Abstract
Background
For women with hormone receptor positive breast cancer, long-term endocrine therapy (ET) can greatly reduce the risk of recurrence, yet adherence is low- particularly among traditionally underserved populations.
Methods
The Carolina Breast Cancer Study oversampled Black and young women (<50 years of age). Participants answered an ET-specific medication adherence questionnaire assessing reasons for non-adherence. We used principal factor analysis to identify latent factors describing ET non-adherence. We then performed multivariable regression to determine clinical and demographic characteristics associated with each ET non-adherence factor.
Results
1,231 women were included in analysis, 59% reported at least one barrier to ET adherence. We identified three latent factors which we defined as: habit - challenges developing medication-taking behavior; tradeoffs - high perceived side effect burden and medication safety concerns; and resource barriers - challenges related to cost or accessibility. Older age (50+) was associated with less reporting of habit (Adjusted Risk Ratio (aRR) 0.54[95% CI: 0.43-0.69] and resource barriers (aRR 0.66[0.43-0.997]), but was not associated with tradeoff barriers. Medicaid-insured women were more likely than privately-insured to report tradeoff (aRR:1.53 [1.10-2.13]) or resource barriers (aRR:4.43[2.49-6.57]). Black race was associated with increased reporting of all factors (habit: aRR 1.29[1.09-1.53]; tradeoffs: 1.32[1.09-1.60], resources: 1.65[1.18-2.30]).
Conclusion
Barriers to ET adherence were described by three distinct factors, and strongly associated with sociodemographic characteristics. Barriers to ET adherence appear inadequately addressed for younger, Black, and publicly-insured breast cancer survivors. These findings underscore the importance of developing multi-faceted, patient-centered interventions that address a diverse range of barriers to ET adherence.
Keywords: Cancer, Oncology, Breast Cancer, Endocrine Therapy, Medication Adherence, Cancer Disparities, Cancer Equity, Factor Analysis
Introduction
For women with Endocrine Receptor positive breast cancer, endocrine therapy (ET) medication taken for five to ten years after completion of primary treatments can reduce the risk of cancer recurrence by up to 50%.1,2 However, even among those who initiate ET, many women struggle to maintain the daily regimen and some discontinue the medication completely, with estimates of non-adherence rates ranging from 20% to 50%.3-5 Breast cancer recurrence is higher among Black women6, young women7 and low-income women;8 ET adherence is also lower in these vulnerable groups.4,9,10
Previous studies have evaluated the extent of ET non-adherence in self-report 4,11,12 or claims data9,13 and have assessed reasons for non-adherence qualitatively.14-16 Qualitative studies highlight a need to improve provider communication, particularly around the benefit of ET in recurrence risk reduction and need to help patients to better manage potential ET side effects.15,16 Quantitative studies have identified a number of reasons for non-adherence and patient characteristics associated with non-adherence3,4,16, but reasons for non-adherence have not been mapped to thematic domains that can be targeted by potential interventions. Further, it is unknown how ET barriers vary for younger, Black, and uninsured or publicly insured women -- groups particularly at risk for ET under-use.4,17
To date, efforts to support ET adherence have largely been focused on educational interventions, and have shown little success.18 Better understanding of the multifaceted reasons for ET non-adherence, particularly among vulnerable groups, may help inform more targeted intervention design. Our analysis, based on a diverse population-based patient cohort, used an ET-specific questionnaire of medication adherence to identify latent factors in ET non-adherence and assess whether non-adherence associated with each factor varies by patient characteristics.
Methods
Data
The Carolina Breast Cancer Study Phase III (CBCS-III), a population-based study, recruited 2,998 women diagnosed with breast cancer in 44 North Carolina counties using rapid case ascertainment between 2008 and 2013. The study was designed to oversample Black women and young (<50) women, with each group making up 50% of the study population (25% of participants were both Black and under 50 years of age).19 After providing written, informed consent, detailed demographic, socioeconomic, and medical history data were collected at baseline through an in-person survey and medical record abstraction. Women completed a second survey by mail, completed an average of 25 months after their initial diagnosis, including questions about reasons for ET non-adherence and the presence of ET-associated side effects.
Inclusion
A total of 2,328 women received the ET questionnaire (546 eligible women had already completed the follow-up questionnaire when the instrument was added); among those who received the ET questionnaire, 2,015 (89%) responded. We restricted our analysis to women with stage I-III cancer who completed the follow-up survey and reported currently taking ET medication at the time of follow-up (n=1,231). Study activities were approved by the University’s Institutional Review Board (IRB #92-0410 and #13-0736).
Measures
We used ET-specific survey questions assessing reasons for ET non-adherence (See online supplemental materials). These questions were based on existing medication adherence intruments20-22 and previous qualitative assessments of reasons for ET non-adherence.15,23,24 Questions were cognitively tested with 12 breast cancer patients and revised iteratively based on patient input. Age at diagnosis, stage at diagnosis, surgery type (breast conserving surgery or mastectomy), and receipt of chemotherapy, radiation, trastuzumab were determined through medical record abstraction. Treatments were not mutually exclusive. Type of ET medication in use at time of survey completion (tamoxifen or aromatase inhibitor) was also determined through medical record abstraction. Participants self-reported source of insurance coverage at diagnosis (baseline). For ease of analysis, insurance was grouped into mutually exclusive categories: women with any private insurance (including women on Medicare with supplemental insurance plans) were categorized as privately insured; those covered by Medicaid (including dual Medicaid/Medicare beneficiaries) were categorized as on Medicaid; those with Medicare but no supplemental insurance were categorized as Medicare; and those who identified no source of public or private insurance were categorized as uninsured. As part of the follow-up questionnaire, women reported whether the decision to begin ET was provider-led, patient-led, made through a shared decision-making process, or whether ET was not discussed prior to initiation.
Race was self-reported at baseline and was dichotomized as Black/African American vs. non-Black/African American. Non-Black/African American was comprised of women who reported Race and Ethnicity as White Non-Hispanic (93%), White Hispanic (3%), and other Race/ethnicity (4%) In keeping with recommendations from the Institute of Medicine27, we report both unadjusted and adjusted analyses and controlled for insurance status but not for other socioeconomic moderators. We conceptualized race in our analysis as a social construct which captures the total effect of Black race on health, including the potential effects of individual disadvantage, structural inequity, and provider bias.28
Analysis
Exploratory Factor Analysis
To identify salient domains of ET non-adherence, questions assessing reasons for non-adherence were first evaluated using exploratory principal factor analysis. To allow for correlations between factors, an oblique rotation (promax) was used in the calculations of the factor solution.29 We identified unique factors through visual assessment of the scree plot as well as interpretability of the factors. Once the number of factors was identified, factor loadings were used to identify the items with highest association within each latent factor (factor loading >0.4). The overarching theme described by each latent factor was agreed upon through consensus of the study team.
Bivariable and Multivariable Analysis
Due to a small number of items and clustering of responses around zero, index scores within each latent factor were determined to be impractical. Therefore, we created dichotomized variables assessing whether a woman responded affirmatively to any of the observed items clustered within each latent factor (for binary questions: “yes”, for Likert-scale questions “sometimes” or “often”). We used the indictor of positive response in each non-adherence factor as a binary outcome (dependent variable). We first assessed the prevalence of reporting each latent factor by clinical and demographic characteristics (independent variables), which were unadjusted but weighted to account for oversampling. We then ran multivariable generalized linear models to assess the adjusted effect of all characteristics, using a Poisson family and log link to produce adjusted risk ratios.30 Covariates included race (binary), age (continuous), stage (categorical), treatments received (binary), ET decision-making process (categorical) and insurance status (categorical).
Combined missingness across all outcomes and covariates was low (<5%), therefore complete case analysis was performed. Statistical significance of between-group differences was assessed using a Wald test evaluated with an alpha value of 0.05. Analysis was performed with Stata 15 (College Station, Tx).
Results
Patient characteristics
Table 1 presents characteristics of the final sample. By design, the sample over-represents both younger (<50) and Black breast cancer survivors. One half of participants were diagnosed with stage I cancer (49.0%), the majority received radiation (72.5%) and just over half (54.6%) received chemotherapy as part of their first treatment course. The majority of participants (52.2%) report that taking ET was a joint decision between themselves and their doctor, while 22.3% felt that their doctor led the decision, 17.2% felt that they led the decision, and 6.9% report no discussion of ET prior to being prescribed the medication.
Table 1:
Characteristics of ET-initiators in the Carolina Breast Cancer Study
| n | % | ||
|---|---|---|---|
| Overall | 1231 | ||
| Age | 53.19 (10.91) | ||
| mean (sd) | |||
| Race | Non-Black | 709 | 57.6% |
| Black/African American | 522 | 42.4% | |
| Stage | Stage 1 | 603 | 49.0% |
| Stage 2 | 451 | 36.6% | |
| Stage 3 | 156 | 12.7% | |
| Stage Unknown | 21 | 1.70% | |
| Chemotherapy | Received Chemo | 672 | 54.6% |
| Radiation | Received Radiation | 892 | 72.5% |
| Surgery Type | Breast Conserving | 599 | 48.7% |
| Mastectomy | 596 | 48.4% | |
| Trastuzumab | Received trastuzumab | 153 | 12.4% |
| Insurance | Private Insurance | 783 | 63.6% |
| Medicare | 288 | 23.4% | |
| Medicaid | 97 | 7.9% | |
| Uninsured | 62 | 5.0% | |
| ET Type | Tamoxifen | 513 | 41.7% |
| Aromatase Inhibitor | 710 | 57.7% | |
| Decision Process | Shared | 642 | 52.2% |
| Provider-led | 275 | 22.3% | |
| Patient-led | 212 | 17.2% | |
| No Discussion | 85 | 6.9% | |
Reason-specific ET non-adherence
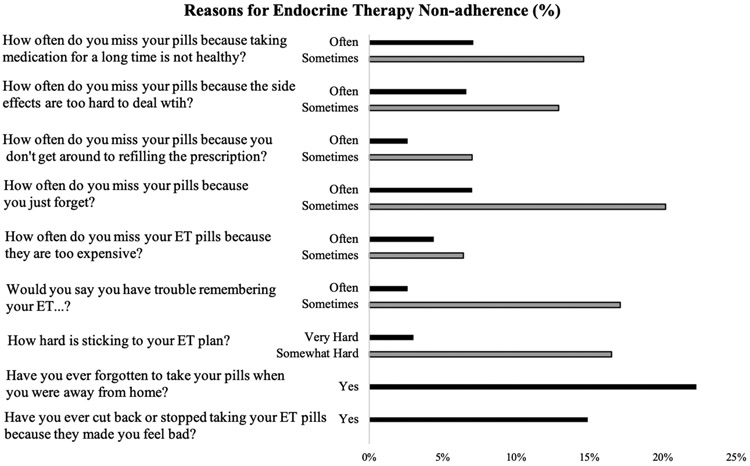
Overall, 58% of women reported experiencing at least one barrier to ET adherence. (Figure 1) Women were most likely to say they miss pills because they “just forget” (27.2% reporting “sometimes” or “often”) or because they forget their pills when they are away from home (22.7%). Least commonly endorsed was missing pills because they are too expensive (10.8% reporting “sometimes” or “often”) and missing pills because they don’t get around to refilling the prescription (9.6% reporting “sometimes” or “often).
Figure 1:
Frequency of reason-specific endocrine therapy non-adherence
Principal factor analysis was performed to identify items with strong clusters of related responses and suggested the presence of three relevant factors. (See online supplemental materials) All items loaded onto their corresponding factor at 0.4 or higher. (See online supplemental materials) Items comprising the first factor included forgetting medication when away from home, difficulty sticking to the treatment plan, and having trouble remembering medication. This latent factor was identified as barriers related to medication-taking habits. The second latent factor included missing pills because they made the patient feel bad, had too many side effects, and concerns about the safety of long-term medication use. The common theme among these items was identified as concerns about the risk/benefit tradeoff associated with ET medication. Finally, the last latent factor is comprised of only two items; reporting that medication is too expensive and reporting missed doses due to failure to refill the prescription. This domain was identified as resource barriers to ET adherence.
Correlates of adherence barriers
Once these three latent factors were identified, we assessed both unadjusted (Table 2) and adjusted (Table 3) correlates of positive response to at least one of the questions within each factor. Importantly, all latent factors of reason-specific non-adherence were more strongly associated with sociodemographic, rather than clinical characteristics notably age, race, insurance status, and the decision-making process around ET. Yet we found variation in the specific factors associated with each of these three adherence barriers, with only Black race associated with increased likelihood of reporting across all three non-adherence themes.
Table 2:
Prevalence of Latent Factors of Non-Adherence among ET-initiators in the Carolina Breast Cancer Study
| Habit | Tradeoffs | Resource | ||
|---|---|---|---|---|
| Overall | 31.3% [28.4%,34.4%] | 27.6% [24.7%,30.7%] | 12.1% [10.2%,14.3%] | |
| Age | <50 | 48.6% [44.1%,53.1%] | 31.6% [27.7%,35.8%] | 16.9% [13.9%,20.4%] |
| 50+ | 25.5% [22.0%,29.4%] | 26.3% [22.7%,30.2%] | 10.5% [8.2%,13.2%] | |
| Race | Non-Black | 29.4% [26.0%,33.0%] | 25.9% [22.5%,29.6%] | 10.1% [8.0%,12.7%] |
| Black/AA | 40.6% [36.4%,44.8%] | 35.9% [31.8%,40.2%] | 21.4% [18.1%,25.3%] | |
| Stage | Stage 1 | 31.3% [27.2%,35.8%] | 26.0% [22.1%,30.4%] | 10.3% [7.9%,13.2%] |
| Stage 2 | 31.6% [26.9%,36.8%] | 29.9% [25.0%,35.3%] | 12.7% [9.7%,16.5%] | |
| Stage 3 | 32.1% [24.0%,41.4%] | 30.6% [22.6%,40.1%] | 15.9% [10.2%,24.0%] | |
| Chemo | No Chemo | 29.4% [25.2%,33.9%] | 25.6% [21.7%,30.1%] | 10.9% [8.3%,14.1%] |
| Chemo | 33.3% [29.3%,37.6%] | 29.7% [25.7%,34.1%] | 13.4% [10.8%,16.5%] | |
| Radiation | No Radiation | 29.4% [25.2%,33.9%] | 25.6% [21.7%,30.1%] | 10.9% [8.3%,14.1%] |
| Radiation | 33.3% [29.3%,37.6%] | 29.7% [25.7%,34.1%] | 13.4% [10.8%,16.5%] | |
| Surgery | BCS | 33.6% [29.3%,38.2%] | 26.2% [22.3%,30.6%] | 13.1% [10.3%,16.5%] |
| Mastectomy | 27.1% [23.1%,31.4%] | 27.4% [23.2%,31.9%] | 10.7% [8.3%,13.8%] | |
| Herceptin | No Herceptin | 30.0% [26.9%,33.4%] | 26.7% [23.6%,30.1%] | 12.2% [10.1%,14.6%] |
| Herceptin | 31.5% [23.6%,40.6%] | 27.5% [19.7%,37.0%] | 9.4% [5.8%,14.7%] | |
| ET Type | Tamoxifen | 37.3% [32.6%,42.3%] | 28.4% [24.0%,33.2%] | 13.3% [10.4%,16.9%] |
| Aromatase Inhibitor | 28.4% [24.8%,32.4%] | 27.3% [23.6%,31.3%] | 11.5% [9.2%,14.4%] | |
| Insurance | Private Insurance | 33.0% [29.3%,36.9%] | 27.9% [24.2%,31.8%] | 9.4% [7.4%,11.9%] |
| Medicare | 24.4% [19.2%,30.6%] | 23.8% [18.6%,29.9%] | 9.8% [6.6%,14.3%] | |
| Medicaid | 42.1% [30.4%,54.8%] | 49.2% [36.3%,62.2%] | 47.0% [34.2%,60.2%] | |
| Uninsured | 47.5% [32.3%,63.1%] | 28.2% [17.8%,41.6%] | 32.4% [19.2%,49.1%] | |
| Decision | Shared | 29.6% [25.7%,33.9%] | 24.7% [20.9%,28.9%] | 9.9% [7.6%,12.7%] |
| Process | Provider-led | 28.4% [21.9%,35.9%] | 26.9% [20.4%,34.6%] | 11.8% [7.9%,17.2%] |
| Patient-led | 37.3% [30.8%,44.4%] | 34.6% [28.0%,41.8%] | 15.9% [11.6%,21.4%] | |
| No Discussion | 37.2% [26.1%,49.9%] | 32.8% [22.3%,45.3%] | 20.2% [11.7%,32.5%] |
AA- African American; AJCC- American Joint Committee on Cancer; BCS- Breast Conserving Surgery. n=1231.
Responses are survey-weighted to account for designed oversampling.
Bolded values represent the prevalence of a factor is statistically significantly different (p<.05) from the referent group (the first level listed in each category) using a Wald test.
Table 3:
Multivariable Associations of Domain-Specific ET Non-Adherence among ET-initiators in the Carolina Breast Cancer Study
| Habit | Tradeoffs | Resource | |||||||
|---|---|---|---|---|---|---|---|---|---|
| aRR | LCL | UCL | aRR | LCL | UCL | aRR | LCL | UCL | |
| Age | 0.54 | 0.43 | 0.69 | 0.96 | 0.74 | 1.25 | 0.66 | 0.43 | 0.997 |
| Stage (ref: 1) | |||||||||
| Stage 2 | 0.78 | 0.61 | 0.996 | 0.98 | 0.73 | 1.31 | 1.04 | 0.68 | 1.59 |
| Stage 3 | 0.79 | 0.53 | 1.18 | 0.90 | 0.58 | 1.38 | 0.66 | 0.67 | 2.36 |
| Chemotherapy (ref: none) | |||||||||
| Received | 1.07 | 0.83 | 1.38 | 1.18 | 0.87 | 1.59 | 0.92 | 0.61 | 1.39 |
| Radiation (ref: none) | |||||||||
| Received | 1.09 | 0.81 | 1.46 | 1.23 | 0.86 | 1.77 | 1.05 | 0.60 | 1.84 |
| Surgery (ref: BCS) | |||||||||
| Mastectomy | 0.81 | 0.62 | 1.05 | 0.95 | 0.70 | 1.29 | 0.95 | 0.59 | 1.54 |
| Herceptin (ref: none) | |||||||||
| Received | 1.00 | 0.74 | 1.35 | 0.86 | 0.60 | 1.24 | 0.78 | 0.49 | 1.24 |
| Insurance (ref: Private) | |||||||||
| Medicare | 1.00 | 0.67 | 1.23 | 1.00 | 0.59 | 1.10 | 0.78 | 0.65 | 1.75 |
| Medicaid | 1.20 | 0.86 | 1.66 | 1.53 | 1.10 | 2.13 | 4.04 | 2.49 | 6.57 |
| Uninsured | 1.37 | 0.98 | 1.93 | 0.87 | 0.54 | 1.40 | 2.83 | 1.60 | 4.99 |
| Race (Ref: non-Black) | |||||||||
| Black race | 1.29 | 1.09 | 1.53 | 1.32 | 1.09 | 1.60 | 1.65 | 1.18 | 2.30 |
| ET Decision Process (ref: Shared Decision) | |||||||||
| Provider-led | 1.17 | 0.93 | 1.48 | 1.31 | 1.00 | 1.71 | 1.51 | 1.02 | 2.22 |
| Patient-led | 0.90 | 0.66 | 1.21 | 1.03 | 0.75 | 1.42 | 1.36 | 0.85 | 2.19 |
| No Discussion | 1.39 | 0.95 | 2.03 | 1.37 | 0.91 | 2.08 | 2.55 | 1.38 | 4.70 |
| ET Type (ref: AI) | |||||||||
| Tamoxifen | 0.91 | 0.67 | 1.23 | 0.80 | 0.59 | 1.10 | 1.07 | 0.65 | 1.75 |
AA- African American; AJCC- American Joint Committee on Cancer; BCS- Breast Conserving Surgery; AI- Aromatase Inhibitor. n=1149; Bolded values represent the relative risk of reporting a factor is significantly different from the referent group.
Overall, 31.3% of women identified at least one item in the habit factor that affected adherence. Among women under the age of 50, however, the rate was 48.6% [95% CI: 44.1%-53.1%] compared to 25.5% [22.0%-29.4%] among those 50 years of age an older (Table 2). Black women were also more likely to report habit-related nonadherence (40.6% [36.4%-44.8%]) than non-Black women (29.4% [26.0%-33.0%]). In multivariable analysis (Table 3) relationships remained significant with those over 50 years of age at a lower adjusted relative risk (0.54 [0.43-0.69]) compared to those under 50 and of reporting and Black women at higher relative risk (1.29 [1.09-1.53]) of reporting habit-related non-adherence.
A large percentage of women also reported challenges related to risk/benefit tradeoffs of ET, with 27.6% endorsing at least one tradeoff item. Reporting of tradeoff-related non-adherence among Black women was 35.9% [31.8%-40.2%] relative to 25.9% [22.5%-29.6%] for non-Black women. Women on Medicaid were also more likely to report tradeoff-related non-adherence (49.2% [36.3%-62.2%]) compared to women with private insurance (27.9% [24.2%-31.8%]). When controlling for clinically relevant differences in these populations, both Black women (aRR: 1.32 [1.09, 1.60]) and women insured by Medicaid (aRR: 1.53 [1.10, 2.13]) were more likely to report tradeoff-related barriers.
Resource barriers were the least common reason for ET non-adherence, with only 12.1% of women responding that either medication cost or the refilling of prescriptions was a barrier- however the prevalence of this barrier varied widely between groups. Reporting of resource-related barriers was higher for women under 50 years of age (16.9% [13.9%-20.4%]) than those age 50 and up (10.5% [8.2%-13.2%]). Resource barriers were reported more than twice as often among Black women (21.4% [18.1%-25.3%]) as among non-Black women (10.1% [8.0%-12.7%]). Finally, resource barriers were three to four times as common among uninsured women (32.4% [19.2%-49.1%]) and Medicaid insured women (47.0% [34.2%-60.2%) as the privately insured (9.4% [7.4%-11.9%]). In adjusted analysis, these relationships remained significant, although the magnitude of these differences decreased after observable differences between these groups were accounted for. Additionally, after controlling for clinical differences, the decision-making process for starting ET was a statistically significant correlate, with those who reported a provider-led decision (aRR: 1.51 [1.02, 2.22]) or no discussion prior to initiating ET (aRR: 2.55 [1.38, 4.70]) more likely to report resource barriers than those who had a joint decision-making process.
Discussion
Our study identified three latent factors representing key themes of ET non-adherence among women with hormone-receptor positive breast cancer, as well as clinical and demographic characteristics associated with each of these factors. Understanding the specific challenges that women taking ET face is important for designing effective interventions to increase use of this preventive medication. The three latent non-adherence factors include: habit (difficulty habituating medication-taking behavior), risk/benefit tradeoffs (difficulty with side effect burden and perceived medication benefit) and resource barriers (difficulty paying for medication or picking up a prescription). Habit and resource barriers were more common among younger women and the frequency of all three barriers was higher in black women. Further, we found that lack of insurance and certain public insurance types were associated with higher risk of reporting resource barriers. The association between insurance and resource barriers was quite strong, with more than 32% of the uninsured and 47% of those on Medicaid reporting non-adherence due to resource concerns, relative to 9.4% of the privately insured.
Elements of the non-adherence themes we identify here are consistently seen in the existing ET literature, though they have not previously been categorized into larger themes. Much of the previous work in ET adherence has focused on the risk/benefit tradeoffs associated with ET. In particular, they find that women reporting greater side-effect burden are more likely to take medication sporadically or discontinue completely.12,16 We find risk/benefit tradeoffs are greatest among Black women, consistent with literature describing higher side effect burden, lower recurrence risk perception, and less continuous care during survivorship for this population.4,31
Studies have also reported on the prevalence of cost-based resource barriers, focusing on the direct burden via the out-of-pocket costs from ET medication.32,33 However, we are aware of no studies that have reported on other resource barriers- including the time and transportation- that may make refilling a prescription harder for low-resource women. As a proxy for these challenges, however, the use of mail-order pharmacies and use of 90-day rather than 30-day refills has been shown to positively correlate with increased adherence3. Our finding that resource barriers to ET adherence are larger in younger, Black and uninsured women are consistent with previous work showing these groups are more likely to experience financial barriers to care across the cancer continuum.34,35
Finally- few studies focus on the habit-formation challenges that were the most common source of non-adherence in our study. A qualitative study of ET use found several patients reported difficulty remembering their medication, and those who were adherent described the use of a routine time for taking medication and reminders (such as alarms or pill-boxes) as a facilitator.14 Importantly, studies examining racial difference in ET utilization have described racial differences in perceptions of tradeoffs as well as resource barriers36, but have generally not described significant differences by age and race in habit formation, which we observe is nearly as large as the other, more commonly described, barriers.
For interventions designed to address ET non-adherence, particularly among vulnerable populations, a “one-size-fits-all” strategy may miss the unique combination of barriers faced by different groups. These findings suggest that interventions to improve equity in ET use should use multi-faceted approaches that can address the compounding barriers faced by patients with a history of breast cancer. Importantly interventions focused singularly on reminders to take medication, on offering low-cost medication, or on mitigating side effects may leave women- particularly vulnerable women- with unaddressed adherence barriers. Multi-component interventions such as motivational interviewing and cognitive behavioral therapy have been proven effective for numerous targets of behavior change in cancer patients and survivors.37-39 These strategies have also been successfully used to improve medication adherence in individuals with HIV.40-43 These interventions allow individuals to self-identify barriers to medication-taking and build skills and self-efficacy for addressing these barriers, which can accommodate the diversity of barriers present for younger, Black, and uninsured patients with breast cancer as well as the potentially compounded challenges for those patients who exist at the intersection of these categories.
Limitations
Women who failed to initiate or discontinued medication completely (non-persistent) were not included in our analysis. Therefore, these findings are only representative of women actively taking endocrine therapy and may not reflect challenges faced by the full population of women who are prescribed endocrine therapy. This may result in an under-estimation of the scope of our described barriers, since women who discontinued completely may report many of the same concerns as their motivation for stopping.
While CBCS is a longitudinal cohort, our analysis is cross-sectional at an average 25-months post-diagnosis. As a result, there is low variation in time on ET and we are unable to explore duration on medication as a contributing factor. Follow-up is ongoing and we hope to further explore adherence changes over time as more data are available. Our ET questionnaire was developed specifically for CBCS, as existing medication adherence scales do not capture the unique challenges associated with adherence to endocrine therapy. While the items were derived from existing quantitative and qualitative literature and cognitively tested in patients with breast cancer, we note that the measures have not undergone formal tests for validity and reliability.
Finally, we note that these results are from exploratory factor analysis and that adjusted models do not represent causal pathways. Only two items describe the resource factor which may lead to instability in this factor. Further, deeper understanding of the structural and psychological causes of non-adherence in vulnerable populations is important for addressing the upstream factors which may contribute to increased risk of barriers and the downstream factors which may increase the salience of these barriers for underserved women.
Clinical Implications
This study used an ET-specific adherence instrument to assess non-adherence in a large and racially diverse group of breast cancer survivors across North Carolina, offering important insight on vulnerable populations that are often under-represented in breast cancer studies. Our analysis identified three broad domains of barriers to ET adherence, including habit-formation, perceptions of low risk/benefit tradeoffs, and inadequate resources. We found that reasons for non-adherence may vary by sociodemographic characteristics, with high but incompletely overlapping burden among young, minority, and Medicaid-insured or uninsured women. As doctors and researchers continue to develop strategies to support women with a history of breast cancer, it will be important to develop patient-centered, multi-faceted strategies to improve ET adherence across the full breast cancer population.
Supplementary Material
Acknowledgements:
This research was supported by the University of North Carolina’s (UNC) Cancer Care Quality Training Program (T32 CA116339) and by the American Cancer Society (MRSG-13-157-01-CPPB, PI: Wheeler). This research was also funded in part by the University Cancer Research Fund of North Carolina and the National Cancer Institute Specialized Program of Research Excellence (SPORE) in Breast Cancer (NIH/NCI P50-CA58223). Additional support has been obtained from National Cancer Institute (P01CA151135) and the Susan G Komen Foundation (CCR 15333140).
We would like to thank Drs. Carol Golin, Joanne Earp, Michael Bowling, and all of the breast cancer patients and staff who made the Carolina Breast Cancer Study possible.
Disclosure: SBW receives unrelated grant funding to her institution from Pfizer
Footnotes
Data Availability Statement: The data that support the findings of this study are available from The Carolina Breast Cancer Study. Restrictions apply to the availability of these data, which were used under license for this study. Further information about data as well as applications for data use are available at http://cbcs.web.unc.edu.
References:
- 1.Abe O, Abe R, Enomoto K, et al. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0 [DOI] [PubMed] [Google Scholar]
- 2.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–816. doi: 10.1016/S0140-6736(12)61963-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134(2):459–478. doi: 10.1007/s10549-012-2114-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wheeler SB, Spencer J, Pinheiro LC, et al. Endocrine Therapy Nonadherence and Discontinuation in Black and White Women. J Natl Cancer Inst. 2018;111:1–11. doi: 10.1093/jnci/djy136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hershman DL, Kushi LH, Shao T, et al. Early Discontinuation and Nonadherence to Adjuvant Hormonal Therapy in a Cohort of 8,769 Early-Stage Breast Cancer Patients. J Clin Oncol. 2010;28(27):4120–4128. doi: 10.1200/jco.2009.25.9655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard-McNatt M, Lawrence J, Melin SA, Levine EA, Shen P, Stewart JH. Race and recurrence in women who undergo neoadjuvant chemotherapy for breast cancer. Am J Surg. 2013;205(4):397–401. doi: 10.1016/j.amjsurg.2013.01.009 [DOI] [PubMed] [Google Scholar]
- 7.Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver KE, Camacho F, Hwang W, Anderson R, Kimmick G. Adherence to Adjuvant Hormonal Therapy and Its Relationship to Breast Cancer Recurrence and Survival Among Low-income Women. Am J Clin Oncol. 2013;36(2):181–187. doi: 10.1097/COC.0b013e3182436ec1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26(4):556–562. doi: 10.1200/JCO.2007.11.5451 [DOI] [PubMed] [Google Scholar]
- 10.Roberts MC, Wheeler SB, Reeder-Hayes K. Racial/Ethnic and socioeconomic disparities in endocrine therapy adherence in breast cancer: a systematic review. Am J Public Heal. 2015;105 Suppl:e4–e15. doi: 10.2105/AJPH.2014.302490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brett J, Fenlon D, Boulton M, et al. Factors associated with intentional and unintentional non-adherence to adjuvant endocrine therapy following breast cancer. Eur J Cancer Care (Engl). 2018;27(1):e12601. doi: 10.1111/ecc.12601 [DOI] [PubMed] [Google Scholar]
- 12.Demissie S, Silliman RA, Lash TL. Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. J Clin Oncol. 2001;19(2):322–328. [DOI] [PubMed] [Google Scholar]
- 13.Partridge AH. Nonadherence to Adjuvant Tamoxifen Therapy in Women With Primary Breast Cancer. J Clin Oncol. 2003;21(4):602–606. doi: 10.1200/jco.2003.07.071 [DOI] [PubMed] [Google Scholar]
- 14.Wells KJ, Pan TM, Vázquez-Otero C, et al. Barriers and facilitators to endocrine therapy adherence among underserved hormone-receptor-positive breast cancer survivors: a qualitative study. Support Care Cancer. 2016;24(10):4123–4130. doi: 10.1007/s00520-016-3229-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheeler S, Roberts M, Bloom D, et al. Oncology providers’ perspectives on endocrine therapy prescribing and management. Patient Prefer Adherence. 2016;Volume 10:2007–2019. doi: 10.2147/PPA.S95594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bright EE, Petrie KJ, Partridge AH, Stanton AL. Barriers to and facilitative processes of endocrine therapy adherence among women with breast cancer. Breast Cancer Res Treat. 2016;158(2):243–251. doi: 10.1007/s10549-016-3871-3 [DOI] [PubMed] [Google Scholar]
- 17.Wheeler SB, Kohler RE, Reeder-Hayes KE, et al. Endocrine therapy initiation among Medicaid-insured breast cancer survivors with hormone receptor-positive tumors. J Cancer Surviv. 2014;8(4):603–610. doi: 10.1007/s11764-014-0365-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekinci E, Nathoo S, Korattyil T, et al. Interventions to improve endocrine therapy adherence in breast cancer survivors: what is the evidence? J Cancer Surviv. 2018;12(3):348–356. doi: 10.1007/s11764-017-0674-4 [DOI] [PubMed] [Google Scholar]
- 19.Parada H, Sun X, Fleming JM, et al. Race-associated biological differences among luminal A and basal-like breast cancers in the Carolina Breast Cancer Study. Breast Cancer Res. 2017;19(1):131. doi: 10.1186/s13058-017-0914-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim CJ, Schlenk EA, Ahn JA, Kim M, Park E, Park J. Evaluation of the Measurement Properties of Self-reported Medication Adherence Instruments Among People at Risk for Metabolic Syndrome: A Systematic Review. Diabetes Educ. 2016;42(5):618–634. doi: 10.1177/0145721716655400 [DOI] [PubMed] [Google Scholar]
- 21.Clifford S, Perez-Nieves M, Skalicky AM, Reaney M, Coyne KS. A systematic literature review of methodologies used to assess medication adherence in patients with diabetes. Curr Med Res Opin. 2014;30(6):1071–1085. doi: 10.1185/03007995.2014.884491 [DOI] [PubMed] [Google Scholar]
- 22.Lavsa SM, Holzworth A, Ansani NT. Selection of a validated scale for measuring medication adherence. J Am Pharm Assoc. 2011;51(1):90–94. doi: 10.1331/JAPhA.2011.09154 [DOI] [PubMed] [Google Scholar]
- 23.Wheeler SB, Roberts MC, Bloom D, et al. Understanding barriers and facilitators to endocrine therapy use: A qualitative study of breast cancer patients and providers. [Abstract]. Acad Annu Res Meet. 2014;June. [Google Scholar]
- 24.Harrow A, Dryden R, McCowan C, et al. A hard pill to swallow: a qualitative study of women’s experiences of adjuvant endocrine therapy for breast cancer. BMJ Open. 2014;4(6):e005285–e005285. doi: 10.1136/bmjopen-2014-005285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Victorson D, Barocas J, Song J, Cella D. Reliability across studies from the functional assessment of cancer therapy-general (FACT-G) and its subscales: a reliability generalization. Qual Life Res. 2008;17(9):1137–1146. doi: 10.1007/s11136-008-9398-2 [DOI] [PubMed] [Google Scholar]
- 26.Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570 [DOI] [PubMed] [Google Scholar]
- 27.Smedley BD, Stith AY, Nelson AR (Alan R, Institute of Medicine (U.S.). Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care Unequal Treatment : Confronting Racial and Ethnic Disparities in Health Care. National Academies Press; 2003. [PubMed] [Google Scholar]
- 28.VanderWeele TJ, Robinson WR. On the causal interpretation of race in regressions adjusting for confounding and mediating variables. Epidemiology. 2014;25(4):473–484. doi: 10.1097/EDE.0000000000000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Browne MW. An Overview of Analytic Rotation in Exploratory Factor Analysis. Multivariable Behav Res. 2001;36(1):111–150. doi: 10.1207/S15327906MBR3601_05 [DOI] [Google Scholar]
- 30.Zou G A Modified Poisson Regression Approach to Prospective Studies with Binary Data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 31.Husain M, Nolan TS, Foy K, Reinbolt R, Grenade C, Lustberg M. An overview of the unique challenges facing African-American breast cancer survivors. Support Care Cancer. 2019. doi: 10.1007/s00520-018-4545-y [DOI] [PubMed] [Google Scholar]
- 32.Neugut AI, Subar M, Wilde ET, et al. Association Between Prescription Co-Payment Amount and Compliance With Adjuvant Hormonal Therapy in Women With Early-Stage Breast Cancer. J Clin Oncol. 2011;29(18):2534–2542. doi: 10.1200/JCO.2010.33.3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126(2):529–537. doi: 10.1007/s10549-010-1132-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wheeler SB, Spencer JC, Pinheiro LC, Carey LA, Olshan AF, Reeder-Hayes KE. Financial impact of breast cancer in black versus white women. J Clin Oncol. 2018;36(17):1695–1701. doi: 10.1200/JCO.2017.77.6310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yabroff KR, Dowling EC, Guy GP, et al. Financial hardship associated with cancer in the United States: Findings from a population-based sample of adult cancer survivors. J Clin Oncol. 2016. doi: 10.1200/JCO.2015.62.0468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts MC, Wheeler SB, Reeder-Hayes K. Racial/Ethnic and socioeconomic disparities in endocrine therapy adherence in breast cancer: a systematic review. Am J Public Health. 2015;105 Suppl 3(S3):e4–e15. doi: 10.2105/AJPH.2014.302490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20–28. doi: 10.1016/j.smrv.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 38.Ye M, Du K, Zhou J, et al. A meta-analysis of the efficacy of cognitive behavior therapy on quality of life and psychological health of breast cancer survivors and patients. Psychooncology. 2018;27(7):1695–1703. doi: 10.1002/pon.4687 [DOI] [PubMed] [Google Scholar]
- 39.Spencer JC, Wheeler SB. A systematic review of Motivational Interviewing interventions in cancer patients and survivors. Patient Educ Couns. 2016;99(7):1099–1105. doi: 10.1016/j.pec.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 40.Parsons JT, John SA, Millar BM, Starks TJ. Testing the Efficacy of Combined Motivational Interviewing and Cognitive Behavioral Skills Training to Reduce Methamphetamine Use and Improve HIV Medication Adherence Among HIV-Positive Gay and Bisexual Men. AIDS Behav. 2018;22(8):2674–2686. doi: 10.1007/s10461-018-2086-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Safren SA, Bedoya CA, O’Cleirigh C, et al. Cognitive behavioural therapy for adherence and depression in patients with HIV: a three-arm randomised controlled trial. lancet HIV. 2016;3(11):e529–e538. doi: 10.1016/S2352-3018(16)30053-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golin CE, Earp J, Tien H-C, Stewart P, Porter C, Howie L. A 2-arm, randomized, controlled trial of a motivational interviewing-based intervention to improve adherence to antiretroviral therapy (ART) among patients failing or initiating ART. J Acquir Immune Defic Syndr. 2006;42(1):42–51. doi: 10.1097/01.qai.0000219771.97303.0a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adamian MS, Golin CE, Shain LS, DeVellis B. Brief motivational interviewing to improve adherence to antiretroviral therapy: development and qualitative pilot assessment of an intervention. AIDS Patient Care STDS. 2004;18(4):229–238. doi: 10.1089/108729104323038900 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.