Abstract
Knee meniscus is a wedge-shaped fibrocartilaginous tissue, playing important roles in maintaining joint stability and function. Injuries to meniscus, particularly with the avascular inner third zone, hardly heal and frequently progress into structural breakdown, followed by initiation of osteoarthritis. As the importance of meniscus in joint function and diseases is being recognized, the field of meniscus research is growing. Not only development, biology and metabolism but also injury, repair and healing of meniscus are being actively investigated. As meniscus functions as an integrated unit of a knee joint, in vivo models with various species have been the predominant method for studying meniscus pathophysiology and for testing healing/regeneration strategies. However, in vivo models for meniscus studies suffer from low reproducibility and high-cost. To complement the limitations of in vivo animal models, several types of meniscus explants have been applied as highly controlled, standardized in vitro models to investigate meniscus metabolism, pathophysiology, and repair or regeneration process. This review summarizes and compares the existing meniscus explant models. We also discuss advantages and disadvantages of each explant model.
Keywords: Meniscus explant, meniscus metabolism, meniscus injury, tissue engineering
INTRODUCTION
Knee menisci are crescent and wedge-shaped fibrocartilaginous tissues residing between the distal femoral condyle and the proximal tibial plateaus, playing indispensable roles in joint congruence and stability, shock absorption, and stress transmission (1–3). Meniscus is featured by its regionally variant biochemical composition and structure (2, 4–6). The outer third region of meniscus is vascularized and constituted of dense fibrous matrix populated with fibroblast-like cells, whereas the inner third region is avascular cartilaginous tissue with chondrocyte-like cells. The middle region is fibrocartilaginous tissue with a mixed population of fibroblasts and chondrocytes. Menisci show a unique collagen arrangement with a randomly oriented superficial layer and circumferentially oriented fibers with a small fraction of radial fibers in the deep zone. The regionally variant, anisotropic collagen fiber orientation is optimally designed to transfer vertical compressive load into circumferential “hoop” stresses (1–3). Proteoglycan is accounted for a smaller fraction in meniscus than in hyaline cartilage and is primarily concentrated in the inner 2/3 zone, playing major roles in load bearing as it correlates with the surface nature in the avascular portion of the tissue (1–3).
Meniscus is susceptible to sports injury and age- or disease-related degenerative breakdown (7–9). Over one million patients undergo surgical repair or meniscectomy annually in the U.S. alone (7–9). Tears in vascularized outer third region of meniscus can be surgically repaired by suturing torn parts. In contrast, tears in the inner avascular region are hardly repaired due to poor intrinsic healing capacity and are frequently extended into the middle-third region, followed by meniscus deterioration. To alleviate symptoms caused by such irreparable meniscus injuries, partial or total meniscectomy is often performed. However, meniscectomy significantly increases the incidence of osteoarthritis (OA) later in life by elevating joint contact stress (3, 10). Approximately 50% of all patients with meniscal injuries develop OA within 10 to 20 years of injury (3, 10). Allograft transplantation from cadavers may be considered after meniscectomy to prevent the increase in joint contact pressure, but the procedure suffers from donor shortage, pathogen transmission, immunorejection and tissue mismatch (2, 3, 10).
Given its critical roles in joint function and in the initiation and progress of degenerative joint diseases, knee meniscus has become the focus of a growing body of research. Various studies have investigated not only repair strategies and tissue engineering approaches to replace degenerated menisci but also their development, biology, and metabolism. As meniscus functions as an integrated unit of a knee joint, in vivo animal surgical models have been predominant in tissue pathophysiology studies and in tests for new surgical solutions, devices, and engineered implants for meniscus regeneration. Although they cannot be replaced, the in vivo animal models for studying meniscus metabolism, injuries, repair or regeneration have outstanding challenges because of their low reproducibility, interspecies variables, and high cost. In particular, there is a concerning level of low reproducibility in large animal studies due to variables embedded in a surgical model and uncontrolled, complex bio-chemical responses in vivo (11). As an alternative to the in vivo animal model, various meniscus explant models have been adopted to study meniscus pathophysiology, injury, repair and healing (1, 12–16). Although the complex in vivo process of injuries, inflammation, repair or healing cannot be fully recapitulated in an explant model, it has several benefits over in vivo models by allowing a standardized test with highly controlled variables. This review summarizes and compares the existing meniscus explant models. We also discuss the advantages and disadvantages of each model in order to support applications of well-controlled explant models for various meniscus studies.
Explant models to study meniscus metabolism
Metabolic events of the knee meniscus include synthesis, resorption and remodeling of tissue matrix, and secretion of growth factors or cytokines. Such metabolic events are mostly regulated by biochemical and/or mechanical cues in connection with a number of physiological or pathological events in vivo. Despite the complex metabolic processes in vivo, there are several tissue explant models showing potential to recapitulate selected aspects of meniscus metabolism ex vivo.
Tissue responses to pro-inflammatory stimuli have been studied extensively in various meniscus explant models. For example, treatment of IL-1α, one of the major pro-inflammatory cytokines, led to aggrecan catabolism in cylindrical meniscus explants prepared from pigs (17). Exogenous application of IL-1α induced secretion of sulfated glycosaminoglycan (sGAG) mediated by aggrecanases (17). IL-1β, another subtype of IL-1, provoked prostaglandin E2 (PGE2), IL-6, IL-8, keratinocyte-derived chemokine (KC) and monocyte chemotactic protein-1 (MCP-1) in a menisci fragment (18). In addition to IL-1, tumor necrosis factor alpha (TNFα) showed inflammatory reaction in degradation of meniscus explants (19, 20). TNFα treatment to meniscus explants significantly increased secretion of sGAG and nitric oxide (NO) and expressions of MMP-3, a disintegrin and metalloproteinase with thrombospondin motifs 4 (ADAMTS-4), and inducible nitric oxide synthase (iNOS) (19). In a study with bovine explants, TNFα stimulation increased NO release, which in turn led to sustained expressions of pro-inflammatory cytokines (19). IL-1 and TNFα also inhibited ex vivo meniscus healing by inducing cell apoptosis through increasing iNOS (19, 20). Similarly, NO and PEG2 induced by IL-1β prevented synthesis of collagen and proteoglycan that consequently serve as an intermediary between the inflammation and meniscus deterioration, followed by progression of osteoarthritis (18).
Effects of IL-1 on meniscus metabolism ex vivo were discovered as age-dependent. Matrix metalloproteinase (MMPs) expression induced by pro-inflammatory cytokines shows different patterns between explants from young and old animals (15). Expressions of IL-1β, MMPs, ADAMTS-5, upon IL-1 stimulation, were significantly higher in young explants than old explants (15). Similarly, meniscus explants from older velvet monkeys secreted more MMP-1, MMP-3, and MMP-8 as compared to young healthy animals, which is likely correlated with age-related osteoarthritic changes (20). Even without osteoarthritis, older menisci secreted more IL-7 than healthy young menisci while older osteoarthritic menisci secreted more IL-7 and granulocyte-macrophage colony-stimulating factor than healthy older menisci (20). The abovementioned studies collectively suggest that tissue matrix degradation and associated pro-inflammatory responses of meniscus can be partially reconstructed in tissue explants.
An explant model was also adopted to investigate the interaction between infrapatellar fat pad (IFP) and knee meniscus (21). Co-culture of meniscus explants with IFP increased cumulative sGAG release and sGAG release rate but total sGAG content, indicating that fat co-culture stimulates production of sGAG (21). Meniscus explants stimulated by adipokines such as leptin, visfatin, adiponectin or resistin showed sGAG and NO release (22). Meniscus co-culture with IFP significantly increased NO release and decreased MMP-2 production, suggesting IFP may modulate meniscus homeostasis (21). Besides co-culture with other joint tissues, effects of mechanical stimuli on meniscus metabolism have been investigated. Application of static and dynamic compressive stresses in cylindrical meniscus explants resulted in decreases in mRNA expression of ECM proteins, including decorin, aggrecan, biglycan, COL-I and COL-II, likely indicating that metabolic roles of mechanical stimuli on meniscus can be observed ex vivo (23).
In summary, meniscus explants from various animal sources showed a notable potential as controlled ex vivo experimental models that mimic in vivo metabolic responses to pro-inflammatory signals, biochemical cues and mechanical loading. Ex vivo tissue culture model may serve as an efficient tool to investigate bio-molecular and biomechanical processes regulating meniscus metabolism as related to matrix remodeling, degradation, and disease development. Table 1 lists meniscus explant models applied for metabolic studies.
Table 1.
Explant models for meniscus metabolism
| Explant model | Source | Treatment | Outcome | Ref |
|---|---|---|---|---|
| 3-mm cylindrical tissue explants from middle region and 2-mm thick tissue from superficial tissue site | Bovine | IL-1, aggrecan-selective inhibitor, MMP-selective inhibitor, and broad-spectrum metalloproteinase | IL-1α induced secretion of sGAG via aggrecanases and decreased mechanical properties | (17) |
| 4-mm cylindrical explants from medial and lateral zones | Bovine | IL-1α (0, 1.25, 5, or 20 ng/mL) to juvenile and adult explants | IL-1α induced sGAG release and tissue degradation in an age-dependent manner | (15) |
| 5×1-mm cylindrical explants from peripheral 1/2 zone | Porcine | Static (0.1 MPa for 24 hours) and dynamic (0.5 Hz; 0.08 – 0.16 MPa; 24 hours) | Mechanical stimuli decreased mRNA expressions of matrix protein with difference between static and dynamic loading. | (23) |
| 4-mm cylindrical explants from middle and lateral region | Bovine | Adipokines (leptin, visfatin, adiponectin or resistin at 0.02, 0.2, or 2 μg/ml) | Adipokines induced sGAG and NO release. | (24) |
| 4-mm cylindrical explants from the central zone | Canine | IL-1β (0.1 ng/ml) with an impact loading at 25% - 75% strain | IL-1β or 75% loading increased pro-inflammatory responses. IL-1β and 75% loading together showed synergistic effect on PGE2 production. | (18) |
| Whole meniscus explants from animals with various ages and OA grades | Primate | MMPs and cytokines secretion measured without a treatment | Older menisci with OA secreted more MMP-1, MMP-3, and MMP-8 than young healthy menisci. Older menisci without OA secreted more IL-7 than healthy young menisci. | (20) |
| 5×1-mm cylindrical explants from outer half of meniscus | Porcine | Compressive loads (0.1 MP at 0.5 Hz) wit IL-1α (1 ng/ml) and NOS2 inhibitor (2 mM) | Dynamic compressions increased protein and proteoglycan synthesis, which was prevented by IL-1α via NOS2. | (58) |
| 3×1-mm cylindrical explants from unspecified region | Bovine | TNFα (10 ng/ml) and sodium selenite (6.7, 40, or 100 ng/ml) | TNFα induced sGAG release and NO production that can be reduced by 6.7 and 40 ng/ml sodium selenite. | (19) |
Injury and healing of meniscus explants
Meniscus tears are primarily caused by either excessive force exerted on a normal meniscus or normal forces on a degenerative meniscus (24). Commonly described patterns of meniscal tear include longitudinal (also known as vertical or circumferential), horizontal, radial (or transverse), flap, and complex tear (24, 25). A longitudinal tear usually occurs in the longitudinal direction along the meniscus periphery. A longitudinal tear with centrally displaced inner fragment is referred as a “bucket handle” tear that is highly associated with pain, perceived instability, and mechanical locking. A horizontal tear is a cleavage of the meniscus tissue that runs horizontally, parallel to the tibial plateau. A radial tear is oriented perpendicular to the long axis of the meniscus, representing a perpendicular tear to the circumferential collagen fibers. A flap tear is an oblique vertical/horizontal cleavage. Tears occurring in multiple planes are termed as complex or degenerative tear. As per a previous study followed up total 198 patients, longitudinal tear is the most prevalent, accounting for ~46% of all tears, whereas radial, flap, and complex tears account for ~7%, ~4.5% and ~13%, respectively (10, 26). Another study with a radiographic follow-up of total 155 patients reported that traumatic longitudinal tears are accounted for 42% and degenerative tears are 45% of all meniscus injuries (25). Of the degenerative tears, total 32% were flap tears and horizontal and other tears were 13% (25). It is apparent that the incident rate of each type of tear varies among the tested patient populations.
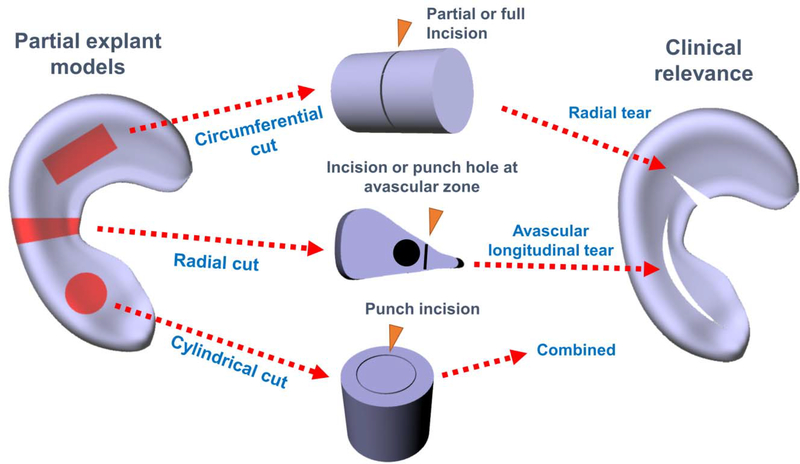
Most of ex vivo meniscus healing models adopted the radial and longitudinal tears because of the simplicity of these tears compared to others. A very common type of injury healing model is a cylindrical or circular defect, which is typically created using a biopsy punch (12, 16, 18, 27–35). The circular cavity from the biopsy punch can be left empty or filled with various materials supplemented with growth factors (GF) and cells (12, 31, 36, 37), or it can be glued back using bioadhesives, without additional supplements (27, 30, 35, 38). A radial or longitudinal incision has been frequently made directly in whole meniscus explants or sliced/fragmented meniscus (5, 6, 16, 39–41). Various hydrogels, bio-adhesives, and scaffolds with and without cells and GFs are then applied in the gap created by the radial or longitudinal cut. Types of explant injury and healing model are depicted in Fig. 1.
Figure 1.
Explant models for meniscus injuries and healing.
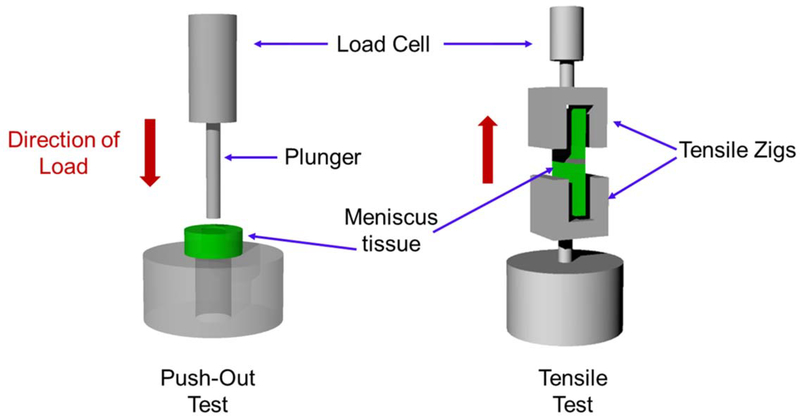
Cylindrical defects created by biopsy punch represent a simple and straightforward injury model. Tissue explants punched out and reinserted into defect cavity maintain tight junction on the incised interface, which makes this model easy to handle in the laboratory and the most suitable for compression or push-out shear test. This model also minimizes the potential influence by varied gap size because of the ease of control over defect dimension. These aspects have made the cylindrical or circular defects a widely-adopted injury model in meniscus explants. Despite the advantages, the cylindrical defect model hardly represents a specific clinical injury, as a circular incision created by biopsy punch includes cuts both in circumferential and radial fibers covering at least two different radial zones of meniscus. In contrast, the radial or longitudinal defects better represent clinically relevant meniscus injuries. However, it is often difficult to control the defect dimension and integrity at the healing junction with such radial or longitudinal incision. Consequently, the radial and longitudinal injury model may require additional fixation (e.g. suture repair) unless applied with strong adhesives. Moreover, it is often challenging to secure the explant specimen with radial or longitudinal incisions in tensile jigs due to the thin, wedged, and highly lubricated avascular zone tissues, which results in technical difficulties in achieving an accurate and reproducible tensile analysis. Schematic representation of the push-out test using a cylindrical healing model and tensile test of meniscus explant healing model is shown in Fig. 2. For the tensile tests, thin strip-shaped specimens are frequently prepared with a high ratio of length to width to ensure a tight grip with specially designed tensile jigs for soft tissues, and tears are aligned perpendicularly to the pulling direction (Fig. 2). Thus, it is imperative to consider the preparation of tensile specimen in determination of injury model. For example, a small sized meniscus explant model is not likely technically eligible tensile tests for longitudinal defect healing as the specimens are to be elongated to the radial direction.
Figure 2.
Schematic representation of the push-out and the tensile test for meniscus healing model.
Bioactive cues for explant injury healing
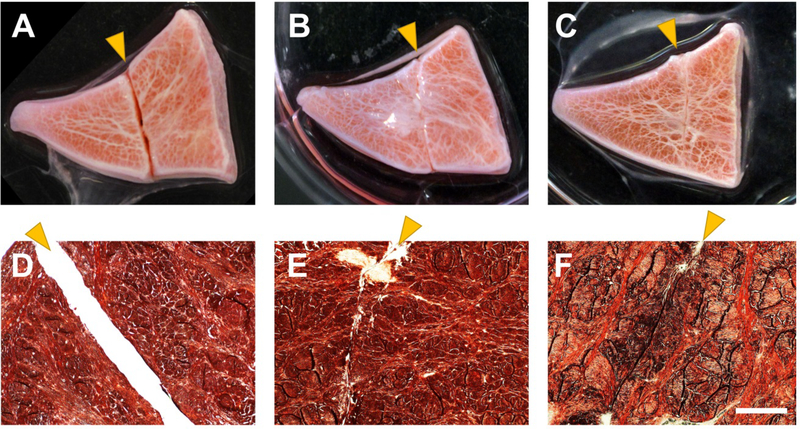
A wide range of bioactive cues and growth factors (GFs) have been tested for meniscus explant injury healing models (5, 12, 32, 36). GFs mixed with hydrogels or bioadhesives can be used as bioglue or defect filler for the injury healing. Our recent studies with sequential delivery approach of CTGF and TGF-β3 showed that a fast CTGF release, followed by sustained TGF-β3 release resulted in seamless healing of avascular meniscus tears by stem cell recruitment in 8 weeks (5). Radially sliced, wedged explants from inner one-third avascular zone of bovine menisci showed ex vivo healing of full-thickness incisions made at the avascular zone by treatment with bio-adhesives and control-delivered GFs (Fig. 3) (5).
Figure 3.
Ex vivo healing of full-thickness meniscus incision in the avascular zone of wedge-sliced bovine meniscus. Control with no treatment (A, D) ended up with remaining gap, while explants treated with bioadhesives and GFs improved healing by 8 wks ex vivo (B, C, E, and F) (scale = 200 μm; arrows indicate defects).
With some exceptions, various GFs favor tissue healing through proteoglycan-rich extracellular matrix deposition. When platelet-derived growth factor (PDGF) and hepatocyte growth factor (HGF) were loaded in collagen gel and sponge, a rapid release of PDGF and a slow release of HGF from collagen was observed, which resulted in organized collagen fiber in the 2 mm cylindrical defect area (12). Besides growth factors, chemical inhibitors for a wide spectrum of MMPs showed potential to improve ex vivo healing of meniscus in the presence of IL-1 (30). Application of platelet-rich plasma (PRP) with ECM-based decellularized scaffolds (dECM) has also shown to increase cell proliferation and infiltration into defect site (16). A previous work showed that effects of GFs on meniscus healing can be diminished by a static compression, due to inhibition of matrix accumulation (29). Despite the essential roles of mechanical loading in meniscus function and healing, no previous work has investigated how dynamic mechanical stimulations can orchestrate the meniscus explant healing by GFs treatment.
Cell-based approaches for ex vivo healing of meniscus
Cells can be delivered in the injury site by directly seeding on the scaffold or mixing with the hydrogel or scaffold material in order to accelerate the healing process. The idea behind cell delivery is that dense meniscus matrix may hinder migration of enough progenitor cells to the injury site, followed by initiation of the healing process (42, 43). Meniscal fibrochondrocytes (MFC)-seeded electrospun nanofibrous scaffolds improved healing of a radial defect in a cylindrical explant through facilitating migration and differentiation of MFCs (34). Similarly, a micropatterned methacrylated gelatin (Gel-MA) scaffolds-loaded with human meniscus cells promoted tissue integration between new and native tissue when applied into a longitudinal defect, although there was no difference in the tensile modulus between Gel-MA with cells and without cells (44). Freshly isolated chondrocytes from cartilage and minced cartilage fragments also promoted matrix deposition at the injury site and significantly increased push-out strength in comparison with acellular polyurethane implants (37).
Application of GFs in such cell-loaded scaffolds further improved healing of explant injuries. Delivery of TGF-β3 on adipose-derived stem cells (ASCs)-seeded methacrylated gelatin (mGL) hydrogels resulted in a higher load to failure, stiffness, and Young modulus in a radial tear model by inducing robust chondrogenic differentiation (45). When equine fibroblast-like synoviocytes (FLS) were delivered into cylindrical defects in the avascular zone via PGA/PLLA scaffolds, co-delivery with basic fibroblast growth factor (bFGF), TGF-β1 and/or insulin-like growth factor (IGF-1) played essential roles in leading tissue integration at the healing region (36).
Meniscus explant healing via bioadhesives and digestive enzymes
Instead of cells or growth factors, various biomaterials have been applied as bioadhesives to support integration of torn meniscus. A number of bioadhesives have been tested in meniscus explant injury and healing models, including but not limited to fibrin glue, Gel-MA, Chondroitin Sulfate (CS) BM adhesive, and 3-armed- and hyper-branched tissue adhesives (6, 13, 46, 47). The previously tested bioadhesives showed potential to support initial bonding of torn meniscal tissues, cell migration, and healing in varying degrees (6, 13, 46, 47). Fibrin is a widely used natural biomaterial as a bio-glue for meniscus healing. Despite its promising biocompatibility, instant gelation and easy handling, fibrin often suffers from weak adhesive strength and fast degradation in vivo. As another example, chondroitin sulphate (CS) bone marrow tissue adhesive showed promising potential to promote fibrochondrocytes viability, proliferation and migration as exhibiting an enhanced adhesive strength (49). Despite the promising research progress, currently no existing bio-glue provides mechanical properties and bonding strength sufficient to enable functional repair of torn menisci as well as supports cells and tissue ingrowth and remodeling.
Given the high density of meniscus tissue, matrix digesting enzymes were often applied with or without such bioadhesives to further facilitate tissue integration by loosening the dense meniscal matrix. For example, a short exposure of the defect edges to collagenase eased the recruitment of progenitor cells from the dense matrix of the meniscus into the injury, followed by improved healing ex vivo (48). The collagenase-delivered nanofibrous scaffolds also resulted in significant improvement in avascular meniscus healing in vivo (48). In another study, collagenase pre-treatment was combined with TGF-β3 and a block copolymer-based bioadhesive for circular injury explant model (46). The experimental data supported that the pre-treatment with collagenase improves tissue healing stimulated by bioadhesives and TGF-β3. Despite the promising outcome, an excessive application of collagenase can cause damages to other joint tissues (Collagenase treatment for OA). Thus, a precise control of collagenase treatment is imperishable for in vivo application. Nonetheless, effects of doses and duration of collagenase seems to be marginal in meniscus explant injury and healing models (41, 46, 48). As compared with collagenase, Matrix Metalloproteinases (MMPs) showed somewhat distinct aspects in explant healing. In presence of IL-1, inhibition of wide spectrum of MMPs significantly enhanced healing of cylindrical, punched defects in disc-shaped meniscus explants, likely associated with detrimental effects of MMPs induced by IL-1 (30).
Cell source for meniscus healing ex vivo
An investigation to identify the cell sources responsible for ex vivo meniscus healing suggested that a relatively higher population of migrating meniscus progenitor cells (MPC) are present in the red vascular zone than the white avascular zone (49). As a result, significantly higher number of cells migrated in the red zone than in the white zone in response to injury. The migrating cell population was found more similar to chondrogenic progenitor cells (CPCs) than other meniscus cells. As potential cell sources for meniscus healing in vivo, chondroprogenitor cells (CPCs) and bone-marrow mesenchymal stem cells (BM-MSCs) for meniscus tissue repair have also been investigated (50). Previous works demonstrated that CPCs, unlike BM-MSCs, do not undergo terminal hypertrophic differentiation during the tissue repair process. Both BM-MSCs and CPCs are responsive to the chemokine stromal cell-derived factor-1 (SDF-1), and they can promote tissue repair by migrating to the injured or damaged meniscal tissues through SDF-1/CXCR4 signaling pathway (50). In addition, the cells residing inside the meniscus may migrate through collagenase-digested tissue matrix and participate in ex vivo meniscus healing (48).
Animal sources for meniscus explants
Although human meniscus tissue would be the most representative explant model for meniscus injury healing, live tissue specimen from human source are very limited. Moreover, human patients who suffer from meniscus injuries are mostly of a young, active population. Only fragmented explant is likely available from young patient undergoing partial meniscectomy. Whole meniscus explants, possibly obtained through total knee joint replacement surgeries, are very likely in an age- or OA-associated degradation process. Therefore, meniscus obtained from various animals are the primary source for explant injury healing models. Meniscus from bovine, pig, canine, equine, and caprine are commonly used as explants to study injury repair and healing. Despite some difference in contents and ratio of meniscus matrices among species, no study has directly compared meniscus explants from different animal sources in regard to repair or healing capacity (27, 30, 35, 36). A selection of animal sources for meniscus explants likely have been determined based on the tissue size and accessibility. Table 2 below summarizes literature for explant models adopted for meniscus injuries, repair and healing.
Table 2.
Meniscus explant models for injury repair and healing
| Injury Model | Source | Treatment | Outcomes | Ref. |
|---|---|---|---|---|
| 5-mm radial tears in the mid body of the whole meniscus including both the red-white and white-white zones. | Canine | ECM based decellularized scaffolds (dECM) with platelet-rich plasma (PRP) | Cell infiltration and proliferation enhanced by dECM and PRP in contrast to the suture-repaired defect only group by 40 days. | (16) |
| 2-mm full thickness cylindrical defects in the whole meniscus | Canine | HGF and PDGF released from collagen sponge and collagen gel | Higher cell numbers around the defect after repair with collagen gel than with collagen sponge treated with HGF or PDG. HGF and PDGF delivery showed organized collagen formation after 4 wks. | (12) |
| 4×2 mm cylindrical tissue was punched out from 8 mm disc and re-inserted back with tissue adhesives. | Porcine | Biodegradable block copolymeric tissue adhesives with collagenase | Significantly higher adhesive strength of new copolymer glue than fibrin glue. No improvement observed by collagenase treatment after 4 wks. | (27) |
| 10-mm meniscal fragment: 5-mm longitudinal tears in central avascular zone | Human | Transplantation of meniscus cells transfected with 18 genes | Both juvenile and adult human meniscal fibrochondrocytes were safely transfected with nonviral genes. Transgenes expression was maintained for at least 5 days after seeding cells around the meniscal defect ex vivo. No tissue repair outcome was reported | (40) |
| Sliced strips with full-depth longitudinal incisions in avascular zone | Porcine | Sequential delivery of CTGF and TGF-β3 | Fast release of CTGF, followed by TGF-β3 release resulted in seamless healing of avascular meniscus tears by stem cell recruitment in 8 wks. | (6) |
| A high CTGF dose and slow TGF-β3 release are most effective for integrated healing of avascular meniscus. | (5) | |||
| 4-mm cylindrical tissues punched out and reinserted into 8 mm disc from the outer 2/3 zone. | Porcine | Matrix Metalloproteinases (MMPs) inhibition | Enhanced tissue repair at the interface enhanced by inclusion of the broad-spectrum MMP inhibitor in presence of IL-1 in 2 wks. | (30) |
| Wedge-shaped nit explants prepared by radial cut; radial defect leaving 1mm gap | Bovine | Cells digested from meniscus with different duration of collagenase | Rapid dissociation in collagenase resulted in cells expressing a higher level of collagen type II expression than the overnight dissociation group over 8 wks. No difference in tissue healing between rapid and slow digestion. | (41) |
| 2-mm cylindrical defects in the 5-mm thick radially cut meniscus strips. | Human | High mannuronic acid content (BioMVM) alginate spheres containing human meniscal fibrochondrocytes | Fibrochondrocytes encapsulated BioMVM alginate produced and retained significant amounts of proteoglycans in vitro for 21 days. Ex vivo culture for 3 dyas only showed the use potential of this system for meniscus tissue healing. Tissue healing outcome was not assessed here. | (31) |
| 5×10 mm cylindrical disc punched out from the avascular zone of radially cut meniscus strips followed by a 2.5-mm cut along the long axis of the cylindrical disc. | Bovine | Adipose-derived stem cells (ASCs) seeded methacr ylated gelatin (mGL) hydrogels with TGF-β3 | ASC-seeded hydrogels with preloaded TGF-β3 resulted in enhanced chondrogenic differentiation and improved healing with higher load to failure, stiffness, and Young modulus than control without TGF-β3 over 8 wks. | (32) |
| 2×2 mm cylindrical hole in the intermediate zone | Caprine | Fresh chondrocyte (FC) isolates vs minced cartilage (MC) fragments loaded polyurethane scaffolds (Actifit®). | Both enzymatically isolated FC and MC showed improved matrix deposition and enhanced push-out strength after 4 wks compared with the acellular implant. | (37) |
| 4-mm cylindrical defects | Bovine | Fibrin gel | Significantly higher cell migration in the red zone than in the white zone in response to injury. Migrating population is more similar to chondrogenic progenitor cells (CPCs) than other meniscus cells. | (33) |
| 5×10 mm cylindrical disc punched out from the avascular zone of radially cut meniscus strips followed by around 2.5-mm cut along the long axis of the cylindrical disc. | Bovine | Meniscal fibrochondrocytes (MFC)-seeded electrospun nanofibrous scaffold wrapped around the defect | Improved healing by 8 wks through recruiting cells, and promoting their differentiation into defect site, and recovered mechanical properties up to 40% of native meniscus | (34) |
| 4-mm full thickness cylindrical explants from outer two third zone were sliced into 1 to 2 mm thick tissue slices. | Bovine | Multiple growth factors on meniscus tissue with static mechanical compression | TGF-β1 was the most potent stimulator of both protein and proteoglycan production, whereas bFGF was the least effective stimulator among bFGF, IGF-I, PDGF, and TGF-β1 as revealed by 2 wks explant culture. | (29) |
| 8-mm full thickness cylindrical defect from outer one third region with 4-mm core defects punched out and reinserted | Porcine | Application of interleukin-1 (IL-1) on the integrative repair of the meniscus | Explant culture over 4 wks time period showed exposure to IL-1α even for 1 day can significantly reduce cell accumulation, tissue repair and integration. | (35) |
| 4-mm cylindrical defects in the avascular zone of the meniscus fragments. | Ovine | Fibroblast-like synoviocytes (FLS) seeded PGA/PLLA scaffolds with multiple growth factors (e.g. FGF, TGF-β1, IGF-1) | FLS-seeded scaffold constructs failed to integrate into avascular meniscal tissue even after 6 wks. | (36) |
| Full thickness (dimension not known) longitudinal defect into meniscus tissue fragments. | Human | Micropatterned methacrylated gelatin (Gel-MA) scaffold | Improved integration between the new tissue and the native meniscus tissue was observed with relatively poor tensile mechanical properties after 4 wks. | (44) |
| 4-mm full thickness cylindrical defect made into 8-mm cylindrical explants from the inner and outer zones | Porcine | No treatment; Intrinsic repair response differences between the inner and outer zone | Repair strength and tissue integration increased significantly over time (6 wks) in both zones with no significant difference between the zones. | (38) |
| Whole meniscus with radial tear (size not reported) in the inner anterior horn | Rodent | Chondroprogenitor cells (CPCs), bone-marrow mesenchymal stem cells and SDF-1 | Unlike BM-MSCs, CPCs do not undergo terminal hypertrophic differentiation during the tissue repair process. Like BM-MSCs, CPCs are responsive to the chemokine stromal cell-derived factor-1 (SDF-1) and they can promote tissue repair by migrating to the injured/damaged meniscal tissue through SDF-1/CXCR4 signaling pathway. | (50) |
| Longitudinal meniscal tear was created by a full length cut in the meniscus strips along the circumferential direction of the collagen bundles in the avascular zone. | Bovine | Human meniscus avascular cells seeded electrospun collagen scaffold | Collagen scaffolds showed no significant difference from defect only control, whereas cell-seeded collagen scaffolds led to improved tissue repair and integration after 3 wks. | (39) |
Consideration of meniscus mechanobioloy
Under physiological loading, the meniscus deforms radially which is constrained by anchors at the anterior and posterior horns (2). Given the regionally and directionally variant collagen orientation and the unique wedge-shape, compressive loads exerted by the femur pressing down, a combination of tensile, compressive, and shear forces are generated. Radial deformation generate a tensile hoop stress, whereas the femur pressing down creates vertical and horizontal forces on the curved surface (2). Meniscal cells are predicted to experience 7% and 2 – 4% tensile strains under the normal physiological loading in the inner and outer zones, respectively (51). Such mechanical stimulation is believed to play essential roles in normal meniscus cell functions given that joint immobilization decreases aggrecan gene expression by 2- to 5-fold (52).
Previous studies collectively suggest that meniscus cells differently respond to various loading conditions. For example, static compression attenuated gene expression for matrix molecules and increase expression for MMPs, while dynamic compression decreased collagen and decorin expression (23). With biaxial strains of 5% in vitro, meniscus cells increased total protein synthesis and nitric oxide levels (51). Under 10% cyclic tension at 1 Hz, meniscal cells in 3D fibrin gels inhibited matrix synthesis as indicated by 3H-proline and 35S-sulfate incorporation (53). Cyclic tensile strain of 5% – 20% significantly reduced iNOS mRNA induced by IL-1β in magnitude- and frequency-dependent manners, suggesting anti-inflammatory roles of tensile stimulation (54). Static hydrostatic pressure at 4 MPa suppressed mRNA expressions of MMP-1 and MMP-13, whereas cyclic hydrostatic pressure at 4 MPa and 1 Hz significantly increased mRNA expression of Col-I, TIMP-1 and −2 (55). Besides the direct stimulation to meniscal cells, effects of mechanical stimulations on meniscus explants have been extensively investigated. When 25 – 50% static compression was applied to disc-shaped bovine meniscus explants, the protein and proteoglycan production stimulated by various growth factors were significantly reduced (29). Dynamic compressions ranging from 10% up to 40% were also applied to disc-shaped meniscus explants that resulted in various tissue-level responses including expressions of pro- or anti-inflammatory cytokines, and matrix synthesis or breakdown depending on magnitude of stimulation and/or associated biochemical stimulations (56–59).
Unlike compressive loading, other types of mechanical stimulations, including tensile and shear forces have rarely been investigated in meniscus explant models. Previous studies with tensile stimulation have been limited to meniscal cells either in 2D or encapsulated in 3D scaffolds (60, 61). As physiological compressive load is transferred to circumferential loop stress in meniscus, understanding roles of tensile stress or strain in tissue level is a remaining task. Moreover, most of previous works applied a selected type of mechanical stimulation to isolated tissue explants. As abovementioned, meniscus physiologically experience a cluster of complex loadings including compression, tension, and shear. Such complex loading conditions were more closely simulated for meniscus tissue engineering by applying dynamic compression on anatomically shaped scaffolds or engineered tissue constructs (62–65). For example, dynamic compressive loading of anatomically shaped tissue engineered constructs significant promoted meniscus matrix formation and maturation (62–65). However, effects of the combined loadings have not been investigated on meniscus explants in regard to biosynthesis, metabolism or healing.
Summary and perspective
Meniscus explants have been adopted in a number of previous studies for pathophysiology, repair, healing, or tissue engineering of knee meniscus. Meniscus explants derived from various animal species were proved as a viable model to study metabolic responses to cytokines, growth factors, and co-culture with other types of cells. Meniscus healing regulated by cytokines, enzymes, bio-adhesives, stem cells, scaffolds, and tissue engineered constructs were successfully reconstructed in explant models. Various injury types such as longitudinal and radial tears are available in the meniscus explant models, with experimental data supporting a partial replication of in vivo healing process.
Despite the advantages, meniscus explant models have several limitations. First, mechanical stimuli exerted on meniscus by physiological loading have been comprehensively incorporated in the existing explant models. Some previous works have applied mechanical forces to understand complex biomechanical behaviors and properties of meniscus tissues. However, meniscus pathophysiology, repair or healing is under complex physiological loadings has rarely been investigated.
Second, the existing meniscus explants lack potential cross-talks with other components in knee joints such as synovial fluid, synovial membrane, articular cartilage and cruciate ligaments. Meniscus tissue in the knee joint is in intimate contact with the synovial fluid, articular cartilage and the ligaments in the synovial joint cavity. Synovial fluid, secreted by the cells present in the synovial membrane, lubricates the joint cavity and nourishes the tissues present there. Synovial cells can also produce different cytokines in response to injury or trauma (66, 67). Several previous studies suggested that meniscus lesion not only a cause of cartilage degeneration but also a consequence of OA (68, 69). Degenerative changes in articular cartilage can cause meniscus lesion likely through inflammatory cytokines (68, 69). In the possible process of meniscus lesion caused by cartilage degeneration, involvement of recruitment and activation of immune cells such as macrophages and T cells is highly likely, which has been rarely adopted in meniscus explant healing or degeneration model (70).
In addition, articular fat pad playing important roles in joint homeostasis that may interact with other joint tissues including articular cartilage and meniscus (71). For example, adipose tissue secretes leptin that stimulates inflammatory cytokine production, followed by expression of matrix degradative peptides (71). Given the existence of leptin receptor on chondrocytes and possibly in meniscal cells, the cross-talk with fat pad is likely involved with meniscus injury and degeneration (71). Therefore, any impact on any of the component in the joint cavity will greatly influence the function and structural integrity of other components as well. The cross-talks between these components is thus crucial for tissue engineering and regenerative applications.
Lastly, the most of injuries applied to meniscus explants represent an acute injury rather than a chronic injury. Given the inevitable time interval (> 2 ~ 3 weeks) between the meniscus injury and the first treatment, the injured meniscus to be treated will likely have been undergoing pathological changes, such as lubricin infiltration and inflammation-induced matrix breakdown.
Lubricin (proteoglycan 4) serves as a lubricant on articular cartilage and knee meniscus that reduces friction-induced damage and prevents cell and protein adhesion. However, an exposure to synovial fluid containing lubricin likely has harmful effect on healing of torn meniscal tissues in clinics. As the lubricin expression penetrates deeply into the torn menisci in human patients upon exposure to synovial fluids (72), it is imperative to understand effects of lubricin infiltration into torn menisci on their healing that can better simulate the healing or repair process in human patients.
Various designs may be applied to meniscus explant models to overcome the outstanding limitations. For example, a state-of-art bioreactor system has potential to enable application of physiological loadings while meniscus explants undergo injuries and healing. Such bioreactors have been widely applied to tissue-engineer functional blood vessels, articular cartilage and bone, allowing for application of various mechanical stresses simultaneously with supplying and refreshment of culture media and necessary supplements (73). A bioreactor system can also be used to pre-treat meniscus explants with lubricin coating, pro-inflammatory cytokines, and/or physiological loading to simulate pathological conditions prior to testing repair or healing strategies. In conclusion, meniscus explant models have potential to serve as an effective tool for investigations of meniscus metabolism, injury, repair and healing.
Acknowledgments
Funding
This manuscript was supported by NIH grants, 5R01AR065023-05 and 5R01AR071316-02, to C.H.L.
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Reference
- 1.Abraham AC, et al. , Regional and fiber orientation dependent shear properties and anisotropy of bovine meniscus. Journal of the Mechanical Behavior of Biomedical Materials, 2011. 4(8): p. 2024–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Athanasiou KA and Sanchez-Adams J, Engineering the Knee Meniscus. Synthesis Lectures on Tissue Engineering, ed. Athanasiou KA and Kent Leach JK. 2009: Morgan and Claypool Publishers. [Google Scholar]
- 3.Baker BM, et al. , Meniscus tissue engineering on the nanoscale: from basic principles to clinical application. J Knee Surg, 2009. 22(1): p. 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee CH, et al. , Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Science Translational Medicine, 2014. 6(266): p. 266ra171–266ra171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarafder S, et al. , Effect of dose and release rate of CTGF and TGFbeta3 on avascular meniscus healing. J Orthop Res, 2019. 37(7): p. 1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarafder S, et al. , Engineered Healing of Avascular Meniscus Tears by Stem Cell Recruitment. Sci Rep, 2018. 8(1): p. 8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC, Center for Disease Control and Prevention Report. 2011. [Google Scholar]
- 8.Cheung HS, Distribution of type I, II, III and V in the pepsin solubilized collagens in bovine menisci. Connect Tissue Res, 1987. 16(4): p. 343–56. [DOI] [PubMed] [Google Scholar]
- 9.Cook JL and Fox DB, A novel bioabsorbable conduit augments healing of avascular meniscal tears in a dog model. Am J Sports Med, 2007. 35(11): p. 1877–87. [DOI] [PubMed] [Google Scholar]
- 10.Noyes FR and Barber-Westin SD, Repair of complex and avascular meniscal tears and meniscal transplantation. J Bone Joint Surg Am, 2010. 92(4): p. 1012–29. [PubMed] [Google Scholar]
- 11.Voelkl B, et al. , Reproducibility of preclinical animal research improves with heterogeneity of study samples. PLoS Biol, 2018. 16(2): p. e2003693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhargava MM, et al. , Effects of Hepatocyte Growth Factor and Platelet-Derived Growth Factor on the Repair of Meniscal Defects in vitro. In Vitro Cellular & Developmental Biology. Animal, 2005. 41(8/9): p. 305–310. [DOI] [PubMed] [Google Scholar]
- 13.Bochyńska AI, et al. , Development of a fast curing tissue adhesive for meniscus tear repair. Journal of Materials Science: Materials in Medicine, 2016. 28(1): p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemke AK, et al. , Interleukin-1α treatment of meniscal explants stimulates the production and release of aggrecanase-generated, GAG-substituted aggrecan products and also the release of pre-formed, aggrecanase-generated G1 and m-calpain-generated G1-G2. Cell and Tissue Research, 2010. 340(1): p. 179–188. [DOI] [PubMed] [Google Scholar]
- 15.Ling CH-Y, et al. , Bovine meniscal tissue exhibits age- and interleukin-1 dose-dependent degradation patterns and composition-function relationships. Journal of Orthopaedic Research, 2016. 34(5): p. 801–811. [DOI] [PubMed] [Google Scholar]
- 16.Monibi FA, et al. , Development of a Micronized Meniscus Extracellular Matrix Scaffold for Potential Augmentation of Meniscal Repair and Regeneration. Tissue Eng Part C Methods, 2016. 22(12): p. 1059–1070. [DOI] [PubMed] [Google Scholar]
- 17.Wilson CG, et al. , Aggrecanolysis and in vitromatrix degradation in the immature bovine meniscus: mechanisms and functional implications. Arthritis Research & Therapy, 2009. 11(6): p. R173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook AE, et al. , Metabolic responses of meniscal explants to injury and inflammation ex vivo. Journal of Orthopaedic Research, 2018. 36(10): p. 2657–2663. [DOI] [PubMed] [Google Scholar]
- 19.Häfelein K, et al. , Selenium Reduces Early Signs of Tumor Necrosis Factor Alpha-Induced Meniscal Tissue Degradation. Biological Trace Element Research, 2017. 177(1): p. 80–89. [DOI] [PubMed] [Google Scholar]
- 20.Stone AV, et al. , Osteoarthritic changes in vervet monkey knees correlate with meniscus degradation and increased matrix metalloproteinase and cytokine secretion. Osteoarthritis Cartilage, 2015. 23(10): p. 1780–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishimuta JF, Bendernagel MF, and Levenston ME, Co-culture with infrapatellar fat pad differentially stimulates proteoglycan synthesis and accumulation in cartilage and meniscus tissues. Connective Tissue Research, 2017. 58(5): p. 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishimuta JF and Levenston ME, Meniscus is more susceptible than cartilage to catabolic and anti-anabolic effects of adipokines. Osteoarthritis and Cartilage, 2015. 23(9): p. 1551–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Upton ML, et al. , Differential effects of static and dynamic compression on meniscal cell gene expression. Journal of Orthopaedic Research, 2003. 21(6): p. 963–969. [DOI] [PubMed] [Google Scholar]
- 24.Greis PE, et al. , Meniscal injury: I. Basic science and evaluation. J Am Acad Orthop Surg, 2002. 10(3): p. 168–76. [DOI] [PubMed] [Google Scholar]
- 25.Englund M, Roos EM, and Lohmander LS, Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: a sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum, 2003. 48(8): p. 2178–87. [DOI] [PubMed] [Google Scholar]
- 26.Rubman MH, Noyes FR, and Barber-Westin SD, Arthroscopic repair of meniscal tears that extend into the avascular zone. A review of 198 single and complex tears. Am J Sports Med, 1998. 26(1): p. 87–95. [DOI] [PubMed] [Google Scholar]
- 27.Bochynska AI, et al. , Evaluation of novel biodegradable three-armed- and hyper-branched tissue adhesives in a meniscus explant model. J Biomed Mater Res A, 2017. 105(5): p. 1405–1411. [DOI] [PubMed] [Google Scholar]
- 28.Coluccino L, et al. , Porous Poly(vinyl alcohol)-Based Hydrogel for Knee Meniscus Functional Repair. ACS Biomaterials Science & Engineering, 2018. 4(5): p. 1518–1527. [DOI] [PubMed] [Google Scholar]
- 29.Imler SM, Doshi AN, and Levenston ME, Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarthritis Cartilage, 2004. 12(9): p. 736–44. [DOI] [PubMed] [Google Scholar]
- 30.McNulty AL, Weinberg JB, and Guilak F, Inhibition of Matrix Metalloproteinases Enhances In Vitro Repair of the Meniscus. Clinical Orthopaedics and Related Research®, 2009. 467(6): p. 1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rey-Rico A, et al. , Biomedical-grade, high mannuronic acid content (BioMVM) alginate enhances the proteoglycan production of primary human meniscal fibrochondrocytes in a 3D microenvironment. Sci Rep, 2016. 6: p. 28170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasaki H, et al. , In Vitro Repair of Meniscal Radial Tear With Hydrogels Seeded With Adipose Stem Cells and TGF-β3. . The American Journal of Sports Medicine, 2018. [DOI] [PubMed] [Google Scholar]
- 33.Seol D, et al. , Characteristics of Meniscus Progenitor Cells Migrated From Injured Meniscus. Journal of Orthopaedic Research, 2017. 35(9): p. 1966–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimomura K, et al. , In Vitro Repair of Meniscal Radial Tear Using Aligned Electrospun Nanofibrous Scaffold. . Tissue Engineering Part A, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilusz RE, et al. , Inhibition of integrative repair of the meniscus following acute exposure to interleukin-1 in vitro. Journal of Orthopaedic Research, 2008. 26(4): p. 504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fox DB, et al. , Effects of growth factors on equine synovial fibroblasts seeded on synthetic scaffolds for avascular meniscal tissue engineering. Research in Veterinary Science, 2010. 88(2): p. 326–332. [DOI] [PubMed] [Google Scholar]
- 37.Vedicherla S, et al. , Chondrocyte-based intraoperative processing strategies for the biological augmentation of a polyurethane meniscus replacement. . Connective Tissue Research, 2018. [DOI] [PubMed] [Google Scholar]
- 38.Hennerbichler A, et al. , Repair Response of the Inner and Outer Regions of the Porcine Meniscus in Vitro. The American Journal of Sports Medicine, 2007. 35(5): p. 754–762. [DOI] [PubMed] [Google Scholar]
- 39.Baek J, et al. , Repair of Avascular Meniscus Tears with Electrospun Collagen Scaffolds Seeded with Human Cells. Tissue Engineering Part A, 2016. 22(5–6): p. 436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee HP, et al. , Nonviral gene transfer to human meniscal cells. Part I: transfection analyses and cell transplantation to meniscus explants. Int Orthop, 2014. 38(9): p. 1923–30. [DOI] [PubMed] [Google Scholar]
- 41.Numpaisal PO, et al. , Rapidly dissociated autologous meniscus tissue enhances meniscus healing: An in vitro study. Connect Tissue Res, 2017. 58(3–4): p. 355–365. [DOI] [PubMed] [Google Scholar]
- 42.Qu F, et al. , Repair of dense connective tissues via biomaterial-mediated matrix reprogramming of the wound interface. Biomaterials, 2015. 39: p. 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forriol F, Growth factors in cartilage and meniscus repair. Injury, 2009. 40: p. S12–S16. [DOI] [PubMed] [Google Scholar]
- 44.Grogan SP, et al. , Digital micromirror device projection printing system for meniscus tissue engineering. Acta Biomater, 2013. 9(7): p. 7218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sasaki H, et al. , In Vitro Repair of Meniscal Radial Tear With Hydrogels Seeded With Adipose Stem Cells and TGF-β3. The American Journal of Sports Medicine, 2018. 46(10): p. 2402–2413. [DOI] [PubMed] [Google Scholar]
- 46.Bochynska AI, et al. , The effect of tissue surface modification with collagenase and addition of TGF-beta3 on the healing potential of meniscal tears repaired with tissue glues in vitro. J Mater Sci Mater Med, 2017. 28(1): p. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simson JA, et al. , Bonding and fusion of meniscus fibrocartilage using a novel chondroitin sulfate bone marrow tissue adhesive. Tissue Eng Part A, 2013. 19(15–16): p. 1843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qu F, et al. , Biomaterial-mediated delivery of degradative enzymes to improve meniscus integration and repair. Acta Biomater, 2013. 9(5): p. 6393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seol D, et al. , Characteristics of meniscus progenitor cells migrated from injured meniscus. Journal of orthopaedic research : official publication of the Orthopaedic Research Society, 2017. 35(9): p. 1966–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jayasuriya CT, et al. , Human Cartilage-Derived Progenitors Resist Terminal Differentiation and Require CXCR4 Activation to Successfully Bridge Meniscus Tissue Tears. STEM CELLS, 2019. 37(1): p. 102–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Upton ML, et al. , Finite element modeling predictions of region-specific cell-matrix mechanics in the meniscus. Biomech Model Mechanobiol, 2006. 5(2–3): p. 140–9. [DOI] [PubMed] [Google Scholar]
- 52.Djurasovic M, et al. , Knee joint immobilization decreases aggrecan gene expression in the meniscus. Am J Sports Med, 1998. 26(3): p. 460–6. [DOI] [PubMed] [Google Scholar]
- 53.Vanderploeg EJ, et al. , Oscillatory tension differentially modulates matrix metabolism and cytoskeletal organization in chondrocytes and fibrochondrocytes. J Biomech, 2004. 37(12): p. 1941–52. [DOI] [PubMed] [Google Scholar]
- 54.Ferretti M, et al. , Anti-inflammatory effects of continuous passive motion on meniscal fibrocartilage. J Orthop Res, 2005. 23(5): p. 1165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki T, et al. , Hydrostatic pressure modulates mRNA expressions for matrix proteins in human meniscal cells. Biorheology, 2006. 43(5): p. 611–22. [PubMed] [Google Scholar]
- 56.Aufderheide AC and Athanasiou KA, A direct compression stimulator for articular cartilage and meniscal explants. Ann Biomed Eng, 2006. 34(9): p. 1463–74. [DOI] [PubMed] [Google Scholar]
- 57.Gupta T, et al. , IL-1 and iNOS gene expression and NO synthesis in the superior region of meniscal explants are dependent on the magnitude of compressive strains. Osteoarthritis Cartilage, 2008. 16(10): p. 1213–9. [DOI] [PubMed] [Google Scholar]
- 58.Shin SJ, et al. , Regulation of matrix turnover in meniscal explants: role of mechanical stress, interleukin-1, and nitric oxide. J Appl Physiol (1985), 2003. 95(1): p. 308–13. [DOI] [PubMed] [Google Scholar]
- 59.Zielinska B, et al. , Meniscal tissue explants response depends on level of dynamic compressive strain. Osteoarthritis Cartilage, 2009. 17(6): p. 754–60. [DOI] [PubMed] [Google Scholar]
- 60.Ferretti M, et al. , Dynamic biophysical strain modulates proinflammatory gene induction in meniscal fibrochondrocytes. Am J Physiol Cell Physiol, 2006. 290(6): p. C1610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McNulty AL and Guilak F, Mechanobiology of the meniscus. J Biomech, 2015. 48(8): p. 1469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ballyns JJ and Bonassar LJ, Dynamic compressive loading of image-guided tissue engineered meniscal constructs. J Biomech, 2011. 44(3): p. 509–16. [DOI] [PubMed] [Google Scholar]
- 63.Huey DJ and Athanasiou KA, Tension-compression loading with chemical stimulation results in additive increases to functional properties of anatomic meniscal constructs. PLoS One, 2011. 6(11): p. e27857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Puetzer JL, Ballyns JJ, and Bonassar LJ, The effect of the duration of mechanical stimulation and post-stimulation culture on the structure and properties of dynamically compressed tissue-engineered menisci. Tissue Eng Part A, 2012. 18(13–14): p. 1365–75. [DOI] [PubMed] [Google Scholar]
- 65.Zhang ZZ, et al. , Orchestrated biomechanical, structural, and biochemical stimuli for engineering anisotropic meniscus. Sci Transl Med, 2019. 11(487). [DOI] [PubMed] [Google Scholar]
- 66.de Lange-Brokaar BJ, et al. , Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage, 2012. 20(12): p. 1484–99. [DOI] [PubMed] [Google Scholar]
- 67.Sohn DH, et al. , Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res Ther, 2012. 14(1): p. R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Atik OS, et al. , Is there crosstalk between subchondral bone, cartilage, and meniscus in the pathogenesis of osteoarthritis? Eklem Hastalik Cerrahisi, 2016. 27(2): p. 62–7. [DOI] [PubMed] [Google Scholar]
- 69.Englund M, Guermazi A, and Lohmander SL, The role of the meniscus in knee osteoarthritis: a cause or consequence? Radiol Clin North Am, 2009. 47(4): p. 703–12. [DOI] [PubMed] [Google Scholar]
- 70.Orlowsky EW and Kraus VB, The role of innate immunity in osteoarthritis: when our first line of defense goes on the offensive. J Rheumatol, 2015. 42(3): p. 363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Labusca L and Zugun-Eloae F, The Unexplored Role of Intra-articular Adipose Tissue in the Homeostasis and Pathology of Articular Joints. Front Vet Sci, 2018. 5: p. 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang D, et al. , Lubricin distribution in the torn human anterior cruciate ligament and meniscus. J Orthop Res, 2011. 29(12): p. 1916–22. [DOI] [PubMed] [Google Scholar]
- 73.Song L, et al. , Successful development of small diameter tissue-engineering vascular vessels by our novel integrally designed pulsatile perfusion-based bioreactor. PLoS One, 2012. 7(8): p. e42569. [DOI] [PMC free article] [PubMed] [Google Scholar]