Summary
The COVID-19 pandemic commands a major reorganisation of the entire French healthcare system. In France, general rules have been issued nationally and implemented by each healthcare centre, both public and private, throughout France. Guidelines drafted by an expert group led by the French-speaking Association of Endocrine Surgery (AFCE) propose specific surgical management principles for thyroid, parathyroid, endocrine pancreas and adrenal surgery during and after the COVID-19 epidemic.
Keywords: Coronavirus, COVID-19, Endocrine surgery, Thyroid, Parathyroid, Adrenal, Neuroendocrine tumour
Introduction
The ongoing COVID-19 pandemic commands a major reorganisation of the entire French healthcare system [1]. To respond to the present and expected influx of patients needing a period of intensive care [2], the short-term priority has been directing available material and human resources toward sectors dispensing care for COVID-19 patients [3], [4]. This policy has entailed the almost complete descheduling of non-urgent surgery [5]. More than a month now after the start of the epidemic, there is a pressing need to manage other health disorders not linked to COVID-19, but for which deferral of surgery until after the epidemic is over, could worsen prognosis or be life-threatening. It is also important to be thinking now about the conditions under which surgery can be resumed at a normal pace after the epidemic. General rules have been put out nationally and implemented by each healthcare centre, both public and private, throughout France. Specific guidelines have been proposed for visceral surgery [6]. Likewise, to meet their need for specific guidelines, the French-speaking Association of Endocrine Surgery (AFCE) brought together a group of experts to propose principles for the surgical management of thyroid, parathyroid, endocrine pancreas and adrenal pathologies during the COVID-19 epidemic and afterwards, for when surgical activity will be able to return gradually to its normal pattern. These guidelines were drafted in the light of the existing literature. They will be updated as knowledge advances.
General principles for scheduling surgery during and after the COVID-19 epidemic
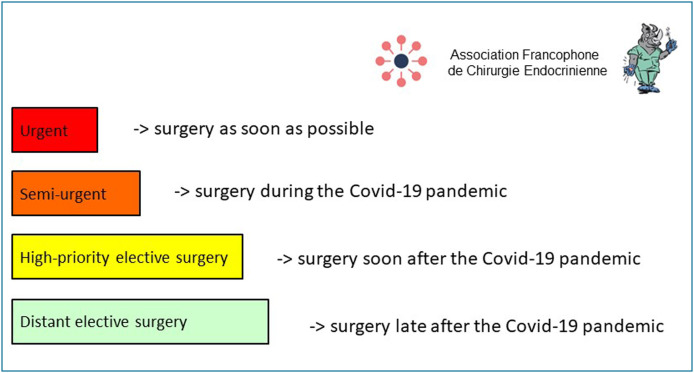
Four scheduling levels were defined to help prioritise patients (these levels may change according to how the epidemic setting evolves) (Fig. 1 ):
-
•
urgent surgery that must be carried out as soon as possible because even a short deferral would be life-threatening;
-
•
semi-urgent surgery that can be deferred for a few weeks but not beyond 3 months without threat to life or adverse effects on cancer or functional prognosis;
-
•
high-priority elective surgery that can wait for several months but must be given scheduling priority as soon as the epidemic is over;
-
•
distant elective surgery that can be deferred until well after the epidemic is over, even more than 6 months, without compromising the indication.
Figure 1.
General principles for scheduling endocrine surgery during and after the COVID-19 epidemic.
For urgent surgery, the ratio of the benefits expected from surgery to the risks incurred by scheduling it during the epidemic must always be evaluated according to how both the national and local contexts are evolving, in particular the resources available: operating room, consumables and hospital capacities, particularly if intensive care may be needed. When surgery is prescribed in the epidemic setting, short hospital stays or outpatient care is recommended [7], provided this does not increase the risk of rehospitalisation. To limit operating time and the risk of postoperative complications, the surgery should also be performed by one or more experienced surgeons. Even if no symptoms of COVID-19 are apparent, the risk of infection should be assessed beforehand as it may be associated with unfavourable prognosis [8], [9]. Any surgery on a patient infected or suspected of being infected must be performed according to the rules laid down by the hospital's hygiene teams and infectiologists [10].
Thyroid and parathyroid surgery
Thyroid cancers
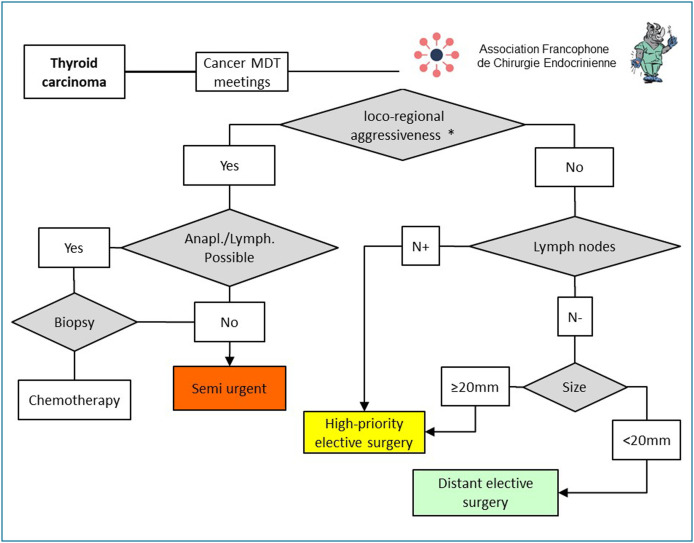
Differentiated thyroid cancers most often have an excellent prognosis [11], and there is no high level of proof supporting an optimal surgery deferral time (Fig. 2 ). During the epidemic, any thyroid tumour suspected of malignancy (Bethesda 5 or 6) must be discussed at a multidisciplinary team (MDT) meeting. When there are clinical or paraclinical signs pointing to an aggressive form of cancer (recurrent nerve palsy, local invasion with esophageal, vascular or tracheal involvement, massive lymph nodes infiltration [12]), surgery must be programmed as semi-urgent. If anaplastic, poor differentiated thyroid carcinoma or lymphoma is suspected, a surgical biopsy must be performed before any surgery is undertaken. If either of these diagnoses is confirmed, an appropriate treatment with corticoids and/or chemotherapy must be proposed as first line therapy [13]. If there is no loco-regional aggressiveness, deferral of surgery for differentiated carcinoma will depend on lymph node and tumour size. A tumour size greater than or equal to 2 cm and/or associated with lymph nodes can be deferred without risk until the epidemic is over but must then be given priority and scheduled within the following 3 months. Tumours smaller than 2 cm in size, with no ganglion metastases, can be deferred until well after the end of the epidemic. Although the prognosis of medullary thyroid carcinoma (MTC) is slightly less favourable than that of follicular differentiated thyroid cancer [14], the care deferral guidelines given above still hold.
Figure 2.
Principles for scheduling thyroid cancers during and after the COVID-19 epidemic. * Loco-regional aggressiveness defined as the presence of recurrent nerve paralysis, local invasion with esophageal, vascular or tracheal involvement, or massive ganglion infiltration. (Anapl = anaplastic, Lymph: lymphoma; MDT: multidisciplinary team).
Benign thyroid disorders
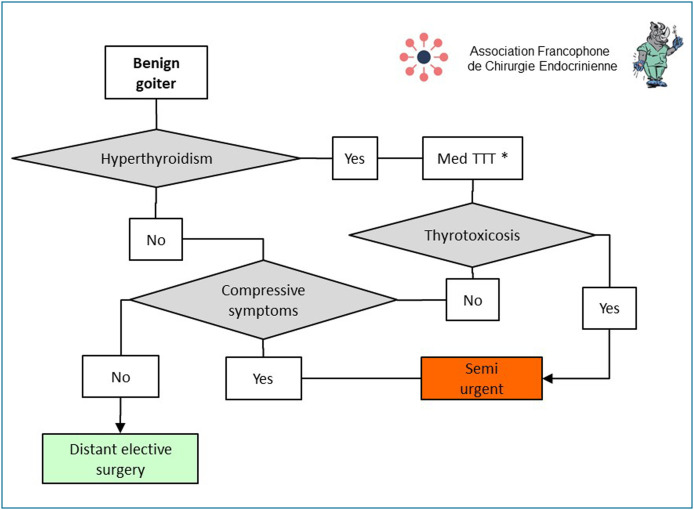
Most thyroidectomies for benign lesions can be deferred without risk (Fig. 3 ). However, specific situations can require semi-urgent scheduling, e.g. thyrotoxicosis (Graves's disease, toxic nodules, toxic goiters, iatrogenic hyperthyroidism) resistant to or poorly controlled by synthetic anti-thyroid (SAT) agents [15]. In this situation, lithium, potassium perchlorate, and if this fails, plasmapheresis may be useful to normalise levels of triiodothyronine (T3) and control those of thyroxine (T4) at the time of surgery. Non-suspect goiters responsible for severe compressive symptoms (inspiratory dyspnea due to tracheal compression, dysphagia due to esophageal compression, superior vena cava syndrome due to deep vein compression) must also be scheduled for semi-urgent surgery before the epidemic ends.
Figure 3.
Principles for scheduling surgery for benign thyroid disorders during and after the COVID-19 epidemic. * Synthetic anti-thyroid agents, lithium, potassium perchlorate, plasmapheresis. (Med TTT = medical treatment).
Hyperparathyroidism
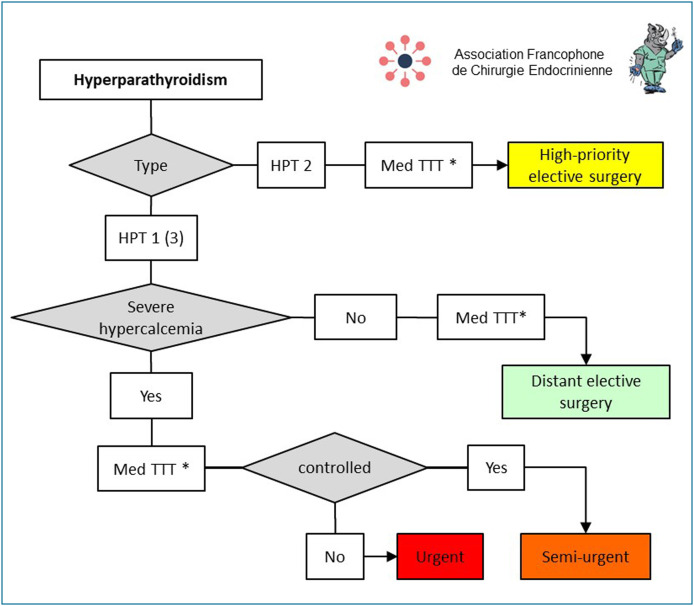
Surgical treatment of primary hyperparathyroidism (HPT) is generally not urgent (Fig. 4 ) [16]. In the COVID-19 epidemic setting, its scheduling depends on the presence or absence of severe hypercalcemia, defined by a very high level of blood calcium > 3.5 mmol/L (140 mg/L) [17], and/or the presence of clinical complications–acute pancreatitis secondary to HPT, brown tumour, calciphylaxis, fracture osteopenia, heart rhythm disorders (QT shortening on ECG, bradycardia with risk of asystole) with cardiac insufficiency [17], [18], [19], [20]. In all cases, hypocalcemic treatment must first be given. In the epidemic setting, the use of cinacalcet is recommended [21]. In cases of severe hypercalcemia, surgery must be scheduled as semi-urgent, without waiting for the epidemic to end, or as urgent when it escapes control by the medical treatment. If there is no severe hypercalcemia, surgery can be deferred without risk until the epidemic is over. These guidelines are valid for cases of genetically determined primary HPT. For tertiary HPT, the blood calcium threshold defining severe hypercalcemia must be lowered to 2.8 mmol/L to protect renal grafts (nephrocalcinosis, acute tubular necrosis, lithiasis) and bone and vascular impact [22], [23]. For secondary HPT, surgical treatment is not recommended during the epidemic because of the higher risk of COVID-19 infection in dialysed patients [24]. When indicated, surgery must be scheduled as a priority in the three months following the epidemic in cases of disabling bone pain, brown tumour or temporary contraindication for renal transplant [25].
Figure 4.
Principles for scheduling parathyroid surgery during and after the COVID-19 epidemic. Blood calcium > 3.5 mmol/L and/or presence of clinical complications (acute pancreatitis secondary to HPT, brown tumour, calciphylaxis fracture osteopenia, cardiac rhythm disorder with heart failure). * Hydration, calcimimetic (Med TTT = medical treatment).
Technical aspects of neck surgery
Uni- or bilateral cervicotomy is the approach recommended for the surgical treatment of thyroid or parathyroid pathologies in the epidemic setting, so as to limit operating time and complication risk [26]. Surgery requiring a thoracic or mediastinal approach and/or postoperative intensive care [27] must be deferred whenever possible until after the epidemic is over. Endoscopic surgical approaches, with or without robotic assistance, are not recommended [28]. If there is no dysphonia, laryngoscopy before or after surgery is not recommended [29] because of the high risk of airborne SARS-CoV-2 transmission during such examination [30]. Neuromonitoring of the inferior laryngeal nerve is recommended:
-
•
to make sure the neuromuscular apparatus of the larynx is functioning before and after surgery [31];
-
•
to reduce the risk of bilateral recurrent nerve palsy that can require intensive care and interruption of surgery if the signal is lost during the dissection of the first side [32].
Peroperative PTH assay is possible but must not increase time spent in the operating room. The usual guidelines for the prevention and/or management of postoperative hypocalcemia remain unchanged during the epidemic [33].
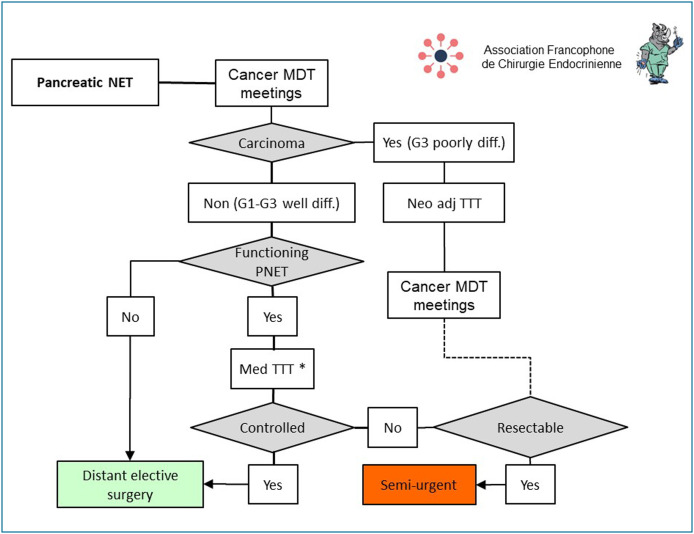
Neuroendocrine tumours of the pancreas
In the epidemic setting, the indication for the surgical treatment of a neuroendocrine tumour of the pancreas must be discussed in an MDT meeting to assess the balance between the risks of surgery and its oncological and/or secretory benefits (Fig. 5 ) [34]. The management of Grade 3 carcinomas (poorly differentiated) has already been the subject of guidelines as part of the French National Digestive Oncology Thesaurus [35], and the COVID-19 epidemic does not change the usual guidelines favouring chemotherapy or neoadjuvant radio-chemotherapy [36]. A pancreatectomy may be indicated when a curative resection can be considered after clinical and morphological reassessment [37], in which case surgery is scheduled as semi-urgent before the epidemic has ended. Patients with a well-differentiated neuroendocrine tumour of the pancreas (Grades G1, G2 or G3), that is non-secretory, can be deferred until well after the epidemic is over. If there is an associated secretory syndrome, a medical treatment should first be given [38]. If this treatment fails to control the secretory syndrome satisfactorily, pancreatectomy must be scheduled as semi-urgent before the end of the epidemic. If the medical treatment is effective, surgery can be deferred until well after the epidemic has ended. When technically possible, laparoscopy is recommended for left pancreatectomies and enucleations to minimise postoperative impact on respiratory function and hospital length of stay [6], [10], [19].
Figure 5.
Principles for scheduling surgery for neuroendocrine tumours of the pancreas during and after the COVID-19 epidemic. * Diazoxide, proton pump inhibitor, somatostatin analogues, (TTT med: medical treatment; MDT: multidisciplinary team).
Adrenal lesions
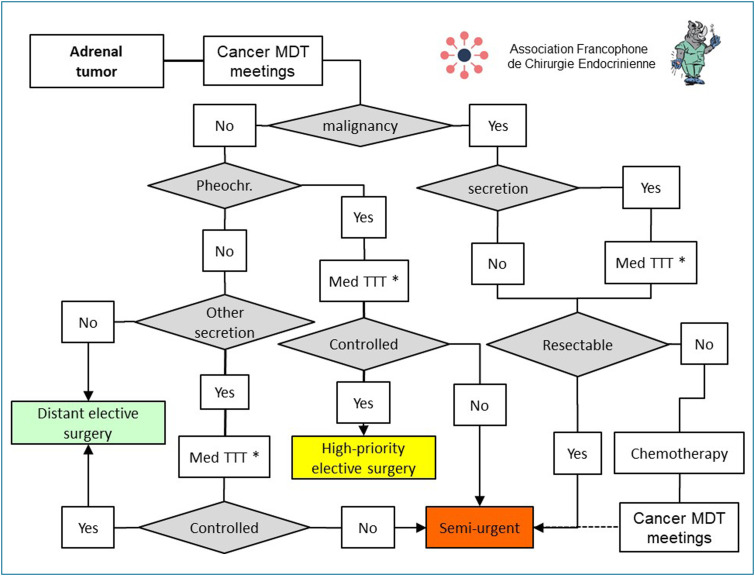
In the epidemic setting, the indication for the surgical treatment of an adrenal lesion must be discussed at an MDT meeting to assess the balance of risk and its oncological and/or secretory benefits (Fig. 6 ).
Figure 6.
Principles for scheduling surgery for adrenal tumours during and after the COVID-19 epidemic. * Steroidogenesis inhibitors (metyrapone, ketoconazole), anti-hypertensive agents (alpha-blocking drugs, beta-blocking drugs, calcium inhibitors), anti-aldosterone diuretics, (Med TTT:medical treatment, pheochr: pheochromocytoma; MDT: multidisciplinary team).
Lesions suspected to be malignant (adrenal cortical carcinoma, metastases) must undergo surgery when they are considered resectable [39], [40]. In cases of secretory syndrome, prior management by a medical treatment is recommended (metyrapone, ketoconazole). Surgery must be scheduled as semi-urgent, before the end of the epidemic, in an expert centre [41]. Chromaffin lesions (pheochromocytoma and/or paraganglioma) must first receive an appropriate anti-hypertension treatment (alpha-blocking agents, beta-blocking agents, calcium inhibitors), and be monitored by an experienced care team [42]. If this treatment controls the secretory syndrome, close monitoring can be continued until the adrenalectomy, which will be scheduled as a priority when the epidemic ends. If this treatment fails to control the secretory syndrome satisfactorily, surgery can be scheduled as semi-urgent, before the epidemic ends, in an experienced centre.
For other secretory adrenal lesions (in particular, hypercorticism and hyperaldosteronism), an appropriate medical treatment (steroidogenesis inhibitors, anti-aldosterone) must first be implemented. If the secretory syndrome is not controlled or if impact is marked, adrenalectomy can be scheduled as semi-urgent during the epidemic. In other cases, adrenalectomy can be scheduled well after the epidemic has ended. During the epidemic, laparoscopy remains the preferred approach for adrenalectomy. Conversely, for suspect lesions and/or those larger than 10 cm, laparotomy is recommended [43].
Postoperative follow-up in the epidemic setting
Postoperative follow-up consultations must be maintained during the epidemic. Tele-consultation is recommended to ensure continuity of care while limiting the risks of coronavirus propagation in healthcare centres. For a consultation in which a diagnosis of cancer or a therapeutic strategy is to be announced, some form of video exchange is recommended. Whenever possible, blood tests and imaging must be performed outside hospitals.
In a situation where medical drugs of major therapeutic importance may be in short supply, patients, who are dependent on a hormone substitution treatment, should be reminded never to interrupt their treatment longer than 24 h for corticoids [44], longer than 48 h for calcium [45], and longer than one week for thyroid hormones [46].
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Heymann D.L., Shindo N., Scientific W.H.O., Technical Advisory Group for Infectious H. COVID-19: what is next for public health? Lancet. 2020;395(10224):542–545. doi: 10.1016/S0140-6736(20)30374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A. High prevalence of obesity in severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020 doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G., Pesenti A., Cecconi M. Critical care utilisation for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020 doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 4.Zarzaur B.L., Stahl C.C., Greenberg J.A., Savage S.A., Minter R.M. Blueprint for restructuring a department of surgery in concert with the health care system during a pandemic: the University of Wisconsin experience. JAMA Surg. 2020 doi: 10.1001/jamasurg.2020.1386. [DOI] [PubMed] [Google Scholar]
- 5.Iacobucci G. COVID-19: all non-urgent elective surgery is suspended for at least three months in England. BMJ. 2020;368:m1106. doi: 10.1136/bmj.m1106. [DOI] [PubMed] [Google Scholar]
- 6.Tuech J.J., Gangloff A., Di Fiore F., Michel P., Brigand C., Slim K. Strategy for the practice of digestive and oncological surgery during the COVID-19 epidemic. J Visc Surg. 2020 doi: 10.1016/j.jviscsurg.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunaud L., Zarnegar R., Mirallie E. At last a step forward toward ambulatory care for endocrine surgery in France? J Visc Surg. 2018;155(4):251–252. doi: 10.1016/j.jviscsurg.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Lei S., Jiang F., Su W., Chen C., Chen J., Mei W. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinMed. 2020 doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang W., Guan W., Chen R., Wang W., Li J., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montravers P. 2020. Propositions pour la prise en charge anesthésique d’un patient suspect ou infecté à Coronavirus COVID-19. [Available from: https://sfar.org/propositions-pour-la-prise-en-charge-anesthesique-dun-patient-suspect-ou-infecte-a-coronavirus-covid-19/] [Google Scholar]
- 11.Cabanillas M.E., McFadden D.G., Durante C. Thyroid cancer. Lancet. 2016;388(10061):2783–2795. doi: 10.1016/S0140-6736(16)30172-6. [DOI] [PubMed] [Google Scholar]
- 12.Haugen B.R., Alexander E.K., Bible K.C., Doherty G.M., Mandel S.J., Nikiforov Y.E. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Besic N., Auersperg M., Us-Krasovec M., Golouh R., Frkovic-Grazio S., Vodnik A. Effect of primary treatment on survival in anaplastic thyroid carcinoma. Eur J Surg Oncol. 2001;27(3):260–264. doi: 10.1053/ejso.2000.1098. [DOI] [PubMed] [Google Scholar]
- 14.Wells S.A., Jr., Asa S.L., Dralle H., Elisei R., Evans D.B., Gagel R.F. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartes B., Rodien P. The Graves’ disease, consensus of the French Society of Endocrinology. Ann Endocrinol (Paris) 2018;79(6):597–598. doi: 10.1016/j.ando.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Silverberg S.J., Shane E., Jacobs T.P., Siris E., Bilezikian J.P. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341(17):1249–1255. doi: 10.1056/NEJM199910213411701. [DOI] [PubMed] [Google Scholar]
- 17.Cannon J., Lew J.I., Solorzano C.C. Parathyroidectomy for hypercalcemic crisis: 40 years’ experience and long-term outcomes. Surgery. 2010;148(4):807–812. doi: 10.1016/j.surg.2010.07.041. [discussion 12–3] [DOI] [PubMed] [Google Scholar]
- 18.Roy R., Lee J.A. Calciphylaxis due to hyperparathyroidism. Endocr Pract. 2011;17(Suppl 1):54–56. doi: 10.4158/EP10349.RA. [DOI] [PubMed] [Google Scholar]
- 19.Bai H.X., Giefer M., Patel M., Orabi A.I., Husain S.Z. The association of primary hyperparathyroidism with pancreatitis. J Clin Gastroenterol. 2012;46(8):656–661. doi: 10.1097/MCG.0b013e31825c446c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraser W.D. Hyperparathyroidism. Lancet. 2009;374(9684):145–158. doi: 10.1016/S0140-6736(09)60507-9. [DOI] [PubMed] [Google Scholar]
- 21.Peacock M., Bilezikian J.P., Klassen P.S., Guo M.D., Turner S.A., Shoback D. Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2005;90(1):135–141. doi: 10.1210/jc.2004-0842. [DOI] [PubMed] [Google Scholar]
- 22.Pitt S.C., Panneerselvan R., Chen H., Sippel R.S. Tertiary hyperparathyroidism: is less than a subtotal resection ever appropriate? A study of long-term outcomes. Surgery. 2009;146(6):1130–1137. doi: 10.1016/j.surg.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto T., Tominaga Y., Okada M., Hiramitsu T., Tsujita M., Goto N. Characteristics of persistent hyperparathyroidism after renal transplantation. World J Surg. 2016;40(3):600–606. doi: 10.1007/s00268-015-3314-z. [DOI] [PubMed] [Google Scholar]
- 24.Ikizler T.A., Kliger A.S. Minimising the risk of COVID-19 among patients on dialysis. Nat Rev Nephrol. 2020 doi: 10.1038/s41581-020-0280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ketteler M., Block G.A., Evenepoel P., Fukagawa M., Herzog C.A., McCann L. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what's changed and why it matters. Kidney Int. 2017;92(1):26–36. doi: 10.1016/j.kint.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Daher R., Lifante J.C., Voirin N., Peix J.L., Colin C., Kraimps J.L. Is it possible to limit the risks of thyroid surgery? Ann Endocrinol (Paris) 2015;76(1 Suppl 1):1S16–1S26. doi: 10.1016/S0003-4266(16)30010-5. [DOI] [PubMed] [Google Scholar]
- 27.Tabchouri N., Anil Z., Marques F., Michot N., Dumont P., Arnault V. Morbidity of total thyroidectomy for substernal goiter: a series of 70 patients. J Visc Surg. 2018;155(1):11–15. doi: 10.1016/j.jviscsurg.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Sephton B.M. Extracervical approaches to thyroid surgery: evolution and review. Minim Invasive Surg. 2019;2019:5961690. doi: 10.1155/2019/5961690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kowalski L.P., Sanabria A., Ridge J.A., Ng W.T., de Bree R., Rinaldo A. COVID-19 pandemic: effects and evidence-based recommendations for otolaryngology and head and neck surgery practice. Head Neck. 2020 doi: 10.1002/hed.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirallie E., Caillard C., Pattou F., Brunaud L., Hamy A., Dahan M. Does intraoperative neuromonitoring of recurrent nerves have an impact on the postoperative palsy rate? Results of a prospective multicentre study. Surgery. 2018;163(1):124–129. doi: 10.1016/j.surg.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 32.Schneider R., Randolph G.W., Dionigi G., Wu C.W., Barczynski M., Chiang F.Y. International neural monitoring study group guideline 2018 part I: staging bilateral thyroid surgery with monitoring loss of signal. Laryngoscope. 2018;128(Suppl 3):S1–S17. doi: 10.1002/lary.27359. [DOI] [PubMed] [Google Scholar]
- 33.Genser L., Tresallet C., Godiris-Petit G., Li Sun Fui S., Salepcioglu H., Royer C. Randomised controlled trial of alfacalcidol supplementation for the reduction of hypocalcemia after total thyroidectomy. Am J Surg. 2014;207(1):39–45. doi: 10.1016/j.amjsurg.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Delle Fave G., O’Toole D., Sundin A., Taal B., Ferolla P., Ramage J.K. ENETS consensus guidelines update for gastroduodenal neuroendocrine neoplasms. Neuroendocrinology. 2016;103(2):119–124. doi: 10.1159/000443168. [DOI] [PubMed] [Google Scholar]
- 35.Di Fiore F., Bouché O., Lepage C., Sefriou D., Gangloff A., Schwarz L. Propositions of alternatives in digestive cancers management during the COVID-19 epinemic period: A French Intergroup clinical point of view (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, SFR) Dig Liver Dis. 2020 doi: 10.1016/j.dld.2020.03.031. [Thésaurus National de Cancérologie Digestive (http://www.tncd.org)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia-Carbonero R., Sorbye H., Baudin E., Raymond E., Wiedenmann B., Niederle B. ENETS consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumours and neuroendocrine carcinomas. Neuroendocrinology. 2016;103(2):186–194. doi: 10.1159/000443172. [DOI] [PubMed] [Google Scholar]
- 37.Hill J.S., McPhee J.T., McDade T.P., Zhou Z., Sullivan M.E., Whalen G.F. Pancreatic neuroendocrine tumours: the impact of surgical resection on survival. Cancer. 2009;115(4):741–751. doi: 10.1002/cncr.24065. [DOI] [PubMed] [Google Scholar]
- 38.Jensen R.T., Cadiot G., Brandi M.L., de Herder W.W., Kaltsas G., Komminoth P. ENETS consensus guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumour syndromes. Neuroendocrinology. 2012;95(2):98–119. doi: 10.1159/000335591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaujoux S., Mihai R., Joint working group of E, Ensat. European Society of Endocrine Surgeons (ESES) and European Network for the Study of Adrenal Tumours (ENSAT) recommendations for the surgical management of adrenocortical carcinoma. Br J Surg. 2017;104(4):358–376. doi: 10.1002/bjs.10414. [DOI] [PubMed] [Google Scholar]
- 40.Mirallie E., Blanchard C., Caillard C., Rodien P., Briet C., Mucci S. Adrenocortical carcinoma: impact of surgical treatment. Ann Endocrinol (Paris) 2019;80(5–6):308–313. doi: 10.1016/j.ando.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Caiazzo R., Marciniak C., Lenne X., Clement G., Theis D., Menegaux F. Adrenalectomy Risk Score: an original preoperative surgical scoring system to reduce mortality and morbidity after adrenalectomy. Ann Surg. 2019;270(5):813–819. doi: 10.1097/SLA.0000000000003526. [DOI] [PubMed] [Google Scholar]
- 42.Neumann H.P.H., Young W.F., Jr., Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381(6):552–565. doi: 10.1056/NEJMra1806651. [DOI] [PubMed] [Google Scholar]
- 43.Donatini G., Caiazzo R., Do Cao C., Aubert S., Zerrweck C., El-Kathib Z. Long-term survival after adrenalectomy for stage I/II adrenocortical carcinoma (ACC): a retrospective comparative cohort study of laparoscopic versus open approach. Ann Surg Oncol. 2014;21(1):284–291. doi: 10.1245/s10434-013-3164-6. [DOI] [PubMed] [Google Scholar]
- 44.Arlt W., Allolio B. Adrenal insufficiency. Lancet. 2003;361(9372):1881–1893. doi: 10.1016/S0140-6736(03)13492-7. [DOI] [PubMed] [Google Scholar]
- 45.Reber P.M., Heath H., 3rd. Hypocalcemic emergencies. Med Clin North Am. 1995;79(1):93–106. doi: 10.1016/s0025-7125(16)30086-4. [DOI] [PubMed] [Google Scholar]
- 46.Clyde P.W., Harari A.E., Getka E.J., Shakir K.M. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomised controlled trial. JAMA. 2003;290(22):2952–2958. doi: 10.1001/jama.290.22.2952. [DOI] [PubMed] [Google Scholar]