Abstract
The novel severe acute respiratory syndrome coronavirus 2 is causing a worldwide pandemic that may lead to a highly morbid and potentially fatal coronavirus disease 2019 (COVID-19). There is currently no drug that has been proven as an effective therapy for COVID-19. Several candidate drugs are being considered and evaluated for treatment. This includes clinically available drugs, such as chloroquine, hydroxychloroquine, and lopinavir/ritonavir, which are being repurposed for the treatment of COVID-19. Novel experimental therapies, such as remdesivir and favipiravir, are also actively being investigated for antiviral efficacy. Clinically available and investigational immunomodulators, such as the interleukin 6 inhibitors tocilizumab and sarilumab and the anti–granulocyte-macrophage colony-stimulating factor lenzilumab, are being tested for their anticipated effect in counteracting the pro-inflammatory cytokine environment that characterizes severe and critical COVID-19. This review article examines the evidence behind the potential use of these leading drug candidates for the treatment of COVID-19. The authors conclude, based on this review, that there is still no high-quality evidence to support any of these proposed drug therapies. The authors, therefore, encourage the enrollment of eligible patients to multiple ongoing clinical trials that assess the efficacy and safety of these candidate therapies. Until the results of controlled trials are available, none of the suggested therapeutics is clinically proven as an effective therapy for COVID-19.
Abbreviations and Acronyms: ACE2, angiotensin-converting enzyme 2; ARDS, acute respiratory distress syndrome; CC, 50% cytotoxic concentration; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; EC50, half-maximal effective concentration; FDA, US Food and Drug Administration; GM-CSF, granulocyte-macrophage colony-stimulating factor; HIV, human immunodeficiency viruses; IFN-α, interferon-alpha; IFN-β, interferon-beta; IL-6, interleukin 6; LPV, lopinavir; LPV/r, lopinavir/ritonavir; MERS-CoV, Middle East respiratory syndrome–related coronavirus; SARS, severe acute respiratory syndrome; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Article Highlights.
-
•
Antiviral and immunomodulatory therapies, investigational and re-purposed, for the management of coronavirus disease-2019 are reviewed. The use of these drugs should be under clinical trial protocols.
-
•
The mechanisms of action for treatment options being considered for coronavirus disease 2019 and their potential toxicities are discussed.
-
•
A hypothetical protocol that may serve as a starting point for health care providers to develop institution-specific protocols based on available resources.
In December 2019, a novel coronavirus outbreak was reported from Wuhan in the Hubei province of China. As of April 16, 2020, the virus has infected more than 2,090,000 people worldwide, resulting in more than 139,000 deaths.1 Initially called novel coronavirus, it was officially named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) because of its similarities with the agent that caused the severe acute respiratory syndrome (SARS) outbreak in 2003.2 Severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome–related coronavirus (MERS), and SARS-CoV-2 belong to the Genus betacoronavirus and are agents of respiratory infections in humans.
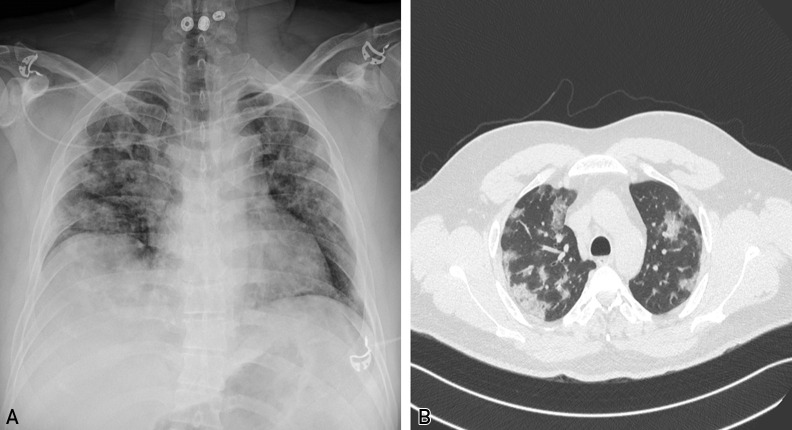
The infection caused by SARS-CoV-2 manifests as coronavirus disease 2019 (COVID-19). The majority of patients infected with SARS-CoV-2 either remain asymptomatic or demonstrate mild symptoms and recover from their illness. The most common clinical presentation is pneumonia with symptoms of fever, dry cough, and shortness of breath.3 Anosmia, dysgeusia, and diarrhea have been reported.4 Approximately 20% to 30% of patients require intensive care for respiratory support, and more than 15% of patients with severe pneumonia develop acute respiratory distress syndrome (ARDS) (Figure 1 ).5 , 6
Figure 1.
A, Chest x-ray showing diffuse bilateral opacities in a patient with coronavirus disease 2019 and acute respiratory distress syndrome. B, Computed tomography scan of the chest showing peripherally based ground-glass opacities and pulmonary infiltrates.
There is currently no proven drug for the treatment of COVID-19. The standard of care is supportive measures, aimed at managing fever, dehydration, and constitutional and other clinical symptoms. Because of the morbid and potentially fatal nature of COVID-19, there have been efforts to repurpose other clinically available drugs based on antiviral activities that have either been shown in vitro or proposed due to the agents’ mechanism of action. Novel and experimental drugs with potential antiviral properties are also being considered. Finally, therapies that modulate the hyperinflammatory response of the host are being investigated (Table ).7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 Antiviral drugs are believed to be useful early in the course of the disease when it is mediated by active viral replication. Immunomodulating agents are generally being evaluated for use during the later pro-inflammatory process, usually manifesting as clinical deterioration in the second week after symptom onset.
Table.
Candidate Therapies for the Management of Coronavirus Disease 2019a
| Drug | Proposed mechanism of action | Evidence for SARS-CoV-2 | Clinical trials7 | |
|---|---|---|---|---|
| Hydroxychloroquine and chloroquine | Blocks viral entry by increasing endosomal pH and inhibiting viral fusion to the cell membrane Decreases affinity of ACE2 receptor for SARS-Cov-2 by impairing terminal glycosylation of ACE2 |
In vitro Clinical |
Wang et al: Chloroquine affected entry and post-entry stages of infection. 500 mg per day of chloroquine would achieve EC50.8 Yao et al: Hydroxychloroquine was more potent (EC50 of 0.72 μM) than chloroquine (EC50 of 5.47 μM).9 Liu et al: Chloroquine had lower EC50 compared with hydroxychloroquine.10 Gao et al: Preliminary data from 100 patients demonstrated chloroquine was better than control (peer review data not available).11 Gautret et al: Hydroxychloroquine was better than control at viral eradiation. This effect was magnified by addition of azithromycin. There are major limitations in the study analysis.12 Gautret et al: Confirmed the efficacy of hydroxychloroquine and azithromycin combination but the study did not have a control group.13 Molina et al: 8/10 (80%) of patients had positive SARS-CoV-2 PCR on days 5-6 after treatment with a combination of hydroxychloroquine and azithromycin. The study did not have a control group.14 Chen et al: Hydroxychloroquine was associated with better clinical outcome compared with control group (80.6% versus 54.8%, respectively). The study is pending peer-review.15 |
NCT04261517NCT04308668NCT04323527NCT04304053NCT04307693 |
| Favipiravir | RNA-dependent RNA polymerase inhibitor | In vitro Clinical |
Wang et al: EC50 of favipiravir was 61.88 which was higher than EC50 for remdesivir and chloroquine.8 Cai et al: The study has been temporarily removed. Open label study comparing favipiravir and LPV/r. Favipiravir was associated with shorter time to viral clearance (median 4 d vs 11 d, P<.001) and significant improvement in chest imaging (improvement rate 91.43% versus 62.22%, P=.004).16 |
NCT04310228 NCT04303299 |
| Lopinavir/ritonavir | Lopinavir is a viral protease inhibitor that blocks viral replication Ritonavir blocks CYP3A4 thereby boosting concentration of lopinavir |
Clinical | Bhatnagar et al, Young et al, Han et al, Lim et al, Wang et al: Case reports or case series of patients treated with LPV/r along with other therapies including Chinese herbal therapies.17, 18, 19, 20, 21 Cao et al: Randomized open label study that showed that LPV/r combination was not significantly better than standard of care.22 Deng et al: combination of arbidol with LPV/r was significantly better than LPV/r alone for nasopharyngeal swab test conversion.23 |
NCT04307693 NCT04276688 |
| Remdesivir | RNA-dependent RNA polymerase inhibitor | In vitro Clinical |
Wang et al: Remdesivir has potent activity against SARS-CoV-2 infected Vero cells8 Holshue et al: First successful use of remdesivir in COVID-19 patient in the United States.24 Kujawski et al: Compassionate use of remdesivir in three of the first 12 cases of COVID-19 in the United States.25 Grein et al: Compassionate use of remdesivir in severe COVID-19 led to improvement in oxygen status in 36 of 53 (67.9%) of patients. The study is limited by absence of control arm.26 |
NCT04323761 NCT04302766 NCT04315948 NCT04252664 NCT04257656 NCT04292899 NCT04292730 |
| IL-6 inhibitors | Curbs cytokine release syndrome by inhibiting IL-6 receptors | Clinical | Xu et al: Defervescence and reduction in supplemental oxygen requirement in 21 patients after tocilizumab use.27 |
NCT04315298 NCT04320615 NCT04317092 NCT04306705 NCT04322188 |
| Anti–GM-CSF | Reduce severity of cytokine release syndrome by inhibiting GM-CSF pathway | No clinical data available as of yet. | ||
| Convalescent plasma | Antibody neutralization of virus | Clinical | Shen et al: Improvement in clinical status following administration of convalescent plasma in addition to antiviral agents in 5 patients with ARDS due to COVID-19.28 |
NCT04323800 NCT04292340 NCT04264858 |
ACE2 = angiotensin-converting enzyme; ARDS = acute respiratory distress syndrome; COVID-19 = coronavirus disease 2019; CYP3A4 = cytochrome P450 3A4; EC50 = half-maximal effective concentration; GM-CSF = granulocyte-macrophage colony-stimulating factor; Il-6 = interleukin 6; LPV/r = lopinavir/ritonavir; PCR = polymerase chain reaction; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
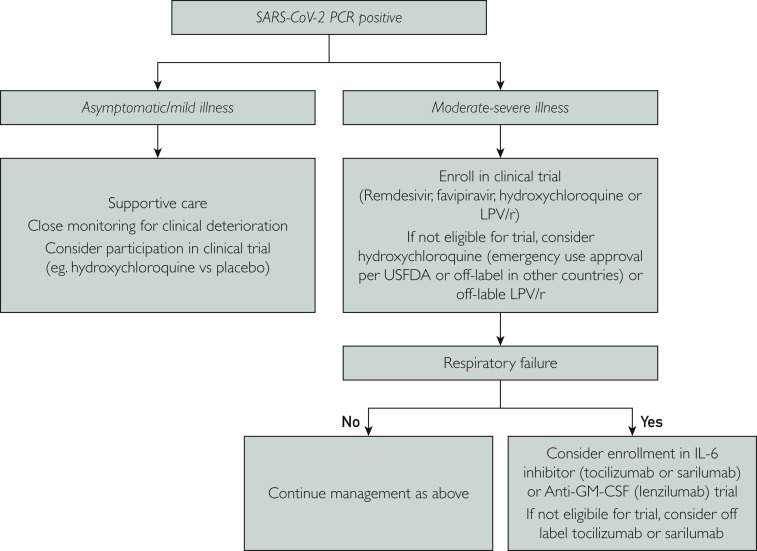
In this article, we aim to provide an objective review of the evidence behind the proposed drug therapies for COVID-19. Electronic search in PubMed, LitCovid, Embase, Google Scholar, and clinicaltrials.gov databases was conducted. Search terms included SARS-CoV-2, COVID-19, 2019-nC0V, and individual drug names. Articles published in English were reviewed and relevant references were checked. The information in this review is only as current as of the time this article is written (April 16, 2020) since new data is anticipated to emerge during this rapidly evolving pandemic. Based on the currently available information, health care institutions are encouraged to create local protocols based on available resources. A hypothetical protocol that may serve as a starting point is depicted in Figure 2 .
Figure 2.
Hypothetical algorithm for treatment of coronavirus disease 2019. GM-CSF = granulocyte-macrophage colony-stimulating factor; IL-6 = interluekin-6; LPV/r = lopinavir/ritonavir; PCR = polymerase chain reaction; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; USFDA = US Food and Drug Administration.
Strategies Directed Against the Virus
Chloroquine and Hydroxychloroquine
Chloroquine is an aminoquinoline antimalarial drug discovered in 1934.29 In addition to antimalarial activity, it has antiviral, anti-inflammatory, and immunomodulatory effects. These properties have led to its use in inflammatory rheumatologic diseases. Various mechanisms have been proposed for its antiviral activity (Table). Chloroquine can rapidly increase the endosomal pH, thereby reducing the fusion between SARS-CoV-2 and the endosome. By impairing terminal glycosylation of angiotensin-converting enzyme 2 (ACE2), it decreases the affinity of SARS-CoV-2 with ACE2, which serves as its main entry point into the cell.
Chloroquine’s antiviral activity has been tested against SARS-CoV, MERS-CoV, human immunodeficiency virus (HIV), dengue, and chikungunya.29, 30, 31 However, it failed to show any benefit in clinical or animal models.32 Paton et al33 tested chloroquine as a prophylactic agent against influenza. In this randomized double-blind placebo-controlled trial, chloroquine failed to prevent influenza infection. Similarly, after showing a promising in vitro effect, chloroquine failed to reduce the duration of dengue viremia and nonstructural protein 1 antigenemia.34 Moreover, chloroquine did not improve acute disease and was associated with more chronic arthralgia in patients with chikungunya infection.35 , 36
Assessing its activity against SARS-CoV-2, Wang et al8 showed, in in vitro studies on Vero cells, that chloroquine affected the entry and post-entry stages of infection. They suggested that a daily dose of 500 mg of chloroquine would achieve the half-maximal effective concentration (EC50) in clinical scenarios. Subsequently, Gao et al11 reported their preliminary finding on the use of chloroquine in 100 patients in Wuhan, China, which concluded that chloroquine prevented exacerbation of pneumonia, improved findings on lung imaging, and shortened the course of the disease. However, the full, peer-reviewed publication of this clinical trial is not available.
Because of a shortage of chloroquine and a superior safety profile, hydroxychloroquine has been proposed as an alternative for the treatment of COVID-19. Yao et al9 compared the EC50 for hydroxychloroquine and chloroquine and found that hydroxychloroquine was more potent (EC50 of 0.72 μM) than chloroquine (EC50 of 5.47 μM). However, in a subsequent analysis, Liu et al10 found chloroquine to show a lower EC50 across four different multiplicities of infection. The antiviral effects of hydroxychloroquine were tested in a small open-label non-randomized trial by Gautret et al.12 In this study, viral eradication was faster in the hydroxychloroquine group compared with the untreated control group; this finding was further magnified in six patients who received azithromycin for prevention of bacterial infection. However, there are major limitations of the study that raise caution in the interpretation of the results. Indeed, the International Society of Antimicrobial Chemotherapy released a statement that the study does not meet the Society’s expected standards.37 Only 20 of 26 patients treated with hydroxychloroquine were included in the final analysis. The SARS-CoV-2 polymerase chain reaction (PCR) cycle threshold (which is inversely related to the viral load) was higher in patients treated with hydroxychloroquine and azithromycin combination (therefore, they have lower viral load and expected to clear sooner). The majority of the patients treated with hydroxychloroquine had milder disease (only 22% patients had lower respiratory tract infection). The comparator group was from a different hospital that may have different clinical practices.38 A follow-up to this study by the same investigators reported the promising clinical and virologic outcomes of 80 patients managed with the combination of hydroxychloroquine and azithromycin. Although the authors confirmed the efficacy of this combination therapy, this single-arm observational report lacks an all-important control group, thereby limiting the strength of their conclusion.13 Molina et al14 also investigated the use of hydroxychloroquine and azithromycin and reported that 8 of 10 (80.0%) patients remain positive for SARS-CoV-2 PCR on days 5 to 6 after treatment initiation. In another study that is yet to be peer-reviewed, clinical improvement was observed in 25 of 31 (80.6%) of patients treated with hydroxychloroquine as compared to 17 of 31 (54.8%) in the control group that received standard treatment (including antivirals other than hydroxychloroquine).15 Collectively, the suggestion of clinical benefit for the use of chloroquine and hydroxychloroquine for SARS-CoV-2 should be interpreted with caution, as it is supported only by limited, and often times conflicting, clinical data. Accordingly, the use of chloroquine or hydroxychloroquine for COVID-19 should be under clinical trials or treatment registries, and their off-label use outside of these trials is not currently recommended.
Before starting hydroxychloroquine or chloroquine, patients should have corrected QT measured.39 Certain patient groups must be tested for glucose-6-phosphate dehydrogenase deficiency, which is associated with hemolytic anemia with chloroquine use. Although chloroquine and hydroxychloroquine are considered safe, the therapeutic index for chloroquine is narrow, and it has been associated with gastrointestinal symptoms, retinopathy, and deafness/tinnitus, as well as life-threatening toxicity, including cardiomyopathy and arrhythmias and methemoglobinemia.40 , 41
Several clinical trials are underway to provide real-world clinical data on the efficacy of chloroquine and hydroxychloroquine for the treatment of COVID-19 (Supplemental Table).11 Based on current limited data, strong recommendations for widespread off-label clinical use cannot be made. However, the US Food and Drug Administration (FDA) has issued an emergency-use authorization of chloroquine and hydroxychloroquine to treat hospitalized patients with COVID-19.42 Clinicians considering the use of these agents are advised to enroll their patients in clinical trials. In the event that trial participation is not possible, careful consideration of the risks versus potential benefits is emphasized. Detailed information on these clinical trials is available in the Supplemental Table (available online at http://www.mayoclinicproceedings.org) and on clinicaltrials.gov.7
Favipiravir
Favipiravir is a non-nucleoside RNA polymerase inhibitor that was developed for the treatment of influenza. It has also been tested against Ebola, Marburg, Nipah, Lassa fever, and Zika viruses.43, 44, 45 Favipiravir was used alone or in combination with monoclonal antibodies as post-exposure prophylaxis for Ebola in at least five health care workers.46 Its use, in combination with ribavirin, potentiated its effect in vitro.45 , 47
There are limited published studies regarding in vitro and clinical data on the use of favipiravir in coronavirus infections. In a study that was previously shared before peer-review but has since been temporarily removed, favipiravir and lopinavir/ritonavir (LPV/r) were compared in an open-label study of 80 patients during the current SARS-CoV-2 pandemic in China.16 When compared with LPV/r, favipiravir was associated with shorter time-to-viral-clearance (median 4 versus 11 days, P<.001) and significant improvement in chest imaging (improvement rate 91.43% versus 62.22%, P=.004). Additionally, the Japanese Health Ministry commented that the use of favipiravir in 70 to 80 patients did not produce similar clinical benefit.48 These contrasting findings call for a randomized controlled trial to assess the safety and efficacy of favipiravir for the treatment of COVID-19 (Table).
Lopinavir/Ritonavir
Lopinavir (LPV) is a protease inhibitor approved for use, in a fixed-dose combination with ritonavir, as part of antiretroviral therapy for HIV infection. LPV/r was used for the treatment of SARS-CoV during the 2003 outbreak. Chu et al49 showed the cytopathic effect of LPV/r on SARS-CoV by in vitro antiviral susceptibility testing, although it showed weak antiviral activity. In a subsequent study of 41 patients who received LPV/r or standard of care, there was a significant difference in the incidence of ARDS or mortality at day 21 (LPV/r = 2.4% versus control = 28.8%, P<.001). A multivariate analysis showed that lack of LPV/r use was independently associated with worse outcomes. Notably, ribavirin was used concurrently with LPV/r because of its known broad-spectrum antiviral activity and possibly an indirect immunomodulatory effect.50 During the 2003 SARS outbreak, Chan et al51 expanded the study to 75 patients treated with LPV/r and compared those with matched controls. All patients also received steroids and ribavirin. In the initial-therapy group of 44 patients, LPV/r was initiated within a median of 5.5 days. When compared with controls (n=634), there was significant decrease in mortality (2.3% versus 15.6%, P<.05), intubation rate (0% versus 11%, P<.05), and the proportion requiring methylprednisolone rescue (27.3% versus 55.4%, P<.05). However, in the rescue-therapy group of 31 patients, LPV/r was administered at a median of 18 days after symptom onset (when patients had been off ribavirin). No statistical difference was seen between LPV/r and the control group (n=343) in mortality, intubation rate, and steroid use. These observations suggest that the efficacy of LPV/r may be best achieved when it is given early in the course of coronavirus infection. The MERS outbreak reignited interest in the anti-coronavirus activity of LPV/r. In vitro data and animal model showed therapeutic potential in human MERS infection.52, 53, 54 A clinical trial, MERS-CoV Infection Treated With a Combination of Lopinavir/Ritonavir and Interferon Beta-1b, (MIRACLE) is ongoing to evaluate the efficacy of LPV/r in combination with interferon beta (IFN-β).55
Because of anecdotal clinical data from the previous coronavirus outbreaks, LPV/r has been used for the SARS-CoV-2 outbreak in China and has been part of the Chinese national guidelines.56 Reports of use in individual cases or series are available; however, it is difficult to critically appraise the data because of concomitant use of Chinese herbal and other therapies.17, 18, 19, 20, 21 In a retrospective cohort study, Deng et al23 has shown that combination of arbidol with LPV/r was significantly better than LPV/r alone for nasopharyngeal swab PCR conversion to negative after 7 d (75% versus 35%, P<.05), after 14 d (94% versus 52.9%, P<.05), and improvement on computed tomography scan of the chest (69% versus 29%, P<.05).23 However, in a randomized controlled open-label study, LPV/r combination was not significantly better than the standard of care for the management of severe COVID-19.22 This randomized controlled trial has some noteworthy limitations. First, the mortality rate was high, which suggests the enrollment of patients with severe COVID-19. Second, the median time from symptom onset to randomization was 13 days, which suggests that the drug may have been administered too late, when viral replication may no longer be the major factor. A total of 42% of patients remained positive at day 28, raising the concern about its antiviral efficacy. An absolute difference of 6.8% in 28-day mortality was observed, but this did not reach statistical significance. Collectively, the data on the use of LPV/r for the treatment of COVID-19 is very limited. Larger controlled clinical studies are needed to evaluate the role of LPV/r (potentially in combination with ribavirin) in COVID-19 (Table).
Remdesivir
Remdesivir is an investigational nucleoside analog that works as an RNA-chain terminator by inhibiting RNA-dependent viral RNA polymerase. It is a monophosphoramidate prodrug of adenosine C-nucleoside,57 and its structure is similar to tenofovir alafenamide, which is active against HIV and hepatitis B virus.58 It was developed by Gilead Sciences (Foster City, CA) for treatment of Ebola during the 2013–2016 outbreak. The use of remdesivir in the rhesus monkey model showed that once-daily intravenous administration resulted in suppression of Ebola viral replication and protected animals from lethal disease.59 Subsequently, it was used in two clinical cases (in combination with other therapies) with a successful outcome.60 , 61 However, a randomized controlled trial in patients with Ebola showed that remdesivir-treated patients had higher mortality compared with three monoclonal antibodies (53.1% versus 33.5%, 35.1%, and 49.7%, respectively).62
Remdesivir was shown to have in vitro activity against SARS-CoV and MERS-CoV.57 , 63, 64, 65 Sheehan et al54 showed that remdesivir, when combined with IFN-β had superior antiviral properties as compared with LPV/r-IFN-β.54 Accordingly, there has been renewed interest in remdesivir for treatment of SARS-CoV-2, especially after early anecdotal use in China suggested that it may offer benefit. However, peer-reviewed clinical data for remdesivir use in COVID-19 is still not available. In vitro data shows that remdesivir has potent activity against SARS-CoV-2–infected Vero cells.8 Remdesivir had higher selectivity index (ratio of half-cytotoxic concentration [CC50] and EC50) when compared with ribavirin, penciclovir, favipiravir, nafamostat, and nitazoxanide (>129.87 and >88.50 versus >3.65, >4.17, >6.46, >4.44, and >16.76, respectively). Holshue et al24 reported the first successful use of remdesivir, given on a compassionate basis, in a 35-year-old US man with COVID-19. Kujawski et al25 further reported that 3 of the first 12 cases of COVID-19 in the United States received compassionate-use remdesivir as treatment. The three patients received remdesivir for 4 to 10 days, which resulted in improvement in respiratory symptoms. All three patients had gastrointestinal symptoms and elevation of serum transaminases. Lescure et al66 reported the outcomes of the first five cases of COVID-19 in Europe, including three who received remdesivir. One of these three, a 31-year-old patient, discontinued remdesivir on day 5 of therapy due to elevated alanine aminotransferase, but recovered. A second, 48-year-old patient, received 10 days of therapy and recovered; and a third, an 80-year-old patient, received 10 days of therapy but died on day 24.
The study of 53 patients who received compassionate remdesivir for severe COVID-19 showed improvement in the oxygen status of 36 (68%) patients.26 The reported mortality rate was 13%, which is lower than the anticipated mortality rate of severe COVID-19, albeit the variability in supportive care and available resources could also account for the difference in mortality. Moreover, the results of this cohort study are limited by the lack of a control group, the exclusion of eight (13%) patients from the final analysis, and a relatively small sample size. There are several ongoing and expanding randomized controlled trials to evaluate the safety and antiviral activity in patients with COVID-19. Currently, the use of remdesivir is restricted to controlled clinical trials, although it is also available through an expanded-access program and a compassionate-use program for individuals who are not eligible to participate in controlled clinical trials. Only results of these controlled clinical trials will provide the data on its true efficacy and safety.
Based on in vitro data, it is known that remdesivir has wide therapeutic index, high genetic barrier to resistance, and long intracellular half-life.38 , 58 , 59 Remdesivir is also highly selective for viral polymerases and has a low propensity for human toxicity. Gilead has now stopped supplying remdesivir for compassionate use in adults but has opened an expanded access program. Most of its drug supply is now reserved for patients enrolled in several ongoing randomized controlled trials. The dose in clinical trials is 200 mg intravenously on day 1 followed by 100 mg intravenously daily for a total of a 5- or 10-day course (Table).
Other Candidate Drugs
Several compounds that are also being proposed, but with much more limited experimental or clinical data, are nitazoxanide (an antiparasitic drug with broad-spectrum antiviral activity), ribavirin (a broad-spectrum antiviral drug), baloxavir (an anti-influenza drug that is undergoing clinical trials in China), among many others. Data on these drugs are scant and very limited and will not be discussed further in this review.
Immunomodulation: Strategies Directed Against Inflammatory Cytokine Storm
A pro-inflammatory stage of COVID-19 that often presents later in the course of infection is believed to be mediated by cytokines, including granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin 6 (IL-6). This is clinically manifested by the development of severe pneumonia leading to ARDS and the requirement for mechanical ventilation.67 This pro-inflammatory storm is associated with a high risk of multiorgan failure and mortality. Accordingly, there has been an interest in the use of pharmacologic compounds directed against these cytokines.
Anti–GM-CSF
GM-CSF is believed to be a key cytokine mediator of the pro-inflammatory state in patients with COVID-19. Although there has been no clinical data on its use in patients with COVID-19, mechanistically, blocking the GM-CSF pathway is expected to reduce the severity of cytokine-induced inflammation. Based on this, a randomized controlled clinical trial is planned to assess the efficacy and safety of lenzilumab, a humaneered recombinant monoclonal antibody against GM-CSF. Lenzilumab has undergone phase I and II studies when it was assessed for the treatment of cytokine release syndrome associated with chimeric antigen receptor T cell (CAR-T cell) therapy. Riovant Sciences (Basel, Switzerland) also announced that they developed an anti–GM-CSF monoclonal antibody (gimsilumab) to treat COVID-19 patients with ARDS. Gimsilumab has been tested in a phase I study of healthy volunteers (results unpublished). Binding of the monoclonal antibody to GM-CSF receptor will block the signaling pathway that leads to cytokine release syndrome, which is also believed to characterize the pro-inflammatory stage of COVID-19.
IL-6 Inhibitors
Severe COVID-19 patients are characterized by a higher baseline IL-6 level compared with nonsevere infections.68 In critically ill patients with COVID-19, IL-6 levels were almost 10-fold higher.69 Higher IL-6 level is closely associated with SARS-CoV-2 RNAaemia.69 Although it is not clear whether elevation in IL-6 has a causal association with pro-inflammatory damage of the lungs or is just a consequence of the lung infection, attempts at blocking IL-6 by using monoclonal antibodies directed against IL-6 receptors have garnered interested as a potential therapeutic option.
Tocilizumab is a humanized monoclonal antibody that is used to manage cytokine release syndrome associated with CAR-T cell therapy. Sarilumab is a human immunoglobulin G1 (IgG1) monoclonal antibody approved for the treatment of rheumatoid arthritis. These IL-6 blockers bind to soluble and membrane-bound IL-6 receptors. A clinical trial involving sarilumab for the treatment of severe COVID-19 is ongoing, where the efficacy and safety of sarilumab 200-mg and 400-mg doses administered intravenously over 1 hour are being compared with standard of care (Table). Because measurement of IL-6 levels is not readily available in most institutions, C-reactive protein (CRP) levels may be used as surrogate markers of the increased pro-inflammatory state. IL-6 inhibitors rapidly decrease CRP levels after administration; therefore, CRP levels may be used to monitor the response to therapy.
During the current COVID-19 outbreak, a cohort of 21 febrile patients (including 17 severe and 4 critical patients) received tocilizumab (including 3 patients who received a second dose), and this led to defervescence, reduction in supplemental oxygenation, and improvement in clinical symptoms. However, the absence of a control group cautions against the optimistic interpretation of this promising data. It is unclear if the patients would have improved otherwise with aggressive standard of care, even if they had not received tocilizumab.27 Clinical trials are underway to evaluate the efficacy and safety of sarilumab and tocilizumab for IL-6 inhibition in the management of COVID-19 (Table).
Blocking IL-6 receptors may curb fever and inflammation, but this approach also blunts the host defense against infection. IL-6 inhibition is associated with increased risk of infections, albeit this is more common after chronic use and in combination with other immunosuppressive drugs. Ongoing clinical trials are excluding patients with active or untreated tuberculosis and systemic bacterial and fungal infections.
Convalescent Plasma and Neutralizing Antibodies
The US FDA has approved the emergency investigational use of convalescent plasma for the treatment of critically ill patients with COVID-19.70 Convalescent plasma is collected from COVID-19–recovered individuals who are eligible for blood donation. The traditional screening protocol for blood donation should be satisfied. In addition, the plasma should be collected from people with a prior confirmed diagnosis of COVID-19 who have resolved their symptoms at least 14 days before donation. In addition, they should have negative PCR for SARS-CoV-2 and high SARS-CoV-2 neutralizing antibody titers. Takeda Pharmaceuticals (Tokyo, Japan) is also developing an anti–SARS-CoV-2 polyclonal hyperimmune globulin (TAK-888).
The use of convalescent plasma resulted in a shorter hospital stay and reduced mortality in SARS71 and H1N1 influenza.72 It has also been used during Ebola and MERS outbreaks.73 A preliminary report of a series of five patients with severe COVID-19 pneumonia complicated by ARDS showed that administration of convalescent plasma containing neutralizing antibody (SARS-CoV-2 IgG titers greater than 1:1000 by enzyme-linked immunosorbent assay and neutralizing antibody titer >40) led to clinical improvement.28 There was a normalization of body temperature in four patients within 3 days, the sequential organ failure assessment score decreased, and viral load declined to negativity by day 12. ARDS resolved within 12 days in four patients. These preliminary findings are promising, but the small number and the uncontrolled nature of this study calls for further evaluation of this treatment modality in randomized controlled clinical trials. Several groups, including investigators from our institution and other academic institutions, are collaborating to achieve a common goal of developing convalescent plasma as a viable treatment of critically ill patients with COVID-19.
Conclusion
To date, there is no proven effective drug or immunomodulator therapy for the management of COVID-19. As this SARS-CoV-2 pandemic escalates, the search for an effective treatment regimen is at the forefront of clinical medicine and research.
Two major approaches are being actively pursued. First, strategies directed against the virus have led to the use of repurposed drugs (such as chloroquine, hydroxychloroquine, LPV/r, and ribavirin) and novel investigational compounds (such as favipiravir and remdesivir). We do not yet know which, if any, of these compounds will eventually be proven effective against SARS-COV-2. Thus, the collaboration of the health care community to engage patients for participation in clinical trials is essential. In addition to the single-center and multicenter trials, the World Health Organization recently embarked on a large mega-trial that will compare the clinical outcome of patients treated with chloroquine or hydroxychloroquine, LPV/r, and remdesivir. Second, there are numerous clinical trials that are aimed to determine if curbing the pro-inflammatory state produced during COVID-19 with drugs such as IL-6 inhibitors (eg, sarilumab or tocilizumab) or anti–GM-CSF compounds (eg, lenzilumab and gimsilumab) will lead to better clinical outcomes by preventing or reversing ARDS and multiorgan failure.
In addition, convalescent plasma and neutralizing antibodies are being pursued for treatment (and maybe prevention). Vaccine development (which is outside the scope of this review) is also given the utmost priority. The world is eagerly awaiting the outcomes of these clinical trials, as they will inform and guide the medical community with the best evidence to fight this pandemic. The authors strongly encourage health care providers to use these off-label and experimental therapies under the direction of clinical trials and treatment registries. Until the results of these properly conducted studies are available, there is no drug that is considered to be of proven clinical benefit for the treatment of COVID-19.
Footnotes
Potential Competing Interests: Dr Razonable serves as the principal investigator on clinical trials for tocilizumab and sarilumab. The remaining authors report no competing interests.
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;(20):S1473–S3099. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome–related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah A., Kashyap R., Tosh P., Sampathkumar P., O'Horo J.C. Guide to understanding the 2019 novel coronavirus. Mayo Clin Proc. 2020;95(4):646–652. doi: 10.1016/j.mayocp.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AAO-HNS: Anosmia, Hyposmia, and Dysgeusia Symptoms of Coronavirus Disease. https://www.entnet.org/content/aao-hns-anosmia-hyposmia-and-dysgeusia-symptoms-coronavirus-disease
- 5.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ClinicalTrials.gov. U.S. National Library of Medicine Listed clinical studies related to the coronavirus disease (COVID-19) https://clinicaltrials.gov/ct2/results?cond=COVID-19
- 8.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao X., Ye F., Zhang M. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [published online ahead of print] Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J., Cao R.Y., Xu M.Y. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:1–4. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 12.Gautret P., Lagier J.C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial [published online ahead of print] Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Gautret P., Lagier J.-C., Parola P. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: an observational study 2020 [published online ahead of print] Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molina J.M., Delaugerre C., Goff J. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection [published online ahead of print] Méd Mal Infect. 2020 doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z., Hu J., Zhang Z. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv. 2020 doi: 10.1101/2020.03.22.20040758. [DOI] [Google Scholar]
- 16.Cai Q., Yang M., Liu D. Experimental treatment with favipiravir for COVID-19: An open-label control study [published online ahead of print] Engineering. 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatnagar T., Murhekar M.V., Soneja M. Lopinavir/ritonavir combination therapy amongst symptomatic coronavirus disease 2019 patients in India: protocol for restricted public health emergency use [published online ahead of print] Indian J Med Res. 2020 doi: 10.4103/ijmr.IJMR_502_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young B.E., Ong S.W.X., Kalimuddin S. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323(15):1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han W., Quan B., Guo W. The course of clinical diagnosis and treatment of a case infected with coronavirus disease 2019. J Med Virol. 2020;92(5):461–463. doi: 10.1002/jmv.25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim J., Jeon S., Shin H.Y. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci. 2020;35(6):e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z., Chen X., Lu Y., Chen F., Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14(1):64–68. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- 22.Cao B., Wang Y., Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19 [published online ahead of print] N Engl J Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng L., Li C., Zeng Q. Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study [published online ahead of print] J Infect. 2020 doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kujawski S.A., Wong K.K., Collins J.P. First 12 patients with coronavirus disease 2019 (COVID-19) in the United States. medRxiv. 2020 doi: 10.1101/2020.03.09.20032896. [DOI] [PubMed] [Google Scholar]
- 26.Grein J., Ohmagari N., Shin D. Compassionate use of remdesivir for patients with severe COVID-19 [published online ahead of print] N Engl J Med. 2020 doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu X., Han M., Li T. Effective treatment of severe COVID-19 patients with tocilizumab. ChinaXiV. 2020;202003:v1. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen C., Wang Z., Zhao F. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colson P., Rolain J.M., Lagier J.C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55(4):105932. doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keyaerts E., Li S., Vijgen L. Antiviral Activity of Chloroquine against Human Coronavirus OC43 Infection in Newborn Mice. Antimicrob Agents Chemother. 2009;53(8):3416–3421. doi: 10.1128/AAC.01509-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Touret F., de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020;177:104762. doi: 10.1016/j.antiviral.2020.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paton N.I., Lee L., Xu Y. Chloroquine for influenza prevention: a randomised, double-blind, placebo controlled trial. Lancet Infect Dis. 2011;11(9):677–683. doi: 10.1016/S1473-3099(11)70065-2. [DOI] [PubMed] [Google Scholar]
- 34.Tricou V., Minh N.N., Van T.P. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Negl Trop Dis. 2010;4(8):e785. doi: 10.1371/journal.pntd.0000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roques P., Thiberville S.D., Dupuis-Maguiraga L. Paradoxical effect of chloroquine treatment in enhancing chikungunya virus infection. Viruses. 2018;10(5):268. doi: 10.3390/v10050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Lamballerie X., Boisson V., Reynier J.C. On chikungunya acute infection and chloroquine treatment. Vector Borne Zoonotic Dis. 2008;8(6):837–839. doi: 10.1089/vbz.2008.0049. [DOI] [PubMed] [Google Scholar]
- 37.Official Statement from International Society of Antimicrobial Chemotherapy (ISAC) https://www.isac.world/news-and-publications/official-isac-statement
- 38.McCreary E.K., Pogue J.M. COVID-19 treatment: a review of early and emerging options. Open Forum Infect Dis. 2020;7(4):ofaa105. doi: 10.1093/ofid/ofaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giudicessi J.R., Noseworthy P.A., Friedman P.A., Ackerman M.J. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19) Mayo Clin Proc. 2020 doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frisk-Holmberg M., Bergqvist Y., Englund U. Chloroquine intoxication. Br J Clin Pharmacol. 1983;15(4):502–503. doi: 10.1111/j.1365-2125.1983.tb01540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fearing coronavirus, Arizona man dies after taking a form of chloroquine used to treat aquariums. https://www.cnn.com/2020/03/23/health/arizona-coronavirus-chloroquine-death/index.html
- 42.Request for Emergency Use Authorization For Use of Chloroquine Phosphate or Hydroxychloroquine Sulfate Supplied From the Strategic National Stockpile for Treatment of 2019 Coronavirus Disease. https://www.fda.gov/media/136534/download Vol March 30, 2020.
- 43.Best K., Guedj J., Madelain V. Zika plasma viral dynamics in nonhuman primates provides insights into early infection and antiviral strategies. Proc Natl Acad Sci U S A. 2017;114(33):8847–8852. doi: 10.1073/pnas.1704011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madelain V., Guedj J., Mentre F. favipiravir pharmacokinetics in nonhuman primates and insights for future efficacy studies of hemorrhagic fever viruses. Antimicrob Agents Chemother. 2016;61(1):e01305–e01316. doi: 10.1128/AAC.01305-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oestereich L., Rieger T., Ludtke A. Efficacy of favipiravir alone and in combination with ribavirin in a lethal, immunocompetent mouse model of lassa fever. J Infect Dis. 2016;213(6):934–938. doi: 10.1093/infdis/jiv522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischer W.A., 2nd, Vetter P., Bausch D.G. Ebola virus disease: an update on post-exposure prophylaxis. Lancet Infect Dis. 2018;18(6):e183–e192. doi: 10.1016/S1473-3099(17)30677-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westover J.B., Sefing E.J., Bailey K.W. Low-dose ribavirin potentiates the antiviral activity of favipiravir against hemorrhagic fever viruses. Antiviral Res. 2016;126:62–68. doi: 10.1016/j.antiviral.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Japanese flu drug 'clearly effective' in treating coronavirus, says China. https://www.theguardian.com/world/2020/mar/18/japanese-flu-drug-clearly-effective-in-treating-coronavirus-says-china
- 49.Chu C.M., Cheng V.C., Hung I.F. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ning Q., Brown D., Parodo J. Ribavirin inhibits viral-induced macrophage production of TNF, IL-1, the procoagulant fgl2 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. J Immunol. 1998;160(7):3487–3493. [PubMed] [Google Scholar]
- 51.Chan K.S., Lai S.T., Chu C.M. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. 2003;9(6):399–406. [PubMed] [Google Scholar]
- 52.Chan J.F., Yao Y., Yeung M.L. Treatment with lopinavir/ritonavir or interferon-beta1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212(12):1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Wilde A.H., Jochmans D., Posthuma C.C. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58(8):4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheahan T.P., Sims A.C., Leist S.R. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11(1):222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arabi Y.M., Asiri A.Y., Assiri A.M. Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-beta1b (MIRACLE trial): statistical analysis plan for a recursive two-stage group sequential randomized controlled trial. Trials. 2020;21(1):8. doi: 10.1186/s13063-019-3846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th edition) http://kjfy.meetingchina.org/msite/news/show/cn/3337.html
- 57.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Gotte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020;295(15):4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ko W.C., Rolain J.M., Lee N.Y. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents. 2020;55(4):105933. doi: 10.1016/j.ijantimicag.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warren T.K., Jordan R., Lo M.K. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531(7594):381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacobs M., Rodger A., Bell D.J. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet. 2016;388(10043):498–503. doi: 10.1016/S0140-6736(16)30386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dornemann J., Burzio C., Ronsse A. First newborn baby to receive experimental therapies survives Ebola virus disease. J Infect Dis. 2017;215(2):171–174. doi: 10.1093/infdis/jiw493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mulangu S., Dodd L.E., Davey R.T., Jr. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N Engl J Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agostini M.L., Andres E.L., Sims A.C. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9(2):e00221-18. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Wit E., Feldmann F., Cronin J. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A. 2020;117(12):6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sheahan T.P., Sims A.C., Graham R.L. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9(396):eaal3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lescure F.-X., Bouadma L., Nguyen D. Clinical and virological data of the first cases of COVID-19 in Europe: a case series [published online ahead of print] Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou Y., Fu B., Zheng X. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+ CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. bioRxiv. 2020 2020.2002.2012.945576. [Google Scholar]
- 68.Liu T., Zhang J., Yang Y. The potential role of IL-6 in monitoring severe case of coronavirus disease 2019. medRxiv. 2020 doi: 10.15252/emmm.202012421. 2020.2003.2001.20029769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen X., Zhao B., Qu Y. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. medRxiv. 2020 doi: 10.1093/cid/ciaa449. 2020.2002.2029.20029520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Food and Drug Administration. Investigational COVID-19 Convalescent Plasma - Emergency INDs. https://www.fda.gov/media/136470/download. Accessed April 16, 2020.
- 71.Lai S.T. Treatment of severe acute respiratory syndrome. Eur J Clin Microbiol Infect Dis. 2005;24(9):583–591. doi: 10.1007/s10096-005-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hung I.F., To K.K., Lee C.-K. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arabi Y., Balkhy H., Hajeer A.H. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springerplus. 2015;4:709. doi: 10.1186/s40064-015-1490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.