Introduction
In late December 2019, clusters of patients with interstitial pneumonia of unknown cause were reported by some local health facilities in Wuhan (China). The Chinese Centre for Disease Control conducted an epidemiologic and etiologic investigation, leading to the identification of a novel coronavirus (SARS-CoV-2).1, 2 On March 11th, the World Health Organization (WHO) declared the novel coronavirus disease (COVID-19) a pandemic. In the area of Wuhan, COVID-19 mainly affected male patients (around 60%), with a median age of about 50 years; 40% of patients developed Acute Respiratory Distress Syndrome (ARDS) 5% requiring intensive care. The mortality rate was around 2%.3, 4 However, Grasselli et al. found that the mortality was 26% in ICU. The death rate was higher among those who were older.5
In a more recent report from Italy including 22512 patients, COVID-19 has infected 2026 healthcare workers, with a total case fatality rate of 7.2%. Patients were predominantly older than 60 years, 46.1% had mild severity, while 24.9% severe disease.6
To date (April 16th) the cases are 1991562 with more than 130 000 deaths.7 In a certain percentage of patients, COVID-19 is a viral interstitial pneumonia8 characterized by fever, dry cough, dyspnoea, and bilateral ground-glass opacities,9 with about 67% of patients evolving to a severe pneumonia.10, 11
However, preliminary observations reported that COVID-19 patients, compared to conventional ARDS, are characterized by moderate to severe hypoxaemia despite a relatively high pulmonary compliance.12, 13 A potential mechanism may be loss of hypoxic vasoconstriction, explaining the observed severe hypoxaemia and the effect of very high levels of positive end-expiratory pressure (PEEP) on oxygenation not depending on lung recruitment.13 High levels of PEEP may adjust redistribution of perfusion diverting flow towards high ventilation-perfusion (Va/Q) areas increasing arterial oxygen tension (PaO2); however, over-distension of the healthy lung areas and an increased right cardiac afterload is possible.13
In patients with mild to moderate ARDS, with a PaO2 to inspired oxygen fraction (PaO2/FiO2) >150, different modalities of non-invasive respiratory support (NIRS) might be attempted in order to avoid intubation.14, 15 However, NIRS could potentially lead to intubation delay and cause a self-inflicted lung-injury (SILI),16 due to the high transpulmonary pressures. SILI in turn would lead to a severe decrease in lung compliance.17 Continuous Positive Airway Pressure (CPAP) is a form of NIRS during which a fixed level of PEEP is applied to the airways, while the entire work of breathing is generated by the patient's respiratory muscles (i.e. no pressure assist is provided during inspiration). This would reduce the likelihood of generating high transpulmonary pressure and tidal volume compared to non-invasive intermittent positive pressure ventilation.18
Due to the enormous number of COVID-19 patients with acute respiratory failure and to the shortage of ICU beds and ventilators, helmet CPAP (hCPAP) is widely used in Italy.5, 19
In particular, in a scenario of a discrepancy between facilities and a large number of casualties, as with COVID-19 pandemic, the application of hCPAP might be useful as an “easy to perform” supportive strategy.
Prone position sessions may adjust pulmonary perfusion diverting flow towards high Va/Q areas, and allowing a redistribution of aerated and non-aerated areas whenever present.20, 21 Furthermore, as opposed to non-invasive intermittent positive pressure, hCPAP does not necessarily need a ventilator (potentially in short supply in case of mass casualties) and it is not affected by patient-ventilator asynchrony, a determinant of discomfort and treatment failure.22, 23, 24
The hypothesis is that in case of a pandemic, selected COVID-19 patients may benefit from the combination of early hCPAP and prone position sessions, in order to reduce the need for intubation and invasive mechanical ventilation, “buying time” for the disease to heal.
Evaluation of the hypothesis
Prone position
Prone position was first described in 1976 in patients with ARDS.25 First, prone position modifies respiratory mechanics. In particular, the ventral chest wall cannot expand, because it is in contact with the firm surface of the bed.26 In patients with ARDS, the lung weight increases by 4–5 times, pulmonary tissue becomes stiffer and compliance decreases, in association with compression atelectasis.26, 27 During prone position, decreased chest wall compliance improves the redistribution of lung density from dorsal to ventral areas, and increases lung aeration from ventral to atelectatic dorsal regions, improving gas exchange.28 Nowadays, the application of prone position is recommended in most severely ill patients. Guerin et al. showed that, in patients with severe ARDS, the application of prolonged (17 h) prone position sessions for approximately 4 days reduced the absolute mortality risk by 17% and the relative risk by 50%.29 However, other studies have not shown outcome benefits of prone position.30, 31, 32, 33
These differences might be explained by the fact that patients included were not so severely ill, periods of use were shorter and the use of protective ventilation strategies were less strictly enforced.34 Early application of prone position for prolonged (up to 16 h) periods has been also demonstrated to improve the survival rate29 in other clinical settings.
It has been suggested that prone position in COVID-19 patients may lead to overwork of professionals with scarce clinical efficacy in terms of recruitment.13 However, preliminary data suggest that COVID-19 patients undergoing CPAP may benefit from this treatment with even the most severe forms of hypoxaemic respiratory failure characterized by a refractory hypoxaemia (i.e. PaO2/FiO2 < 150).5
Our hypothesis is that, selected COVID-19 patients may benefit from the combination of early hCPAP at moderate levels of PEEP (i.e. 10 cmH2O) and prone position, to avoid overdistension of the healthy lung areas thus slowing the progression of the disease and allowing patients to “buy time” to heal. Indeed, in these instances, hCPAP is likely be effective by keeping the lung open20 and reducing venous admixture by diverting flow towards better high Va/Q areas.21
Non-invasive respiratory support
Although life-saving, invasive mechanical ventilation is associated with side effects and complications leading to increased morbidity and mortality. Therefore, alternative strategies have been proposed, especially for those patients with less severe forms of ARDS. Among these strategies, NIRS might play a role in reducing intubation rate.14 Application of positive pressure to the airway may open collapsed alveoli, increases functional residual capacity and improve the Va/Q match and lung compliance. As a result, oxygenation and respiratory workload improve, with the potential benefit of avoiding intubation and invasive mechanical ventilation.14 More recently, the combination of non-invasive intermittent positive pressure with prone position was shown to prevent the need for intubation in up to half of the patients with moderate to severe ARDS. In addition, patients failing NIRS and requiring intubation were more severe, as compared to those succeeded.35
The use of CPAP may provide the application of a stable level of positive airway pressure throughout the entire respiratory cycle. Therefore, it may result in effective recruitment of closed alveoli, with an increase in the functional residual capacity and improvement of oxygenation.36, 37 During NIRS, comfort is one of the determinants of treatment success or failure.38 CPAP may be delivered through different interfaces, such as masks or helmets. Compared to masks, helmets are more comfortable,39 they allow longer continuous application of the treatment and lower complications correlated to the interface (i.e. eye irritation, gastric distension and skin necrosis).39 As during NIRS, comfort is one of the determinants of treatment success or failure38, 39 it is important to note that unintentional leaks are kept to a minimum during hCPAP.40, 41, 42
The “helmet bundle” in COVID-19 patients has recently been published to optimize treatment.19
Precautions when using NIRS
It is worth noting that the recent guidelines on the use of NIRS in de novo hypoxaemic acute respiratory failure do not provide any recommendation, due to uncertain and conflicting evidence.43 Very recently, the Surviving Sepsis Campaign has provided some guidelines on the management of critically ill COVID-19 patients.44 The panel of experts suggested a trial of NIRS, recommending close short-interval monitoring for worsening of respiratory status and early intubation in a controlled setting if worsening occurs,44 although the main risk of using NIRS in de novo ARF is delay in intubation44 with the risk of developing SILI.16, 17
NIRS in the era of COVID-19
Non-invasive intermittent positive pressure requires the use of mechanical ventilators, of there is currently a shortage due to the pandemic COVID-19.3, 6 Furthermore, non-invasive intermittent positive pressure may also worsen patient-ventilator interaction and synchrony, which might be detrimental for patients’ comfort, leading to treatment failure.22, 23, 24 For these reasons, CPAP might be a valid alternative. In addition, the use of an interface such as the helmet may be advantageous, compared to a facial mask.45, 46, 47 In fact, the helmet improves comfort of the patient, assures prolonged continuous application of the treatment and it is characterized by very low air-leaks,40, 45, 46, 47, 48 limiting the spread of the virus in the environment. Interestingly, when a patient coughs, he/she generates a peak cough flow up to 400 L/min, theoretically creating less contamination for the operators and environment. High flow nasal therapy could be also combined with hCPAP.49, 50 However, experimental studies of exhaled air dispersion by mannequins have demonstrated greater exhaled air dispersion with conventional low flow nasal cannula at 5 l/min, compared with HFNC.51 Furthermore, the helmet's ports can be protected with two antimicrobial filters, further reducing air dispersion. This is of utmost importance in cases of infections transmitted by aerosolization, such as COVID-19.41
Based on these facts, we have hypothesized that, in the current COVID-19 pandemic emergency, an attempt to combine early hCPAP and prone positioning sessions might improve oxygenation in selected patients. Criteria to attempt hCPAP (8–12 cmH2O) and prone position in fully collaborating symptomatic patients are listed in Table 1 . The presence of dyspnoea, as defined by a Borg scale >3,52 is not deemed necessary because dyspnoea is not always clinically evident in these patients. Patients with chronic obstructive pulmonary disease or with an arterial partial pressure of carbon dioxide >50 mmHg will be excluded.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria | Esclusion criteria |
|---|---|
| Cough, fever, sign of interstitial pneumonia on chest X-Ray, lung ultrasound and/or CT-scan | Chronic obstructive pulmonary disease |
| History of previous contact with COVID-19 patients | Arterial partial pressure of carbon dioxide >50 mmHg |
| Patients with a PaO2/FiO2 between 200 and 300, while breathing in room air or through Venturi mask measured after 1 h from hospital admission | Pregnancy |
| Pulse oxymetry (SpO2) <95% in room air | Contraindication to CPAP |
| Dyspnoea, as defined by a Borg scale >3 |
Settings for CPAP in prone position
Prone position during hCPAP requires some precautions, in order to avoid discomfort, skin and eyes lesion and treatment failure. First, the use of helmet without armpit braces is preferable, although not mandatory, since in the literature it has been reported to be more comfortable.48, 53 Another important precaution is to prevent the rigid collar from generating skin lesions by direct pressure on skin due to ischaemia or shearing forces and mechanical stress to the neck. Interestingly awake patients during hCPAP may assume prone position with minimal assistance. Fig. 1 shows one patient switched from supine to prone position using hCPAP and continuous tidal volume measurement using a dedicated software built into a turbine driven ventilator.54
Figure 1.
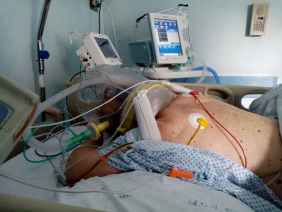
The figure shows a patient in prone position receiving hCPAP. One small pillow is positioned under the chest and another one under the head, to raise the head and to leave some free space around the neck.
Consequence of the hypothesis and discussion
This is the first proposal of a study aimed at investigating the possibility of combining hCPAP and prone position in order to avoid deterioration of gas exchange and intubation in patients affected by COVID-19 pneumonia.
The application of early sessions of pronation in patients with mild-to-moderate ARDS might improve gas exchange without further increasing PEEP.26, 27 This experimental plan has several strengths. First, a thorough literature review and straight-forward protocol definition will guarantee the best possibilities for the intervention. The experimental treatment has notable possibilities of being effective and helpful, in particular in a setting of mass-casualties happening now in Italy and rest of Europe. Second, the ability to limit the treatment to selected patients may amplify the potential benefits reducing the failure rate. Third, if the combination of hCPAP and prone position reduced the intubation rate, the health care system could improve the allocation of ICU beds, granting better treatment to all patients needing ventilatory assistance.
Furthermore, our preliminary results (Gregoretti et al., unpublished data) from an ongoing pilot study in COVID-19 patients, measuring tidal volume during hCPAP,54 showed that a low mean tidal volume coupled with high pulmonary compliance and a low respiratory rate, which suggests that transpulmonary pressure is kept low.
Our hypothesis has also some major limitations. First of all, the real effect of hCPAP from the pathophysiological point of view in this disease is unknown. In healthy patients, CPAP in prone position causes a Va/Q mismatch for a more uniform ventilation distribution, despite a higher perfusion in dependent parts of the lung.33, 55 CPAP may increase alveolar pressures and resistance in small vessels of the lungs (zone 1)56 especially in nondependent lung regions, explaining the higher perfusion in dependent parts when the subject is prone. This finding suggests the redistribution of perfusion could improve oxygenation in patients lacking hypoxic vasoconstriction. Second, inclusion criteria for this treatment are untested; in addition, time in prone position from our preliminary data is shorter than in sedated patients. Third, strict monitoring by trained personnel, in a step-down unit or in a monitored unit, would be required to early identify a treatment failure and avoid any intubation delay. Lastly, patient's tolerance may play a fundamental role in the treatment. In addition, the lack of patient-ventilator asynchronies during CPAP together with the use of the “helmet bundle” should ameliorate tolerance.41
In conclusion, if our hypothesis is valid, physicians may reduce the need for endotracheal intubation and invasive mechanical ventilation, shortening the hospital length of stay and improving survival rates. Furthermore, the need for ICU beds may be reduced, in favour of sub-intensive beds. This setting does not exclude the need of implementation in areas where prompt intubation can be easily performed. In addition, this strategy may also be an adjunctive tool for those patients who are not recommended for endotracheal intubation and care is limited to hCPAP as “ceiling of treatment”.
Otherwise, patients treated with hCPAP presenting with clinical signs of excessive inspiratory effort, should be promptly intubated to avoid too injurious transpulmonary pressure leading to SILI.16
Conflicts of interest
Dr. Navalesi's research laboratory has received equipment and grants from Maquet Critical Care, Draeger and Intersurgical S.p.A. He also received honoraria/speaking fees from Maquet Critical Care, Orionpharma, Philips, Resmed, MSD and Novartis. Dr. Navalesi contributed to the development of the helmet Next, whose licence for patent belongs to Intersurgical S.P.A., and receives royalties for that invention. Dr. Longhini and Dr. Navalesi contributed to the development of a new device, whose patent is in progress (European Patent application number EP20170199831). Dr. Cortegiani, Dr. Accurso, and Dr. Gregoretti declare a patent pending, in association with the University of Palermo – Italy (No. 102019000020532 – Italian Ministry of Economic Development) related to the content of this manuscript. Dr. Gregoretti received fees for lectures by Philips, and received payments by Philips for consultancies in the developing process of the EVO Ventilator and fees for lectures or consultancies from Resmed, Vivisol and Air Liquide not related to the present work. The remaining authors have no conflict of interest to disclose.
Sources of support
None.
Funding
None declared.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Froes F. And now for something completely different: from 2019-nCoV and COVID-19 to 2020-nMan. Pulmonology. 2020;26:114–115. doi: 10.1016/j.pulmoe.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Livingston E., Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization . 2020. Coronavirus disease (COVID-2019) situation reports. [Google Scholar]
- 8.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020 doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K. Covid-19 in critically ill patients in the seattle region – case series. N Engl J Med. 2020 doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gattinoni L., Coppola S., Cressoni M., Busana M., Chiumello D. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demoule A., Hill N., Navalesi P. Can we prevent intubation in patients with ARDS? Intensive Care Med. 2016;42:768–771. doi: 10.1007/s00134-016-4323-6. [DOI] [PubMed] [Google Scholar]
- 15.Cortegiani A., Madotto F., Gregoretti C., Bellani G., Laffey J.G., Pham T. Immunocompromised patients with acute respiratory distress syndrome: secondary analysis of the LUNG SAFE database. Crit Care. 2018;22:157. doi: 10.1186/s13054-018-2079-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brochard L., Slutsky A., Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 17.Grieco D.L., Menga L.S., Raggi V., Bongiovanni F., Anzellotti G.M., Tanzarella E.S. Physiological comparison of high-flow nasal cannula and helmet noninvasive ventilation in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2020;201:303–312. doi: 10.1164/rccm.201904-0841OC. [DOI] [PubMed] [Google Scholar]
- 18.Carteaux G., Millan-Guilarte T., De Prost N., Razazi K., Abid S., Thille A.W. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: role of tidal volume. Crit Care Med. 2016;44:282–290. doi: 10.1097/CCM.0000000000001379. [DOI] [PubMed] [Google Scholar]
- 19.Lucchini A., Giani M., Isgro S., Rona R., Foti G. The “helmet bundle” in COVID-19 patients undergoing non invasive ventilation. Intensive Crit Care Nurs. 2020;1028:59. doi: 10.1016/j.iccn.2020.102859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cammarota G., Vaschetto R., Turucz E., Dellapiazza F., Colombo D., Blando C. Influence of lung collapse distribution on the physiologic response to recruitment maneuvers during noninvasive continuous positive airway pressure. Intensive Care Med. 2011;37:1095–1102. doi: 10.1007/s00134-011-2239-8. [DOI] [PubMed] [Google Scholar]
- 21.Lindner K.H., Lotz P., Ahnefeld F.W. Continuous positive airway pressure effect on functional residual capacity, vital capacity and its subdivisions. Chest. 1987;92:66–70. doi: 10.1378/chest.92.1.66. [DOI] [PubMed] [Google Scholar]
- 22.Garofalo E., Bruni A., Pelaia C., Liparota L., Lombardo N., Longhini F. Recognizing, quantifying and managing patient-ventilator asynchrony in invasive and noninvasive ventilation. Expert Rev Respir Med. 2018;12:557–567. doi: 10.1080/17476348.2018.1480941. [DOI] [PubMed] [Google Scholar]
- 23.Cortegiani A., Ippolito M., Lujan M., Gregoretti C. Tidal volume and helmet: is the never ending story coming to an end? Pulmonology. 2020 doi: 10.1016/j.pulmoe.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Bruni A., Garofalo E., Pelaia C., Messina A., Cammarota G., Murabito P. Patient-ventilator asynchrony in adult critically ill patients. Minerva Anestesiol. 2019;85:676–688. doi: 10.23736/S0375-9393.19.13436-0. [DOI] [PubMed] [Google Scholar]
- 25.Piehl M.A., Brown R.S. Use of extreme position changes in acute respiratory failure. Crit Care Med. 1976;4:13–14. doi: 10.1097/00003246-197601000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Pelosi P., D’Andrea L., Vitale G., Pesenti A., Gattinoni L. Vertical gradient of regional lung inflation in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149:8–13. doi: 10.1164/ajrccm.149.1.8111603. [DOI] [PubMed] [Google Scholar]
- 27.Gattinoni L., Caironi P., Cressoni M., Chiumello D., Ranieri V.M., Quintel M. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354:1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 28.Pelosi P., Tubiolo D., Mascheroni D., Vicardi P., Crotti S., Valenza F. Effects of the prone position on respiratory mechanics and gas exchange during acute lung injury. Am J Respir Crit Care Med. 1998;157:387–393. doi: 10.1164/ajrccm.157.2.97-04023. [DOI] [PubMed] [Google Scholar]
- 29.Guerin C., Reignier J., Richard J.C., Beuret P., Gacouin A., Boulain T. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 30.Gattinoni L., Tognoni G., Pesenti A., Taccone P., Mascheroni D., Labarta V. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345:568–573. doi: 10.1056/NEJMoa010043. [DOI] [PubMed] [Google Scholar]
- 31.Guerin C., Gaillard S., Lemasson S., Ayzac L., Girard R., Beuret P. Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA. 2004;292:2379–2387. doi: 10.1001/jama.292.19.2379. [DOI] [PubMed] [Google Scholar]
- 32.Taccone P., Pesenti A., Latini R., Polli F., Vagginelli F., Mietto C. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2009;302:1977–1984. doi: 10.1001/jama.2009.1614. [DOI] [PubMed] [Google Scholar]
- 33.Mure M., Nyren S., Jacobsson H., Larsson S.A., Lindahl S.G. High continuous positive airway pressure level induces ventilation/perfusion mismatch in the prone position. Crit Care Med. 2001;29:959–964. doi: 10.1097/00003246-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Gattinoni L., Taccone P., Carlesso E., Marini J.J. Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. Am J Respir Crit Care Med. 2013;188:1286–1293. doi: 10.1164/rccm.201308-1532CI. [DOI] [PubMed] [Google Scholar]
- 35.Ding L., Wang L., Ma W., He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24:28. doi: 10.1186/s13054-020-2738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cosentini R., Brambilla A.M., Aliberti S., Bignamini A., Nava S., Maffei A. Helmet continuous positive airway pressure vs oxygen therapy to improve oxygenation in community-acquired pneumonia: a randomized, controlled trial. Chest. 2010;138:114–120. doi: 10.1378/chest.09-2290. [DOI] [PubMed] [Google Scholar]
- 37.Delclaux C., L’Her E., Alberti C., Mancebo J., Abroug F., Conti G. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA. 2000;284:2352–2360. doi: 10.1001/jama.284.18.2352. [DOI] [PubMed] [Google Scholar]
- 38.Elliott M.W. The interface: crucial for successful noninvasive ventilation. Eur Respir J. 2004;23:7–8. doi: 10.1183/09031936.03.00115903. [DOI] [PubMed] [Google Scholar]
- 39.Antonelli M., Conti G., Pelosi P., Gregoretti C., Pennisi M.A., Costa R. New treatment of acute hypoxemic respiratory failure: noninvasive pressure support ventilation delivered by helmet – a pilot controlled trial. Crit Care Med. 2002;30:602–608. doi: 10.1097/00003246-200203000-00019. [DOI] [PubMed] [Google Scholar]
- 40.Cammarota G., Olivieri C., Costa R., Vaschetto R., Colombo D., Turucz E. Noninvasive ventilation through a helmet in postextubation hypoxemic patients: physiologic comparison between neurally adjusted ventilatory assist and pressure support ventilation. Intensive Care Med. 2011;37:1943–1950. doi: 10.1007/s00134-011-2382-2. [DOI] [PubMed] [Google Scholar]
- 41.Hui D.S., Chow B.K., Lo T., Ng S.S., Ko F.W., Gin T. Exhaled air dispersion during noninvasive ventilation via helmets and a total facemask. Chest. 2015;147:1336–1343. doi: 10.1378/chest.14-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cabrini L., Landoni G., Zangrillo A. Minimise nosocomial spread of 2019-nCoV when treating acute respiratory failure. Lancet. 2020;395:685. doi: 10.1016/S0140-6736(20)30359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rochwerg B., Brochard L., Elliott M.W., Hess D., Hill N.S., Nava S. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017:50. doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 44.Alhazzani W., Møller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020 doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Longhini F., Abdalla K., Navalesi P. Non-invasive ventilation in hypoxemic patients: does the interface make a difference? Ann Transl Med. 2016;4:359. doi: 10.21037/atm.2016.09.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Longhini F., Liu L., Pan C., Xie J., Cammarota G., Bruni A. Neurally-adjusted ventilatory assist for noninvasive ventilation via a helmet in subjects with copd exacerbation: a physiologic study. Respir Care. 2019;64:582–589. doi: 10.4187/respcare.06502. [DOI] [PubMed] [Google Scholar]
- 47.Vaschetto R., Longhini F., Persona P., Ori C., Stefani G., Liu S. Early extubation followed by immediate noninvasive ventilation vs. standard extubation in hypoxemic patients: a randomized clinical trial. Intensive Care Med. 2019;45:62–71. doi: 10.1007/s00134-018-5478-0. [DOI] [PubMed] [Google Scholar]
- 48.Cammarota G., Longhini F., Perucca R., Ronco C., Colombo D., Messina A. New setting of neurally adjusted ventilatory assist during noninvasive ventilation through a helmet. Anesthesiology. 2016;125:1181–1189. doi: 10.1097/ALN.0000000000001354. [DOI] [PubMed] [Google Scholar]
- 49.Mauri T., Spinelli E., Mariani M., Guzzardella A., Del Prete C., Carlesso E. Nasal high flow delivered within the helmet: a new noninvasive respiratory support. Am J Respir Crit Care Med. 2019;199:115–117. doi: 10.1164/rccm.201806-1124LE. [DOI] [PubMed] [Google Scholar]
- 50.Garofalo E., Bruni A., Pelaia C., Cammarota G., Murabito P., Biamonte E. Evaluation of a new interface combining high-flow nasal cannula and CPAP. Respir Care. 2019;64:1231–1239. doi: 10.4187/respcare.06871. [DOI] [PubMed] [Google Scholar]
- 51.Greenland J.R., Michelow M.D., Wang L., London M.J. COVID-19 infection: implications for perioperative and critical care physicians. Anesthesiology. 2020 doi: 10.1097/ALN.0000000000003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borg G.A. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 53.Olivieri C., Longhini F., Cena T., Cammarota G., Vaschetto R., Messina A. New versus conventional helmet for delivering noninvasive ventilation: a physiologic, crossover randomized study in critically ill patients. Anesthesiology. 2016;124:101–108. doi: 10.1097/ALN.0000000000000910. [DOI] [PubMed] [Google Scholar]
- 54.Cortegiani A., Navalesi P., Accurso G., Sabella I., Misseri G., Ippolito M. Tidal volume estimation during helmet noninvasive ventilation: an experimental feasibility study. Sci Rep. 2019;9:17324. doi: 10.1038/s41598-019-54020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nyren S., Mure M., Jacobsson H., Larsson S.A., Lindahl S.G. Pulmonary perfusion is more uniform in the prone than in the supine position: scintigraphy in healthy humans. J Appl Physiol (1985) 1999;86:1135–1141. doi: 10.1152/jappl.1999.86.4.1135. [DOI] [PubMed] [Google Scholar]
- 56.Benumof J.L. Anesthesia. WB Saunders; Philadelphia: 1994. Respiratory physiologyand respiratory function during anesthesia; pp. 577–620. [Google Scholar]


