Highlights
-
•
Pulmonary Nocardiosis is a rare bacterial disease which predominantly affects the immunocompromised patients.
-
•
The coexistence of Pulmonary Nocardiosis and Aspergillosis is rarely reported in the literature.
-
•
Pulmonary Nocardiosis and Aspergillosis can occur concomitantly in an immunocompetent patient with COPD or other structural lung diseases alone as a risk factor.
Keywords: Nocardia, Aspergillosis, COPD, Old Tuberculosis
Abstract
Pulmonary Nocardiosis and invasive Aspergillosis are well documented in immunocompromised patients. The coexistence of both infections is a diagnostic rarity, especially in patients with underlying structural lung diseases. We describe this rare association in a 46-year-old female with a history of pulmonary tuberculosis and COPD. The diagnosis of pulmonary Nocardiosis is challenging due to non-specific clinical features, inherent ability to mimic malignancy, tuberculosis and difficulty in the cultivation of the organism. The treating physicians should aware of the rare occurrence of such co-infections in order to prevent misdiagnosis and prompt treatment.
Introduction
Immunocompromised patients are prone to opportunistic infections. Nocardiosis is an uncommon gram-positive bacterial infection with the ability to cause local or systemic suppurative disease in human [1]. Nocardiosis usually occurs in immunocompromised patients with known risk factors like solid organ or hematopoietic cell transplantation, HIV infection (CD4 count <100 cells/mm3), malignancy, glucocorticoid therapy and diabetes mellitus. Co-infection of Nocardiosis and Aspergillosis is rare, even more so in an immunocompetent patient. Herein, we highlight a coexistence of pulmonary Nocardiosis and Aspergillosis in a 46-yr-old COPD patient. Due to a broad variety of clinical manifestations and indolent course, Nocardiosis can masquerade other opportunistic infections or malignancy. Patient with nonresolving pulmonary symptoms in the setting of structural lung disease, the possibility of Nocardiosis should be sought.
Case Presentation
A 46-yr-old female patient presented to us with a history of low-grade fever, cough with expectoration, and increasing breathlessness for last 2 months. There was no history of weight loss, chest pain or pedal edema. There is a history of pulmonary tuberculosis 20 years back. She was taking bronchodilators for COPD with no exacerbation in the last 6 months. She denied taking systemic corticosteroids for her respiratory symptoms. She also denied any history of diabetes, hypertension, alcohol or other drug abuse. On physical examination, she was febrile, with a pulse rate of 100/min, blood pressure of 100/68 mm hg, oxygen saturation of 92% on room air, and respiratory rate of 22/min with use of respiratory accessory muscles. Respiratory examination revealed bibasilar inspiratory crackles and bronchial breath sound on the left infra-axillary region. On haematological investigations, complete blood counts revealed leukocytosis (22470/mm3). Other biochemical investigations were within normal limit except increased CRP (182.4, normal <1 ng/ml). Her viral markers (HIV, HbsAg, Anti HCV) were negative. The chest radiograph showed (Fig. 1, A) multifocal patchy consolidation in bilateral lower, mid and right upper zone. The patient was given empirically broad-spectrum antibiotics (piperacillin-tazobactam and amikacin) with bronchodilators. A CT of thorax was performed which revealed multiple cavitary consolidations in bilateral lung parenchyma with the largest measuring 6 x 4 cm in left lower lobe (Fig. 1, B, C, D). CT thorax also showed Centrilobular pulmonary micro-nodules noted predominantly in right upper, bilateral lower lobe.
Fig. 1.
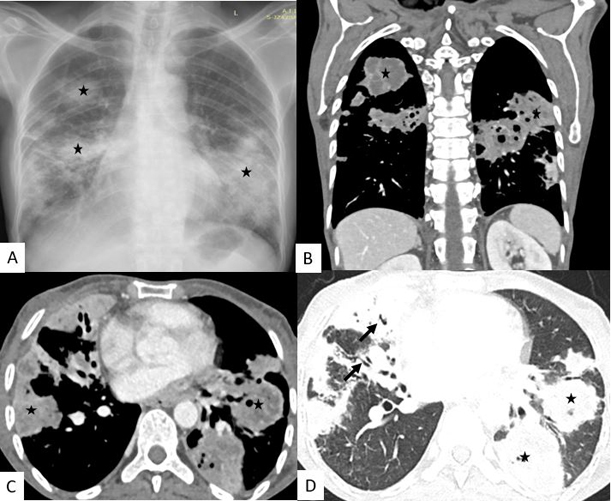
Chest Radiograph posteroanterior view (A) showing multifocal patchy consolidation (asterix) in bilateral lower, mid and right upper zone. Contrast Enhanced Computed tomography (CECT) of thorax coronal (B) and axial (C) with showing multiple consolidation (asterix) in bilateral lower and right middle and upper lobes with internal hypodensities suggestive of necrosis. Lung window axial sections (D) also showing similar multiple consolidations with internal air-bronchogram (black arrow).
For the initial 7 days, our patient did not respond with progressive worsening of respiratory symptoms. Repeated blood cultures were sterile. Sputum for modified acid-fast gram staining (1% sulphuric acid) revealed long, branching, filamentous acid-fast bacilli suggestive of Nocardia species (Fig. 2). Lacto-phenol cotton blue (LPCB) mount staining of sputum showed hyaline septate hyphae, conidiophore with oval vesicle, and biserriate phialide with a chain of conidia suggestive of Aspergillus species (Fig. 3, A, B). By 9th day of admission, the fungal culture grew Aspergillus terreus. Subsequently, delayed growth of Nocardia species from bronchoalveolar lavage fluid was also confirmed by day 14. Galactomannan testing of bronchoalveolar lavage fluid was found to be positive, which further consolidate the presence of pulmonary Aspergillosis. After confirmation of Co-infection of Aspergillosis and Nocardiosis, our patient was started on oral Trimethoprim-sulfamethoxazole (480 mg/2400 mg) per day in divided doses. In a view of invasive pulmonary Aspergillosis, voriconazole (200 mg twice a day) was also added. Within a week of starting Trimethoprim-sulfamethoxazole and voriconazole, the patient became symptomatically well and discharged. At one month of follow-up, the patient became asymptomatic with antifungal and Cotrimoxazole were planned to be continued for further 6 months.
Fig. 2.
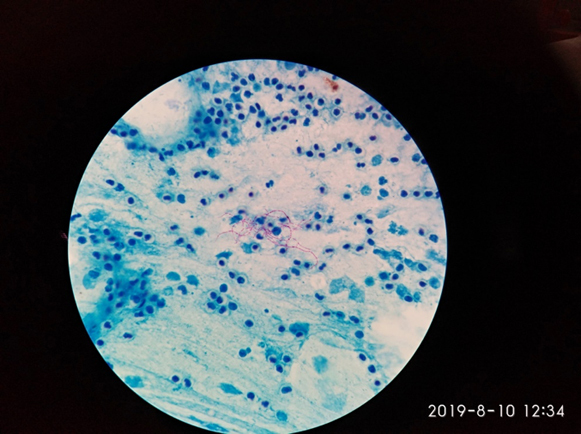
Modified ZN-Staining: Showing Acid Fast Branching filamentous bacilli suggestive of Nocardia species.
Fig. 3.
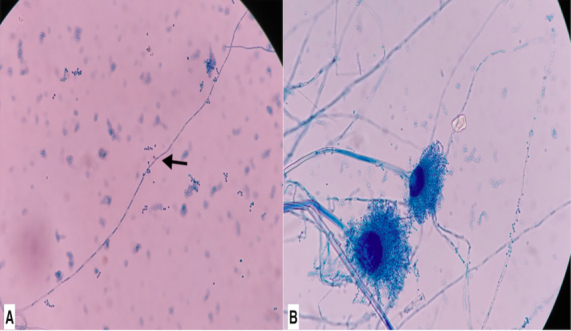
3A and 3B. Lacto-phenol cotton blue (LPCB) mount.
(3A). Hyaline septate hyphae with pedunculated Aleurioconidia (black arrow) suggestive of Aspergillus.
(3B). Hyaline septate hyphae, conidiophore with oval vesicle, biserriate phialide with chain of conidia.
Discussion
Nocardia are aerobic actinomycetes, with a branching, beaded, filamentous appearance on microscopy. Nocardia can be differentiated from Actinomyces by acid-fast staining, as they exhibit varying degree of acid-fastness due to mycolic acid composition of cell wall and type of stain used [2]. The taxonomy of Nocardia continues to evolve with more than 50 species have been identified. Most human infections are caused by members of formerly called Nocardia asteroids complex. Evolving molecular methods have led to the reclassification of Nocardia asteroids complex with new species will continue to emerge [2]. Aspergillus species are ubiquitous in nature with pulmonary infection can occur with inhalation of infectious conidia. Both Nocardiosis and Aspergillosis usually occur with the underlying immunocompromised state however, can develop in immunocompetent patients as well [[3], [4], [5]]. The co-infection of Nocardia and Aspergillus is a rare occurrence with some reports in immunocompromised patients [6,7]. The clinical presentation of pulmonary Nocardiosis can be acute, subacute or chronic with fever, night sweats, cough, anorexia, dyspnea, hemoptysis are common symptoms. Our patient was having similar symptoms for the last 2 months. Pulmonary Nocardiosis may mimic malignancy, tuberculosis or sometimes as an exacerbation of a known or underlying lung disease. Our patient did not have any features of malignancy, tuberculosis or other immunocompromised states except the underlying history of COPD. Concurrent pulmonary Aspergillosis and Nocardiosis in an immunocompetent patient is rarely reported in the literature [[8], [9]]. Patient with structural lung diseases like bronchiectasis, old pulmonary tuberculosis with fibrocavitary lesion, COPD, cystic fibrosis, history of trauma or surgery may predispose to opportunistic infections like Nocardia and Aspergillus. Several theories have been proposed for the increased susceptibility of these infections in COPD patients such as alteration in bronchial architecture, frequent use of antibiotics and hospitalization, use of steroids, alcoholism and diabetes [10,11]. In a patient of bronchiectasis, bacterial colonization may impair the ciliary motility, resulting in the escalation of Nocardiosis [12].
Similar to clinical features, the radiographic findings of Nocardiosis are also non-specific, which may include large irregular nodules (with cavitation), consolidation, diffuse alveolar pulmonary infiltrates, lung abscess or pleural effusion. In invasive pulmonary Aspergillosis, characteristic radiological findings are nodules with surrounding ground-glass infiltrates (the Halo sign), which reflects hemorrhage into the area surrounding the organism [13]. Over time, cavitation of these nodules (characterized by an intracavitary mass composed of sloughed tissue with a surrounding rim of air) leads to the development of the air crescent sign. The definitive diagnosis of Nocardiosis always required isolation of organism from a clinical specimen. The inherent difficulty in culture and non-pathognomic clinical presentation may lead to delay in diagnosis of Nocardia infections. According to one report, the mean time from development of symptoms to diagnosis was 42 days [12]. Blood culture should be incubated for 3-4 weeks and microbiologist must be notified when Nocardiosis is suspected, to maximize the isolation of organism.
Due to the lack of prospective randomization studies, treatment regimen of Nocardiosis is based on retrospective clinical experiences. Trimethoprim-sulfamethoxazole is usually given as a first-line therapy. Other treatment regimen includes amikacin, imipenem, third-generation cephalosporins, moxifloxacin and linezolid based on in vitro susceptibility [14,15]. Therapy duration should be prolonged (6-12 months) to prevent relapse. The mortality rate in pulmonary Nocardiosis is high, (ranges from 14-40%), [12] though our patient responded well on Trimethoprim-sulfamethoxazole with Voriconazole (for invasive Aspergillosis).
In conclusion, the concurrent Nocardiosis and Aspergillosis should be considered in the setting of structural lung diseases, as both infections can mimic malignancy, pulmonary tuberculosis and other infections. Timely diagnosis and treatment can prevent significant morbidity and mortality.
Disclosures
The authors have no conflicts of interest to disclose. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Authors contribution
Conception and design of study: Durga Shankar Meena, Deepak Kumar, Gopal Krishana Bohra, Mahendra Kumar Garg, Anuradha Sharma, Pawan Garg
Acquisition of Data: Durga Shankar Meena, Deepak Kumar, Kumar S Abhishek, Prakrati Yadav, Jaya Pamnani
Analysis and/or Interpretation of Data: Mahendra Kumar Garg, Deepak Kumar, Pawan Garg, Anuradha Sharma
Drafting of Manuscript: Durga Shankar Meena, Deepak Kumar, Gopal Krishana Bohra
Revising the manuscript critically for intellectual content: Mahendra Kumar Garg, Anuradha Sharma, Gopal Krishana Bohra
All the authors have approved the final version of the manuscript to be published. All authors meet the ICMJE authorship criteria.
Acknowledgements
None.
References
- 1.Brown-Elliott B.A., Brown J.M., Conville P.S., Wallace R.J., Jr. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19(April(2)):259–282. doi: 10.1128/CMR.19.2.259-282.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valdezate S., Garrido N., Carrasco G., Medina-Pascual M.J., Villalón P., Navarro A.M. Epidemiology and susceptibility to antimicrobial agents of the main Nocardia species in Spain. J Antimicrob Chemother. 2017;1(March(3)):754–761. doi: 10.1093/jac/dkw489. 72. [DOI] [PubMed] [Google Scholar]
- 3.Singh I., West F.M., Sanders A., Hartman B., Zappetti D. Pulmonary Nocardiosis in the Immunocompetent Host: Case Series. Case Rep Pulmonol. 2015;2015 doi: 10.1155/2015/314831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreno-González G., Ricart de Mesones A., Tazi-Mezalek R., Marron-Moya M.T., Rosell A., Mañez R. Invasive Pulmonary Aspergillosis with Disseminated Infection in Immunocompetent Patient. Can Respir J. 2016;2016 doi: 10.1155/2016/7984032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohara Y., Ito T., Ito M., Yamashita K., Toyokuni S. Acute fulminant invasive pulmonary aspergillosis in an immunocompetent host: An autopsy case report. Med Mycol Case Rep. 2018;9(Feburary(20)):39–42. doi: 10.1016/j.mmcr.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Misra D.P., Parida J.R., Chowdhury A.C., Agarwal V. Pulmonary co-infection with Nocardia and Aspergillus in a patient with adult-onset Still’s disease receiving steroids and tacrolimus. BMJ Case Rep. 2014;14(November(2014)) doi: 10.1136/bcr-2014-207335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alonso-Sierra M., Calvo M., González-Lama Y. Nocardia and Aspergillus Coinfection in a Patient with Ulcerative Colitis during Golimumab Therapy. J Crohns Colitis. 2016;10(September(9)):1127–1128. doi: 10.1093/ecco-jcc/jjw064. [DOI] [PubMed] [Google Scholar]
- 8.Balasubramanian V., Singh A., Gupta P., Prasad R. A rare coexistence of pulmonary nocardiosis and aspergillosis in patient of COPD. Egyptian J of Chest Dis Tuberc. 2016;65(2):405–409. [Google Scholar]
- 9.Mohan A., Sharma S.K., Arora V.K., Sharma S., Prakash J. Concurrent pulmonary Aspergillosis and Nocardiosis in an old tubercular cavity masquerading as malignancy in an immunocompetent individual. Respir. Med. CME. 2008;1:231–234. [Google Scholar]
- 10.Ader F., Nseir S., Le Berre R., Leroy S., Tillie-Leblond I., Marquette C.H. Invasive pulmonary aspergillosis in chronic obstructive pulmonary disease: an emerging fungal pathogen. Clin Microbiol Infect. 2005;11(June(6)):427–429. doi: 10.1111/j.1469-0691.2005.01143.x. [DOI] [PubMed] [Google Scholar]
- 11.Rivie`re F., Billhot M., Soler C., Vaylet F., Margery J. Pulmonary nocardiosis in immunocompetent patients: can COPD be the only risk factor? Eur Respir. Rev. 2011;1(September(121)):210–212. doi: 10.1183/09059180.00002211. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez Tomás R., Menéndez Villanueva R., Reyes Calzada S., Santos Durantez M., Vallés Tarazona J.M., Modesto Alapont M. Pulmonary nocardiosis: risk factors and outcomes. Respirology. 2007;12(May(3)):394–400. doi: 10.1111/j.1440-1843.2007.01078.x. [DOI] [PubMed] [Google Scholar]
- 13.Georgiadou S.P., Sipsas N.V., Marom E.M., Kontoyiannis D.P. The diagnostic value of halo and reversed halo signs for invasive mold infections in compromised hosts. Clin Infect Dis. 2011;52(May(9)):1144–1155. doi: 10.1093/cid/cir122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cercenado E., Marín M., Sánchez-Martínez M., Cuevas O., Martínez-Alarcón J., Bouza E. In vitro activities of tigecycline and eight other antimicrobials against different Nocardia species identified by molecular methods. Antimicrob Agents Chemother. 2007;51(March(3)):1102–1104. doi: 10.1128/AAC.01102-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson J.W. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87(April(4)):403–407. doi: 10.1016/j.mayocp.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


