Abstract
The study’s objective was to determine the effectiveness of a task-sharing psychological treatment for perinatal depression using non-specialist community health workers. A double-blind individual randomised controlled trial was conducted in two antenatal clinics in the peri-urban settlement of Khayelitsha, Cape Town. Adult pregnant women who scored 13 or above on the Edinburgh Postnatal Depression rating Scale (EPDS) were randomised into the intervention arm (structured six-session psychological treatment) or the control arm (routine antenatal health care and three monthly phone calls). The primary outcome was response on the Hamilton Depression Rating Scale (HDRS) at three months postpartum (minimum 40% score reduction from baseline) among participants who did not experience pregnancy or infant loss (modified intention-to-treat population) (registered on Clinical Trials: NCT01977326). Of 2187 eligible women approached, 425 (19.4%) screened positive on the EPDS and were randomised; 384 were included in the modified intention-to-treat analysis (control: n=200; intervention: n=184). There were no significant differences in response on the HDRS at three months postpartum between the intervention and control arm. A task-sharing psychological treatment was not effective in treating depression among women living in Khayelitsha, South Africa. The findings give cause for reflection on the strategy of task-sharing in low-resource settings.
Keywords: Randomised controlled trial, perinatal depression, task-sharing, counselling, community health workers, South Africa
Introduction
Perinatal depression, which occurs during pregnancy or the first 12 months postpartum, is a major public health problem, particularly in low- and middle-income countries (LMIC). Pooled prevalence estimates in LMIC are 19.2-25.3% and 18.7-19.0% for antenatal and postnatal depression, respectively (Gelaye, Rondon, Araya, & Williams, 2016; Woody, Ferrari, Siskind, Whiteford, & Harris, 2017), consistently higher than high-income countries. In poor urban settings in South Africa, such as Khayelitsha in the Western Cape, 21.5% and 34.7% of antenatal and postnatal women have been diagnosed with depression, respectively (Cooper et al., 1999; Van Heyningen et al., 2016). Perinatal depression in this community has been associated with low socio-economic status, unemployment, violence, crime, HIV status, poor health care, poor emotional and practical support from partners, social isolation, and interpersonal disputes (Hartley et al., 2011). Despite adverse consequences of perinatal depression for mothers and their babies in LMIC (Gelaye et al., 2016; Rahman, Iqbal, Bunn, Lovel, & Harrington, 2004; Senturk et al., 2012), there is a large treatment gap for perinatal depression in South Africa (Williams et al., 2008) and other African countries (Azale, Fekadu, & Hanlon, 2016).
With the dearth of mental health professionals to narrow this treatment gap, the World Health Organization (WHO) and other mental health advocates have proposed a strategy of task-sharing (sometimes referred to as task-shifting): the use of non-specialists to provide mental health care under the training and supervision of specialists (Kakuma et al., 2011). Previous meta-analyses on task-shared psychosocial interventions for perinatal common mental disorders in LMIC have found pooled effect sizes of −0.38 (Rahman et al., 2013) and −0.34 (Clarke, King, & Prost, 2013). Often these interventions were relatively lengthy, such as the Thinking Healthy Programme (16 sessions) (Rahman, Malik, Sikander, Roberts, & Creed, 2008), or required substantial training. A key question remains whether such interventions can be delivered in a scalable, brief and effective way in routine low-resource settings in sub-Saharan Africa.
The primary objective of this study was to determine the effectiveness of a task-sharing psychological treatment for perinatal depression using non-specialist community health workers (CHWs) in South Africa. Secondary objectives were to assess predictors of response to, and the cost-effectiveness of, the task-shared psychological treatment.
Methods
Trial design
The study was an individual level randomised controlled trial (RCT); methods have been presented previously (Lund et al., 2014) and are described briefly here.
Participants
Pregnant women were recruited at two antenatal clinics in community health centres (CHCs) in the peri-urban settlement of Khayelitsha in Cape Town, South Africa, an area marked by high HIV prevalence, high levels of poverty, and unemployment (Statistics, 2011). The majority of perinatal women from the Khayelitsha community attend these two antenatal clinics (Midwife Obstetric Units) for antenatal care, delivery and postnatal care . Women were recruited during their first antenatal visit and were eligible if: aged 18 years or older; spoke isiXhosa; were resident in Khayelitsha; were no more than 28 weeks pregnant; did not require urgent medical or psychiatric attention; and were able to give informed consent. Women with a diagnosis of schizophrenia or bipolar disorder were excluded. Fieldworkers who conducted the assessments for all four of the data collection points were trained to identify symptoms of schizophrenia and bipolar disorder and refer possible cases to the mental health nurse in the facility for further assessment.
Eligible participants were screened using the Edinburgh Postnatal Depression Scale (EPDS) (Cox, Holden, & Sagovsky, 1987). Those who scored 13 or more on the EPDS (De Bruin, Swartz, Tomlinson, Cooper, & Molteno, 2004) were enrolled into the study and randomised into the intervention or control arm. The EPDS was selected for use in our study as it had been validated with an acceptable sensitivity and specificity in South Africa (Lawrie, Hofmeyr, De Jager, & Berk, 1998) and its factor structure has been evaluated in Khayelitsha (De Bruin et al., 2004).
Intervention
Participants allocated to the treatment arm received a structured, manualised psychological treatment comprising six counselling sessions (Nyatsanza, Schneider, Davies, & Lund, 2016). The intervention was adapted for this population after conducting research on isiXhosa-speaking women’s experience of perinatal depression, in consultation with clinical experts (Davies, Schneider, Nyatsanza, & Lund, 2016; Nyatsanza et al., 2016). Among other aspects, this qualitative formative research indicated a strong association between perinatal women’s experience of depression symptoms and stressors associated with poverty, unemployment, lack of support from partners, abuse, loss of loved ones, unwanted or unplanned pregnancies and the discovery of HIV status at antenatal clinic appointments. We also identified a number of local idioms of distress, including “stress (unxunguphalo), thinking too much (ucingakakhulu), being sad or unhappy (ukudakumba), and being scared (ukoyika)” (Davies et al., 2016), p7.
In this context, the content of the counselling sessions was designed to promote resilience and support perinatal women’s capacity to cope with their adverse life circumstances. Sessions included psycho-education, problem solving, behavioural activation, healthy thinking, relaxation training, and birth preparation. We hypothesised that the specific focus on psycho-education (explaining the common causes of low mood), problem solving skills, healthy thinking and behavioural activation would help to build resilience and social support for women who experience low mood in the context of social and interpersonal adversity in Khayelitsha. The content of the counselling manual included specific idioms of distress that had been identified in the formative research (mentioned above). At each session, participants’ health and suicidal risk were assessed with the use of a checklist. Sessions were provided in addition to the routine antenatal health care provided by the clinic. The intended duration of the sessions was between 45 and 60 minutes.
The intervention was provided by six CHWs who were recruited from a local non-governmental organization (NGO) and worked full-time on the study. The CHWs received five days of training by a clinical social worker in basic counselling and delivery of the intervention. Subsequently, the CHWs received weekly group-based supervision from the clinical social worker (Munodawafa, Lund, & Schneider, 2017). A fidelity checklist was developed by the trial team and included 10 items, divided into three sections: (i) the introduction to each session (ii) exploration of the topic of each session, and lastly (iii) ending. Each item on the checklist was scored by a three tiered scoring system: “not done” = 0, “needs improvement” = 1, and “well done” = 2 (Munodawafa et al., 2017).
Counselling sessions were initiated within two weeks of enrolment and continued, usually on a weekly basis, until all six sessions were completed. If participants could not attend weekly, some sessions extended into the postnatal period in a pragmatic fashion, to allow for variation in timing of session delivery in the real world. Sessions were conducted either at the clinic or in the participant’s home, depending on her preference.
Control
Participants allocated to the control arm received enhanced usual care (EUC), which involved monthly phone calls for three months, in addition to the routine antenatal health care provided by the clinic. The phone calls followed a set protocol with the use of a checklist, which included items such as participant’s health, major life changes, mental health support received, and experience of depressive symptoms or suicidal ideation. Two CHWs recruited from another NGO were trained to conduct the phone calls, but were not trained in any counselling techniques used in the intervention arm.
Randomisation and blinding
Randomisation was conducted using a computer generated random number sequence stratified by clinic of recruitment, in blocks of 60 (30 control and 30 intervention). The data management system automatically allocated numbers from the random number list to study participants. Once the baseline assessment was completed, the fieldworker informed the participant that she would either receive an appointment to attend the first session with the CHW counselor, or receive a phone call to check on her progress. The system then sent a text message with the participant’s contact details to one of the six CHW counselors in a rotating manner (if the participant was allocated to the intervention arm) or to one of the two telephone CHWs in an alternating sequence (if the participant was allocated to the control arm), instructing them to set up their first appointment or phone call with the relevant study participant.
Fieldworkers were blinded to participants’ allocation arm and were managed by different team leaders to the CHWs. The fieldworkers, control and intervention CHWs did not have interactions during the course of the trial. The fieldwork supervisor, counseling trainer/supervisor and the CHWs were the only team members who were unblinded to arm allocation. Investigators were blinded to the allocation arm until the completion of the final follow-up assessments, the finalisation of the data analysis plan and lock down of the data. Participants were not informed of the study hypothesis. All staff employed on the study were trained in the importance of adhering to the study protocol.
Retention in care
Control and intervention CHWs were trained in a standardised protocol to follow up participants who missed sessions or phone calls. Drop out from care was defined as a participant who missed three consecutive scheduled sessions or phone calls. After this no further attempts were made to engage the participant in either intervention.
Outcome measures
Participants were assessed by trained fieldworkers using handheld electronic devices at enrolment, eight months gestation, and at three and 12 months postpartum.
All assessments included basic socio-demographic and economic measures, and HIV status. An index of asset-based welfare was created, using multiple correspondence analysis, to measure socio-economic status. This included the following variables: education, employment status, main income source, whether the participant owned a house or a flat, type of dwelling they lived in, whether household income was fixed, whether they had access to electricity, drinking water, and type of toilet facilities, where they shopped for groceries, whether they had a bank account, an automatic teller machine card, a credit card or an informal saving scheme.
The primary outcome measure was the 17-item Hamilton Depression Rating Scale (HDRS), which we adapted and validated for administration by non-clinicians (Davies, Baron, Schneider, & Lund, Under review). The adapted HDRS had excellent inter-rater reliability (intra-class correlation coefficient: 0.97-0.98) and acceptable internal reliability (Cronbach’s α=0.76). The primary outcome (response) was defined as at least 40% reduction in score at 3 months postpartum compared to baseline, while the secondary outcome (recovery) was defined as a score below eight at both three-month and 12-month postpartum.
Additional secondary outcome measures were the EPDS (Cox et al., 1987); World Health Organization Disability Assessment Schedule (WHODAS) 2.0 Üstün, Kostanjsek, Chatterji, & Rehm, 2010); Cape Town Functional Assessment Instrument for Perinatal depression (FAI) (Marguerite Schneider, Baron, Davies, Bass, & Lund, 2015); Multidimensional Scale of Perceived Social Support (MSPSS) (Zimet, Dahlem, Zimet, & Farley, 1988); Household Food Insecurity Access scale (HFIAS) (Coates, Swindale, & Bilinsky, 2006); and the Alcohol Use Disorders Identification Test (AUDIT) (Saunders, Aasland, Babor, De la Fuente, & Grant, 1993), as well as the Major Depressive Episode and Suicidality modules of the Mini International Neuropsychiatric Interview 6.0.0 (MINI) (Sheehan et al., 1998).
Birth and child outcome measures at three- and 12-months postpartum included preterm birth or birth complications, Apgar scores, anthropometric measures, duration of breastfeeding, number of postnatal visits, number of immunizations completed, and prevalence of diarrheal disease and respiratory tract infections.
Health care utilization and costs were measured with the Health Care Utilization Questionnaire, adapted from the Client Socio-Demographic and Service Receipt Inventory – European Version (Chisholm, 2000), and the Client Service Receipt Inventory (Beecham & Knapp, 1992). Country specific provider unit costs were obtained (Western Cape Department of Health Annual Report 2014/2015).
All instruments were translated into isiXhosa and independently back-translated into English. Previously translated instruments (EPDS, MINI, HFIAS and WHODAS) were checked by our translation team. We reviewed the performance of all instruments during the pilot phase.
Sample size
We assumed that 40% of the control arm would show a response on HDRS score at three months postpartum, an absolute effect size in the intervention arm of 20% (corresponding to a risk ratio of 1.5), and two-sided testing at α=0.05 and 90% power. Attrition was estimated at 10% based on previous trials in this community (Cooper et al., 2002), and we assumed 5% contamination between the two arms. Based on these assumptions, we estimated that 420 women (210 in each arm) would be required.
Statistical methods
A data management and statistical analysis plan was developed in preparation for the trial implementation, and finalised before the data lockdown. The plan set out the procedures for data quality assurance and quality control (including standard operating procedures), data entry, management and cleaning, and data analysis (including a priori comparisons, interim analyses and Data and Safety Monitoring Board (DSMB) instructions). Data were analysed using STATA version 14 (Statacorp, 2015). All statistical tests were two-sided at α=0.05. Bivariate comparisons employed Fisher’s exact or rank-sum tests, as appropriate. The Benjamini-Hochberg procedure was used to account for multiple comparisons with a false discovery rate of 0.20 (Benjamini & Hochberg, 1995).
The primary analysis was on a modified intention-to-treat population, defined as all participants who did not experience pregnancy loss or infant death during the study period. Analyses were conducted on observed data. Sensitivity analyses were conducted using multiple imputation with chained equations to assess the potential impact of missing data for the primary outcome measure. Secondary analyses focused on the per protocol population, defined as participants who completed all sessions in either arm of the trial.
The primary outcome to assess effectiveness of the psychological treatment was response on the HDRS at three months postpartum (Lund et al., 2014). This was assessed using unadjusted log binomial regression, with risk ratios (RR) reported. Response on the HDRS and secondary outcomes were also assessed in unadjusted models for all three follow-up assessments: all continuous measures were non-normally distributed and so were modelled using negative binomial models. Binary, nominal and count variables were assessed using log binomial, multinomial logistic and Poisson regressions, respectively. Cohen’s d effect size for mean change in HDRS scores at three months postpartum was also calculated (Sullivan & Feinn, 2014).
Socio-economic, clinical, and intervention-related variables were entered in a series of log binomial regressions to identify predictors of response on the HDRS among the intervention arm at each follow-up assessment. Models were adjusted for the number of sessions received at the time of the assessment.
Intervention and health service utilisation costs were calculated to assess the cost-effectiveness of the intervention. The health utilization costs of a task-shared psychological intervention and enhanced usual care were analysed from a societal view that includes both health system, patient and caregiver costs. Costs were calculated for a 1-year time horizon thus not discounted. The South African currency (Rand) was converted to the US Dollar (US$) using the average exchange rate for the period 2014-2016 (US$1 =12.77 ZAR) for all costs. Multivariate analyses with negative binomial models were used to calculate mean annual costs, opportunity (time) costs, health system costs and mean clinical outcomes. When the residuals of the regression model were non-normally distributed, bootstrap methods were used. The relationships between costs and outcomes were assessed using further regression models for the main clinical outcome measures.
Ethical procedures
The trial was subjected to rigorous oversight by the Data and Safety Monitoring Board (DSMB), appointed by the National Institute for Mental Health, which conducted reviews of the trial protocol and manual of operating procedures, together with independent clinical site monitoring visits and ethical oversight. The DSMB and Human Research Ethics Committee (HREC) approved standardised protocols to manage risk of suicide and self-harm. Participants who were at high risk for suicide were referred to the psychiatric nurse in the adjacent community health centre facility. All study participants gave written voluntary informed consent to participate; provision was made for illiterate participants. The study protocol was approved by the HREC of the University of Cape Town Faculty of Health Sciences (HREC Ref: 226/2011) and the DSMB.
Results
Participant flow
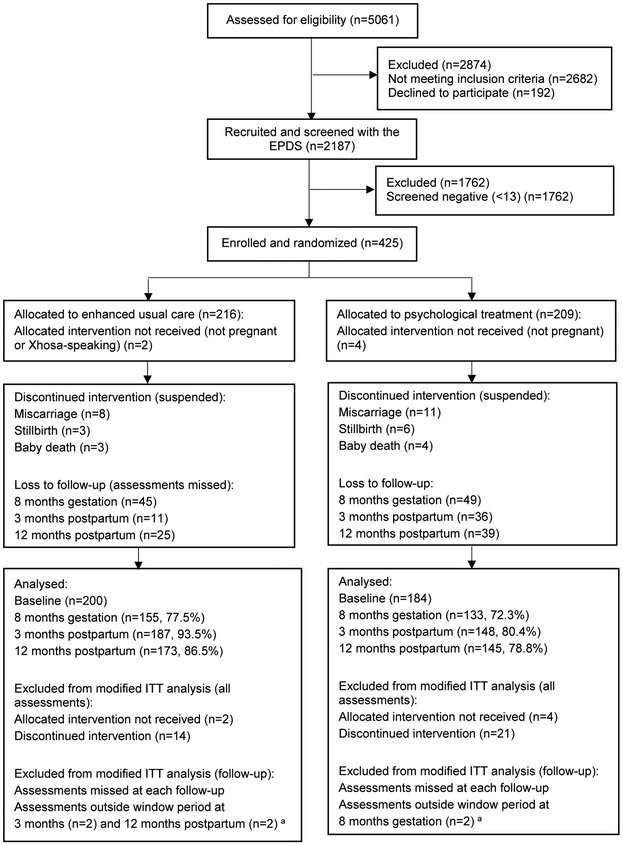
Participants were enrolled from October 2013 to October 2014, and followed up until May 2016. A total of 5061 women were approached and more than half were ineligible or refused to participate (n=2874, 56.8%), with the main reason for ineligibility relating to area of residence (Figure 1). Of the 2187 eligible women who were screened with the EPDS, 425 (19.4%) screened positive and were enrolled into the study. Altogether, 209 participants were randomised into the intervention arm, and 216 randomised into the control arm. Baseline demographic characteristics are presented in Table 1. No differences were noted between the two arms in baseline demographic, socio-economic, or clinical measures, in either recruitment sites (Supplementary Table 1).
Figure 1. Consort chart of recruitment and follow-up process.
a Defined as over 75 days before or 75 days after due date of assessment
Table 1.
Baseline characteristics of the participants recruited in the randomised controlled trial
| Enhanced usual care (N=216) |
Psychological treatment (N=209) |
|
|---|---|---|
| Age | 27 (23-30) | 27 (23-32) |
| Gestation (weeks) | 18 (15-22) | 18 (14-22) |
| Marital status | ||
| Lives with partner | 71 (32.9) | 77 (36.8) |
| Doesn’t live with a partner | 145 (67.1) | 132 (63.2) |
| Educational level | ||
| Grade 0-11 | 132 (61.1) | 119 (56.9) |
| Grade 12 or more | 84 (38.9) | 90 (43.1) |
| Employment | ||
| Employed | 95 (44.0) | 98 (46.9) |
| Unemployed/studying | 121 (56.0) | 111 (53.1) |
| Economic status | ||
| Lowest wealth | 42 (19.4) | 43 (20.6) |
| Low wealth | 46 (21.3) | 39 (16.7 |
| Middle wealth | 40 (18.5) | 45 (21.5) |
| High wealth | 45 (20.8) | 40 (19.1) |
| Highest wealth | 43 (19.9) | 42 (20.1) |
| Food status | ||
| Food secure | 52 (24.1) | 55 (26.3) |
| Mildly food insecure | 59 (27.3) | 38 (18.2) |
| Moderately food insecure | 47 (21.8) | 47 (22.5) |
| Severely food insecure | 58 (26.9) | 69 (33.0) |
| HIV status a | ||
| Negative | 140 (67.6) | 142 (69.6) |
| Positive | 67 (32.4) | 62 (30.4) |
| MINI diagnosis | ||
| Not depressed | 131 (60.7) | 118 (56.5) |
| Depressed | 85 (39.3) | 91 (43.5) |
| Suicide risk | ||
| Low | 183 (84.7) | 168 (80.4) |
| High | 33 (15.3) | 41 (19.6) |
| HDRS score | 15 (12-19) | 15 (12-18) |
| EPDS score | 17 (14-19) | 17 (15-20) |
| AUDIT score | 0 (0-6.5) | 0 (0-5) |
| WHODAS score | 27.8 (15.3-41.7) | 27.8 (13.9-41.7) |
| FAI score | 0.7 (0.4-1.1) | 0.7 (0.4-1.3) |
| MSPSS score | 61 (50-69) | 60 (53-68) |
| Support from family | 22 (17-24) | 22 (18-24) |
| Support from special person | 24 (22-25) | 24 (20-24) |
| Support from friends | 17 (10.5-21) | 17 (12-21) |
Median (IQR) or N (%) are presented; AUDIT = Alcohol Use Disorder Identification Test; EPDS = Edinburgh Postnatal Depression Scale; FAI = Functional Assessment Instrument; HDRS = Hamilton Depression Rating Scale; IQR = Interquartile range; MSPSS = Multidimensional Scale of Perceived Social Support; WHODAS = WHO Disability Assessment Schedule;
Data available for 411 participants only
After randomisation, six participants (1.4%) were excluded from the study as they were found to not fit some inclusion criteria (not pregnant and not isiXhosa-speaking). The allocated intervention and assessments were discontinued for a further 35 participants (8.2%) across both arms, due to miscarriage or baby death. The analysis was conducted among the remaining 384 participants recruited, referred to as the “modified intention-to-treat” population (200 and 184 participants in the control and intervention arms, respectively). These participants did not differ from those excluded from the analysis, besides reporting lower baseline levels of functioning (Supplementary Table 2).
Follow-up rates at three months postpartum were 93.5% and 80.4% in the control and intervention arms, respectively. There were no notable differences in baseline characteristics between these participants and those lost to follow-up in either arm.
Of the 184 women assigned to the intervention arm, 147 (80.0%) received at least one session and 98 (53.3%) completed all six sessions. The median duration of sessions was 40 minutes (interquartile range (IQR)=30-50); the first counselling session was conducted at a median of 20 days after randomisation (IQR=10-49) and the intervention lasted for a median of 2.5 months (IQR=0.9-4.2). A total of 51 participants (27.7%) received some or all sessions in the postnatal period. Unadjusted models indicated that none of the socio-demographic, clinical, or intervention characteristics were associated with number of counselling sessions attended (Supplementary Table 3).
Of the 200 participants assigned to the control arm, 187 (93.5%) received all three phone calls. The first call was conducted on average 13 days (IQR=6-30) after enrolment; 69 (34.8%) participants received some or all calls in the postnatal period. The mean duration of phone calls was 8 minutes (IQR=5-10). There were no significant harms associated with the intervention, and no notable differences between the two arms in the number of adverse events.
Primary objective: effectiveness
The unadjusted log binomial regressions indicated no significant difference in the proportion of participants who showed a response on the HDRS at three months postpartum between the intervention (n=82, 55.4%) and the control arm (n=89, 47.6%; RR=1.16; 95% Confidence Interval (CI) 0.94, 1.43) (Table 2). Response at 12 months postpartum was also not different between the two arms.
Table 2.
Unadjusted analyses of HDRS outcomes among modified intention-to-treat and per-protocol populations
| Enhanced usual care |
Psychological treatment |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) or N (%) |
Mean change (95%CI) from BL |
N | Mean (SD) or N (%) |
Mean change (95%CI) from BL |
RR (95%CI) | p | |
| Modified intention-to-treat population | ||||||||
| HDRS response | ||||||||
| 8 months gestation | 155 | 34 (21.9) | - | 133 | 32 (24.1) | - | 1.10 (0.72 to 1.68) | 0.669 |
| 3 months postpartum | 187 | 89 (47.6) | - | 148 | 82 (55.4) | - | 1.16 (0.94 to 1.43) | 0.153 |
| 12 months postpartum | 173 | 71 (41.0) | - | 145 | 75 (51.7) | - | 1.26 (0.99 to 1.60) | 0.057 |
| HDRS Recovery | ||||||||
| 12 months postpartum | 173 | 25 (14.5) | - | 144 | 31 (21.5) | - | 1.49 (0.92 to 2.40) | 0.102 |
| HDRS score a | ||||||||
| Baseline | 200 | 15.5 (4.69) | - | 184 | 15.7 (4.82) | - | - | |
| 8 months gestation | 155 | 12.8 (4.53) | −2.74 (−3.56 to −1.92) | 133 | 12.6 (5.51) | −2.92 (−3.86 to −1.98) | 0.97 (0.86 to 1.09) | 0.617 |
| 3 months postpartum | 187 | 10.1 (4.97) | −5.27 (−6.10 to −4.45) | 148 | 9.1 (4.58) | −6.46 (−7.37 to −5.55) | 0.89 (0.79 to 1.00) | 0.053 b |
| 12 months postpartum | 173 | 10.8 (5.07) | −4.71 (−5.65 to −3.76) | 145 | 9.5 (4.32) | −6.01 (−6.95 to −5.08) | 0.87 (0.78 to 0.99) | 0.028 c |
| Per protocol population | ||||||||
| HDRS response | ||||||||
| 8 months gestation | 83 | 17 (20.5) | - | 30 | 6 (20.0) | - | 0.98 (0.43 to 2.24) | 0.955 |
| 3 months postpartum | 151 | 76 (50.3) | - | 82 | 46 (56.1) | - | 1.11 (0.87 to 1.43) | 0.392 |
| 12 months postpartum | 163 | 68 (41.7) | - | 91 | 44 (48.4) | - | 1.16 (0.88 to 1.53) | 0.300 |
| HDRS Recovery | ||||||||
| 12 months postpartum | 90 | 14 (15.6) | - | 34 | 7 (20.6) | - | 1.32 (0.58 to 3.00) | 0.501 |
| HDRS score a | ||||||||
| 8 months gestation | 83 | 12.8 (4.83) | −2.81 (−3.96 to −1.65) | 30 | 12.3 (5.41) | −2.40 (−4.14 to −0.66) | 1.01 (0.80 to 1.26) | 0.964 |
| 3 months postpartum | 151 | 10.0 (4.94) | −5.32 (−6.27 to −4.38) | 82 | 8.5 (4.07) | −6.64 (−7.78 to −5.51) | 0.88 (0.73 to 1.06) | 0.187 |
| 12 months postpartum | 163 | 10.8 (5.07) | −4.74 (−5.73 to −3.76) | 91 | 9.1 (3.82) | −6.02 (−7.19 to −4.85) | 0.88 (0.73 to 1.06) | 0.167 |
BL=Baseline; CI = confidence intervals; HDRS=Hamilton Depression Rating Scale; IRR=incidence rate ratio; RR=risk ratio; SD= standard deviation;
Incident rate ratio (95%CI) reported;
Cohen’s d=0.21
p-value no longer significant after using the Benjamini-Hochberg procedure to adjust for multiple comparisons.
The unadjusted negative binomial regressions indicated that there were no notable differences in the mean change in HDRS scores from baseline to three or 12 months postpartum. Among participants who scored above seven on the HDRS at baseline, there was no notable difference in recovery at 12 months postpartum between the two arms (Table 2). The sensitivity analysis using multiple imputation yielded essentially the same results (not presented here).
Differences in secondary outcomes between both arms at three months postpartum are shown in Table 3, and at eight months gestation and 12 months postpartum in Supplementary Table 4. Change in mean EPDS scores from baseline to three months postpartum was significantly greater in the intervention (mean=−10.0, 95%CI −11.04, −9.06) compared to the control arm (mean=−7.6, 95%CI −8.40, −6.75; RR=0.78; 95%CI 0.67, 0.91). A similar difference was found at 12 months postpartum (intervention: mean=−9.8, 95%CI −10.79, −8.82; control: mean=−7.4, 95%CI −8.34, −6.49; RR=0.79; 95%CI 0.68, 0.92). There were no other differences between the two arms at eight months gestation, three months, or 12 months postpartum. Among the per protocol population, response, recovery, and mean change in HDRS scores did not differ between the two arms (Table 2).
Table 3.
Unadjusted analyses of secondary outcomes at 3 months postpartum in the modified intention-to-treat population
| EUC (N=187) | PSY (N=148) | RR (95%CI) | p | |
|---|---|---|---|---|
| Maternal outcomes | ||||
| MINI diagnosis | ||||
| Not depressed | 153 (81.8) | 125 (84.5) | ref | - |
| Depressed | 34 (18.2) | 23 (15.5) | 0.78 (0.45 to 1.33) | 0.358 |
| Suicide risk | ||||
| Low | 181 (96.8) | 145 (98.0) | - | - |
| High | 6 (3.2) | 3 (2.0) | 0.49 (0.12 to 2.05) | 0.326 |
| EPDS score a | 9.5 (5.70) | 7.6 (5.20) | 0.78 (0.67 to (0.91) | 0.001 c |
| WHODAS score a | 20.8 (14.16) | 19.0 (13.37) | 0.93 (0.74 to 1.17) | 0.554 |
| FAI score a | 0.6 (0.47) | 0.6 (0.43) | 0.84 (0.60 to 1.19) | 0.328 |
| AUDIT score a | 2.1 (4.14) | 2.1 (4.17) | 0.90 (0.45 to 1.81) | 0.766 |
| MSPSS score a | 57.6 (12.66) | 59.7 (11.53) | 1.03 (0.96 to 1.10) | 0.414 |
| Food status b | ||||
| Food secure | 26 (13.9) | 24 (16.2) | ref | - |
| Mildly food insecure | 38 (20.3) | 37 (25.0) | 2.02 (0.80 to 5.08) | 0.135 |
| Moderately food insecure | 54 (28.9) | 38 (25.7) | 0.95 (0.38 to 2.37) | 0.917 |
| Severely food insecure | 69 (36.9) | 49 (33.1) | 0.79 (0.33 to 1.86) | 0.583 |
| Economic status b | ||||
| Highest wealth | 30 (16.0) | 35 (23.7) | ref | - |
| High wealth | 29 (15.5) | 22 (14.9) | 0.66 (0.25 to 1.75) | 0.405 |
| Middle wealth | 50 (26.7) | 33 (22.3) | 0.48 (0.19 to 1.21) | 0.118 |
| Low wealth | 54 (28.9) | 30 (20.3) | 0.51 (0.20 to 1.28) | 0.150 |
| Lowest wealth | 24 (12.8) | 28 (19.9) | 0.94 (0.36 to 2.49) | 0.904 |
| Birth outcomes | ||||
| Timing of delivery b | ||||
| At term | 135 (72.2) | 116 (78.4) | ref | - |
| Preterm (live birth) | 24 (12.8) | 18 (12.2) | 0.87 (0.45 to 1.69) | 0.686 |
| Over term | 28 (15.0) | 14 (9.5) | 0.58 (0.29 to 1.16) | 0.123 |
| Problematic delivery | ||||
| No | 161 (86.1) | 119 (80.4) | ref | - |
| Yes | 26 (13.9) | 29 (19.6) | 1.41 (0.87 to 2.29) | 0.164 |
| Apgar scores a | 9.7 (0.70) | 9.8 (0.53) | 1.01 (0.94 to 1.09) | 0.802 |
| Birthweight (kg) a | 3.1 (0.54) | 3.1 (0.46) | 0.99 (0.88 to 1.13) | 0.919 |
| Birth height (cm) a | 49.1 (3.84) | 49.2 (3.48) | 1.00 (0.97 to 1.03) | 0.933 |
| Head circumference (cm) a | 33.8 (1.70) | 33.9 (1.68) | 1.00 (0.96 to 1.04) | 0.896 |
| Child outcomes | ||||
| Weight (kg) a | 5.9 (1.06) | 6.1 (1.00) | 1.03 (0.94 to 1.12) | 0.589 |
| Height (cm) a` | 59.4 (6.17) | 59.8 (5.59) | 1.00 (0.98 to 1.04) | 0.619 |
| Head circumference (cm) a | 42.8 (4.70) | 43.9 (5.93) | 1.03 (0.99 to 1.06) | 0.163 |
| Number of postnatal visits a | 2.3 (0.90) | 2.3 (0.87) | 0.97 (0.84 to 1.12) | 0.690 |
| Number of immunisations completed a | 8.6 (2.15) | 8.7 (1.93) | 1.02 (0.94 to 1.10) | 0.664 |
| Duration of breastfeeding (weeks) a | 8.6 (4.93) | 9.5 (4.58) | 1.10 (0.95 to 1.28) | 0.199 |
| Suffered from (in past 2 weeks): | ||||
| Diarrhoea | 35 (18.7) | 38 (25.7) | 1.37 (0.91 to 2.06) | 0.126 |
| Difficulty breathing | 57 (30.5) | 40 (27.0) | 0.89 (0.63 to 1.25) | 0.491 |
| Cough | 87 (46.5) | 77 (52.0) | 1.12 (0.90 to 1.39) | 0.315 |
| Admitted to hospital for difficult breathing | 16 (14.7) | 8 (9.4) | 0.64 (0.29 to 1.43) | 0.276 |
Means (SD) or N (%) are reported; AUDIT = Alcohol Use Disorder Identification Test; CI = confidence intervals; EPDS = Edinburgh Postnatal Depression Scale; EUC= Enhanced usual care; FAI = Functional Assessment Instrument; HDRS = Hamilton Depression Rating Scale; MSPSS = Multidimensional Scale of Perceived Social Support; PSY = Psychological treatment; RR= risk ratios; WHODAS = WHO Disability Assessment Schedule;
incident rate ratio reported;
relative risk ratio reported;
p-value still significant after using the Benjamini-Hochberg procedure to adjust for multiple comparisons.
Secondary objective: predictors of response
Table 4 presents the results of models adjusted for the number of sessions attended, to identify baseline factors associated with response on the HDRS at three months postpartum. The risk of showing a response increased by 3% with each point increase in the baseline HDRS score (adjusted RR (aRR)=1.03; 95%CI 1.01, 1.05). The risk increased by 10% at eight months gestation (aRR=1.10; 95%CI 1.08, 1.12) and by 4% at 12 months postpartum (aRR=1.04; 95%CI 1.03, 1.06) (Supplementary Table 5). No other variables were associated with response.
Table 4.
Adjusted analysis of predictors of response on the HDRS at 3 months postpartum in the modified intention-to-treat population (intervention arm only)
| No (N=66) | Yes (N=82) | aRR (95%CI) | p | |
|---|---|---|---|---|
| Baseline socio-demographics | ||||
| Age (at baseline) | 27.7 (5.94) | 27.9 (5.73) | 1.00 (0.98 to 1.03) | 0.820 |
| Education (at baseline) | ||||
| Grade 0-11 | 35 (53.0) | 45 (54.9) | ref | - |
| Grade 12 or more | 31 (47.0) | 37 (45.1) | 0.97 (0.72 to 1.30) | 0.830 |
| Marital status | ||||
| Lives with partner | 25 (37.9) | 27 (32.9) | ref | - |
| Doesn’t live with a partner | 41 (62.1) | 55 (67.1) | 1.11 (0.81 to 1.51) | 0.527 |
| Employment | ||||
| Employed | 29 (43.9) | 43 (52.4) | ref | - |
| Unemployed/studying | 37 (56.1) | 39 (47.6) | 0.86 (0.64 to 1.15) | 0.299 |
| Socio-economic status | ||||
| Lowest wealth | 13 (19.7) | 18 (22.0) | ref | - |
| Low wealth | 14 (21.2) | 19 (23.2) | 0.98 (0.64 to 1.50) | 0.929 |
| Middle wealth | 15 (22.7) | 15 (18.3) | 0.85 (0.53 to 1.37) | 0.508 |
| High wealth | 14 (21.2) | 16 (19.5) | 0.92 (0.58 to 1.44) | 0.705 |
| Highest wealth | 10 (15.2) | 14 (17.1) | 1.00 (0.63 to 1.57) | 0.985 |
| Baseline clinical characteristics | ||||
| HDRS score | 14.4 (4.73) | 16.5 (4.62) | 1.03 (1.01 to 1.05) | 0.001 c |
| EPDS score | 17.4 (3.62) | 17.9 (3.81) | 1.01 (0.98 to 1.05) | 0.445 |
| AUDIT score | 4.3 (6.70) | 3.7 (6.29) | 0.99 (0.97 to 1.02) | 0.584 |
| WHODAS score | 27.9 (17.64) | 29.6 (17.86) | 1.00 (0.99 to 1.01) | 0.530 |
| FAI score | 0.8 (0.54) | 0.8 (0.50) | 1.13 (0.87 to 1.48) | 0.359 |
| MSPSS score | 59.3 (10.30) | 59.9 (12.39) | 1.00 (0.99 to 1.02) | 0.666 |
| MINI diagnosis | ||||
| Not depressed | 34 (51.5) | 50 (61.0) | ref | - |
| Depressed | 32 (48.5) | 32 (39.0) | 0.84 (0.62 to 1.14) | 0.262 |
| Suicide risk | ||||
| Low | 52 (78.8) | 66 (80.5) | ref | - |
| High | 14 (21.2) | 16 (19.5) | 0.96 (0.66 to 1.39) | 0.832 |
| Treatment correlates | ||||
| Number of sessions a | 4.0 (2.56) | 4.2 (2.36) | 1.01 (0.95 to 1.07) | 0.768 |
| Recruitment clinic b | ||||
| CHC 1 | 46 (50.6) | 45 (49.4) | ref | - |
| CHC 2 | 20 (35.1) | 37 (64.9) | 1.31 (0.99 to 1.74) | 0.061 |
| Counsellors b | ||||
| Counsellor 1 | 12 (46.2) | 14 (53.8) | ref | - |
| Counsellor 2 | 13 (50.0) | 13 (50.0) | 0.93 (0.55 to 1.58) | 0.796 |
| Counsellor 3 | 16 (59.3) | 11 (40.7) | 0.75 (0.43 to 1.35) | 0.345 |
| Counsellor 4 | 4 (21.1) | 15 (79.0) | 1.45 (0.93 to 2.24) | 0.099 |
| Counsellor 5 | 7 (38.9) | 11 (61.1) | 1.12 (0.67 to 1.88) | 0.666 |
| Counsellor 6 | 7 (30.4) | 16 (69.6) | 1.31 (0.84 to 2.04) | 0.241 |
Means (SD) or N (%) are reported; aRR= risk ratio adjusted for number of sessions attended at the time of assessment; AUDIT = Alcohol Use Disorder Identification Test; CHC= Community Health Centre; CI = confidence intervals; EPDS = Edinburgh Postnatal Depression Scale; FAI = Functional Assessment Instrument; HDRS = Hamilton Depression Rating Scale; MSPSS = Multidimensional Scale of Perceived Social Support; WHODAS = WHO Disability Assessment Schedule;
univariate analysis conducted;
percentages are rows;
p-value still significant after using the Benjamini-Hochberg procedure to adjust for multiple comparisons.
There were no significant differences in participants’ unit costs and service utilization patterns between the two arms, or in mean costs per visit to a healthcare provider. However, the psychological treatment was more costly per participant per year (US$117.16, 95%CI 94.05, 140.26) compared to EUC (US$85.30, 95%CI 55.98, 114.62; p=0.04) (Supplementary Tables 6, 7, and 8). Because no notable differences were found in mean HDRS scores at 3 months postpartum between the two arms, this resulted in negative incremental cost-effectiveness ratios for the intervention arm in relation to patient, health system and total costs.
Discussion
Results of this RCT indicate that a task-sharing psychological intervention was neither effective nor cost-effective in treating perinatal depression in an adverse low-resource South African setting. The effect size of the intervention in our study was substantially smaller than that found in other trials of task-sharing psychological treatments for perinatal common mental disorders in LMIC (Clarke et al., 2013; Gureje et al., 2019; Rahman et al., 2013).
There are several potential explanations for our findings. First, despite our efforts to develop a targeted psychological treatment, the format and content of the intervention may not have been optimal. In seeking to develop a treatment that could be realistically scaled up within our setting, we designed an intervention comprising only six sessions, and covering several aspects including problem solving, behavioural activation and cognitive reframing. In comparison, Rahman et al. (2008)’s highly effective Thinking Healthy Program in Pakistan comprised 16 sessions and focused mostly on cognitive reframing. Our intervention may therefore have covered too many complex components in too few sessions, leaving participants with insufficient time to practise their newly acquired skills. In addition, the adverse social and economic conditions experienced by the participants in this trial (including high levels of food insecurity, intimate partner violence and poverty) may have over-ridden the potential benefit of this brief psychological intervention.
Second, the low dose of the intervention may have been inadequate to show an effect, as only 53% of women in the intervention arm completed all six sessions. Nevertheless, the per protocol analysis also yielded a non-significant difference between the arms, reducing the likelihood of this ‘dose’ explanation.
Third, the delivery of the intervention may not have been optimal. Our previous process evaluation of the intervention delivery indicated a moderately good fidelity rating of 62.8% (range 55-70%) (Munodawafa et al., 2017). This indicates that there was perhaps room for improvement in the duration or quality of the CHW training and supervision.
Fourth, the small effect size may be partially explained by measurement factors. It is important to note that significantly greater reductions in EPDS scores were found among women in the intervention group compared to the control group at three and 12 months postpartum. The lack of effect of the intervention could thus be a result of the different measures used to recruit women in the trial and to assess their improvement over time. Indeed, although all participants recruited into the trial were distressed, 60% screened below the cut-off of 17 on the HDRS suggesting moderate depressive symptoms (Zimmerman, Martinez, Young, Chelminski, & Dalrymple, 2013), and more than 50% did not fulfil criteria for major depression on the MINI. The lack of effect of the intervention on the HDRS may therefore reflect floor effects, which is consistent with the finding that the intervention had a greater impact on women with higher baseline HDRS scores, a finding also noted in pharmacological trials (Kilts, Wade, Andersen, & Schlaepfer, 2009).
Fifth, the EUC intervention may have been effective in reducing symptoms, above and beyond what would be expected given the natural course of depressive symptoms during the perinatal phase (Baron, Bass, Murray, Schneider, & Lund, 2017). Concerns about suicide risk and safety of all participants meant that we instituted an EUC protocol that provided more psychological support than was available in routine settings in Khayelitsha, which is usually limited to care for perinatal women who are actively suicidal or who experience psychosis. Thus, the EUC may have been a substantial intervention in its own right, and the psychological intervention may have appeared to be less effective than it actually was in relation to real world usual care. This is a phenomenon that has commonly been reported in RCTs assessing behavioural interventions (Gold et al., 2017), and was also evident in a recent trial of a psychological treatment for perinatal depression in Nigeria (Gureje et al., 2019).
Despite these limitations, a robust evaluation design was used in this study, with relatively large sample size, good follow-up rates, and a range of validated measures covering health, social, and economic outcomes. It was beyond the scope of this study to investigate the mediating factors contributing to improved outcomes over time. However, given the trial findings and the range of measures assessed, it is essential that further analyses be conducted to gain a better understanding of the potential mechanisms of change in task-sharing psychological therapies in LMIC. We are planning further analysis to qualitatively explore themes from transcripts of the counseling sessions (including fidelity and therapeutic alliance), and quantitatively examine the effect of potential mediating factors including fidelity, therapeutic alliance and dose on the primary outcome.
With growing policy attention to global mental health, there is increasing pressure to deliver interventions that are less intensive and less costly. The findings of this study give cause for reflection on the strategy of task-sharing in low-resource settings and highlight the need to adequately resource psychological interventions, particularly in the context of task-sharing. More specifically, the study demonstrates the importance of carefully considering the content of the intervention, selection and recruitment of interventionists, as well as linking the content and methods of the intervention with the skill level of the interventionists.
The study also prompts reflection on the design of control group interventions in trials addressing mental disorders in LMIC. The higher adherence to a phone-based approach to engagement (as administered to the control group) may be more feasible in this population. However, we would be cautious to advocate that this is an effective approach given that only 47.6% of women showed a clinical response in the control group.
In circumstances of social and economic adversity, depression is intrinsically linked to broader societal factors (Lund et al., 2018), and it is important to both address the social determinants of depression and treat its symptoms. Our psychological intervention was developed specifically to strengthen perinatal women’s resilience and mental health by providing them with problem solving skills to cope with daily stressors including poverty, HIV and intimate partner violence (Lund et al., 2018; Schneider, Baron, Davies, Munodawafa, & Lund, 2018). However, future studies assessing interventions for depression in adverse low-resource settings should consider including both a psychosocial component that addresses symptoms of depression, and components to address the social determinants of depression more directly, for example through poverty alleviation interventions such as cash transfer programmes or interventions to reduce gender-based violence.
Conclusions
Further research is needed on developing short but effective perinatal mental health interventions, on implementation challenges in the delivery of these interventions in low-resource contexts, and on difficult choices regarding control conditions for RCTs in these settings.
Supplementary Material
Highlights.
In South Africa, prevalence of perinatal depression ranges between 21.5 and 34.7%
Task-shared psychosocial interventions for perinatal depression can be effective
Can such interventions be effective for routine use in low-resource settings?
A brief task-shared psychological treatment was not effective in South Africa
Less intense interventions in low resource routine care might dilute effectiveness
Acknowledgements
This research was conducted as part of the Africa Focus on Intervention Research for Mental Health (AFFIRM). The NIMH DSMB contributed to the quality assurance and fidelity of the research, as well as protocol approval, ethical approval, and data collection recommendations. We are particularly grateful for the technical support of Pamela Collins, Adam Haim, LeShawndra Price and Beverly Pringle from the NIMH in the process of designing and implementing this study. We would like to thank Dan Chisholm for work in support of health economic aspects of the trial. We are grateful to Maia Lesosky for her review of the statistical analysis. We would like to thank the fieldworkers and counsellors for their dedication and hard work. We are grateful to the study participants for generously sharing their time and experiences during the conduct of this study. GT is supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South London at King’s College London NHS Foundation Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. GT acknowledges financial support from the Department of Health via the National Institute for Health Research (NIHR) Biomedical Research Centre and Dementia Unit awarded to South London and Maudsley NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust. GT is supported by the European Union Seventh Framework Programme (FP7/2007-2013) Emerald project. GT also receives support from the National Institute of Mental Health of the National Institutes of Health under award number R01MH100470 (Cobalt study). GT is also supported by the UK Medical Research Council in relation the Emilia and Indigo Partnership awards. MT is a Lead Investigator in the Centre of Excellence in Human Development, University Witwatersrand, South Africa. AA, CH, CL, IP, MP and GT are also funded through the ASSET research programme, supported by the UK's National Institute of Health Research (NIHR) using Official Development Assistance (ODA) funding (NIHR Global Health Research Unit on Health Systems Strengthening in Sub-Saharan Africa at King's College London (16/136/54)). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care, England. CH receives support from AMARI as part of the DELTAS Africa Initiative [DEL-15-01].
Funding
This work was supported by the National Institute of Mental Health of the National Institutes of Health [Award Number U19MH095699]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Prof Stein is supported by the Medical Research Council of South Africa. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Conflicts of interests
None.
Data sharing
The data collected for this study is available from the corresponding author upon reasonable request. Deidentified participant data will be made publicly available from March 2020.
References
- Azale T, Fekadu A, & Hanlon C (2016). Treatment gap and help-seeking for postpartum depression in a rural African setting. BMC Psychiatry, 16, 196. doi: 10.1186/s12888-016-0892-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron E, Bass J, Murray SM, Schneider M, & Lund C (2017). A systematic review of growth curve mixture modelling literature investigating trajectories of perinatal depressive symptoms and associated risk factors. Journal of Affective Disorders, 223, 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecham J, & Knapp M (1992). Costing psychiatric interventions In Thornicroft G, Brewin C, & Wing J (Eds.), Measuring Mental Health Needs. London: Gaskell. [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 289–300. [Google Scholar]
- Chisholm D (2000). Client socio-demographic and service receipt inventory - European version: development of an instrument for international research: EPSILON study 5. Br J Psychiatry, 177. doi: 10.1192/bjp.177.39.s28 [DOI] [PubMed] [Google Scholar]
- Clarke K, King M, & Prost A (2013). Psychosocial interventions for perinatal common mental disorders delivered by providers who are not mental health specialists in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med, 10(10), e1001541. doi: 10.1371/journal.pmed.1001541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates J, Swindale A, & Bilinsky P (2006). Household Food Insecurity Access Scale (HFIAS) for Measurement of Food Access: Indicator Guide. Washington DC: Fanta III. [Google Scholar]
- Cooper P, Landman M, Tomlinson M, Molteno C, Swartz L, & Murray L (2002). Impact of mother-infant intervention in an indigent peri-urban South African context. British Journal of Psychiatry, 180, 76–81. [DOI] [PubMed] [Google Scholar]
- Cooper P, Tomlinson M, Swartz L, Woolgar M, Murray L, & Molteno C (1999). Post-partum depression and the mother-infant relationship in a South African peri-urban settlement. Br J Psychiatry, 175. doi: 10.1192/bjp.175.6.554 [DOI] [PubMed] [Google Scholar]
- Cox JL, Holden JM, & Sagovsky R (1987). Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry, 150. doi: 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- Davies T, Baron E, Schneider M, & Lund C (Under review). Adaptation and validation of a non-clinician administered version of the Hamilton Depression Rating Scale. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies T, Schneider M, Nyatsanza M, & Lund C (2016). “The sun has set even though it is morning”: Experiences and explanations of perinatal depression in an urban township, Cape Town. Transcultural Psychiatry, 53(3), 286–312. doi: 10.1177/1363461516632389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruin G, Swartz L, Tomlinson M, Cooper P, & Molteno C (2004). The factor structure of the Edinburgh Postnatal Depression scale in a South African peri-urban settlement. South African Journal of Psychology, 34, 113–121. [Google Scholar]
- Gelaye B, Rondon M, Araya R, & Williams MA (2016). Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. The lancet. Psychiatry, 3(10), 973–982. doi: 10.1016/S2215-0366(16)30284-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold SM, Enck P, Hasselmann H, Friede T, Hegerl U, Mohr DC, & Otte C (2017). Control conditions for randomised trials of behavioural interventions in psychiatry: a decision framework. The Lancet Psychiatry, 4(9), 725–732. [DOI] [PubMed] [Google Scholar]
- Gureje O, Oladeji BD, Montgomery AA, Araya R, Bello T, Chisholm D, … Zelkowitz P (2019). High- versus low-intensity interventions for perinatal depression delivered by non-specialist primary maternal care providers in Nigeria: cluster randomised controlled trial (the EXPONATE trial). Br J Psychiatry, 1–8. doi: 10.1192/bjp.2019.4 [DOI] [PubMed] [Google Scholar]
- Hartley M, Tomlinson M, Greco E, Comulada WS, Stewart J, le Roux I, … Rotheram-Borus MJ (2011). Depressed mood in pregnancy: prevalence and correlates in two Cape Town peri-urban settlements. Reprod Health, 8, 9. doi: 10.1186/1742-4755-8-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuma R, Minas H, van Ginneken N, dal Poz M, Desiraju K, Morris J, … Scheffler R (2011). Human resources for mental health care: current situation and strategies for action. Lancet, 378. doi: 10.1016/s0140-6736(11)61093-3 [DOI] [PubMed] [Google Scholar]
- Kilts CD, Wade AG, Andersen HF, & Schlaepfer TE (2009). Baseline severity of depression predicts antidepressant drug response relative to escitalopram. Expert Opinion on Pharmacotherapy, 10(6), 927–936. [DOI] [PubMed] [Google Scholar]
- Lawrie T, Hofmeyr G, De Jager M, & Berk M (1998). Validation of the Edinburgh Postnatal Depression Scale on a cohort of South African women. South African Medical Journal, 88, 1340–1344. [PubMed] [Google Scholar]
- Lund C, Brooke-Sumner C, Baingana F, Baron EC, Breuer E, Chandra P, … Saxena S (2018). Social determinants of mental disorders and the Sustainable Development Goals: a systematic review of reviews. Lancet Psychiatry, 5(4), 357–369. doi: 10.1016/S2215-0366(18)30060-9 [DOI] [PubMed] [Google Scholar]
- Lund C, Schneider M, Davies T, Nyatsanza M, Honikman S, Bhana A, … Susser E (2014). Task sharing of a psychological intervention for maternal depression in Khayelitsha, South Africa: study protocol for a randomized controlled trial. Trials, 15(1), 457. doi: 10.1186/1745-6215-15-457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munodawafa M, Lund C, & Schneider M (2017). A process evaluation exploring the lay counsellor experience of delivering a task shared psycho-social intervention for perinatal depression in Khayelitsha, South Africa. BMC Psychiatry, 17(1), 236. doi: 10.1186/s12888-017-1397-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyatsanza M, Schneider M, Davies T, & Lund C (2016). Filling the treatment gap: developing a task sharing counselling intervention for perinatal depression in Khayelitsha, South Africa. BMC Psychiatry, 16(1), 164. doi: 10.1186/s12888-016-0873-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Fisher J, Bower P, Luchters S, Tran T, Yasamy MT, … Waheed W (2013). Interventions for common perinatal mental disorders in women in low- and middle-income countries: a systematic review and meta-analysis. Bull World Health Organ, 91(8), 593–601I. doi: 10.2471/BLT.12.109819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Iqbal Z, Bunn J, Lovel H, & Harrington R (2004). Impact of Maternal Depression on Infant Nutritional Status and Illness: A Cohort Study. Archives of General Psychiatry, 61, 946–952. [DOI] [PubMed] [Google Scholar]
- Rahman A, Malik A, Sikander S, Roberts C, & Creed F (2008). Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised control trial. Lancet, 372, 902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J, Aasland O, Babor T, De la Fuente J, & Grant M (1993). Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption - II. Addiction, 88. doi: 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- Schneider M, Baron E, Davies T, Bass J, & Lund C (2015). Making assessment locally relevant: measuring functioning for maternal depression in Khayelitsha, Cape Town. Social Psychiatry and Psychiatric Epidemiology, 50(5), 797–806. doi: 10.1007/s00127-014-1003-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Baron E, Davies T, Munodawafa M, & Lund C (2018). Patterns of intimate partner violence among perinatal women with depression symptoms in Khayelitsha, South Africa: a longitudinal analysis. Global Mental Health, 5(e13). doi:doi: 10.1017/gmh.2018.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senturk V, Hanlon C, Medhin G, Dewey M, Araya M, Alem A, … Stewart R (2012). Impact of perinatal somatic and common mental disorder symptoms on functioning in Ethiopian women: the P-MaMiE population-based cohort study. J Affect Disord, 136(3), 340–349. doi: 10.1016/j.jad.2011.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D, Lecrubier Y, Sheehan K, Amorim P, Janavs J, Weiller E, … Dunbar G (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry, 59. [PubMed] [Google Scholar]
- Statacorp. (2015). Stata Statistical Software: Release 14. College Station, TX: Statacorp LP. [Google Scholar]
- Statistics, S. A. (2011). City of Cape Town - 2011 Census Suburb Overview. Cape Town, SA: Strategic Development Information and GIS Department (SDI and GIS). [Google Scholar]
- Sullivan GM, & Feinn R (2014). Using effect size - or why the P value is not enough. Journal of Graduate Medical Education, 4(3), 279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Üstün TB, Kostanjsek N, Chatterji S, & Rehm J (2010). Measuring Health and Disability Manual for WHO Disability Assessment Schedule. Geneva: World Health Organization. [Google Scholar]
- Van Heyningen T, Myer L, Onah MN, Tomlinson M, Field S, & Honikman S (2016). Antenatal depression and adversity in urban South Africa. Journal of Affective Disorders, 203, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Herman A, Stein DJ, Heeringa SG, Jackson PB, Moomal H, & Kessler RC (2008). Prevalence, Service Use and Demographic Correlates of 12-Month Psychiatric Disorders in South Africa: The South African Stress and Health Study. Psychological Medicine, 38(2), 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody CA, Ferrari AJ, Siskind DJ, Whiteford HA, & Harris MG (2017). A systematic review and meta-regression of the prevalence and incidence of perinatal depression. Journal of Affective Disorders, 219, 86–92. doi: 10.1016/j.jad.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Zimet G, Dahlem N, Zimet S, & Farley G (1988). The multidimensional scale of perceived social support. J Pers Assess, 52. doi: 10.1207/s15327752jpa5201_2 [DOI] [Google Scholar]
- Zimmerman M, Martinez JH, Young D, Chelminski I, & Dalrymple K (2013). Severity classification on the Hamilton depression rating scale. Journal of Affective Disorders, 150(2), 384–388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.