX-770 therapy. Extrapolating our results suggests that at least half of the more than 10% of CF patients for which VX-770 mono-therapy has been approved could benefit from the development of a dual potentiator treatment.
Supplementary Material
Highlights.
A subset of gating mutants are only partially potentiated by VX-770 (Ivacaftor).
These mutant activities are increased by rationally selected dual potentiators.
Dual potentiators conferred WT-like G551D-CFTR function in human nasal epithelia.
Acknowledgement
We thank A.S. Verkman (University of California San Francisco) for the potentiator A15, W.E. Finkbeiner (University of California San Francisco) for supplying HBE and HNE cells, G.R. Cutting and the CFTR2 study group (Johns Hopkins University) for providing the 470M/V CFTR assignments in collaboration with Counsyl Inc., Ambry Genetics, and Quest Diagnostics, D. Faubert (Institut de Recherches Cliniques de Montréal) for his help in the phospho-occupancy studies, the late D. Gruenert (University of California San Francisco) for the parental CFBE41o-cell line and R. J. Bridges (Rosalind Franklin University of Medicine and Science) and Cystic Fibrosis Foundation Therapeutics (CFFT) Inc. for providing CFTR potentiators.
Funding
This work was supported by the Canadian Institutes of Health Research (MOP-142221 to G.L.L. and PJT-153095 to G.V., E.M., and G.L.L.), National Institute of Diabetes & Digestive & Kidney Diseases (5R01DK075302 to G.L.L.), the Cystic Fibrosis Foundation Therapeutics to G.L.L., as well as Cystic Fibrosis Canada to G.L.L. R.G.A. was a recipient of the Fonds de Recherche du Québec - Santé (FRQS) Doctoral Training Scholarship. A.P. is supported by Cystic Fibrosis Canada and FQRS postdoctoral fellowships. G.L.L. is a Canada Research Chair.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures
GLL is member of the Proteostasis Inc. scientific advisory board. All other authors declare that they have no competing interests.
References
- [1].Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet. 2015;16:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Veit G, Avramescu RG, Chiang AN, Houck SA, Cai Z, Peters KW, et al. From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol Biol Cell. 2016;27:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Skilton M, Krishan A, Patel S, Sinha IP, Southern KW. Potentiators (specific therapies for class III and IV mutations) for cystic fibrosis. Cochrane Database Syst Rev. 2019;1:CD009841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jih KY, Hwang TC. Vx-770 potentiates CFTR function by promoting decoupling between the gating cycle and ATP hydrolysis cycle. Proc Natl Acad Sci U S A. 2013;110:4404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A. 2009;106:18825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lin WY, Sohma Y, Hwang TC. Synergistic Potentiation of Cystic Fibrosis Transmembrane Conductance Regulator Gating by Two Chemically Distinct Potentiators, Ivacaftor (VX-770) and 5-Nitro-2-(3-Phenylpropylamino) Benzoate. Mol Pharmacol. 2016;90:275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Haggie PM, Phuan PW, Tan JA, Xu H, Avramescu RG, Perdomo D, et al. Correctors and Potentiators Rescue Function of the Truncated W1282X-Cystic Fibrosis Transmembrane Regulator (CFTR) Translation Product. J Biol Chem. 2017;292:771–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Phuan PW, Son JH, Tan JA, Li C, Musante I, Zlock L, et al. Combination potentiator (‘co-potentiator’) therapy for CF caused by CFTR mutants, including N1303K, that are poorly responsive to single potentiators. J Cyst Fibros. 2018;17:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cho DY, Zhang S, Lazrak A, Grayson JW, Pena Garcia JA, Skinner DF, et al. Resveratrol and ivacaftor are additive G551D CFTR-channel potentiators: therapeutic implications for cystic fibrosis sinus disease. Int Forum Allergy Rhinol. 2019;9:100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dekkers JF, Van Mourik P, Vonk AM, Kruisselbrink E, Berkers G, de Winter-de Groot KM, et al. Potentiator synergy in rectal organoids carrying S1251N, G551D, or F508del CFTR mutations. J Cyst Fibros. 2016;15:568–78. [DOI] [PubMed] [Google Scholar]
- [12].Yu YC, Miki H, Nakamura Y, Hanyuda A, Matsuzaki Y, Abe Y, et al. Curcumin and genistein additively potentiate G551D-CFTR. J Cyst Fibros. 2011;10:243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Avramescu RG, Kai Y, Xu H, Bidaud-Meynard A, Schnur A, Frenkiel S, et al. Mutation-specific downregulation of CFTR2 variants by gating potentiators. Hum Mol Genet. 2017;26:4873–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Veit G, Bossard F, Goepp J, Verkman AS, Galietta LJ, Hanrahan JW, et al. Proinflammatory cytokine secretion is suppressed by TMEM16A or CFTR channel activity in human cystic fibrosis bronchial epithelia. Mol Biol Cell. 2012;23:4188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Veit G, Xu H, Dreano E, Avramescu RG, Bagdany M, Beitel LK, et al. Structure-guided combination therapy to potently improve the function of mutant CFTRs. Nat Med. 2018;24:1732–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schnur A, Premchandar A, Bagdany M, Lukacs GL. Phosphorylation-dependent modulation of CFTR macromolecular signalling complex activity by cigarette smoke condensate in airway epithelia. Sci Rep. 2019;9:12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Veit G, Avramescu RG, Perdomo D, Phuan PW, Bagdany M, Apaja PM, et al. Some gating potentiators, including VX-770, diminish ΔF508-CFTR functional expression. Sci Transl Med. 2014;6:246ra97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cuppens H, Lin W, Jaspers M, Costes B, Teng H, Vankeerberghen A, et al. Polyvariant mutant cystic fibrosis transmembrane conductance regulator genes. The polymorphic (Tg)m locus explains the partial penetrance of the T5 polymorphism as a disease mutation. J Clin Invest. 1998;101:487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Murakami K, Cheng Y, Bradbury NA, Bridges RJ. Differential Pharmacological Stability of M470-F508del-Cftr against Cftr Modulators. Pediatr Pulm. 2015;50:221–. [Google Scholar]
- [20].Han ST, Rab A, Pellicore MJ, Davis EF, McCague AF, Evans TA, et al. Residual function of cystic fibrosis mutants predicts response to small molecule CFTR modulators. JCI Insight. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sabusap CM, Wang W, McNicholas CM, Chung WJ, Fu L, Wen H, et al. Analysis of cystic fibrosis-associated P67L CFTR illustrates barriers to personalized therapeutics for orphan diseases. JCI Insight. 2016;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bagdany M, Veit G, Fukuda R, Avramescu RG, Okiyoneda T, Baaklini I, et al. Chaperones rescue the energetic landscape of mutant CFTR at single molecule and in cell. Nat Commun. 2017;8:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Phuan PW, Veit G, Tan JA, Finkbeiner WE, Lukacs GL, Verkman AS. Potentiators of Defective DeltaF508-CFTR Gating that Do Not Interfere with Corrector Action. Mol Pharmacol. 2015;88:791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Matthes E, Goepp J, Carlile GW, Luo Y, Dejgaard K, Billet A, et al. Low free drug concentration prevents inhibition of F508del CFTR functional expression by the potentiator VX-770 (ivacaftor). Br J Pharmacol. 2016;173:459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cholon DM, Quinney NL, Fulcher ML, Esther CR Jr., Das J, Dokholyan NV, et al. Potentiator ivacaftor abrogates pharmacological correction of DeltaF508 CFTR in cystic fibrosis. Sci Transl Med. 2014;6:246ra96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yeh HI, Sohma Y, Conrath K, Hwang TC. A common mechanism for CFTR potentiators. J Gen Physiol. 2017;149:1105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yeh HI, Qiu L, Sohma Y, Conrath K, Zou X, Hwang TC. Identifying the molecular target sites for CFTR potentiators GLPG1837 and VX-770. J Gen Physiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu F, Zhang Z, Levit A, Levring J, Touhara KK, Shoichet BK, et al. Structural identification of a hotspot on CFTR for potentiation. Science. 2019;364:1184–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Eckford PD, Li C, Ramjeesingh M, Bear CE. Cystic fibrosis transmembrane conductance regulator (CFTR) potentiator VX-770 (ivacaftor) opens the defective channel gate of mutant CFTR in a phosphorylation-dependent but ATP-independent manner. J Biol Chem. 2012;287:36639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cui G, Stauffer BB, Imhoff BR, Rab A, Hong JS, Sorscher EJ, et al. VX-770-mediated potentiation of numerous human CFTR disease mutants is influenced by phosphorylation level. Sci Rep. 2019;9:13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu X, Krawczyk E, Suprynowicz FA, Palechor-Ceron N, Yuan H, Dakic A, et al. Conditional reprogramming and long-term expansion of normal and tumor cells from human biospecimens. Nat Protoc. 2017;12:439–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Clarke LA, Awatade NT, Felicio VM, Silva IA, Calucho M, Pereira L, et al. The effect of premature termination codon mutations on CFTR mRNA abundance in human nasal epithelium and intestinal organoids: a basis for read-through therapies in cystic fibrosis. Hum Mutat. 2019;40:326–34. [DOI] [PubMed] [Google Scholar]
- [33].Gees M, Musch S, Van der Plas S, Wesse AS, Vandevelde A, Verdonck K, et al. Identification and Characterization of Novel CFTR Potentiators. Front Pharmacol. 2018;9:1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kirwan L, Fletcher G, Harrington M, Jeleniewska P, Zhou S, Casserly B, et al. Longitudinal Trends in Real-World Outcomes after Initiation of Ivacaftor. A Cohort Study from the Cystic Fibrosis Registry of Ireland. Ann Am Thorac Soc. 2019;16:209–16. [DOI] [PubMed] [Google Scholar]
- [35].Sawicki GS, McKone EF, Pasta DJ, Millar SJ, Wagener JS, Johnson CA, et al. Sustained Benefit from ivacaftor demonstrated by combining clinical trial and cystic fibrosis patient registry data. Am J Respir Crit Care Med. 2015;192:836–42. [DOI] [PubMed] [Google Scholar]
- [36].Heltshe SL, Mayer-Hamblett N, Burns JL, Khan U, Baines A, Ramsey BW, et al. Pseudomonas aeruginosa in cystic fibrosis patients with G551D-CFTR treated with ivacaftor. Clin Infect Dis. 2015;60:703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Berkers G, van Mourik P, Vonk AM, Kruisselbrink E, Dekkers JF, de Winter-de Groot KM, et al. Rectal Organoids Enable Personalized Treatment of Cystic Fibrosis. Cell Rep. 2019;26:1701–8 e3. [DOI] [PubMed] [Google Scholar]
- [38].Liu W, Zhai Y, Heng X, Che FY, Chen W, Sun D, et al. Oral bioavailability of curcumin: problems and advancements. J Drug Target. 2016;24:694–702. [DOI] [PubMed] [Google Scholar]
- [39].Yang Z, Kulkarni K, Zhu W, Hu M. Bioavailability and pharmacokinetics of genistein: mechanistic studies on its ADME. Anticancer Agents Med Chem. 2012;12:1264–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang Z, Liu F, Chen J. Conformational Changes of CFTR upon Phosphorylation and ATP Binding. Cell. 2017;170:483–91 e8. [DOI] [PubMed] [Google Scholar]
- [41].Csanady L, Vergani P, Gadsby DC. Structure, Gating, and Regulation of the Cftr Anion Channel. Physiol Rev. 2019;99:707–38. [DOI] [PubMed] [Google Scholar]
- [42].Moran O, Galietta LJ, Zegarra-Moran O. Binding site of activators of the cystic fibrosis transmembrane conductance regulator in the nucleotide binding domains. Cell Mol Life Sci. 2005;62:446–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang W, Bernard K, Li G, Kirk KL. Curcumin opens cystic fibrosis transmembrane conductance regulator channels by a novel mechanism that requires neither ATP binding nor dimerization of the nucleotide-binding domains. J Biol Chem. 2007;282:4533–44. [DOI] [PubMed] [Google Scholar]
- [44].Bernard K, Wang W, Narlawar R, Schmidt B, Kirk KL. Curcumin cross-links cystic fibrosis transmembrane conductance regulator (CFTR) polypeptides and potentiates CFTR channel activity by distinct mechanisms. J Biol Chem. 2009;284:30754–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.