Abstract
Purpose
The aim of the present study was to investigate the effect of a three-month dietary supplementation with a methylfolate formulation on homocysteine plasma concentrations and ocular blood flow parameters in patients with diabetes.
Methods
Twenty-four patients with diabetes received a dietary supplement (Oculofolin, Aprofol AG, Switzerland) containing 900 µg L‑methylfolate (levomefolate calcium or [6S]-5-methyltetrahydrofolic acid, calcium salt), methylcobalamin, and other ingredients for three consecutive months. The patients’ plasma homocysteine concentration and retinal blood flow were assessed at baseline and after three months of folate intake. Retinal blood flow was measured using a custom-built dual-beam Doppler optical coherence tomography (OCT) system. In addition, flicker-induced retinal vasodilatation was assessed by means of a commercially available dynamic vessel analyzer (IMEDOS, Jena, Germany).
Results
Supplementation was well tolerated by all patients. After three months, plasma homocysteine concentration significantly decreased from 14.2 ± 9.3 to 9.6 ± 6.6 µmol/L (p < 0.001). In addition, a tendency toward an increased total retinal blood flow from 36.8 ± 12.9 to 39.2 ± 10.8 µl/min was observed, but this effect did not reach the level of significance (p = 0.11). Supplementation had no effect on retinal vessel diameter or flicker-induced vasodilatation.
Conclusions
The present data show that a three-month intake of a dietary supplement containing methylfolate can significantly reduce blood homocysteine levels in patients with diabetes. This is of importance because higher homocysteine plasma levels have been found to be associated with an increased risk of vascular associated systemic diseases and eye diseases. Whether systemic methylfolate supplementation affects retinal perfusion must be studied in a larger population.
Introduction
Homocysteine (Hcy) is a non-protein amino acid naturally occurring in the human body. There is accumulating evidence that increased Hcy plasma levels play an important role in several systemic and ocular vascular-related conditions. As such, observational studies consistently report an association between total plasma Hcy concentrations and the risk of systemic microvascular complications, as well as with cardiovascular events [1,2]. Further, high plasma Hcy levels have been shown to be associated with coronary heart disease, stroke, peripheral artery stenosis, and venous thrombosis [3].
With respect to ocular conditions, recently published data support the hypothesis that increased Hcy levels also play an important role in ocular diseases. A meta-analysis pooling the data of 11 clinical studies reports that age-related macular degeneration (AMD) is associated with increased Hcy levels and decreased vitamin B12 concentration [4]. Further, a recently published study shows that elevated Hcy levels were associated with an increased risk of diabetic retinopathy, especially in type 2 diabetic patients [5]. These data indicate that high plasma Hcy concentrations may also play a role in the pathogenesis of vascular-related ocular diseases, which makes folate a potential candidate for dietary supplementation.
However, studies investigating the value of interventions using folic acid to lower Hcy and prevent vascular complications in the eye are sparse. A recent study investigating the association between folic acid supplementation and retinal atherosclerosis in patients with diabetes showed that folic acid supplementation was significantly associated with reduced risk of retinal atherosclerosis in females with hyperhomocysteinemia [6].
The current study was designed to further investigate the effect of a dietary supplement containing L-methylfolate on systemic Hcy concentrations and its potential effects on ocular blood flow. However, because the potential effect size of a folate supplementation on blood flow and systemic blood parameters is unclear, creating a proper statistical design for a large, controlled, randomized study is difficult. Thus, the present pilot study should (1) provide information about the Hcy-lowering potential of the formulation under study and (2) identify potential vascular-related outcome parameters for further, larger, placebo-controlled studies, as well as provide sufficient data to allow for proper statistical planning of such a study.
The primary objective of the study was to investigate whether a three-month supplementation with L-methylfolate and B vitamins can lower systemic plasma Hcy levels in patients with diabetes. Further, as a secondary outcome, ocular blood flow parameters and flicker-induced vasodilatation in the ocular microcirculation were assessed at baseline and after the three-month supplementation period.
Methods
Patients
The study protocol was approved by the Ethics Committee of the Medical University of Vienna. The study was performed in adherence to the guidelines of the Declaration of Helsinki and Good Clinical Practice. Twenty-five patients with either type 1 or type 2 diabetes and mild or no diabetic retinopathy participated in this study. All patients passed a screening examination, including physical examination, blood pressure measurement, and ophthalmic examination. Exclusion criteria were an age < 18 years, ametropia > 6 dpt, best corrected visual acuity < 0.8, presence of any ocular pathologies except mild diabetic retinopathy, untreated systemic hypertension (defined as either systolic blood pressure > 150 mmHg or diastolic blood pressure > 95 mmHg), clinically relevant illness before the study, pregnancy or lactation, and participation in a clinical study or intake of dietary supplements containing folate within the three months before the screening visit. Pupil dilation was achieved instilling one drop of 0.5% tropicamide (Mydriaticum “Agepha,” Agepha, Vienna, Austria). Measurements were taken after a resting period of 20 min to achieve stable hemodynamic conditions. Measurements were taken in the right eye of all patients in one major artery and one major vein. In addition, total retinal blood flow was measured in all retinal vessels.
Dietary supplement
Ocufolin™ forte, Aprofol AG, Switzerland
Dose: one capsule per day
Ocufolin™ forte is registered as a dietary supplement (DS) or as food for special medical purposes (FSMP), depending on the regulation of the country where it is registered. Its ingredients are listed in Table 1.
Table 1. Formulation of the dietary supplement used.
| Ingredient | Amount |
|---|---|
| Folate (as (6S)-5-methyltetrahydrofolic acid, calcium salt) |
900 µg |
| Vitamin C (Ca-Ascorbate) |
45 mg |
| Vitamin D (as Cholecalciferol) |
37.5 µg |
| Vitamin E Natural Tocopherols (as Alpha, Beta, Gamma, & Delta) |
5 mg |
| Vitamin B1 (As Thiamine Hydrochloride) |
1.5 mg |
| Vitamin B2 (Riboflavin) |
10 mg |
| Vitamin B6 (as Pyridoxal-5-Phosphate) |
3 mg |
| Vitamin B12 (as Methylcobalamin) |
500 µg |
| Pantothenic Acid (as Calcium-D-Pantothenate) |
5 mg |
| Zinc (as Zinc Acetate) |
25 mg |
| Selenium (as L- Selenomethionine) |
20 µg |
| Copper (as Cupric Gluconate) |
667 µg |
| N-Acetyl Cysteine (NAC) |
180 mg |
| Lutein |
10 mg |
| Zeaxanthin | 2 mg |
Doppler optical coherence tomography measurements
A dual-beam bidirectional Doppler optical coherence tomography (D-OCT) system was used in the present study, as described in detail previously [7–9]. The device is based on a broadband superluminescent diode with a central wavelength of 840 nm (spectral bandwidth, 54 nm) and two charge-coupled device (CCD) cameras, which provides a tissue resolution of approximately 6 μm and 18 μm in the axial and transversal directions, respectively. The retinal vessel under study was illuminated by two probe beams separated by their polarization properties under a known angle Δα. Light backscattered and back-reflected from the sample (i.e., the retinal vessel under study) was spectrally detected by two identical spectrometers. Signal post-processing, including segmentation of vessels and calculation of the phase shift due to moving erythrocytes within the vessels, was semiautomated using custom software (written in LabVIEW; National Instruments; Austin, TX) as outlined previously [7].
D-OCT gives readings of blood flow (µl per min) as well as blood flow velocity (mL per s). Measurements of vessel caliber (in µm) were obtained from the OCT phase images, as described in detail elsewhere [10]. With respect to vessel caliber measurements using this method, Fondi et al. recently demonstrated high interobserver reproducibility and a high level of agreement with vessel caliber measurements as obtained from fundus images [10]. Diffuse luminance flicker was applied during the measurements for 60 s.
Measurement of retinal vessel diameters and flicker-induced vasodilatation
For the assessment of retinal vessel diameters, a commercially available dynamic vessel analyzer (DVA, Imedos, Jena, Germany) was used. The DVA comprises a fundus camera, a video camera, a real-time monitor, and a personal computer with analyzing software for accurate determination of retinal arterial and venous diameters [11]. Every second, a maximum of 25 readings of vessel diameters can be obtained. For the purpose of diameter measurement, the fundus is imaged onto the CCD chip of the video camera. The consecutive fundus images are digitized using a frame grabber. In addition, the fundus image can be inspected on the real-time monitor and digitally stored for further analysis. Due to the absorptive properties of hemoglobin, each blood vessel has a specific transmittance profile. The measurement of retinal vessel diameters is based on adaptive algorithms using these specific profiles. Whenever a specific vessel profile is recognized, the DVA is able to follow this vessel as long as it appears within the measurement window. This means that the system automatically corrects for alterations in luminance induced, for instance, by slight eye movements. If the requirements for the assessment of retinal vessel diameters are not fulfilled, as during blinks, the system automatically stops measuring the vessel diameter. As soon as an adequate fundus image is achieved again, the measurement of vessel diameters restarts automatically.
The built-in stimulation system of the DVA was used for flicker stimulation. This system uses a shutter that interrupts the observation illumination to the fundus camera, which generates a square wave stimulation pattern with a frequency of 12.5 Hz and a modulation depth of 100%. Vessel diameter measurements were performed for 60 s at baseline and for 60 s during flicker stimulation.
Measurement of blood pressure and pulse rate
Systolic, diastolic and mean arterial blood pressures (SBP, DBP, MAP) were measured in the upper arm by an automated oscillometric device (Infinity Delta, Draeger Medical Systems, Telford, PA). The pulse rate was automatically recorded with a finger oxymetric device connected to the same device.
Determination of plasma homocysteine levels
After an overnight fast, blood was drawn into ethylenediaminetetraacetic acid (EDTA) tubes from all patients at the beginning of days one and two of the study. The tubes were immediately put on ice and sent to the central laboratory of the Medical University of Vienna. Determination of Hcy levels was done by the chemiluminescent one-step microparticle immunoassay method [12].
Intraocular pressure
Intraocular pressure was measured using a slit lamp–mounted Goldmann applanation tonometer. Before each measurement, one drop of oxybuprocain hydrochloride combined with sodium fluorescein was used for local anesthesia of the cornea.
Experimental paradigm
Two study days were scheduled for all patients. On the first study day, blood was drawn to obtain plasma Hcy levels and capillary blood glucose levels. Afterwards, one drop of mydriaticum was instilled into the eye under study for pupil dilatation. After a resting period of at least 20 min to ensure constant hemodynamic conditions, retinal vessel diameters and oxygen saturation were measured with a DVA, and retinal blood flow was measured using D-OCT. Blood pressure, heart rate, and intraocular pressure were assessed after blood flow was measured. The same procedures were performed on the second study day (day 84 ± 7) as on day one.
Statistical analysis
Data are presented as mean ± standard deviation (SD). Statistical analysis was done per-protocol analysis. The plasma Hcy level was defined as the main outcome variable. To detect differences between the two study days, a paired t test was applied for the analysis of all outcome variables. The values during flicker stimulation were calculated as the average of the last 20 s of the stimulation period. Flicker responses in retinal vessel diameters were expressed as the percentage of change over baseline values [(flicker − baseline) × 100/baseline]. A p value < 0.05 was considered as the level of significance.
Results
A total of 25 patients between 18 and 75 years (mean: 49.4 ± 18.9 years) with diabetes type 1 (n = 10) and diabetes type 2 (n = 15) were included. Among these, one patient was excluded because of a serious adverse event unrelated to the study. For a second patient, the second visit did not occur within the proposed timeline but three weeks ahead of schedule. As this patient completed both study visits, the patient was not excluded from analysis. For all other patients, the second visit was performed 84 ± 7 days after the first (mean time between visits: 84 ± 6 days). In summary, 24 patients (10 male and 14 female) were included in the statistical analysis.
Plasma homocysteine concentration
The mean duration of diabetes in the whole study group was 14.6 ± 12.3 years. After three months of Ocufolin forte intake, plasma Hcy levels significantly decreased from 14.2 ± 9.3 to 9.6 ± 6.6 µmol/L (p < 0.001 versus baseline, Figure 1). This corresponds to a mean decrease of 32 ± 19%.
Figure 1.
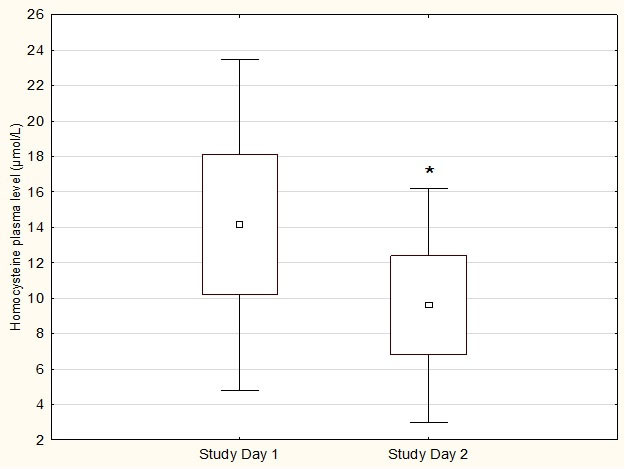
Plasma Hcy levels on both study days. * Significant on study day 2 vs. study day 1 (p<0.001). Data are presented as mean ± 0.95 CI ± SD (n=24).
Retinal hemodynamics
No changes in retinal arterial or venous vessel diameters were observed after Ocufolin administration. Stimulation with flickering light increased retinal arterial diameter by 2.4 ± 2.7%, from 127.7 ± 18.1 to 130.9 ± 19.4 µm on study day one (p < 0.001). On study day two, a similar response was observed (1.8 ± 3.3% increase in diameter, from 126.7 ± 18.3 to 128.9 ± 18.2 µm, p = 0.01). No difference between the two study days in the increase in arterial diameter during stimulation with flickering light was observed (p = 0.46).
Retinal venous diameter also showed a significant increase during stimulation with flickering light on study day one (from 159.3 ± 20.5 to 167.6 ± 22.0µm, p < 0.001) as well as on study day two (from 160.4 ± 22.1 to 166.0 ± 21.8 µm, p < 0.001). The increase on the two study days was 5.2 ± 3.2% and 3.7 ± 3.1%, respectively (p = 0.10).
Stimulation with flickering light increased retinal arterial blood flow from 9.1 ± 4.3 to 13.7 ± 6.4 µl/min on study day one (p < 0.001). On study day two, a similar response was observed (increase in blood flow from 9.2 ± 4.7 to 11.9 ± 5.0 µl/min, p < 0.01). No difference was observed between the two study days (p = 0.68).
Retinal venous blood flow also showed a significant increase during stimulation with flickering light on study day one (from 9.9 ± 5.2 to 13.7 ± 7.7 µl/min, p < 0.01) as well as on study day two (from 10.1 ± 6.2 to 14.5 ± 7.4 µl/min, p < 0.01).
In 13 patients, measurements of total retinal blood flow could be obtained for both study days (Figure 2), while in the other patients, not all measurements were evaluable. After a three-month supplementation, a tendency toward an increased total retinal blood flow was observed, from 36.8 ± 12.9 µl/min to 39.2 ± 10.8 µl/min, but this effect did not reach the level of significance (p = 0.11).
Figure 2.
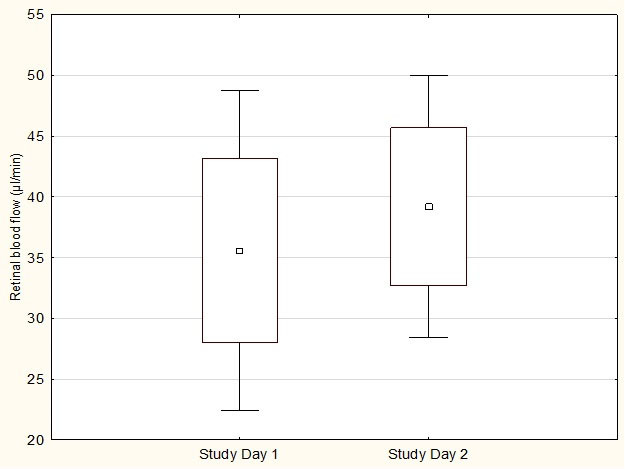
Total retinal blood flow on both study days. Data are presented as mean ± 0.95 CI ± SD, n=13.
In addition, a small but significant decrease in intraocular pressure was observed, from 14.8 ± 3.0 to 13.4 ± 2.2 mmHg (p < 0.02).
Discussion
The aim of the present study was to investigate whether a combination of L-methylfolate and other supplements could alter systemic Hcy plasma concentration in patients with diabetes. The study met its primary goal and showed that, after a three-month supplementation, plasma Hcy levels significantly decreased from 14.2 ± 9.3 µmol/L to 9.6 ± 6.6 µmol/L, corresponding to a mean decrease of 32% ± 19%. As a secondary outcome, the current study indicates that there is a tendency toward an increased total retinal blood flow.
Chemically speaking, Hcy is a key intermediate in the metabolism of methionine and is converted by transsulfuration to cysteine or by methionine synthase back to methionine. Given that these enzymatic reactions depend on an adequate supply of folate and other micronutrients, a lack of folate results in the accumulation of Hcy and its active metabolite Hcy-thiolactone in the blood. Conversely, it has been shown that high dietary folate intake is associated with lower plasma Hcy levels and that supplementation with folate and vitamin B12 lowers plasma Hcy levels [13,14].
There is increasing evidence that Hcy plays a role in the pathogenesis of diabetic retinopathy. Higher Hcy levels have consistently been found in the plasma and the vitreous of patients with diabetic retinopathy compared to those of non-diabetic subjects [15–17]. Further, patients with proliferative diabetic retinopathy show higher Hcy concentrations compared to patients with non-proliferative diabetic retinopathy or diabetics without retinopathy, indicating that Hcy levels are associated with the severity of diabetic retinopathy [18]. Along this line of thought, human donor retinas with established diabetic retinopathy have been shown to have considerably higher Hcy levels than those of age-matched non-diabetic donors. In addition, more recent evidence indicates that the observed increased retinal Hcy levels may be attributed to compromised Hcy recycling in diabetic retinopathy [19].
Our data shows that, in patients with diabetes, a three-month supplementation with L-methylfolate in combination with other ingredients leads to an average decrease of plasma Hcy of 4.6 µmol/l, or 32%. This pronounced decrease in plasma Hcy is at the upper end of effects found in studies using other formulations containing folate or folic acid. In particular, a previous study showed that a 2.5 mg folic acid administration in women led to an 18% reduction in Hcy levels [20]. Further, a meta-analysis comprising 25 studies, including more than 2,500 subjects, found that co-administration of folic acid (0.8–5 mg) and vitamin B12 (0.4 mg) resulted in an Hcy-lowering effect of approximately 30% in a population with a typical Hcy concentrations of 10–12µmol/L [13].
Previous studies have also found Hcy-lowering effects in patients with diabetes. In a study using 3 mg L-methylfolate calcium supplementation in patients with type 2 diabetes, a decrease in Hcy levels of 2.68 µmol/l was found after a 24-week treatment [21]. Daily intake of 800 µg folic acid for eight weeks reduced Hcy levels by 22% in women with type 2 diabetes [22]. In men with type 2 diabetes, a reduction of 20% was reported [23].
Considering these previously published studies, the observed decrease in plasma Hcy might be of particular clinical interest. As stated previously, higher Hcy levels are a consistent risk factor for ocular diseases such as AMD [4,24], diabetic retinopathy [18,25], and glaucoma [26-28]. Meta-analyses investigating the effect of folic acid supplementation on stroke prevention, as well as on reducing the risk for cardiovascular or cerebrovascular events, found a greater benefit in patients with a decrease in Hcy levels of 25% or more, depending on the baseline values [29,30]. Thus, one could hypothesize that supplementation with folate to lower Hcy levels might be beneficial in this group of patients. However, longitudinal, randomized, controlled studies are warranted to confirm the clinical effect of folate supplementation.
As a secondary outcome, the study aimed to investigate the effect of folate substitution on ocular perfusion parameters. Our data show that a three-month supplementation had no effect on baseline retinal vessel diameters and flicker-induced vasodilatation. To assess neurovascular coupling, flicker-induced vasodilatation was measured. Although a significant response of about 2% in the retinal arterial diameter and about 4.5% in the retinal veins were found, this response is lower than that reported for healthy subjects in the literature [31-33]. This response is in line with the results found other studies investigating in patients with diabetes with no or mild non-proliferative diabetic retinopathy, corresponding to the present study population [31,32]. However, folate substitution had no influence on flicker-induced vasodilatation.
Finally, in a subgroup of 13 patients, measurements of the total retinal blood flow were obtained on both study days. Our data show a numerical increase in total retinal blood flow from 36.8 ± 12.9 to 39.2 ± 10.8 µl/min. Although this effect did not reach the level of significance, our data could serve as a basis to calculate the sample size for a larger study with a double-masked, randomized controlled study design. As a blood flow–increasing effect of folate supplementation has not yet been identified, further studies need to be performed to assess its potential effect on retinal blood flow.
Several limitations must be mentioned when considering the current study’s results. First, as this was a pilot study, a non-controlled design was chosen and relatively few patients were included. As this study was designed mainly to assess changes in subjects’ plasma Hcy, this approach was acceptable to estimate the effect size of the intervention and for the statistical planning of subsequent trials. Based on this data, larger trials will be planned to assess the dose-relationship and the possible effect of folate administration on retinal blood flow. Larger sample sizes will also allow for the analysis of specific subgroups to investigate the effect of supplementation in relation to age and sex, which was not possible in the current study due to the small sample size.
Second, administration of the supplement was limited to three months. Although we observed a pronounced decrease in plasma Hcy levels, we cannot exclude that a longer administration would have led to an even stronger decrease. Third, the formulation used in the current study included a variety of vitamins and minerals. Thus, in the strictest sense, the conclusions drawn hold true for the vitamin combination outlined in Table 1 and may not reflect the effect of other combinations differing in dosing and composition. Finally, baseline folate levels were not measured in the current experiment. As the effect of folate substitution may depend on baseline folate levels, information regarding baseline folate plasma levels might help to explain differences in plasma Hcy in response to supplementation.
In conclusion, the present data show that a three-month oral supplementation of L-methylfolate and additional dietary supplements is safe and capable of significantly reducing blood Hcy levels. This may be of clinical importance, since higher Hcy plasma levels have been found to be associated with an increased risk for vascular-related systemic and ocular diseases. Further studies are required to assess the clinical effect of a long-term administration of folate.
Acknowledgments
Conflicts of interest: DS has received financial support for attending symposia by Aprofol AG, Switzerland. The authors declare that they have no conflict of interest. Funding statement: The study was sponsored by Aprofol AG, Switzerland. Clinicaltrials.gov registry: NCT03997032. This work was presented at the Annual Meeting of the Swiss Society of Ophthalmology in 2018.
References
- 1.Khandanpour N, Loke YK, Meyer FJ, Jennings B, Armon MP. Homocysteine and peripheral arterial disease: systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2009;38:316–22. doi: 10.1016/j.ejvs.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Peng HY, Man CF, Xu J, Fan Y. Elevated homocysteine levels and risk of cardiovascular and all-cause mortality: a meta-analysis of prospective studies. J Zhejiang Univ Sci B. 2015;16:78–86. doi: 10.1631/jzus.B1400183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai WK, Kan MY. Homocysteine-Induced Endothelial Dysfunction. Ann Nutr Metab. 2015;67:1–12. doi: 10.1159/000437098. [DOI] [PubMed] [Google Scholar]
- 4.Huang P, Wang F, Sah BK, Jiang J, Ni Z, Wang J, Sun X. Homocysteine and the risk of age-related macular degeneration: a systematic review and meta-analysis. Sci Rep. 2015;5:10585. doi: 10.1038/srep10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lei X, Zeng G, Zhang Y, Li Q, Zhang J, Bai Z, Yang K. Association between homocysteine level and the risk of diabetic retinopathy: a systematic review and meta-analysis. Diabetol Metab Syndr. 2018;10:61. doi: 10.1186/s13098-018-0362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng Y, Li J, Chen X, She H, Zhao L, Peng Y, Zhang J, Shang K, Li H, Yang W, Zhang Y, Gu X, Li J, Qin X, Wang B, Xu X, Hou F, Tang G, Liao R, Yang L, Huo Y. Association Between Folic Acid Supplementation and Retinal Atherosclerosis in Chinese Adults With Hypertension Complicated by Diabetes Mellitus. Front Pharmacol. 2018;9:1159. doi: 10.3389/fphar.2018.01159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doblhoff-Dier V, Schmetterer L, Vilser W, Garhofer G, Groschl M, Leitgeb RA, Werkmeister RM. Measurement of the total retinal blood flow using dual beam Fourier-domain Doppler optical coherence tomography with orthogonal detection planes. Biomed Opt Express. 2014;5:630–42. doi: 10.1364/BOE.5.000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werkmeister RM, Dragostinoff N, Pircher M, Gotzinger E, Hitzenberger CK, Leitgeb RA, Schmetterer L. Bidirectional Doppler Fourier-domain optical coherence tomography for measurement of absolute flow velocities in human retinal vessels. Opt Lett. 2008;33:2967–9. doi: 10.1364/ol.33.002967. [DOI] [PubMed] [Google Scholar]
- 9.Werkmeister RM, Palkovits S, Told R, Groschl M, Leitgeb RA, Garhofer G, Schmetterer L. Response of retinal blood flow to systemic hyperoxia as measured with dual-beam bidirectional Doppler Fourier-domain optical coherence tomography. PLoS One. 2012;7:e45876. doi: 10.1371/journal.pone.0045876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fondi K, Aschinger GC, Bata AM, Wozniak PA, Liao L, Seidel G, Doblhoff-Dier V, Schmidl D, Garhofer G, Werkmeister RM, Schmetterer L. Measurement of Retinal Vascular Caliber From Optical Coherence Tomography Phase Images. Invest Ophthalmol Vis Sci. 2016;57:OCT121–9. doi: 10.1167/iovs.15-18476. [DOI] [PubMed] [Google Scholar]
- 11.Blum M, Bachmann K, Wintzer D, Riemer T, Vilser W, Strobel J. Noninvasive measurement of the Bayliss effect in retinal autoregulation. Graefes Arch Clin Exp Ophthalmol. 1999;237:296–300. doi: 10.1007/s004170050236. [DOI] [PubMed] [Google Scholar]
- 12.Shipchandler MT, Moore EG. Rapid, fully automated measurement of plasma homocyst(e)ine with the Abbott IMx analyzer. Clin Chem. 1995;41:991–4. [PubMed] [Google Scholar]
- 13.Homocysteine Lowering Trialists C. Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr. 2005;82:806–12. doi: 10.1093/ajcn/82.4.806. [DOI] [PubMed] [Google Scholar]
- 14.Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. Homocysteine Lowering Trialists’ Collaboration. BMJ. 1998;316:894–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Cho HC. The Relationship among Homocysteine, Bilirubin, and Diabetic Retinopathy. Diabetes Metab J. 2011;35:595–601. doi: 10.4093/dmj.2011.35.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aydemir O, Turkcuoglu P, Guler M, Celiker U, Ustundag B, Yilmaz T, Metin K. Plasma and vitreous homocysteine concentrations in patients with proliferative diabetic retinopathy. Retina. 2008;28:741–3. doi: 10.1097/IAE.0b013e31816079fb. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein M, Leibovitch I, Yeffimov I, Gavendo S, Sela BA, Loewenstein A. Hyperhomocysteinemia in patients with diabetes mellitus with and without diabetic retinopathy. Eye (Lond) 2004;18:460–5. doi: 10.1038/sj.eye.6700702. [DOI] [PubMed] [Google Scholar]
- 18.Malaguarnera G, Gagliano C, Giordano M, Salomone S, Vacante M, Bucolo C, Caraci F, Reibaldi M, Drago F, Avitabile T, Motta M. Homocysteine serum levels in diabetic patients with non proliferative, proliferative and without retinopathy. BioMed Res Int. 2014;2014:191497. doi: 10.1155/2014/191497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kowluru RA, Mohammad G, Sahajpal N. Faulty homocysteine recycling in diabetic retinopathy. Eye Vis (Lond) 2020;7:4. doi: 10.1186/s40662-019-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christen WG, Cook NR, Van Denburgh M, Zaharris E, Albert CM, Manson JE. Effect of Combined Treatment With Folic Acid, Vitamin B6, and Vitamin B12 on Plasma Biomarkers of Inflammation and Endothelial Dysfunction in Women. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.008517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fonseca VA, Lavery LA, Thethi TK, Daoud Y, DeSouza C, Ovalle F, Denham DS, Bottiglieri T, Sheehan P, Rosenstock J. Metanx in type 2 diabetes with peripheral neuropathy: a randomized trial. Am J Med. 2013;126:141–9. doi: 10.1016/j.amjmed.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 22.Vijayakumar A, Kim EK, Kim H, Choi YJ, Huh KB, Chang N. Effects of folic acid supplementation on serum homocysteine levels, lipid profiles, and vascular parameters in post-menopausal Korean women with type 2 diabetes mellitus. Nutr Res Pract. 2017;11:327–33. doi: 10.4162/nrp.2017.11.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aghamohammadi V, Gargari BP, Aliasgharzadeh A. Effect of folic acid supplementation on homocysteine, serum total antioxidant capacity, and malondialdehyde in patients with type 2 diabetes mellitus. J Am Coll Nutr. 2011;30:210–5. doi: 10.1080/07315724.2011.10719962. [DOI] [PubMed] [Google Scholar]
- 24.Pinna A, Zaccheddu F, Boscia F, Carru C, Solinas G. Homocysteine and risk of age-related macular degeneration: a systematic review and meta-analysis. Acta Ophthalmol. 2018;96:e269–76. doi: 10.1111/aos.13343. [DOI] [PubMed] [Google Scholar]
- 25.Xu C, Wu Y, Liu G, Liu X, Wang F, Yu J. Relationship between homocysteine level and diabetic retinopathy: a systematic review and meta-analysis. Diagn Pathol. 2014;9:167. doi: 10.1186/s13000-014-0167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ajith TA. Ranimenon. Homocysteine in ocular diseases. Clin Chim Acta. 2015;450:316–21. doi: 10.1016/j.cca.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Junemann A, Rejdak R, Hohberger B. Significance of Homocysteine in Glaucoma. . Klin Monatsbl Augenheilkd. 2018;235:163–74. doi: 10.1055/s-0044-101621. [DOI] [PubMed] [Google Scholar]
- 28.Lee JY, Kim JM, Kim IT, Yoo CK, Won YS, Kim JH, Kwon HS, Park KH. Relationship between Plasma Homocysteine Level and Glaucomatous Retinal Nerve Fiber Layer Defect. Curr Eye Res. 2017;42:918–23. doi: 10.1080/02713683.2016.1257728. [DOI] [PubMed] [Google Scholar]
- 29.Tian T, Yang KQ, Cui JG, Zhou LL, Zhou XL. Folic Acid Supplementation for Stroke Prevention in Patients With Cardiovascular Disease. Am J Med Sci. 2017;354:379–87. doi: 10.1016/j.amjms.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Wang WW, Wang XS, Zhang ZR, He JC, Xie CL. A Meta-Analysis of Folic Acid in Combination with Anti-Hypertension Drugs in Patients with Hypertension and Hyperhomocysteinemia. Front Pharmacol. 2017;8:585. doi: 10.3389/fphar.2017.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lasta M, Pemp B, Schmidl D, Boltz A, Kaya S, Palkovits S, Werkmeister R, Howorka K, Popa-Cherecheanu A, Garhofer G, Schmetterer L. Neurovascular dysfunction precedes neural dysfunction in the retina of patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 2013;54:842–7. doi: 10.1167/iovs.12-10873. [DOI] [PubMed] [Google Scholar]
- 32.Pemp B, Weigert G, Karl K, Petzl U, Wolzt M, Schmetterer L, Garhofer G. Correlation of flicker-induced and flow-mediated vasodilatation in patients with endothelial dysfunction and healthy volunteers. Diabetes Care. 2009;32:1536–41. doi: 10.2337/dc08-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharifizad M, Witkowska KJ, Aschinger GC, Sapeta S, Rauch A, Schmidl D, Werkmeister RM, Garhofer G, Schmetterer L. Factors Determining Flicker-Induced Retinal Vasodilation in Healthy Subjects. Invest Ophthalmol Vis Sci. 2016;57:3306–12. doi: 10.1167/iovs.16-19261. [DOI] [PubMed] [Google Scholar]


