Abstract
Stomatal closure is one of the main physiological responses to increasing CO2 concentration, which leads to a reduction in plant water loss. This response has the potential to trigger changes in the climate system by regulating surface energy budgets—a phenomenon known as CO2 physiological forcing. However, its remote impacts on the Arctic climate system are unclear. Here we show that vegetation at high latitudes enhances the Arctic amplification via remote and time-delayed physiological forcing processes. Surface warming occurs at mid-to-high latitudes due to the physiological acclimation-induced reduction in evaporative cooling and resultant increase in sensible heat flux. This excessive surface heat energy is transported to the Arctic ocean and contributes to the sea ice loss, thereby enhancing Arctic warming. The surface warming in the Arctic is further amplified by local feedbacks, and consequently the contribution of physiological effects to Arctic warming represents about 10% of radiative forcing effects.
Subject terms: Biogeochemistry, Climate sciences, Ecology
Plants respond to increasing CO2 concentrations in the atmosphere by stomatal closure which causes a reduction of evapotranspiration and thus latent heat flux. Here, the authors show that this CO2 physiological forcing strengthens Arctic warming through increasing sea ice loss and local feedbacks.
Introduction
The increase in atmospheric CO2 concentration has an influence on plant physiology. Physiological responses to increasing CO2 include changes in leaf area index (LAI) and stomatal conductance, and those affect the plant transpiration in opposite ways. First, one of the main physiological responses is the CO2 fertilization effect—that is, an increase in the rate of photosynthesis1–3. This effect accounts for the largest contribution to the positive trends in the LAI detected by satellite data sets4 and can also lead to an increase in plant transpiration, resulting in cooling effects5. Second, another plant response is a reduction in stomatal conductance. In other words, stomatal apertures open less widely under elevated CO2 concentrations. These CO2-induced reductions in the stomatal conductance have been confirmed through experiments and from reconstruction data2,3,6,7.
The stomatal closure resulting from elevated CO2 levels can decrease the rate of transpiration by diminishing the amount of water loss from plants. This reduction in plant transpiration can lead to an increase of near-surface air temperature by decreasing the evaporative cooling effect and simultaneously increasing the sensible heat flux above the land surface8–10. This nonradiative effect from physiological acclimation is known as CO2 physiological forcing11. Previous studies using model experiments have investigated how the physiological forcing will affect the future climate in vegetation-covered regions, through influences such as amplified heat extremes12, intensified zonal asymmetry of rainfall over tropical land13, drying over the Eastern Amazon14 and Sahel greening15.
This physiological effect has a potential to remotely alter the entire climate system through the redistribution of the surface energy and disturbance of hydrological cycle, but still, the remote impacts of physiological effect on the climate system are unclear especially in the Arctic region (north of 70°N). The Arctic is the region most sensitive to greenhouse warming and has experienced warming faster than the global average, a phenomenon known as Arctic amplification16. Many mechanisms have been suggested to explain the Arctic amplification including a role of diminishing sea ice17,18, seasonal storage and release of the absorbed shortwave (SW) radiation coupling with sea-ice loss19–21, enhanced downward longwave (LW) radiation due to an increase in water vapor and cloud fraction22,23, ocean biogeochemical feedback24,25, increased poleward energy transport26,27 and other processes28,29. However, their relative contributions are still under debate and also many alternative mechanisms are under investigation. Here, we suggest that the CO2 physiological forcing has a remote forcing on the Arctic climate and can intensify the Arctic amplification through the enhanced atmospheric poleward heat transport and the physical processes coupling with the Arctic sea-ice change.
To examine the impacts of physiological acclimation under elevated CO2 on the future climate system, we analyzed eight Earth system models (ESMs), which can simulate interactions between the physical climate system and the biogeochemical processes, from the Coupled Model Intercomparison Project Phase 5 (CMIP5)30 (Supplementary Tables 1 and 2). In line with previous studies12,13,31–34, we respectively quantified the physiological forcing (Phy), which includes the CO2 fertilization effect and the dependency of stomatal conductance on CO2, and CO2 radiative forcing (Rad) (average CO2 concentrations ~823 ppm) using carbon–climate feedback experiments (see the Methods section and Supplementary Table 3).
Results
Land surface warming by plant physiological effects
Figure 1 shows changes in the annual mean evapotranspiration (ET), Bowen ratio (the ratio of sensible to latent heat fluxes), and near-surface air temperature resulting from the CO2 physiological forcing. In contrast with the radiative effect inducing the increase in ET due to enhanced water-demand from the temperature rise (Supplementary Fig. 1), physiological effects cause a conspicuous and significant reduction in the annual mean ET in densely vegetated areas of the tropics and mid-to-high latitudes (Fig. 1a) in line with previous studies12,31,32,34–37. In this idealized experiment for evaluating the CO2 physiological forcing, the fertilization effect plays a role in increasing ET due to the resulting increased LAI, but the effect of stomatal closure works in the opposite way at the same time5,10,37. Therefore, this overall drop in ET suggests that the stomatal closure have a greater influence in controlling the total ET than the CO2 fertilization, when only the physiological effects is considered under elevated CO2 levels, in consistency with the argument in previous studies12,31,34,37.
Fig. 1. Change in evapotranspiration, Bowen ratio and temperature resulting from CO2 physiological forcing.

Multimodel mean change of the annual mean evapotranspiration (a), Bowen ratio (sensible heat flux/latent heat flux) (b), and near-surface air temperature (c) resulting from CO2 physiological forcing. Only significant values at the 95% confidence level based on a bootstrap method are shown.
The physiological effects change the surface energy budgets by reducing the evaporative cooling and simultaneously increasing the sensible heat flux (Fig. 1b and Supplementary Table 4). These heat flux changes induce surface and near-surface air warming around regions where ET is significantly decreased. Interestingly, significant surface warming occurs in the Arctic Ocean under the influence of CO2 physiological forcing, despite the fact that vegetation obviously does not exist in the Arctic Ocean (Fig. 1c). Another interesting point is that a synergy effect, a nonlinear interaction of physiological forcing with the radiative forcing15,37, additionally contributes to the surface warming (see the Methods section and Supplementary Table 5). The magnitude of synergy effect in Arctic region is equivalent to ~24% of annual mean temperature change resulting from physiological forcing. These results imply that the global warming signal by the radiation forcing plays a role in amplifying the physiological effect through their interactions. Meanwhile, the physiological forcing excluding a synergy effect still induces the statistically significant Arctic warming, which confirms the consistency and robustness of our findings (Supplementary Fig. 2).
Seasonal changes caused by the physiological effects (Fig. 2 and Supplementary Fig. 3) were further examined. While the variations of ET remain almost constant throughout the year in tropical regions, the reduction in ET at mid-to-high latitudes (40°–70°N) exhibits a strong seasonality. As a result, the changes in the surface energy fluxes and temperature in mid-to-high latitudes also show a strong seasonality. In summer (June–July–August; JJA) when photosynthesis is most active, the maximum decline in ET and the resulting strongest continental warming occur. Unlike this continental warming (40°–70°N), however, the maximum warming in the Arctic regions due to physiological effects, an increase of +0.99 K, occurs in winter with a time lag (Supplementary Table 6). The mechanisms of this remotely induced Arctic warming are discussed in the next section.
Fig. 2. Impacts of the physiological forcing on the evapotranspiration and temperature.
Zonally and monthly averaged change in the evapotranspiration (a) and surface air temperature (b). The shading represents the change resulting from CO2 physiological forcing. The contouring represents the change resulting from CO2 radiative forcing. The contour intervals for radiative forcing are 0.1 mm day–1 in (a) and 1.5 K in (b).
Arctic warming remotely induced by CO2 physiological forcing
As illustrated in Fig. 1, changes in plant physiology lead to a statistically significant temperature rise in the Arctic Ocean. The continental warming in JJA resulting from the physiological responses seems to be propagated to the polar region with time (Fig. 2b, shading), whereas this pattern was not observed in the CO2 radiative forcing experiment (Fig. 2b, contour). The Arctic warming resulting from the physiological effects is most distinctive during the boreal winter (December–January–February; DJF). In addition, the magnitude of this Arctic warming in DJF is comparable with that of continental warming during JJA.
It is important to understand how this continental warming resulting from the physiological effects can remotely cause the distinctive delayed warming in the Arctic Ocean. A previous study demonstrated that mid- and high-latitude forcing can remotely contribute to the Arctic warming through various physical processes38. In particular, the increase in atmospheric northward energy transport (NHTATM) due to the continental warming can be responsible for the delayed Arctic warming. It is evident that the northward energy transport significantly increases during the warm season from April to July (see the Methods section and Supplementary Fig. 4a). Furthermore, this NHTATM continuously increases during the whole period of simulations due to the intensified CO2 physiological forcing with increasing CO2 levels (Supplementary Fig. 4b). These results imply that the NHTATM plays a role in connecting the extratropical continental warming to the Arctic warming under an influence of the physiological effect, which shows the similarity with previous studies, suggesting that the high-latitude greening and mid-latitude afforestation can enhance the Arctic amplification through an increase in poleward energy transport39–42. The increase in NHTATM is associated with sea-ice melting and the resultant newly open waters in the Arctic allow it to absorb more sunlight during the warm season (Supplementary Table 6). Most of this energy is released to the atmosphere through the longwave radiative flux, and sensible and latent heat flux in the Arctic Ocean during autumn and winter, thereby inducing the Arctic warming (Supplementary Table 6). These mechanisms, the seasonal storage and release of the absorbed shortwave radiation coupling with an Arctic sea-ice change, have already been proposed in previous studies to explain the Arctic amplification19–21. Nonetheless, our results suggest that the plant physiological forcing as well as radiative forcing can contribute to the Arctic amplification under elevated CO2 levels.
A previous study has shown that the mid-troposphere in the Arctic sensitively responds to the energy advection across the Arctic boundary26. The bottom-heavy warming profile has been attributed to increased upward turbulent heat fluxes by the loss of sea ice in previous studies17,19. The vertical structure of atmospheric warming shows that the mid-tropospheric warming first occurred with a large vertical extent in the Arctic region during summer, and then this warming was propagated to the lowermost region of the atmosphere with time (Supplementary Fig. 5). These results support our hypothesis that the remote and lagged effects of plant physiological acclimation can intensify Arctic warming through an enhancement of NHTATM and the resulting Arctic sea-ice change. However, there is a large inter-model diversity in the magnitude of Arctic warming, which seems to be closely related to the strength of local feedback related to Arctic sea ice, but further research is needed to confirm this (Supplementary Figs. 6 and 7).
In summary, the surface warming resulting from physiological effect enhances an atmospheric energy convergence into the Arctic basin and this increases the net SW absorption during the warm season in the Arctic Ocean by melting sea ice. Subsequent energy release to the atmosphere increases the air temperature and ice-free waters in the Arctic, thereby intensifying the Arctic amplification during the cold season. As a result, the CO2 physiological forcing accounts for 27.7% of the continental warming in summer and 9.7% of the annual surface warming in Arctic region resulting from CO2 radiative forcing (Fig. 3). These results emphasize that the contribution of the plant physiological effects to the Arctic warming is quite significant.
Fig. 3. Ratio between changes of temperature in response to CO2 physiological forcing and radiative forcing.

Ratio of the change in the near-surface air temperature resulting from CO2 physiological effects to that resulting from CO2 radiative forcing ([CO2 physiological forcing/CO2 radiative forcing] × 100) in the continents (40°–70°N) and Arctic region (70°–90°N), respectively. Each bar shows the area-weighted average of multimodel ensemble. The black dots represent the individual results from ESMs. The error bar for each column indicates the range of the 95% confidence level on the basis of a bootstrap method.
Intensified and continued surface warming by local feedback
Besides the direct heating from the enhanced sensible heat flux, an increase in net shortwave absorption (4.58 W m−2 in JJA) additionally heats the air above the land surface in JJA (Supplementary Table 4). In this experimental design, the net SW absorption can be largely affected by these two factors: An increase in LAI resulting from CO2 fertilization effect can alter the surface albedo and increase the net SW absorption, thereby contributing to the temperature rise. The decrease in cloud fractions caused by physiological acclimation-driven reduction of relative humidity35,43,44 can also be a cause of surface warming because it enhances downward SW radiative flux42. From their relative contributions, we found that vegetation-cloud feedback has a dominant role in the increased net SW absorption during summer (Supplementary Fig. 8), thereby contributing the continental warming (40°–70°N) (Fig. 4 and Supplementary Fig. 9) particularly in summer (Supplementary Table 4). Furthermore, the relative magnitude of the vegetation-cloud feedback in ESMs seems to explain the inter-model diversity of the land surface warming (40°–70°N) in JJA (r = −0.79, P = 0.02) (Supplementary Fig. 7). Specifically, two models, HadGEM2-ES and MPI-ESM-LR, show the greatest warming in JJA due to this greatest cloud effect despite the moderate reduction of ET (Supplementary Figs. 10–12).
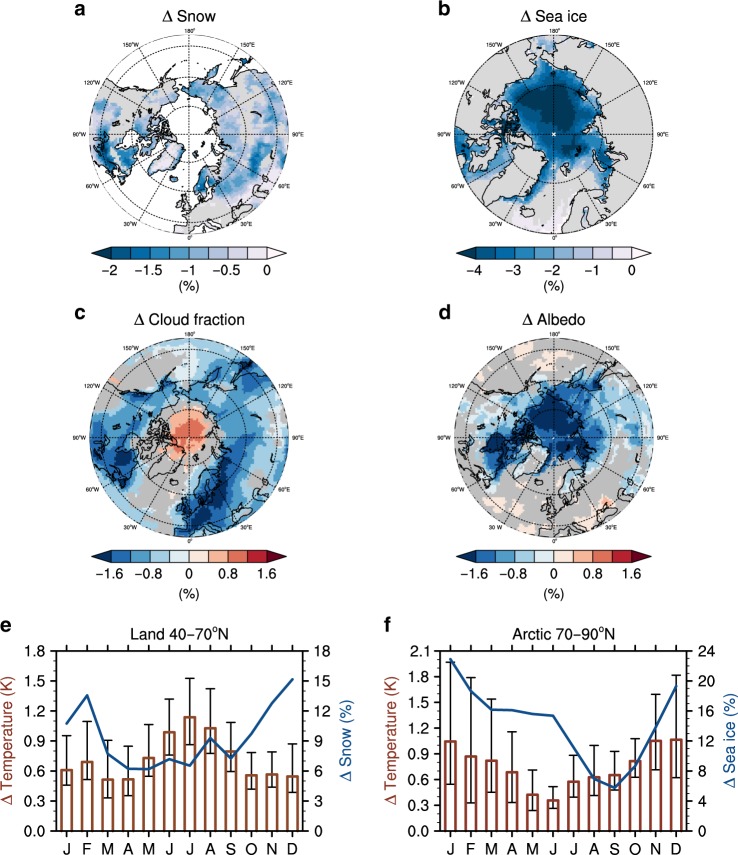
Fig. 4. Local feedback triggered by CO2 physiological forcing.
Multimodel mean change of the annual mean snow concentration (a), sea-ice concentration (b), total cloud fraction (c) and surface albedo (d). Only significant values at the 95% confidence level based on a bootstrap method are shown. e, f Annual cycle of change in the surface air temperature (red bar) resulting from CO2 physiological effect with the ratio between change in the snow concentration (blue line) in response to physiological forcing and radiative forcing in continents (40°–70°N) (e). The same as in (e), but in the Arctic region (70°–90°N), with the ratio between change in the sea-ice concentration (blue line) in response to physiological forcing and radiative forcing (f). The error bars represent a range of the 95% confidence level on the basis of a bootstrap method. All of the values are area-weighted averages of eight ESMs, except for the snow concentration, which is an average of five ESMs, because GFDL-ESM2M, HadGEM2-ES, and IPSL-CM5A-LR do not provide the surface snow area fraction data.
In contrast to the change of cloud cover over the continents (40°–70°N), the cloud formation is enhanced in the Arctic region especially during winter (Fig. 4 and Supplementary Table 6). This increased cloud fraction additionally intensifies the surface warming by decreasing the outgoing longwave radiation especially in non-summer season45,46 (Supplementary Table 6). Although it is difficult to prove the causality in this experiment, it is conceived that this increase in cloud formation contributes to the Arctic sea-ice loss, which in turn causes the increase in water vapor from the newly opened Arctic waters, as proposed previously21,46. In summary, the cloud feedback in the Arctic can enhance the surface warming by increasing a downward LW radiation, and in turn, the enhanced surface warming can accelerate the sea-ice loss, thereby causing positive feedback during the cold season.
Another local feedback might be triggered by physiological forcing over the continents (40°–70°N). As shown in Fig. 4, a snow concentration and a surface albedo in high latitudes significantly decline in response to the CO2 physiological forcing. The warming resulting from the physiological effects presumably melts snow and the resultant less-snow-covered surface absorbs more solar radiation (Supplementary Table 4). Furthermore, an increase of LAI from the fertilization effects and the land cover change in models with interactive vegetation might partially contribute to the surface warming by altering the surface albedo independently of a change in temperature and would melt the snow as noticed previously47,48 (Supplementary Figs. 13 and 14). Consequently, this snow–albedo feedback may help enhance and maintain the land surface warming throughout the year, especially in high latitudes where the surface albedo is relatively high due to the high snow cover (Fig. 4). On the whole, our results suggest that the local feedbacks triggered by physiological effects might additionally contribute to the amplified and maintained surface warming in both continents and Arctic Ocean.
Discussion
So far, it has been shown based on a multimodel mean that distinctive Arctic warming occurs due to the physiological effects, but this conclusion can be model-dependent because the structure of models and the parameterization schemes are different from each other. The magnitudes and spatial patterns of change in ET and temperature are diverse and HadGEM2-ES seems to greatly contribute to the multimodel ensemble mean temperature change (Supplementary Figs. 10 and 11). Nevertheless, most models consistently simulate the reduction in ET, the resulting surface warming over the continents and enhanced Arctic warming as a result of physiological effect (Supplementary Fig. 6), which suggests that the results are not sensitive to a subsampling of the models. In addition, multimodel ensemble results excluding HadGEM2-ES are not much different with those including HadGEM2-ES and still statistically significant though the magnitude is a bit altered (Supplementary Fig. 15). These again attest to the robustness of our findings and also suggest that ensemble mean is not controlled by an outlier.
This study shows that the physiological effects amplify Arctic warming by 9.7% compared with that from the radiative forcing. This surface warming in the Arctic region resulting from the physiological response might have the potential ramifications of future changes in the carbon and hydrological cycles by intensifying the interaction between the Arctic climate and Arctic biological system47,48. Considering the physiological effects of CO2 might be helpful for understanding the inter-model diversity in future climate change. A previous study has reported that the stomatal conductance schemes in the current ESMs do not consider various plant water use strategy49, which can lead to the underestimation of the surface warming across Northern Eurasia50. This result raises a possibility that Arctic warming may be greater than that in the current projections. Furthermore, there are still the limitations of land surface models in simulating LAI and the albedo dynamics and the stomatal conductance schemes in ESMs are rather static and semi-empirical (see the Supplementary Notes 1 and 2). These factors make it hard to simulate the realistic plant behavior to elevated CO2 levels and also increase the uncertainty in the quantification of climate change caused by the physiological forcing. These point to the need for improvement of land models’ schemes based on a fundamental understanding of the involved processes.
Methods
CMIP5 data and experimental design
Eight ESMs (bcc-csm1-1, CanESM2, CESM1-BGC, GFDL-ESM2M, HadGEM2-ES, IPSL-CM5A-LR, MPI-ESM- LR, and NorESM1-ME) were used, which were coupled with the full carbon cycle and used in idealized experiments designed to assess carbon–climate feedback, from the CMIP5 archive30 (see Supplementary Tables 1 and 2). These experiments were run for 140 years with 1% per year increase in atmospheric CO2 concentration from preindustrial levels to quadrupling (285−1140 ppm) for both radiation and biogeochemistry (1pctCO2), radiation only (esmFdbk1) and biogeochemistry only (esmFixClim1) (see Supplementary Table 3). In GFDL-ESM2M, the atmospheric CO2 levels were prescribed to increase from their initial mixing ratio level of 286.15 ppmv at a rate of 1% per year until year 70 (the point of doubling, 2 × CO2) and thereafter CO2 concentrations were kept at a constant for the remainder of the run.
To quantify the CO2 physiological forcing (Phy) (average CO2 concentrations ~823 ppm), we calculated the difference between the final 70 years of two simulations data: Full CO2 simulation (1pctCO2) that includes the fully interactive radiative, physiological, and fertilization effects in response to increasing CO2 and radiation simulation (esmFdbk1) that includes only radiative effects in response to increasing CO2. Following the previous study12, we extracted the physiological forcing (Phy) from full CO2 simulation rather than using the physiology simulation directly to evaluate CO2 physiological forcing (Phy) relative to future CO2 radiative forcing (Rad). Since a nonlinear interaction between CO2 radiative forcing and physiological forcing exists in full CO2 simulation, the physiological forcing (Phy), defined as 1pctCO2-esmFdbk1, includes this nonlinear interaction, or synergy effect, as well as the pure physiological forcing. We additionally assessed the physiological forcing in a different way by calculating the difference between the average of the final 70 years of physiology simulation (esmFixClim1) and averaged values of preindustrial control simulation (piControl) over the whole period to verify the robustness of our finding. Unlike the previous method, this alternative physiological forcing does not include an interaction between physiology and radiation. We evaluated a synergy effect by calculating the difference between the two physiological forcing with the different definition. We quantified the CO2 radiative forcing (Rad) (average CO2 concentrations ~823 ppm) by calculating the difference between the average of the final 70 years of radiation simulation (esmFdbk1) and averaged values of preindustrial control simulation (piControl) over the whole period.
The multimodel ensemble was derived by re-gridding the outputs from ESMs to a common 1° × 1° grid, then averaging together. The bootstrap method was used to test the statistical significance of the difference between the simulations. For MME, eight values were randomly selected from eight ESMs with replacements, and then their average was computed. By repeating this process 1000 times, the confidence intervals were determined, and only significant values were shown to show the model agreement. For each individual model, we randomly selected 70 years with replacements from year 71 to 140, calculated their average and finally computed the confidence intervals by repeating this process 1000 times.
Atmospheric northward energy transport calculation
The atmospheric energy convergence into the Arctic basin for transient conditions was estimated using energy budgets and residual methods51,52. Following the framework in the previous studies53,54, the energy budget of an atmospheric column can be denoted as:
| 1 |
where is the time change of atmospheric energy storage (W m–2), is the sum of the net radiation budget at the top of atmosphere (W m–2), is the net surface energy flux (W m–2) and is the vertically integrated northward heat transport (W m–2). All terms are defined as positive when they increase the atmospheric energy, hence positive downward for the TOA net radiation, positive upward for the net surface energy flux and positive for northward heat transport.
Based on Eq. (1), the transient vertically integrated atmospheric northward heat transport can be expressed as:
| 2 |
The atmospheric energy storage is written as:
| 3 |
where is pressure (Pa), is the reference surface pressure (hPa), is gravitational acceleration (m s–2), is the specific heat of the atmosphere at constant pressure (J K–1 kg–1), is temperature (K), is the kinetic energy (J kg–1), is the latent heat of evaporation (J kg–1), is the specific humidity (kg kg–1), and is the surface geopotential which is not a function of pressure54. The contribution of kinetic energy, , is ignored here due to its comparatively small magnitude53.
The net radiation at the TOA, , is defined as:
| 4 |
where is the net shortwave (solar) and is the longwave (thermal) radiation, both in W m–2.
The net surface energy budget at the surface, , is defined as:
| 5 |
where and are the net surface shortwave and longwave surface radiative fluxes, and and are the net surface sensible and latent heat fluxes, all in W m–2.
Supplementary information
Acknowledgements
We acknowledge the World Climate Research Programme’s Working Group on Coupled Modelling, which is responsible for CMIP, and the climate modelling groups (listed in Supplementary Table 1) for producing and making available their model output. This work was supported by the National Research Foundation of Korea (NRF-2017R1A2B3011511). J.-S. Kim was supported by University of Zurich Research Priority Programme “Global Change and Biodiversity” (URPP GCB).
Author contributions
S.-W.P. compiled the data, conducted analyses, prepared the figures, and wrote the manuscript. J.-S.Kug and J.-S.Kim designed the research and wrote majority of the manuscript content. All of the authors discussed the study results and reviewed the manuscript.
Data availability
All CMIP5 data30 that support the findings of this study are publicly available on Earth System Grid Federation website: https://esgf-node.llnl.gov/.
Code availability
Processed data, products, and code produced in this study are available from the corresponding authors upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Justin Mankin and other, anonymous, reviewers for their contributions to their peer review of this work. Peer review reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jin-Soo Kim, Email: jinsoo.kim@uzh.ch.
Jong-Seong Kug, Email: jskug@postech.ac.kr.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-15924-3.
References
- 1.Gunderson CA, Wullschleger SD. Photosynthetic acclimation in trees to rising atmospheric CO2: a broader perspective. Photosynth. Res. 1994;39:369–388. doi: 10.1007/BF00014592. [DOI] [PubMed] [Google Scholar]
- 2.Drake BG, Gonzàlez-Meler MA, Long SP. More efficient plants: a consequence of rising atmospheric CO2? Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997;48:609–639. doi: 10.1146/annurev.arplant.48.1.609. [DOI] [PubMed] [Google Scholar]
- 3.Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. N. Phytol. 2005;165:351–372. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Z, et al. Greening of the Earth and its drivers. Nat. Clim. Change. 2016;6:791–795. doi: 10.1038/nclimate3004. [DOI] [Google Scholar]
- 5.Zeng Z, et al. Climate mitigation from vegetation biophysical feedbacks during the past three decades. Nat. Clim. Change. 2017;7:432–436. doi: 10.1038/nclimate3299. [DOI] [Google Scholar]
- 6.Medlyn BE, et al. Stomatal conductance of forest species after long‐term exposure to elevated CO2 concentration: a synthesis. N. Phytol. 2001;149:247–264. doi: 10.1046/j.1469-8137.2001.00028.x. [DOI] [PubMed] [Google Scholar]
- 7.Lammertsma EI, et al. Global CO2 rise leads to reduced maximum stomatal conductance in Florida vegetation. Proc. Natl. Acad. Sci. USA. 2011;108:4035–4040. doi: 10.1073/pnas.1100371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Field CB, Jackson RB, Mooney HA. Stomatal responses to increased CO2: implications from the plant to the global scale. Plant Cell Environ. 1995;18:1214–1225. doi: 10.1111/j.1365-3040.1995.tb00630.x. [DOI] [Google Scholar]
- 9.Sellers PJ, et al. Comparison of radiative and physiological effects of doubled atmospheric CO2 on climate. Science. 1996;271:1402–1406. doi: 10.1126/science.271.5254.1402. [DOI] [Google Scholar]
- 10.Betts RA, Cox PM, Lee SE, Woodward FI. Contrasting physiological and structural vegetation feedbacks in climate change simulations. Nature. 1997;387:796–799. doi: 10.1038/42924. [DOI] [Google Scholar]
- 11.Betts RA, et al. The role of ecosystem−atmosphere interactions in simulated Amazonian precipitation decrease and forest dieback under global climate warming. Theor. Appl. Climatol. 2004;78:157–175. doi: 10.1007/s00704-004-0050-y. [DOI] [Google Scholar]
- 12.Skinner CB, Poulsen CJ, Mankin JS. Amplification of heat extremes by plant CO2 physiological forcing. Nat. Commun. 2018;9:1094. doi: 10.1038/s41467-018-03472-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kooperman GJ, et al. Forest response to rising CO2 drives zonally asymmetric rainfall change over tropical land. Nat. Clim. Change. 2018;8:434–440. doi: 10.1038/s41558-018-0144-7. [DOI] [Google Scholar]
- 14.Richardson TB, et al. Carbon dioxide physiological forcing dominates projected eastern amazonian drying. Geophys. Res. Lett. 2018;45:2815–2825. doi: 10.1002/2017GL076520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bathiany S, Claussen M, Brovkin V. CO2-induced Sahel greening in three CMIP5 Earth system models. J. Clim. 2014;27:7163–7184. doi: 10.1175/JCLI-D-13-00528.1. [DOI] [Google Scholar]
- 16.Hartmann, D. L. et al. in Climate Change 2013: The Physical Science Basis (eds Stocker, T. F. et al.) 159–254 (IPCC, Cambridge University Press, 2013).
- 17.Serreze MC, Barrett AP, Stroeve JC, Kindig DM, Holland MM. The emergence of surface-based Arctic amplification. Cryosphere. 2009;3:11–19. doi: 10.5194/tc-3-11-2009. [DOI] [Google Scholar]
- 18.Screen JA, Simmonds I. The central role of diminishing sea-ice in recent Arctic temperature amplification. Nature. 2010;464:1334–1337. doi: 10.1038/nature09051. [DOI] [PubMed] [Google Scholar]
- 19.Screen JA, Simmonds I. Increasing fall-winter energy loss from the Arctic Ocean and its role in Arctic temperature amplification. Geophys. Res. Lett. 2010;37:L16707. doi: 10.1029/2010GL044136. [DOI] [Google Scholar]
- 20.Bintanja R, van der Linden EC. The changing seasonal climate in the Arctic. Sci. Rep. 2013;3:1556. doi: 10.1038/srep01556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai A, Luo D, Song M, Liu J. Arctic amplification is caused by sea-ice loss under increasing CO2. Nat. Commun. 2019;10:121. doi: 10.1038/s41467-018-07954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burt MA, Randall DA, Branson MD. Dark warming. J. Clim. 2016;29:705–719. doi: 10.1175/JCLI-D-15-0147.1. [DOI] [Google Scholar]
- 23.Gong T, Feldstein S, Lee S. The role of downward infrared radiation in the recent Arctic winter warming trend. J. Clim. 2017;30:4937–4949. doi: 10.1175/JCLI-D-16-0180.1. [DOI] [Google Scholar]
- 24.Park JY, Kug JS, Bader J, Rolph R, Kwon MH. Amplified Arctic warming by phytoplankton under greenhouse warming. Proc. Natl. Acad. Sci. USA. 2015;112:5921–5926. doi: 10.1073/pnas.1416884112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim HG, Kug JS, Park JY. Biogeophysical feedback of phytoplankton on Arctic climate. Part II: Arctic warming amplified by interactive chlorophyll under greenhouse warming. Clim. Dyn. 2019;53:3167–3180. doi: 10.1007/s00382-019-04693-5. [DOI] [Google Scholar]
- 26.Graversen RG, Mauritsen T, Tjernström M, Källén E, Svensson G. Vertical structure of recent Arctic warming. Nature. 2008;451:53–56. doi: 10.1038/nature06502. [DOI] [PubMed] [Google Scholar]
- 27.Hwang Y-T, Frierson DMW, Kay JE. Coupling between Arctic feedbacks and changes in poleward energy transport. Geophys. Res. Lett. 2011;38:L17704. [Google Scholar]
- 28.Serreze MC, Barry RG. Processes and impacts of Arctic amplification: a research synthesis. Glob. Planet. Change. 2011;77:85–96. doi: 10.1016/j.gloplacha.2011.03.004. [DOI] [Google Scholar]
- 29.Pithan F, Mauritsen T. Arctic amplification dominated by temperature feedbacks in contemporary climate models. Nat. Geosci. 2014;7:181–184. doi: 10.1038/ngeo2071. [DOI] [Google Scholar]
- 30.Taylor KE, Stouffer RJ, Meehl GA. An overview of CMIP5 and the experiment design. Bull. Am. Meteorol. Soc. 2012;93:485–498. doi: 10.1175/BAMS-D-11-00094.1. [DOI] [Google Scholar]
- 31.Swann ALS, Hoffman FM, Koven CD, Randerson JT. Plant responses to increasing CO2 reduce estimates of climate impacts on drought severity. Proc. Natl. Acad. Sci. USA. 2016;113:10019–10024. doi: 10.1073/pnas.1604581113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemordant L, Gentine P, Swann AS, Cook BI, Scheff J. Critical impact of vegetation physiology on the continental hydrologic cycle in response to increasing CO2. Proc. Natl. Acad. Sci. USA. 2018;115:4093–4098. doi: 10.1073/pnas.1720712115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemordant L, Gentine P. Vegetation response to rising CO2 impacts extreme temperatures. Geophys. Res. Lett. 2019;46:1383–1392. doi: 10.1029/2018GL080238. [DOI] [Google Scholar]
- 34.Hong T, et al. The response of vegetation to rising CO2 concentrations plays an important role in future changes in the hydrological cycle. Theor. Appl. Climatol. 2019;136:135–144. doi: 10.1007/s00704-018-2476-7. [DOI] [Google Scholar]
- 35.Cao L, Bala G, Caldeira K, Nemani R, Ban-Weiss G. Importance of carbon dioxide physiological forcing to future climate change. Proc. Natl. Acad. Sci. USA. 2010;107:9513–9518. doi: 10.1073/pnas.0913000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrews T, Doutriaux-Boucher M, Boucher O, Forster PM. A regional and global analysis of carbon dioxide physiological forcing and its impact on climate. Clim. Dyn. 2011;36:783–792. doi: 10.1007/s00382-010-0742-1. [DOI] [Google Scholar]
- 37.Skinner CB, Poulsen CJ, Chadwick R, Diffenbaugh NS, Fiorella RP. The role of plant CO2 physiological forcing in shaping future daily-scale precipitation. J. Clim. 2017;30:2319–2340. doi: 10.1175/JCLI-D-16-0603.1. [DOI] [Google Scholar]
- 38.Stuecker MF, et al. Polar amplification dominated by local forcing and feedbacks. Nat. Clim. Change. 2018;8:1076–1081. doi: 10.1038/s41558-018-0339-y. [DOI] [Google Scholar]
- 39.Swann ALS, Fung IY, Chiang JCH. Mid-latitude afforestation shifts general circulation and tropical precipitation. Proc. Natl. Acad. Sci. USA. 2012;109:712–716. doi: 10.1073/pnas.1116706108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeong JH, et al. Intensified Arctic warming under greenhouse warming by vegetation–atmosphere–sea ice interaction. Environ. Res. Lett. 2014;9:1–10. doi: 10.1088/1748-9326/9/9/094007. [DOI] [Google Scholar]
- 41.Laguë MM, Swann ALS. Progressive midlatitude afforestation: impacts on clouds, global energy transport, and precipitation. J. Clim. 2016;29:5561–5573. doi: 10.1175/JCLI-D-15-0748.1. [DOI] [Google Scholar]
- 42.Cho MH, et al. Vegetation-cloud feedbacks to future vegetation changes in the Arctic regions. Clim. Dyn. 2018;50:3745–3755. doi: 10.1007/s00382-017-3840-5. [DOI] [Google Scholar]
- 43.Doutriaux‐Boucher M, Webb MJ, Gregory JM, Boucher O. Carbon dioxide induced stomatal closure increases radiative forcing via a rapid reduction in low cloud. Geophys. Res. Lett. 2009;36:L02703. doi: 10.1029/2008GL036273. [DOI] [Google Scholar]
- 44.de Arellano JVG, Van Heerwaarden CC, Lelieveld J. Modelled suppression of boundary-layer clouds by plants in a CO2-rich atmosphere. Nat. Geosci. 2012;5:701–704. doi: 10.1038/ngeo1554. [DOI] [Google Scholar]
- 45.Eastman R, Warren SG. Interannual variations of arctic cloud types in relation to sea ice. J. Clim. 2010;23:4216–4232. doi: 10.1175/2010JCLI3492.1. [DOI] [Google Scholar]
- 46.Goosse H, et al. Quantifying climate feedbacks in polar regions. Nat. Commun. 2018;9:1919. doi: 10.1038/s41467-018-04173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chapin FS, et al. Role of land-surface changes in Arctic summer warming. Science. 2005;310:657–660. doi: 10.1126/science.1117368. [DOI] [PubMed] [Google Scholar]
- 48.Pearson RG, et al. Shifts in Arctic vegetation and associated feedbacks under climate change. Nat. Clim. Change. 2013;3:673–677. doi: 10.1038/nclimate1858. [DOI] [Google Scholar]
- 49.Lin Y-S, et al. Optimal stomatal behaviour around the world. Nat. Clim. Change. 2015;5:459–464. doi: 10.1038/nclimate2550. [DOI] [Google Scholar]
- 50.Kala J, et al. Impact of the representation of stomatal conductance on model projections of heatwave intensity. Sci. Rep. 2016;6:23418. doi: 10.1038/srep23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Porter DF, Cassano JJ, Serreze MC, Kindig DN. New estimates of the large-scale Arctic atmospheric energy budget. J. Geophys. Res. 2010;115:D08108. doi: 10.1029/2009JD012653. [DOI] [Google Scholar]
- 52.Kay JE, et al. The influence of local feedbacks and northward heat transport on the equilibrium Arctic climate response to increased greenhouse gas forcing. J. Clim. 2012;25:5433–5450. doi: 10.1175/JCLI-D-11-00622.1. [DOI] [Google Scholar]
- 53.Nakamura N, Oort AH. Atmospheric heat budgets of the polar regions. J. Geophys. Res. 1988;93:9510–9524. doi: 10.1029/JD093iD08p09510. [DOI] [Google Scholar]
- 54.Trenberth KE, Caron JM, Stepaniak DP. The atmospheric energy budget and implications for surface fluxes and ocean heat transports. Clim. Dyn. 2001;17:259–276. doi: 10.1007/PL00007927. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All CMIP5 data30 that support the findings of this study are publicly available on Earth System Grid Federation website: https://esgf-node.llnl.gov/.
Processed data, products, and code produced in this study are available from the corresponding authors upon reasonable request.