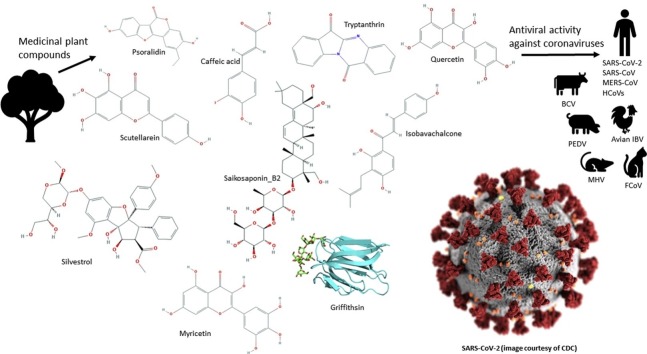
Graphical abstract
Keywords: Coronaviridae, Severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), Traditional medicine, COVID-19, SARS-CoV-2
Highlights
-
•
Naturally derived compounds provide a potential wealth of antiviral agents.
-
•
We reviewed the literature on phytochemicals against different human and animal coronaviruses.
-
•
Compounds showing the greatest potential for drug development are highlighted.
-
•
All promising compounds contain a conjugated ring structure.
-
•
Most are polyphenols and/or contain a substituted fused ring.
Abstract
Coronaviruses are responsible for a growing economic, social and mortality burden, as the causative agent of diseases such as severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), avian infectious bronchitis virus (IBV) and COVID-19. However, there is a lack of effective antiviral agents for many coronavirus strains. Naturally existing compounds provide a wealth of chemical diversity, including antiviral activity, and thus may have utility as therapeutic agents against coronaviral infections. The PubMed database was searched for papers including the keywords coronavirus, SARS or MERS, as well as traditional medicine, herbal, remedy or plants, with 55 primary research articles identified. The overwhelming majority of publications focussed on polar compounds. Compounds that show promise for the inhibition of coronavirus in humans include scutellarein, silvestrol, tryptanthrin, saikosaponin B2, quercetin, myricetin, caffeic acid, psoralidin, isobavachalcone, and lectins such as griffithsin. Other compounds such as lycorine may be suitable if a therapeutic level of antiviral activity can be achieved without exceeding toxic plasma concentrations. It was noted that the most promising small molecules identified as coronavirus inhibitors contained a conjugated fused ring structure with the majority being classified as being polyphenols.
1. Introduction
The dramatic change of events with the recent unprecedented coronavirus pandemic declared by the World Health Organisation (WHO) has prompted an exponential increase of scientific interest in coronaviruses globally. As of April 22nd 2020, the pandemic has resulted in 2,553,112infections, with 177,286 deaths worldwide, which continues to drastically increase as we write (https://www.who.int/emergencies/diseases/novel-coronavirus-2019).
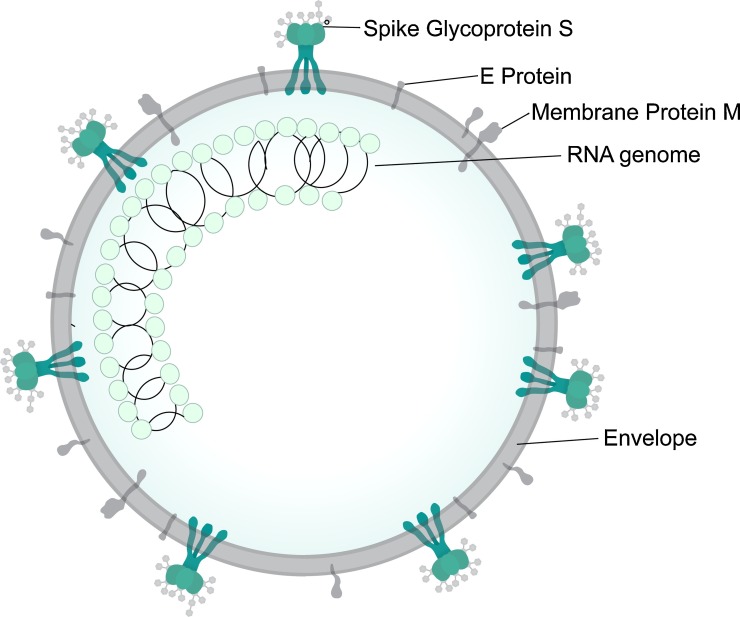
Coronaviruses (CoVs) belong to the family Coronaviridae, subfamily Coronavirinae and are large (genome size 26−32 kb; Wu et al., 2020a), enveloped, positive-sense single-stranded ribonucleic acid (RNA) viruses that can infect both animals and humans (Fig. 1 ). Based on their genotypic and serological characteristics, the viruses are subdivided into four genera: Alpha-, Beta-, Gamma-, and Deltacoronavirus (Chu et al., 2020; Lu et al., 2015). At present, all identified CoVs that are capable of infecting humans belong to the first two genera. These include the alphacoronaviruses (αCoVs) HCoV-NL63 (Human CoV-NL63) and HCoV-229E and the betacoronaviruses (βCoVs) HCoV-OC43 (Human CoV-OC43), HKU1 (Human CoV), SARS-CoV (Severe Acute Respiratory Syndrome CoV), and MERS-CoV (Middle Eastern Respiratory Syndrome CoV) (Lu et al., 2015). In the past two decades there have been three epidemics caused by the betaCoVs, namely SARS in 2002−03, MERS in 2012 and COVID-19, first identified in 2019 (Yang et al., 2020b).
Fig. 1.
The general structure of a coronavirus (reproduced from Wikipedia under CC licence 4.0). E protein = envelope protein.
SARS-CoV emerged in 2002−03 in Southern China, causing a global threat and infecting more than 8000 people, with approximately 800 fatalities recorded, largely in China and the surrounding regions (Lu et al., 2015; Paraskevis et al., 2020). MERS-CoV emerged in the Middle East, spreading to several countries to infect close to 2300 individuals, resulting in 845 deaths as of July 2019 (World Health Organization, 2019). The present CoV pandemic resulting from SARS-CoV-2, which causes COVID-19 (coronavirus disease), was identified in Wuhan City, in the Hubei province of southern mainland China on the 31st December 2019 (Sohrabi et al., 2020). The genome of SARS-CoV-2 is approximately 70 % identical to that of SARS-CoV (Hui et al., 2020), hence leading to its current name.
The major druggable targets of SARS-CoV-2 include 3-chymotrypsin-like protease (3CLpro), papain like protease (PLpro), RNA-dependent RNA polymerase, and spike (S) proteins (Wu et al., 2020b). The S proteins interact directly with human angiotensin-converting enzyme (ACE) 2, allowing the virus to enter the cells. At present, no preventive vaccines or established antiviral therapies are available for coronaviruses (Sohrabi et al., 2020). However, several synthetic compounds have shown promise, including hydroxychloroquine and choloroquine phosphate (Cortegiani et al., 2020; Gao et al., 2020), which act through several mechanisms, including alkalisation of the host cell phagolysosomes. Newer antiviral medications such as lopinavir (Yao et al., 2020), remdesivir (Holshue et al., 2020; Wang et al., 2020), and arbidol (Khamitov et al., 2008) also show promise. Other suggested treatment options include lopinavir/ritonavir, nucleoside analogues, neuraminidase inhibitors, and peptide EK1 (Lu, 2020). A detailed list of current and planned clinical trials investigating various drugs for the treatment of SARS-CoV-2 was provided by Pang et al. (2020), with updated results available from ClinicalTrials.gov (2020).
In addition, traditional herbal medicines and purified natural products may guide the development of novel antiviral drugs. In other words, more efficient drugs can often be designed based on the structure of natural compounds that exhibit the desired activity. Classic examples of this drug discovery pathway include emetine, an isoquinoline alkaloid isolated from Cephaelis ipecacuanha and used as an amoebicidal drug; quinine, derived from the bark of Cinchona trees; and numerous other drugs modified from natural compounds, such aspirin, morphine and paclitaxel, an antineoplastic drug used for the treatment of cancer (Ganjhu et al., 2015). Indeed, half of all drugs approved between 1981 and 2014 were derived from or mimicked a natural compound (Newman and Cragg, 2016). Furthermore, in the current outbreak of COVID-19, many patients appear to be turning to complementary or traditional medicinal therapies, albeit using them almost exclusively in conjunction with western medicine. For example, one study suggested that almost 92 % of 135 hospitalised patients in northeast Chonqing (China) received traditional Chinese medicine in addition to western medicine (Wan et al., 2020). However, based on the many studies conducted on this topic, it is hard to separate the potential effects of, and interaction between, traditional Chinese herbal medicine and western medicine. Recent reviews have suggested that traditional Chinese medicine could be used for the prevention (Luo et al., 2020) or treatment (Yang et al., 2020a) of COVID-19; while still acknowledging that many studies involving clinical trials are poorly designed or controlled, and the choice of treatments is largely empirically based. As previous work has highlighted the potential of traditional Chinese medicines as a source of potential novel drugs (Ling, 2020), we have not included details on such studies investigating the antiviral activity of remedies comprising portions of numerous plant species in this review. Rather, our aim is to collate data on the broad spectrum of natural phytochemicals from individual plant species that may have therapeutic potential.
Naturally occurring antiviral agents acting against general coronaviruses were briefly reviewed by Lin et al. (2014) six years ago, while more recent reviews by Pang et al. (2020) and Lu (2020) on therapies for COVID-19 made only brief mention of natural therapeutics and did not explore the active compounds or their mechanism of action. In light of the current COVID-19 pandemic, this review aims to gather and consolidate information on extracts and compound(s) derived from natural products which show potential antiviral bioactivity for the inhibition of coronaviruses. It is hoped that the information presented may guide the naturally-derived drug discovery process in finding a treatment for SARS-CoV-2.
2. Methods
The PubMed database (www.ncbi.nlm.nih.gov/pubmed/) was used to locate articles including the following combination of terms: (coronavirus, SARS OR MERS) AND (traditional medicine, herbal, remedy OR plants). Papers primarily focussed on the antiviral activity of prepared Chinese traditional medicines, which typically comprise multiple plant species, were considered out of scope of this review. All articles up to and including 25 March 2020 were considered, yielding a total of 659 results. Two of the authors independently screened the results and identified relevant articles, yielding a total of 35 primary articles on human coronaviruses and 22 on animal coronaviruses were found to be pertinent and thus included in this review (total = 58 papers). Of these, two papers (6 %) were on SARS-CoV-2 (COVID-19). The majority of studies on human coronaviruses (69 %; n = 24) included SARS-CoV, with only 3 (9 %) including MERS-CoV and 8 (23 %) other human coronaviruses. It should be noted that one study included both SARS-CoV and MERS-CoV (O’Keefe et al., 2010) while another included both MERS-CoV and HCoV-229E (Müller et al., 2018), hence these percentages do not add to 100 %.
3.
Table 1 summarises the studies reporting the inhibition of various human coronavirus strains using compounds derived from plant sources. The table is arranged by viral strain in order to better compare the bioactivity of compounds from different studies upon the same viral genotypes. Where identified, the key compounds responsible for the antiviral activity and their identified mechanisms of action are presented. It should be noted the term EC50 (effective concentration) applies to cell-based assays, while IC50 (inhibitory concentration) applies to enzyme- or biochemical-based assays.
Table 1.
Studies reporting antiviral activity of natural products or isolates against human coronavirus strains.
| Viral strain | Assay method | Plant species | Plant part/ isolate | EC50 or IC50 (μM unless otherwise stated) | SI | Key compounds present (if identified) | Biological action | Reference |
|---|---|---|---|---|---|---|---|---|
| SARS-CoV-2 | Computer modelling | Present in: Camellia sinensis | – | ND | ND | Theaflavin | Binding to RNA-dependent RNA polymerase | (Lung et al., 2020) |
| SARS-CoV-2 | Computer modelling | Compounds previously identified in a range of Chinese traditional medicines | n/a | ND | ND | Betulinic acid | Replication & 3CLpro | (Zhang et al., 2020) |
| Coumaroyltyramine | PLpro & 3CLpro | |||||||
| Cryptotanshinone | PLpro & 3CLpro | |||||||
| Desmethoxyreserpine | Replication, 3CLpro & entry | |||||||
| Dihomo-c-linolenic | 3CLpro | |||||||
| Dihydrotanshinone | Entry & spike protein | |||||||
| Kaempferol | PLpro & 3CLpro | |||||||
| Lignan | Replication & 3CLpro | |||||||
| Moupinamide | PLpro | |||||||
| N-cis-feruloyltyramine | PLpro & 3CLpro | |||||||
| Quercetin | PLpro & 3CLpro | |||||||
| Sugiol | Replication & 3CLpro | |||||||
| Tanshinone IIa | PLpro & 3CLpro | |||||||
| SARS-CoV | CPE assay | Boenninghausenia sessilicarpa | Isolated compound from ethanol extract | ∼450 | ND | Leptodactylone | Not determined | (Yang et al., 2007) |
| SARS-CoV | CPE assay | Hippeastrum hybrid | Lectins isolated from diaminopropane extracts | 3.2 ± 2.8 | >31.3 | Agglutinins: mannose-specific | Inhibit viral attachment and another target at end of replication cycle | (Keyaerts et al., 2007) |
| Galanthus nivalis | 6.2 ± 0.6 | >16.1 | ||||||
| Narcissus pseudonarcissus | 5.7 ± 4.4 | >17.5 | ||||||
| Lycoris radiata | 48 | >2.1 | ||||||
| Allium porrum | 0.45 ± 0.0 | >222.2 | ||||||
| Allium ursinum | 8 | >5.5 | ||||||
| Cymbidium hybrid | 18 ± 4 | >20 | ||||||
| Listera ovata | 4.9 ± 0.8 | >45.5 | ||||||
| Epipactis helleborine | 2.2 ± 1.3 | >55.5 | ||||||
| Tulipa hybrid | 1.8 ± 0.3 | >2.3 | ||||||
| Morus nigra | 22 ± 6 | >62.5 | ||||||
| Nicotiana tabacum | 1.6 ± 0.5 | >58.8 | GlcNAc-specific | |||||
| Urtica dioica | 1.7 ± 0.3 | >76.9 | (GlcNAc)n-specific | |||||
| Morus nigra | 1.3 ± 0.1 | >2 | Gal-specific | |||||
| Cladastris lutea | 50 ± 13 | >13.5 | Man/Glc-specific | |||||
| Polygonatum | 7.4 ± 0.2 | >5.5 | Gal/GalNAc-specific | |||||
| multiflorum | 18 ± 13 | >12.6 | GalNAc(>Gal) specific | |||||
| Iris hybrid | 28 ± 11 | 22.7 | GalNAcα(1.3)Gal > Gal NAc > Gal-specific | |||||
| 2.2 ± 0.9 | 8.2 | |||||||
| 4.4 ± 3.1 | 16.2 | |||||||
| Tulipa hybrid | 3.4 ± 2.0 | >1.3 | Man/GalNAc-specific | |||||
| 38 ± 0 (all μg/mL) | ||||||||
| SARS-CoV (BJ001 and BJ006) | CPE/MTS assay | Artemisia annua | 95 % EtOH extract | 34.5−39.2 | 27−31 | In L. radiata: lycorine | Not determined | (Li et al., 2005) |
| Pyrrosia lingua | Chloroform | 40.5−43.2 | 55−59 | |||||
| Lindera aggregate | 95 % EtOH | 80.6−88.2 | 16−17 | |||||
| Lycoris radiata | 95 % EtOH | 2.1−2.4 (all μg/mL) | 370−422 | |||||
| Isolated lycorine | 48.8 ± 3.6 nM | 954 | ||||||
| Commercial lycorine | 15.7 ± 1.2 nM | 885 | ||||||
| SARS-CoV BJ01 | MTT cytotoxicity assay | Galla chinensis | Isolated compounds from 85 % ethanol extract | 10.6 | 14.622 | Luteolin | Binds with S2 subunit and preventing entry | (Yi et al., 2004) |
| 4.5 | 40.0 | Tetra-O-galloyl-β-D-glucose | ||||||
| SARS-CoV FFM1 | CPE assay | Toona sinensis | Boiled water extract of leaves | 30−43 μg/mL | 12−17 | Not determined | Not determined | (Chen et al., 2008) |
| SARS-CoV FFM1 | CPE assay | Glycyrrhizin and glycyrrhetinic acid found in: Glycyrrhiza radix | Chemical standards | 365 ± 12 | >65 | Glycyrrhizin | Not determined | (Hoever et al., 2005) |
| >20 | – | 18β-glycyrrhetinic acid | ||||||
| 40 ± 13 | >75 | Selected synthetic derivatives | ||||||
| 35 ± 7 | 41 | |||||||
| 139 ± 20 | 2 | |||||||
| 8 ± 2 | 6 | |||||||
| 50 ± 10 | 5 | |||||||
| 5 ± 3 | 3 | |||||||
| 16 ± 1 | 4 | |||||||
| SARS-CoV FFM1 | CPE assay | Laurus nobilis | Essential oil | 120 ± 1.2 μg/mL | 4.2 | L. nobilis: β-ocimene, 1,8-cineole, α-pinene, β-pinene | Inhibition of viral replication | (Loizzo et al., 2008) |
| Thuja orientalis | 130 ± 0.4 μg/mL | 3.8 | T. orientalis: α-pinene, δ-3-carene, α-cedrol | |||||
| SARS-CoV (Hong Kong strain) | CPE assay | Cibotium barometz | 75 % ethanol | 8.42->10 | >59.4 | Not determined | Not determined | (Wen et al., 2011) |
| Gentiana scabra | extract | 8.70 | >57.5 | Secoiridoid & glycosides? | ||||
| Dioscorea batatas | 8.06 | >62.0 | Polysaccharides? | |||||
| Cassia tora | 8.43 | >59.3 | Emodin? | |||||
| Taxillus chinensis | 5.39 (all μg/mL) | >92.8 | Quercetin? | |||||
| SARS-CoV PUMC01 F5 | Plaque reduction assay | Cinnamomi sp. | Water extraction followed by phase extraction | 10.7 ± 0.4 μg/mL (EtOH fraction) | 16.9 | Procyanidin A2 | Early stage inhibition of viral entry (clathrin-dependent endocytosis pathway) | (Zhuang et al., 2009) |
| 7.8 ± 0.3 μg/mL | 23.1 | |||||||
| (butanol fraction) | ||||||||
| Isolated compound | 29.9 ± 3.3 μM | 37.35 | ||||||
| SARS-CoV urbani strain (200,300,592) | Neutral red uptake assay SARS-CoV-infected BALB/c mouse model | Found in: Urtica dioica | Chemical standard used | 2.6 ± 3.7 μg/mL | 10.2 ± 5.6 | Urtica dioica agglutinin | Dose-dependent inhibition of viral replication, likely in adsorption or penetration stages. Binds to SARS-CoV spike glycoprotein and N-acetylglucosamine-like residues on the glycosylated envelope | (Kumaki et al., 2011) |
| SARS-CoV | CPE assay | Found in Griffithsia sp. | Chemical standard used | (μg/mL) | Griffithsin | Direct binding to surface envelope glycoprotein spike | (O’Keefe et al., 2010) | |
| Urbani strain | 0.61 | >164 | ||||||
| Tor-II strain | 0.61 | >164 | ||||||
| CuHK strain | 0.78 | >128 | ||||||
| Frank strain | 1.19 | >83 | ||||||
| SARS-CoV helicase nsP13 | Fluorometric helicase activity assay | n/a | Chemical standard | 2.71 ± 0.19 | ND | Myricetin | Inhibit ATPase activity of SARS-CoV helicase nsP13 | (Yu et al., 2012) |
| Scutettaria baicalensis | Isolated compounds | 0.86 ± 0.48 | Scutellarein | |||||
| SARS-CoV S protein | Immunofluorescence assay (IFA) | Rheum officinale | Water extracts (at 40 °C) of roots | ∼5 μg/mL | ND | Emodin | Inhibited binding of S protein to ACE2 | (Ho et al., 2007) |
| Polygonum multiflorum | Synthetic emodin standard | 1−5 μg/mL | ||||||
| 200 μM | ||||||||
| SARS-CoV 3CLpro | Computer modelling | Compounds from marine natural products database and traditional Chense medicines database | n/a | n/a | n/a | 18 compounds identified: M3927, M4367, M4890, M5410, M5789, M6601, M6602, T1434, T1441, T2826, T2831, T4744, T537, T5656, T6791, T8593, T3091, T5242 | Inhibition of 3CLpro | (Liu and Zhou, 2005) |
| SARS-CoV CLpro | Computer modelling | Identified via computer modelling. Found in: Veratrum sabadilla | n/a | ND | ND | Sabadinine | Inhibition of CoV protease | (Toney et al., 2004) |
| SARS-CoV CLpro | Computer modelling for compounds docking in cathepsin-L protease | Found in: Artemisia annua | n/a | ND | ND | Aurantiamide acetate | Inhibition of active pocket of CoV protease | (Wang et al., 2007) |
| SARS-CoV 3CLpro | 3CLpro cleavage assay | Isatis indigotica | Water extract of roots | 53.8 ± 4.2 μg/mL | >92.9 | Inhibition of 3CLpro | (Lin et al., 2005) | |
| Isolated compounds | 121 μM | >99.4 | Sinigrin | |||||
| 300 μM | 24.6 | Indigo | ||||||
| 115 μM | 12.8 | β-sitosterol | ||||||
| 132 μM | 87.8 | Aloe-emodin | ||||||
| 60 μM | 45.3 | Hesperetin | ||||||
| SARS-CoV 3CLpro | 3CLpro inhibition test | Rheum palmatum | 75 % ethanol | 13.76 ± 0.03 μg/mL | ND | Possibly anthraquinones | Inhibition of 3CLpro | (Luo et al., 2009) |
| SARS-CoV CLpro | CLpro inhibition assay | Salvia miltiorrhiza | Isolated compounds from ethanol extract | 89.1 ± 5.2 | ND | Tanshinone IIA | Non-competitive enzyme isomerization inhibitor of protease (except for rosmariquinone which exhibits simple reversible slow-binding inhibition) | (Park et al., 2012) |
| 24.8 ± 0.8 | Tanshinone IIB | |||||||
| 21.1 ± 0.8 | Methyl tanshinonate | |||||||
| 226.7 ± 6.2 | Cryptotanshinone | |||||||
| 38.7 ± 8.2 | Tanshinone I | |||||||
| 14.4 ± 0.7 | Dihydrotanshinone I | |||||||
| 21.1 ± 0.8 | Rosmariquinone | |||||||
| SARS-CoV CLpro | CLpro inhibition assay | Torreya nucifera | Isolated compounds from ethanol extract | 8.3 ± 1.2 | ND | Amentoflavone | Non-competitive inhibition of CoV CLpro | (Ryu et al., 2010a) |
| 72.3 ± 4.5 | Bilobetin | |||||||
| 32.0 ± 1.7 | Ginkgetin | |||||||
| 38.4 ± 0.2 | Sciadopitysin | |||||||
| SARS-CoV CLpro | CLpro inhibition assay | Tripterygium regelii | Isolated compounds from 95 % methanol extract of bark | 10.3 ± 0.2 | ND | Celastrol | Competitive inhibition of CoV protease | (Ryu et al., 2010b) |
| 5.5 ± 0.7 | Pristimererin | |||||||
| 9.9 ± 0.1 | Tingenone | |||||||
| 2.6 ± 0.3 | Iguesterin | |||||||
| SARS-CoV 3CLpro | Fluorogenic 3CLpro inhibition assay | Houttuynia cordata | Boiled water extract | ∼1000 μg/mL | ND | Not determined | Minor 3CLpro inhibition. May inhibit pivotal enzymes and trigger negative feedback control in immune systems | (Lau et al., 2008) |
| SARS-CoV PLpro | PLpro inhibition assay | Salvia miltiorrhiza | Isolated compounds from ethanol extract | 1.6 ± 0.5 | ND | Tanshinone IIA | Non-competitive enzyme isomerization inhibitor of protease (except for rosmariquinone which exhibits simple reversible slow-binding inhibition) | (Park et al., 2012) |
| 10.7 ± 1.7 | Tanshinone IIB | |||||||
| 9.2 ± 2.8 | Methyl tanshinonate | |||||||
| 0.8 ± 0.2 | Cryptotanshinone | |||||||
| 8.8 ± 0.4 | Tanshinone I | |||||||
| 4.9 ± 1.2 | Dihydrotanshinone I | |||||||
| 30.0 ± 5.5 | Rosmariquinone | |||||||
| SARS-CoV PLpro | PLpro inhibition assay |
Broussonetia papyrifera |
Isolated compounds from ethanol extract | 3.7 ± 1.6 | ND | 3′-(3-methylbut-2-enyl)-3′,4,7-trihydroxyflavane | Non-competitive inhibition of CoV PLpro | (Park et al., 2017) |
| SARS-CoV PLpro | Fluorogenic PLpro inhibition assay | Psoralea corylifolia | Ethanol extract of seeds | 15 μg/mL | Mixed inhibitor of SARS-CoV PLpro (isobavachalcone and psoralidin also reversible) | (Kim et al., 2014) | ||
| 38.4 ± 2.4 | Bavachinin | |||||||
| 18.3 ± 1.1 | Neobavaisoflavone | |||||||
| 7.3 ± 0.8 | Isobavachalcone | |||||||
| 10.1 ± 1.2 | 4′-O-methylbavachalcone | |||||||
| 4.2 ± 1.0 | Psoralidin | |||||||
| 32.3 ± 3.2 (rest in μM) | Corylifol A | |||||||
| SARS-CoV urbani strain PLpro | Fluorogenic protease activity assay | Paulownia tomentosa | Methanol extracts of fruit | 6.2 ± 0.04 | ND | Tomentin A | Reversible, mixed-type (allosteric) inhibitors of PLpro | (Cho et al., 2013) |
| 6.1 ± 0.02 | Tomentin B | |||||||
| 11.6 ± 0.13 | Tomentin C | |||||||
| 12.5 ± 0.22 | Tomentin D | |||||||
| 5.0 ± 0.06 | Tomentin E | |||||||
| MERS-CoV EMC/2012 | Luciferase assay | Found in Griffithsia sp. | Chemical standard/pure isolate used | ∼0.125 μg/mL | ND | Griffithsin | Direct inhibition of protein spikes preventing viral binding | (Millet et al., 2016) |
| MERS-CoV EMC/2012 | Cellular dual luciferase reporter assay | Found in Aglaia sp. | Chemical standard used | 1.3 | >7690 | Silvestrol | Specific inhibitor of RNA helicase eIF4A | (Müller et al., 2018) |
| MERS-COV PLpro | PLpro inhibition assay | Broussonetia papyrifera | Isolated compounds from ethanol extract | 39.5 ± 5.1 | ND | Kazinol F | Non-competitive inhibition of CoV PLpro | (Park et al., 2017) |
| 42.1 ± 5.0 | Broussochalcone A | |||||||
| HCoV-229E | XTT assay | Calophyllum blancoi | Isolated compounds from acetone extract of roots | 3 | ND | Blancoxanthone | Not determined | (Shen et al., 2005) |
| 15 | Pyranojacareubin | |||||||
| HCoV-229E | XTT assay | Found in: Bupleurum spp., Heteromorpha spp. and Scrophularia scorodonia | Chemical standards used | 8.6 ± 0.3 | 26.6 | Saikosaponin A | Possible interference in early stage of viral replication, e.g. absorption and penetration | (Cheng et al., 2006) |
| 1.7 ± 0.1 | 221.9 | Saikosaponin B2 | ||||||
| 19.9 ± 0.1 | 19.2 | Saikosaponin C | ||||||
| 13.2 ± 0.3 | 13.3 | Saikosaponin D | ||||||
| HCov-229E | CPE assay | Pelargonium sidoides | EPs® 7630 (proprietary extract using 11 % ethanol) | 44.50 ± 15.84 μg/mL | >2.3 | Not determined | Possibly interference of virus surface resulting in viral inactivation | (Michaelis et al., 2011) |
| HCoV-229E | Cellular dual luciferase reporter assay | Found in Aglaia sp. | Chemical standard used | 3 | >3330 | Silvestrol | Specific inhibitor of RNA helicase eIF4A | (Müller et al., 2018) |
| HCoV-NL63 | Plaque viricidal assay | Strobilanthes cusia leaf | Methanol extract | 0.64 μg/mL | >156 | Blocking viral RNA genome synthesis and papain-like protease 2 activity | (Tsai et al., 2020) | |
| Isolated compounds | 0.06 | >6600 | Tryptanthrin | |||||
| 2.09 | >191 | Indigodole B | ||||||
| HCoV-NL63 | Virus yield reduction assay | Sambucus formosana | Ethanol extract of stem | 1.17 ± 0.75 (μg/mL) | ∼154 | (Weng et al., 2019) | ||
| Isolated compounds | 3.54 ± 0.77 | >141 | Caffeic acid | Inhibits cell docking | ||||
| 43.5 ± 6.0 | >11 | Chlorogenic acid | Not determined | |||||
| 71.5 ± 18.4 | >7 | Gallic acid | Not determined | |||||
| HCoV-OC43 | CPE assay and neutral red assay | Found in Griffithsia sp. | Chemical standard used | 0.048−0.16 | 320->2100 | Griffithsin | Direct binding to surface envelope glycoprotein spike | (O’Keefe et al., 2010) |
| HCoV-299E | 0.18−0.33 | >30−56 | ||||||
| HCoV-NL63 | <0.0032 (all μg/mL) | >3100 | ||||||
| HCoV-OC43 | MTS assay and qRT-PCR | Found in: Stephania tetrandra and related species | Chemical standards used | 0.33 ± 0.03 | >40.2 | Tetrandrine | Inhibit viral replication and expression of viral S and N protein | (Kim et al., 2019) |
| 1.01 ± 0.07 | 11.5 | Fangchinoline | ||||||
| 0.83 ± 0.07 | 13.6 | Cepharanthine |
CLpro = chymotrypsin-like protease; CPE assay = cytopathogenic effect assay; n/a = not applicable to this study; ND = no data; PLpro = papain-like protease.
3.1. Inhibitors of SARS-CoV-2 (COVID-19)
Few studies report on SARS-CoV-2, as expected given the short time since its emergence. However, a number of studies report on use of computer modelling for screening purposes (Liu and Zhou, 2005; Lung et al., 2020; Toney et al., 2004; Wang et al., 2007; Zhang et al., 2020). Typically, these models determine the free binding of energy between a ligand and a receptor (Forli et al., 2016), with a lower free binding energy indicating a stronger bond between the ligand and receptor. Although obtaining consistent results via different modelling approaches can be challenging (Aldeghi et al., 2016), computer modelling nevertheless allows for comparison of the relative binding affinity of bank of molecules toward the receptor in question.
In addition to reducing the high costs and length of time associated with physically screening large banks of compounds or plant extracts for bioactivity (Chen et al., 2017), the speed and versatility of this method may be particularly valuable for rapidly finding a potent inhibitor of SARS-CoV-2. Compounds that are highlighted through this method can then be forwarded on cell-based assays to assess their in vitro effectiveness and toxicity, before continuing to animal and clinical trials.
Lung et al. (2020) virtually screened 83 compounds found in Chinese traditional medicines for activity against the RNA-dependent RNA polymerase of SARS-CoV-2, identifying theaflavin, an antioxidant polyphenol, as a potential inhibitor. Similarly, Zhang et al. (2020) virtually screened 115 compounds found in Chinese traditional medicines, highlighting 13 for further studies. Several of these were naturally occurring polyphenolic compounds such as quercetin and kaempferol, which have already received considerable interest for the treatment of other disease types (Cassidy et al., 2019; Khan et al., 2019; Tome-Carneiro and Visioli, 2016).
3.2. Inhibitors of SARS-CoV
Given the relatively large amount of research that has been performed searching for inhibitors of SARS-CoV, antiviral agents that successfully inhibit this viral strain may provide a good starting point for identifying compounds that are active against SARS-CoV-2.
3.2.1. Virtual screening
Several authors have utilised virtual computer docking models to screen for potential compounds that could bind to and inhibit key proteins present in SARS-CoV (Liu and Zhou, 2005; Toney et al., 2004; Wang et al., 2007), highlighting the potential antiviral activity of compounds such as sabadinine and aurantiamide acetate. Compounds may be screened against a number of binding sites in order to test for potential inhibition of coronaviruses; the main sites that are typically used are the chymotrypsin-like protease (3CLpro), papain like protease (PLpro), spike proteins and RNA-dependent RNA polymerase.
3.2.2. Compound library screening
Several large in vitro screening studies searching for inhibitory activity of naturally occurring compounds against SARS-CoV have been performed, mainly on Chinese medicinal herbs (Li et al., 2005; Wang et al., 2003). While the results highlight the potential of selected plant extracts against SARS-CoV, they also demonstrate that such work can be akin to searching for a ‘needle in a haystack’. For example, Li et al. (2005) screened over 200 ethanol/chloroform extracts of Chinese medicinal herbs and found only four (Lycoris radiata, Artemisia annua, Pyrrosia lingua and Lindera aggregata) with moderate to high antiviral activity using a CPE assay (EC50 values ranging from 2.4 ± 0.2–88.2 ± 7.7 μg/mL). Of these, a single compound (lycorine) from one plant species (L. radiata) was earmarked as a potential drug candidate against SARS-CoV. The antiviral efficacy of lycorine was quite high (EC50 of 15.7 ± 1.2 nM), with a selectivity index greater than 900. Although the authors did not make mention of this fact, lycorine can cause toxic effects at low dosage levels (around 1 mg/kg in dogs) (Kretzing et al., 2011), hence great caution would be required for further development of this compound as a drug candidate.
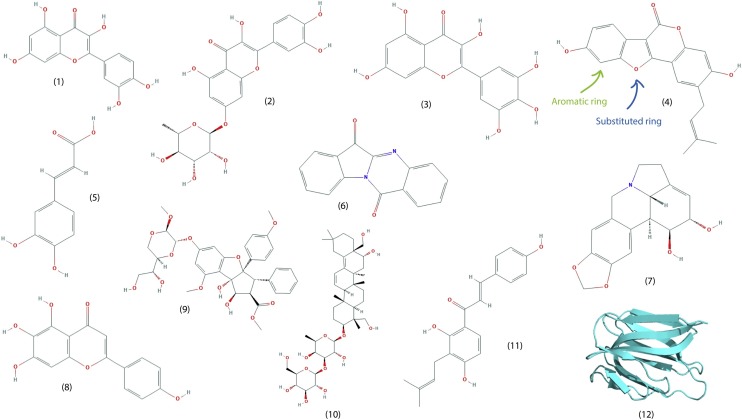
Yu et al. (2012) screened the activity of a library of 64 naturally occurring compounds against SARS-CoV helicase, which plays a key role in the viral genome replication, transcription, and translation. The polyphenolics myricetin and scutellarein (Fig. 2 ) were identified as the most promising candidates (IC50 values of 2.71 ± 0.19 and 0.86 ± 0.48 μM, respectively). Although the antiviral activity of the compounds was not assessed in cell-based assays, the authors did report that neither compound was toxic to normal (non-tumorigenic) breast epithelial cells. Myricetin is found in reasonably high concentrations in fruits such as cranberry (Häkkinen et al., 1999) as well as in several vegetables such as Calamus scipionum and garlic (Miean and Mohamed, 2001). Scutellarein was isolated from Scutellaria baicalensis (Chinese Skullcap), which has been traditionally used in the treatment of inflammation and respiratory infections, amongst other uses (Zhao et al., 2016). Both compounds were found to inhibit SARS-CoV helicase (nsP13) through the inhibition of ATPase activity, but did not directly inhibit helicase activity. This appeared to be the only publication identifying naturally occurring compounds as inhibitors of SARS-CoV helicase.
Fig. 2.
The structure of selected naturally occurring compounds that demonstrate promising anti-coronavirus activity. (1) Quercetin (2) Quercetin 7-rhamnoside (3) Myricetin (4) Psoralidin (5) Caffeic acid (6) Tryptanthrin (7) Lycorine (8) Scutellarein (9) Silvestrol (10) Saikosaponin B2 (11) Isobavachalcone (12) Griffithsin. As annotated on (4), note the aromatic rings and substituted fused rings present in most compounds.
The need to isolate and synthesise more active agents as part of the development pipeline was highlighted in a study by Runfeng et al. (2020). The authors reported that the Chinese herbal medicine Lianhuaqingwen (comprised of a mixture of plant species) showed antiviral activity against SARS-CoV-2; however, the EC50 was quite high (∼411 μg/mL). For comparison, the commercial drug remdesivir showed an EC50 of 0.651 μM (approx. 0.39 μg/mL) using the same assay (Runfeng et al., 2020). This also underscores the potential lack of potency in some traditional medicines promoted for the treatment of coronavirus symptoms.
3.2.3. Polyphenols
One family of compounds that demonstrate antiviral activity across a number of studies is the polyphenols. For example, quercetin (Fig. 3 ) showed an IC50 of 8.6 ± 3.2 μM against SARS-CoV PLpro (Park et al., 2017). No cell-based assay of antiviral activity was performed. Quercetin (Fig. 2) is a flavonoid found in many foods, but in particularly high levels in certain berries and herbs (Justesen and Knuthsen, 2001; Kaack and Austed, 1998). As previously mentioned (section 3.2.2), the structurally similar polyphenolics myricetin and scutellarein (Fig. 2) display reasonable levels of inhibitory activity against SARS-CoV helicase (Yu et al., 2012).
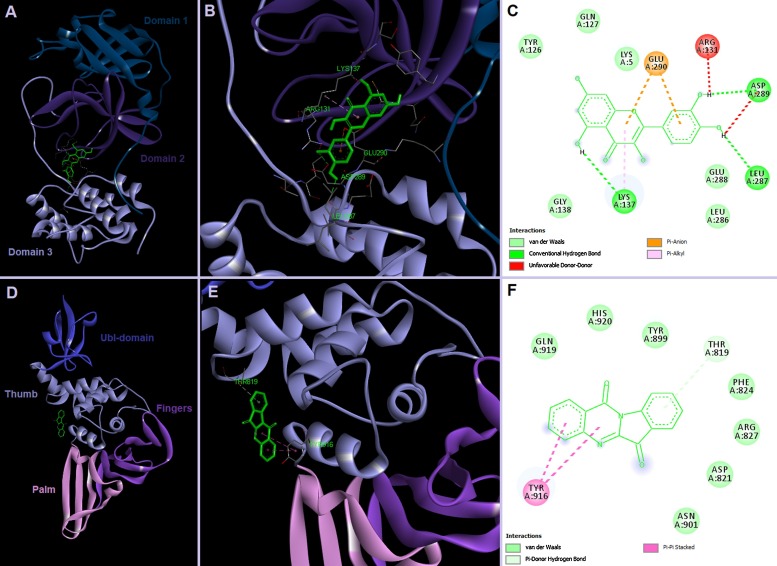
Fig. 3.
Possible binding sites of quercetin in SARS-CoV-2 3CLpro and tryptanthrin in PLpro. Docking was performed in AutoDock Vina 1.1.2 (Trott and Olson, 2010) against target proteins generated by SWISS-MODEL (https://swissmodel.expasy.org/repository/species/2697049).
Bioassay-guided fractionation of the ethanolic extract obtained from Psoralea corylifolia seeds has also identified polyphenolics as the bioactive compounds responsible for the activity of this plant species against SARS-CoV PLpro (Kim et al., 2014). Furthermore, six phenolic phytochemicals were isolated from the ethanolic extracts - identified as bavachinin, neobavaisoflavone, isobavachalcone, 4′-O-methylbavachalcone, psoralidin and corylifol A - with their antiviral activity varying widely (IC50 values between 4.2–38.4 μM). Again, no cell-based antiviral assays were performed. Isobavachalcone and psoralidin (Fig. 2) showed the greatest antiviral activity, with both found to be mixed, reversible inhibitors of PLpro through a type I mechanism (i.e. preferentially bind to the free enzyme, rather than the enzyme substrate complex) (Kim et al., 2014).
3.2.4. Lectins
Plant lectins, which are proteins that can bind specifically and reversibly to carbohydrate groups (Mitchell et al., 2017), are another group of naturally occurring compounds that may inhibit SARS-CoV. Lectins have shown promise as antiviral agents against viruses such as influenza and herpes simplex virus (Hwang et al., 2020), as well as Ebola (Covés-Datson et al., 2019; Michelow et al., 2011). Remarkably, elevating the plasma levels of recombinant human mannose-binding lectin in mice allowed them to survive otherwise fatal Ebola infections (Michelow et al., 2011). Keyaerts et al. (2007) screened the activity of a broad range of plant lectins (33 in total) against SARS-CoV using a cytopathicity effect (CPE) assay, finding EC50 values as low as 0.45 ± 0.08 μg/mL for Lycoris radiata agglutinin. Although the exact mechanism of action was not determined, activity at the stage of viral attachment or the end of the infectious viral cycle were deemed to be the most likely targets. In clinical trials, other lectins have demonstrated reasonable to good tolerability (Petersen et al., 2006), hence with further testing, they may prove to be one of the more promising classes of naturally derived compound(s) for the treatment of SARS-CoV-2 and other coronavirus infections.
3.2.5. Increasing potency via chemical modification
Whilst many natural derived compounds show considerable promise for the inhibition of SARS-CoV and other human coronaviruses, few approach the level of efficacy and/or selectivity required for commercial drugs. For example, the calpain inhibitor MDL28170 has been shown to have an IC50 value of just 2.5 nM (∼1 ng/mL) in its activity against the SARS-CoV enzyme cathepsin-L (Simmons et al., 2005). Cathepsin-L is a cysteine protease which is an important lysosomal endopeptidase enzyme involved in the initiation of protein degradation (Sudhan and Siemann, 2015) and mediates entry and infection of SARS-CoV in its host cells (Huang et al., 2006). Chemical modification of naturally-derived compounds may be required to increase the potency of their antiviral activity to levels suitable for therapeutic application. For the drug discovery process, beginning with the understanding of the structural conformity (i.e. structure including potential isomers) of the naturally derived compound(s) can reduce timelines and greatly increase the chance of finding effective viral inhibitors.
Working on this principle, Hoever et al. (2005) found that modification of glycyrrhizin, naturally found in liquorice and previously used for the treatment of SARS-CoV (Haiying et al., 2003), could increase its viral inhibition activity. For example, adding 2-acetamido-β-D-glucopyranosylamine to the glycoside chain of glycyrrhizin increased its antiviral activity in a CPE assay by 10-fold (decrease in EC50 from 365 ± 12 μM to 40 ± 13 μM), through increased attraction to the S proteins. Amides and conjugates with amino acid residues and free COOH increased activity of glycyrrhizin by up to 70-fold (EC50 for CPE assay ranging from 5 ± 3 μM to 139 ± 20 μM), albeit with significantly reduced selectivity. Cho et al. (2013) demonstrated that tomentins A–E (all naturally occurring compounds) showed viral inhibition activity against SARS-CoV greater as compared to their non-geranylated precursor compounds. Similarly, quercetin-7-rhamnoside (Fig. 2) demonstrates over 100 times higher antiviral activity against an animal coronavirus strain compared to its parent compound, quercetin (Choi et al., 2009).
While the examples of modifications presented here vary, it should not be considered that substitutions are added in a random fashion, but rather performed in the context of targeting a specific receptor or biochemical pathway. For example, the addition of glycosides and specific amino acid residues to glycyrrhizin was performed with the intent of increasing its affinity for the highly glycosylated spike proteins of SARS-CoV. With this in mind, future studies that identify an effective natural inhibitor of coronavirus should also consider the design and testing of potential modifications or synthetic derivatives that could increase its desired activity.
3.2.6. The significance of viral strains
A difference in the efficacy of natural therapeutics, mirroring that observed for commercially/synthetically available drugs, has been observed between coronavirus species/strains. For example, Park et al. (2017) found that the compound 3′-(3-methylbut-2-enyl)-3′,4,7-trihydroxyflavane, isolated from Broussonetia papyrifera, showed good inhibition of PLpro from SARS-CoV (IC50 of 3.7 ± 1.6 μM), but not against PLpro from MERS-CoV (IC50 of 112.5 ± 7.3 μM). In contrast, the polyphenolics kazinol F and broussochalcone A isolated from the same species showed better efficacy against MERS-CoV PLpro. Similarly, O'Keefe et al. (2010) found that the efficacy of the compound griffithsin against SARS-CoV was highest for the Urbani and Tor-II strains (EC50 of 0.61 μg/mL using a CPE inhibition assay) and lowest for the Frank strain (EC50 of 1.19 μg/mL). Griffithsin, isolated from the red alga Griffithsia sp., is a lectin possessing three large identical carbohydrate-binding domains orientated as an equatorial triangle, which enable multivalent binding (O’Keefe et al., 2010). Although the source of these observed differences in the inhibition activity of griffithsin against different SARS-CoV strains was not explored by O’Keefe et al. (2010), it may be attributable to genomic differences in the amino acid sequences of the spike proteins between SARS-CoV strains, leading to varying multivalent interactions with the carbohydrates-binding domains and thus differing affinity for binding to the spike proteins. Nevertheless, these studies highlight the importance of conducting in vitro tests of potential naturally-derived therapeutics on SARS-CoV-2 prior to animal or clinical trials.
3.3. Inhibitors of MERS-CoV
Only a handful of studies have investigated the potential of natural products as therapeutic agents against MERS-CoV. Silvestrol (Fig. 2), a phytochemical from Aglaia sp., was found to be a more potent inhibitor of MERS-CoV replication (EC50 of 1.3 nM) (Müller et al., 2018). However, no cell-based antiviral assays were performed. Silvestrol is a specific inhibitor of RNA helicase eIF4A, thus inhibiting viral replication and leading to the inhibition of expression of CoV proteins and preventing the formation of replication/transcription complexes (Müller et al., 2018).
Griffithsin (Fig. 2), a 12.7 kDa lectin found in the Griffithsia genus (red algae), is one of the most promising inhibitors of MERS-CoV. It comprises three carbohydrate-binding domains which allow it to bind specifically to glycans on CoV protein spikes and inhibit viral attachment to host cells, with high potency found in in vitro trials against MERS-CoV (EC50 of ∼0.125 μM) (O'Keefe et al., 2010) and several HCoV strains (EC50 of 0.0032−0.33 μM) (Millet et al., 2016). Griffithsin also appears to have a low systematic toxicity, with its specificity index against HCoV cells (compared to human colorectal adenocarcinoma or fibroblast cell lines) has been estimated at between 30–3100 (O’Keefe et al., 2010), hence showing the potential to be considered as one of the prime candidates for animal and clinical trials against SARS-CoV-2.
3.4. Inhibitors of other human coronoviruses
A recent study highlighted the potential anti-HCoV-NL63 activity of the methanolic extract of Strobilanthes cusia leaf and its major phytochemicals (Tsai et al., 2020). The S. cusia extract effectively reduced virus yield (EC50 = 0.64 μg/mL) in cells infected with HCoV-NL63 in a dose-dependent manner. Of the six key compounds isolated, purified and identified via NMR spectroscopy, two exhibited the most potent antiviral activity against HCoV, namely tryptanthrin (Fig. 2), a natural alkaloid containing the basic indoloquinazoline moiety, and indigodole B (5aR-ethyltryptanthrin), an indole alkaloid derivative. The EC50 values against virus yield from infected cells were 1.52 μM and 2.60 μM for tryptanthrin and indigodole B, respectively. The increased antiviral activity of tryptanthrin in comparison to indigodole B may result from the double bond at C5a in the former, as compared to the additional ethyl moiety in the latter. This highlights that for compounds based on a tryptanthrin structural conformity, addition of a double bond in the quinazoline ring could significantly increase their antiviral activity. Tryptanthrin has previously been found to have a broad spectrum of biological activities including anticancer, anti-inflammatory, antiprotozoal, antiallergic, antioxidant and antimicrobial action (Kaur et al., 2017). Through manipulation of varying modes of time-of-addition/removal assay, it was found that tryptanthrin prevented the early and late stages of HCoV-NL63 replication, particularly by blocking the viral RNA genome synthesis and papain-like protease 2 activity (Fig. 3), and inhibiting the post-entry stage of HCoV replication (Tsai et al., 2020). Intriguingly, both tryptanthrin (EC50 = 0.06 μM) and indigodole B (EC50 = 2.09 μM) exhibited strong viricidal activity directly against HCoV-NL63. As the HCoV-NL63 spike protein (S protein) targets the ACE2 receptor, showing a highly conserved sequence and structural similarity to SARS-CoV and SARS-CoV-2 (Letko et al., 2020), tryptanthrin has the potential to be explored as a possible bioactive agent against SARS-CoV-2 and other human coronaviruses.
Another recent study on HCoV-OC43 focussed on the antiviral activities of the bis-benzylisoquinoline alkaloids tetrandrine, fangchinoline, and cepharanthine (CPE assay EC50 values of 0.33 ± 0.03, 1.01 ± 0.07 and 0.83 ± 0.07 μM, respectively) (Kim et al., 2019). These compounds were the primary bioactive phytochemicals identified in Stephania tetrandra and related species. All compounds were found to inhibit virus-induced cell death, through suppressing viral replication, expression of viral S and N proteins (nucleocapsid protein), and the virus-induced host response. However, the effective inhibitory concentrations were not reported for the inhibition of viral protein expression or host response. In addition, tetrandrine activated the p38MAPK pathway in MRC-5 cells, improving the aforementioned host response.
Weng et al. (2019) tested the ethanolic extracts of Sambucus formosana (elderberry) stems against the human coronavirus strain HCoV-NL63, finding quite high efficacy (EC50 of 1.17 ± 0.75 μg/mL) for viral yield reduction. With further investigation of the phenolic composition of the extracts and antiviral assays performed on the main individual phenolic acids present, caffeic acid (Fig. 2) was identified as the most potent compound present (EC50 of 3.54 ± 0.77 μM; or ∼0.64 ± 0.14 μg/mL). Although no specific binding sites were identified, caffeic acid was found to inhibit the attachment of HCoV to host cells, indicating potential binding to S proteins. Caffeic acid has also been found to inhibit other viruses such as hepatitis B (Wang et al., 2009). Notably, extracts of Sambucus nigra (black elderberry), another species in the same genus as S. formosana, have been commercialised for the treatment of cold and flu symptoms. Therefore, it is likely that extracts from S. formosana would prove similarly nontoxic and suitable for human use, although clinical trials would be required to confirm this. Nevertheless, the bioavailability/delivery mechanism of such extracts would need to be considered in order to reach therapeutic plasma concentrations for viral inhibition (Wittemer et al., 2005).
Cheng et al. (2006) examined the anticoronaviral activity of saikosaponins (A, B2, C and D) and their mode of action against HCoV-229E in vitro. Saikosaponins represent a group of pentacyclic triterpenoid derivatives, usually present as glycosides, that have been isolated from medicinal plants such as Bupleurum spp., Heteromorpha spp. and Scrophularia scorodonia, previously found to possess efficacy against several viruses, including human immunodeficiency virus (HIV) (Chiang et al., 2003; Ushio and Abe, 1992). All saikosaponins tested showed good to moderate anti-coronavirus activity, with saikosaponin B2(Fig. 2) showing the greatest potency (EC50 of 1.7 ± 0.1 μM). Subsequent time-of-addition studies indicated that saikosaponin B2 inhibited viral attachment and penetration. Interestingly, the same compound has been found to inhibit hepatitis C entry (Lin et al., 2015) as well as multidrug resistance-associated drug transporters present on the cell surface (Zhao et al., 2019), indicating that it has potential to display a broad spectrum of bioactivity. This may be advantageous in developing nations where hepatitis C prevalence is high (Pawlotsky, 2014).
Finally, two compounds that have shown efficacy against MERS-CoV (section 3.3) also show promise against HCoV. Müller et al. (2018) found that silvestrol (Fig. 2) inhibited the translation of HCoV-229E proteins, with an EC50 of 3 nM. A follow-up study confirmed that silvestrol can inhibit HCoV-229E in an ex vivo bronchial epithelial cell system, with its mechanism of action being the specific inhibition of RNA helicase eIF4A (Müller et al., 2020). Another compound active against MERS-CoV, griffithsin, also has shown great efficacy against several HCoV strains (EC50 of 0.0032−0.33 μM) (Millet et al., 2016). This again underscores the prospect of exploring griffithsin for antiviral activity against SAR-CoV-2.
4. Animal coronaviruses
Animal coronavirus strains are responsible for severe morbidity events across a wide range of domestic animals and livestock, incurring major economic demise worldwide (Jackwood et al., 2010; Lelesius et al., 2019; McCutcheon et al., 1995). The genomic diversity, coupled with the ability of coronaviruses to rapidly adapt and mutate, presents unique challenges in the development of novel antiviral agents, hence exploring alternative methods of controlling these viruses could potentially be effective across many or all serotypes. As natural phytochemicals have been shown to have activity across a wide range of viral pathogens (Chen et al., 2014), they form the basis of the studies reviewed here. The majority of plant-derived compounds considered in this review are direct-targeting antivirals, acting through direct inhibition of some part of the virus, such as proteases or spike proteins. For example, silvestrol prevents viral replication occurring through the specific inhibition of RNA helicase eIF4A (Müller et al., 2018), while griffithsin binds directly to the S protein, preventing viral entry to the host cell (O’Keefe et al., 2010). However, host-targeting antivirals form another important group of antiviral compounds. For example, extracts of Cinnamomi sp. Inhibit the clathrin-dependent endocytosis pathway, thus preventing viral entry to the host cells (Zhuang et al., 2009). Other host-targeting antiviral compounds may stimulate the immune response (Lau et al., 2008).
Table 2 presents a summary of studies reporting anti-viral activity of plant-derived isolates against a range of animal coronavirus strains. Where available, the key compounds responsible for the antiviral activity and their mechanism of action are provided.
Table 2.
Studies reporting antiviral activity of natural products or isolates against non-human coronavirus strains.
| Viral strain | Assay method | Plant species | Plant part/ isolate | EC50 or IC50 (μM unless otherwise stated) | SI | Key compounds present (if identified) | Biological action | Reference |
|---|---|---|---|---|---|---|---|---|
| Avian IBV | Plaque assay | Alstonia scholaris | Isolated from 50 % ethanol extract | 35 | >2.8 | Alstotide 1 | As1: interferes with membrane and spike proteins but not nucleocapsid proteins | (Nguyen et al., 2015) |
| 55 | >1.8 | Alstotide 3 | ||||||
| Avian IBV Beaudette strain | CPE and plaque assays | Sambucus nigra | 70 % ethanol extract | ND | ND | Possibly flavonols or lectins | Disrupts virion structure and compromises membrane integrity | (Chen et al., 2014) |
| Avian IBV Beaudette strain | CPE assay | n/a | QR448(a) (emulsion of oleoresins and essential oils | ∼1 × 10−4 dilution of extract | ND | Not determined | Possibly disrupt viral membrane or interfere with viral envelope proteins involved in host cell attachment | (Jackwood et al., 2010) |
| In vivo study in chickens | ||||||||
| Avian IBV Beaudette strain | CPE (MTT) assay | Mentha piperita | 40 % ethanol | 0.004 μg/mL | 67.5 | Not determined | Possibly direct inactivation of virus envelope structures | (Lelesius et al., 2019) |
| Thymus vulgaris | extract | 0.010 μg/mL | 63.1 | |||||
| Desmodium canadense | 0.017 μg/mL | 17.1 | ||||||
| Avian IBV Beaudette strain | Plaque assay | Houttuynia cordata | H. cordata solution (essential oils and sodium chloride solution) | 0.97 mg/mL | >257 | Main component: methyl-nonyl-ketone | Not determined | (Yin et al., 2011) |
| In ovo and in vivo trials | ||||||||
| Avian IBV Gray strain | MTT assay | Found in numerous plants, e.g eucalypts | Chemical standard used | 0.61 ± 0.07 mM | >16.39 | Eucalyptol (1,8-cineole) | Interferes with binding between RNA and IBV nucleocapsid protein | (Yang et al., 2010) |
| Avian IBV Gray strain | MTT assay | Found in coniferous trees. Produced as by-products of the pulp industry | Chemical standards used | 0.98 ± 0.25 mM | >10.20 | (-)-α-pinene | May suppress N-protein, hindering binding process between RNA and IBV N-protein | (Yang et al., 2011) |
| 1.32 ± 0.11 mM | >7.58 | (-)-β-pinene | ||||||
| Avian IBV M41 | CPE assay and RT-qPCR | Found in: Forsythia suspensa | Chemical standard used | 0.64 mM (complete inhibition) | ND | Forsythoside A | Not clear. Appears to affect cell signalling | (Li et al., 2011) |
| Bovine coronavirus (BCV) | CPE assay | Rosa nutkana | Methanol extract (branches) | <200 μg/mL for both | ND | R. nutkana: ND | Not determined | (McCutcheon et al., 1995) |
| Amelanchier alnifolia | A. alnifolia: possibly prunasin | |||||||
| FCoV NTU156 | CPE assay | Galanthus nivalis | Commercial standard | 0.0088 nM | >218 | Galanthus nivalis agglutinin | Binds to spike and membrane proteins | (Hsieh et al., 2010) |
| FIPV1146 and FECV1683 (FCoVs) | CPE assay | n/a | Commercial standards | >>10 | ND | Quercetin | Not determined | (McDonagh et al., 2014) |
| >>10 | Curcumin | |||||||
| >>25 | Rutin | |||||||
| >>25 | Glycyrrhizic acid | |||||||
| >>50 | Hesperidin | |||||||
| >>50 | Hesperitin | |||||||
| >>10 | Baicalin | |||||||
| >>25 | Artemisinin | |||||||
| FIPV1146 (FCoV) | Virtual screening followed by 3CLpro inhibition assay | Found in: several lichen and plant species | Commercial standards | 29.4 ± 4.6 | n/a | Stictic acid | Inhibition of 3CLpro | (Theerawatanasirikul et al., 2020) |
| 28.5 ± 4.2 | 7-Methylluteolin | |||||||
| 77.2 ± 13.8 | Quercetin 7-rhamnoside | |||||||
| >500 | 7-benzyl luteolin | |||||||
| >500 | Steviol | |||||||
| MHV-A59 | Plaque assay | Cimicifuga racemosa | Methanol extracts | 19.4 ± 7.0 | 12.3 | Ferulic & isoferulic acid? | Inhibit replication of MHV | (Kim et al., 2008) |
| Melia sp. | 13.0 ± 1.4 | 25.6 | Toosendanin? | |||||
| Coptidis sp. | 2.0 ± 0.5 | 34.9 | Berberine? | |||||
| Phellodendron sp. | 10.4 ± 2.2 | 13.4 | Protoberberine alkaloids? | |||||
| Sophora subprostrata | 27.5 ± 1.1(μg/mL) | 11.1 | Matrine, oxymatrine, sophoranone & sophocarpine? | |||||
| MHV-A59 | Plaque assay | Sophorae sp. | Methanol | 0.8 ± 0.2 | 696.0 | Not determined | Possibly inhibitors of RNA-dependent RNA polymerase or other protease activity | (Kim et al., 2010) |
| Also inhibited MHV-JHM | Acanthopanacis sp. | extracts | 0.9 ± 0.1 | 188.9 | ||||
| Sanguisorbae sp. | 3.7 ± 1.4 | 105.0 | ||||||
| Torilis sp. | 0.8 ± 0.0(μg/mL) | 195.6 | ||||||
| MHV-A59 | Plaque assay | Punica granatum | Pomegranate juice and ethanol/water extract of powder | ≥ 200 μg/mL | ND | Possibly polyphenols | May interact with surface glycoproteins spikes | (Sundararajan et al., 2010) |
| MHV-A59 | RT-qPCR | Nigella sativa | Ethanol extract | ND | ND | Not determined | Inhibits viral replication via undetermined mechanism | (Ulasli et al., 2014) |
| Anthemis hyaline | ||||||||
| Citrus sinensis | ||||||||
| PEDV | CPE assay | Ziziphus jujuba | Compounds isolated from methanol extract | 13.41 ± 1.13 | >30.0 | Jubanine G | Not determined | (Kang et al., 2015) |
| 4.49 ± 0.67 | 47.11 | Jubanine H | ||||||
| 6.17 ± 0.50 | 26.75 | Nummularine B | ||||||
| PEDV CV 777 | CPE assay | Ginkgo biloba | Polysaccharides purified from 98 % ethanol extract | 1.7 ± 1.3 μg/mL | >58.8 | Mixture of polysaccharides | Dose-dependent inhibition, apparently at viral attachment and entry steps | (Lee et al., 2015) |
| PEDV CV 777 | CPE assay | Houttuynia cordata | Compound isolated from methanol extract | ND | ND | Quercetin 7-rhamnoside | Uncertain. Doesn’t obstruct viral mRNA production or interact directly with PEDV | (Song et al., 2011) |
| PEDV CV 777 | CPE assay | Houttuynia cordata | Compounds isolated from methanol extract | ∼0.03 ± 0.01 | 7143 | Quercetin 7-rhamnoside | Not determined | (Choi et al., 2009) |
| ∼5.6 ± 2.6 | 215 | Quercetin | ||||||
| ∼0.4 ± 0.4 | 370 | Apigenin | ||||||
| ∼0.7 ± 0.7 | 32.7 | Luteolin | ||||||
| PEDV (KPEDV-9) | CPE assay | Prunus serrulata var. spontanea | 80 % methanol extract | 1.95 μg/mL | ND | Possibly polyphenols | Not determined | (Yook et al., 2010) |
| PEDV (NJ-PEDV) | Immunofluorescence assay and RT-qPCR | Found in: Griffithsia sp. | Purified compound used | ∼0.08 | ND | Griffithsin | Prevents viral attachment to host cells | (Li et al., 2019) |
BCV = bovine coronavirus; CPE assay = cytopathogenic effect assay; FCoV = feline coronavirus; IBV = (avian) infectious bronchitis virus; MHV = mouse hepatitis virus; n/a = not applicable to this study; ND = no data; PEDV = porcine epidemic diarrhoea virus.
4.1. Avian infectious bronchitis virus (IBV)
The antiviral activity of extracts from plant species against the avian IBV viral strains have been extensively studied (Chen et al., 2014; Jackwood et al., 2010; Lelesius et al., 2019; Li et al., 2011; Nguyen et al., 2015; Yang et al., 2010, 2011; Yin et al., 2011). Considering all studies on avian IBV that have established the mechanism of viral inhibition, the main mechanisms of action appear to be through viral envelope disruption or interference with the spike protein (Table 2). A notable exception are the terpenoid compounds 1,8-cineole, (-)-α-pinene and (-)-β-pinene, which bind to the IBV nucleocapsid protein (N-protein) (Yang et al., 2010, 2011), inhibiting its interaction with the viral genomic RNA and breaking the IBV replication cycle. These observations were reinforced by conformational models of the terpenoids binding to the active site at the N terminus of the N protein, which indicated that these compounds have the potential to bind strongly to five amino acid residues at this location (TyrA92, ProA134, PheA137, AspA138 and TyrA140) (Yang et al., 2010, 2011). As these residues are highly conserved between IBV strains (Yang et al., 2010), these terpenoids would be expected to show high efficacy against most or all IBV strains, making them a sensible target for further research.
Nevertheless, the envelope protein (E protein) of avian IBV remains the key target for most researchers. The coronavirus E protein is integral to many stages of the viral life cycle, including assembly, budding, envelope formation, and pathogenesis (Schoeman and Fielding, 2019). Interestingly, Chen et al. (2014) reported the inactivation of two distinct enveloped viruses (avian IBV and influenza) following treatment with Sambucus nigra extract, suggesting that S. nigra extract may have the potential to display a broad spectrum of antiviral effects against many other enveloped viruses. Although the authors raised the possibility of synergistic action of inhibitory compounds within the extract, no fractionation, identification or characterisation of the components was performed. However, flavonoids or lectins were suggested as the likely aetiological agents of the antiviral activity.
4.2. Feline coronavirus
Feline coronavirus is another coronavirus in the Alphacoronavirus group, causing a fatal disease in cats with no effective antiviral treatments available. In one study, Galanthus nivalis agglutinin, another lectin, was identified as a potent inhibitor of feline coronavirus (FCoV) (Hsieh et al., 2010). With an EC50 of just 0.0088 nM and a high selectivity index (>218), this carbohydrate-binding protein outperformed all synthetic antiviral agents tested for comparison purposes. For example, the protease inhibitor nelfinavir displayed an EC50 of 8.19 μM and a selectivity index of just 1.4. As with other agglutinins, Galanthus nivalis agglutinin binds to the spike and membrane proteins of FCoV, preventing their attachment to the host cells.
McDonagh et al. (2014) conducted a screening study against 19 compounds, focusing on those demonstrated to have previous antiviral effects against coronaviruses or other RNA viruses. Several naturally occurring compounds were included in this study, such as quercetin, curcumin, rutin, glycyrrhizic acid, hesperidin, hesperitin, baicalin and artemisinin. However, none of these compounds reached EC50 at the concentrations tested. Glycyrrhizic acid was the most promising, causing a 26.7 % reduction in CPE at a concentration of 25 μM. As only a single concentration was tested for each compound (ranging from 10 to 50 μM, depending on the compound), further investigation of these compounds against FCoV is required.
Theerawatanasirikul et al. (2020) adopted a computer-aided approach to their screening study against feline infectious peritonitis virus (FIPV), a mutated form of the parental enteric form of FCoV. Firstly, compounds from a chemical library were virtually screened for potential binding to the protease 3CL. The 15 most promising compounds were then evaluated in vitro using a protease inhibitor assay against FIPV 3CLpro. Of the naturally occurring compounds tested, the lowest IC50 values were shown by 7-methylluteolin (28.5 ± 4.2 μM) and stictic acid (29.4 ± 4.6 μM). Quercetin 7-rhamnoside (Fig. 2) also showed moderate inhibition (IC50 of 77.2 ± 13.8 μM); however 7-benzyl luteolin and steviol showed no inhibition (IC50 > 500 μM). Subsequent testing of the active compounds using a cytopathic effect (CPE) assay indicated that only stictic acid protected cells from viral-induced death (EC50 for pre-viral entry treatment of 16.24 ± 1.33 μM; selectivity index of 23).
4.3. Porcine endemic diarrhoea virus (PEDV)
Porcine endemic diarrhoea virus is another serious coronavirus from the Alphacoronavirus group. Several studies have investigated the activity of phytochemicals against murine coronaviruses (strain MHV-A59), the most extensive of which were performed by Kim et al. (2008) and Kim et al. (2010). Nevertheless, none of the studies of this viral strain have conclusively managed to determine the specific compounds responsible for viral inhibition, but rather suggested possible compounds or classes of compounds based on their abundance in the extracts tested. The low inhibitory concentrations (<1 μg/mL) of extracts obtained from Sophorae sp., Acanthopanacis sp. and Torilis sp. appear to show significant potential. In particular, the high viral specificity (SI = 696) of Sophorae sp. root extracts suggests that it could be considered as a prime candidate for future studies on the screening and isolation of compounds from the aforementioned species. The authors suggested that the antiviral activity of these three species are likely to be occurring through inhibition of RNA-dependent RNA polymerase or other protease activity.
Six studies considered natural agents active against porcine endemic diarrhea virus (PEDV), with the majority of these identifying the compounds with for their antiviral activity. In particular, the anti-PEDV activity of griffithsin and quercetin (Fig. 2) and its derivatives deserve particular discussion, given that these compounds have also been found to have antiviral activity against human CoVs (Millet et al., 2016; O'Keefe et al., 2010; Wen et al., 2011). Notably, quercetin was also one of the compounds shortlisted by Zhang et al. (2020) in their virtual screening of inhibitors of SARS-CoV-2 proteases. Against PEDV, quercetin-7-rhamnoside, a disaccharide glucoside, provided 50 % inhibition of viral activity at a concentration of just 0.03 μM, approximately 187 times lower than quercetin alone (Choi et al., 2009). Significantly, the specificity of quercetin-7-rhamnoside was exceptionally high (SI = 7143), indicating its potential for application in future animal or clinical trials. This study again highlights the importance of considering all possible structural conformities of a compound, including glycosides, in order to identify the most bioactive chemical species. Although the mechanism of action was not determined for quercetin or quercetin-7-rhamnoside against PEDV, previous computer modelling work has indicated that quercetin binds to and inhibits the SARS-CoV proteases PLpro and 3CLpro (Zhang et al., 2020). As previously determined, griffithsin binds to the spike protein of CoV, preventing attachment to host cells (Millet et al., 2016; O'Keefe et al., 2010).
In general, most authors recommend testing multiple coronavirus strains when searching for antiviral activity in naturally occurring compounds. Similarly important is determining their specific mechanism of action. As highlighted by several studies, a selection of compounds that are active against animal coronaviruses are also active against human coronavirus strains (e.g. griffithsin, quercetin). This underscores the potential of utilising compounds with identified activity against animal coronaviruses to guide the discovery of drugs against human coronaviruses, in addition to identifying drugs for the treatment of economically significant animal coronaviruses such as PEDV and avian IBV.
5. The significance of chemical polarity
Across both human and animal CoV strains, a clear trend toward the use of chemically polar compounds is evident (Fig. 2). Of the 30 studies that specified the solvent extraction protocols used, ethanol or an ethanol/water combination was the most commonly used (50 % of all studies), followed by methanol or a methanol/water combination (27 %). A further 17 % of relevant literature used a water-based extraction protocol, with only three studies using relatively non-polar solvents (diaminopropane, chloroform and acetone). This is consistent with a wide body of research indicating that relatively polar extracted fractions generally contain a greater level of bioactive and antimicrobial compounds compared to their nonpolar counterparts (Han et al., 2007; Tian et al., 2009; Wigmore et al., 2016). This may also be indicative of increased bioactivity of highly polar glycosylated compounds, similar to the vast difference in antiviral activity observed between quercetin and quercetin-7-rhamnoside (Choi et al., 2009).
In the case of compounds being administered orally, more polar compounds are subject to compartmentalisation within the body, reducing their rate of elimination. However, the potential chemical changes that could occur as a result of highly acidic stomach conditions and the level of absorption in the intestine would need to be considered individually for each compound.
6. Conclusions
Naturally occurring phytochemicals provide a valuable and powerful resource of chemical compounds displaying antiviral properties. Further chemical modification of these structures, guided by computer-based docking simulations, may also increase their potency and/or selectivity. Some of the key compounds that show promise for the treatment of coronavirus in humans include scutellarein, silvestrol, tryptanthrin, saikosaponin B2, lectins such as griffithsin, lycorine and polyphenolics – including quercetin, myricetin, caffeic acid, psoralidin and isobavachalcone. Needless to mention, these compounds may be toxic at certain levels, and hence in vitro and in vivo testing is required to determine safe and therapeutic levels for each compound before clinical trials in humans can be performed. Initial studies could focus on compounds which have previously been approved for drug use, or are generally regarded as safe by the FDA or other national organisations, as is the case for some polyphenolic compounds. It is hoped that researchers will be guided by this information presented here in the process of developing safe, effective anti-coronavirus therapeutic agents from naturally derived compounds.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that no conflict of interest exists.
References
- Aldeghi M., Heifetz A., Bodkin M.J., Knapp S., Biggin P.C. Accurate calculation of the absolute free energy of binding for drug molecules. Chem. Sci. 2016;7(1):207–218. doi: 10.1039/c5sc02678d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy L., Fernandez F., Johnson J.B., Naiker M., Owoola A.G., Broszczak D.A. Oxidative stress in alzheimer’s disease: a review on emergent natural polyphenolic therapeutics. Complement. Ther. Med. 2019:102294. doi: 10.1016/j.ctim.2019.102294. [DOI] [PubMed] [Google Scholar]
- Chen C.J., Michaelis M., Hsu H.K., Tsai C.C., Yang K.D., Wu Y.C., Cinatl J., Jr., Doerr H.W. Toona sinensis Roem tender leaf extract inhibits SARS coronavirus replication. J. Ethnopharmacol. 2008;120(1):108–111. doi: 10.1016/j.jep.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Zuckerman D.M., Brantley S., Sharpe M., Childress K., Hoiczyk E., Pendleton A.R. Sambucus nigra extracts inhibit infectious bronchitis virus at an early point during replication. BMC Vet. Res. 2014;10:24. doi: 10.1186/1746-6148-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., de Bruyn Kops C., Kirchmair J. Data resources for the computer-guided discovery of bioactive natural products. J. Chem. Inf. Model. 2017;57(9):2099–2111. doi: 10.1021/acs.jcim.7b00341. [DOI] [PubMed] [Google Scholar]
- Cheng P.W., Ng L.T., Chiang L.C., Lin C.C. Antiviral effects of saikosaponins on human coronavirus 229E in vitro. Clin. Exp. Pharmacol. Physiol. 2006;33(7):612–616. doi: 10.1111/j.1440-1681.2006.04415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang L.-C., Ng L.T., Liu L.-T., Shieh D.-e., Lin C.-C. Cytotoxicity and anti-hepatitis B virus activities of saikosaponins from Bupleurum species. Planta Med. 2003;69(8):705–709. doi: 10.1055/s-2003-42797. [DOI] [PubMed] [Google Scholar]
- Cho J.K., Curtis-Long M.J., Lee K.H., Kim D.W., Ryu H.W., Yuk H.J., Park K.H. Geranylated flavonoids displaying SARS-CoV papain-like protease inhibition from the fruits of Paulownia tomentosa. Bioorg. Med. Chem. 2013;21(11):3051–3057. doi: 10.1016/j.bmc.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.-J., Kim J.-H., Lee C.-H., Ahn Y.-J., Song J.-H., Baek S.-H., Kwon D.-H. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Research. 2009;81(1):77–81. doi: 10.1016/j.antiviral.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D.K., Pan Y., Cheng S., Hui K.P., Krishnan P., Liu Y., Ng D.Y., Wan C.K., Yang P., Wang Q. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin. Chem. 2020;66(4):549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials.gov . U.S. National Library of Medicine; 2020. COVID-19 - List Results.https://clinicaltrials.gov/ct2/results?cond=COVID-19 [Google Scholar]
- Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care. 2020 doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covés-Datson E.M., Dyall J., DeWald L.E., King S.R., Dube D., Legendre M., Nelson E., Drews K.C., Gross R., Gerhardt D.M. Inhibition of Ebola virus by a molecularly engineered banana lectin. PLoS Negl. Trop. Dis. 2019;13(7) doi: 10.1371/journal.pntd.0007595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forli S., Huey R., Pique M.E., Sanner M.F., Goodsell D.S., Olson A.J. Computational protein–ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016;11(5):905. doi: 10.1038/nprot.2016.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganjhu R.K., Mudgal P.P., Maity H., Dowarha D., Devadiga S., Nag S., Arunkumar G. Herbal plants and plant preparations as remedial approach for viral diseases. VirusDisease. 2015;26(4):225–236. doi: 10.1007/s13337-015-0276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- Haiying L., Na H., Xiaoyuan X. The curative effects of glycyrrhizin on patients with SARS. Annual Meeting of The Society of Infectious and Parasitic Diseases; Chinese Medical Association, Wuhan, China; 2003. pp. 18–22. [Google Scholar]
- Häkkinen S.H., Kärenlampi S.O., Heinonen I.M., Mykkänen H.M., Törrönen A.R. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food Chem. 1999;47(6):2274–2279. doi: 10.1021/jf9811065. [DOI] [PubMed] [Google Scholar]
- Han T., Li H.-L., Zhang Q.-Y., Han P., Zheng H.-C., Rahman K., Qin L.-P. Bioactivity-guided fractionation for anti-inflammatory and analgesic properties and constituents of Xanthium strumarium L. Phytomedicine. 2007;14(12):825–829. doi: 10.1016/j.phymed.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Ho T.Y., Wu S.L., Chen J.C., Li C.C., Hsiang C.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. 2007;74(2):92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoever G., Baltina L., Michaelis M., Kondratenko R., Baltina L., Tolstikov G.A., Doerr H.W., Cinatl J., Jr. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J. Med. Chem. 2005;48(4):1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh L.E., Lin C.N., Su B.L., Jan T.R., Chen C.M., Wang C.H., Lin D.S., Lin C.T., Chueh L.L. Synergistic antiviral effect of Galanthus nivalis agglutinin and nelfinavir against feline coronavirus. Antiviral Res. 2010;88(1):25–30. doi: 10.1016/j.antiviral.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I.C., Bosch B.J., Li F., Li W., Lee K.H., Ghiran S., Vasilieva N., Dermody T.S., Harrison S.C., Dormitzer P.R., Farzan M. SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. J. Biol. Chem. 2006;281(6):3198–3203. doi: 10.1074/jbc.M508381200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., I Azhar E., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., McHugh T.D., Memish Z.A., Drosten C., Zumla A., Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health — the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang H.-J., Han J.-W., Jeon H., Cho K., Kim J.-h., Lee D.-S., Han J.W. Characterization of a novel mannose-binding lectin with antiviral activities from red alga, Grateloupia chiangii. Biomolecules. 2020;10(2):333. doi: 10.3390/biom10020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackwood M.W., Rosenbloom R., Petteruti M., Hilt D.A., McCall A.W., Williams S.M. Avian coronavirus infectious bronchitis virus susceptibility to botanical oleoresins and essential oils in vitro and in vivo. Virus Res. 2010;149(1):86–94. doi: 10.1016/j.virusres.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justesen U., Knuthsen P. Composition of flavonoids in fresh herbs and calculation of flavonoid intake by use of herbs in traditional Danish dishes. Food Chem. 2001;73(2):245–250. [Google Scholar]
- Kaack K., Austed T. Interaction of vitamin C and flavonoids in elderberry (Sambucus nigra L.) during juice processing. Plant Foods Hum. Nutr. 1998;52(3):187–198. doi: 10.1023/a:1008069422202. [DOI] [PubMed] [Google Scholar]
- Kang K.B., Ming G., Kim G.J., Ha T.K., Choi H., Oh W.K., Sung S.H. Jubanines F-J, cyclopeptide alkaloids from the roots of Ziziphus jujuba. Phytochemistry. 2015;119:90–95. doi: 10.1016/j.phytochem.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R., Manjal S.K., Rawal R.K., Kumar K. Recent synthetic and medicinal perspectives of tryptanthrin. Bioorg. Med. Chem. 2017;25(17):4533–4552. doi: 10.1016/j.bmc.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Keyaerts E., Vijgen L., Pannecouque C., Van Damme E., Peumans W., Egberink H., Balzarini J., Van Ranst M. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res. 2007;75(3):179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamitov R., Loginova S., Shchukina V., Borisevich S., Maksimov V., Shuster A. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures. Vopr. Virusol. 2008;53(4):9–13. [PubMed] [Google Scholar]
- Khan H., Sureda A., Belwal T., Çetinkaya S., Süntar İ., Tejada S., Devkota H.P., Ullah H., Aschner M. Polyphenols in the treatment of autoimmune diseases. Autoimmun. Rev. 2019;18(7):647–657. doi: 10.1016/j.autrev.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.Y., Shin H.S., Park H., Kim Y.C., Yun Y.G., Park S., Shin H.J., Kim K. In vitro inhibition of coronavirus replications by the traditionally used medicinal herbal extracts, Cimicifuga rhizoma, Meliae cortex, Coptidis rhizoma, and Phellodendron cortex. J. Clin. Virol. 2008;41(2):122–128. doi: 10.1016/j.jcv.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.Y., Eo E.Y., Park H., Kim Y.C., Park S., Shin H.J., Kim K. Medicinal herbal extracts of Sophorae radix, Acanthopanacis cortex, Sanguisorbae radix and Torilis fructus inhibit coronavirus replication in vitro. Antivir. Ther. (Lond.) 2010;15(5):697–709. doi: 10.3851/IMP1615. [DOI] [PubMed] [Google Scholar]
- Kim D.W., Seo K.H., Curtis-Long M.J., Oh K.Y., Oh J.W., Cho J.K., Lee K.H., Park K.H. Phenolic phytochemical displaying SARS-CoV papain-like protease inhibition from the seeds of Psoralea corylifolia. J. Enzyme Inhib. Med. Chem. 2014;29(1):59–63. doi: 10.3109/14756366.2012.753591. [DOI] [PubMed] [Google Scholar]
- Kim D.E., Min J.S., Jang M.S., Lee J.Y., Shin Y.S., Song J.H., Kim H.R., Kim S., Jin Y.H., Kwon S. Natural Bis-Benzylisoquinoline Alkaloids-Tetrandrine, Fangchinoline, and Cepharanthine, inhibit human coronavirus OC43 infection of MRC-5 human lung cells. Biomolecules. 2019;9(11):696. doi: 10.3390/biom9110696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzing S., Abraham G., Seiwert B., Ungemach F.R., Krügel U., Regenthal R. Dose-dependent emetic effects of the Amaryllidaceous alkaloid lycorine in beagle dogs. Toxicon. 2011;57(1):117–124. doi: 10.1016/j.toxicon.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Kumaki Y., Wandersee M.K., Smith A.J., Zhou Y., Simmons G., Nelson N.M., Bailey K.W., Vest Z.G., Li J.K., Chan P.K., Smee D.F., Barnard D.L. Inhibition of severe acute respiratory syndrome coronavirus replication in a lethal SARS-CoV BALB/c mouse model by stinging nettle lectin, Urtica dioica agglutinin. Antiviral Res. 2011;90(1):22–32. doi: 10.1016/j.antiviral.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K.M., Lee K.M., Koon C.M., Cheung C.S., Lau C.P., Ho H.M., Lee M.Y., Au S.W., Cheng C.H., Lau C.B., Tsui S.K., Wan D.C., Waye M.M., Wong K.B., Wong C.K., Lam C.W., Leung P.C., Fung K.P. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J. Ethnopharmacol. 2008;118(1):79–85. doi: 10.1016/j.jep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Park J.S., Lee S.W., Hwang S.Y., Young B.E., Choi H.J. Porcine epidemic diarrhea virus infection: inhibition by polysaccharide from Ginkgo biloba exocarp and mode of its action. Virus Res. 2015;195:148–152. doi: 10.1016/j.virusres.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Lelesius R., Karpovaite A., Mickiene R., Drevinskas T., Tiso N., Ragazinskiene O., Kubiliene L., Maruska A., Salomskas A. In vitro antiviral activity of fifteen plant extracts against avian infectious bronchitis virus. BMC Vet. Res. 2019;15(1):178. doi: 10.1186/s12917-019-1925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5(4):562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.Y., Chen C., Zhang H.Q., Guo H.Y., Wang H., Wang L., Zhang X., Hua S.N., Yu J., Xiao P.G., Li R.S., Tan X. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res. 2005;67(1):18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wu J., Zhang Z., Ma Y., Liao F., Zhang Y., Wu G. Forsythoside a inhibits the avian infectious bronchitis virus in cell culture. Phytother. Res. 2011;25(3):338–342. doi: 10.1002/ptr.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Yu X., Zhang H., Cheng H., Hou L., Zheng Q., Hou J. In vitro antiviral activity of Griffithsin against porcine epidemic diarrhea virus. Virus Genes. 2019;55(2):174–181. doi: 10.1007/s11262-019-01633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.W., Tsai F.J., Tsai C.H., Lai C.C., Wan L., Ho T.Y., Hsieh C.C., Chao P.D. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antiviral Res. 2005;68(1):36–42. doi: 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L.-T., Hsu W.-C., Lin C.-C. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014;4(1):24–35. doi: 10.4103/2225-4110.124335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L.-T., Chung C.-Y., Hsu W.-C., Chang S.-P., Hung T.-C., Shields J., Russell R.S., Lin C.-C., Li C.-F., Yen M.-H. Saikosaponin b2 is a naturally occurring terpenoid that efficiently inhibits hepatitis C virus entry. J. Hepatol. 2015;62(3):541–548. doi: 10.1016/j.jhep.2014.10.040. [DOI] [PubMed] [Google Scholar]
- Ling C.-q. Traditional Chinese medicine is a resource for drug discovery against 2019 novel coronavirus (SARS-CoV-2) J. Integr. Med. 2020;18(2):87–88. doi: 10.1016/j.joim.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Zhou J. SARS-CoV protease inhibitors design using virtual screening method from natural products libraries. J. Comput. Chem. 2005;26(5):484–490. doi: 10.1002/jcc.20186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizzo M.R., Saab A.M., Tundis R., Statti G.A., Menichini F., Lampronti I., Gambari R., Cinatl J., Doerr H.W. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem. Biodivers. 2008;5(3):461–470. doi: 10.1002/cbdv.200890045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Trends. 2020;14(1):69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- Lu G., Wang Q., Gao G.F. Bat-to-human: spike features determining ‘host jump’of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. 2015;23(8):468–478. doi: 10.1016/j.tim.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung J., Lin Y.S., Yang Y.H., Chou Y.L., Shu L.H., Cheng Y.C., Liu H.T., Wu C.Y. The potential chemical structure of anti-SARS-CoV-2 RNA-dependent RNA polymerase. J. Med. Virol. 2020;92(6):693–697. doi: 10.1002/jmv.25761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Su X., Gong S., Qin Y., Liu W., Li J., Yu H., Xu Q. Anti-SARS coronavirus 3C-like protease effects ofRheum palmatum L. extracts. Biosci. Trends. 2009;3(4):124–126. [PubMed] [Google Scholar]
- Luo H., Tang Q.-l., Shang Y.-x., Liang S.-b., Yang M., Robinson N., Liu J.-p. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin. J. Integr. Med. 2020;26(4):243–250. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon A.R., Roberts T.E., Gibbons E., Ellis S.M., Babiuk L.A., Hancock R.E., Towers G.H. Antiviral screening of British Columbian medicinal plants. J. Ethnopharmacol. 1995;49(2):101–110. doi: 10.1016/0378-8741(95)90037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonagh P., Sheehy P.A., Norris J.M. Identification and characterisation of small molecule inhibitors of feline coronavirus replication. Vet. Microbiol. 2014;174(3–4):438–447. doi: 10.1016/j.vetmic.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis M., Doerr H.W., Cinatl J., Jr. Investigation of the influence of EPs(R) 7630, a herbal drug preparation from Pelargonium sidoides, on replication of a broad panel of respiratory viruses. Phytomedicine. 2011;18(5):384–386. doi: 10.1016/j.phymed.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelow I.C., Lear C., Scully C., Prugar L.I., Longley C.B., Yantosca L.M., Ji X., Karpel M., Brudner M., Takahashi K. High-dose mannose-binding lectin therapy for Ebola virus infection. J. Infect. Dis. 2011;203(2):175–179. doi: 10.1093/infdis/jiq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miean K.H., Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001;49(6):3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- Millet J.K., Seron K., Labitt R.N., Danneels A., Palmer K.E., Whittaker G.R., Dubuisson J., Belouzard S. Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. Antiviral Res. 2016;133:1–8. doi: 10.1016/j.antiviral.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C.A., Ramessar K., O’Keefe B.R. Antiviral lectins: selective inhibitors of viral entry. Antiviral Res. 2017;142:37–54. doi: 10.1016/j.antiviral.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C., Schulte F.W., Lange-Grunweller K., Obermann W., Madhugiri R., Pleschka S., Ziebuhr J., Hartmann R.K., Grunweller A. Broad-spectrum antiviral activity of the eIF4A inhibitor silvestrol against corona- and picornaviruses. Antiviral Res. 2018;150:123–129. doi: 10.1016/j.antiviral.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C., Obermann W., Schulte F.W., Lange-Grünweller K., Oestereich L., Elgner F., Glitscher M., Hildt E., Singh K., Hans-Guido W., Hartmann R.K. Comparison of broad-spectrum antiviral activities of the synthetic rocaglate CR-31-B (−) and the eIF4A-inhibitor silvestrol. Antiviral Res. 2020;175 doi: 10.1016/j.antiviral.2020.104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79(3):629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Nguyen P.Q., Ooi J.S., Nguyen N.T., Wang S., Huang M., Liu D.X., Tam J.P. Antiviral cystine knot alpha-amylase inhibitors from Alstonia scholaris. J. Biol. Chem. 2015;290(52):31138–31150. doi: 10.1074/jbc.M115.654855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe B.R., Giomarelli B., Barnard D.L., Shenoy S.R., Chan P.K., McMahon J.B., Palmer K.E., Barnett B.W., Meyerholz D.K., Wohlford-Lenane C.L., McCray P.B., Jr. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J. Virol. 2010;84(5):2511–2521. doi: 10.1128/JVI.02322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J., Wang M.X., Ang I.Y.H., Tan S.H.X., Lewis R.F., Chen J.I.-P., Gutierrez R.A., Gwee S.X.W., Chua P.E.Y., Yang Q. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel Coronavirus (2019-ncoV): a systematic review. J. Clin. Med. 2020;9(3):623. doi: 10.3390/jcm9030623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevis D., Kostaki E.G., Magiorkinis G., Panayiotakopoulos G., Sourvinos G., Tsiodras S. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect. Genet. Evol. 2020;79 doi: 10.1016/j.meegid.2020.104212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.Y., Kim J.H., Kim Y.M., Jeong H.J., Kim D.W., Park K.H., Kwon H.J., Park S.J., Lee W.S., Ryu Y.B. Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases. Bioorg. Med. Chem. 2012;20(19):5928–5935. doi: 10.1016/j.bmc.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.Y., Yuk H.J., Ryu H.W., Lim S.H., Kim K.S., Park K.H., Ryu Y.B., Lee W.S. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J. Enzyme Inhib. Med. Chem. 2017;32(1):504–515. doi: 10.1080/14756366.2016.1265519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlotsky J.-M. New hepatitis C virus (HCV) drugs and the hope for a cure: concepts in anti-HCV drug development. Semin. Liver Dis. 2014;34:022–029. doi: 10.1055/s-0034-1371007. [DOI] [PubMed] [Google Scholar]
- Petersen K.A., Matthiesen F., Agger T., Kongerslev L., Thiel S., Cornelissen K., Axelsen M. Phase I safety, tolerability, and pharmacokinetic study of recombinant human mannan-binding lectin. J. Clin. Immunol. 2006;26(5):465–475. doi: 10.1007/s10875-006-9037-z. [DOI] [PubMed] [Google Scholar]
- Runfeng L., Yunlong H., Jicheng H., Weiqi P., Qinhai M., Yongxia S., Chufang L., Jin Z., Zhenhua J., Haiming J., Kui Z., Shuxiang H., Jun D., Xiaobo L., Xiaotao H., Lin W., Nanshan Z., Zifeng Y. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020;156 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu Y.B., Jeong H.J., Kim J.H., Kim Y.M., Park J.-Y., Kim D., Naguyen T.T.H., Park S.-J., Chang J.S., Park K.H. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CLpro inhibition. Bioorg. Med. Chem. 2010;18(22):7940–7947. doi: 10.1016/j.bmc.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu Y.B., Park S.J., Kim Y.M., Lee J.Y., Seo W.D., Chang J.S., Park K.H., Rho M.C., Lee W.S. SARS-CoV 3CLpro inhibitory effects of quinone-methide triterpenes from Tripterygium regelii. Bioorg. Med. Chem. Lett. 2010;20(6):1873–1876. doi: 10.1016/j.bmcl.2010.01.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16(1):69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y.C., Wang L.T., Khalil A.T., Chiang L.C., Cheng P.W. Bioactive pyranoxanthones from the roots of Calophyllum blancoi. Chem Pharm Bull (Tokyo). 2005;53(2):244–247. doi: 10.1248/cpb.53.244. [DOI] [PubMed] [Google Scholar]
- Simmons G., Gosalia D.N., Rennekamp A.J., Reeves J.D., Diamond S.L., Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. 2005;102(33):11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabi C., Alsafi Z., O’Neill N., Khan M., Kerwan A., Al-Jabir A., Iosifidis C., Agha R. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.H., Shim J.K., Choi H.J. Quercetin 7-rhamnoside reduces porcine epidemic diarrhea virus replication via independent pathway of viral induced reactive oxygen species. Virol. J. 2011;8:460. doi: 10.1186/1743-422X-8-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhan A.R., Siemann D.W. Cathepsin L targeting in cancer treatment. Pharmacol. Ther. 2015;155:105–116. doi: 10.1016/j.pharmthera.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundararajan A., Ganapathy R., Huan L., Dunlap J.R., Webby R.J., Kotwal G.J., Sangster M.Y. Influenza virus variation in susceptibility to inactivation by pomegranate polyphenols is determined by envelope glycoproteins. Antiviral Res. 2010;88(1):1–9. doi: 10.1016/j.antiviral.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theerawatanasirikul S., Kuo C.J., Phetcharat N., Lekcharoensuk P. In silico and in vitro analysis of small molecules and natural compounds targeting the 3CL protease of feline infectious peritonitis virus. Antiviral Res. 2020;174 doi: 10.1016/j.antiviral.2019.104697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F., Li B., Ji B., Yang J., Zhang G., Chen Y., Luo Y. Antioxidant and antimicrobial activities of consecutive extracts from Galla chinensis:the polarity affects the bioactivities. Food Chem. 2009;113(1):173–179. [Google Scholar]
- Tome-Carneiro J., Visioli F. Polyphenol-based nutraceuticals for the prevention and treatment of cardiovascular disease: review of human evidence. Phytomedicine. 2016;23(11):1145–1174. doi: 10.1016/j.phymed.2015.10.018. [DOI] [PubMed] [Google Scholar]
- Toney J.H., Navas-Martin S., Weiss S.R., Koeller A. Sabadinine: a potential non-peptide anti-severe acute-respiratory-syndrome agent identified using structure-aided design. J. Med. Chem. 2004;47(5):1079–1080. doi: 10.1021/jm034137m. [DOI] [PubMed] [Google Scholar]
- Tsai Y.C., Lee C.L., Yen H.R., Chang Y.S., Lin Y.P., Huang S.H., Lin C.W. Antiviral action of tryptanthrin isolated from Strobilanthes cusia leaf against human coronavirus NL63. Biomolecules. 2020;10(3) doi: 10.3390/biom10030366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulasli M., Gurses S.A., Bayraktar R., Yumrutas O., Oztuzcu S., Igci M., Igci Y.Z., Cakmak E.A., Arslan A. The effects of Nigella sativa (Ns), Anthemis hyalina (Ah) and Citrus sinensis (Cs) extracts on the replication of coronavirus and the expression of TRP genes family. Mol. Biol. Rep. 2014;41(3):1703–1711. doi: 10.1007/s11033-014-3019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio Y., Abe H. Inactivation of measles virus and herpes simplex virus by saikosaponin d. Planta Med. 1992;58(2):171–173. doi: 10.1055/s-2006-961422. [DOI] [PubMed] [Google Scholar]
- Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y., Lang C., Huang D., Sun Q., Xiong Y., Huang X., Lv J., Luo Y., Shen L., Yang H., Huang G., Yang R. Clinical features and treatment of COVID-19 patients in Northeast Chongqing. J. Med. Virol. 2020 doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.M., Zhu X.X., Cui X.L., Liang A.H., Du G.H., Ruan J.X., Sun J.N., Li P.T., Wang S.R. Screening of traditional Chinese remedies for SARS treatment. Zhongguo Zhong Yao Za Zhi. 2003;28(6):484–487. [PubMed] [Google Scholar]
- Wang S.Q., Du Q.S., Zhao K., Li A.X., Wei D.Q., Chou K.C. Virtual screening for finding natural inhibitor against cathepsin-L for SARS therapy. Amino Acids. 2007;33(1):129–135. doi: 10.1007/s00726-006-0403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.-F., Shi L.-P., Ren Y.-D., Liu Q.-F., Liu H.-F., Zhang R.-J., Li Z., Zhu F.-H., He P.-L., Tang W. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antiviral Res. 2009;83(2):186–190. doi: 10.1016/j.antiviral.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C.C., Shyur L.F., Jan J.T., Liang P.H., Kuo C.J., Arulselvan P., Wu J.B., Kuo S.C., Yang N.S. Traditional Chinese medicine herbal extracts of Cibotium barometz, Gentiana scabra, Dioscorea batatas, Cassia tora, and Taxillus chinensis inhibit SARS-CoV replication. J. Tradit. Complement. Med. 2011;1(1):41–50. doi: 10.1016/S2225-4110(16)30055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J.R., Lin C.S., Lai H.C., Lin Y.P., Wang C.Y., Tsai Y.C., Wu K.C., Huang S.H., Lin C.W. Antiviral activity of Sambucus formosana Nakai ethanol extract and related phenolic acid constituents against human coronavirus NL63. Virus Res. 2019;273 doi: 10.1016/j.virusres.2019.197767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigmore S.M., Naiker M., Bean D.C. Antimicrobial activity of extracts from native plants of temperate Australia. Pharmacogn. Commun. 2016;6(2):80–84. [Google Scholar]
- Wittemer S.M., Ploch M., Windeck T., Müller S.C., Drewelow B., Derendorf H., Veit M. Bioavailability and pharmacokinetics of caffeoylquinic acids and flavonoids after oral administration of Artichoke leaf extracts in humans. Phytomedicine. 2005;12(1):28–38. doi: 10.1016/j.phymed.2003.11.002. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2019. WHO MERS Global Summary and Assessment of Risk, July 2019. [Google Scholar]
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J., Sheng J. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M., Chen L., Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020 doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q.Y., Tian X.Y., Fang W.S. Bioactive coumarins from Boenninghausenia sessilicarpa. J. Asian Nat. Prod. Res. 2007;9(1):59–65. doi: 10.1080/10286020500382397. [DOI] [PubMed] [Google Scholar]
- Yang Z., Wu N., Fu Y., Yang G., Wang W., Zu Y., Efferth T. Anti-infectious bronchitis virus (IBV) activity of 1,8-cineole: effect on nucleocapsid (N) protein. J. Biomol. Struct. Dyn. 2010;28(3):323–330. doi: 10.1080/07391102.2010.10507362. [DOI] [PubMed] [Google Scholar]
- Yang Z., Wu N., Zu Y., Fu Y. Comparative anti-infectious bronchitis virus (IBV) activity of (-)-pinene: effect on nucleocapsid (N) protein. Molecules. 2011;16(2):1044–1054. doi: 10.3390/molecules16021044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional chinese medicine in the treatment of patients infected with 2019-New coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 2020;16(10):1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Peng F., Wang R., Guan K., Jiang T., Xu G., Sun J., Chang C. The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao T.T., Qian J.D., Zhu W.Y., Wang Y., Wang G.Q. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus–A possible reference for coronavirus Disease‐19 treatment option. J. Med. Virol. 2020;92(6):556–563. doi: 10.1002/jmv.25729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L., Li Z., Yuan K., Qu X., Chen J., Wang G., Zhang H., Luo H., Zhu L., Jiang P., Chen L., Shen Y., Luo M., Zuo G., Hu J., Duan D., Nie Y., Shi X., Wang W., Han Y., Li T., Liu Y., Ding M., Deng H., Xu X. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J. Virol. 2004;78(20):11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Li G., Li J., Yang Q., Ren X. In vitro and in vivo effects of Houttuynia cordata on infectious bronchitis virus. Avian Pathol. 2011;40(5):491–498. doi: 10.1080/03079457.2011.605107. [DOI] [PubMed] [Google Scholar]
- Yook H.S., Kim K.H., Park J.E., Shin H.J. Antioxidative and antiviral properties of flowering cherry fruits (Prunus serrulata L. Var. spontanea). Am J Chin Med. 2010;38(5):937–948. doi: 10.1142/S0192415X10008366. [DOI] [PubMed] [Google Scholar]
- Yu M.-S., Lee J., Lee J.M., Kim Y., Chin Y.-W., Jee J.-G., Keum Y.-S., Jeong Y.-J. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorg. Med. Chem. Lett. 2012;22(12):4049–4054. doi: 10.1016/j.bmcl.2012.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.H., Wu K.L., Zhang X., Deng S.Q., Peng B. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J. Integr. Med. 2020;18(2):152–158. doi: 10.1016/j.joim.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Chen X.-Y., Martin C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. (Beijing) 2016;61(18):1391–1398. doi: 10.1007/s11434-016-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Feng L., Liu L., Zhao R. Saikosaponin b2 enhances the hepatotargeting effect of anticancer drugs through inhibition of multidrug resistance-associated drug transporters. Life Sci. 2019;231 doi: 10.1016/j.lfs.2019.116557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang M., Jiang H., Suzuki Y., Li X., Xiao P., Tanaka T., Ling H., Yang B., Saitoh H., Zhang L., Qin C., Sugamura K., Hattori T. Procyanidins and butanol extract of Cinnamomi Cortex inhibit SARS-CoV infection. Antiviral Res. 2009;82(1):73–81. doi: 10.1016/j.antiviral.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]